Abstract
Background
Regenerative therapy using platelet-rich plasma (PRP), a rich source of growth factors, has become popular in orthopedic sports medicine. Elite athletes prefer PRP therapy for their injured muscles and tendons primarily to avoid the possible risks of surgical treatment. However, the clinical effectiveness of PRP therapy in elite athletes compared to that in non-athletes remains unknown. Therefore, to investigate the effectiveness of PRP therapy in professional athletes (pro-athletes), we focused on the quality of PRP preparations and compared the levels of bioactive molecules between pro-athletes and non-athletes.
Methods
PRP was prepared from healthy, non-smoking male professional soccer players (pro-athletes) (n = 22) and non-athletes (VEGF: n = 34, others: n = 38). The levels of TGFβ1, PDGF-BB, VEGF, and PF4 were determined using ELISA kits. Polyphosphate was probed with 4’,6-diamidino-2-phenylindole and monitored using a fluorometer. The body composition of the donors was determined using a bathroom weighing scale.
Results
The levels of TGFβ1 and VEGF were significantly lower in pro-athletes than in non-athletes, whereas PF4 levels were significantly higher in pro-athletes. No significant difference was found in PDGF-BB levels between these groups. Biomolecule levels were not correlated with polyphosphate levels.
Conclusion
TGFβ1, VEGF, and PDGF-BB levels in pro-athletes were not higher than those in non-athletes. These findings suggest that growth factor levels in PRP may not be a predominant determinant of the clinical effectiveness of PRP therapy in pro-athletes. Increased PF4 levels in pro-athletes suggest an immunological function of PRP that may positively influence tissue regeneration.
Keywords: Platelet-rich plasma, Athlete, Growth factors, Polyphosphate
Introduction
Platelet-rich plasma (PRP) was first reported as a promising tool for regenerative therapy by Marx in the late 1990s [1]. PRP therapy is based on the process of wound healing that involves the release of growth factors from activated, aggregated platelets [2]. It is hypothesized that the efficacy of PRP is due to the amount of growth factors in the individual PRP preparations. Several studies have supported this hypothesis by demonstrating a correlation between the concentrated levels of growth factors, such as platelet-derived growth factor (PDGF) and transforming growth factor β1 (TGFβ1) and platelet counts [2–5]. To date, however, an increasing number of systematic reviews and meta-analyses have reported that the positive effects of PRP application are not only limited to tissue regeneration but also provide symptomatic relief in the treatment of several orthopedic indications, such as rotator cuff tears [6–9], chronic lumber pain, long bone fracture [10, 11], knee osteoarthritis [12], lateral epicondylitis [13], and tendon and ligament [14]. However, a non-negligible number of review articles has expressed skepticism through their meta-analyses of similar orthopedic indications and suggested further randomized clinical trials with qualified PRP preparations in similar clinical indications [15–25]. Such a controversial understanding is due to the quality of individual PRP preparations as well as the condition of individual recipients.
PRP therapy is widely accepted among elite athletes and is the primary choice of medical treatment. The main reason behind this trend is that pro-athletes hope to achieve faster recovery by avoiding surgical risks. Faster recovery could be attributed to early detection and treatment by dedicated medical teams; however, the influence of athlete-specific physical conditions cannot be ruled out by the current understanding. For example, well-trained muscles and superior cardiopulmonary function could contribute to the higher regenerative potential of elite athletes than that of non-athletes.
Athlete-specific physical conditions, i.e., higher physical activity, can be summarized by their higher muscular strength, energy metabolism, motor reflexes, and cardiorespiratory endurance [26, 27]. Interestingly, a recent study indicated that skeletal muscle cells that have had circulating platelets, are known to possess higher energy-metabolic activity than other major nucleated cell types [28]. In this case, because their platelets have higher potential, PRP preparations derived from elite athletes may have higher therapeutic efficacy than those derived from non-athletes. Although reliable cohort studies have not yet been reported, we, like many other sports doctors, have experienced that PRP therapy seems more effective in elite athletes than in non-athletes.
As Marx mentioned in his early publications [29], there is no doubt that the major factors contributing to tissue regeneration are the growth factors stored in platelets. However, we believe that several other factors modulate the therapeutic potential of PRP through potentiation and suppression and are present in individual PRP preparations [30]. The candidate factor that we have recently paid attention to is polyphosphate (polyP) [31–37]. PolyP is an ancient, but still mysterious, biopolymer that is produced in the mitochondria in the extension of the ATP production line [33, 34, 38] and stored in the dense granules of platelets. Upon platelet activation, polyP is released alongside growth factors, and this impacts the immunological functions of platelets in addition to hemostatic function [32]. Thus, polyP could be recognized as a potent bioactive factor as well as a biomarker of platelet energy metabolism, which is the quality of functional platelets. When platelet mitochondria are damaged or dysfunctional, it is speculated that platelets cannot control their response to external stimuli [39, 40]. In the case of knee osteoarthritis, mutations in mitochondrial DNA were proposed as contributors to the risk of progression [41, 42].
To provide evidence for PRP therapy efficacy in elite athletes and to further investigate the possible involvement of polyP in PRP therapy, the levels of bioactive factors (TGFβ1, PDGF-BB, and VEGF) and their correlations with polyP levels in PRP preparations from professional male athletes and male non-athletes of the same age group were compared.
Materials and methods
Study design
A cross-sectional study was performed in two independent groups of healthy male Japanese adults (age:19–36 years): the first group (non-athletes: control) comprised ordinary healthy adults (VEGF: n = 34, mean age = 26.0 ± 4.9; others: n = 38, mean age = 26.6 ± 5.1), whereas the second group (pro-athletes: n = 22; mean age = 26.2 ± 4.7) comprised professional soccer players who played in a local team, Albirex Niigata, belonging to the domestic professional soccer league (J. League: https://www.jleague.co/). In the 2022 season, Albirex Niigata won the J2 championship and will automatically be promoted to the J1 league, the top league of the three hierarchized leagues, the next season after six seasons in the J2 league.
