Abstract
Objectives
To correlate immune responses following a two-dose regimen of mRNA anti-SARS-CoV-2 vaccines in patients with rheumatoid arthritis (RA) to the development of a potent neutralising antiviral activity.
Methods
The RECOVER study was a prospective, monocentric study including patients with RA and healthy controls (HCs). Assessments were performed before, and 3, 6, 12 and 24 weeks, after the first vaccine dose, respectively, and included IgG, IgA and IgM responses (against receptor binding domain, S1, S2, N), IFN-γ ELISpots as well as neutralisation assays.
Results
In patients with RA, IgG responses developed slower with lower peak titres compared with HC. Potent neutralising activity assessed by a SARS-CoV-2 pseudovirus neutralisation assay after 12 weeks was observed in all 21 HCs, and in 60.3% of 73 patients with RA. A significant correlation between peak anti-S IgG levels 2 weeks after the second vaccine dose and potent neutralising activity against SARS-CoV-2 was observed at weeks 12 and 24. The analysis of IgG, IgA and IgM isotype responses to different viral proteins demonstrated a delay in IgG but not in IgA and IgM responses. T cell responses were comparable in HC and patients with RA but declined earlier in patients with RA.
Conclusion
In patients with RA, vaccine-induced IgG antibody levels were diminished, while IgA and IgM responses persisted, indicating a delayed isotype switch. Anti-S IgG levels 2 weeks after the second vaccine dose correlate with the development of a potent neutralising activity after 12 and 24 weeks and may allow to identify patients who might benefit from additional vaccine doses or prophylactic regimen.
Keywords: Rheumatoid Arthritis, COVID-19, Vaccination
What is already known on this topic.
mRNA SARS-CoV-2 vaccine-induced immune and antiviral responses are impaired in patients with rheumatoid arthritis (RA).
There is uncertainty whether and when monitoring of anti-S responses might guide individual patient management.
What this study adds
Longitudinal data up to 24 weeks demonstrate impaired humoral immune responses in patients with RA versus healthy controls while T cell responses are largely maintained.
Immunoglobulin isotype kinetics demonstrate that partial versus full seroconversion occurs more frequently in patients with RA compared with healthy controls suggesting a delayed isotype switch.
A significant proportion of patients with RA do not develop potent neutralising antibody responses.
Postvaccination anti-S responses predict the subsequent development of potent neutralising antibody activity up to 24 weeks.
How this study might affect research, practice or policy
The determination of anti-S titres early after the second vaccine dose allows to identify patients with a low likelihood of a protective humoral immune response.
Optimisation of SARS-CoV-2 vaccine strategies, depending on the DMARD regimen, is needed and may include pausing DMARD therapy, application of an early third vaccine dose or the use of prophylactic antibodies.
Introduction
The development of safe and effective COVID-19 vaccines has been a milestone in controlling the pandemic.1 2 While longitudinal studies in SARS-CoV-2 convalescent and vaccinated individuals have revealed the dynamics of humoral and cellular immune responses in healthy individuals, data on long-term vaccine-induced immune responses in patients with rheumatic diseases are still scarce.3–5 Two doses of mRNA-based anti-SARS-CoV-2 vaccines result in durable SARS-CoV-2 antibody binding that correlate to potent neutralisation responses in healthy volunteers.6 7 Most patients with rheumatic diseases seroconvert following mRNA vaccination but anti-S titres may develop with a delayed kinetic and lower magnitude compared with healthy controls (HCs) depending on the immunomodulatory therapies used.8–10
Lower anti-S levels in patients on immunomodulatory therapies may translate into a lower level of protection. The observation that vaccine effectiveness for prevention of symptomatic COVID-19 in immunosuppressed patients is significantly lower compared with immunocompetent controls supports this hypothesis.11 12 Immunomodulatory therapies may reduce or prevent antibody responses following SARS-CoV-2 vaccination. In patients with rheumatoid arthritis (RA), the use of JAK inhibitors (tsDMARDs), abatacept or rituximab has been associated with reduced anti-S responses.13–16 Reports on decreasing antibody titres over time and the emergence of variants of concern contribute to the uncertainty about the potency and durability of the vaccine induced protection in immunocompromised patients.17–19 Efforts seem warranted to optimise vaccine strategies in immunocompromised patients.
The American College of Rheumatology (ACR) and the updated European League against Rheumatism recommendations support a third mRNA vaccine dose in all patients with autoimmune diseases.20 21 Although currently not recommended in daily practice, the determination of vaccine-induced antibody responses may help to guide management in patients who are considered to be at risk for an impaired immune response.
The aim of our study was to assess vaccine-induced immune responses following a two-dose regimen of mRNA-based anti-SARS-CoV-2 vaccines over 24 weeks and to analyse the development of neutralising activity against SARS-CoV-2 in patients with RA on DMARD therapies compared with HCs.
Methods
Study participants
The RECOVER trial (Rheumatoid COVID-19 Vaccine Immune Response) is a non-randomised, prospective, observational, monocentric control group trial. The vaccination itself was not part of the study and performed according to Swiss federal regulations. Consecutive patients with RA on DMARD therapy willing to undergo vaccination were included in our trial. HC mainly consisted of healthcare workers. All participants provided written informed consent before enrolment.
