Abstract
Alleviation of localised, sustained tissue loads and microclimate management are the most critical performance criteria for materials in use for pressure ulcer prevention, such as in prophylactic dressings, padding or cushioning. These material performance criteria can be evaluated by calculating the extents of matching between the material stiffness (elastic modulus) and the thermal conductivity of the protective dressing, padding or cushioning with the corresponding properties of native skin, separately or in combination. Based on these bioengineering performance criteria, hydrocolloids, which are commonly used for prophylaxis of medical device‐related pressure ulcers, exhibit poor stiffness matching with skin. In addition, there is remarkable variability in the modulus and thermal conductivity matching levels of different material types used for pressure ulcer prevention, however, it appears that among the materials tested, hydrogels provide the optimal matching with skin, followed by gels and silicone foams. The stiffness matching for hydrocolloids appears to be inferior even to that of gauze. This article provides quantitative performance criteria and metrics for these evaluations, and grades commonly used material types to biomechanically guide clinicians and industry with regards to the selection of dressings for pressure ulcer prevention, both due to bodyweight forces and as a result of applied medical devices.
Keywords: biomechanical properties, laboratory testing, padding and cushioning, pressure injury, prophylactic dressings
1. INTRODUCTION
Pressure ulcers (PUs, also known as pressure injuries) are silent but deadly condition, which affects millions of patients across different populations, not just the elderly but also, young individuals such as those affected by central nervous system trauma or neuromuscular diseases. Prior to the coronavirus 2019 pandemic, PUs were reported as a cause of death among ~3.8 per 100 000 person‐years, and as the underlying cause of death in ~19% of these deaths cases; patients acquiring PUs in acute care settings have 12‐weeks and 26‐weeks mortality rates of 66% and 75%, respectively. 1 , 2 , 3 As healthcare resources are spread thinner globally since the breakout of the pandemic, these rates are unlikely to improve in the foreseeable future, and are expected to continue to impact healthcare as an ongoing, and perhaps as an increasing burden to the quality of life of patients and family members, and as a major expenditure item in budgets of medical facilities and governments. Just prior to the pandemic, the United States annual expenditure on treating PUs was reported to be $26.8‐billion. 4 Cost of individual patient care ranges from $20 900 to $151 700 per PU case; Medicare estimated that each PU case adds $43 180 in costs to a hospital stay. 5 Prolonged sedentary stays in the intensive care unit (ICU) or long surgical procedures can also drive PU development in patients who were initially considered to not be at immediate risk for these wounds. In addition, the prolonged use of skin‐contacting medical devices such as continuous positive airway pressure (CPAP) masks, oxygen nasal cannulas, endotracheal tubes, cervical collars or intravenous drips can also result in medical device‐related pressure ulcers (MDRPUs), the recently reported incidence of which is approximately 28%. 6 Whilst the above data typically refer to the more serious PU categories (ie, the deeper wounds), and do not reflect the entire range of possible PU severities and clinical settings, this information overall points to the fact that PUs continue to be a significant burden to patients, care providers and healthcare systems, considering the epidemiological, economic and socio‐familial impacts of these wounds altogether.
It is commonly accepted that many of the bodyweight‐induced Pus, as well as the MDRPUs, can be prevented with proper care and the use of effective preventative technologies, employing adequate skin‐contacting materials. In particular, the risk for developing bodyweight‐induced PUs or MDRPUs can be substantially reduced by providing additional soft and flexible cushioning materials at the susceptible body sites, 7 , 8 however, these prophylactic interventions result in variable physiological and clinical outcomes 9 , 10 , 11 depending on the specific structure and composition of the applied dressing, cushioner or pad, which differ fundamentally across manufacturers. For example, the application of different foam‐based dressings resulted in different extents of changes in skin surface roughness, the profile of interleukin 1‐α (IL‐1α) release from skin sebum, and skin temperatures at the dressing application site under the sacrum. 9 , 10 However, there are other commonly used materials in the prophylaxis of PUs, such as hydrocolloids or gels, or sometimes, gauzes that are used in low‐resource facilities, which belong to inherently different material families and so, are expected to induce an even larger variation in clinical performance. The concept of local padding or cushioning applied to a susceptible anatomical site directly addresses the underlying biomechanical factors eventually leading to a PU, 12 that is, smoothening the stiffness gradient between the support surface or device materials and the skin, and increasing the contact area for transferring mechanical loads, which results in redistribution and reduction in the intensities of the tissue stress concentrations. Nevertheless, different materials having distinct physical and engineering properties will, by definition, lead to diverse physiological responses and clinical outcomes.
This article analyses the alternatives for materials in use for dressings, padding, and cushioning in the prophylaxis of PUs or MDRPUs, focusing on two key performance aspects: Alleviation of the sustained mechanical loads in the skin and the management of microclimate conditions at the skin‐device contact sites. Our objective was to formulate quantitative criteria and metrics for such performance evaluations, as well as a systematic material grading methodology for existing and potentially new material types utilised for PU prevention. The proposed criteria, metrics, and methodology can biomechanically guide clinicians and industry in selecting materials for prophylaxis of PUs caused by either bodyweight or device‐induced forces.
2. THE BIOMECHANICAL COMPATIBILITY CONCEPT AND ITS ROLE IN PRESSURE ULCER PREVENTION
The biomechanical compatibility of dressing, padding or cushioning materials with skin can be assessed in terms of the mechanical stiffness matching and the thermodynamic matching between the skin and an applied (interfacing) material for the purpose of tissue protection. 13 , 14 , 15 The earlier criterion is based on the engineering principle that material stiffness gradients cause mechanical stress concentrations, hence, for example, a dressing that is considerably stiffer than skin will provide poor prophylactic performance, because of the large difference between the dressing versus the skin stiffness will promote focal loading under the dressing generated by the dressing‐skin stiffness differences, as indeed demonstrated in bioengineering prophylactic dressing studies. 16 , 17 The latter, thermodynamic criterion is similarly based on the engineering principle that large differences in thermal conductance between interfacing materials will create a temperature gradient between the two contacting materials, and hence, correspondingly, a dressing that will have a substantially lower thermal conductance with respect to the skin will not allow effective heat transfer from the skin surface to the environment through the dressing (ie, the dressing will act as an insulator), which may be a warranted effect if the dressing is applied onto an open wound, but not for prophylaxis on intact skin where excess perspiration moisture on the skin should be avoided to maintain the skin strength. 15 These bioengineering considerations in fact indicate that the theoretically ideal preventative dressing, padding or cushioning material for application onto the skin is – more skin – but as this is not feasible in the real world, the optimal is a synthetic or semi‐biological biocompatible material that has the closest stiffness and thermal matching with skin, that is, E/E skin ➔ 1 and k/k skin ➔ 1, where E is the elastic modulus * (stiffness measure) parameter of the material under consideration, and k is its thermal conductivity. †
The fundamental principle in materials science and engineering is to correlate the structure–function relations of certain materials of interest, and, thereby, to control the structure and microstructure which directly affect the physical and engineering properties of that material (Figure 1A). In the context of dressings, padding and cushioning materials used for PU prevention, “structure” typically refers to the microstructure of the material, such as the porosity of foam or the water content in a hydrogel. “Function” refers to the physical, mechanical, and thermal properties resulting from the microstructure, such as the stiffness, which, in foams, for example, relates to the micro‐pore sizes (smaller pores indicate a denser material which typically results in increased stiffness and strength, however, larger pores in foams contain more air which acts as a thermal insulator, and hence, there is an interaction between the properties resulting from a certain structure). Generally, the measurable, physical, and engineering set of properties characterising a certain material, such as the elastic modulus as a measure of the mechanical stiffness, or thermal conductivity as a measure of the thermal conductance/insulation performance, are therefore the quantitative metrics characterising the function of a material structure. This triangle of structure–function‐property relations (Figure 1A) has not been studied sufficiently in the context of the performance of materials used in PU prevention products. Consider for example foam‐based dressings which are commonly used for PU prevention. A foam material that has excessively small micro‐pores, that is, is too dense, will make an overly stiff dressing, which, rather than largely deforming under bodyweight forces or the loads applied by a medical device (and thereby, alleviating the loads in the underlying skin and subdermal tissue), will be displaced by the bodyweight or external loads into the skin, imprinting the skin and thus contributing to a potential PU or MDRPU (Figure 1B). Likewise, consider a foam‐based dressing with excessively large pores, which may be soft and compliant, but, due to the high air contents, have critically low thermal conductivity, which will then cause the dressing to act as a thermal insulator, preventing the release of metabolic heat from the skin to the external surface of the dressing and from there, to the environment, for example, through convection (Figure 1C). These examples, referring to foam‐based dressings, can be extended to non‐foam dressings, and indicate that not only that ideally, we should require that PU prevention materials exhibit E/E skin ➔ 1 and k/k skin ➔ 1 as explained above, but also, that the elastic modulus E and thermal conductivity k of the dressing, padding or cushioning material will result from their microstructure and composition.
