Abstract
Background/Objectives
Implantable cardioverter defibrillators are used to prevent sudden cardiac death. The subcutaneous implantable cardioverter-defibrillator was newly developed to overcome the limitations of the conventional implantable cardioverter defibrillator-transvenous device. The subcutaneous implantable cardioverter defibrillator is indicated for young patients with heart disease, congenital heart defects, and poor venous access, who have an indication for implantable cardioverter defibrillator without the need for anti-bradycardic stimulation. We aimed to compare the efficacy and complications of subcutaneous with transvenous implantable cardioverter-defibrillator devices.
Methodology
A systematic review was conducted using different databases. The inclusion criteria were observational and clinical randomized trials with no language limits and no publication date limit that compared subcutaneous with transvenous implantable cardioverter-defibrillators. The selected patients were aged > 18 years with complex ventricular arrhythmia.
Results
Five studies involving 2111 patients who underwent implantable cardioverter defibrillator implantation were included. The most frequent complication in the subcutaneous device group was infection, followed by hematoma formation and electrode migration. For the transvenous device, the most frequent complications were electrode migration and infection. Regarding efficacy, the total rates of appropriate shocks were 9.04% and 20.47% in the subcutaneous and transvenous device groups, respectively, whereas inappropriate shocks to the subcutaneous and transvenous device groups were 11,3% and 10,7%, respectively.
Conclusion
When compared to the transvenous device, the subcutaneous device had lower complication rates owing to lead migration and less inappropriate shocks due to supraventricular tachycardia; nevertheless, infection rates and improper shocks due to T wave oversensing were comparable for both devices CRD42021251569.
Keywords: Subcutaneous implantable cardioverter defibrillator, transvenous implantable cardioverter defibrillator, efficacy, complications, supraventricular tachycardia, bruise, infection
1. INTRODUCTION
The estimated incidence of sudden cardiac death (SCD) in the United States is between 180,000 and 400,000 cases/year, whereas, in Brazil, DATASUS data indicate ~250,000 cases/year [1, 2]. The main causes of SCD are ventricular arrhythmias (AVs), particularly ventricular fibrillation (VF), which accounts for ~80% of SCDs [3]. Antiarrhythmic drugs and defibrillators are used to reestablish or reorganize the heart rhythm to reverse this condition; the latter are considered more effective [3]. With regard to defibrillators, there are two types of implantable cardioverter defibrillators (ICDs): transvenous (conventional) and subcutaneous.
Conventional ICD uses transvenous defibrillator leads implanted into the right ventricle and is indicated for primary and secondary prevention of sudden death, based on results from large multicenter and randomized clinical trials [4-6]. Although effective and relatively safe, ICD-transvenous (ICD-TV) presents acute and chronic limitations to the implant, difficulty in extraction, and complications in the short and long term. Major complications include in-hospital death, cardiac perforation, cardiac valve injury, hemothorax, pneumothorax, deep phlebitis, transient ischemic attack, stroke, myocardial infarction, cardiac tamponade, and arteriovenous fistula [4-7]. Defibrillation occurs at a rate of 20% - 40% in 8-10 years, mainly due to defects related to the insulation, which occur more frequently in young patients. In addition, late extraction of the electrode cable is difficult and is associated with significant morbidity and mortality [8].
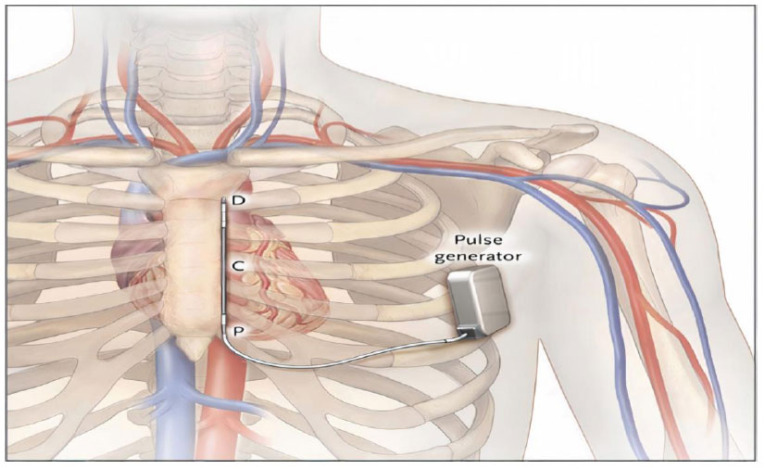
In view of these challenges, there was the need to create an ICD without endovascular electrodes to overcome the limitations related to the implantation of this system. Therefore, a subcutaneous cardioverter defibrillator (ICD) was developed. One of the components of the subcutaneous ICD system is a 3-mm tripolar parasternal electrode (polycarbonate urethane 55D) that is linked to an electrically active pulse generator. The electrode is located on the left side of the sternum, parallel to it, and 1 to 2 cm to the left of the sternal midline, and the pulse generator is positioned above the sixth rib at the juncture of the midaxillary line and the anterior axillary line (Fig. 1). On one side of the coil are two sensing electrodes, while on the other is an 8-cm shocking coil. The distal sensing electrode is placed at the manubrium sternum junction, while the proximal sensing electrode is placed near the xiphoid process [ 3, 4, 7, 9, 10].
Fig. (1).
The location of the subcutaneous implantable cardioverter-defibrillator (ICD-S) components is shown below. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
The distal and proximal sensing electrodes (D and P, respectively) of the LGen-S8 device are shown, with the left lateral pulse generator and an 8-cm parasternal coil electrode (C). From Bardy GH, Smith W.M, Hood M.A., et al. An entirely subcutaneous implantable cardioverter-defibrillator N Engl J Med, 363 (2010), pp. 36-44. Copyright © (2021) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.”
ICD-S is indicated for young patients with electrical cardiac diseases, congenital heart diseases, and poor venous access, who need an ICD but do not require anti-bradycardic stimulation and anti-tachycardia pacing (ATP) [ 4, 5]. However, ICD-S is being increasingly used in older patients with more serious heart diseases and associated comorbidities. The ICD-S was developed to avoid problems associated with transvenous leads, but it lacks pacing capability and so can only be used in patients who do not require pacing [10].