Blood sampling was performed at six study visits: baseline (T0; before first vaccine dose), 3 (T1), 6 (T2, 2 weeks after the second vaccination), 12 (T3) and 24 (T4) weeks after the first vaccine dose. Demographics, medication and clinical data were recorded at all time points.
Serological testing and neutralisation assays
Antibodies to the receptor binding domain (RBD) within the SARS-CoV-2 S1 protein (S) were measured with the Roche Elecsys Anti-SARS-CoV-2-S assay (range 0.4–2500 U/mL, cut-off >0.8 U/mL) and to SARS-CoV-2 nucleocapsid (N) to exclude patients with previously unnoticed COVID-19 infection.
The multiplex bead assay ABCORA was used to measure IgG, IgA and IgM reactivity to four SARS-CoV-2 antigens (RBD, spike glycoprotein subunits S1 and S2, and nucleocapsid protein (N)) as described elsewhere.22 In brief, EDTA plasma is diluted (1/100) and incubated with antigen-loaded MagPlex beads (Luminex Corporation, Austin, Texas, USA). To detect bound immunoglobulins (Ig), secondary phycoerythrin-labelled detector antibodies for IgG, IgA or IgM were used and median fluorescence intensity of the single dilution measurements was corrected for background binding (fold over empty beads). To distinguish SARS-CoV-2-specific from cross-reactive antibodies, signal over cut-off (SOC) values were defined for each of the 12 SARS-CoV-2 antigen and Ig class combinations. Results are presented as the sum of S1 SOC values of IgG, IgA and IgM.
Antibody-mediated neutralisation at week 12 was assessed against Wuhan-Hu-1 pseudovirus (HIV-based) as described.23 Particles of the env-inactivated HIV-1 reporter construct pHIV-1NL4-3 ΔEnv-NanoLuc (pHIV-1Nanoluc; provided by P. Bieniasz, Rockefeller University, New York, USA) were pseudotyped with codon optimised, truncated SARS-CoV-2 spike expression plasmid (P_CoV2_Wuhan) by co-expression in 293 T cells. Infection of human ACE2 stable HeLa cells (Biogene, Shirley, New York, USA) with SARS-CoV-2 pseudoparticles was detected by measuring the NanoLuc luciferase reporter activity in cell lysates 48 hours post infection using the Nano-Glo Luciferase Assay System (Promega, Fitchburg, Wisconsin, USA) on a Perkin Elmer EnVision reader. Neutralisation tests of diluted plasma were performed and neutralisation titres causing 50% reduction in viral infectivity (NT50) compared with controls without plasma were calculated using GraphPad Prism with constraints (bottom=0, top=100). If 50% inhibition was not achieved at the lowest plasma dilution of 1/100, a ‘≤100’ value was recorded. All measurements were conducted as single measurements.
T cell responses
IFN-γ ELISpots detecting SARS-CoV-2 Spike glycoprotein-reactive T cells after in vitro stimulation with a spike glycoprotein peptide mix were performed as described elsewhere (detailed in online supplemental file 1).24
rmdopen-2022-002575supp001.pdf (8.1MB, pdf)
Statistical analysis
Baseline demographics and laboratory parameters were analysed using descriptive statistics. Comparisons of serological response between patients and controls and between different treatment groups were performed by the Wilcoxon paired samples test and Mann-Whitney test for unpaired samples. χ2 test was applied for the comparison of frequencies. Tests were two-sided and done at the 0.05 significance level. Statistical analyses were performed in R (V.4.0.5). Figures were made using the ggplot2 package. Spearman’s correlation test was used to detect associations between continuous variables using GraphPad Prism V.9.0. Receiver operating characteristic (ROC) curve analysis was performed to assess predictability and results expressed as area under the curve (AUC). Comparisons of antigen-specific T cells were performed using GraphPad Prism V.9.0. using repeated measures two-way ANOVAs with Fisher’s LSD multiple comparison test.
Results
Cohort characteristics
Seventy-seven patients with RA on DMARD therapy and 21 HCs were enrolled. Participants with clinically suspected or confirmed previous COVID-19 infection and patients with RA treated with rituximab were excluded.
Baseline characteristics of patients and HC are summarised in table 1. Patients with RA were older than HC (mean age 64±12.5 vs 44.1±13.8 years, p<0.0001). At baseline, 22 of 77 (28.6%) patients with RA received monotherapy with conventional (cs) DMARDs, the majority of them with methotrexate (MTX) (15/22, 68.2%) with a median weekly dose of 15 mg (10–20) or leflunomide (6/22). Thirty-five of 77 (45.5%) patients with RA received biological (b)DMARDs (19 TNF inhibitors, 5 IL-6 receptor inhibitors, 10 abatacept, 1 anakinra), 14 (40%) as monotherapy. JAK inhibitors (tsDMARDs) were used in 20/77 (26%) patients, in 8 (40%) as monotherapy. Medication was continued throughout the vaccination period. Four (5.2%) patients with RA had antibodies to nucleoprotein at baseline in line with a clinically unnoticed COVID-19 infection and were excluded from the immunogenicity analyses. After 12 weeks, three patients with absent anti-S antibodies received a third vaccine dose, one of these patients had underlying RA-ILD, developed COVID-19 3 months after the third dose and died. Two patients declined further participation. Sixty-eight of the remaining 73 (93.2%) patients were followed until week 24 after vaccination.