FIGURE 1.
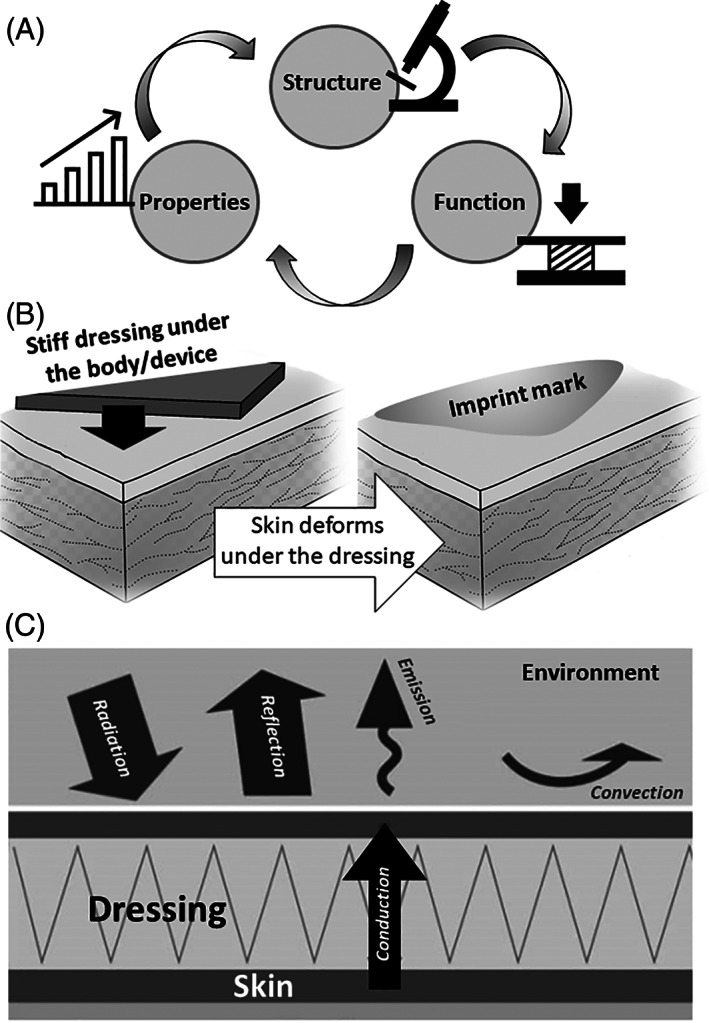
The structure–function‐property principle in material science (A) and its key implications concerning the performance of dressings used for pressure ulcer prophylaxis: (B) the material stiffness (elastic modulus) determines the extent of deformation of a dressing when it is subjected to the forces of the bodyweight or a medical device, and thereby, whether the dressing will deform under the device, hence alleviating skin deformations, or be displaced with little self‐deformation onto the skin, to imprint it. (C) The thermal conductivity of the dressing determines the level of heat transfer from the skin surface to the environment. A dressing with low thermal conductivity, that is, an insulator, will cause accumulation of heat at the skin‐dressing interface and hinder heat release to the surface of the dressing and from there, to the environment, such as through convection. These illustrations demonstrate how the physical and engineering characteristics (“properties”) of a dressing material (“structure”) define its biomechanical protective efficacy (“function”) (A) against pressure ulcers, both of the bodyweight‐induced type and medical device‐related injuries
3. MATERIALS FOR PRESSURE ULCER PREVENTION AND THEIR REPORTED CLINICAL EFFECTIVENESS
The primary material types currently used in dressing, padding, and cushioning structures for PU prevention are foams or silicone foams, gels, and hydrocolloids, the latter being particularly popular for prophylactic dressings applied for the prevention of MDRPUs. These materials are inherently different in their chemical composition and physical and engineering properties, yet, they are all used in clinical practice for the prevention of either bodyweight‐induced PUs or MDRPUs. Studies comparing the prophylactic efficacy of one material type to none (typically, to regular skin care) are abundant. For example, Cai and colleagues 18 conducted a meta‐analysis encompassing 80 publications, out of which 22 met the meta‐analysis inclusion criteria (totalling N = 2519 patients). They reported that the facial MDRPU incidence associated with CPAP usage was lower for patients who received hydrocolloid‐based dressings for skin protection compared to the gauze or regular skin care, but had also noted a risk of bias. A related study by Bishopp and colleagues 19 was focused on protecting the nasal bridge in patients requiring CPAP masks for a period of a year. These patients were assessed daily and new MDRPUs were defined as category‐2 or above. A total of 295 patients were included, of which 161 were historical controls. Their conclusion was that the prophylactic use of hydrocolloid dressing cuts (BeneHold Bordered by Aspen Medical) placed over the nasal bridge effectively reduced the risk of category‐2 CPAP‐related PUs, as the incidence in their pre‐intervention group was 6%, but there were no evident MDRPUs in the intervention group who received the hydrocolloid dressings. Similarly, Swan 20 studied an alternative gel pad (Dermisplus Prevent, Frontier Medical) in a 4‐week non‐comparative audit (N = 37 patients) to investigate the effect of this product on PU incidence in the ICU and found that no new PUs developed during the study period with the new gel pad, although there was also no reduction in the overall PU incidence compared with the previous 3 months. None of the four patients in their study who presented blanching erythema (11%) developed a category‐1 PU. 20 Of note, all these recent studies compared the application of hydrocolloid or gel‐based products to regular skin care (with no protective dressings), or to use of gauze as a cushioner under the medical device at best, and none had included matched groups of patients receiving different types of (non‐gauze) dressing materials for prophylaxis of MDRPUs.