The inclusion criteria for the control group were:
Healthy male young adults
Non-smokers
No systemic diseases regardless of medical intervention
No daily physical training
Exclusion criteria for both groups were:
Acute or chronic inflammatory conditions reflected in blood cell counts
Current or former thrombotic or platelet disorders
The indices of body composition and platelet counts of both groups are summarized in Table 1 [34].
Table 1.
Basic body composition and platelet characteristics of non-athlete and pro-athlete groups
| Index | Control (non-athlete) | Pro-athlete | P |
|---|---|---|---|
| Age | 26.6 ± 5.1 | 26.2 ± 4.7 | 0.981* |
| BMI | 23.3 ± 2.5 | 23.4 ± 1.1 | 0.375* |
| BMR | 1564.1 ± 121.1 | 1663.1 ± 96.2 | 0.00125** |
| BFP | 21.0 ± 3.7 | 21.8 ± 2.0 | 0.259** |
| MPV | 9.70 ± 0.65 | 9.72 ± 0.66 | 0.890** |
| TPC | 24.9 ± 4.3 | 23.7 ± 4.7 | 0.337** |
Control (non-athletes) (n = 38) and pro-athletes (n = 22)
*Mann–Whitney U test, **Welch’s test (Two-tailed)
BMI, body mass index; BMR, basal metabolic rate; BFP, body fat percentage; MPV, mean platelet volume; TPC, total platelet count
The study design and consent forms for all procedures (approval no. 2021–0126) were approved by the Ethics Committee for Human Participants at Niigata University (Niigata, Japan) and complied with the Helsinki Declaration of 1964 as revised in 2013.
Blood collection and preparation of platelet-rich plasma
Blood samples were collected in glass vacuum tubes containing acid-citrate-dextrose-formula A (ACD-A; Vacutainer, Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and examined after a 24 h-incubation period, as described previously [34]. From a blood collection tube (approximately 9 mL whole blood including ACD-A), 0.4 mL PRP was prepared using the double-spin method, immediately frozen, and stored at − 80 °C until use.
Pro-athlete blood samples were collected immediately after the regular season when a medical check was performed. Thus, they were free from physical stress such as hard physical training or regular games. Blood collection was done in the afternoon, approximately 2 − 4 h after lunch, and this was conducted once. For non-athletes as well, blood samples were collected in the afternoon.
Blood cell counting
The total platelet count and mean platelet volume were determined from whole blood and PRP using an automated hematology analyzer (pocHiV-diff, Sysmex Corporation, Kobe, Japan), as described previously [34].
Determination of body Composition
Prior to blood collection, the body composition of donors was determined using a bathroom weighing scale (HCS-FS03; ECLEAR, ELECOM, Osaka, Japan), which was installed with a unique MRI-based program [43] that enables a more accurate determination of an individual’s body mass index, body fat percentage, and basal metabolic rate based on measured body weight and bioelectrical impedance [34].
For body composition measurement, because of the direct measurement-based evaluation, densitometry has gained widespread use and was considered as “gold standard” until recently [44, 45]. Compared with these modalities, the measurement reliability of bioelectrical impedance analysis (BIA) is influenced by various factors, such as electrodes, operators, subjects, and the environment [46]. However, owing to its advantages of speed, non-invasiveness, inexpensiveness, and portability, BIA currently seems to be the most feasible body composition measurement technique for bedside use [47] and was suitable for our measurement conditions.
Quantification of platelet polyP levels
As previously described [32, 35], platelets fixed with ThromboFix (Thermo Fisher) were probed in Milli-Q water containing 4 μg/mL DAPI (Dojin, Kumamoto, Japan), and the fluorescence intensity was measured using a fluorometer (FC-1; Tokai Optical Co., Ltd.) with excitation and emission wavelengths of 425 and 525 nm, respectively.
Determination of growth factor and cytokine levels by ELISA
As previously described [48], the concentrations of TGF-β1, platelet-derived growth factor-BB (PDGF-BB), and platelet factor 4 (PF4) in frozen PRP were determined using human TGF-β1, PDGF-BB, and PF4 Quantikine ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA). Concentrations of vascular endothelial growth factor (VEGF) were determined using the LBIS Human VEGF ELISA Kit (FUJIFILM Wako Chemicals, Osaka, Japan).
Statistical analysis
To compare each biomolecule level between the two groups, the data are expressed as box plots in Fig. 1. Body indices are expressed as the mean ± SD in Table 1. Mann–Whitney U test or Welch’s t-test was performed to confirm statistical differences (SigmaPlot version 14.5; Systat Software, Inc., Systat Software, Inc., San Jose, CA, USA). Differences were considered statistically significant at P < 0.05. Spearman’s correlation analysis was performed to compare each correlation between the two indices, and correlation coefficients were calculated using SigmaPlot software (Fig. 2). Differences were considered statistically significant at P < 0.05.
