Abstract
Slow or non‐healing wounds caused by full‐thickness skin wounds of various origins have become a difficult challenge in clinical wound treatment. In particular, large full‐thickness skin wounds often lead to serious chronic skin wounds that do not heal. Electrospinning technology and stem cell treatment for wound repair have attracted much attention due to its unique advantages. In the current study, we electrospun polyvinyl alcohol (PVA) and bone marrow–derived stem cells (BMSCs) by a handheld electrospinning device, the distribution and interaction of cells and fibres were determined by light and electron microscopy and the cell viability and proliferation were determined by live/dead cell staining. The tissues were analysed by histology with Haematoxylin and Eosin (H&E) and Masson staining and immunohistochemical staining. We found that the fibres were distributed uniformly and BMSCs were distributed between the fibres. Cytotoxicity and cell proliferation tests proved its good biocompatibility. Histological staining shows it can accelerate wound healing and appendages regeneration by promoting granulation tissue repair. The instant PVA/stem cell fibres prepared by a handheld electrospinning device strongly promote the repair of full‐thickness skin wounds in rats. The proposed electrospinning technology is expected to have great potential in household, outdoor and battlefield first aid.
Keywords: BMSCs, cell electrospun, PVA, tissue engineering, wound
1. INTRODUCTION
Slow or non‐healing wounds caused by full‐thickness skin wounds of various origins have become a difficult challenge in clinical wound treatment in recent years. In particular, large, full‐thickness skin wounds often lead to serious chronic skin wounds that do not heal. 1 Traditional wound dressings, including inorganic nanomaterials (silver, zinc and copper) 2 and organic molecules (quaternary ammonium salt and alkylated polyethyleneimine) can be used to treat wounds. 3 , 4 However, the cellular compatibility and blood compatibility of these biomaterials are not ideal for wound healing, and large skin wounds often require skin transplantation, which has become a clinical problem.
Electrospinning technology for preparing wound dressings has attracted much attention due to its unique advantages and the hope it brings to the above problems. The fibre membrane produced by electrospinning has a large surface area and an interconnected porous structure and provides effective wound protection and gas exchange, which are especially important for absorbing wound exudate and promoting free breathing. Wound dressings prepared by electrospinning, such as polymer dressings, 5 polysaccharide dressings and protein‐derived dressings, 6 have shown good application potential and can provide a moist wound‐healing environment with ideal mechanical properties.
However, most of the current fibre dressings are prepared by electrospinning first and then applied to the wound, but this can lead to several problems: the dressing cannot fit the wound completely, especially when the wound surface is uneven; practical application of the dressings is inconvenient, such that the dressings cannot be used under certain circumstances, especially when there is no power supply in the field, and the electrospinning device is bulky and not portable.
To prepare dressings that can be directly applied to wounds, researchers have developed portable devices that can deposit nanofibers directly on the wound surface in situ to better fit over the wound surface and reduce pain. The dressing can be customised according to the individual needs of the patient. As this technology has advanced, handheld electrospinning devices have shown their potential for advanced and individualised wound care.
Zhao et al prepared an in situ wound dressing with a self‐powered portable melt‐electrospinning device and used it to successfully repair the back skin wounds of Sprague–Dawley (SD) rats. 7 Yan et al prepared a polyurethane nanofiber dressing with antibacterial activity through a portable electrospinning device and confirmed its strong antibacterial activity. 8 The previous reports on portable electrospinning devices did not involve cell electrospinning, and the effect of adding stem cells to the in situ dressings prepared by a handheld electrospinning device to repair wounds has not been studied.
Stem cell therapy has become a promising new approach in the field of regenerative medicine. Stem cells have the ability to self‐renew and differentiate into a variety of cell types, which can be highly valuable in the renewal and regeneration of physiological tissues after injury. 9 Bone marrow‐derived stem cells (BMSCs) are excellent seed cells for the treatment of different types of wounds. 10 Preclinical studies using autologous BMSCs have reported the potential therapeutic effects of these cells in dermal reconstruction and scar reduction in chronic wounds. 11 BMSCs can improve indices related to wound healing by increasing the re‐epithelialisation and the thickness of the epidermis. 12 Transplantation of allogeneic BMSCs can promote wound healing in diabetic rats. BMSCs enhance the wound healing ability of non‐diabetic and diabetic mice by promoting re‐epithelialisation, cell infiltration and angiogenesis. 12 , 13
Polyvinyl alcohol (PVA) is a safe organic polymer with good biocompatibility, biodegradability and electrospinning properties. We have studied PVA in wound healing. 14 , 15 In this study, PVA was loaded with rat BMSCs, and a handheld cell electrospinning device applied PVA/cell scaffolds to skin wounds to repair them by in situ tissue engineering (Figure 1). The cell viability, biocompatibility and proliferation of the dressings supplied by cell electrospinning were examined, and the effects of the dressings on the repair of full‐thickness skin wounds of SD rats were assessed.
FIGURE 1.