Table 1.
Baseline characteristics, vaccination type and schedule of patients with RA and healthy controls (HCs)
| Patients with RA (n=77) |
Healthy controls (n=21) | P value | |
| Age (years), mean (±SD) | 64 (12.5) | 44.1 (13.8) | <0.0001 |
| Female sex, n (%) | 46 (59.7) | 15 (71.4) | 0.45 (NS) |
| Vaccination type/schedule | |||
| mRNA-1273, n (%) | 12 (15.6) | 0 (0) | 0.06 |
| BNT162b2, n (%) | 65 (84.4) | 21 (100) | |
| Mean interval between 1st vaccination and sampling (days±SD) | 21.4±2.3 | 21.8±2 | 0.33 (NS) |
| Mean interval between 2nd vaccination and sampling (days±SD) | 14.9±2.5 | 15.1±1.6 | 0.22 (NS) |
| Mean interval between 1st and 2nd vaccination (days±SD) | 34.5±4 | 32.9±5.9 | 0.15 (NS) |
| RA disease characteristics | |||
| ACPA±RF, n (%) | 48/77 (62.3) | NA | |
| ACPA+RF+, n (%) | 37/77 (48.1) | NA | |
| ACPA+, n (%) | 38/77 (49.4) | NA | |
| RF+, n (%) | 47/77 (61.0) | NA | |
| Disease activity (CDAI) at baseline | |||
| Remission (≤2.8), n (%) | 17/77 (22.1) | NA | |
| Low disease activity (2.9–10), n (%) | 40/77 (51.9) | NA | |
| Moderate disease activity (10.1–22.0), n (%) | 15/77 (19.5) | NA | |
| High disease activity (≥22.1), n (%) | 5/77 (6.5) | NA | |
| DMARD therapy | |||
| csDMARDs-mono, n (%) | 22/77 (28.6) | NA | |
| bDMARDs-mono/combo, n (%) | 35/77 (45.5) | NA | |
| bDMARDs-mono, n (%) | 14/35 (40) | NA | |
| tsDMARDs-mono/combo, n (%) | 20/77 (26) | NA | |
| tsDMARDs-mono, n (%) | 8/20 (40) | NA | |
| Prednisone, n (%) | 25/77 (32.5) | NA | |
| Mean daily dose prednisone (mg±SD) | 5.6±3.6 | NA |
Data are presented as n (%) or mean as indicated. Anti-cytokine bDMARDs and abatacept are summarised as bDMARDs. Targeted synthetic (ts)DMARDs included tofacitinib, baricitinib and upadacitinib.
RA, rheumatoid arthritis.
RA disease activity was prospectively assessed using CDAI until week 12. At baseline, 17/77 (22.1%) and 40/77 (51.9%) patients with RA were in remission or low disease activity. Significant increases in CDAI were noted after the first and second vaccination (p<0.0001) and after 12 weeks (p<0.001), and RA treatment was modified in 11/77 (14.3%) patients with RA (online supplemental figure 1).
Anti-S IgG responses to mRNA SARS-CoV-2 vaccine
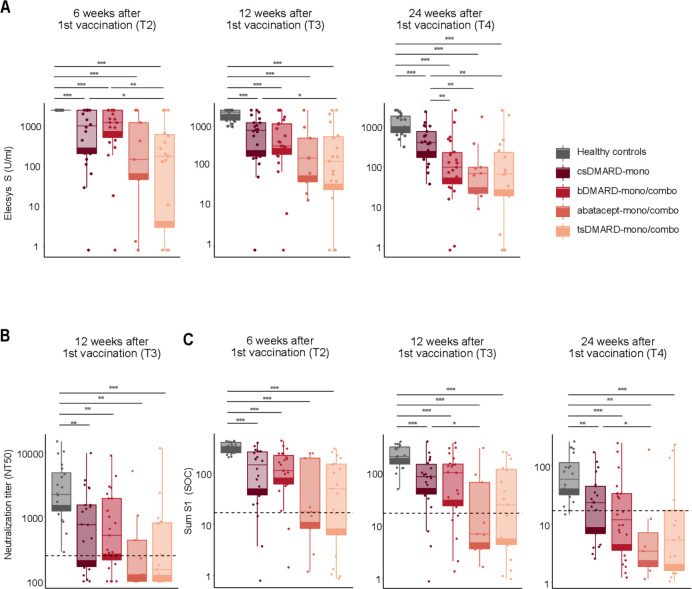
We longitudinally assessed the antibody response to SARS-CoV-2 S antigen detected by the Elecsys Anti-SARS-CoV-2 assay (anti-S) in patients with RA and HC. The majority of patients with RA (92%) seroconverted 12 weeks after the first vaccine dose (T3), nine patients did not mount a response even after two doses. Anti-S levels were significantly lower in patients with RA compared with HC at all time points after two vaccine doses (T2, T3, T4) (figure 1A, online supplemental figure 2). Anti-S levels of patients with RA treated with csDMARD-mono versus anti-cytokine bDMARDs-mono/combo were comparable at week 12. At week 24, patients receiving anti-cytokine bDMARDs-mono/combo showed significantly lower anti-S levels compared with patients on csDMARD-mono (median 94 (37–218) vs 388 (165–734) U/mL), p=0.02. Patients using anti-cytokine bDMARDs-mono had numerically higher anti-S levels (median 152 (42–507) U/mL) compared with patients on anti-cytokine bDMARDs-combo (median 70 (28–192) U/mL).
