Abstract
The prevention of hospital‐acquired pressure injuries (HAPIs) in children undergoing long‐duration surgical procedures is of critical importance due to the potential for catastrophic sequelae of these generally preventable injuries for the child and their family. Long‐duration surgical procedures in children have the potential to result in high rates of HAPI due to physiological factors and the difficulty or impossibility of repositioning these patients intraoperatively. We developed and implemented a multi‐modal, multi‐disciplinary translational HAPI prevention quality improvement program at a large European Paediatric University Teaching Hospital. The intervention comprised the establishment of wound prevention teams, modified HAPI risk assessment tools, specific education, and the use of prophylactic dressings and fluidized positioners during long‐duration surgical procedures. As part of the evaluation of the effectiveness of the program in reducing intraoperative HAPI, we conducted a prospective cohort study of 200 children undergoing long‐duration surgical procedures and compared their outcomes with a matched historical cohort of 200 children who had undergone similar surgery the previous year. The findings demonstrated a reduction in HAPI in the intervention cohort of 80% (p < 0.01) compared to the comparator group when controlling for age, pathology, comorbidity, and surgical duration. We believe that the findings demonstrate that it is possible to significantly decrease HAPI incidence in these highly vulnerable children by using an evidence‐based, multi‐modal, multidisciplinary HAPI prevention strategy.
Keywords: double protection strategy (DPS), paediatric pressure ulcers, prevention, prophylactic dressings
1. INTRODUCTION
1.1. Problem description
The increasing complexity of paediatric patients in terms of both acute morbidity and associated comorbidities, combined with the requisite technological interventions and devices that are in contact with the skin resulting in a high risk of multiple sites of skin breakdown, device, and pressure injuries. These wounds are classified as hospital‐acquired pressure injuries (HAPI) and within this group of wounds, we decided to focus our attention specifically on HAPI developed in the operating room (OR) and critical care areas. Our hospital is a major European paediatric center for cardiothoracic and neurosurgery and we have observed that our paediatric patients undergoing long‐duration surgical procedures (LDSP) had a relatively high rate of OR acquired PIs. As already described in several studies, patients undergoing LDSP with limited possibilities for repositioning because of surgical positioning constraints, are more prone to developing PI and friction‐shearing injuries. 1 , 2
In a paediatric population, the risk of HAPI development is magnified due to the fragility of the skin due to a reduced thickness of the dermal‐epidermal complex and the presence of minimal adipose tissue. Furthermore, assisted mechanical ventilation, both in the OR and in the intensive care unit (ICU), coupled with a precarious metabolic balance sometimes precludes our ability to optimally reposition patients to minimise pressure on high‐risk anatomical sites such as bony prominences. 1 , 3 Finally, the physiological edema of the skin mantle and the instability of the microclimate at the skin/surface interface as well as the immature thermoregulatory system are all co‐responsible for an increased propensity to develop skin breakdown and related major injuries.
2. BACKGROUND AND AVAILABLE KNOWLEDGE
The prevention of pressure injuries in adults and the elderly has been explored by many studies, whereas prevention focused on children is a pioneering field. The fact that children with complex health needs are more prone to develop pressure injuries due to their skin fragility has already been noted by many studies and expert panels. 4 Our most recent understanding of anatomophysiopathology of the skin in the early phases of life correlates this fragility with the reduced cohesion between epidermis and dermis, a true immature dermal instability, an alkaline skin surface, and some transient deficiencies of zinc and subcutaneous fat. 5 The incidence of HAPI in paediatric population is remarkable, with the highest prevalence among children hospitalised in intensive care with some authors reporting incidence rates of 20% to 30% in NICU and PICU settings both characterised by highest patient's complexity. 4 , 5 Furthermore, patients' complexity and critical illness is directly proportional to the presence of devices in contact with child's skin. 6 , 7 These are life‐saving devices, however, when not correctly managed, they have the potential to severely harm the child's skin resulting in medical device‐related injuries (MDRI). 8
The prevention of MDRI in children is an under‐investigated problem and should be pursued through careful review of the materials used in the devices' manufacture, strict repositioning protocols as well as preventing direct contact between child's skin and device itself by interposing protective materials between the skin and the device. 9 , 10 We highlight that serious pressure injuries acquired in childhood may have long‐term consequences requiring further surgical management of potentially disfiguring scars. These injuries also often require long‐term psychological support, especially during adolescence and may result in life‐long negative sequelae. 11 , 12 , 13
It must be remembered that the word “child” refers to a unique stage in the lifespan encompassing enormous physiological change and variability. A paediatric patient may be a 600‐g premature neonate or a 100 Kg adolescent. Obviously, the pressure injury prevention needs of these individuals would vary greatly based on their age, physiology, maturity of tissues such as the skin, and presenting pathology. These and many other factors must be considered when matching preventative measures to the age‐dependent needs of the child. 11
The different body weight proportions and anatomical topography of children result in different pressure injury risk profiles and incidence characteristics compared to adults. Children experience relatively more occipital pressure injuries whereas adults are more likely to develop more sacral and heel injuries. 12
Several risk assessment scales have been proposed in order to identify and stratify patients based on their predicted risk of developing a pressure injury as well as the nature and intensity of preventative measures required to prevent these injuries, however paediatric populations require specifically designed and validated risk assessment instruments which vary from those commonly used in adult populations. 13 The unique physiological characteristics of children make the use of adult risk assessment scales inappropriate for paediatric practice. The introduction of the Braden QD scale represented an important advance in paediatric HAPI prevention by focusing not only on children's risk factors but also including the consideration of medical devices that may be present. 14 More recently, a metanalysis reported “moderate accuracy” for the Braden Q scale in predicting pressure ulcer risk in the PICU 15 suggesting the need to improve scale's performance. Expert opinion suggests that current risk assessment scales available for paediatric use are only partially reliable and consequently there have been suggestions to integrate physiological parameters which are specific to different ages within the paediatric spectrum. 16 , 17
We note the recent emergence of artificial networks for the prediction the risk of developing intraoperative pressure injuries, but their routine application is, in our opinion, very limited at this time. 18 , 19
The prevention of paediatric HAPI has primarily focused on settings considered as constituting the highest risk for children, such as the ICU. There has been limited focus on the risk to children of intraoperative HAPI. Long duration surgical procedures (LDSP) result in the child's body lying immobile for many hours on an OR table surface not specifically designed for the minimization of pressure and shear forces generated during LDSP. Often the child's position cannot be changed due to the requirements of surgical procedure being undertaken such as in neurosurgery, additionally, skin checks cannot be performed due to the need to preserve the sterile field. Consequently, pressure injuries arising during an LDSP, may not be detected immediately after surgery because of the patient's conditions often limiting timely post‐operative skin checks. The common consequence of this situation is that the HAPI will be discovered later in the receiving unit and likely to be misdiagnosed as not being related to the time in surgery. 19
If the paediatric patient, then requires prolonged post‐operative intensive care due to physiological instability, further delays may occur in risk assessment and repositioning. These compounding factors can result in the development of more severe HAPI. The risks of developing an intraoperative HAPI have predominantly been investigated in adult surgical patients and linked to pre‐op, perioperative, and post‐op factors. Preoperative risk factors that have been identified include chronic comorbidities such as diabetes, cardiovascular disease, lean body mass, lower level of albumin, and elevated levels of lactate have been significantly associated with pressure injuries development. 19 , 20 , 21 Perioperative risk factors associated with pressure injury development are surgery duration (>6 h), the application of external forces, prone positioning, intraoperative cardio‐pulmonary bypass, significant blood loss, and corticosteroids administration. 17 , 22
The intraoperative‐related risk of developing a pressure injury has a dose‐response association with surgery duration and was found to have a linear relationship, increasing with the duration of the procedure. 23 Post‐operative risk factors have been identified as relating to the length of post‐operative hospitalisation (>5 days), prolonged post‐op immobility and post‐op ICU stay. 3
In paediatric patients, the duration of surgery risk of pressure injury varies according to child's age. This is due to the interplay of many factors such as age‐dependent or comorbidity‐dependent soft tissues composition, different pressure thresholds able to cause tissue damage as well as metabolic status and stability. It should be noted that babies' metabolic stability is significantly more variable compared to older children and this may increase the risk for babies to develop a perioperative HAPI.