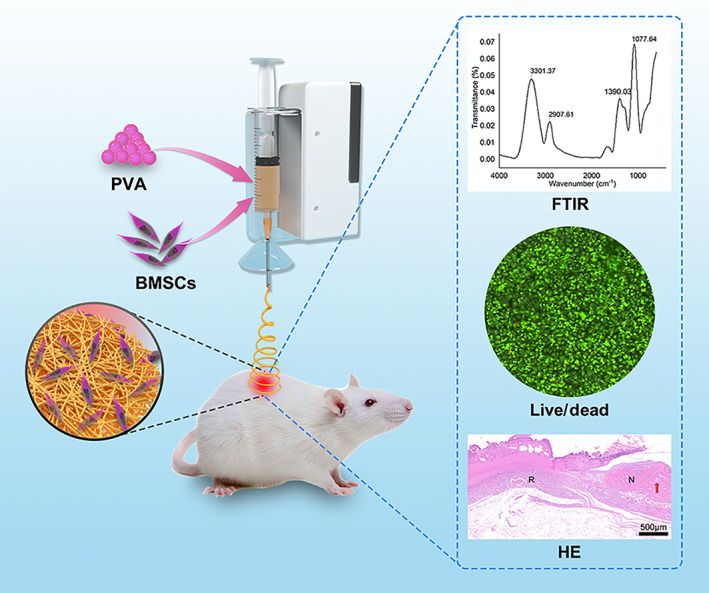
Schematic diagram of preparation of polymer fibres for wound dressing by in situ cell electrospun using a portable handheld electrospinning apparatus
2. MATERIALS AND METHODS
2.1. Materials
The main materials and device used in this study are as follows: PVA (molecular weight = 186 000, Institute of Polymer Science & Engineering, Tsinghua University), handheld electrospinning device (Junada, Qingdao, China), electron microscope (Hitachi, Japan) and cell incubator (Thermo, China). The chemicals and reagents used in this study were purchased from Sigma‐Aldrich (St. Louis, MO, USA) unless otherwise specified.
2.2. Cell extraction and culture
The primary BMSCs were extracted from 1‐ to 2‐week‐old rats (30‐50 g). After SD rats were sacrificed under anaesthesia, the femoral bone marrow was placed in a culture flask, and cells were in low‐glucose Dulbecco's Modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) (BI, South America), 100 units/mL penicillin and 100 mg/mL streptomycin and were cultured in an incubator at 37°C with 5% CO2 and saturated humidity. When the primary cells grew adherently and reached 80% confluence, the cells were passaged using a mixed solution of 0.2% trypsin and 0.02% ethylenediaminetetraacetic acid. Cells at the 3rd passage were used for subsequent experiments.
2.3. Preparation of cell scaffolds
Cell scaffolds were prepared through a handheld electrospinning device. Sterile 6%, 8% and 10% PVA solutions were prepared using sterilised phosphate‐buffered saline (PBS), and a PVA solution containing 1 × 107 BMSCs was obtained after compounding the PVA solution with rat passage‐3 BMSCs. The prepared solutions were placed into a 5‐ml syringe equipped with a nozzle with a diameter of 0.1 mm and then loaded into the handheld portable electrospinning apparatus. The high voltage of this device is about 10 kv fixed. During the in situ electrospinning process, one can firstly operate the device and then press the syringe by a finger. The electrospun fibres can be fabricated and then deposited onto the collector, as suggested in Figure 2. The cell scaffolds were collected for subsequent experiments.
FIGURE 2.
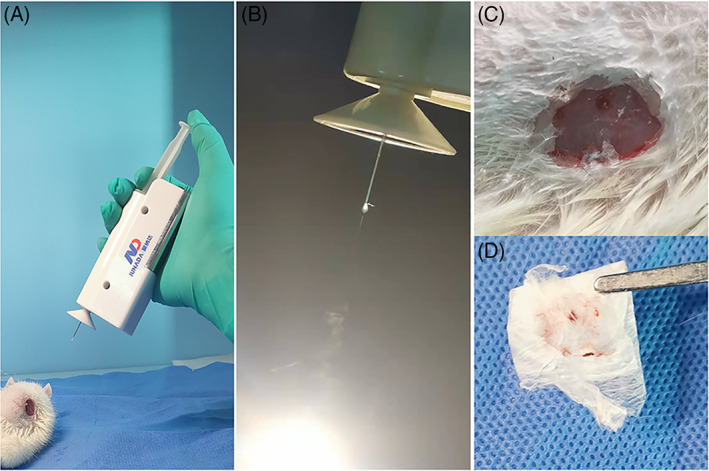
In situ applications of the handheld apparatus. (A) In situ electrospinning on the wound of rat. (B) The electrospinning jets can be seen from the spinneret. (C) The electrospun mats. (D) Electrospun film taken from rat wound
2.4. Scanning electron microscopy
The fibre membranes obtained through electrospinning in each group were dried and coated with gold, and the fibre filament formation was examined by scanning electron microscopy (SEM). The diameter of the fibre filaments was measured by ImageJ (N = 100).
2.5. Characterisation
The Fourier transform infrared spectroscopy (FTIR) spectrums were measured by a Thermo Scientific Nicolet iN10 spectrometer, the transmittance of PVA membrane was detected at the wavelength of 400 to 4000 cm−1. The scanning resolution was 4 cm−1, and the scanning time was 100 seconds. The stress–strain behaviours of the PVA electrospun fibres were tested using a universal electronic testing machine (Meters Industrial Systems, Inc., China). The air permeability under a pressure drop of 200 Pa was tested by an air permeability tester (Textest FX3300). Pore sizes of the as‐spun fibrous meshes were examined by PSM 165 (Germany, Topas GmbH, PSM 165) at pressure of 200 Pa.
2.6. Cell survival rate
To measure the survival of electrospun BMSCs, all cells were maintained in DMEM supplemented with 10% FBS (BI) and grown to the third generation. The cell/fibre scaffolds prepared as above were evenly collected onto the cell slides and cultured in an incubator at 37°C with 5% CO2 and saturated humidity for 24 hours. The live and dead cells were stained with live/dead dye, and the survival rate (%) after electrospinning was calculated as number of live cells/(number of live cells + number of dead cells) × 100%.
