Abstract
The parasitic loads of mouse livers experimentally infected with Leishmania infantum were determined using a double real-time quantitative PCR test targeted to the parasite DNA polymerase gene and to the mouse brain-derived neutrophic factor gene. The Leishmania DNA copy number was normalized to the number of mouse gene copies in order to quantify the former independently of liver weight. The correlation coefficient with the microtitration method was 0.66. This PCR assay can be considered for experimental pharmaceutical studies.
The leishmaniases are a group of parasitic diseases of major and growing public health importance (9). Standard therapies include pentavalent antimonials and amphotericin B. These drugs cause secondary side effects, and relapses are frequent. Therefore, other antileishmanial compounds (11) or new formulations of existing ones (14) are needed.
Mouse inoculation is the most used in vivo model of visceral leishmaniasis for the evaluation of anti-Leishmania drugs (5–7, 16). Assessment of parasitic burdens is usually based on microscopic enumerations of amastigotes against host cell nuclei on liver imprints (15). This type of assay is time-consuming and subjective and is not reliable when the parasites are not equally dispersed on the slides. More recently, culture microtitrations have been developed (2, 17). These techniques are more sensitive than the imprint method, but the assays remain labor-intensive.
Since recurrences of leishmaniasis are associated with tissue loads of residual, latent parasites after treatment, nonquantitative PCR tests (3, 12, 13) are of little value in indicating a positive or negative result. A recent approach for quantitation of DNA copy number is based on the 5′ nuclease activity of Taq polymerase for fragmentation of a dual-labeled fluorogenic hybridization probe (8). A real-time quantitative TaqMan PCR assay for measuring the copy numbers of Leishmania infantum DNA in mouse liver was developed. A first possibility was to use absolute quantitation. This requires the design of standards known by independent means. Several critical points must be considered, such as the reliability of the serial dilutions of the parasites, the accuracy of pipetting, and the stability of the diluted standards. For the present purpose, i.e., to quantify L. infantum in mouse tissues, very precise weighing of liver biopsy specimens is also necessary. Another possibility was to use relative quantification using the ΔΔCt method (1). In this system, each sample tested is normalized on the basis of its mouse DNA content, and the result is independent of the quantity of the DNA tested.
Infections were conducted with 5-week-old BALB/c female mice and the L. infantum MON1 (MHOM/FR92/LEM 2385) strain. Comparative studies of three techniques of counting the parasites were performed as part of experimental studies of different drug regimens (antimonial pentavalent compounds versus liposomal amphotericin B). The Guiding Principles for Biomedical Research involving animals, published by the Council for International Organizations of Medical Sciences, were followed for all procedures. Mice were inoculated via the tail vein with 107 L. infantum promastigotes in a 0.1-ml volume. The livers of 33 control or treated mice were weighed and used for each titration method.
Imprints from each liver were stained with Giemsa stain, and amastigotes were enumerated against hepatic nuclei at a magnification of ×1,000. At least 100 microscopic fields were examined before an imprint was reported as negative. Each positive result was expressed as the number of amastigotes per 500 hepatic cell nuclei.
Culture microtitration was performed as previously described (2). Briefly, a piece of liver was excised, weighed, and homogenized. Serial fourfold dilutions ranging from 1 to 1/4 10−6 were distributed into 96-well microtitration plates (Becton Dickinson). After 7 and 15 days at 27°C, the presence or absence of mobile promastigotes was recorded in each well. The final titer was the last dilution for which the well contained at least one parasite.
DNA was extracted from about 200 μg of liver biopsy specimens using the High Pure DNA Extraction kit (Boehringer-Roche, Grenoble, France) according to the manufacturer's recommendations. Ten microliters of the 50 μl final elution was used for each PCR test, and each test was duplicated.
Two TaqMan systems were developed: the Leishmania TaqMan system and the mouse TaqMan system. For the Leishmania TaqMan system, the target DNA was the DNA polymerase of L. infantum (GenBank accession number AF009147), which is a single-copy-number gene (4). The Leishmania fluorogenic PCR system consisted of the amplification primers (forward primer, 5′-TGTCGCTTGCAGACCAGATG-3′; reverse primer, 5′-GCATCGCAGGTGTGAGCAC-3′) designed to amplify a 90-bp fragment and the fluorogenic probe (5′FAM-CAGCAACAACTTCGAGCCTGGCACC-3′TAMRA).
For the mouse TaqMan system, the target was the mouse brain-derived neutrophic factor (BDNF) gene (GenBank accession number NM007540), a single-copy-number housekeeping gene (10). The amplification primers (5′-TTGGATGCCGCAAACATGTC-3′ [forward] and 5′-CTGCCGCTGTGACCCACTC-3′ [reverse]) were designed to amplify a 196-bp fragment. The fluorogenic probe sequence was 5′FAM-TCACACACGCTCAGCTCCCCACGG-3′TAMRA.