The SCD-S post-approval registry (SICD-PAS), which is the biggest registry of SICD patients in the United States, was just made public. Using this registry, researchers were able to characterize the characteristics and acute outcomes of individuals who had a SICD implanted in a real-world context outside of an exploratory trial. More than 1600 patients were registered in this registry. The mean age of the cohort was 52 years and 15 years, which is consistent with the current trend of implanting SICDs in younger patients. A total of 98.7 percent of induced VT/VF were effectively converted in the SICD-PAS study.
Studies reveal that ICD-S has similar effectiveness as ICD-TV in reversing ventricular-induced fibrillation, but it has some short-term limitations, such as requiring a mandatory defibrillation test at the time of implantation and infection of the device with a low risk of systemic dissemination [5, 6, 10]. Further, long-term complications are not yet fully elucidated. Commercial use is approved in the European Union, New Zealand, and the United States, but not yet in Brazil [3, 5, 10].
To provide clinical guidance in therapeutic decision-making, to contribute to the planning of new research on the subject, and because of the paucity of data regarding the future repercussions of the ICD-S, a systematic review of observational studies was conducted to compare the efficacy and complications associated with ICD-TV and ICD-S in the prevention of SCD in patients with ventricular arrhythmias.
2. METHODOLOGY
2.1. Research Strategy and Study Eligibility
The present study was based on the PICOS strategy, which addresses five essential components for the elaboration of a systematic review: population, intervention, comparison, outcome, and study type. A bibliographic search was performed in the databases: PUBMED, LILACS, Google academic, Cochrane Central Register of Controlled Trials (CENTRAL), on November 7, 2018, using the search terms “Subcutaneous ICD” AND “ICD Implantation” AND “Ventricular Arrhythmia.” The evaluation for possible inclusion was independently performed by two reviewers based on the titles and abstracts. The inclusion criteria for this review were published studies comparing ICD-S and ICD-TV with no language limits and no publication date limit. Clinical studies, case reports, reviews, and book chapters were excluded. The patients selected were aged > 18 years with complex ventricular arrhythmia; patients aged < 18 years of age were excluded. Recently, on August 5, 2020, a prospective, randomized multicenter study evaluated the advantages and disadvantages of ICD-S; and because it enriches the knowledge about this topic, it was included in the review.
2.2. Extraction and Analysis of Data
Data from the independent analysis included the type of study and the year of publication, clinical characteristics of the patients, indications for primary and secondary prevention, presence of previous heart disease, efficacy, and complications. The relationship and grouping of the data were performed and summarized in the Tables. A joint analysis of all collected data was performed. To arrive at a single result that is common to all studies, an association r was made between the total number of events and the sum of participating patients, obtaining a combined rate that is expressed as a percentage.
3. RESULTS
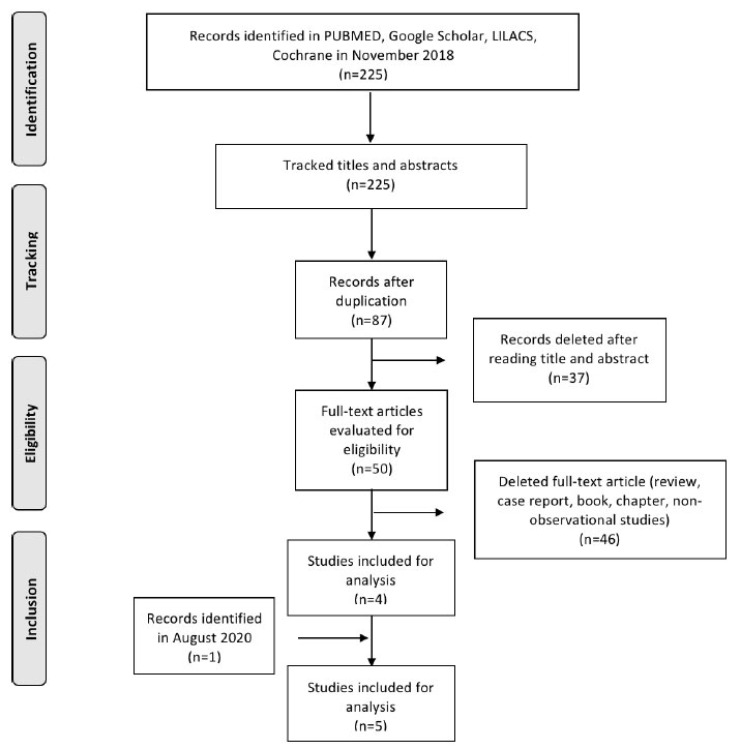
Five studies were included, and the phases of study selection are shown in Fig. (2) [11-24].
Fig. (2).
Flowchart of selected studies.
3.1. Characteristics of Selected Studies
The five studies included were performed between 2013 and 2020, and the sample size ranged from 138 to 849 participants, with a total cohort of 2111 patients who underwent ICD implantation. Of these, 864 and 1247 patients received subcutaneous and transvenous devices, respectively (Table 1). The largest study of 849 individuals was a non-inferiority trial in which patients with an indication for an ICD, but no indication for pacing, were assigned to receive a subcutaneous ICD or a transvenous ICD [15-24]. The second largest analysis of 510 participants was obtained from the registration of Italian centers invited by the Italian Society of Heart Rhythm (AIAC), which reported the number of patients subjected to the ICD TV (update or replacement) and ICD-S in Italy [11].
Table 1.