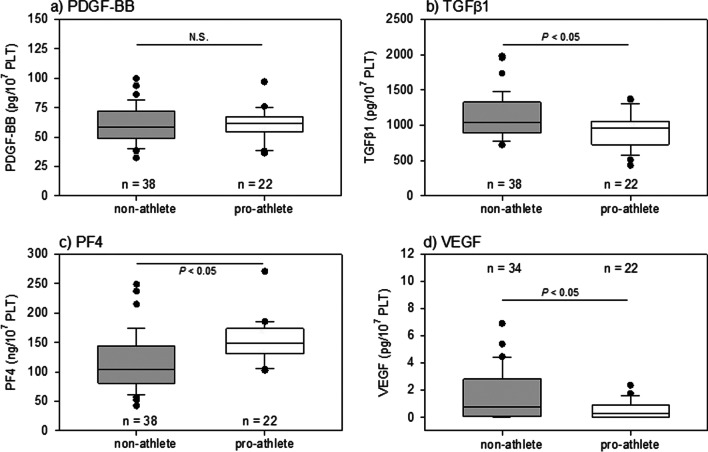
Fig. 1.
Levels of PDGF-BB, TGFβ1, PF4, and VEGF in PRP preparations from young non-athletes and pro-athletes
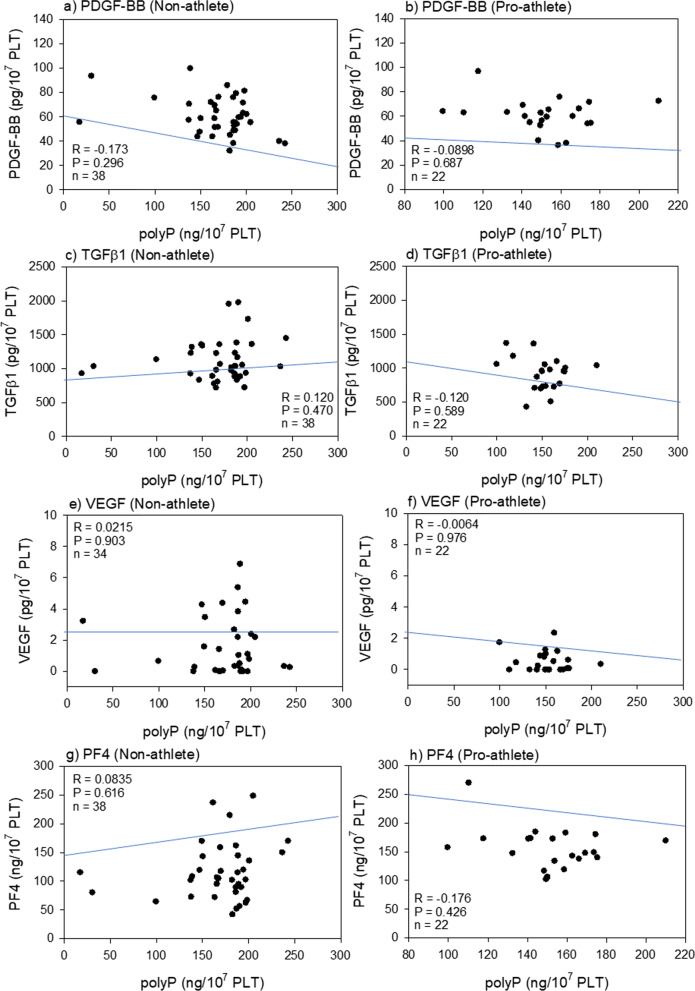
Fig. 2.
Correlations between polyP levels and PDGF-BB (a, b), TGFβ1 (c, d), VEGF (e, f) or PF4 levels (g, h) in the non-athlete (a, c, e, g) and the pro-athlete (b, d, f, h) groups. polyP, polyphosphate
Results
We compared the levels of representative bioactive factors per unit platelet count in PRP preparations derived from pro-athletes and non-athletes. As shown in Fig. 1, the levels of TGFβ1 and VEGF were higher in non-athletes than those in pro-athletes, whereas those of PF4 were higher in pro-athletes. No significant differences were found in PDGF-BB levels between these groups.
The correlation between bioactive factor levels and polyP levels were analyzed in pro-athletes and non-athletes. In our working hypothesis, because bioactive factors stored in intra-platelet granules are released along with polyP upon activation, the levels of bioactive factors are assumed to correlated with those of polyP. However, as shown in Fig. 2, no moderate or strong correlations were found between any of the bioactive factors.
Discussion
To our knowledge, this is the first report to compare the bioactive factor levels in PRP between elite athletes and non-athletes. In anuclear platelets, bioactive factors are released from intracellular storage sites, such as α-granules [49], without new synthesis from genomic DNA. Blood samples were collected during medical checks for pro-athletes and at intervals between business or classes for non-athletes; thus, the influence of heavy acute physical or mental strain could be excluded. Therefore, it is conceivable that the differences in bioactive peptide factor levels (TGFβ1, VEGF, PDGF-BB, and PF4) are due to the original storage levels or tolerance variability among individuals.
Growth factors and PF4 storage in platelet α-granules
Storage levels are primarily determined by protein synthesis from genomic DNA in megakaryocytes. As this is a fundamental aspect, we have not discussed this in detail in this study. However, platelet behavior may be influenced by general conditions such as physical trauma, strain, or injury, Platelet sensitivity to external stimuli may be higher in pro-athletes than in non-athletes as the former is more prone to develop venous thromboembolism [50, 51]. During physical stress the sympathetic nervous system enters a state of dormancy, and circulating platelets are potentially exposed to adrenergic stimuli, leading to aggregation and activation with an increased release of stored growth factors [52–54]. Consequently, stored growth factor levels decrease.
Decreased levels of TGFβ1 and VEGF confirm this scenario; however, no statistical difference in PDGF-BB was identified. This unexpected result may be due to the dimeric configuration of PDGF. PDGF-BB is an isoform of the five dimeric isoforms derived from the four gene products in the PDGF family [55]. Therefore, for a more accurate comparison, the total levels of all these isoforms need to be determined, wherein differences, similar to those in TGFβ1 and VEGF, could be detected.