A number of intraoperative support surfaces have been tested in adults for PI prophylaxis in specific anatomical sites such as the occiput or applied to the whole body or dedicated to a special class of high‐risk patients such as those affected by spinal pathology. 24 , 25 The use of silicone foam dressings during spinal surgery has been advocated to reduce HAPI rates. 26 , 27 , 28 As already noted, wide paediatric age‐dependent differences exist in relation to intraoperative HAPI risk, the study of support surfaces has frequently been supplemented by pressure mapping technology focused on redistributing age‐dependent pressure on specific body areas such as the occiput. 29 Bioengineering studies, comparing paediatric tissues and support surface properties stress that paediatric support surfaces should be characterised by adjustability and adaptability. 30 In children with disabilities, these characteristics are even more important as the surface needs to adapt to abnormal and potentially worsening biometry. 31
The choice of support surfaces to be used in the OR for the prevention of HAPI must also meet several important safety criteria including being hypoallergenic, latex and DEHP‐free, not electrically conductive, impermeable to fluids, easily able to be decontaminated, and should be reusable.
Several studies have evaluated the intraoperative effectiveness of different prophylactic dressings on adult anatomical high‐risk sites (sacrum, heels), among the dressings investigated, the foams and 5‐layer silicone bordered foam dressings appeared the most protective against PI development. 32 , 33 , 34 , 35 Very few studies have investigated the prevention of intraoperative paediatric pressure injuries and they are limited by only testing the effectiveness of prophylactic foam dressings during OR positioning. 36 We believe that prophylactic intraoperative dressings for paediatric populations need to be skin‐friendly, flexible, able to mould to anatomical contours, not easily dislodged and be available in a range of sizes.
As noted by Razmus et al. most of preventative interventions in paediatrics reported to date are the use of skin assessment and pressure redistributing surfaces. 33 , 37 , 38
2.1. Rationale for the study
The field of paediatric pressure injury prevention is very complex there is a need to explore new and innovative methods to protect children from avoidable injury. Thus, the rationale for the conduct of our study was to attempt to determine if we could improve the safety of children undergoing LDSP at our hospital by bringing together the best available current evidence on paediatric intraoperative PI prevention into one cohesive, evidence‐based, institution‐wide prevention strategy for children at high risk of developing intraoperative PI whilst under our care.
3. METHODS
3.1. Aim
The aim of the study was to investigate the effectiveness of a quality improvement method for preventing or reducing the number and severity of intraoperative and post‐surgical HAPI in children undergoing long duration surgical procedures (LDSPs). We postulated that for a HAPI prevention strategy to be effective in this high‐risk paediatric population would require an institution‐wide multidisciplinary and multi‐modal translational quality improvement approach.
3.2. Objectives
Establish a multidisciplinary team for HAPI prevention involving nurses and physicians working in operating rooms and in critical care areas, experienced in positioning and repositioning, and aware of the clinical risk represented by exposure to unrelieved pressure and shear forces.
Minimise pressure injuries and device‐related pressure injuries in paediatric patients undergoing LDSP, regardless of the patient's age and comorbidities.
Avoid an increase in hospitalisation due to pressure injuries and subsequent direct and indirect costs (future cost due to the treatment of sequelae).
Prevent permanent injuries such as scarring, scarring alopecia, retracting scars, disfigurement, and the necessity for supplemental surgical procedures due to HAPI.
Reduce/eliminate the delay in rehabilitation procedures due to HAPI.
Reduce the potential for litigation due to HAPI.
3.3. Study design
The study was designed as a monocentric, interdepartmental, interventional (non‐pharmacological) prospective cohort quality improvement study with historical comparators of children who had previously undergone long duration surgical procedure in the preceding 12‐months.
3.4. Study setting and context
The study was conducted at Bambino Gesu' Children's Hospital located in Rome, Italy from 2018 to 2019. The hospital is a major specialist referral paediatric and research, university hospital for Italy and Europe. The operating rooms, critical and intensive care units were the main settings for the study.
3.5. Definition of long duration surgical procedures (LDSP)
Due to the variability in the maturation of children across the paediatric age spectrum we define the duration of LDSP according to the child's age as follows.
Over 3 h for children 0 to 3 years of age.
Over 5 h for up to 10 years of age.
Over 6 h for 10 to 18 years of age.
3.6. Study population
The study population (n = 200) was composed of a cohort of paediatric inpatients aged 0 to 18, presenting with any comorbidities and diseases, admitted to any department of our institution, undergoing long duration surgical procedures (Table 2) and protected intraoperatively with DPS protocol (Diagram 1). The comparator group (n = 200) was composed of a cohort of historical paediatric in‐patients who had undergone LDSPs prior to the introduction of the DPS intervention. Both groups (case and comparator) were homogenous in the presenting diagnoses and ages. Very complex and unique patients were excluded from the final analysis when no historical comparator was available, for example, genodermatosis of bullous type, complex autoimmune disorders, rare syndromes with a complex skin involvement were excluded from eligibility as were children presenting with an existing pressure injury (Table 1).