2.7. In vivo wound‐healing study
SD rats were provided by the Laboratory Animal Center of Guizhou Medical University, including 10 adult male or female rats, 2 to 3 months old, weighing 280 to 320 g. All procedures were carried out in accordance with the guidelines of the Laboratory Animal Center, were approved by the Animal Ethics Committee of Guizhou Medical University and were also in line with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health in 1996. The SD rats were fed routinely for 1 week and then anaesthetised with 10% chloral hydrate at the dose of 3.5 mL/kg. Three 1‐cm‐diameter full‐thickness skin wounds were made on the back of each rat. In the PVA/cell group, the PVA/stem cell scaffold was electrospun onto the defect by the handheld electrospinning device. In the PVA group, only PVA was electrospun onto the defect, and in the control group, no special treatment was performed. After the surgery, all wounds were covered with gauzes, and penicillin was injected intramuscularly in the 3 days following the surgery. The healing of the defect was observed as below.
2.8. Histological staining
The animals were anaesthetised and sacrificed at day 3, 7 or 14 after the wound was made. All tissue samples were fixed in 4% paraformaldehyde, dehydrated in gradient ethanol and embedded in paraffin. The samples were then cross‐sectioned at a thickness of 5 μm for histological analysis. The sections were deparaffinised, rehydrated and subjected to Haematoxylin and Eosin (HE) staining, Masson staining and immunohistochemical (IHC) staining for vascular endothelial growth factor (VEGF) and proliferating cell nuclear antigen (PCNA). The stained sections were examined by light microscopy.
2.9. Statistical analysis
All results are expressed as mean ± standard deviation and were subjected to one‐way analysis of variance in SPSS 18.0. *P < .05 and **P < .01 indicate that the differences were statistically significant.
3. RESULTS
3.1. SEM
Scaffolds with 6%, 8% and 10% PVA fibres and 8% PVA/cell fibres were prepared by the handheld electrospinning device (Figure 3). Examination of the 6%, 8% and 10% fibres showed that the group with 6% PVA had many bead‐like structures (Figure 3A). Fibres in the group with 8% PVA were distributed evenly, with good filament formation (Figure 3B). Fibres in the group with 10% PVA had droplets, and the thickness of the fibres was uneven (Figure 3C). Therefore, only 8% PVA was used for subsequent experiments. SEM of the BMSCs added to the PVA fibres showed that the stem cells were wrapped by fibre filaments and were covered by the fibres (Figure 3D). Under the light microscope, the cells were evenly distributed throughout the fibre filaments (Figure 3E), and the fibre diameter was 133.4 ± 29.6 μm.
FIGURE 3.
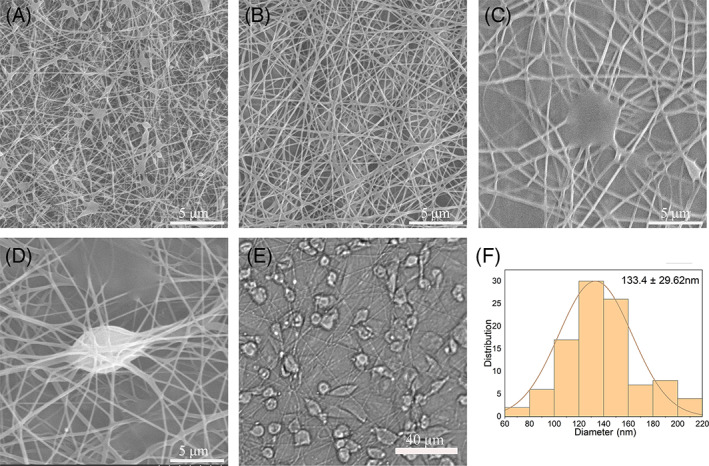
Scanning electron microscope (SEM) images of the nanofiber membranes. (A) Fibres with 6% polyvinyl alcohol (PVA) spun with a handheld electrospinning device. (B) Fibres with 8% PVA. (C) Fibres with 10% PVA. (D) Fibres with 8% PVA and cells, spun with a handheld electrospinning device. (E) Examination of cells after cell electrospinning under a light microscope. (F) Diameter distribution of the electrospun fibres
3.2. Characterisation
The FTIR spectrum of the PVA nanofibers showed absorption bands at 3301 cm−1 (OH, stretching vibration), 2907 cm−1 (C–H, stretching vibration), 1390 cm−1 (C–H, bond stretching) and 1077 cm−1 (C–O, stretching vibration). The above peaks are characteristic absorption of PVA (Figure 4A). The stress–strain curve tests show that the material broke at approximately a 200% elongation, and the fibre film could withstand stress of about 3 MPa before breaking (Figure 4B). The air permeability and average pore size of electrospun fibrous mats were 71.3 ± 10.69 mm/s and 2.52 μm (1.76‐2.77 μm).
FIGURE 4.
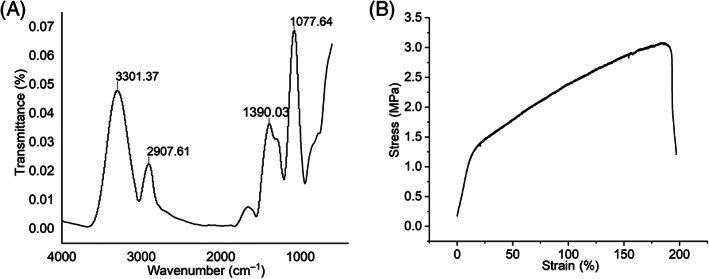
Characterisation of polyvinyl alcohol (PVA) fibre. (A) Fourier transform infrared spectroscopy (FTIR) spectra and (B) stress–strain curve of electrospun PVA nanofiber membrane
3.3. Cell survival rate after cell electrospinning with the handheld electrospinning device
The cell proliferation and biocompatibility after cell electrospinning were examined. Dead/live staining showed that the cell survival rate immediately after cell electrospinning was 90.15% (Figure 5A). After culturing the electrospun fibres for 24 hours, it was 92.98% (Figure 5B), and after 7 days, it was 99.21% (Figure 5C). The number of cells increased significantly over time.
FIGURE 5.