Figure 1.
Antibody kinetics and neutralizing activity is influenced by DMARD regimen. (A) Boxplots showing anti-S antibodies as determined by Elecsys Anti-SARS-CoV-2 (S) assay at 6, 12 and 24 weeks following the first vaccine dose in HC and RA patients stratified by different DMARD regimen. (B) Boxplots showing NT50 values as assessed by a pseudovirus neutralization assay of HC compared to RA patients stratified by DMARD regimen at 12 weeks after first vaccination. The dashed line corresponds to an NT50 of 250. (C) Boxplots showing sum S1 reactivity (SOC) in HC compared to RA patients stratified by different DMARD regimen at 6, 12 and 24 weeks after first vaccination. Dashed lines correspond to a sum S1 of 17.3 that represents the threshold for prediction of neutralizing activity against Wuhan-Hu-1 387 *p<0.05, **p<0.01, ***p<0.001. T1=3 weeks after 1st vaccination, T2=2 weeks after 2nd vaccination, T3=12 weeks after 1st vaccination, T4= 24 weeks after 1st vaccination
Patients treated with abatacept showed numerically lower anti-S levels at week 12 compared with patients with csDMARD-mono (median 158 (41–498) vs 782 (176–1216) U/mL), p=0.08, and significantly lower levels at week 24 (median 66 (21–94) vs 388 (165–734) U/mL), p<0.01. In patients on tsDMARDs, anti-S levels were significantly lower compared with patients on csDMARD-mono at 12 and 24 weeks (median 132 (29–542) and 64 (18–224) vs 782 (176–1216) and 388 (165–734) U/mL), p<0.05 and p<0.01, respectively.
There was no association between disease activity as monitored by CDAI or seropositivity and anti-S levels at any timepoint.
As the mean age of patients with RA was higher compared with HC, we matched patients with RA with HCs regarding age and type of vaccination. Patients with RA revealed significantly lower anti-S levels at all timepoints following vaccination whereas T cell responses did not differ (online supplemental figure 3A, B).
Neutralising activity against SARS-CoV-2
The neutralising potency of the measured antibody response is a critical correlate of protective immunity after vaccination. We measured the neutralising activity in patients with RA and HCs using a pseudovirus-neutralisation assay against Wuhan-Hu-1 at week 12. All HCs developed high neutralisation titres compared with patients with RA. 39.7% of patients with RA did not mount a potent neutralising response (NT≤250) (p=0.0003) and the absence of neutralising activity at week 12 (NT50≤100) was noted in 23.3% patients with RA. The absence of neutralising activity was noted more frequently in patients with RA on abatacept or tsDMARDs, compared with patients with RA on csDMARDs-mono or anti-cytokine bDMARDs-mono/combo (figure 1B).
Seroprofiling with the ABCORA assay allows to predict whether infected individuals develop high (NT50>250) or low neutralisation titres (NT50≤250), respectively, by the sum of S1 signal over cut-off (SOC) values for IgG, IgA, IgM (sum S1) against different viral antigens. The sum S1 threshold of 17.3 reliably predicts neutralisation activity against the vaccine strain Wuhan-Hu-1 with a specificity of 94% and a sensitivity of 67%.22 All HCs in our study exceeded the sum S1 predicting potent neutralisation at all timepoints following two vaccination doses. In contrast, patients with RA had significantly lower sum S1 levels at all timepoints (figure 1C).