In contrast, PF4 levels were significantly higher in pro-athletes than in non-athletes, despite being stored in α-granules like TGFβ1 and VEGF [56, 57]. The differences between PF4 and growth factors are the much higher levels of storage and the recycling system at the megakaryocyte stage [56, 57]. Immune function is suppressed in pro-athletes [58]; however, it is possible that this hypofunction is compensated for by the upregulated network between platelets and other immune cells through the intermediation of increased levels of PF4. As further information is not currently available in literature, it is interesting to establish this phenomenon and clarify the mechanism for a better understanding of the possible immunological functions of PRP.
Polyphosphate stored in dense granules
Polyphosphate was vigorously studied and discussed in the 1980s and 90s [36, 37], however, the role of eukaryotic polyP as a bioactive factor remains unclear. All of the suggested functions, except the hemostatic action of polyP, have not yet been clearly demonstrated [59]. Nevertheless, a recent study demonstrated that PF4 forms complexes with polyP and thereby activates platelets through an autocrine loop [60], suggesting a synchronized behavior of these factors. However, we found in the previous [34] and current studies demonstrate that PF4 and polyP behave differently. Considering another possible function of platelet polyP as an alternative energy stock [34], the “seemingly-conflicting” discrepancy between PF4 and polyP levels may be acceptable but needs to be further studied to precisely evaluate the quality of PRP.
To our knowledge, the involvement of polyP in orthopedic and sports medicine is poorly understood. However, in addition to studies implying the involvement of mutated mitochondrial DNA in exacerbated knee osteoarthritis [61], some review articles indicated a relationship between age-related mitochondrial dysfunction and sarcopenia [62, 63]. Thus, considering the correlation between platelet and muscle energetics, even though polyP is not directly involved in muscle function, it might be helpful to evaluate the status of mitochondria through their products, i.e., polyP and ATP, and DNA for the diagnosis of recipient body conditions, identification of responder patients for PRP therapy, and eventual establishment of personalized treatment algorithms in the future [61].
Limitations
This is a pilot study that requires further investigation. First, the sample size, especially of pro-athletes, was too small to perform statistical analysis. Second, blood collection from the same donors was not repeated to determine the reproducibility or diurnal variation of the data. Thus, the data may not necessarily reflect the actual status or reality. However, the protocol of blood collection from this study correlates with autologous PRP preparations used in clinical settings as these are derived from one-time collected samples. Third, for several reasons, the study adopted the BIA technique to measure body composition. However, the BIA technique is inferior to densitometry and other X-ray-based techniques in terms of accuracy and reliability. Thus, to further explore the relationship between PRP quality or recipient conditions and body composition, gold-standard techniques should be adopted to acquire more reliable data.
Prospective view and further studies
Regenerative therapy using PRP has become widespread over the last two decades. However, it is difficult to appreciate that the clinicians’ understanding has reached a standard level, as it notably varies among individuals [64]. Using a series of original and review articles [65–70], this study proposed important factors to focus on for better PRP therapy. However, to the best of our knowledge, no breakthrough advances have been achieved in this decade.
The goal of this study is to make PRP therapy more predictable by improving the diagnostic procedure to discern the responder from the non-responder to save athletes’ careers. Thus, we plan to overcome the previously discussed limitations using a step-by-step approach and combine basic research with clinical research to analyze the possible correlation between PRP quality and clinical outcome. Simultaneously, reliable cohort studies that address the longstanding question by comparing the responsiveness of pro-athletes to PRP therapy with that of non-athletes are essential.
Clinical relevance
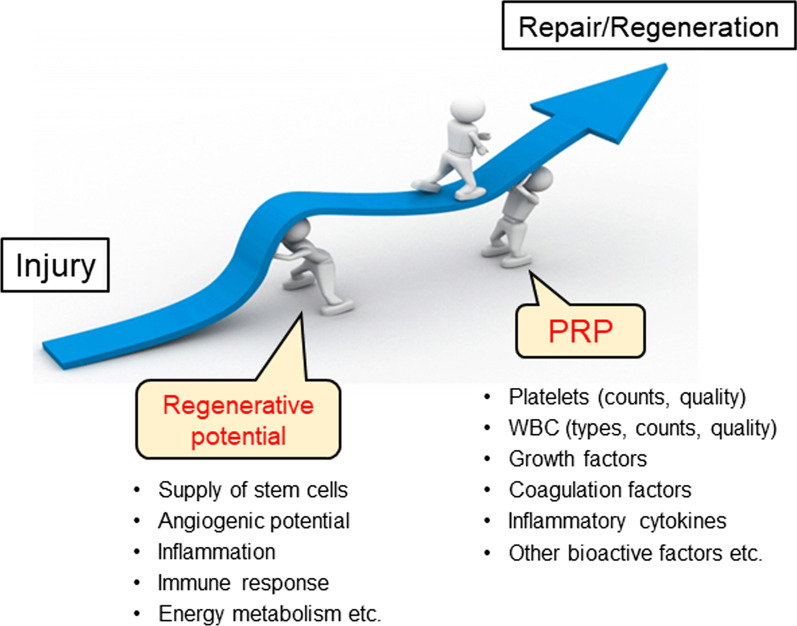
According to clinical experience, many sports doctors have a similar impression that PRP therapy enables elite athletes to return from medical leave due to injury faster than non-athletes or non-professional (amateur athletes). If this is true, unlike the widely accepted understanding of PRP therapy, growth factor levels are not the predominant determinant of clinical outcomes. As previously claimed [65], the outcome of PRP therapy can be determined by the balance between recipient conditions and PRP quality based on the adjuvant principle (Fig. 3) [65]. It is assumed that PRP therapy works successfully only when the recipient’s condition, especially the regeneration activity, is maintained at moderate to high levels. If the recipients’ regenerative activity is too low, for example, in elderly individuals, the expected outcome of PRP therapy could not be induced. However, it should be noted that if the recipients’ regenerative activity is sufficiently high, for example in younger schoolchildren, the expected outcome of PRP therapy may be hidden by their spontaneous regenerative response.