Design of studies and characteristics of participants.
| First Author [Ref. #] | Type of Study, Country, Year | Criteria for Inclusion of Participants | Number of Patients | Male Gender | Age (Years) | Ejection Fraction (%) | Indications | Underlying Heart Disease | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary Prevention | Secondary Prevention | CM (Ischemic, Non Ischemic, Dilated) |
CAD/ IHD | HCM | Others | |||||||||||||||||
| ICD-S | ICD-TV | ICD-S (%) | ICD-TV (%) | ICD-S | ICD-TV | ICD-S | ICD-TV | ICD-S | ICD-TV | ICD-S | ICD-TV | ICD-S | ICD-TV | ICD-S | ICD-TV | ICD-S | ICD-TV | ICD-S | ICD-TV | |||
| Köbe et al. [13] | Case-control, Germany, 2013 | Primary and secondary prevention | 69 | 69 | 50 (72) | 50 (72) |
45.7 | 47.7 | 46.2 | 40.6 | 41 (59) |
34 (49) |
28 (41) |
35 (51) |
25 (36) |
32 (46) |
11 (16) |
13 (19) |
10 (14) |
4 (6) |
24 (35) |
22 (32) |
| Pedersen et al. [14] | Case-control, Czech Republic, Denmark, Germany, Italy, Netherlands, New Zealand, Portugal, United Kingdom, 2016 | Primary and secondary prevention | 167 | 167 | 122 (73) | 120 (72) |
54 | 55 | NR | NR | 123 (74) |
115 (69) |
44 (26) | 52 (31) | 37 (22) |
51 (30) |
- | - | 22 (13) |
18 (11) |
76 (45) |
82 (49) |
| Brouwer et al. [12] | Case-control, Netherlands, 2016 | Primary and secondary prevention | 140 | 140 | 84 (60) | 87 (62) |
41 | 42 | 50 | 49 | 93 (66) |
86 (61) |
47 (34) | 54 (39) | 54 (39) |
71 (51) |
- | - | - | - | 84 (61) |
68 (49) |
| Botto et al. [11] | Cohort, Italy, 2017 | Primary and secondary prevention | 62 | 448 | 45 (73) |
354 (79) |
47 | 67 | 49 | 34 | 30 (48) | 357 (80) | 32 (52) |
91 (20) |
21 (34) |
362 (81) |
18 (29) |
275 (61) |
9 (14) |
16 (4) |
9 (14) |
47 (10) |
| Knops et al. [15,24] | Non-inferiority trial, Germany, Netherlands, United Kingdom, United States, 2020 | Primary and secondary prevention | 426 | 423 | 337 (79.1) | 345 (81.6) | 63 | 64 | 30 | 30 | 346 (81.2) | 339 (80.1) | 80 (18.8) | 84 (19.9) | 388 (91) | 396 (93.6) | NR | NR | 15 (3.5) | 7 (1.7) | 38 (8.9) | 27 (6.38) |
CM= cardiomyopathy; CAD= coronary artery disease; IHD= ischemic Heart Disease; HCM= hypertrophic cardiomyopathy; ICD-S= subcutaneous implantable cardioverter defibrillator; ICD-TV= transvenous implantable cardioverter defibrillator.
Following the description of each research that was selected for inclusion, just one of the studies included in the meta-analysis found a statistically significant difference in age [ 11 ]: That is, the study by Köbe et al. [ 13 ]: ICD S 45.7±15.7 and ICD TV 47.7±14.7 (p=0.433); Pedersen et al. [ 14 ]: ICD S 54± 16 years and ICD TV 55 ± 13 years (p=0.8831); Brouwer et al. [ 12 ]: ICD S 41(26-52) years and ICD TV 42 (32-50) years (p=0.33); Botto et al. [ 11 ]: ICD S 47±11 years and ICD TV 67±13 years (p < 0.001); Knops et al [ 15, 24]: ICD S 63 (54-69) years and ICD TV 64 (56-70) years (p>0.05). Most of the patients were male, representing a range of 60%-79% for ICD-S and 62%-82% for ICD-TV. There were significant differences between the two groups in that S-ICD patients were younger, had a higher systolic function and functional status were less likely to present with structural cardiomyopathy and were more likely to have hereditary channelopathies. S-ICD recipients also had less coronary artery disease and comorbidities, and they were more likely to have their device implanted as a means of secondary prevention of sudden cardiac death (SCD). Patients with arrhythmic genetic syndrome, asymmetric septal hypertrophy, and idiopathic ventricular fibrillation are more likely to be diagnosed with ICD-S, while patients with ICD-TV are more likely to be diagnosed with nonischemic dilated cardiomyopathy, ischemic cardiomyopathy, and ventricular tachycardia (with or without myocardial infarction) [11-24].
Table 1 . Design of studies and characteristics of participants
3.2. Efficacy in the Treatment of Ventricular Arrhythmias
Treatment efficacy was assessed using appropriate and inappropriate shock rates. Appropriate therapy consisted of anti-tachycardia pacing and shock (whether preceded by ATP or not) for ventricular tachycardia (VT) or ventricular fibrillation (VF). Meanwhile, inappropriate therapy consisted of ATP and shocks to ventricular arrhythmias other than VT or VF [12]. The extracted data are reported in Table 2.
Table 2.
Efficacy of ICD-S and ICD-TV.
|
First Author
[Ref. #] |
Inappropriate Shocks | Appropriate Shocks | Supraventricular Tachycardia | Oversensing T-wave | ||||
|---|---|---|---|---|---|---|---|---|
| ICD-S | ICD-TV | ICD-S | ICD-TV | ICD-S | ICD-TV | ICD-S | ICD-TV | |
| Köbe et al. [13] | 3 | 2 | 3 | 9 | 0 | 2 | 3 | 0 |
| Pedersen et al. [14] | NR | NR | 19 | 29 | NR | NR | NR | NR |
| Brouwer et al. [12] | 20 | 22 | 12 | 39 | 3 | 21 | 17 | 1 |
| Botto et al. [11] | NR | NR | NR | NR | NR | NR | NR | NR |
| Knops et al. [15] | 41 | 29 | 83 | 57 | 11 | 27 | 24 | 2 |
| Rate (%) | 7.4 | 4.25 | 11.34 | 10.74 | 1.62 | 4 | 5.09 | 0.24 |
ICD-S= subcutaneous implantable cardioverter defibrillator; ICD-TV= transvenous implantable cardioverter defibrillator; NR= not reported.