Each amplification was performed in duplicate, in a 50-μl reaction mixture using the components of the TaqMan PCR Core Reagents Kit (Perkin-Elmer, les Ulis, France). The reaction mixture included: 1× PCR TaqMan buffer; 3 mM MgCl2; 0.2 mM each dATP, dGTP, and dCTP; 0.4 mM dUTP; 20 pmol each of either L. infantum primers or mouse BDNF primers (Perkin-Elmer, les Ulis, France); 0.5 U of uracyl-N-glycosylase (Perkin-Elmer, les Ulis, France), 1.25 U of AmpliTaq Gold (Perkin-Elmer, Roissy, France), and 10 μl of eluted sample. The samples were initially incubated for 2 min at 50°C for optimum uracyl-N-glycosylase activity. This reaction was followed by a 10-min incubation at 95°C to denature the DNA and to activate the AmpliTaq Gold. The temperature cycling (50 cycles at 95°C for 15 s and 65°C for 1 min each) was performed in a 96-well thermal cycler (Perkin-Elmer Applied Biosystems) in the same run for both the L. infantum and the mouse gene amplifications. Each amplification run contained several negative controls (buffer and primers alone). Amplification data collected by the 7700 Sequence Detector and stored in the MacIntosh computer were then analyzed by use of the Sequence Detection System software developed by Perkin-Elmer Applied Biosystems. The threshold of detection was set at 10 times the standard deviation above the mean baseline fluorescence calculated from cycles 1 to 15. The fractional cycle number reflecting a positive PCR result is called the cycle threshold (Ct). Both PCR tests were performed on liver biopsy specimens by individuals blinded to the results of the other titration techniques.
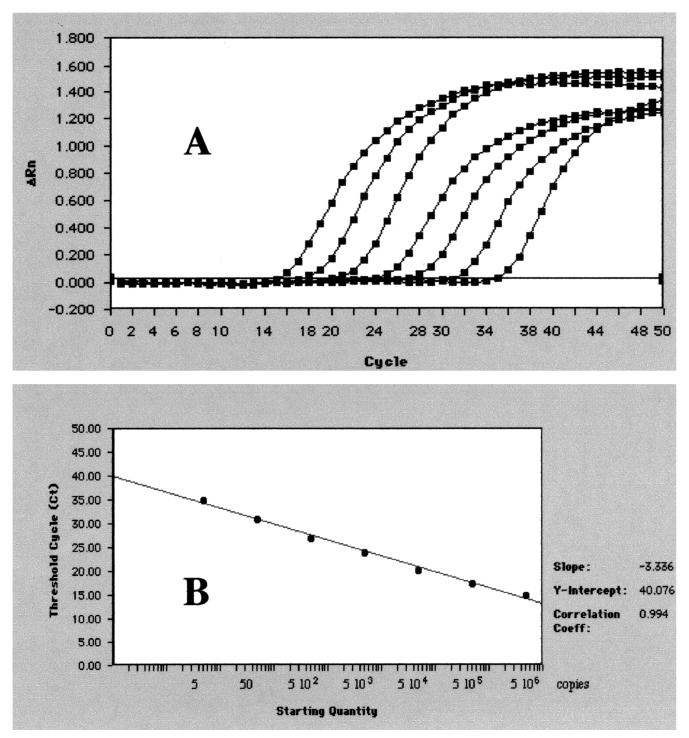
In initial experiments, we determined the dynamic range of the real-time quantitative Leishmania TaqMan PCR test by making serial dilutions of L. infantum DNA in water, consisting of the DNA equivalent from 5 × 106 to 5 cells. The dilutions were subjected to analysis by the Leishmania TaqMan system (Fig. 1). The efficiency of the amplification was close to 1. The intra-assay coefficient of variation was below 1% for the high-concentration DNA and 1.6% for the low-concentration DNA. Reproducibility was estimated by testing the 10-fold dilution 10 times in independent runs. The interassay coefficient of variation was 6.4, 12.3, 13.8, and 36% for 103, 102, 10, and 1 parasite, respectively. Similar results were obtained with the mouse BDNF TaqMan system (data not shown).
FIG. 1.
Amplification plots and a standard curve obtained with the TaqMan Leishmania infantum PCR test. (A) Serial 10-fold dilution of L. infantum DNA from 1 to 106 ng per reaction (5 to 5 × 106 copies/reaction); the amplification curves shift to the right as the input target quantity is reduced, since reactions with fewer target molecules require more amplification cycles to produce a detectable quantity of reporter molecules than do reactions with more target molecules. (B) Standard curve obtained by plotting the Ct against the input target quantity, with the latter plotted on a common log scale. Ct represents the fractional cycle number reflecting a positive PCR result differentiated from the background noise.