Studies comparing different products applied to similar groups of participants using the same experimental protocols are much less frequent. Amrani et al 9 used infrared thermography (IRT) to measure local skin temperatures at the buttocks of supine healthy subjects (N = 3), to quantitatively determine how skin microclimate conditions associated with a weight‐bearing Fowler's position are affected by the application of PolyMem (Ferris Mfg. Corp.) dressing versus placebo foam, with a no‐dressing case used as reference. They reported that the PolyMem dressing allowed a more effective and homogenous clearance of locally accumulated body heat with respect to the simple foam. 9 Lechner et al 10 conducted an exploratory randomised crossover trial with intra‐individual evaluations to compare the effects of three different multi‐layer foam‐based dressings (Mepilex Border Sacrum by Mölnlycke; ALLEVYN Life Sacrum by Smith & Nephew; and Optifoam Gentle Sacrum by Medline), applied to the sacral skin of elderly female volunteers (N = 12) for 3.5 hours while lying supine on a standard hospital mattress and regularly performing standardised movements to increase the sacral shear loads. The parameters for comparison across the dressing types included the skin surface temperature, stratum corneum hydration, erythema, skin roughness, and the IL‐1α concentration per total protein. They reported a significant decrease of the mean skin roughness for the Optifoam Gentle Sacrum dressing, increased erythema index for the ALLEVYN Life Sacrum dressing, and the highest releases of IL‐1α for the ALLEVYN Life Sacrum and Optifoam Gentle Sacrum dressings, as opposed to the Mepilex Border Sacrum group for which the changes in these skin parameters were minor. 10 Importantly, Lechner et al 10 concluded that different dressing types cause different physiological responses of skin during sustained mechanical loading. In a related study, Beeckman and colleagues 11 compared clinical outcomes of applying the Allevyn brand (n = 539) against the Mepilex brand (n = 538) and versus controls who received standard skin care in a multi‐centre study conducted in Belgian hospitals (including 1633 at‐risk patients in total). Their primary trial endpoint was the proportion of patients who developed at least one new category‐2 PU or worse on the sacrum, heels or greater trochanter during a 2‐weeks period. 11 Their results showed an overall low PU incidence, with a decrease in PUs of category‐2 or worse for the sacrum in the groups who received dressings (from 4.8% to 2.8%), but no statistically significant effect for the heels (decrease from 1.9% to 1.4%). 11 Beeckman et al 11 did not find statistical differences between the two investigated aforementioned foam‐based dressing brands, however, their overall incidence rates were relatively small. Overall, it appears that even among the foam‐based dressings in prophylactic use, there are significant dressing‐type‐dependent differences in the physiological responses of skin subjected to sustained loading under the dressing, 10 which must be attributed to the different material characteristics used in dressings (or padding and cushioning) products. 16 , 21 This naturally indicates that some materials may perform better than others in minimising the alteration of normal skin physiology under sustained loading conditions, and thereby, in reducing the risk for bodyweight‐related PUs or MDRPUs. 13
As reflected from the literature reviewed above, the mainstream of PU prevention practice by means of dressings employs hydrocolloids, foams, and silicone foams, however, recently published work points to the potential of other wound dressing materials (ie, which already comply with biocompatibility requirements and possibly also include integrated skin attachment mechanisms) for prophylactic applications. Specifically, Gefen and colleagues 22 developed an experimental‐computational analysis framework for evaluating the biomechanical protective efficacy of a dressing technology based on cellulose fibres used as the core matrix (Zetuvit Plus Silicone Border by Paul Hartmann AG). Using an anatomically‐realistic computer model of a supine female patient to whom this sacral dressing was virtually applied, Gefen et al 22 evaluated the protective efficacy index, the protective endurance, and the prophylactic trade‐off design parameter of new and post‐use dressings. Their results demonstrated that the above dressing type, with its fluff core, is at least as‐good as silicone foams, but importantly, provides the best balance between protective biomechanical performances at its “new” condition and the performance after being exposed to moisture. Grigatti and Gefen 23 , 24 similarly performed an integrated experimental‐computational evaluation of a hydrogel‐based dressing, HydroTac Transparent (also by Paul Hartmann AG) to determine the facial tissue loading state under a CPAP mask while using cuts of this hydrogel‐based dressing for preventing CPAP‐related MDRPUs. For this purpose, they measured the compressive mask‐skin contact forces at the nasal bridge, cheeks, and chin with versus without these dressing cuts and fed these data to a computer model of an adult head. They found that the dry (new) dressing cuts reduced to skin and underlying tissue exposures to loads (above the median loading level) by at least 30% at the nasal bridge and by up to 99% at the cheeks, across the tissue depth. 24 Hence, while most of the published work, including the 2019 International Guideline for Pressure Ulcer Prevention and Treatment, 25 refer to foams and silicone foams for prophylaxis of MDRPUs and PUs in general, other materials and dressing or padding technologies may not be inferior to foams, and in some aspects, particularly concerning microclimate management (as manifested through the thermal conductivity property), can be superior to foams.
Compressive elastic moduli of different commercial wound dressing products that are commonly used in the clinical practice of PU prevention, for preventing both bodyweight‐induced PUs and MDRPUs, and which were measured historically at the author's laboratory 26 following a protocol modified from the ASTM D3574‐11 test standard 27 (as detailed below), are shown in Figure 2. These compression tests were performed using an electromechanical testing apparatus equipped with a load cell that is capable of measuring forces in the range of 2 N to 2 kN at an accuracy of ±0.5% (model 5944, Instron Co., Norwood MA, USA); the Bluehill testing software V3.68 was used to generate the force‐displacement curves. Rigid flat anvils larger than our largest test specimen were used to apply the displacement and support the specimens at their inferior and superior faces, respectively. After the undeformed diameter and thickness of each product were measured using a Vernier calliper with a resolution of 0.05 mm, specimens were placed, one at a time, between the aforementioned anvils in preparation for the uniaxial compression testing. The measurement method consisted of two different individual compression tests for each specimen, the first being a preconditioning (pre‐testing) phase, and the second generated the force‐displacement curve used for the data analysis and calculation of the elastic modulus values (Figure 2). Pre‐testing was performed by raising and lowering the compression anvil at a deformation rate of 250 ± 25 mm/min in order to pre‐compress the specimen twice, up to ~80% of its original thickness, and then relax the specimen. Next, we allowed specimens to rest for a period of 6 ± 1 minute, before running the final tests to obtain the load–displacement results for the data analyses. Final mechanical testing was performed by bringing the compression anvil into contact with the specimen and determining its thickness after applying an initial contact load of 140 Pa to the specimen area. We compressed the specimens until reaching 50% of their initial thickness at the deformation rate of 50 ± 5 mm/min. The elastic moduli were obtained by linearisation of the stress–strain curves over the 30%–50% strain domain. Furthermore, the corresponding tangent elastic moduli at the 5% and 20% strain levels were calculated from the stress–strain curves of each product, to capture potential stiffness variations associated with non‐linear stress–strain (stress stiffening) behaviour of the tested dressing products if such behaviour had occurred.
FIGURE 2.
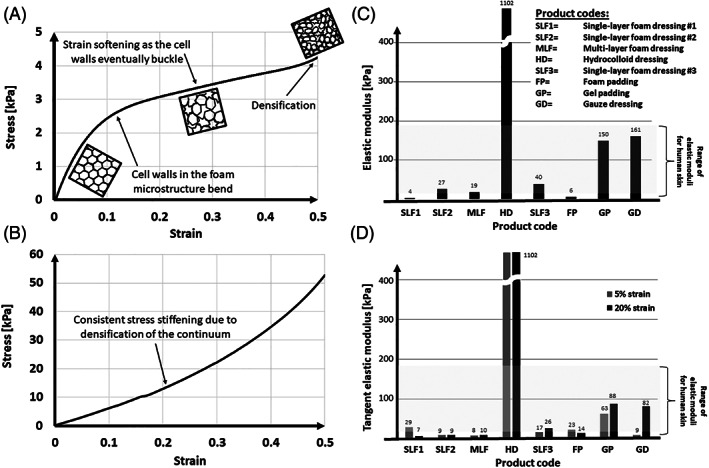
Examples of compressive stress–strain curves and elastic moduli of different commercial wound dressing products that are commonly used in the clinical practice of pressure ulcer prophylaxis, for preventing both bodyweight‐induced and medical device‐related pressure ulcers, and which were measured historically at the author's laboratory 26 following a protocol modified from the ASTM D3574‐11 test standard, 27 as described in the body of the text: (A) Example stress–strain curve for a single‐layer foam dressing #1 (SLF1); and an additional example (B) of a stress–strain curve for gel padding (GP) material. Note the stress softening behaviour of the foam (where the slope of its stress–strain curve decreases at the higher strains), in contrast to the stress stiffening behaviour of the gel (where the corresponding slope increases). (C) Elastic moduli obtained by linearisation of the stress–strain curves for protective materials over the 30%–50% strain domain; and (D) the corresponding tangent elastic moduli at the 5% and 20% strain levels, to capture stiffness variations associated with potential non‐linear stress–strain (stress stiffening) behaviours of the tested dressing products. Both bar graphs demonstrate that the hydrocolloid dressing product is remarkably stiffer than the range of adult skin stiffnesses under compression (which is up to 200 kPa; see the literature reviewed in the text), whereas the foam‐based and the gel‐based products generally fall within that range for skin. N = 3 specimens per product type; the standard deviations around the mean values were negligible
The results of the above stiffness measurements are shown in Figure 2, and a review of published research, which analysed each of the following materials: Foams, hydrocolloids, gel, and gauze for their elastic moduli and thermal conductivities is provided in Table 1. Example stress–strain curves for a single‐layer foam dressing (Figure 2A) and for gel padding (Figure 2B) demonstrate strong non‐linearity of the compressive behaviours of both of these materials, and further exhibit a stress softening behaviour of the foam (ie, the slope of its stress–strain curve decreases for the higher strains), likely due to buckling of the cell walls in the microstructure, in contrast to a stress stiffening behaviour of the studied gel (where the corresponding slope consistently increases). Noteworthy, for the highest strains applied to the foam, a region of stress stiffening is identifiable (post the stress softening), probably due to densification where cell walls crush together, resulting in an increase of the compressive stress (Figure 2A). In addition, both bar graphs of Figure 2C,D demonstrate a large variability in stiffness properties of the products under investigation, and in particular, these data show that the hydrocolloid dressings are remarkably stiffer than the range of adult skin stiffnesses under compression (which is up to 200 kPa as described in the next section below), whereas the foam‐based and the gel‐based products generally fall within that range of skin (see also Table 1). Of note, even though hydrocolloids are highly popular among clinicians for the prevention of MDRPUs, their stiffness properties are the furthest from those of native skin (Figure 2C,D, Table 1).