TABLE 2.
Long duration surgical procedures (LDPS) criteria
| Age | Surgery duration |
|---|---|
| a | >1 or ½ h |
| 0 to 3 years | >3 h |
| 3 to 10 years | >5 h |
| 10 to 18 years | >6 h |
Patients presenting comorbidities such as prematurity, very low BMI (<16), obesity (>30), haemodynamic instability, very low 02saturation (<90 pO2), ECMO, skin diseases (GVHD, HVGD) acquired post‐septic skin disorders, pre‐existing pressure injuries; are considered at major risk if undergoing surgery lasting more than 1 or ½ h.
DIAGRAM 1.
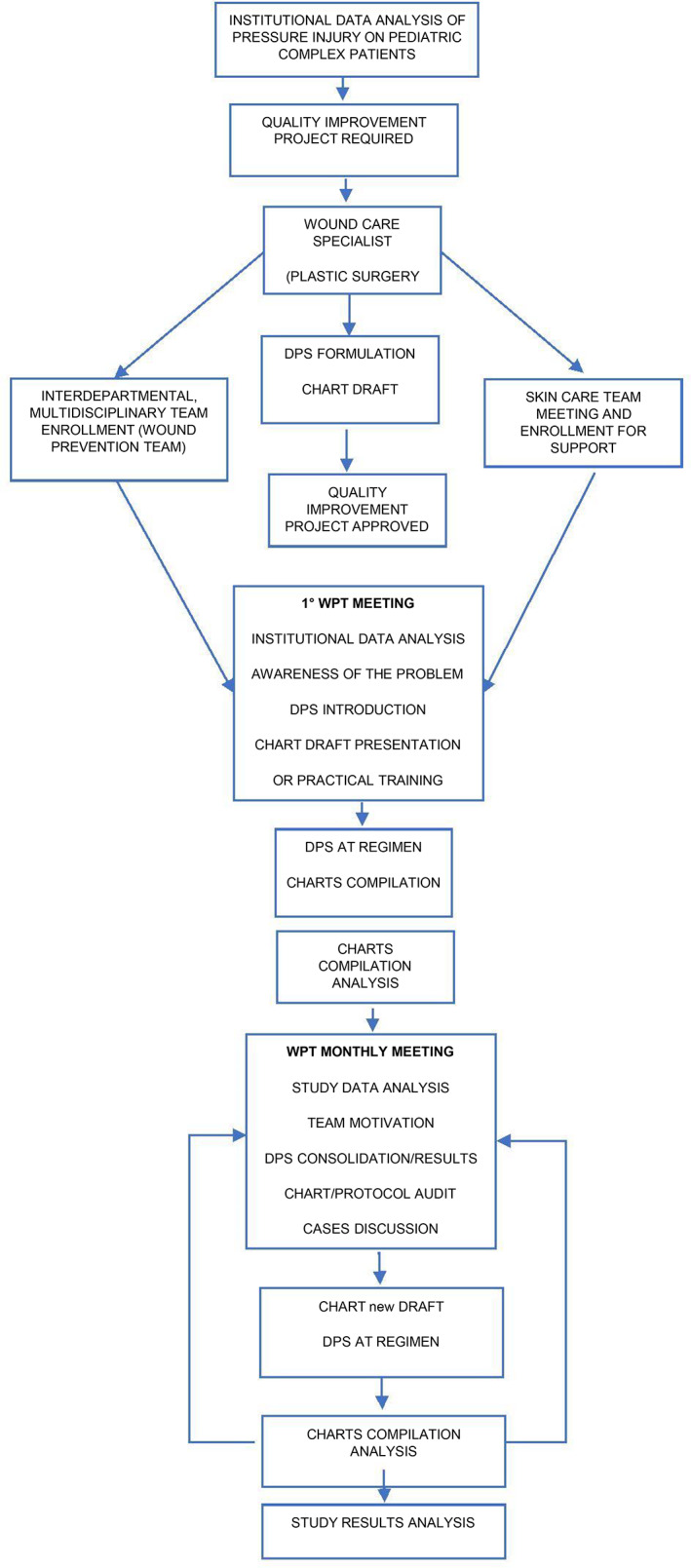
DPS study flow chart
TABLE 1.
Case and control populations
| Group A: Double protection strategy (DPS) | Group B: Standard care (No DPS) |
|---|---|
| N200 | N200 |
| Admitted since January 2019 | Admitted up to December 2018 |
| Age 0 to 18 years | Age 0 to 18 years |
| Inpatients | Inpatients |
| Undergoing long duration surgical procedures a | Undergoinglong duration surgical procedures a |
| (LDSP) | (LDSP) |
| Any comorbidities b | Any comorbidities b |
| No pre‐existing pressure injuries | No pre‐existing pressure injuries |
LDSPs have been defined according to duration and patient's age: Over 3 h for children from 0 to 3 years, over 5 h up to 10 years, over 6 h for patients aged from 10 to 18 years.
Patients' presenting comorbidities such as prematurity, very low or BMI (<16), obesity (>30), hemodynamic instability, very low saturation (<90 pO2), ECMO, skin diseases (GVHD, HVGD) acquired post‐septic skin disorders, pre‐existing pressure ulcers, were considered at major risk if undergoing surgery lasting more than 1 or 1.5 (Table 2).
Stratification of patients was performed according to age, surgical procedure, and surgical duration. Based on these parameters, 3 main ORs were included in the study;1. Cardiothoracic and Hemodynamic OR, 2. General‐Neonatal Neurosurgical and Transplantation OR and 3. Plastic, Facial, Gastrointestinal OR.
3.7. Intervention
As previously noted, it is clear from the current literature that the development of an intraoperative HAPI is a complex and highly variable process and particularly so in children. We believed that to achieve our goal of reducing HAPI rates and improving the safety of children undergoing LDSP at our hospital we would need to design a multi‐modal, evidence‐based, interventional model including all departments involved in the care of these vulnerable patients. Our intervention comprised the following elements.
A Wound Prevention Team (WPT),
Collaboration with existing Wound Ostomy Continence Nurses (WOCNs) and the Skin Care Team,
The provision of specific HAPI prevention education,
Development of a specific PI risk assessment scale based on the Braden Q and Braden QD scales, and
Development of a HAPI prevention protocol known as the Double Protection Strategy (DPS) for the intraoperative and the post‐operative periods in ICU.