Fig. 3.
Proposed relationship, the “adjuvant principle”, between recipient’s regenerative potential and PRP therapy during tissue repair and regeneration
A similar concept has been proposed more specifically by Maffulli and his coworkers [61, 71, 72], and the clinical effectiveness of PRP is a result of its interaction with recipient tissue. The status of the recipient tissue, primarily demographic factors, immune status, metabolic diseases, and concomitant medications, could influence the effectiveness of PRP. Nevertheless, the possible relationships between these factors and PRP effectiveness have been poorly explored and remains to be established. It is important for clinicians to acquire the ability to predict which patients will respond positively to PRP. Thus, we again propose that further attention should be paid to recipients’ conditions to obtain an in-depth understanding of PRP therapy [65].
Conclusion
This pilot study suggests that growth factor levels in individual PRP preparations may not be a predominant determinant of the clinical efficacy of PRP therapy, especially in pro-athletes. Increased PF4 levels in pro-athletes suggest an immunological function of PRP that may positively influence tissue regeneration.
Acknowledgements
The authors would like to thank Mr. Suzuki K, Mr. Kamimura M, Dr. Ishiguro H, Dr. Suwabe T, and Dr. Yamamoto N for their technical assistance and participant recruitment. The authors would also like to thank Drs. Sato T, Tanifuji O, Tomiyama Y, and Kawashima H for helpful discussion. Especially, all staff members of nurses, clerks, and assistants belonging to the Department of Orthopedic Surgery in Niigata Rehabilitation Hospital, Niigata Medical Center, and Niigata University are appreciated. In addition, the authors are grateful to the players and staffs of Albirex Niigata Football Team.
Author contributions
TM, TU, and TK conceptualized the study and provided essential resources. TM, TU, SW, GO, and TK did the collection of samples, measurements, and/or the analysis of data. TM, TU, and TK wrote the manuscript with comments from all authors.
Funding
This research was funded by JSPS KAKENHI (#21K09932, #22K11496) and the Japan Agency for Medical Research and Development (AMED) (#21bk0104129h0001).
Availability of data and materials
The data are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study design and consent forms for all procedures (approval no. 2021–0126) were approved by the Ethics Committee for Human Participants at Niigata University (Niigata, Japan) and complied with the Helsinki Declaration of 1964 as revised in 2013. Participants gave informed consent to participate in the study before taking part.
Patient consent for publication
Not applicable.
Competing interests
None declared.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tomoharu Mochizuki and Takashi Ushiki contributed equally to this work.
References
- 1.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(6):638–646. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 2.Kawase T. Platelet-rich plasma and its derivatives as promising bioactive materials for regenerative medicine: basic principles and concepts underlying recent advances. Odontology. 2015;103:126–135. doi: 10.1007/s10266-015-0209-2. [DOI] [PubMed] [Google Scholar]
- 3.El-Sharkawy H, Kantarci A, Deady J, Hasturk H, Liu H, Alshahat M, Van Dyke TE. Platelet-rich plasma: growth factors and pro- and anti-inflammatory properties. J Periodontol. 2007;78(4):661–669. doi: 10.1902/jop.2007.060302. [DOI] [PubMed] [Google Scholar]
- 4.Okuda K, Kawase T, Momose M, Murata M, Saito Y, Suzuki H, Wolff LF, Yoshie H. Platelet-rich plasma contains high levels of platelet-derived growth factor and transforming growth factor-beta and modulates the proliferation of periodontally related cells in vitro. J Periodontol. 2003;74(6):849–857. doi: 10.1902/jop.2003.74.6.849. [DOI] [PubMed] [Google Scholar]
- 5.Qian Y, Han Q, Chen W, Song J, Zhao X, Ouyang Y, Yuan W, Fan C. Platelet-rich plasma derived growth factors contribute to stem cell differentiation in musculoskeletal regeneration. Front Chem. 2017;5:89. doi: 10.3389/fchem.2017.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vavken P, Sadoghi P, Palmer M, Rosso C, Mueller AM, Szoelloesy G, Valderrabano V. Platelet-rich plasma reduces retear rates after arthroscopic repair of small- and medium-sized rotator cuff tears but is not cost-effective. Am J Sports Med. 2015;43(12):3071–3076. doi: 10.1177/0363546515572777. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad Z, Ang S, Rushton N, Harvey A, Akhtar K, Dawson-Bowling S, Noorani A. Platelet-rich plasma augmentation of arthroscopic rotator cuff repair lowers retear rates and improves short-term postoperative functional outcome scores: a systematic review of meta-analyses. Arthrosc Sports Med Rehabil. 2022;4(2):e823–e833. doi: 10.1016/j.asmr.2021.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu W, Xue Q. Application of platelet-rich plasma in arthroscopic rotator cuff repair: a systematic review and meta-analysis. Orthop J Sports Med. 2021;9(7):23259671211016847. doi: 10.1177/23259671211016847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan J, Imbergamo C, Sudah S, Kirchner G, Greenberg P, Monica J, Gatt C. Platelet-rich product supplementation in rotator cuff repair reduces retear rates and improves clinical outcomes: a meta-analysis of randomized controlled trials. Arthroscopy J Arthrosc Rel Surg. 2021;37(8):2608–2624. doi: 10.1016/j.arthro.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaspiris A, Hadjimichael AC, Vasiliadis ES, Papachristou DJ, Giannoudis PV, Panagiotopoulos EC: Therapeutic efficacy and safety of osteoinductive factors and cellular therapies for long bone fractures and non-unions: a meta-analysis and systematic review. J Clin Med 2022; 11(13). [DOI] [PMC free article] [PubMed]