TABLE 1.
Ranges of elastic modulus and thermal conductivity property values for materials which are commonly used in pressure ulcer prophylaxis devices, such as dressings, padding and cushioning
| Material type | Elastic modulus [kPa] | Thermal conductivity [W/mK] | References |
|---|---|---|---|
| Foams a | 4–100 | 0.05–0.11 | 21, 28, 29, 30 |
| Gauze | 40–240 | 0.03–0.07 | 31, 32, 33, 34, 35 |
| Gels b | 3–200 | 0.15 – 0.19 | 36, 37, 38, 39 |
| Hydrocolloids | 1100–1200 | 0.41–0.64 | 40, 41, 42 |
| Hydrogels c | 15–90 | 0.27–0.43 | 23 |
| Silicones | 1–350 | 0.15–0.31 | 43, 44, 45, 46 |
Sieracki and colleagues recently mapped the compressive stiffness properties of foam‐based dressings used for PU prevention (in their Table 1 in Reference 30). Their analysis of elastic moduli of dressings that encompasses many of the foam dressings by lead manufacturers in the dressing industry, and which lists the products that are currently popular for PU prevention, indicated a range of 7–31 kPa for a 50% compressive strain level applied to the dressings during the tests (the specific elastic modulus values attributed to each dressing product type are specified in Reference 30).
The listed properties are for medical‐grade, polyvinylchloride (PVC)‐based gels.
Grigatti and Gefen reported mean elastic moduli between 21 and 74 kPa for the HydroTac Transparent hydrogel‐based dressing (Paul Hartmann AG, Heidenheim, Germany) depending on the moisture absorbed by this dressing in an experimental setup. 23
In contrast, though hydrogel‐based dressings for PU prevention are currently not as popular as the materials analysed in Figure 2 (ie, hydrocolloids, foams, gels, and gauze), they were proposed by Grigatti and Gefen 23 , 24 as being highly effective for the alleviation of tissue loads and microclimate management from a biomechanical perspective. 47 Specifically, the elastic modulus and the thermal conductivity properties of the HydroTac Transparent hydrogel‐based dressing (Paul Hartmann AG) both fall within the respective ranges of skin properties, as depicted in Figure 3. The measurements reported in Figure 3 were acquired for dry and moist dressing conditions, as described by Grigatti and Gefen, 23 and the thermal conductivity data were collected by means of two different methods, namely, a heat‐flow meter and an IRT‐based system. 23 Importantly, the resemblance of the above hydrogel‐based dressing properties to the properties of native skin is not surprising in the context of the structure–function‐property principle (Figure 1A). The skin contains 60%–70% water, 48 , 49 like hydrogels, and similarly to hydrogels, its solid matrix is a chain of long molecules (collagen and elastin proteins) that provide its stiffness and strength properties; in hydrogels, this structural role is fulfilled by the polymer chains. That is, fundamentally, hydrogels better resemble the skin, with respect to foams for example. The thermal conductivity of water (at room temperature), being the main component in hydrogel and skin, is 0.6 W/mK, whereas air, which has a major presence in foams, has a thermal conductivity of only 0.03 W/mK, that is, 20‐times lower, which explains why hydrogels and skin have similar thermal conductivities but foams have a substantially lower thermal matching with skin.
FIGURE 3.
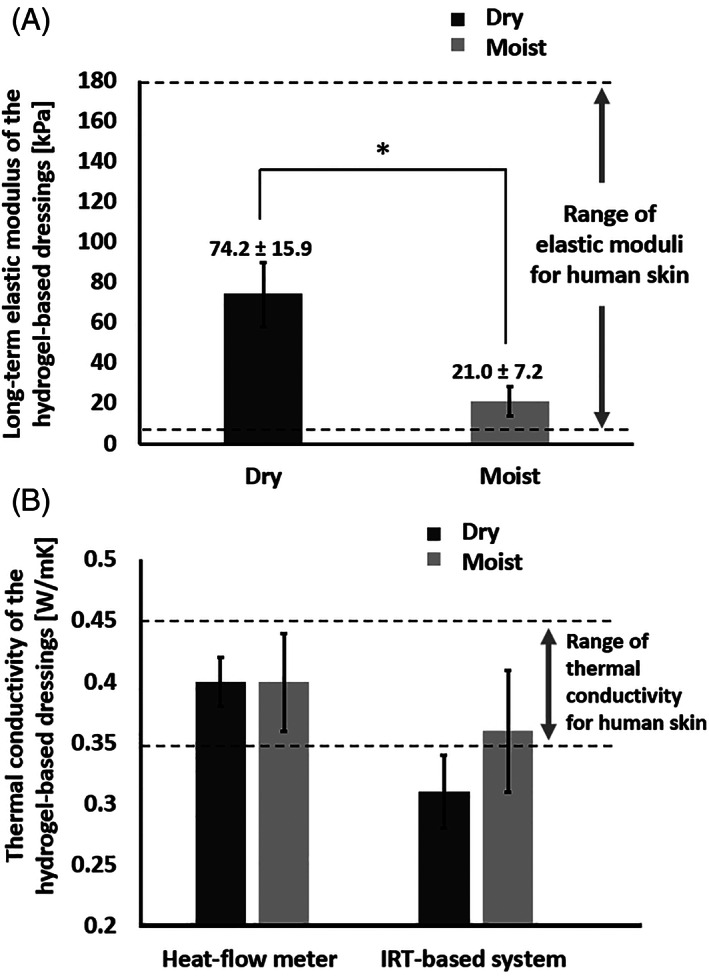
The elastic modulus (A) and thermal conductivity (B) properties of the tested hydrogel‐based dressing (HydroTac Transparent by Paul Hartmann AG), which both fall within the respective ranges of skin properties. These measurements were acquired for dry and moist dressing conditions, as described by Grigatti and Gefen 23 and the thermal conductivity data were collected by means of two different methods, namely, a heat‐flow meter and an infrared thermography (IRT) based system 23
4. QUANTITATIVE CRITERIA FOR THE MATCHING WITH SKIN AND RESULTING PROTECTIVE EFFICACY
The extent of the matching between materials used for PU prevention in adults and skin should be assessed with respect to the properties of native adult human skin. In the context of prevention of MDRPUs, the literature reports that the in vivo elastic moduli of facial skin, measured through indentation (which is the closest loading mode to the real‐world skin‐device interactions) range from 1 to 200 kPa. 50 , 51 , 52 , 53 , 54 , 55 , 56 As noted by McKee et al, 56 the range of stiffnesses reported for human skin studied by means of indentation testing is wide, however, some reported elastic modulus values for skin fall out of the median range, hence, McKee and colleagues 56 conducted an outlier analysis, and after excluding outliers, they concluded that the skin stiffness is within the 6 to 222 kPa range, with an average of 85 kPa, confirming that the mid‐range stiffness of skin subjected to indentation is ~100 kPa. With regards to thermal conductivity, Crawford and colleagues 57 reported anatomical‐site‐specific thermal conductivity data for living adult skin, revealing that the thermal conductivity of adult facial skin at the nose and cheeks is 0.34–0.45 W/mK and 0.39–0.45 W/mK, respectively. These data confirmed the earlier work of Tian et al, 28 who, likewise, found that the thermal conductivity of the nose and cheeks ranges from 0.25 to 0.45 W/mK, depending on the level of skin hydration.