3.8. Wound prevention team
The WPT comprised more than 45 nurses, 5 surgeons and all departmental WOCN who were also part of the hospital Skin Care Team. The aim of this inter‐disciplinary team was to significantly reduce the risk to children undergoing LDSP of developing HAPI intraoperatively. To achieve this aim, the WPT drew on available evidence in the areas of OR HAPI prevention including the use of prophylactic dressings, MDRPI prevention, OR support surfaces, risk assessment scales, education, and hospital‐wide HAPI prevention strategies.
The WPT undertook a series of educational and training meetings aimed at developing and standardising the preventative strategies to be applied to different surgical positions used during LDSP to maximise the protection of at‐risk anatomical areas previously identified for each surgical procedure. The outcome of this planning and iterative development process was the DPS. The DPS consists of the strategic protection of anatomical areas at intraoperative HAPI risk with fluidized positioners coupled with self‐adherent multi‐layer foam dressings. The DPS that was used in the OR was also maintained during the subsequent stay in neonatal or paediatric intensive care units. Another important outcome was the development and deployment of the Stracchini Scale (SS) which was a refinement of Braden Q and Braden QD scales 39 that we believed better met the needs of our high‐risk children.
Ongoing skin integrity monitoring was maintained using a specific chart created to follow the patient from the pre‐operative phase to discharge. The chart allowed the team to report and share patient and procedure‐related risk, recording surgical positions and identifying areas where the DPS was applied.
3.9. Educational component
Effective communication and education strategies are essential to the effectiveness of HAPI prevention at an institutional level. The DPS project was led by the Bambino Gesu' Senior Plastic Surgeon who is also a recognised wound management expert. This leadership involved the coordination of four hospital surgical departments that joined the WPT by nominating one surgeon and two reference nurses from each department. Additionally, 20 WOCN from the hospital Skin Care Team also joined the WPT thus forming a diverse yet cohesive expert group with connections to most clinical staff of the hospital. We strongly believe that this interdisciplinary structure is essential to the effective dissemination and implementation of a HAPI program across a complex large institution.
Educational sessions were conducted with OR staff (Figure 1). These sessions focused on the DPS process and included risk assessment, surgical positions deemed to cause greatest risk for HAPI to children (Figure 2), examples of previous HAPIs developed during LDSP. It was interesting to note that due to the relative isolation of OR staff, many did not appreciate that the OR for LDSP posed such a great PI risk to children. Subsequent sessions covered inclusion and exclusion criteria for the study, the actual procedure of DPS including a review of the fluidized positioners and the most effective method of use and similar detail was provided on the concurrent use of the multi‐layer silicone foam dressings to protect boney prominences for each surgical position (Figure 3). Following the commencement of the study, ongoing sessions were provided to OR staff detailing the changes to HAPI incidence in LDSP patients. We believed that these sessions were very important for staff as they reinforced the effectiveness of the DPS and provided the WPT with valuable feedback to further refine our prevention strategy. Videos of the education sessions were made available to all staff through the institutional intranet to further inform and reinforce the DPS process.
FIGURE 1.

Wound prevention team (WPT) meeting
FIGURE 2.
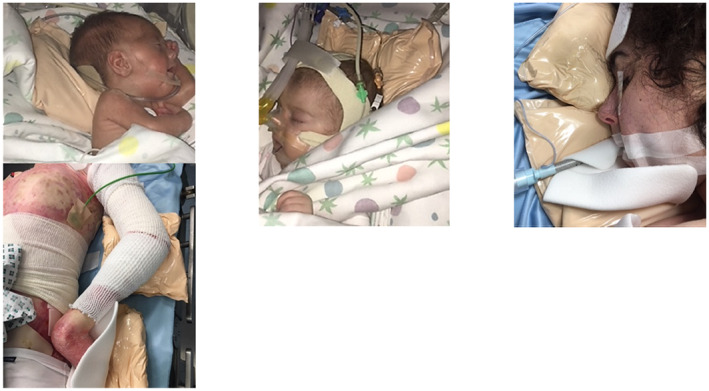
DPS interventions on different paediatric patients
FIGURE 3.
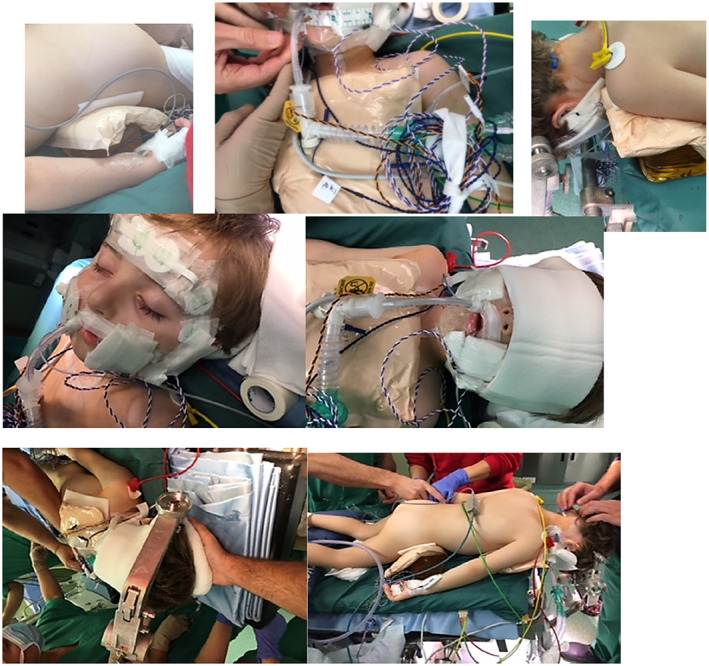
DPS intervention in a neurosurgical patient
The educational process was also augmented through international collaboration with the Professor of Nursing Research, Translational Research from the University of Melbourne who attended meetings at the commencement and completion of the study and shared his experience on the use of prophylactic dressings in the prevention of HAPI.
3.10. Double prevention strategy (DPS)
The DPS consists of the concurrent use of fluidized positioners (small and medium size) latex and DEHP‐free (Sundance Solutions™, Molnlycke Health Care AB, Sweden) and self‐adherent foam dressings in 10 × 10 cm/15 × 15 cm sizes (Mepilex® Molnlycke Health Care AB, Sweden). The fluidized positioners are positioned prior to the commencement of the surgical procedure to achieve maximal offloading. The off‐loading was enhanced by ensuring that the positioner was moulded to match the anatomical characteristics of the child to increase the emersion and envelopment of the body part to be protected. This technique also prevented dislocation during surgical and/or resuscitation manoeuvres and avoided direct contact of the child with the OR table. The Mepilex foam dressings were applied with the adherent inner layer in direct contact with child's skin and the outer layer in contact with the fluidized positioner. The Mepilex foam dressings also functioned as an interface between the child's skin and any medical devices that were present such as tubing or monitoring wires (Figures 2, 3).