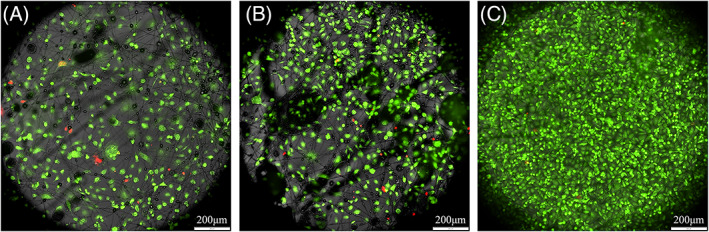
(A) Dead/live staining immediately after cell electrospinning. (B) Dead/live staining at 24 hours after cell electrospinning. (C) Dead/live staining at 7 days after cell electrospinning
3.4. General appearance of wound healing
The electrospun dressing was compared with PVA only and control group in the healing of full‐thickness skin wounds. The results showed that the wound area reduction time and wound closure time were significantly shorter in the PVA/cell group, the wound area was significantly reduced at day 7 (Figure 6). On the other hand, the wounds in the PVA group and the control group were still large at day 7, but they were all almost closed at day 14.
FIGURE 6.
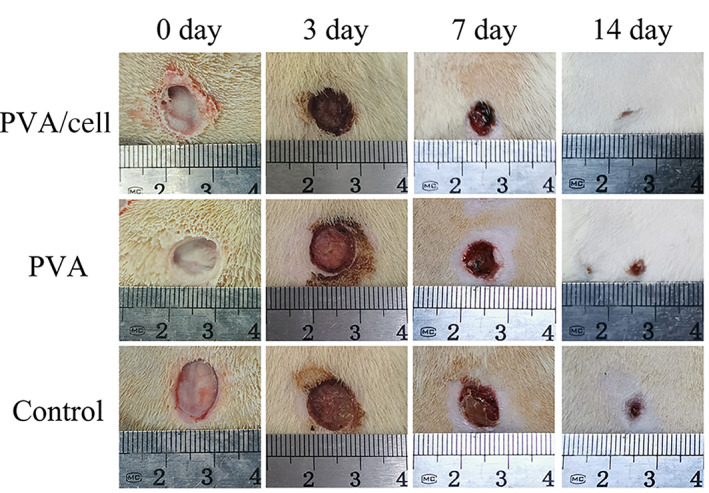
General evaluation of wound healing. Photos of non‐treated, polyvinyl alcohol (PVA)‐treated and PVA/cell fibre membrane–treated wounds at days 0, 3, 7 and 14
3.5. HE staining
In the PVA/cell group, fresh granulation tissue formation, capillary and surrounding fibroblasts and inflammatory cells were detected on the 3rd day, and the fibroblasts were proliferating and hypertrophic (Figure 7). There was less new granulation tissue in the PVA group, and only a large amount of inflammatory necrotic tissue was found in the control group, with no granulation tissue formation. On the 7th day, all wounds had shrunk. The granulation tissue in the PVA/cell group was thicker and fibrotic, the number of capillaries had fallen, the epithelial tissue had migrated to the defect center and a few skin appendages (hair follicles and sebaceous glands) had regenerated. The PVA had much new granulation tissue, many capillaries growing perpendicular to the wound surface and a few new collagen fibres. In the control group, there was a little granulation tissue formation with many capillaries inside, many epithelial tissue defects and few new collagen fibres. So, the wounds of PVA‐cell group were close to primary healing, wounds of PVA group and the control group were healed secondary. On the 14th day, the wound was closed in the PVA/cell group, the epidermis was completely covered by epithelial tissue and was epithelialised, normal skin appendages were found around the wound, the number of subcutaneous fibrocytes was increased and many new collagen fibres were detected. In the PVA group, the non‐fibrotic area and the thickness of the subcutaneous tissues were smaller than those of the PVA/cell group, the epidermis was completely covered by epithelial tissue, the tissue layer was not completely reconstructed and too few fibroblasts and collagen fibres had been generated. The wounds in the control group were still in the granulation tissue repair state, with new granulation tissue and many capillaries, fibroblasts and inflammatory cells; the epidermis still had some crust and was completely replaced by epithelial cells; the number of new fibroblasts and collagen fibres were significantly lower than those in the other groups and a few skin appendages had regenerated.
FIGURE 7.
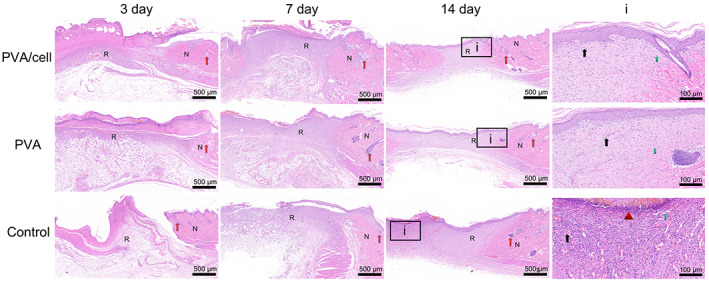
H&E staining showing the wound healing and skin regeneration of the control group, PVA group and PVA/cell group on the 3rd, 7th and 14th days. (N represents normal tissue and R represents repaired tissue, i represents the magnification of day 14, the red arrow represents appendages, the black arrow represents capillary, the green arrow represents fibrocytes, the triangle represents inflammatory cells, scar bar = 500 μm, 100 μm)
3.6. Masson staining
Masson staining reflects the process of collagen deposition during granulation tissue formation and matrix remodelling (Figure 8). The intensity of blue staining corresponds to the relative amount of total collagen fibre deposition, as collagen is bluish purple. During the 3 to 14 days of postoperative skin collagen repair process, the skin wounds gradually shrank. In the early stage, the number of collagen fibres was small, the fibres were thin, their structure was irregular and flocculent and there were many capillaries. Over time, the collagen fibres gradually thickened and arranged themselves in an orderly way, there were fewer capillaries and the regeneration of skin appendages was detected. The wound repair in the PVA‐cell group was faster than that of the PVA and control groups, and its collagen deposition was significantly faster. On post‐operative day 14, a dense and mature collagen fibre structure parallel to the epidermis had formed in the PVA‐cell group, suggesting that the PCA‐cell group had better and faster wound repair.