Based on the above skin property data reported in the literature, the quality of the matching between a dressing (or padding, or cushioning) material (ie, its elastic modulus and thermal conductivity) properties and the corresponding skin properties can be represented by plotting the extent of the overlap (if exists) between the property ranges over the elastic modulus and thermal conductivity domains (Figure 4A ). Further grading of material performance in achieving biomechanical protective efficacy can be formulated by calculating the distance of the normalised property ratios from the unity (1,1) coordinates, as follows. First, we define the coordinates of the protective efficacy performance of a certain dressing/padding/cuhsioning material as (E dressing/E skin, kdressing/k skin) where E dressing and k dressing are the elastic modulus and thermal conductivity of the dressing/padding/cushioning material under investigation and E skin and k skin are the corresponding mid‐range properties of adult skin. Ideally, a dressing for prophylactic use, for instance, would have an elastic modulus that is identical to the mid‐range stiffness of skin (ie, E skin = ~100 kPa), and, likewise, thermal conductivity that equals the mid‐range thermal conductivity of skin (ie, k skin = ~0.4 W/mK), which defines the unity (1,1) coordinates of the property ratios (E dressing/100, k dressing/0.4; where E dressing is in kPa and k dressing is in W/mK). The square distance of any dressing/padding/cushioning material coordinates from the unity coordinates signifying the aforementioned ideal matching with skin, Δ2, can then be calculated as:
| (1) |
The nearer the property ratio coordinates of a certain dressing/padding/cushioning material (E dressing/E skin, k dressing/k skin ) to the unity coordinates, that is, the lower the (dimensionless) Δ2 value, the better is the matching between the material and skin, and hence, the greater is the biomechanical protective efficacy of that dressing/padding/cushioning material.
FIGURE 4.
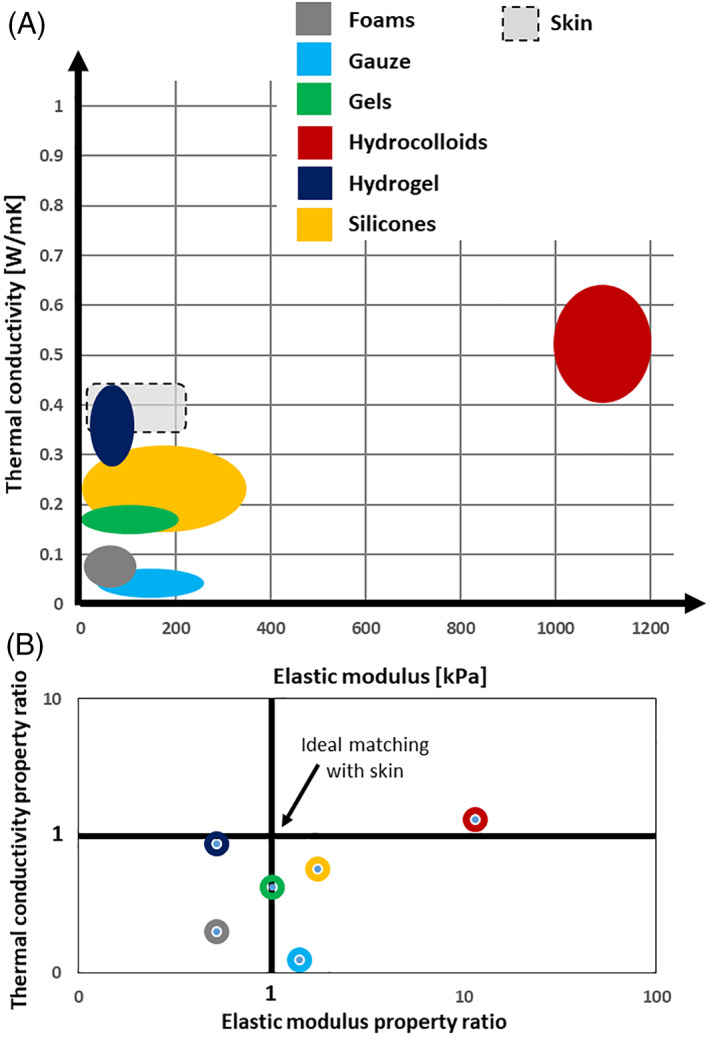
Scatter plots to compare the resemblance of stiffness and thermal conductivity properties of materials that are commonly used in dressings for pressure ulcer prophylaxis, to the corresponding properties of adult human skin. The extent of the matching between the dressing material properties and the corresponding skin properties is represented by the level of the overlap (if any) between property ranges over the elastic modulus and thermal conductivity domains (A); the ideal property range, which is the property range reported for native, living human skin in the literature reviewed here, is depicted in grey shadow with black dash line borders. In addition, the extent of property matching between the investigated materials and human skin is plotted as the distance of the (normalised) property ratios from the unity (1,1) coordinates on a logarithmic scale (B). Please see the text for a detailed description of the calculation method used for generating the results shown in panel (B). Ideally, a dressing for prophylactic use would have an elastic modulus that is identical to the mid‐range stiffness of skin (ie, E skin = ~100 kPa) and thermal conductivity that equals the mid‐range thermal conductivity of skin (ie, k skin = ~0.4 W/mK). Accordingly, the nearer the property ratio coordinates of a certain dressing material (E dressing/E skin, k dressing/k skin) to the unity coordinates, the better is the matching between the dressing material and skin, and hence, the greater is the biomechanical protective efficacy of that dressing. Using this bioengineering grading system of distances from the unity coordinates, the grading of biomechanical protective efficacies of common dressing materials, from best to worst, is: (a) hydrogel‐based dressing; (b) gels; (c) silicones; (d) foams; (e) gauze; and (f) hydrocolloids. Note that the hydrocolloids are distant by two orders of magnitude from the unity coordinates with respect to the other material types, and, hence, from the modulus matching perspective, are inferior even to gauze. The mid‐range properties of skin were taken as E skin = 101 kPa and k skin = 0.4 W/mK for the purpose of the above grading calculations
5. THE MODULUS AND THERMAL MATCHING PERFORMANCE OF MATERIALS FOR PROPHYLAXIS
The stiffness of foam dressing materials is typically within the 5–100 kPa range, 58 which falls within the above range of stiffnesses of adult skin, so in terms of modulus matching, there is a good fit; a large number of prophylactic dressing products will meet this “modulus matching” goal (Figure 2). In terms of the thermal conductivity matching, however, foams are inferior, because, as already mentioned, they contain air (the extent of the air content depends on the microarchitecture of the foam, namely, on the pore size distribution in the specific foam). This makes foams a good thermal insulator, which may be adequate for treatment dressings, to prevent loss of heat and cooling of the wound in the absence of intact skin, but for preventative dressings, this is not the case. Preventative dressings, on the contrary, should allow the effective and continuous release of heat from the skin through the dressing to prevent a perspiration response. The thermal conductivity of foams is in the range of 0.05 to 0.11 W/mK, 21 which, for a mid‐range property ratio, yields k dressing/k skin = 0.08 (foam)/0.4 (skin) = 0.2, and hence, the thermal conductivity matching between skin and foam dressings is poor (Figure 4).
Hydrogel‐based dressing materials are much more promising in this regard, again due to the structure–function‐property principle (Figure 1A), that is, hydrogels resemble the skin in a key structural feature – high water contents, whereas water is a better thermal conductor than air (which is trapped in foams). For the particular hydrogel‐based dressing studied by Grigatti and Gefen, 23 , 24 which was the HydroTac Transparent dressing (Paul Hartmann AG), the compressive stiffness was in the 15 to 90 kPa range (Figure 3A; Table 1), depending on the moisture state of the dressing, 23 which is similar to the aforementioned values for foams, and again, within the range of the compressive elastic moduli of adult skin (Figure 4A). However, in terms of thermal conductivity matching, the hydrogel‐based dressing emerged as being superior to foams, with the thermal conductivity of the hydrogel‐based dressing being in the range of 0.27 to 0.43 W/mK (Figure 3B; Table 1), yielding a mid‐range property ratio of 0.35 (hydrogel)/0.4 (skin) = ~0.9, that is, very close thermal matching between the hydrogel‐based dressing and adult skin (Figure 4A). Accordingly, when comparing a hydrogel‐based dressing technology to a foam dressing, the similarity is expected in the extent of alleviation of skin loads, but the hydrogel‐based technology should perform superiorly in microclimate management (Figure 4A).