3.11. Data collection
Data were acquired during 3 different phases of hospitalisation: Pre‐operative, Intraoperative, Post‐operative in 3 different settings: Originating Pre‐Op Units, Operating Rooms and Receiving Post Op Units.
Data consisted of:
Age
Gender
BMI
Main pathology
Comorbidities
Type of Surgical Procedure
Surgical Position/Duration
Mapping of devices and areas under protection
Determination of HAPI risk according to Braden QD modified risk scale***
Number of positioners and dressings required by each patient
Serial head to toe Skin checks
Staging of any identified PI according to NPUAP/EPUAP classification, updated in 2016.
Pain evaluation (HRF scale) if any HAPI developed
Further interventions required
3.12. Data collection tool
Continuous skin integrity monitoring was supported by a dedicated chart, which followed the patient during each phase of their hospital stay from before, during and after surgery up to discharge (Table 3). The whole prevention team referred to this chart to report and share patients' procedural risk, recording surgical positions and areas protected with the DPS. This skin integrity monitoring was maintained throughout patients' stay in hospital. The chart was composed of a short background detail component plus three check lists and a risk scale that had to be repeated before and after surgery; this repetition was designed to provide a precise detection of critical phases in the child's treatment. HAPI risk was calculated according to a modified Braden Q and Braden QD scale, known as the SS (Table 4) 39 which integrated risks of surgical paediatric patients to the previous scale such as the inclusion of items for duration of surgery and presence of monitoring devices. The SS analysed 16 risk factors in a dichotomous report (+ or −): the final score ranges from a minimum of 2 (deep sedation and device) to a maximum of 16. Any detected pressure injuries were staged according to the EPUAP / NPUAP criteria 2019.
TABLE 3.
Data collection settings and responsible clinician
| Data acquisition | ||
|---|---|---|
| Timing | Setting | Clinician |
| Pre‐operative | Originating unit | WOCN |
| Operating room | OR nurses | |
| Intraoperative | Operating room | OR nurses |
| Post‐operative | Operating room | OR nurses |
| Receiving unit | WOCN | |
TABLE 4.
Stracchini scale for children undergoing LDSPs (Braden QD scale modified)
| RISK FACTORS | Pre Op | Pre‐OP | Post‐OP | t0 | 12 h Post‐OP | 24 h Post‐OP | 48 h Post‐OP | At … day Post‐OP |
|---|---|---|---|---|---|---|---|---|
| Unit | OR | OR | Unit | Unit | Unit | Unit | Unit | |
| PREMATURITY (if children aged <1) | ||||||||
| SKIN DISEASES (BE, burns …) | ||||||||
| SENSORY PERCEPTION (DeepSedation) | ||||||||
| MECHANICAL VENTILATION | ||||||||
| HEMODYNAMICAL INSTABILITY | ||||||||
| HYPOVOLEMIA (bleedings, fluid loss) | ||||||||
| SATURATION (<85) | ||||||||
| HYPOALBUMINEMIA (<3.5 g/dl) nephropathy, hepatopathy, … | ||||||||
| BMI (<16, >30) | ||||||||
| EDEMA | ||||||||
| PROCEDURAL‐RELATED MOISTURES (corporal fluids, cleansing fluids) | ||||||||
| DEVICE (probes, wires…) | ||||||||
| ‐INOTROPES | ||||||||
| SURGERY AT RISK FOR DURATION (LDSP) | ||||||||
| TOTAL RF SCORE | /16 | /16 | /16 | /16 | /16 | /16 | /16 | /16 |
3.13. Data consistency
Consistency and reliability (Inter Rater Reliability) of the data collected were ensured by education provided to clinicians prior to the commencement of the study. The education program focused on the Braden QD risk scale, the SS and PI staging based on the updated EPUAP/NPUAP 2014 to 2019 classification system. 40 , 41
3.14. Statistical analysis
Institutional incidence of HAPI in critical care units from 2007 to 2014 identified 238 HAPI events which represents an incidence rate of 18.2% and rising to 22.5% if adding very complex patients. This incidence equates to 34 children/year developing a HAPI during their treatment in critical areas. We specified a confidence level of 95%, with a precision of 0.01 and determined that the sample size required was a minimum of 134 patients per group. Patients undergoing LDPS in each group were stratified, according to criteria noted in Table 2. Patients were divided into four groups: based on age‐dependent factors (skin development), the fourth group is represented by children‐positive to ae‐independent factors (Table 2). These latter patients were selected and only patients presenting analogous cases in comparator groups were considered eligible.
Differences of experimental treatment have been considered between Group A (cases) and B (comparators). A significant reduction of HAPI was considered a % less than 4 in case group: from 34 patients to 7 children/year. Continuous and categorical variables have been reported as average ± Standard deviation and as a count/percentage. Continue variables were compared with t‐test or ANOVA, whereas percentages were compared using χ2 test.
3.15. Possible sources of bias in data
Improper stratification of patients.
Inappropriate application of DPS.
Operator dependent variables.
Wrong application or lack of knowledge in Braden QD modified scoring.
3.16. Ethical approval
The project was included in the institutional quality improvement plan for years 2018/2019 and received the approval of the Ethics Committee cod. 1868‐Bambino Gesu' Children's Hospital‐2019/20.
4. RESULTS
A total of 421 children undergoing LDSP were reviewed and following exclusions resulted in final cohorts of 200 historical cases and 200 prospective cases provided with the DPS intervention. Table 5. presents the demographic, physiological, and outcome characteristics of the groups.
TABLE 5.