Table 2 . Efficacy of subcutaneous implantable cardioverter defibrillators and implantable cardioverter defibrillators-transvenous
3.2.1. Appropriate Shocks
Appropriate shocks were reported in four studies: Köbe et al. [13], Pedersen et al. [14], Brouwer et al. [12], and Knops et al. [15, 24]. In the first study [13], the average time interval was 10.4 months, with an appropriate shock rate of 4.35% for the ICD-S and 13.04% for the ICD-TV. In the second study [14], the appropriate shock rate was 11.38% in the ICD-S group and 17.36% in the ICD-TV group, with the patients followed up for 6 months. In the third study [12], the subcutaneous rate was 8.57%, whereas the transvenous rate was 27.86%, with a follow-up period of 5 years. The fourth study [15], reported that the subcutaneous and transvenous rates were 19.20% and 11.50%, respectively, with a follow-up period of 49 months. In our analysis, the total rate of appropriate shocks was 11.3% for the subcutaneous device and 10.7% for the transvenous device.
3.2.2. Inappropriate Shocks: Supraventricular Tachycardia and T-wave Oversensing
The prevalence of inappropriate shocks addressed in our review was 7.40% for the ICD-S and 4.25% for the ICD-TV. The extracted data are reported in Table 2. Brouwer et al. [12]. reported this system failure rate of 4,2% in the ICD-S group and 17,6% in the ICD-TV group. Köbe et al. [13]. reported inappropriate shocks at a rate of 4.3% in the subcutaneous device and 2.9% in the transvenous device. In the subcutaneous device, 85% of the inappropriate shocks occurred by T-wave oversensing and only 15% were due to supraventricular tachycardia, whereas in the transvenous device, 94% of these shocks were triggered by supraventricular tachycardia [12]. In our analysis, supraventricular tachycardia occurred in 1.62% of patients who had ICD-S and in 4% of patients who had ICD-TV. The oversensing rates for the subcutaneous and transvenous devices were 5.09% and 0.24%, respectively. Thus, it is evident that ICD-S has less inappropriate shocks due to lower frequency in supraventricular tachycardia episodes when compared to ICD-TV.
3.3. Adverse Events
The identified complications and their frequencies are listed in Table 3. The most frequent complication in the ICD-S group was infection (1.96%, 3 studies), followed by hematoma formation (1.45%, 1 study) and electrode migration (0.48%, 2 studies). Meanwhile, the most frequent complications in the ICD-TV group were electrode migration (9.1%, 2 studies) and infection (2.24%, 3 studies); there were no reports of bruising with the ICD-TV.
Table 3.
Adverse effects of ICD-S and ICD-TV.
|
First Author
[Ref. #] |
Total Patients | Bruise | Electrode Migration | Infection | ||||
|---|---|---|---|---|---|---|---|---|
| ICD-S | ICD-TV | ICD-S | ICD-TV | ICD-S | ICD-TV | ICD-S | ICD-TV | |
| Köbe et al. [13] | 69 | 69 | 1 | 0 | 0 | 2 | 1 | 1 |
| Pedersen et al. [14] | 167 | 167 | NR | NR | NR | NR | NR | NR |
| Brouwer et al. [12] | 140 | 140 | NR | NR | 1 | 17 | 5 | 4 |
| Botto et al. [11] | 62 | 448 | NR | NR | NR | NR | NR | NR |
| Knops et al. [15] | 31 | 44 | 8 | 2 | 2 | 7 | 4 | 8 |
| Rate (%) | 1.04 | 0.16 | 0.35 | 2.08 | 1.16 | 1.04 | ||
ICD-S= subcutaneous implantable cardioverter defibrillator; ICD-TV= transvenous implantable cardioverter defibrillator; NR= not reported.
Table 3 . Adverse effects of subcutaneous implantable cardioverter defibrillators and implantable cardioverter defibrillators-transvenous
3.3.1. Bruise
The two studies by Köbe et al. [13] and Knops et al. [15-24] reported hematoma formation after ICD implantation. A pocket hematoma was observed in patients undergoing anticoagulation therapy after mitral valve replacement surgery, who received a subcutaneous device. This complication is rare, affecting ~2% of patients receiving ICD-TV [13] Knops et al. [15, 24] reported more frequent pocket hematomas in the subcutaneous ICD group.
3.3.2. Electrode Migration
In relation to electrode migration, three of the five articles, specifically those by Köbe et al. [13], Brouwer et al. [12], and Knops et al. [15-24], noted that this complication was more prevalent in those receiving a transvenous implant (2.08%) than in those receiving a subcutaneous implant (0.35%). In the first study, this complication was not reported in relation to the subcutaneous device, whereas in the transvenous device, the resulting rate was 2.90%. In the second study, the complication rates for electrode migration were 0.70% and 12.10% for the ICD-S and ICD-TV groups, respectively. In the third study, the complication rates in the ICD-S and ICD-TV groups were 0.46% and 1.65%, respectively.
3.3.3. Infection
The reported infections were related to the implantation of devices that required revision. Three of the five studies, specifically those by Köbe et al. [13], Brouwer et al. [12], and Knops et al. [15], reported the occurrence of this complication after ICD implantation. Brouwer et al. [12], reported that infections occurred in 3.6% of patients with ICD-S and in 2.9% of patients with ICD-TV. In the study by Köbe et al. [13], both devices obtained a 1.45% infection rate, which required extraction of the electrode (one patient in a total of 69, for each of the groups). In the study by Knops et al. [15], infections occurred in 0.9% of patients with ICD-S and in 1.8% of patients with ICD-TV. In our analysis, the total infection rates in the subcutaneous and transvenous devices were 1.16% and 1.04%, respectively. We, therefore, conclude that there were no significant differences between the infection rates of the two types of cardioverters.