Postvaccination anti-S levels predict neutralising activity after 12 and 24 weeks
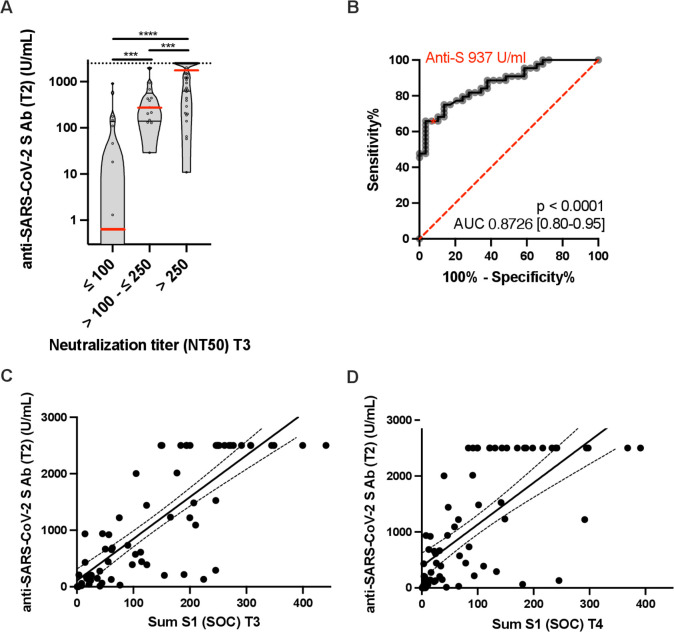
A strong correlation between anti-S antibody levels 2 weeks after the second vaccine dose and a potent neutralising activity (NT50) at week 12 was observed. Patients with RA with NT50>250 at week 12 had significantly higher anti-S levels 2 weeks after the second vaccine dose compared with patients with RA with low or absent neutralising activity (median 1769 (634–2500) vs 131 (0.4–409) and 0.65 (0–129), p<0.0001 and p=0.0001, respectively) (figure 2A). We confirmed the correlation between peak anti-S levels after the second vaccine dose (T2) and NT50 at 12 weeks (T3) by using the spearman correlation coefficient (r=0.5506) and linear regression (F=69.99), p<0.0001. A ROC curve with an AUC (AUC=0.8726, 95% CI 0.80 to 0.95), p<0.0001, revealed a decisive anti-S threshold of 937 U/mL (figure 2B). Sum S1 levels as determined by the ABCORA assay allow to predict neutralising responses by measuring isotype responses to four different viral antigens. Peak anti-S IgG antibody levels correlated to neutralising responses as predicted by ABCORA (sum S1) at week 12 (T3) (r=0.78 (CI 0.67 to 0.86), p<0.0001) and week 24 (T4) (r=0.67 (CI 0.50 to 0.79), p<0.0001) (figure 2C, D).
Figure 2.
Postvaccination anti-S IgG responses predict antiviral neutralization (NT50) at week 12. (A) The violins illustrate the kernel probability density. Red lines indicate the medians, black lines indicate 1st and 3rd IQR. Dots represent individual patients. ***p<0.001, ****p<0.0001. (B) ROC curve peak anti-S IgG at week 2 and NT50 at week 12. Performance of peak anti-S titers in discriminating RA patients with low neutralizing activity (NT50 > 250) at week 12. Anti-S 937 U/ml represents the decisive anti-S threshold value. ROC curve = receiver operating characteristic curves. Sensitivity 66%, specificity 97% (C) Spearman correlation (r = 0.78, p = p < 0.0001; Regression r2 = 0.6814, y = 7.391*x + 108.5, F = 151.8, p < 0.0001) between anti-S IgG at week 2 and sum S1 at week 12. (D) Spearman correlation (r = 0.67, p < 0.0001; Regression r2 = 0.4926, y = 7.477*x + 384.4, F = 68.94, p < 0.0001) between anti-S IgG at week 2 and sum S1 at week 24. T1=3 weeks after 1st vaccination, T2=2 weeks after 2nd vaccination, T3=12 weeks after 1st vaccination, T4= 24 weeks after 1st vaccination.
Analysis of SARS-specific IgG, IgA and IgM isotype responses
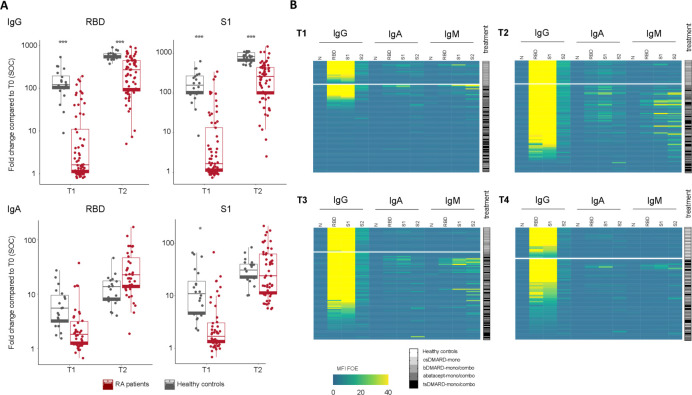
The longitudinal analysis of isotype responses against four SARS-CoV-2 antigens (RBD, S1, S2 and N) using the ABCORA assay allows to differentiate partial (only IgA and IgM responses) and full seroconversion (including IgG responses). Our analysis revealed a differential dynamic of the humoral response between patients with RA and HC (figure 3A). IgG responses to S1 and RBD that show the highest correlation with neutralising activity developed in patients with RA with a slower kinetic and lower magnitude while IgA responses tended to persist (figure 3A). IgM antibodies directed against RBD and S1 persisted longer in patients with RA compared with HCs (7.7% RBD and 12.3% S1 IgM positive vs 0% RBD and 5% S1 IgM positive in HC at week 12). Heatmaps at T1, T2 and T3 reveal the dynamics of isotype evolutions against different viral antigens in patients with RA compared with HC (figure 3B). These data suggest a delayed isotype switch in patients with RA following anti-SARS-CoV-2 vaccination that may underlie the delay in the development of strong IgG responses.