FIGURE 8.
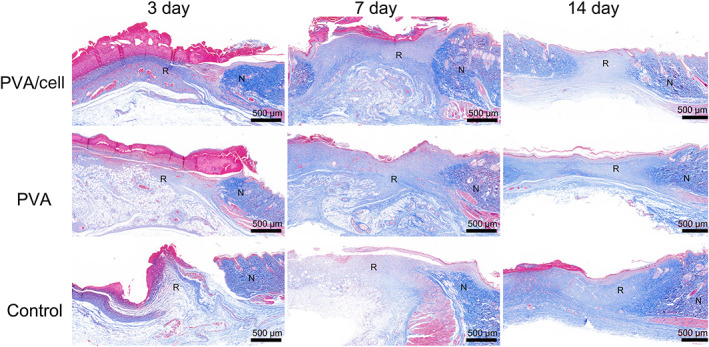
Masson staining showing the wound healing and skin regeneration of the control group, polyvinyl alcohol (PVA) group and PVA/cell group on the 3rd, 7th and 14th days. (N represents normal tissue and R represents repaired tissue, scar bar = 500 μm)
3.7. IHC staining for VEGF and PCNA
IHC staining for VEGF showed that on postoperative day 3, strong VEGF expression was found in the new granulation area in the PVA/cell group, which was around the brown blood vessel tissue of the lesion area (Figure 9). The VEGF expression in the PVA and control groups was weak. On day 7, the VEGF expression in the PVA/cell group was stronger than on day 3 and was mainly distributed in the blood vessel area. On day 14, VEGF immunolabelling in all groups was lower than on days 3 and 7.
FIGURE 9.
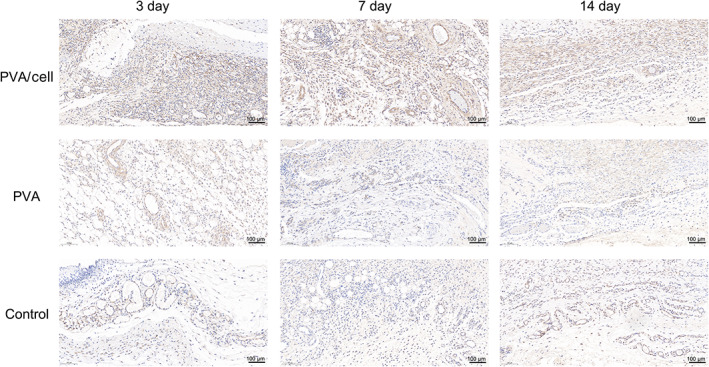
Vascular endothelial growth factor (VEGF) expression in the control group, polyvinyl alcohol (PVA) group and PVA/cell group on the 3rd, 7th and 14th days
PCNA immunolabelling was relatively intensce in the dermis and areas near fibroblasts and sebaceous glands (Figure 10). On the 3rd and 7th days after injury, PCNA immunolabelling in the PVA and control groups was similar, while the PVA/Cell group exhibited the most intense PCNA immunolabelling. The immunolabelling was more intense in all groups on day 7 than day 3. On the 14th day, the immunolabelling in each group was reduced.
FIGURE 10.
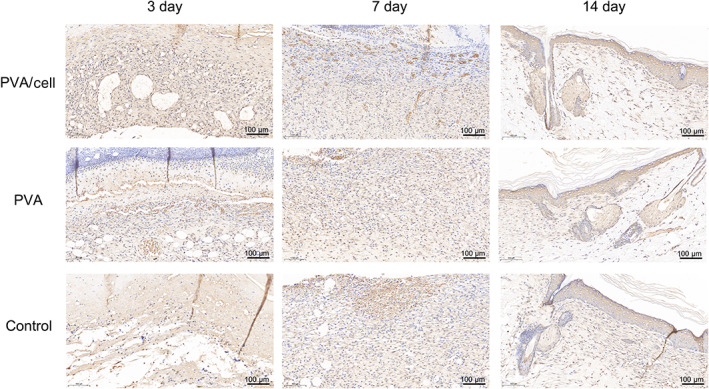
Proliferating cell nuclear antigen (PCNA) expression in the control group, polyvinyl alcohol (PVA) group and PVA/cell group on the 3rd, 7th and 14th days
4. DISCUSSION
Portable electrospinning devices are suitable for depositing fibres directly to a target site. 8 In the dressing applications, fibres are deposited in situ onto wounds, especially traumatic wounds, chronic wounds and irregular wounds because these dressings can be customised for patients, 16 which allows the fast management of the wound site and effectively promotes wound healing. Additionally, a small, portable, and battery‐powered handheld electrospinning device can be used in most locations and situations, including out‐of‐hospital first aid, in‐hospital surgery, clinics, households and outdoors, and it also enables immediate wound dressings in remote areas with poor resources and in war zones.
The cell electrospinning device used in this study is a technology based on existing electrospinning technology and can produce fibres with living cells embedded. 17 The main difference between the two is the use of living cells. To make a cell/fibre composite by cell electrospinning, the cells and fibres are distributed evenly, which solves the problems of poor infiltration, cell growth on the surface of the scaffold, difficulty penetrating inside the scaffold and uneven cell distribution that marred the traditional cell dripping technology. 18 Cell electrospinning can overcome the above shortcomings and can produce fibres containing living cells. Many kinds of cell, such as adipose stem cells, osteoblasts and cardiomyocytes, along with biocompatible materials (including PVA, alginate and collagen) have been used in studies of cell electrospinning. 19 , 20 , 21 Due to their cytotoxicity, organic solvents such as chloroform, tetrahydrofuran and acetone cannot be used for cell electrospinning, especially instant electrospinning by a handheld electrospinning device.