Demographics of all subjects included in the DPS study
| All sites | ||||||
|---|---|---|---|---|---|---|
| 2018 (Pre) | 2019 (Post) | P value | ||||
| Age, years (median, IQR) | 3 | (0.5‐11) | 6 | (1.3‐12) | 0.001 | |
| Surgery duration, hours (median, IQR) | 5 | (2‐8) | 6 | (4‐8) | 0.015 | |
| BMI (median, IQR) | 16 | (13.7‐17.9) | 17 | (14.8‐20.4) | <0.0001 | |
| HB (median, IQR) | 12 | (10.9‐13.4) | 12 | (11.1‐13.4) | 0.916 | |
| GLIC (median, IQR) | 89 | (78‐104) | 97 | (84.5‐112) | <0.0001 | |
| ALB (median, IQR) | 4 | (3.7‐4.5) | 4 | (3.8‐4.7) | 0.145 | |
| ICU stay (days) | 2 | (0‐9.5) | 1 | (0‐3) | <0.0001 | |
| Hospitalisation (days) (median, IQR) | 17 | (7‐65) | 9 | (6‐21) | <0.0001 | |
| Pressure injury, (n, %) | No | 122 | 61.0% | 117 | 88.5% | <0.0001 |
| Yes | 78 | 39.0% | 23 | 11.5% | ||
| No | 133 | 66.5% | 173 | 86.5% | <0.0001 | |
| Urgency, (n, %) | Yes | 67 | 33.5% | 27 | 13.5% | |
The demographic, physiological, and outcomes profile of the 2018 (No DPS) and 2019 (DPS) cohorts of children included in the analysis of the effectiveness of the DPS intervention identified some differences between the cohorts. The 2019 cohort of children had a greater median age, BMI, glycemic index and had a longer duration of surgery than 2018 cohort children. Children in the 2019 cohort spent less days in ICU, had shorter length of stay in hospital and developed significantly fewer pressure injuries than children in the pre‐DPS 2018 cohort.
To further explore the differences between the pre‐DPS and post‐DPS cohorts a logistic regression was performed to determine the effects of treatment, urgency, BMI, ALB, ICU stay (days) and hospitalisation (days) on the likelihood that patients would have a pressure injury. The logistic regression model was statistically significant, χ2(6) = 134.824, p < 0.05. The model explained 42% (Nagelkerke R2) of the variance in pressure injury incidence and correctly classified 82% of cases. The comparator group was 2.67 times more likely to have a pressure injury than the treatment group. Increasing ICU stay (days), as well as hospitalisation (days), were associated with an increased likelihood of having a pressure injury.
5. DISCUSSION
5.1. Summary
Babies and children undergoing LDSP represent a group that is highly vulnerable to the development of intraoperative HAPI. The HAPI risk profile of children varies greatly due to gestational age as well as intrinsic physiological and pathological parameters. 39 This combined with the added risks posed by LDSPs, associated monitoring equipment leads, various tubes, and less than ideal support surfaces on OR tables present clinicians with a complex spectrum of dangers to overcome in protecting these vulnerable children from injury. Our results have demonstrated that an integrated, evidence‐based OR PI prevention program can decrease the incidence of these injuries by more than 2.6 times irrespective of age or comorbidity.
5.2. Interpretation
Our results show that the comparator and intervention cohorts included in the study were generally comparable in terms of physiology and pathology (Table 5) apart from the intervention cohort having a mean age 2.01 years greater than the historical cohort, this difference was initially significant, however, age was not found to be a factor in subsequent multivariate analysis. Similarly, the intervention cohort aged below 12 months was 2.6 months older than controls. The influence of these age differences on HAPI incidence in our study would appear to be important given the possible relationship between the maturation of skin and tissues and pressure injury risk. When we examined this sub‐population more closely, we noted that children less than12‐months old (40%, n = 80 comparators) in both cohorts (23.5%, n = 47 intervention) we found that comparators had an HAPI incidence of 12.5% (n = 10) versus a 14.4% (n = 7) incidence in the intervention cohort. This finding supports the assertion that age is a significant risk factor to HAPI.
When we explored the overall HAPI incidence rate of both cohorts as well as time to injury, comparators had a rate of 39% (n = 78) and a mean of 22.3 days from surgery whereas the intervention cohort rate was 11.5% (n = 23) and 17.77 days from surgery.
Children undergoing LDSP and developing a HAPI within 72 h postoperatively were significantly reduced in the intervention cohort (1%, n = 2) compared to comparators (5%, n = 10). Following our review of the univariate analysis of significant differences between the cohorts we undertook a logistic regression (Table 6) that revealed that group membership was the most influential variable in the regression equation and that children in the historical comparator cohort were 2.6 times more likely to develop a HAPI.
TABLE 6.
Multivariate analysis of risk factors associated with pressure ulcer (n = 400)
| Estimate | Standard error | P‐value | OR | 95% Confidence interval for OR | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Group (1) | 0.981 | 0.308 | 0.001 | 2.667 | 1.459 | 4.875 |
| Urgency (1) | 0.533 | 0.328 | 0.105 | 1.704 | 0.895 | 3.244 |
| BMI | −0.028 | 0.032 | 0.389 | 0.972 | 0.913 | 1.036 |
| ALB | −0.321 | 0.25 | 0.200 | 0.726 | 0.444 | 1.185 |
| ICU stay (days) | 0.048 | 0.018 | 0.009 | 1.049 | 1.012 | 1.088 |
| Hospitalisation (days) | 0.017 | 0.004 | 0.000 | 1.017 | 1.008 | 1.026 |
| Constant | −0.935 | 1.155 | 0.418 | 0.392 | ||
Our findings suggest that the DPS used in the OR to prevent LDSP PI was effective, and we believe that the combination of fluidized positioners and multi‐layer silicone foam dressings provides significant protection of tissues from pressure and shear forces over the long duration of some surgical procedures, particularly where it is not possible to reposition the child. Additionally, the multi‐layer silicone foam dressings provide protection to the skin from pooled fluid at the skin/surface interface. 38 This is of particular importance in the neonate with immature skin. 42 , 43 , 44
5.3. Limitations
A limitation of our study is that it is very difficult to isolate only a few variables that may account for the detected reductions in HAPI. We firmly believe that, in the HAPI prevention puzzle, supporting surfaces, prophylactic dressings, and risk assessment scales do not operate in isolation, what makes an effective HAPI prevention program work effectively is a dedicated and well‐trained interdisciplinary team, devoted to the correct application of different evidence‐based strategies, sophisticated personalised PI risk assessment combined with the minimization of environmental risk in the OR and the post‐operative settings through effective positioning and offloading. 37 , 45
We do not believe that there is one “ideal” structure for a paediatric PI prevention program because individual institutions will have specific strengths and limitations and will therefore require programs designed to maximise those strengths. Therefore, we note that a potential limitation of our study is that of generalizability, however, we note that our interventions and results are consistent with several studies that focused on assessing the impact of pressure injury prevention teams that found a dramatic decrease in the incidence of pressure injuries. 46
Finally, we strongly believe that educational meetings aimed at creating a trained and expert multidisciplinary, interdepartmental team devoted to the application of a protection strategy based on matching support surfaces and prophylactic dressings to meet the PI prevention needs of the whole paediatric age range spectrum during LDSP and the subsequent post‐operative period, are essential and indivisible features of a successful PI prevention program for complex vulnerable children undergoing long duration surgical procedures. 47 , 48 , 49
6. CONCLUSIONS
The WPT together with the DPS reduced by 2.6 times the institutional HAPI incidence rate in children undergoing LDSP regardless of their age or comorbidities. Despite our reported results, the whole DPS prevention intervention requires further evaluation to test its performance over time. The analysis of subpopulations, to better understand surgery‐related risk factors as well as transferring (extending) this quality improvement initiative to non‐surgical patients should also be investigated in future studies.