3.4. Appropriate Shock
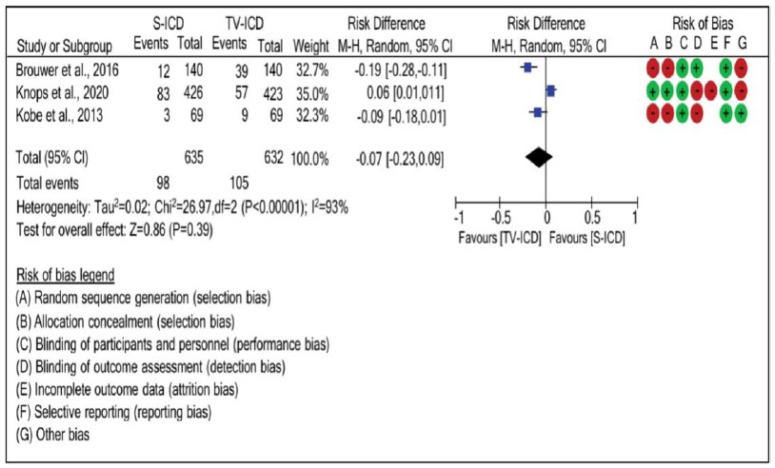
Significant heterogeneity, obtained through a randomized effect of three articles, demonstrated that the potential of pacemakers to cause appropriate shock differed among studies. Through the statistical analysis of risk difference, it was revealed that there was no risk difference (risk difference: -0.07; 95% CI: -0.23, 0.09; Tau2=0.02; χ2=26.97; p<0.00001; I2=93%) between the ICD-S and ICD-TV groups, as seen in Fig. (3).
Fig. (3).
Risk difference between the groups with subcutaneous implantable cardioverter defibrillators and implantable cardioverter defibrillators-transvenous for appropriate shock. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.5. Electrode Migration
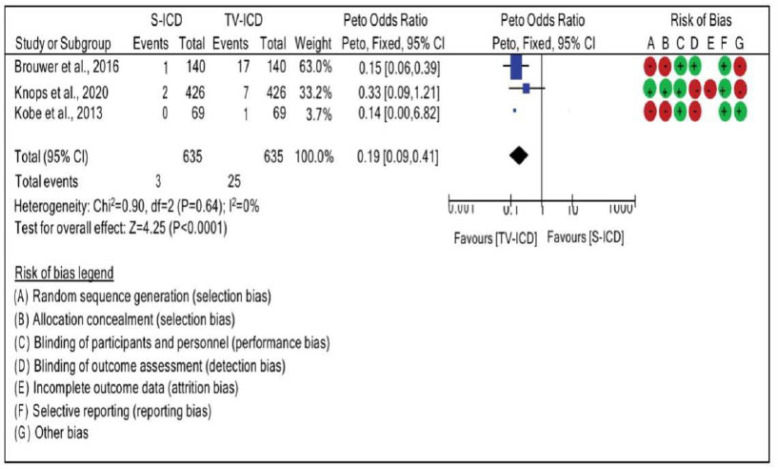
When analyzing electrode migration, no significant heterogeneity between studies (I2=0%), evaluated through the fixed effect, was observed. Moreover, the risk of electrode migration was 0.19 times higher in patients who used ICD-TV than in those who used ICD-S. Electrode migration was compared between the ICD-S and ICD-TV groups using an OR of 0.19 · (95% CI: 0.09, 0.41; χ2=0.90, p=0.64; I2=0%), as seen in Fig. (4).
Fig. (4).
Risk difference between the groups with subcutaneous implantable cardioverter defibrillators and implantable cardioverter defibrillators-transvenous for electrode migration. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
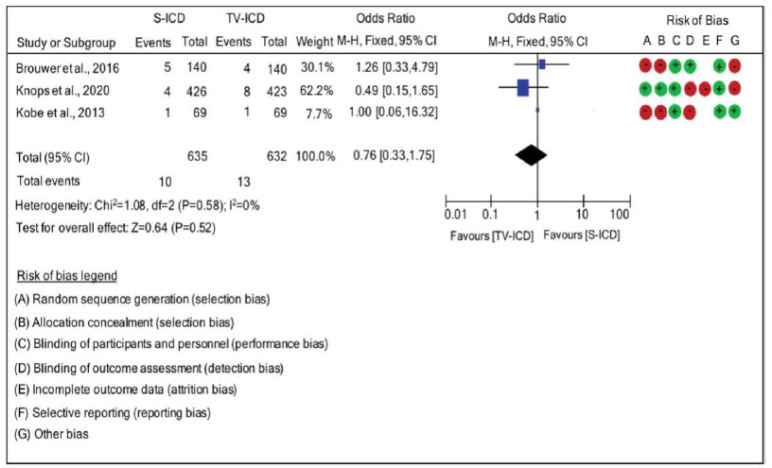
3.6. Infection
There were no significant differences between the presence of subcutaneous and transvenous leads when analyzing the risk of infection. Infection was compared between the ICD-S and ICD-TV groups with an OR of 0.76 · (95% CI: 0.33, 1.75; χ2=1.08; p=0.58; I2=0%). There was no heterogeneity between the results of the studies, and a fixed effect was used to determine heterogeneity, as seen in Fig. (5).
Fig. (5).
Risk difference between the groups with subcutaneous implantable cardioverter defibrillators and implantable cardioverter defibrillators-transvenous for infection. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
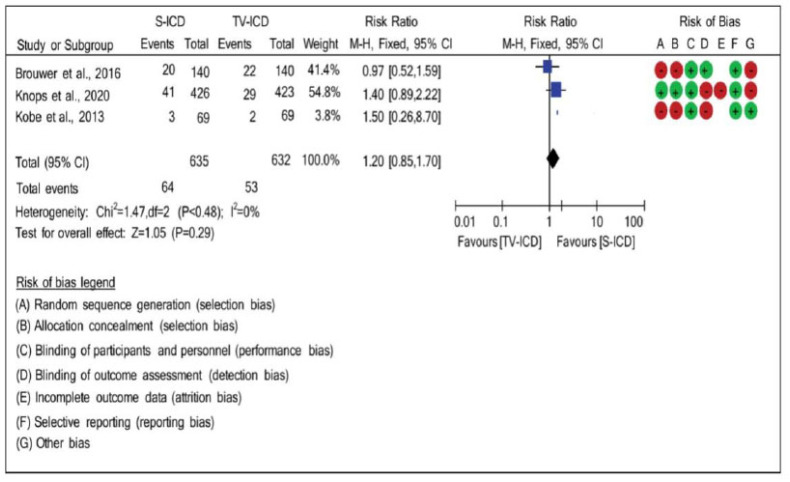
3.7. Inappropriate Shock
There was no heterogeneity (χ2=1.47; p=0.48; I2=0%) when they were compared in terms of the relationship between the effects of inappropriate shock and the two studied types of DHF. The risk ratio (RR: 1.20; 95% CI: 0.85, 1.70) was used to compare the occurrence of inappropriate shock between the ICD-S and ICD-TV groups, and according to the statistical analysis, which used the randomized effect, there was no significant difference between the groups analyzed, as seen in Fig. (6).