The viscosity and surface tension of the solution have an important impact on cell electrospinning. Generally, a solution with low viscosity is advantageous because a higher viscosity could lead to greater shear stress, which will have a negative effect on the cells. 22 Kim et al found that when the collagen content was greater than 7 wt%, the cell viability decreased significantly (<80%), but when the collagen content was less than 5 wt%, the cell viability reached 93%. 23 A too low viscosity will cause spraying of solution droplets instead of generating fibres, especially with handheld electrospinning devices.
The electric field also has an important impact on cell electrospinning. The electric field affects not only fibre generation but also cell viability, as a strong electric field can kill cells. 24 For example, when the electric field range is 0.05 to 0.075 kV/mm, the cell survival rate is as high as 90%, but when the electric field is stronger, the cell viability decreases significantly. A weak electric field (0.1 kV/mm) can result in a high cell survival rate (90%), but the obtained microfiber structure is poor. 25 In this study, PBS was used to dissolve PVA to prepare an 8% PVA solution, and stem cells were mixed with this solution to be spun using the handheld electrospinning device. In this study, the spinnability of the cell electrospinning can be clearly detected, and the stem cells were evenly distributed on the fibre filaments. The low voltage of the handheld electrospinning device did not affect cell viability, and multiple postoperative examinations showed that the cell viability was above 90%.
Wound healing is a dynamic process that includes cell migration, proliferation, angiogenesis, deposition of extracellular matrix and tissue remodelling. 26 In the early stage of wound healing, angiogenesis occurs, capillaries accompanied by fibroblasts and macrophages replace coagulating fibrin, granulation tissue forms and the distribution of type I and type III collagen secreted by fibroblasts increases. VEGF plays an important regulatory role in this process. 27 VEGF stimulates angiogenesis by inducing fibroblasts and endothelial cells to proliferate and promoting angiogenesis, re‐epithelialisation and collagen deposition. 28 In the later stage of wound healing, fibroblasts synthesise and deposit collagen; new epithelial cells, unnecessary blood vessels, fibroblasts and inflammatory cells undergo apoptosis; the scar matures and fibrous repair finally begins. 29 Stem cells can promote collagen synthesis and angiogenesis at the skin wound to accelerate skin healing. 11 When stem cells are used for treating mouse skin wounds, there is less scarring and more tissue regeneration. BMSCs can secrete and release many factors, such as epidermal growth factor, platelet‐derived growth factor, transforming growth factor beta, VEGF, hepatocyte growth factor and insulin‐like growth factor‐1, to promote angiogenesis. 30 BMSCs regulate the proliferation, migration and gene expression of dermal fibroblasts to accelerate wound healing, and they play a role in the orderly transition of matrix and tissue to reduce scar formation. 31 BMSCs produce high amounts of collagen, basic fibroblast growth factor and VEGF to accelerate the healing process. BMSCs promote skin healing by accelerating tissue re‐epithelialisation, increasing angiogenesis and directly differentiating into epithelial cells expressing keratinocyte‐specific markers. 9
This study produced biological dressings by cell electrospinning using a handheld electrospinning device and confirmed that the PVA/cell dressings had excellent biocompatibility in vitro and strongly promoted cell proliferation. The findings from full‐thickness skin wounds in rats confirmed that the composite fibre membrane in the PVA‐cell group achieved excellent wound repair and rapid healing. Compared with the control and PVA groups, the PVA‐cell group showed significantly better healing time of the skin wound and the change in wound area. In the PVA‐cell group, the wound reduction was significantly faster, and the wound closure time was significantly shortened. Evaluation of the wound healing process by histological examination showed that a thick epithelialised area was found in the PVA‐cell group at postoperative day 7, and the epidermis of the closed wound was almost the same as normal skin on the 14th day, indicating a better skin regeneration.
VEGF is involved in wound healing and is secreted by platelets, macrophages, fibroblasts and keratinocytes. 32 Increasing the VEGF level in the granulation tissue early can significantly increase the number of newly formed and mature blood vessels and promote faster dermis regeneration. 33 VEGF has shown promising results as a topical therapy for skin wounds, contributing to the inflammatory phase, the proliferation of fibroblasts and reorganisation of collagen fibres. The VEGF level decreased after 14 days because the skin had entered the structural remodelling phase. PCNA is a marker of cell proliferation, and increased PCNA expression was observed on the 3rd and 7th days in the PVA/cell group, indicating intense repair of the dermis, fibroblasts and sebaceous glands. After the wound had basically closed, on the 14th day, the PCNA expression was lower. In the control group, due to the lack of the regeneration function of stem cells, the repair of granulation tissue was slow, so it was still in the stage of granulation repair on the 14th day, and the expression levels of VEGF and PCNA were still high.
This kind of biological dressing produced by a handheld electrospinning device can be used to cover different types of wounds, can firmly attach to the wound surface without adhesives, can effectively protect the wound surface from bacterial contamination and can absorb wound exudate, thereby providing a suitable environment for wound healing. The use of cell electrospinning enables the effective application of stem cells for skin wound repair, which shortens the wound repair time and promotes wound healing. Some electrospun dressings, such as polymers, 34 , 35 proteins 36 and biohydrogels, 37 have shown good effects in promoting wound healing. Compared with the dressings reported in the literature, this novel PVA/stem cell dressing showed the following advantages in wound healing: 1) it has excellent biocompatibility and promotes cell proliferation; 2) these individualised in situ electrospun dressings are suitable for all types of wounds and 3) stem cell–driven repair is included by the cell electrospinning, which has not been reported in previous studies. Its portable operation, individualised structure, good biocompatibility, stem cell–driven repair and excellent skin regeneration capabilities make it a promising method for wound repair. Other than that, functional wound dressing can be prepared using this system by adding materials with antibacterial, hemostatic and other substances into PVA, such as antibacterial Ag nanoparticles. Then, the composite wound dressings have antibacterial properties. Therefore, we can prepare multifunctional wound dressing by portable handheld electrospinning apparatus conveniently. This research laid the foundation for future animal experiments and clinical trials. And it is expected to play a positive role in wound repair, haemostasis, fracture healing and cartilage regeneration.