Indeed, using the bioengineering grading system of distances from the unity coordinates formulated in Equation (1), and based on the property values listed in Table 1, the grading of biomechanical protective efficacies of common dressing materials, from best to worst, is: (a) hydrogel‐based dressing (Δ2 = 0.24); (b) gels (Δ2 = 0.43); (c) silicones (Δ2 = 0.74); (d) foams (Δ2 = 0.87); (e) gauze (Δ2 = 0.92); and (f) hydrocolloids (Δ2 = 109) (Figure 4B). The mid‐range properties of skin were taken as E skin = 101 kPa and k skin = 0.4 W/mK for the purpose of the above grading calculations. Of note, hydrocolloids are distant by two orders of magnitude from the unity coordinates with respect to the other material types, and hence, particularly from the modulus matching perspective, hydrocolloids are inferior even to gauze (Figure 4). Silicone‐foam composites which are commonly used in prophylactic dressings can be approximated as having a pooled matching performance combining those of silicones and foams separately, and hence, will be rated after hydrogel‐based and gel‐based materials if considering the mechanical and thermal matching requirements altogether.
A potential approach in the design of future products for protecting from MDRPUs can be based on the concept of “graded stiffness.” This biomechanical concept originated in the field of orthopaedics, where attempts were traditionally made to bridge across the stiffness of metallic implants and the substantially lower stiffness of native bone, in order to avoid bone resorption over time due to stress shielding. 59 , 60 A similar approach can be employed for preventing MDRPUs using a gradient of the relevant property, particularly the stiffness, so that the softest material in a sandwich structure (ideally resembling the stiffness of skin) is placed directly against the skin, and increasingly stiffer layers are placed on top of it towards the structure of the device. While this is likely a more complex and expensive solution to implement from a design and manufacturing perspective, it may be required in cases where an inherently stiff device cannot be avoided, such as in the case of a metallic electrode or a cervical collar (where the rigidity is essential for stabilisation of the head and neck).
Only a small number of clinical studies compared different dressing products that were applied prophylactically to similar groups of participants and following a consistent trial protocol; among these papers, 9 , 10 , 11 some of the studied groups were small and/or only included healthy subjects. In fact, the only published large‐scale clinical trial which included head‐to‐head dressing product comparisons in at‐risk patients was the work of Beeckman and colleagues, 11 who compared the protective effects of two commercial silicone adhesive multilayer foam dressing types. Their work focused on the prevention of bodyweight‐induced PUs (at the sacrum, heels, and trochanter areas), but did not test protection against MDRPUs. Clearly, clinical trials of the scale reported by Beeckman et al 11 (who conducted a multicentre, randomised medical device trial in eight different European hospitals, in which data were collected from 1578 patients in total) require vast financial and manpower resources. All the other relevant published literature compared protective dressings against a standard‐of‐care practice that did not include protective dressings, as recently reported in the meta‐analysis by Gong and Xu. 61 The outcome of head‐to‐head product comparisons is, at best, indicative of whether one of the tested products performed better than the others in protecting patients, but such results cannot be extrapolated to any untested product types, either of the same category (eg, silicone foams) or which belong to different categories (eg, hydrogel‐based). Likewise, it is impossible to generalise the findings to new products and technologies that were not available for use at the time of the study, or to variant wound aetiologies that were not directly investigated (eg, to deduce about MDRPUs from findings related to bodyweight‐induced PUs). Even for a large study that required a national public funding program at the scale of the Beeckman research project, the authors stated that their “exploratory data analyses did not demonstrate any major differences in effectiveness between the two brands, considering that the study was not powered to detect such differences.” 11 This indicates, first, that large‐scale clinical trials are likely not the most cost‐effective approach for detecting these product performance differences. In addition, considering for example that no randomised clinical trials exist for hydrogel‐based prophylactic dressings, it cannot be assumed that if differences in protective capacity were not previously detected between two foam‐based dressings, 11 then that will also apply to hydrogels (or to any other theoretically‐promising protective technology in this regard). A feasible way for evaluating and rating technologies and products for PU prevention is, therefore, to combine biomechanical methodology as in the current work with advanced physiological measurements of skin conditions, such as by means of infrared thermography or biocapacitance measurements (as per our published work 9 , 23 , 24 , 62 ), using study designs that are aimed at detecting product performance differences at greater sensitivity than clinical trials. In other words, it is not that large‐scale clinical trials indicate little difference between product type performance metrics, but rather, it is that clinical trials, even the largest and most expensive ones that were reported in the field, are underpowered for detecting such prophylactic product performance differences.
6. CONCLUDING REMARKS
The alleviation of localised, sustained tissue loads and microclimate management are the most critical performance criteria for materials in use for PU prevention, such as in prophylactic dressings or padding. These material performance criteria can be evaluated by calculating the extents of matching between the material stiffness (ie, the elastic modulus) and the thermal conductivity of the protective dressing or pad with the properties of native skin, separately or in combination. Based on these bioengineering performance criteria, hydrocolloids, which are one of the most commonly used material types in the prophylaxis of MDRPUs 18 exhibit poor biomechanical protective efficacy, particularly with regards to the modulus matching criterion (E dressing/E skin), as their compressive elastic modulus is more than 10‐times greater with respect to that of skin. In addition, a remarkable variability exists in the modulus and thermal conductivity matching levels of different material types used for PU prevention (Figure 2; Table 1), however, it appears that among the materials tested and analysed here, hydrogels demonstrate the optimal stiffness/thermal matching (Figures 3), and, thereby, provide the optimal protection to the skin, followed by gels and silicone foams (Figure 4). Based on the quantitative, modulus, and thermal conductivity matching criteria described in this article (Equation (1)), the protective efficacy of hydrocolloids appears to be the worst, inferior even to that of gauze (Figure 4).
In addition to the stiffness and thermal matching metrics discussed here (Figure 4B), prophylactic dressings, cushioning, and padding materials and constructs that come into contact with skin (or mucous membranes) should of course be biocompatible, non‐allergenic, non‐irritating, and should desirably have bacteria‐repellent surfaces. In addition, such materials and constructs must tolerate the sustained or repeated mechanical forces and the wear‐and‐tear scenarios that are experienced in real‐world clinical practice, in the context of their expected role in PU prevention. That is, PU prevention materials and constructs should be evaluated by industry and regulatory bodies similar to the requirements set by test standards for wound dressings used for treatment (eg, the European Norm “EN 13726 Primary Wound Dressing Test Methods”), or by test standards for wheelchair cushions designed for reducing the PU risk (eg, the ISO 16840‐2:2018 standard “Wheelchair seating ‐ Part 2: Determination of physical and mechanical characteristics of seat cushions intended to manage tissue integrity”). Of note, currently, no dedicated and accepted testing standards exist for the class of prophylactic dressings, despite their widespread use and clinically proven effectiveness. In November 2020, the European Pressure Ulcer Advisory Panel and the National Pressure Injury Advisory Panel in the US announced the establishment of an international Task Force Co‐Chaired by the author, in order to develop new performance standards through a multiple‐year workplan involving different stakeholders (clinicians, bioengineers, industry, and test standard experts, professional societies and the like), which is known as the Prophylactic Dressing Standards Initiative (PDSI; www.epuap.org/prophylactic-dressing; last accessed on February 21st, 2022). The new standards to be formed by the PDSI Task Force will generate critically relevant information to guide effective selection and practice of materials and products for PU prevention, as well as benchmarks for development purposes and reimbursement policies for such PU prevention products.