Fig. (6).
Risk difference between the groups with subcutaneous implantable cardioverter defibrillators and implantable cardioverter defibrillators-transvenous for inappropriate shock. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
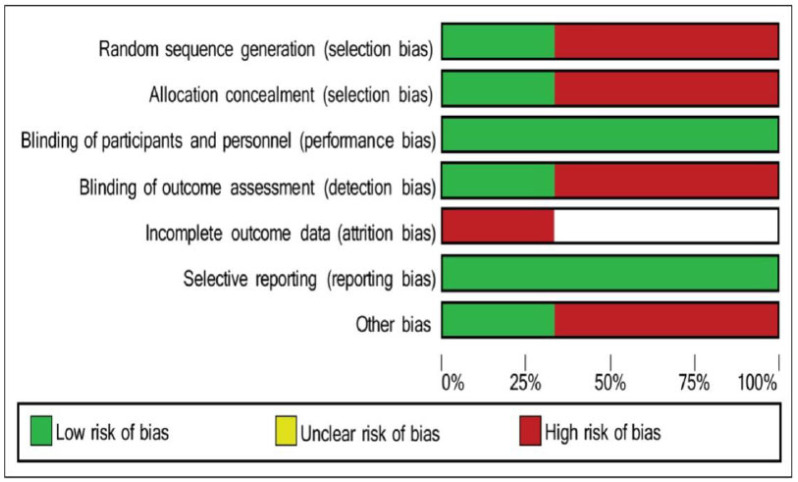
3.8. Risk of Bias
The graph and summary of bias indicate the high risk of bias in the surveys undertaken. Köbe et al [13] and Brouwer et al [12] presented a higher bias index using the applied methodology, with emphasis on the lack of randomization and other biases, as seen in Fig. (7).
Fig. (7).
Risk of bias. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.9. Atrial Tachycardia
In view of the studies that addressed atrial tachycardia as an adverse effect, a low heterogeneity was observed, and these studies were found to have I2=22% and an insignificant p-value (>0.05). Moreover, the odds ratio (OR:0.27; 95% CI: 0.15, 0.49; χ2=2.57, p=0.28; I2=22%) demonstrated that the ICD-TV group is 0.27 times more likely to not present atrial tachycardia than the ICD-S group. Therefore, the TV-ICD group was more capable of detecting atrial tachycardia than the ICD-S group.
4. DISCUSSION
The first tests for temporary implantation of the ICD-S were performed after 2001. In the first 3 years, the first defibrillation test was performed, which identified four electrode configurations for this cardioverter defibrillator. The study involved 78 patients who received one or more of the four electrode configurations associated with the defibrillation threshold test. In 2004, a second study was conducted to compare the best systems tested in the first ICD-TV study. This study involved 49 patients who underwent simultaneous implantation of both types of devices [16].
From these two temporary implantation assays, the permanent implantation of ICD-S was considered, and two clinical trials were performed that were successful in the immediate conversion of two consecutive episodes of induced ventricular fibrillation. However, the comparison between ICD-S and ICD-TV was inconclusive in terms of electrode stability and migration [16].
Several populations have been analyzed from two large prospective studies (IDE and effortless) to determine the safety and efficacy of ICD in patients with primary and secondary indications. The effectiveness of the ICD-S for discriminate spontaneous supraventricular tachycardia and decreasing the incidence of inappropriate shocks was verified [17] Our review is in agreement with the findings of a previous study that demonstrated greater efficacy of this device in the detect episodes of supraventricular tachycardia. In contrast, it presents similar rates of inappropriate shocks between the two devices.
Regarding the complications of ICD-S analyzed in our review, infection of the device had the highest prevalence, according to the study by Lambiase et al. [18]. This study retrospectively and prospectively evaluated data from the effortless registry, with infection being the only significant complication reported (3.9%). Other studies have also reported that the ICD-S does not present with complications related to electrode displacement seen with the transvenous device [19-23].
Other less severe adverse effects, such as heart perforation, cardiac valve damage, hemothorax, pneumothorax, deep phlebitis, transient ischemic attack, stroke, myocardial infarction, cardiac tamponade, and arteriovenous fistula, were also uncommon, in the same way that mortality was. It was stated that there were only 33 bouts of discomfort that required revision and 26 instances of hematoma that required intervention, among other things. Alternatively, under-reporting in the MAUDE (Manufacturer and User Facility Device Experience) [25] database, which likely has a disproportionate effect on the reporting of relatively minor events, rather than a very low event rate, as indicated by the effortless research [26], may be the cause.
Even though ICD-S patients have a lower frequency of supraventricular tachycardia than those who have ICD-TV, [12] patients with ICD-S have a lower frequency of supraventricular tachycardia than those who have ICD-TV. Because there is no endovascular electrode in the right atrium, this should be attributable to-the fact that patients with indications for ICD-S have a lower frequency of arrhythmias and associated comorbidities than those without such indications. Supraventricular tachycardia cannot be treated by an ICD-S alone; however, the decreased number of inappropriate shocks because of the lower frequency of arrhythmia seen may be noticeable when used in combination with an ICD-S The following characteristics are also utilized in the discrimination algorithm: the multiplicity of atrial and ventricular rhythms, the regularity of P-R, and the abrupt start. SMART has recently been enhanced with the inclusion of active detection in the event of 1:1 AV conduction, which enhances the functionality of the original SMART. The counter events and EGMs may be used to estimate the prevalence of SVT in a population [12, 24].
Experimental evidence from pre-clinical studies using premature atrial additional stimuli indicates that a lengthy burst of atrial stimuli is required in order to be able to catch the ventricle in the course of an episode of supraventricular tachycardia. During an episode of supraventricular tachycardia, single isolated stimuli from the auricle (atrial appendage) are usually not able to pass through to the ventricle. It is hazardous to have a lengthy burst in the atrium because it may cause an episode of ventricular tachycardia during an episode of supraventricular tachycardia, which is life-threatening [26-28].