Figure 3.
Longitudinal vaccine-induced antibody responses by isotypes of vaccinated RA patients compared to healthy controls. (A) Comparison of IgA and IgG responses in RA patients capable of mounting an immune response after vaccination (n=64) and HC at visits T1 and T2. Levels of significance are calculated by unpaired t-test. *p<0.05, **p<0.01, ***p<0.001. (B) Heatmaps summarizing the measured MFI signals normalized to empty bead control (MFI FOE) that visualize IgG, IgA and IgM responses to RBD, S1, S2 and N on different DMARD regimen after the first (T1) and second vaccine dose (T2) and after 12 (T3) and 24 weeks (T4) for HC and RA patients. T1=3 weeks after 1st vaccination, T2=2 weeks after 2nd vaccination, T3=12 weeks after 1st vaccination, T4= 24 weeks after 1st vaccination *p<0.05, **p<0.01, ***p<0.001.
SARS-CoV-2 specific IFN-γ release
The number of IFN-γ-spot forming cells (s.f.c) was comparable between HC and patients with RA, with a peak 2 weeks after the second vaccine dose (T2) and decrease thereafter. In patients with RA, a more rapid decline was noted at week 24 compared with HC (online supplemental figure 4). No significant differences regarding the T cell response between patients with RA on csDMARD-mono, anti-cytokine bDMARDs-mono/combo or tsDMARDs were detected. Results are described in detail in online supplemental file 1.
Discussion
The successful development of vaccines for COVID-19 has altered the course and severity of the pandemic, but vaccine efficacy against SARS-CoV-2 variants may lag behind the spread of new variants and anti-S antibodies may wane over time. The importance of a robust humoral immune response is supported by the clinical efficacy of therapeutic and prophylactic antibodies against the spike protein and studies confirmed a reduced occurrence of COVID-19 infections in participants with higher vaccine-induced anti-S or RBD IgG antibodies correlating with higher pseudovirus neutralisation titres.25–28
Patients with rheumatic diseases who receive immunomodulatory therapies may develop blunted vaccine-induced immune responses compared with the healthy population, thus likely reflecting a lower level of protection in these patients.29 Breakthrough SARS-CoV-2 infections in fully vaccinated patients with rheumatic diseases resulting in severe disease courses have been reported.30 Optimising vaccine strategies is therefore of interest in this patient population.
The ACR has recommended a third mRNA vaccine dose to patients with rheumatic diseases, that may be applied at least 28 days after the second dose of the primary vaccination series.20 The optimal timepoint and threshold levels of vaccine-induced immune responses have not yet been determined. The determination of anti-S antibody levels given their correlation to neutralisation therefore represents an attractive option in immunocompromised patients to monitor vaccine effectiveness and guide clinical management.31 32
In this prospective, longitudinal study, patients with RA showed significantly lower anti-S titres at all timepoints than HC. While patients with RA using anti-cytokine bDMARDs in monotherapy showed anti-S levels comparable to those of patients on csDMARD monotherapy after 12 and 24 weeks, we observed lower anti-S levels in patients on tsDMARDs, abatacept or anti-cytokine bDMARDs in combination with csDMARDs as reported by others.33–35 Potent neutralising responses as determined by an HIV-based pseudovirus system (NT50) and the multiplex ABCORA assay were detected in all HC but were absent in a large proportion of patients with RA. Of note, we observed a significant correlation between anti-S responses 2 weeks after the second vaccine dose and the development of potent neutralisation activity as assessed by NT50 after 12 weeks and ABCORA after 12 and 24 weeks. The early determination of anti-S titres 2 weeks after the second vaccine dose may thus allow to identify patients with RA with a higher likelihood of low or absent neutralising antibodies thereafter who would benefit from an earlier third vaccine dose.
Patients with RA demonstrated significantly impaired neutralising responses as assessed by ABCORA at all timepoints following a standard vaccination regimen. Of note, IgG responses to spike antigens show the highest correlation with neutralisation activity.22 The development of a robust IgG response to S1, S2 and RBD was significantly delayed in patients with RA compared with HC. Our findings suggest a delayed isotype switch in vaccine-induced antibody development in patients with RA that might, at least in part, explain these findings. Further studies are needed to corroborate whether the delayed development of a vaccine-induced IgG response is mitigated by the use of certain DMARDs which were continued throughout the vaccination period in our study or whether temporary interruption of DMARDs may promote the rapid development of IgG responses.36 37
Limitations of our study include the observational design, small patient numbers within subgroups on distinct therapies and the inability to differentiate between vaccine regimens using mRNA-1273 and BNT162b2 in patients with RA. The younger age of the HCs (mainly healthcare workers) is a confounding factor that could not be corrected as priorisation for vaccination was performed according to federal regulations.
In conclusion, determination of vaccine-induced anti-S responses 2 weeks after the second dose of an mRNA-based vaccine provides an opportunity to identify patients with an inadequate immune response to SARS-CoV-2 vaccination who are unlikely to develop neutralising activity 3 and 6 months later. The optimal strategies in these patients still need to be explored and may include a timely additional vaccine dose, an interruption of DMARD therapy or the use of pre-exposure monoclonal antibodies.