In closure, this work provided objective, standardised, generic, and quantitative biomechanical performance criteria and metrics for methodological evaluations of materials for PU prevention, and had graded the commonly used material types in a clinically relevant manner. This information should be used as a guide for clinicians and industry with regards to the selection of dressings for PU prophylaxis, concerning both PUs caused due to bodyweight forces and as a result of applied medical devices. Ultimately, the clinical evidence of the effectiveness of a certain material or construct in PU prophylaxis must align with any recommendation that has been based upon laboratory tests and should be used for confirming the pre‐clinical findings and for translating them to the real‐world conditions and implementation in practice. However, for making a good investment of clinical research resources in the field of PU prevention, it is necessary that theoretical biomechanics analyses and laboratory research will precede the conduct of randomised clinical trials, and thereby, guide and focus the clinical studies so that they are informed by the relevant and underlying physical, engineering and physiological principles which are essential for effective PU prevention. 12 , 14
ACKNOWLEDGEMENTS
The basic science and bioengineering laboratory work of Professor Amit Gefen on the topic of pressure ulcer prevention is currently being supported by the Israeli Ministry of Science & Technology (Medical Devices Program Grant no. 3‐17421, awarded to Professor Gefen in 2020). The author acts as a scientific advisor to multiple companies in the field of pressure ulcer/injury prevention, including Paul Hartmann AG (Heidenheim, Germany), whose products are mentioned in this publication. This had no influence on the conclusions from the analyses of the published laboratory findings and test data presented here.
Gefen A. Alternatives and preferences for materials in use for pressure ulcer prevention: An experiment‐reinforced literature review. Int Wound J. 2022;19(7):1797‐1809. doi: 10.1111/iwj.13784
Funding information Ministry of Science, Technology and Space, Grant/Award Number: Medical Devices Program Grant no. 3‐17421; Paul Hartmann AG (Heidenheim, Germany)
Endnotes
The elastic modulus is a quantitative engineering stiffness measure for materials, defined as the ratio of the mechanical force exerted upon a substance to the resultant deformation of that material.
The thermal conductivity is an engineering measure of the rate at which heat passes through a specified material (expressed as the amount of heat that flows per unit time through a unit area with a temperature gradient of one degree per unit distance). In the context of PU prevention, the thermal conductivity of a dressing (or padding, or cushioning) material expresses the extent of metabolic heat transfer from the tissues under the dressing, outwards through the dressing structure, to the environment (Figure 1C). 15
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Brown G. Long‐term outcomes of full‐thickness pressure ulcers: healing and mortality. Ostomy Wound Manage. 2003;49(10):42‐50. [PubMed] [Google Scholar]
- 2. Redelings MD, Lee NE, Sorvillo F. Pressure ulcers: more lethal than we thought? Adv Skin Wound Care. 2005;18(7):367‐372. [DOI] [PubMed] [Google Scholar]
- 3. Khor HM, Tan J, Saedon NI, et al. Determinants of mortality among older adults with pressure ulcers. Arch Gerontol Geriatr. 2014;59(3):536‐541. [DOI] [PubMed] [Google Scholar]
- 4. Padula WV, Delarmente BA. The national cost of hospital‐acquired pressure injuries in the United States. Int Wound J. 2019;16(3):634‐640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Agency for Healthcare Research & Quality . Preventing Pressure Ulcers in Hospitals. Rockville, MD, USA: US Agency for Healthcare Research and Quality; 2014. https://www.ahrq.gov/patient-safety/settings/hospital/resource/pressureulcer/tool/pu1.html, . [Google Scholar]
- 6. Brophy S, Moore Z, Patton D, O'Connor T, Avsar P. What is the incidence of medical device‐related pressure injuries in adults within the acute hospital setting? A systematic review. J Tissue Viability. 2021;29:S0965‐206X(21)00033‐4. doi: 10.1016/j.jtv.2021.03.002 [DOI] [PubMed] [Google Scholar]
- 7. Clark M, Black J, Alves P, et al. Systematic review of the use of prophylactic dressings in the prevention of pressure ulcers. Int Wound J. 2014;11(5):460‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fulbrook P, Mbuzi V, Miles S. Effectiveness of prophylactic sacral protective dressings to prevent pressure injury: a systematic review and meta‐analysis. Int J Nurs Stud. 2019;100:103400. [DOI] [PubMed] [Google Scholar]
- 9. Amrani G, Peko L, Hoffer O, Ovadia‐Blechman Z, Gefen A. The microclimate under dressings applied to intact weight‐bearing skin: infrared thermography studies. Clin Biomech (Bristol, Avon). 2020;75:104994. [DOI] [PubMed] [Google Scholar]
- 10. Lechner A, Rancan F, Hadam S, Vogt A, Blume‐Peytavi U, Kottner J. Comparing the effects of three different multilayer dressings for pressure ulcer prevention on sacral skin after prolonged loading: an exploratory crossover trial. Wound Repair Regen. 2021;29(2):270‐279. [DOI] [PubMed] [Google Scholar]
- 11. Beeckman D, Fourie A, Raepsaet C, et al. Silicone adhesive multilayer foam dressings as adjuvant prophylactic therapy to prevent hospital‐acquired pressure ulcers: a pragmatic noncommercial multicentre randomized open‐label parallel‐group medical device trial. Br J Dermatol. 2021;185(1):52‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gefen A, Brienza DM, Cuddigan J, Haesler E, Kottner J. Our contemporary understanding of the aetiology of pressure ulcers/pressure injuries. Int Wound J. 2022;19(3):692‐704. doi: 10.1111/iwj.13667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bader DL, Worsley PR, Gefen A. Bioengineering considerations in the prevention of medical device‐related pressure ulcers. Clin Biomech (Bristol, Avon). 2019;67:70‐77. [DOI] [PubMed] [Google Scholar]
- 14. Gefen A, Alves P, Ciprandi G, et al. Device‐related pressure ulcers: SECURE prevention. J Wound Care. 2020;29(Sup2a):S1‐S52. [DOI] [PubMed] [Google Scholar]
- 15. Gefen A. The role of the thermal conductivity of dressings in prevention and treatment of wounds. Wounds Int. 2021;12(1):18‐24. [Google Scholar]
- 16. Schwartz D, Gefen A. The biomechanical protective effects of a treatment dressing on the soft tissues surrounding a non‐offloaded sacral pressure ulcer. Int Wound J. 2019;16(3):684‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gefen A, Alves P, Creehan S, Call E, Santamaria N. Computer modeling of prophylactic dressings: an indispensable guide for healthcare professionals. Adv Skin Wound Care. 2019;32(7S Suppl 1):S4‐S13. [DOI] [PubMed] [Google Scholar]
- 18. Cai JY, Zha ML, Chen HL. Use of a hydrocolloid dressing in the prevention of device‐related pressure ulcers during noninvasive ventilation: a meta‐analysis of randomized controlled trials. Wound Manag Prev. 2019;65(2):30‐38. [PubMed] [Google Scholar]
- 19. Bishopp A, Oakes A, Antoine‐Pitterson P, Chakraborty B, Comer D, Mukherjee R. The preventative effect of hydrocolloid dressings on nasal bridge pressure ulceration in acute non‐invasive ventilation. Ulster Med J. 2019;88(1):17‐20. [PMC free article] [PubMed] [Google Scholar]
- 20. Swan J. Use of dermal gel pads in preventing and managing pressure ulcers in ICU: an audit. Br J Nurs. 2018;27(20):S42‐S47. [DOI] [PubMed] [Google Scholar]
- 21. Schwartz D, Gefen A. An integrated experimental‐computational study of the microclimate under dressings applied to intact weight‐bearing skin. Int Wound J. 2020;17(3):562‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gefen A, Krämer M, Brehm M, Burckardt S. The biomechanical efficacy of a dressing with a soft cellulose fluff core in prophylactic use. Int Wound J. 2020;17(6):1968‐1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grigatti A, Gefen A. What makes a hydrogel‐based dressing advantageous for the prevention of medical device‐related pressure ulcers. Int Wound J. 2022;19(3):515‐530. doi: 10.1111/iwj.13650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grigatti A, Gefen A. The biomechanical efficacy of a hydrogel‐based dressing in preventing facial medical device‐related pressure ulcers. Int Wound J. 2021, in press. doi: 10.1111/iwj.13701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walker RM, Ayello EA, Chan SC, et al. Prevention and treatment of pressure ulcers/injuries: clinical practice guideline. In: Haesler E, ed. The International Guideline. Westford, MA: EPUAP/NPIAP/PPPIA; 2019:181‐193. [Google Scholar]
- 26. Amrani G, Gefen A. Considerable variability in stiffness properties of medical products used for prevention of medical device‐related pressure ulcers. Israel Society for Medical & Biological Engineering (ISMBE) 2019 Annual Conference, Haifa Congress Center, Israel, February 25–26, 2019.