We have achieved good experimental results with cell electrospinning using portable handheld electrospinning instrument, but several problems still need to be addressed: (1) Even the skin wound repair reached a similar effect to that of primary healing, but the underlying mechanism is unclear and (2) relative low electrospinning efficiency and inaccurate deposition of fibre were observed in this research using portable handheld electrospinning instrument. In our future studies, it is necessary to further clarify the wound repair promotion mechanism of cell electrospinning.
5. CONCLUSIONS
In this study, the PVA/stem cell fibre dressings prepared by cell electrospinning with a handheld electrospinning device can be deposited onto target sites in situ. By stem cell–driven repair, the dressing had an excellent effect of promoting the repair of full‐thickness skin wounds in rats. This proposed electrospinning technology is expected to have great potential in household, outdoor and battlefield first aid.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
The authors appreciate the finical supports from the Science and Technology Fund Project of Guizhou Health Commission (gzwkj2021‐233).
Xu S, Lu T, Yang L, Luo S, Wang Z, Ye C. In situ cell electrospun using a portable handheld electrospinning apparatus for the repair of wound healing in rats. Int Wound J. 2022;19(7):1693‐1704. doi: 10.1111/iwj.13769
Funding information Science and Technology Fund Project of Guizhou Health Commission, Grant/Award Number: gzwkj2021‐233
DATA AVAILABILITY STATEMENT
Data openly available in a public repository that issues datasets with DOIs.
REFERENCES
- 1. Sorg H, Tilkorn DJ, Hager S, Hauser J, Mirastschijski U. Skin wound healing: an update on the current knowledge and concepts. Eur Surg Res. 2017;58(1–2):81‐94. doi: 10.1159/000454919 [DOI] [PubMed] [Google Scholar]
- 2. Nethi SK, Das S, Patra CR, Mukherjee S. Recent advances in inorganic nanomaterials for wound‐healing applications. Biomater Sci. 2019;7(7):2652‐2674. doi: 10.1039/c9bm00423h [DOI] [PubMed] [Google Scholar]
- 3. Mofazzal Jahromi MA, Sahandi Zangabad P, Moosavi Basri SM, et al. Nanomedicine and advanced technologies for burns: preventing infection and facilitating wound healing. Adv Drug Deliv Rev. 2018;123:33‐64. doi: 10.1016/j.addr.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34(3):599‐610. doi: 10.1007/s12325-017-0478-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feng ZY, Wang CD, Park SJ, Meng W, Meng LY. Polyamide/chitosan/tetraethyl orthosilicate electrospun nanofibers for a novel and promising drug carrier. J Nanosci Nanotechnol. 2021;21(12):5912‐5919. doi: 10.1166/jnn.2021.19511 [DOI] [PubMed] [Google Scholar]
- 6. Lin N, Zuo B. Silk sericin/fibroin electrospinning dressings: a method for preparing a dressing material with high moisture vapor transmission rate. J Biomater Sci Polym Ed. 2021;32(15):1983‐1997. doi: 10.1080/09205063.2021.1952383 [DOI] [PubMed] [Google Scholar]
- 7. Zhao YT, Zhang J, Gao Y, et al. Self‐powered portable melt electrospinning for in situ wound dressing. J Nanobiotechnol. 2020;18(1):111. doi: 10.1186/s12951-020-00671-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yan X, Yu M, Ramakrishna S, Russell SJ, Long YZ. Advances in portable electrospinning devices for in situ delivery of personalized wound care. Nanoscale. 2019;11(41):19166‐19178. doi: 10.1039/c9nr02802a [DOI] [PubMed] [Google Scholar]
- 9. Pang C, Ibrahim A, Bulstrode NW, Ferretti P. An overview of the therapeutic potential of regenerative medicine in cutaneous wound healing. Int Wound J. 2017;14(3):450‐459. doi: 10.1111/iwj.12735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fu X, Liu G, Halim A, Ju Y, Luo Q, Song AG. Mesenchymal stem cell migration and tissue repair. Cell. 2019;8(8):784. doi: 10.3390/cells8080784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang J, Guan J, Niu X, et al. Exosomes released from human induced pluripotent stem cells‐derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49. doi: 10.1186/s12967-015-0417-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hu L, Wang J, Zhou X, et al. Author correction: exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep. 2020;10(1):6693. doi: 10.1038/s41598-020-63068-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kwon DS, Gao X, Liu YB, et al. Treatment with bone marrow‐derived stromal cells accelerates wound healing in diabetic rats. Int Wound J. 2008;5(3):453‐463. doi: 10.1111/j.1742-481X.2007.00408.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ma MX, Liu Q, Ye C, Grottkau B, Guo B, Song YF. Preparation of P3HB4HB/(gelatin + PVA) composite scaffolds by coaxial electrospinning and its biocompatibility evaluation. Biomed Res Int. 2017;2017:9251806. doi: 10.1155/2017/9251806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu T, Zou Q, Zhu K, Yuan D, Ma M, Ye C. Electrospun egg white/polyvinyl alcohol fiber dressing to accelerate wound healing. J Polym Res. 2021;28(2):67. doi: 10.1007/s10965-021-02422-3 [DOI] [Google Scholar]