Acknowledgments
We are grateful to all volunteers and patients for their participation, and we thank all involved study coordinators and nurses for their contribution. We also thank all involved technicians and lab personnel for their work.
Footnotes
KS, IAA, NBP, JvK and AR-R contributed equally.
Contributors: All authors have made substantial contributions to the conception, design of the study, or the acquisition, analysis and interpretation of the data, were involved in drafting the manuscript or critical revision. All authors approved the final version of the manuscript. The authors assume responsibility for the accuracy of the data and analyses and the fidelity of the trial.
Funding: The study was supported by internal institutional grants of the investigators including a Pandemiefond UZH foundation (AT) and a Swiss national Science Foundation Grant (NBP).
Competing interests: ARR received consulting fees from Abbvie, Gilead, Lilly, BMS and Sanofi, honoraria from Abbvie, Pfizer, Sanofi, UCB, BMS, Lilly, Gilead and Roche, payment for expert testimony from Abbvie and Gilead, support for travel or meeting attendance from Sanofi, Roche and Abbvie and compensation for participation on a Data Safety Monitoring Board from R Pharm, outside the submitted work. KS reports support for travel or meeting attendance from Abbvie. JvK reports consulting fees from Abbvie, BMS, Pfizer and Sanofi, honoraria from Lilly and support for travel or meeting attendance from Pfizer outside the submitted work. AT received a research grant from the Gilead foundation, and consulting fees from Roche and Neuroimmun. All other authors declare no competing interests.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study involves human participants. The study was approved by the Cantonal Ethical Committee of St. Gallen, Switzerland (BASEC 2021-00156). Participants gave informed consent to participate in the study before taking part.
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–15. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA Vaccine against SARS-CoV-2 - Preliminary Report. N Engl J Med 2020;383:1920–31. 10.1056/NEJMoa2022483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haberman RH, Um S, Axelrad JE, et al. Methotrexate and TNF inhibitors affect long-term immunogenicity to COVID-19 vaccination in patients with immune-mediated inflammatory disease. Lancet Rheumatol 2022;4:e384–7. 10.1016/S2665-9913(22)00069-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahil SK, Bechman K, Raharja A, et al. Humoral and cellular immunogenicity to a second dose of COVID-19 vaccine BNT162b2 in people receiving methotrexate or targeted immunosuppression: a longitudinal cohort study. Lancet Rheumatol 2022;4:e42–52. 10.1016/S2665-9913(21)00333-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med 2021;384:80–2. 10.1056/NEJMc2032195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doria-Rose N, Suthar MS, Makowski M, et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N Engl J Med 2021;384:2259–61. 10.1056/NEJMc2103916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saleem B, Ross RL, Bissell L-A, et al. Effectiveness of SARS-CoV-2 vaccination in patients with rheumatoid arthritis (rA) on DMARDs: as determined by antibody and T cell responses. RMD Open 2022;8:e002050. 10.1136/rmdopen-2021-002050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 2021;80:1330–8. 10.1136/annrheumdis-2021-220647 [DOI] [PubMed] [Google Scholar]
- 9.Wieske L, van Dam KPJ, Steenhuis M, et al. Humoral responses after second and third SARS-CoV-2 vaccination in patients with immune-mediated inflammatory disorders on immunosuppressants: a cohort study. Lancet Rheumatol 2022;4:e338–50. 10.1016/S2665-9913(22)00034-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun J, Zheng Q, Madhira V, et al. Association between immune dysfunction and COVID-19 breakthrough infection after SARS-CoV-2 vaccination in the US. JAMA Intern Med 2022;182:153–62. 10.1001/jamainternmed.2021.7024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brosh-Nissimov T, Orenbuch-Harroch E, Chowers M, et al. BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clin Microbiol Infect 2021;27:1652–7. 10.1016/j.cmi.2021.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deepak P, Kim W, Paley MA, et al. Effect of Immunosuppression on the Immunogenicity of mRNA Vaccines to SARS-CoV-2 : A Prospective Cohort Study. Ann Intern Med 2021;174:1572–85. 10.7326/M21-1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mrak D, Tobudic S, Koblischke M, et al. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Ann Rheum Dis 2021;80:1345–50. 10.1136/annrheumdis-2021-220781 [DOI] [PubMed] [Google Scholar]