We believe that our study has demonstrated, for the first time, the clinical effectiveness of the DPS for the minimization of OR HAPI in children undergoing LDSP.
FUNDING INFORMATION
This study was supported with an unrestricted grant by Molnlycke Healthcare AB, Sweden which did not have any input into the design, conduct, analysis, or interpretation of the study.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
ACKNOWLEDGEMENTS
This study was facilitated by Bambino Gesù Children's Hospital, Research Institute, and the valuable support of numerous clinical and administrative staff of the hospital.
Ciprandi G, Crucianelli S, Zama M, et al. The clinical effectiveness of an integrated multidisciplinary evidence‐based program to prevent intraoperative pressure injuries in high‐risk children undergoing long‐duration surgical procedures: A quality improvement study. Int Wound J. 2022;19(7):1887‐1900. doi: 10.1111/iwj.13967
Funding information Molnlycke Healthcare AB
DATA AVAILABILITY STATEMENT
Data openly available in a public repository that issues datasets with DOIs.
REFERENCES
- 1. Creehan S, Black J. Defining practices to avoid hospital‐acquired pressure injuries in the operating room. J Wound Ostomy Cont Nurs. 2022;49(1):86‐96. [DOI] [PubMed] [Google Scholar]
- 2. Pittman J, Horvath D, Beeson T, et al. Pressure injury prevention for complex cardiovascular patients in the operating room and intensive care unit. A quality improvement project. J Wound Ostomy Continence Nurs. 2021;48(6):510‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Razmus IS, Keep SM. Neonatal intensive care nursing pressure injury prevention practices. A descriptive survey. J Wound Ostomy Continence Nurs. 2021;48(5):394‐402. [DOI] [PubMed] [Google Scholar]
- 4. Rowe AD, McCartty K, Huett A. Implementation of a nurse driven pathway to reduce incidence of hospital acquired pressure ionjuries in the pediatric intensive care setting. J Pediatr Nurs. 2018;41:104‐109. [DOI] [PubMed] [Google Scholar]
- 5. Baharestani MM, Ratliff CR. Pressure ulcers in neonates and children: an NPUAP white paper. Adv Skin Wound Care. 2007;20(4):208‐220. [DOI] [PubMed] [Google Scholar]
- 6. Visscher MO, Burkes SA, Adams DM, Hammill AM, Wickett RR. Infant skin maturation: preliminary outcomes for color and biomechanical properties. Skin Res Technol. 2017;23(4):545‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Triantafyllou C, Chorianopoulou E, Kourkouni E, Zaoutis TE, Kourlaba G. Prevalence, incidence, length of stay and cost of healthcare‐acquired pressure ulcers in pediatric populations: a systematic review and meta‐analysis. Int J Nurs Stud. 2021;115:103843. [DOI] [PubMed] [Google Scholar]
- 8. Factors RI. Associated with pediatric hospital‐acquired pressure injuries. J Wound Ostomy Continence Nurs. 2018;45(2):107‐116. [DOI] [PubMed] [Google Scholar]
- 9. Freundlich K. Pressure injuries in medically complex children: a review. Children (Basel). 2017;4(4):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nie AM. Pressure injury prevention and treatment in critically ill children. Crit Care Nurs Clin North Am. 2020;32(4):521‐531. [DOI] [PubMed] [Google Scholar]
- 11. Black JM, Cuddigan JE, Walko MA, Didier LA, Lander MJ, Kelpe MR. Medical device related pressure ulcers in hospitalized patients. Int Wound J. 2010;7:358‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gefen A, Alves P, Ciprandi G, et al. Device‐related pressure ulcers: SECURE prevention. J Wound Care. 2020;29(Sup2a):S1‐S52. [DOI] [PubMed] [Google Scholar]
- 13. Ciprandi G, Oranges T, Schluer AB. Pressure ulcers in pediatric patients.Springer‐Verlag London ltd. part of springer nature 2018. In: Romanelli M, Clark M, Gefen A, Ciprandi, G. eds. Science and Practice of Pressure Ulcer Management. doi: 10.1007/978-1-4471-7413-4_10 [DOI] [Google Scholar]
- 14. Ciprandi G, Crucianelli S. Top tips: preventing pressure ulcers in premature babies and neonates. Wounds Int J. 2015;6(4):5‐9. [Google Scholar]
- 15. Kottner J, Hauss A, Schlüer AB, Dassen T. Validation and clinical impact of paediatric pressure ulcer risk assessment scales: a systematic review. Int J Nurs Stud. 2013. Jun;50(6):807‐818. [DOI] [PubMed] [Google Scholar]
- 16. Schroeder J, Sitzer V. Nursing care guidelines for reducing hospital‐acquired nasogastric tube‐related pressure injuries. Crit Care Nurse. 2019;39(6):54‐64. [DOI] [PubMed] [Google Scholar]
- 17. Chamblee TB, Pasek TA, Caillouette CN, Stellar JJ, Quigley SM, Curley MAQ. How to predict pediatric pressure injury risk with the Braden QD scale. Am J Nurs. 2018;118(11):34‐43. [DOI] [PubMed] [Google Scholar]
- 18. Chun X, Lin Y, Ma J, He J, Ye L, Yang H. Predictive efficacy of the Braden Q scale for pediatric pressure ulcer risk assessment in the PICU: a metaanalysis. Pediatr Res. 2019;86(4):436‐443. [DOI] [PubMed] [Google Scholar]
- 19. Kottner J, Dassen T. Pressure ulcer risk assessment in critical care: interrater reliability and validity studies of the Braden and Waterlow scales and subjective ratings in two intensive care units. Int J Nurs Stud. 2010;47:671‐677. [DOI] [PubMed] [Google Scholar]
- 20. Hayes RM, Spear ME, Lee SI, et al. Relationship between time in the operating room and incident pressure ulcers: a matched case‐control study. Am J Med Qual. 2015;30(6):591‐597. [DOI] [PubMed] [Google Scholar]
- 21. Kim KM, Lee H, Ha T, Na S. Perioperative factors associated with pressure ulcer development after major surgery. Korean J Anesthesiol. 2018;71(1):48‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang G, Gao C, Cai J. Prevention of nasal ala pressure injuries with use of hydroactive dressings in patients with nasotracheal intubation of orthognatic surgery: A randomized controlled trial. JWOCN. 2020;7(5):484‐488. [DOI] [PubMed] [Google Scholar]