The L. infantum DNA copies were quantitated using the ΔΔCt method, which has been described in detail elsewhere (1). Briefly, as the precise amount of genomic DNA added to each reaction (based on optical density) is difficult to assess, the L. infantum DNA copies were normalized on the basis of their mouse gene copy content. The L. infantum DNA copies were also normalized to a calibrator, or 1× sample, consisting of the sample among our tested series which contained the fewest L. infantum DNA copies. Final results, expressed as fold differences in L. infantum gene copies relative to the mouse gene copies and the calibrator, termed N L. infantum, were calculated by the equation N L. infantum = 2ΔΔCt = 2(ΔCt sample − ΔCt calibrator), where ΔCt of the sample and the calibrator is the difference, in threshold cycle number, between the average of the duplicate Ct value of the L. infantum gene and the average of the duplicate Ct value of the mouse gene. Although the absolute number of L. infantum gene copies in the calibrator is not known, this method allows one to ascertain that a sample with an N L. infantum of x has x-fold more DNA copies than the calibrator. Since we have initially checked that the efficiencies of the L. infantum and mouse gene amplifications were approximately equal and close to 1, and since, in testing 1/10-diluted DNA liver samples, the relative quantification was similar, the comparative ΔΔCt method was valid for our PCR assays.
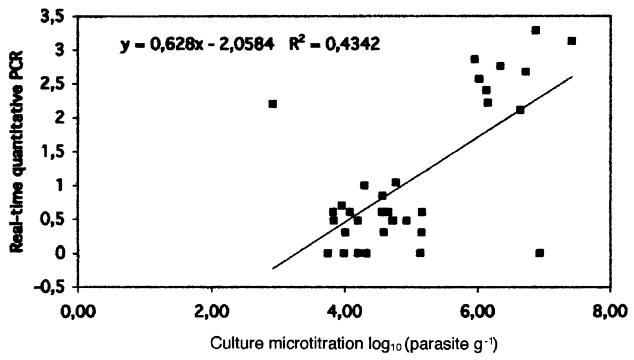
The range of enumeration of amastigotes against hepatic nuclei was 0 to 500 parasites per 500 hepatic cells. A high rate (11 of 33; 33%) of negative results was observed. With the other two methods, negative results were not observed in infected mice, and no organ with positive imprints had a negative culture or a negative PCR result. The TaqMan PCR assay results showed a normalized Leishmania gene copy number between 1 and 1,968. The correlation between the TaqMan PCR assay and the microtitration was calculated with the data expressed as log10 units to assume a normal distribution of the results. The correlation coefficient was 0.66 with a P of <0.01 (Fig. 2). Using the nonparametric Spearman test, the correlation coefficient was 0.52 with a P of <0.01.
FIG. 2.
Correlation between log-transformed individual values of liver parasite burden determined by in vitro micotitration and quantitative PCR test.
To search for any unequal distribution of the parasites in the liver which could explain discrepancies between the techniques, five different liver biopsy specimens from two mice were tested using the TaqMan PCR test. The different liver biopsy specimens gave similar results, showing that the parasites were equally distributed in the liver.
The present work is the first development of TaqMan probes for L. infantum. Instead of quantifying the copy number with a standard curve, we chose a relative quantification according to the liver biopsy. In addition, we determined that liver infestation with Leishmania microorganisms is homogenous. Therefore, the biopsy can be performed anywhere in the liver, and very precise weighing is not necessary in using the double real-time PCR system.
Among the three quantitative techniques tested, the imprints had an extremely high rate of negative results whereas the other techniques gave positive results. Previous studies have shown that the liver imprints were always negative for titers of ≤104 parasites per g (2). Therefore, the imprint technique cannot be used alone in a mouse model.
The correlation coefficient between TaqMan and microtitration was 0.66 (Fig. 2). One could have expected a better figure. However, the techniques could be complementary rather than redundant. Indeed, PCR is unable to distinguish between dead and live parasites. The DNA can come from the liver but also from circulating DNA originating in other cells or organs. The addition of a quantitative PCR test targeted at the mRNA of a housekeeping gene specific to Leishmania should discriminate between live and dead parasites. In contrast to the present TaqMan test, microtitration culture tests only the capability of live amastigotes to transform in vitro into mobile promastigotes. The microtitration technique gives functional information which cannot be directly linked to the number of parasites in the initial tissue. This reasoning may explain some of the aberrant points observed in comparing the microtitration culture method and the real-time quantitative tests. Keeping these limitations in mind, the double PCR TaqMan test developed in this study can give reliable results with a low workload compared with in vitro cultivation to assess the leishmanicidal effect of a given drug in mice.
Acknowledgments
This work was supported by the “Agence Nationale de Recherche contre le SIDA” (grant TIB 9014).