- 11.Li S, Xing F, Luo R, Liu M. Clinical effectiveness of platelet-rich plasma for long-bone delayed union and nonunion: a systematic review and meta-analysis. Front Med (Lausanne) 2021;8:771252. doi: 10.3389/fmed.2021.771252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell KA, Saltzman BM, Mascarenhas R, Khair MM, Verma NN, Bach BR, Jr, Cole BJ. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? a systematic review of overlapping meta-analyses. Arthrosc J Arthrosc Rel Surg. 2015;31(11):2213–2221. doi: 10.1016/j.arthro.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 13.Hardy R, Tori A, Fuchs H, Larson T, Brand J, Monroe E. To improve pain and function, platelet-rich plasma injections may be an alternative to surgery for treating lateral epicondylitis: a systematic review. Arthrosc J Arthrosc Rel Surg. 2021;37(11):3360–3367. doi: 10.1016/j.arthro.2021.04.043. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Jones IA, Park C, Vangsness CT., Jr The Efficacy of platelet-rich plasma on tendon and ligament healing: a systematic review and meta-analysis with bias assessment. Am J Sports Med. 2018;46(8):2020–2032. doi: 10.1177/0363546517743746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcazzan S, Weinstein RL, Del Fabbro M. Efficacy of platelets in bone healing: a systematic review on animal studies. Platelets. 2018;29(4):326–337. doi: 10.1080/09537104.2017.1327652. [DOI] [PubMed] [Google Scholar]
- 16.Roffi A, Di Matteo B, Krishnakumar GS, Kon E, Filardo G. Platelet-rich plasma for the treatment of bone defects: from pre-clinical rational to evidence in the clinical practice A systematic review. Int Orthop. 2017;41(2):221–237. doi: 10.1007/s00264-016-3342-9. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Xing F, Luo R, Duan X. Platelet-rich plasma for bone fracture treatment: a systematic review of current evidence in preclinical and clinical studies. Front Med (Lausanne) 2021;8:676033. doi: 10.3389/fmed.2021.676033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le ADK, Enweze L, DeBaun MR, Dragoo JL. Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med. 2018;11(4):624–634. doi: 10.1007/s12178-018-9527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le ADK, Enweze L, DeBaun MR, Dragoo JL. Platelet-rich plasma. Clin Sports Med. 2019;38(1):17–44. doi: 10.1016/j.csm.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Andersen C, Wragg NM, Shariatzadeh M, Wilson SL. The use of platelet-rich plasma (PRP) for the management of non-union fractures. Curr Osteoporos Rep. 2021;19(1):1–14. doi: 10.1007/s11914-020-00643-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Jones IA, Togashi R, Park C, Vangsness CT., Jr Use of platelet-rich plasma for the improvement of pain and function in rotator cuff tears: a systematic review and meta-analysis with bias assessment. Am J Sports Med. 2020;48(8):2028–2041. doi: 10.1177/0363546519881423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grossen AA, Lee BJ, Shi HH, Shakir HJ, Cornett EM, Kaye AD: Platelet-rich plasma injections: pharmacological and clinical considerations in pain management. Curr Pain Headache Rep 2022. [DOI] [PubMed]
- 23.Jamal MS, Hurley ET, Asad H, Asad A, Taneja T. The role of platelet rich plasma and other orthobiologics in bone healing and fracture management: a systematic review. J Clin Orthop Trauma. 2022;25:101759. doi: 10.1016/j.jcot.2021.101759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunze KN, Pakanati JJ, Vadhera AS, Polce EM, Williams BT, Parvaresh KC, Chahla J. The efficacy of platelet-rich plasma for ligament injuries: a systematic review of basic science literature with protocol quality assessment. Orthop J Sports Med. 2022;10(2):23259671211066504. doi: 10.1177/23259671211066504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Lieshout EMM, Den Hartog D. Effect of platelet-rich plasma on fracture healing. Injury. 2021;52(Suppl 2):S58–s66. doi: 10.1016/j.injury.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Bouchard C, Shephard RJ: Physical activity, fitness and health : the model and key concepts. In: Bouchard C, Shephard R, Stephens T (eds) Physical activity, fitness, and health: International proceedings and consensus statement, Human Kinetics Publishers, Champaign, IL, USA, 1994: 77–88
- 27.Vanhees L, Lefevre J, Philippaerts R, Martens M, Huygens W, Troosters T, Beunen G. How to assess physical activity? How to assess physical fitness? Eur J Cardiovasc Prev Rehabil. 2005;12(2):102–114. doi: 10.1097/01.hjr.0000161551.73095.9c. [DOI] [PubMed] [Google Scholar]
- 28.Braganza A, Corey CG, Santanasto AJ, Distefano G, Coen PM, Glynn NW, Nouraie SM, Goodpaster BH, Newman AB, Shiva S. Platelet bioenergetics correlate with muscle energetics and are altered in older adults. JCI Insight. 2019;5(13):e128248. doi: 10.1172/jci.insight.128248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Kawase T, Okuda K, Saito Y, Yoshie H. In vitro evidence that the biological effects of platelet-rich plasma on periodontal ligament cells is not mediated solely by constituent transforming-growth factor-beta or platelet-derived growth factor. J Periodontol. 2005;76(5):760–767. doi: 10.1902/jop.2005.76.5.760. [DOI] [PubMed] [Google Scholar]