- 23. Higer S, James T. Interface pressure mapping pilot study to select surfaces that effectively redistribute pediatric occipital pressure. J Tissue Viability. 2016;25(1):41‐49. [DOI] [PubMed] [Google Scholar]
- 24. Solis I, Krouskop T, Trainer N, Marburger R. Supine interface pressure in children. Arch Phys Med Rehabil. 1988;69(7):524‐526. [PubMed] [Google Scholar]
- 25. Curley MAQ, Hasbani NR, Quigley SM, et al. Predicting pressure injury risk in pediatric patients: the Braden QDScale. J Pediatr. 2018;192:189‐195. [DOI] [PubMed] [Google Scholar]
- 26. Chen HL, Shen WQ, Liu P, Liu K. Length of surgery and pressure ulcers risk in cardiovascular surgical patients: a dose–response meta‐analysis. Int Wound J. 2017;14(5):864‐869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schoonhoven L, Defloor T, Grypdonck MHF. Incidence of pressure ulcers due to surgery. J Clin Nurs. 2002;11(4):479‐487. [DOI] [PubMed] [Google Scholar]
- 28. Yang T, Shin SH. Effect of soft silicone foam dressings on intraoperatively acquired pressure injuries: a randomized study in patients undergoing spinal surgery. Wound Manag Prev. 2020;66(11):22‐29. [PubMed] [Google Scholar]
- 29. Gao L, Yang L, Li X, et al. The use of a logistic regression model to develop a risk assessment of intraoperatively acquired pressure ulcer. J Clin Nurs. 2018;27(15–16):2984‐2992. [DOI] [PubMed] [Google Scholar]
- 30. Lu CX, Chen HL, Shen WQ, Feng LP. A new nomogram score for predicting surgery‐related pressure ulcers in cardiovascular surgical patient. Int Wound. 2017;14(1):226‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gefen A, Ciprandi G. Biometry impairments: the specific challenges in preventing pressure ulcers in patients with chronic spasticity. J Wound Care. 2019;28(11):699‐700. [DOI] [PubMed] [Google Scholar]
- 32. Chen HL, Yu SJ, Xu Y, et al. Artificial neural network: a method for prediction of surgery‐related pressure injury in cardiovascular surgical patients. Wound Ostomy Continence Nurs. 2018;45(1):26‐30. [DOI] [PubMed] [Google Scholar]
- 33. Huang W, Zhu Y, Qu H. Use of an alternating inflatable head pad in patients undergoing open heart surgery. Med Sci Monit. 2018;24:970‐976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Joseph J, McLaughlin D, Darian V, Hayes L, Siddiqui A. Alternating pressure overlay for prevention of intraoperative pressure injury. J Wound Ostomy Continence Nurs. 2019;46(1):13‐17. [DOI] [PubMed] [Google Scholar]
- 35. Kirkland‐Walsh H, Teleten O, Wilson M, Raingruber B. Pressure mapping comparison of four OR surfaces. AORN J. 2015;102(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Levy A, Kopplin K, Gefen A. Adjustability and adaptability are critical characteristics of pediatric support surfaces. Adv Wound Care. 2015;4(10):615‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Razmus I, Bergquist‐Beringer S. Pressure ulcer risk and prevention practices in pediatric patients: a secondary analysis of data from the National Database of nursing quality indicators. Ostomy Wound Manage. 2017;63(2):28‐32. [PubMed] [Google Scholar]
- 38. Clark M, Black J, Alves P, et al. Systematic review of the use of prophylactic dressings in the prevention of pressure ulcers. Int WoundJ. 2014;11(5):460‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chiari P, Poli M, Magli C, et al. Multicentre prospective cohort study to validate the Italian version of the Braden Q scale for the risk of the pressure sores in newborns and up to 8 years old children. Assist Inferm Ric. 2012;31(2):83‐90. [DOI] [PubMed] [Google Scholar]
- 40. National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel, Pan Pacific Pressure Injury Alliance . In: Haesler E, ed. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. Osborne Park, Western ASustralia: Cambridge Media; 2014. [Google Scholar]
- 41. Delmore B, Deppisch M, Sylvia C, Luna‐Anderson C, Nie AM. Pressure injuries in the pediatric population: a national pressure ulcer advisory panel white paper. Adv Skin Wound Care. 2019;32(9):394‐408. [DOI] [PubMed] [Google Scholar]
- 42. Nie AM. Creating a pediatric and neonatal pressure injury prevention program when evidence was sparse or absent: a view from here. J Wound Ostomy Continence Nurs. 2020;47(4):353‐355. [DOI] [PubMed] [Google Scholar]
- 43. Santamaria N, Alves P, Crucianelli S, Ciprandi G. Pediatric pressure ulcer prevention: positioning and repositioning. Neonatal and pediatric wound care. G. Ciprandi editor. Minerva Med. 2021:87‐102. [Google Scholar]
- 44. Cummins KA, Watters R, Leming‐Lee TS. Reducing pressure injuries in the pediatric intensive care unit. Nurs Clin North Am. 2019;54(1):127‐140. [DOI] [PubMed] [Google Scholar]
- 45. Riemenschneider KJ. Prevention of pressure injuries in the operating room: a quality improvement project. J Wound Ostomy Continence Nurs. 2018;45:141‐145. [DOI] [PubMed] [Google Scholar]
- 46. Black J, Clark M, Dealey C, et al. Dressings as an adjunct to pressure ulcer prevention: consensus panel recommendations. Int Wound J. 2015;12(4):484‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Woodfin KO, Johnson C, Parker R, Mikach C. Use of a novel memory aid to educate perioperative team members on proper patient positioning technique. AORN. 2018;107(3):325‐332. [DOI] [PubMed] [Google Scholar]
- 48. Pasek TA, Geyser A, Sidoni M, et al. Skin care team in the pediatric intensive care unit: a model for excellence. Crit Care Nurse. 2008;28(2):125‐135. [PubMed] [Google Scholar]
- 49. Clay P, Cruz C, Ayotte K, Jones J, Fowler SB. Device related pressure ulcers pre and post identification and intervention. J Pediatr Nurs. 2018;41:77‐79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data openly available in a public repository that issues datasets with DOIs.