The most common cause of the first occurrences of inappropriate shocks in the subcutaneous ICD group was cardiac oversensing (which occurred in 58.5 percent of the patients who received an inappropriate shock), whereas the most common cause of the first occurrences of inappropriate shocks in the transvenous ICD group was supraventricular arrhythmia (which occurred in 58.5 percent of the patients who received an inappropriate shock in 93.1 percent) [24]. However, no patient with ICD-TV developed a hematoma after implantation of the device, whereas one case was observed after implantation of the subcutaneous device [11-14].
CONCLUSION
In conclusion, as compared to the transvenous device, the subcutaneous device reduced complication rates associated with lead migration and inappropriate shocks due to supraventricular tachycardia; nevertheless, infection rates and inappropriate shocks, in general, were comparable for both devices.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
STANDARDS OF REPORTING
This manuscript was prepared according to the PRISMA 2009 statement.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
SUPPLEMENTARY MATERIAL
PRISMA checklist is available as supplementary material on the publisher’s website along with the published article.
REFERENCES
- 1.da Fonseca S.M., Belo L.G., Carvalho H., Araújo N., Munhoz C., Siqueira L., Maciel W., Andréa E., Atié J. Clinical follow-up of patients with implantable cardioverter-defibrillator. Arq. Bras. Cardiol. 2007;88(1):8–16. doi: 10.1590/s0066-782x2007000100002. [DOI] [PubMed] [Google Scholar]
- 2.Braggion-Santos M.F., Volpe G.J., Pazin-Filho A., Maciel B.C., Marin-Neto J.A., Schmidt A. Sudden cardiac death in Brazil: A community-based autopsy series (2006-2010). Arq. Bras. Cardiol. 2015;104(2):120–127. doi: 10.5935/abc.20140178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Markwerth P., Bajanowski T., Tzimas I., Dettmeyer R. Sudden cardiac death-update. Int. J. Legal Med. 2021;135(2):483–495. doi: 10.1007/s00414-020-02481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanghera R., Sanders R., Husby M., Bentsen J.G. Development of the subcutaneous implantable cardioverter-defibrillator for reducing sudden cardiac death. Ann. N. Y. Acad. Sci. 2014;1329(1):1–17. doi: 10.1111/nyas.12550. [DOI] [PubMed] [Google Scholar]
- 5.Bettin M., Reinke F., Rath B., Köbe J., Eckardt L. Recent advances in the entirely subcutaneous ICD System. F1000Prime Rep. 2015;7:46. doi: 10.12703/P7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali H., Lupo P., Cappato R. The entirely subcutaneous defibrillator - A new generation and future expectations. Arrhythm. Electrophysiol. Rev. 2015;4(2):116–121. doi: 10.15420/AER.2015.04.02.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adduci C., Palano F., Francia P. Safety, efficacy and evidence base for use of the subcutaneous implantable cardioverter defibrillator. J. Clin. Med. 2018;7(3):53. doi: 10.3390/jcm7030053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Bie M.K., Thijssen J., van Rees J.B., Putter H., van der Velde E.T., Schalij M.J., van Erven L. Suitability for subcutaneous defibrillator implantation: Results based on data from routine clinical practice. Heart. 2013;99(14):1018–1023. doi: 10.1136/heartjnl-2012-303349. [DOI] [PubMed] [Google Scholar]
- 9.Köbe J., Zumhagen S., Reinke F., Schulze-Bahr E., Eckardt L. Totally subcutaneous cardioverter-defibrillator (S-ICD®) : Recent experience and future perspectives. Herz. 2011;36(7):586–591. doi: 10.1007/s00059-011-3508-6. [DOI] [PubMed] [Google Scholar]
- 10.Lambiase P.D., Srinivasan N.T. Early experience with the subcutaneous ICD. Curr. Cardiol. Rep. 2014;16(8):516. doi: 10.1007/s11886-014-0516-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Botto G.L., Forleo G.B., Capucci A., Solimene F., Vado A., Bertero G., Palmisano P., Pisanò E., Rapacciuolo A., Infusino T., Vicentini A., Viscusi M., Ferrari P., Talarico A., Russo G., Boriani G., Padeletti L., Lovecchio M., Valsecchi S., D’Onofrio A. The Italian subcutaneous implantable cardioverter-defibrillator survey: S-ICD, why not? Europace. 2017;19(11):1826–1832. doi: 10.1093/europace/euw337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brouwer T.F., Yilmaz D., Lindeboom R., Buiten M.S., Olde Nordkamp L.R., Schalij M.J., Wilde A.A., van Erven L., Knops R.E. Long-Term clinical outcomes of subcutaneous versus transvenous implantable defibrillator therapy. J. Am. Coll. Cardiol. 2016;68(19):2047–2055. doi: 10.1016/j.jacc.2016.08.044. [DOI] [PubMed] [Google Scholar]
- 13.Köbe J., Reinke F., Meyer C., Shin D.I., Martens E., Kääb S., Löher A., Amler S., Lichtenberg A., Winter J., Eckardt L. Implantation and follow-up of totally subcutaneous versus conventional implantable cardioverter-defibrillators: A multicenter case-control study. Heart Rhythm. 2013;10(1):29–36. doi: 10.1016/j.hrthm.2012.09.126. [DOI] [PubMed] [Google Scholar]