- 27. ASTM D3574‐11, Standard Test Methods for Flexible Cellular Materials—Slab, Bonded, and Molded Urethane Foams. West Conshohocken, PA: ASTM International; 2011. www.astm.org [Google Scholar]
- 28. Tian L, Li Y, Webb R, et al. Flexible and stretchable 3 omega sensors for thermal characterization of human skin. Adv Funct Mater. 2017;27(26) 1701282:1‐9. [Google Scholar]
- 29. Levy A, Schwartz D, Gefen A. The contribution of a directional preference of stiffness to the efficacy of prophylactic sacral dressings in protecting healthy and diabetic tissues from pressure injury: computational modelling studies. Int Wound J. 2017;14(6):1370‐1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sieracki J, Wilkes R, Bennett ER, McNulty AK. Finite element analysis modeling of a novel silicone dressing. Cureus. 2020;12(9):e10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goldthwait CF, Murphy AL. Special elastic properties of cotton yarn and cloth mercerized without tension. Text Res J. 1955;25(1):47‐57. [Google Scholar]
- 32. Jinyun Z, Lam J, Xuyong C. The Poisson ratio and modulus of elastic knitted fabrics. Text Res J. 2010;80(18):1965‐1969. [Google Scholar]
- 33. Van Amber RR, Wilson CA, Laing RM, Lowe BJ, Niven BE. Thermal and moisture transfer properties of sock fabrics differing in fiber type, yarn, and fabric structure. Text Res J. 2014;85(12):1269‐1280. [Google Scholar]
- 34. Wang W, Hui KT, Kan CW, et al. A study of thermal conductivity property of socks. Mater Sci Forum. 2020;1007:118‐124. [Google Scholar]
- 35. Engineering Toolbox: Young's Modulus, Tensile Strength and Yield Strength Values for some Materials. https://www.engineeringtoolbox.com/young-modulus-d_417.html, last accessed October 20th, 2021.
- 36. Koch RC, Sturza HJ. Polyvinylchloride gel in orthotics and prosthetics part 1: preparation and application of silicone gel. Orthot Prosthet. 1971;25(3):16‐19. [Google Scholar]
- 37. Jaime RAO, Basto RLQ, Lamien B, Orlande HRB, Eibner S, Fudym O. Fabrication methods of phantoms simulating optical and thermal properties. Proced Eng. 2013;59:30‐36. [Google Scholar]
- 38. Xue Y, Lofland S, Hu X. Thermal conductivity of protein‐based materials: a review. Polymers (Basel). 2019;11(3):456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McGarry CK, Grattan LJ, Ivory AM, et al. Tissue mimicking materials for imaging and therapy phantoms: a review. Phys Med Biol. 2020;30:1‐43. doi: 10.1088/1361-6560/abbd17. https://iopscience.iop.org/article/10.1088/1361-6560/abbd17 [DOI] [PubMed] [Google Scholar]
- 40. MacPherson GW, Craig RG, Peyton FA. Mechanical properties of hydrocolloid and rubber impression materials. J Dent Res. 1967;46(4):714‐721. [DOI] [PubMed] [Google Scholar]
- 41. Leal‐Junior A, Guo J, Min R, Fernandes AJ, Frizera A, Marques C. Photonic smart bandage for wound healing assessment. Photon Res. 2021;9(3):272‐280. [Google Scholar]
- 42. Hassan H, Ramaswamy H. Measurement and targeting of thermophysical properties of carrot and meat based alginate particles for thermal processing applications. J Food Eng. 2011;107(1):117‐126. [Google Scholar]
- 43. Egorov V, Tsyuryupa S, Kanilo S, Kogit M, Sarvazyan A. Soft tissue elastometer. Med Eng Phys. 2008;30(2):206‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gutierrez E, Groisman A. Measurements of elastic moduli of silicone gel substrates with a microfluidic device. PLoS One. 2011;6(9):e25534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Licari JL. Coating materials for electronic applications: polymers, processes, reliability, testing. A Volume in Materials and Processes for Electronic Applications. Amsterdam, The Netherlands: Elsevier; 2003. ISBN: 978‐0‐8155‐1492‐3. [Google Scholar]
- 46. Riccucci G, Pezzana L, Lantean S, Tori A, Spriano S, Sangermano M. Investigation of the thermal conductivity of silicon‐base composites: the effect of filler materials and characteristic on thermo‐mechanical response of silicon composite. Appl Sci. 2021;11(12):5663. [Google Scholar]
- 47. Gefen A. Medical device‐related pressure ulcers and the COVID‐19 pandemic: from aetiology to prevention. Wounds UK. 2021;17(3):28‐37. [Google Scholar]
- 48. Mitchell HH, Hamilton TS, Steggerda FR, Bean HW. The chemical composition of the adult human body and its bearing on the biochemistry of growth. J Biol Chem. 1945;158:625‐637. [Google Scholar]
- 49. Forbes RM, Cooper AR, Mitchell HH. The composition of the adult human body as determined by chemical analysis. J Biol Chem 1953. Jul;203(1):359–66. [PubMed] [Google Scholar]
- 50. Zheng YP, Mak A. Effective elastic properties for lower limb soft tissues from manual indentation experiment. IEEE Trans Rehabil Eng. 1999;7(3):257‐267. [DOI] [PubMed] [Google Scholar]
- 51. Pailler‐Mattei C, Bec S, Zahouani H. In vivo measurements of the elastic mechanical properties of human skin by indentation tests. Med Eng Phys. 2008;30(5):599‐606. [DOI] [PubMed] [Google Scholar]
- 52. Zahouani H, Pailler‐Mattei C, Sohm B, Vargiolu R, Cenizo V, Debret R. Characterization of the mechanical properties of a dermal equivalent compared with human skin in vivo by indentation and static friction tests. Skin Res Technol. 2009;15(1):68‐76. [DOI] [PubMed] [Google Scholar]
- 53. Flynn C, Taberner AJ, Nielsen PMF, Fels S. Simulating the three‐dimensional deformation of in vivo facial skin. J Mech Behav Biomed Mater. 2013;28:484‐494. [DOI] [PubMed] [Google Scholar]
- 54. Kalra A, Lowe A, Al‐Jumaily AM. Mechanical behaviour of skin: a review. J Material Sci Eng 2016;5(4):1000254, pp. 1–7. [Google Scholar]
- 55. Dai A, Wang S, Zhou L, Wei H, Wang Z, He W. In vivo mechanical characterization of human facial skin combining curved surface imaging and indentation techniques. Skin Res Technol. 2019;25(2):142‐149. [DOI] [PubMed] [Google Scholar]
- 56. McKee CT, Last JA, Russell P, Murphy CJ. Indentation versus tensile measurements of Young's modulus for soft biological tissues. Tissue Eng Part B Rev. 2011;17(3):155‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Crawford KE, Ma Y, Krishnan S, et al. Advanced approaches for quantitative characterization of thermal transport properties in soft materials using thin, conformable resistive sensors. Extreme Mech Lett. 2018;22:27‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Orlov A, Gefen A. How influential is the stiffness of the foam dressing on soft tissue loads in negative pressure wound therapy? Med Eng Phys. 2021;89:33‐41. [DOI] [PubMed] [Google Scholar]
- 59. Gefen A. Computational simulations of stress shielding and bone resorption around existing and computer‐designed orthopaedic screws. Med Biol Eng Comput. 2002;40(3):311‐322. [DOI] [PubMed] [Google Scholar]
- 60. Gefen A. Optimizing the biomechanical compatibility of orthopedic screws for bone fracture fixation. Med Eng Phys. 2002;24(5):337‐347. [DOI] [PubMed] [Google Scholar]
- 61. Gong X, Xu R. Prophylactic sacral protective dressings' effect on preventing pressure injury: a meta‐analysis. Int Wound J. 2021, in press. doi: 10.1111/iwj.13743 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62. Peko L, Ovadia‐Blechman Z, Hoffer O, Gefen A. Physiological measurements of facial skin response under personal protective equipment. J Mech Behav Biomed Mater. 2021;120:104566. doi: 10.1016/j.jmbbm.2021.104566 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