- 16. Fuenteslópez CV, Ye H. Electrospun fibres with hyaluronic acid‐chitosan nanoparticles produced by a portable device. Nanomaterials (Basel, Switzerland). 2020;10(10):2016. doi: 10.3390/nano10102016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yeo M, Kim G. Micro/nano‐hierarchical scaffold fabricated using a cell electrospinning/3D printing process for co‐culturing myoblasts and HUVECs to induce myoblast alignment and differentiation. Acta Biomater. 2020;107:102‐114. doi: 10.1016/j.actbio.2020.02.042 [DOI] [PubMed] [Google Scholar]
- 18. Hong J, Yeo M, Yang GH, Kim G. Cell‐electrospinning and its application for tissue engineering. Int J Mol Sci. 2019;20(24):6208. doi: 10.3390/ijms20246208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen H, Liu Y, Hu Q. A novel bioactive membrane by cell electrospinning. Exp Cell Res. 2015;338(2):261‐266. doi: 10.1016/j.yexcr.2015.08.007 [DOI] [PubMed] [Google Scholar]
- 20. Jayasinghe SN. Cell electrospinning: a novel tool for functionalising fibres, scaffolds and membranes with living cells and other advanced materials for regenerative biology and medicine. Analyst. 2013;138(8):2215‐2223. doi: 10.1039/c3an36599a [DOI] [PubMed] [Google Scholar]
- 21. Dong C, Lv Y. Application of collagen scaffold in tissue engineering: recent advances and new perspectives. Polymers (Basel). 2016;8(2):42. doi: 10.3390/polym8020042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gopinathan J, Noh I. Recent trends in bioinks for 3D printing. Biomater Res. 2018;22:11. doi: 10.1186/s40824-018-0122-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim YB, Lee H, Kim GH. Strategy to achieve highly porous/biocompatible macroscale cell blocks, using a collagen/genipin‐bioink and an optimal 3D printing process. ACS Appl Mater Interfaces. 2016;8(47):32230‐32240. doi: 10.1021/acsami.6b11669 [DOI] [PubMed] [Google Scholar]
- 24. Yeo M, Kim GH. Anisotropically aligned cell‐laden nanofibrous bundle fabricated via cell electrospinning to regenerate skeletal muscle tissue. Small. 2018;14(48):e1803491. doi: 10.1002/smll.201803491 [DOI] [PubMed] [Google Scholar]
- 25. Yeo MG, Kim GH. Fabrication of cell‐laden electrospun hybrid scaffolds of alginate‐based bioink and PCL microstructures for tissue regeneration. Chem Eng J. 2015;275:27‐35. doi: 10.1016/j.cej.2015.04.038 [DOI] [Google Scholar]
- 26. Sapienza P, Mingoli A, Borrelli V, et al. Inflammatory biomarkers, vascular procedures of lower limbs, and wound healing. Int Wound J. 2019;16(3):716‐723. doi: 10.1111/iwj.13086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173(2):370‐378. doi: 10.1111/bjd.13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kondo T, Ishida Y. Molecular pathology of wound healing. Forensic Sci Int. 2010;203(1–3):93‐98. doi: 10.1016/j.forsciint.2010.07.004 [DOI] [PubMed] [Google Scholar]
- 29. Chitturi RT, Balasubramaniam AM, Parameswar RA, Kesavan G, Haris KT, Mohideen K. The role of myofibroblasts in wound healing, contraction and its clinical implications in cleft palate repair. J Int Oral Health. 2015;7(3):75‐80. [PMC free article] [PubMed] [Google Scholar]
- 30. Harrell CR, Gazdic M, Fellabaum C, et al. Therapeutic potential of amniotic fluid derived mesenchymal stem cells based on their differentiation capacity and immunomodulatory properties. Curr Stem Cell Res Ther. 2019;14(4):327‐336. doi: 10.2174/1574888X14666190222201749 [DOI] [PubMed] [Google Scholar]
- 31. Chen L, Xu Y, Zhao J, et al. Conditioned medium from hypoxic bone marrow‐derived mesenchymal stem cells enhances wound healing in mice. PLoS One. 2014;9(4):e96161. doi: 10.1371/journal.pone.0096161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Borena BM, Martens A, Broeckx SY, et al. Regenerative skin wound healing in mammals: state‐of‐the‐art on growth factor and stem cell based treatments. Cell Physiol Biochem. 2015;36(1):1‐23. doi: 10.1159/000374049 [DOI] [PubMed] [Google Scholar]
- 33. Khalaf AA, Hassanen EI, Zaki AR, Tohamy AF, Ibrahim MA. Histopathological, immunohistochemical, and molecular studies for determination of wound age and vitality in rats. Int Wound J. 2019;16(6):1416‐1425. doi: 10.1111/iwj.13206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao T, Li X, Gong Y, Guo Y, Quan F, Shi Q. Study on polysaccharide polyelectrolyte complex and fabrication of alginate/chitosan derivative composite fibers. Int J Biol Macromol. 2021;184:181‐187. doi: 10.1016/j.ijbiomac.2021.05.150 [DOI] [PubMed] [Google Scholar]
- 35. Fan T, Daniels R. Preparation and characterization of electrospun polylactic acid (PLA) fiber loaded with birch bark triterpene extract for wound dressing. AAPS PharmSciTech. 2021;22(6):205. doi: 10.1208/s12249-021-02081-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mensah RA, Jo SB, Kim H, et al. The eggshell membrane: a potential biomaterial for corneal wound healing. J Biomater Appl. 2021;36(5):912‐929. doi: 10.1177/08853282211024040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Squinca P, Berglund L, Hanna K, et al. Multifunctional ginger nanofiber hydrogels with tunable absorption: the potential for advanced wound dressing applications. Biomacromolecules. 2021;22(8):3202‐3215. doi: 10.1021/acs.biomac.1c00215 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data openly available in a public repository that issues datasets with DOIs.