REFERENCES
- 1.Bièche I, Parfait B, Le Doussal V, Olivi M, Rio M-C, Lidereau R, Vidaud M. Identification of CGA as a novel estrogen receptor-responsive gene in breast cancer: an outstanding candidate marker to predict the response to endocrine therapy. Cancer Res. 2001;61:1652–1658. [PubMed] [Google Scholar]
- 2.Buffet P A, Sulahian A, Garin Y J, Nassar N, Derouin F. Culture microtitration: a sensitive method for quantifying Leishmania infantum in tissues of infected mice. Antimicrob Agents Chemother. 1995;39:2167–2168. doi: 10.1128/aac.39.9.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costa J-M, Durand R, Deniau M, Rivollet D, Izri M, Houin R, Vidaud M, Bretagne S. PCR enzyme-linked immunosorbent assay for diagnosis of leishmaniasis in human immunodeficiency virus-infected patients. J Clin Microbiol. 1996;34:1831–1833. doi: 10.1128/jcm.34.7.1831-1833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Croan D G, Morrison D A, Ellis J T. Evolution of the genus Leishmania revealed by comparison of DNA and RNA polymerase gene sequences. Mol Biochem Parasitol. 1997;89:149–159. doi: 10.1016/s0166-6851(97)00111-4. [DOI] [PubMed] [Google Scholar]
- 5.Durand R, Paul M, Rivollet D, Fessi H, Houin R, Astier A, Deniau M. Activity of pentamidine-loaded poly(d,l-lactide) nanoparticles against Leishmania infantum in a murine model. Parasite. 1997;4:331–336. doi: 10.1051/parasite/1997044331. [DOI] [PubMed] [Google Scholar]
- 6.Durand R, Paul M, Rivollet D, Houin R, Astier A, Deniau M. Activity of pentamidine-loaded methacrylate nanoparticles against Leishmania infantum in a mouse model. Int J Parasitol. 1997;27:1361–1367. doi: 10.1016/s0020-7519(97)00124-0. [DOI] [PubMed] [Google Scholar]
- 7.Gangneux J P, Sulahian A, Garin Y J, Derouin F. Efficacy of aminosidine administered alone or in combination with meglumine antimoniate for the treatment of experimental visceral leishmaniasis caused by Leishmania infantum. J Antimicrob Chemother. 1997;40:287–289. doi: 10.1093/jac/40.2.287. [DOI] [PubMed] [Google Scholar]
- 8.Heid C A, Stevens J, Livak K J, Williams P M. Real-time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 9.Herwaldt B L. Leishmaniasis. Lancet. 1999;354:1191–1199. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- 10.Hofer M, Pagliusi S R, Hohn A, Leibrock J, Barde Y A. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990;9:2459–2464. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jha T K, Sundar S, Thakur C P, Bachmann P, Karbwang J, Fischer C, Voss A, Berman J. Miltefosine, an oral agent, for the treatment of Indian visceral leishmaniasis. N Engl J Med. 1999;341:1795–1800. doi: 10.1056/NEJM199912093412403. [DOI] [PubMed] [Google Scholar]
- 12.Lachaud L, Dereure J, Chabbert E, Reynes J, Mauboussin J-M, Oziol E, Dedet J-P, Bastien P. Optimized PCR using patient blood samples for diagnosis and follow-up of visceral leishmaniasis, with special reference to AIDS patients. J Clin Microbiol. 2000;38:236–240. doi: 10.1128/jcm.38.1.236-240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osman O F, Oskam L, Kroon N C M, Schoone G J, Khalil E T, El-Hassan A M, Zijlstra E E, Kager P A. Use of PCR for diagnosis of post-kala-azar dermal leishmaniasis. J Clin Microbiol. 1998;36:1621–1624. doi: 10.1128/jcm.36.6.1621-1624.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paul M, Durand R, Fessi H, Rivollet D, Houin R, Astier A, Deniau M. Activity of a new liposomal formulation of amphotericin B against two strains of Leishmania infantum in a murine model. Antimicrob Agents Chemother. 1997;41:1731–1734. doi: 10.1128/aac.41.8.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stauber L A, Franchino E M, Grun J. An eight-day method for screening compounds against Leishmania donovani in golden hamster. J Protozool. 1958;5:269–273. [Google Scholar]
- 16.Sulahian A, Garin Y J, Pratlong F, Dedet J-P, Derouin F. Experimental pathogenicity of viscerotropic and dermotropic isolates of Leishmania infantum from immunocompromised and immunocompetent patients in a murine model. FEMS Immunol Med Microbiol. 1997;17:131–138. doi: 10.1111/j.1574-695X.1997.tb01005.x. [DOI] [PubMed] [Google Scholar]
- 17.Titus R G, Marchand M, Boon T, Louis J A. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985;7:545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]