- 31.Sato A, Aizawa H, Tsujino T, Isobe K, Watanabe T, Kitamura Y, Kawase T. Fluorescent cytochemical detection of polyphosphates associated with human platelets. Int J Mol Sci. 2021;22(3):1040. doi: 10.3390/ijms22031040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uematsu T, Sato A, Aizawa H, Tsujino T, Watanabe T, Isobe K, Kawabata H, Kitamura Y, Tanaka T, Kawase T. Effects of SARS-CoV-2 mRNA vaccines on platelet polyphosphate levels and inflammation: a pilot study. Biomed Rep. 2022;16(3):21. doi: 10.3892/br.2022.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ushiki T, Mochizuki T, Suzuki K, Kamimura M, Ishiguro H, Suwabe T, Kawase T. Modulation of ATP production influences inorganic polyphosphate levels in non-athletes; platelets at the resting state. Int J Mol Sci. 2022;23(19):11293. doi: 10.3390/ijms231911293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ushiki T, Mochizuki T, Suzuki K, Kamimura M, Ishiguro H, Watanabe S, Omori G, Yamamoto N, Kawase T. Platelet polyphosphate and energy metabolism in professional male athletes (soccer players): a cross-sectional pilot study. Physiol Rep. 2022;10(15):e15409. doi: 10.14814/phy2.15409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watanabe T, Kitamura Y, Aizawa H, Masuki H, Tsujino T, Sato A, Kawabata H, Isobe K, Nakata K, Kawase T. Fluorometric quantification of human platelet polyphosphate using 4',6-Diamidine-2-phenylindole dihydrochloride: applications in the Japanese population. Int J Mol Sci. 2021;22(14):7257. doi: 10.3390/ijms22147257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kornberg A, Rao NN, Ault-Riche D. Inorganic polyphosphate: a molecule of many functions. Ann Rev Biochem. 1999;68(1):89–125. doi: 10.1146/annurev.biochem.68.1.89. [DOI] [PubMed] [Google Scholar]
- 37.Kornberg A. ATP and inorganic pyro- and polyphosphate. Protein Sci. 1993;2(1):131–132. doi: 10.1002/pro.5560020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baev AY, Angelova PR, Abramov AY. Inorganic polyphosphate is produced and hydrolyzed in F0F1-ATP synthase of mammalian mitochondria. Biochem J. 2020;477(8):1515–1524. doi: 10.1042/BCJ20200042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marcus AJ. Platelet function. N Engl J Med. 1969;280(24):1330–1335. doi: 10.1056/NEJM196906122802405. [DOI] [PubMed] [Google Scholar]
- 40.Verhoeven AJ, Gorter G, Mommersteeg ME, Akkerman JW: The energetics of early platelet responses. Energy consumption during shape change and aggregation with special reference to protein phosphorylation and the polyphosphoinositide cycle. Biochem J 1985; 228(2):451–462. [DOI] [PMC free article] [PubMed]
- 41.Andia I, Maffulli N. Some patients (and some of us) respond better to some biological therapies: the as yet unsolved conundrum. J Orthop Traumatol. 2018;19(1):1. doi: 10.1186/s10195-018-0505-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernández-Moreno M, Soto-Hermida A, Vázquez-Mosquera ME, Cortés-Pereira E, Pértega S, Relaño S, Oreiro-Villar N, Fernández-López C, Blanco FJ, Rego-Pérez I. A replication study and meta-analysis of mitochondrial DNA variants in the radiographic progression of knee osteoarthritis. Rheumatology (Oxford) 2017;56(2):263–270. doi: 10.1093/rheumatology/kew394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.So R, Sasai H, Matsuo T, Tsujimoto T, Eto M, Saotome K, Tanaka K. Multiple-slice magnetic resonance imaging can detect visceral adipose tissue reduction more accurately than single-slice imaging. Eur J Clin Nutr. 2012;66(12):1351–1355. doi: 10.1038/ejcn.2012.147. [DOI] [PubMed] [Google Scholar]
- 44.Schulz LO. Methods of body composition analysis the status of the gold standard. Trends Endocrinol Metab. 1993;4(10):318–322. doi: 10.1016/1043-2760(93)90073-n. [DOI] [PubMed] [Google Scholar]
- 45.Westerterp KR. Basic concepts in nutrition: Body composition and its measurement. Eur e-J Clin Nutrit Metab. 2008;3(3):e126–e129. [Google Scholar]
- 46.Sergi G, De Rui M, Stubbs B, Veronese N, Manzato E. Measurement of lean body mass using bioelectrical impedance analysis: a consideration of the pros and cons. Aging Clin Exp Res. 2017;29(4):591–597. doi: 10.1007/s40520-016-0622-6. [DOI] [PubMed] [Google Scholar]
- 47.Moonen HPFX, Van Zanten ARH. Bioelectric impedance analysis for body composition measurement and other potential clinical applications in critical illness. Curr Opin Crit Care. 2021;27(4):344–353. doi: 10.1097/MCC.0000000000000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Masuki H, Okudera T, Watanabe T, Suzuki M, Nishiyama K, Okudera H, Nakata K, Uematsu K, Su CY, Kawase T. Growth factor and pro-inflammatory cytokine contents in PRP, plasma rich in growth factors (PRGF), advanced-platelet-rich fibrin (A-PRF) and concentrated growth factors (CGF) Int J Implant Dent. 2016;2:19. doi: 10.1186/s40729-016-0052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morrell CN, Aggrey AA, Chapman LM, Modjeski KL. Emerging roles for platelets as immune and inflammatory cells. Blood. 2014;123(18):2759–2767. doi: 10.1182/blood-2013-11-462432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hull CM, Harris JA. Cardiology patient page. Venous thromboembolism and marathon athletes. Circulation. 2013;128(25):e469–471. doi: 10.1161/CIRCULATIONAHA.113.004586. [DOI] [PubMed] [Google Scholar]