- 14.Seror R, Camus M, Salmon J-H, et al. Do JAK inhibitors affect immune response to COVID-19 vaccination? data from the MAJIK-SFR registry. Lancet Rheumatol 2022;4:e8–11. 10.1016/S2665-9913(21)00314-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubbert-Roth A, Vuilleumier N, Ludewig B, et al. Anti-SARS-CoV-2 mRNA vaccine in patients with rheumatoid arthritis. Lancet Rheumatol 2021;3:e470–2. 10.1016/S2665-9913(21)00186-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shrotri M, Navaratnam AMD, Nguyen V, et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet 2021;398:385–7. 10.1016/S0140-6736(21)01642-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med 2021;385:e85. 10.1056/NEJMoa2114228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Liu J, Xia H, et al. BNT162b2-elicited neutralization against new SARS-CoV-2 spike variants. N Engl J Med 2021;385:472–4. 10.1056/NEJMc2106083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curtis JR, Johnson SR, Anthony DD, et al. American College of rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 3. Arthritis Rheumatol 2021;73:e60–75. 10.1002/art.41928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landewé RBM, Kroon FPB, Alunno A, et al. EULAR recommendations for the management and vaccination of people with rheumatic and musculoskeletal diseases in the context of SARS-CoV-2: the November 2021 update. Ann Rheum Dis 2022;0:annrheumdis-2021-222006–-2012. 10.1136/annrheumdis-2021-222006 [DOI] [PubMed] [Google Scholar]
- 21.Abela IA, Pasin C, Schwarzmüller M, et al. Multifactorial seroprofiling dissects the contribution of pre-existing human coronaviruses responses to SARS-CoV-2 immunity. Nat Commun 2021;12:6703. 10.1038/s41467-021-27040-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt F, Weisblum Y, Muecksch F, et al. Measuring SARS-CoV-2 neutralizing antibody activity using pseudotyped and chimeric viruses. J Exp Med 2020;217:e20201181. 10.1084/jem.20201181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lledó A, Retuerto M, Almendro-Vázquez P, et al. SARS-CoV-2-specific T-cell responses after COVID-19 recovery in patients with rheumatic diseases on immunosuppressive therapy. Semin Arthritis Rheum 2021;51:1258–62. 10.1016/j.semarthrit.2021.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abani O, Abbas A, Abbas F, et al. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (recovery): a randomised, controlled, open-label, platform trial. Lancet 2022;399:665–76. 10.1016/S0140-6736(22)00163-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levin MJ, Ustianowski A, De Wit S, et al. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for prevention of Covid-19. N Engl J Med 2022;386:2188–200. 10.1056/NEJMoa2116620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021;27:1205–11. 10.1038/s41591-021-01377-8 [DOI] [PubMed] [Google Scholar]
- 27.Cromer D, Steain M, Reynaldi A, et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe 2022;3:e52–61. 10.1016/S2666-5247(21)00267-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.AHJ K, Immunosuppression SJA. And SARS-CoV-2 breakthrough infections. Lancet Rheumatol 2022;4:e379–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Widdifield J, Kwong JC, Chen S, et al. Vaccine effectiveness against SARS-CoV-2 infection and severe outcomes among individuals with immune-mediated inflammatory diseases tested between March 1 and nov 22, 2021, in Ontario, Canada: a population-based analysis. Lancet Rheumatol 2022;4:e430–40. 10.1016/S2665-9913(22)00096-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goulenok T, Delaval L, Delory N, et al. Pre-Exposure anti-SARS-CoV-2 monoclonal antibodies in severely immunocompromised patients with immune-mediated inflammatory diseases. Lancet Rheumatol 2022;4:e458–61. 10.1016/S2665-9913(22)00099-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albach FN, Burmester GR, Biesen R. Successful BNT162b2 booster vaccinations in a patient with rheumatoid arthritis and initially negative antibody response. Ann Rheum Dis 2021;80:1361–2. 10.1136/annrheumdis-2021-220834 [DOI] [PubMed] [Google Scholar]
- 32.Chen RE, Gorman MJ, Zhu DY, et al. Reduced antibody activity against SARS-CoV-2 B.1.617.2 delta virus in serum of mRNA-vaccinated individuals receiving tumor necrosis factor-α inhibitors. Med 2021;2:1327–41. 10.1016/j.medj.2021.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geisen UM, Sümbül M, Tran F, et al. Humoral protection to SARS-CoV2 declines faster in patients on TNF alpha blocking therapies. RMD Open 2021;7:e002008. 10.1136/rmdopen-2021-002008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jyssum I, Kared H, Tran TT, et al. Humoral and cellular immune responses to two and three doses of SARS-CoV-2 vaccines in rituximab-treated patients with rheumatoid arthritis: a prospective, cohort study. Lancet Rheumatol 2022;4:e177–87. 10.1016/S2665-9913(21)00394-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arumahandi de Silva AN, Frommert LM, Albach FN, et al. Pausing methotrexate improves immunogenicity of COVID-19 vaccination in elderly patients with rheumatic diseases. Ann Rheum Dis 2022;81:881–8. 10.1136/annrheumdis-2021-221876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abhishek A, Boyton RJ, Peckham N, et al. Effect of a 2-week interruption in methotrexate treatment versus continued treatment on COVID-19 booster vaccine immunity in adults with inflammatory conditions (VROOM study): a randomised, open label, superiority trial. Lancet Respir Med 2022;10:840–50. 10.1016/S2213-2600(22)00186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frey S, Chiang TP-Y, Connolly CM, et al. Antibody durability 6 months after two doses of SARS-CoV-2 mRNA vaccines in patients with rheumatic and musculoskeletal disease. Lancet Rheumatol 2022;4:e241–3. 10.1016/S2665-9913(21)00417-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2022-002575supp001.pdf (8.1MB, pdf)
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.