- 14.Pedersen S.S., Mastenbroek M.H., Carter N., Barr C., Neuzil P., Scholten M., Lambiase P.D., Boersma L., Johansen J.B., Theuns D.A. A comparison of the quality of life of patients with an entirely subcutaneous implantable defibrillator system versus a transvenous system (from the effortless S-ICD Quality of Life Substudy). Am. J. Cardiol. 2016;118(4):520–526. doi: 10.1016/j.amjcard.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 15.Olde Nordkamp L.R., Knops R.E., Bardy G.H., Blaauw Y., Boersma L.V., Bos J.S., Delnoy P.P., van Dessel P.F., Driessen A.H., de Groot J.R., Herrman J.P., Jordaens L.J., Kooiman K.M., Maass A.H., Meine M., Mizusawa Y., Molhoek S.G., van Opstal J., Tijssen J.G., Wilde A.A. Rationale and design of the PRAETORIAN trial: A Prospective, RAndomizEd comparison of subcuTaneOus and tRansvenous ImplANtable cardioverter-defibrillator therapy. Am. Heart J. 2012;163(5):753–760.e2. doi: 10.1016/j.ahj.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Bardy G.H., Smith W.M., Hood M.A., Crozier I.G., Melton I.C., Jordaens L., Theuns D., Park R.E., Wright D.J., Connelly D.T., Fynn S.P., Murgatroyd F.D., Sperzel J., Neuzner J., Spitzer S.G., Ardashev A.V., Oduro A., Boersma L., Maass A.H., Van Gelder I.C., Wilde A.A., van Dessel P.F., Knops R.E., Barr C.S., Lupo P., Cappato R., Grace A.A. An entirely subcutaneous implantable cardioverter-defibrillator. N. Engl. J. Med. 2010;363(1):36–44. doi: 10.1056/NEJMoa0909545. [DOI] [PubMed] [Google Scholar]
- 17.Weiss R., Knight B.P., Gold M.R., Leon A.R., Herre J.M., Hood M., Rashtian M., Kremers M., Crozier I., Lee K.L., Smith W., Burke M.C. Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation. 2013;128(9):944–953. doi: 10.1161/CIRCULATIONAHA.113.003042. [DOI] [PubMed] [Google Scholar]
- 18.Lambiase P.D., Barr C., Theuns D.A.M.J., Knops R., Neuzil P., Johansen J.B., Hood M., Pedersen S., Kääb S., Murgatroyd F., Reeve H.L., Carter N., Boersma L. Worldwide experience with a totally subcutaneous implantable defibrillator: Early results from the effortless S-ICD Registry. Eur. Heart J. 2014;35(25):1657–1665. doi: 10.1093/eurheartj/ehu112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brignole M. Are complications of implantable defibrillators under-estimated and benefits over-estimated? Europace. 2009;11(9):1129–1133. doi: 10.1093/europace/eup174. [DOI] [PubMed] [Google Scholar]
- 20.Anderson K.P. Estimates of implantable cardioverter-defibrillator complications: Caveat emptor. Circulation. 2009;119(8):1069–1071. doi: 10.1161/CIRCULATIONAHA.108.841452. [DOI] [PubMed] [Google Scholar]
- 21.van Rees J.B., de Bie M.K., Thijssen J., Borleffs C.J.W., Schalij M.J., van Erven L. Implantation-related complications of implantable cardioverter-defibrillators and cardiac resynchronization therapy devices: A systematic review of randomized clinical trials. J. Am. Coll. Cardiol. 2011;58(10):995–1000. doi: 10.1016/j.jacc.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Hammill S.C., Kremers M.S., Stevenson L.W., Heidenreich P.A., Lang C.M., Curtis J.P., Wang Y., Berul C.I., Kadish A.H., Al-Khatib S.M., Pina I.L., Walsh M.N., Mirro M.J., Lindsay B.D., Reynolds M.R., Pontzer K., Blum L., Masoudi F., Rumsfeld J., Brindis R.G. Review of the registry’s fourth year, incorporating lead data and pediatric ICD procedures, and use as a national performance measure. Heart Rhythm. 2010;7(9):1340–1345. doi: 10.1016/j.hrthm.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Kirkfeldt R.E., Johansen J.B., Nohr E.A., Moller M., Arnsbo P., Nielsen J.C. Risk factors for lead complications in cardiac pacing: A population-based cohort study of 28,860 Danish patients. Heart Rhythm. 2011;8(10):1622–1628. doi: 10.1016/j.hrthm.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Knops R.E., Olde Nordkamp L.R.A., Delnoy P.H.M., Boersma L.V.A., Kuschyk J., El-Chami M.F., Bonnemeier H., Behr E.R., Brouwer T.F., Kääb S., Mittal S., Quast A.B.E., Smeding L., van der Stuijt W., de Weger A., de Wilde K.C., Bijsterveld N.R., Richter S., Brouwer M.A., de Groot J.R., Kooiman K.M., Lambiase P.D., Neuzil P., Vernooy K., Alings M., Betts T.R., Bracke F.A.L.E., Burke M.C., de Jong J.S.S.G., Wright D.J., Tijssen J.G.P., Wilde A.A.M. Subcutaneous or transvenous defibrillator therapy. N. Engl. J. Med. 2020;383(6):526–536. doi: 10.1056/NEJMoa1915932. [DOI] [PubMed] [Google Scholar]
- 25.Zeitler E.P., Friedman D.J., Loring Z., Campbell K.B., Goldstein S.A., Wegermann Z.K., Schutz J., Smith N., Black-Maier E., Al-Khatib S.M., Piccini J.P. Complications involving the subcutaneous implantable cardioverter-defibrillator: Lessons learned from MAUDE. Heart Rhythm. 2020;17(3):447–454. doi: 10.1016/j.hrthm.2019.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westerman S.B., El-Chami M. The subcutaneous implantable cardioverter defibrillator-review of the recent data. J. Geriatr. Cardiol. 2018;15(3):222–228. doi: 10.11909/j.issn.1671-5411.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boersma L., Barr C., Knops R., Theuns D., Eckardt L., Neuzil P., Scholten M., Hood M., Kuschyk J., Jones P., Duffy E., Husby M., Stein K., Lambiase P.D. Implant and midterm outcomes of the subcutaneous implantable cardioverter-defibrillator registry: The effortless study. J. Am. Coll. Cardiol. 2017;70(7):830–841. doi: 10.1016/j.jacc.2017.06.040. [DOI] [PubMed] [Google Scholar]
- 28.Brisben A.J., Burke M.C., Knight B.P., Hahn S.J., Herrmann K.L., Allavatam V., Mahajan D., Sanghera R., Gold M.R. A new algorithm to reduce inappropriate therapy in the S-ICD system. J. Cardiovasc. Electrophysiol. 2015;26(4):417–423. doi: 10.1111/jce.12612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist is available as supplementary material on the publisher’s website along with the published article.