- 51.Hummel C, Geisler PR, Reynolds T, Lazenby T. Posttraumatic deep vein thrombosis in collegiate athletes: an exploration clinical case series. J Athl Train. 2018;53(5):497–502. doi: 10.4085/1062-6050-362-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tschuor C, Asmis LM, Lenzlinger PM, Tanner M, Härter L, Keel M, Stocker R, Stover JF. In vitro norepinephrine significantly activates isolated platelets from healthy volunteers and critically ill patients following severe traumatic brain injury. Crit Care. 2008;12(3):R80. doi: 10.1186/cc6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallén NH, Goodall AH, Li N, Hjemdahl P. Activation of haemostasis by exercise, mental stress and adrenaline: effects on platelet sensitivity to thrombin and thrombin generation. Clin Sci (Lond) 1999;97(1):27–35. [PubMed] [Google Scholar]
- 54.Wang J-S, Cheng L-J. Effect of strenuous, acute exercise on alpha2-adrenergic agonist-potentiated platelet activation. Arterioscler Thromb Vasc Biol. 1999;19(6):1559–1565. doi: 10.1161/01.atv.19.6.1559. [DOI] [PubMed] [Google Scholar]
- 55.Fredriksson L, Li H, Eriksson U. The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 2004;15(4):197–204. doi: 10.1016/j.cytogfr.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 56.Lambert M, Xiao L, Meng R, Marks MS, Poncz M. Platelet factor 4 (PF4) is selectively recycled during megakaryopoiesis. Blood. 2012;120(21):388. [Google Scholar]
- 57.Lambert MP, Meng R, Xiao L, Harper DC, Marks MS, Kowalska MA, Poncz M. Intramedullary megakaryocytes internalize released platelet factor 4 and store it in alpha granules. J Thrombos Haemostasis JTH. 2015;13(10):1888–1899. doi: 10.1111/jth.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nieman DC, Wentz LM. The compelling link between physical activity and the body's defense system. J Sport Health Sci. 2019;8(3):201–217. doi: 10.1016/j.jshs.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maas C, Renné T. Coagulation factor XII in thrombosis and inflammation. Blood. 2018;131(17):1903–1909. doi: 10.1182/blood-2017-04-569111. [DOI] [PubMed] [Google Scholar]
- 60.Cines DB, Yarovoi SV, Zaitsev SV, Lebedeva T, Rauova L, Poncz M, Arepally GM, Khandelwal S, Stepanova V, Rux AH, et al. Polyphosphate/platelet factor 4 complexes can mediate heparin-independent platelet activation in heparin-induced thrombocytopenia. Blood Adv. 2016;1(1):62–74. doi: 10.1182/bloodadvances.2016000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andia I, Maffulli N: Blood-derived products for tissue repair/regeneration. Int J Mol Sci 2019; 20(18). [DOI] [PMC free article] [PubMed]
- 62.Ferri E, Marzetti E, Calvani R, Picca A, Cesari M, Arosio B: Role of age-related mitochondrial dysfunction in sarcopenia. Int J Mol Sci 2020; 21(15). [DOI] [PMC free article] [PubMed]
- 63.Marzetti E, Calvani R, Cesari M, Buford TW, Lorenzi M, Behnke BJ, Leeuwenburgh C. Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials. Int J Biochem Cell Biol. 2013;45(10):2288–2301. doi: 10.1016/j.biocel.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harrison P. The use of platelets in regenerative medicine and proposal for a new classification system: guidance from the SSC of the ISTH. J Thrombos Haemostasis : JTH. 2018;16(9):1895–1900. doi: 10.1111/jth.14223. [DOI] [PubMed] [Google Scholar]
- 65.Kawase T: A Strategic and worldwide cooperative challenge required for the next generation of platelet concentrates. Int J Mol Sci 2022; 23(7). [DOI] [PMC free article] [PubMed]
- 66.Kawase T, Mubarak S, Mourão CF. The platelet concentrates therapy: from the biased past to the anticipated future. Bioengineering (Basel, Switzerland) 2020;7(3):82. doi: 10.3390/bioengineering7030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kawase T, Okuda K. Comprehensive quality control of the regenerative therapy using platelet concentrates: the current situation and prospects in Japan. Biomed Res Int. 2018;2018:6389157. doi: 10.1155/2018/6389157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kawase T, Takahashi A, Watanabe T, Tsujino T. Proposal for point-of-care testing of platelet-rich plasma quality. Int J Growth Factors Stem Cells Dentistry. 2019;2(1):13–17. [Google Scholar]
- 69.Kawase T, Tanaka T. An updated proposal for terminology and classification of platelet-rich fibrin. Regen Therapy. 2017;7:80–81. doi: 10.1016/j.reth.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kobayashi M, Kawase T, Horimizu M, Okuda K, Wolff LF, Yoshie H. A proposed protocol for the standardized preparation of PRF membranes for clinical use. Biologicals. 2012;40(5):323–329. doi: 10.1016/j.biologicals.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 71.Gupta A, Jeyaraman M, Maffulli N: Common medications which should be stopped prior to platelet-rich plasma injection. Biomedicines 2022; 10(9). [DOI] [PMC free article] [PubMed]
- 72.Andia I, Atilano L, Maffulli N: Moving toward targeting the right phenotype with the right platelet-rich plasma (PRP) formulation for knee osteoarthritis. Ther Adv Musculoskelet Dis 2021; 13:1759720x211004336. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available from the corresponding author on reasonable request.