Abstract
Universal lipid screening (ULS) is recommended for all 9- to 11-year-old children. We investigated ULS outcomes and long-term pediatrician management of children with dyslipidemia using a retrospective chart review of well-child visits between 2014 and 2016. Descriptive statistics summarized demographics, ULS results, and follow-up visits/testing. Pearson χ2 test examined differences between those with and without an abnormal screen. A total of 1039 children aged 9 to 11 years were seen for a well-child visit; only 33.3% (343/1039) completed screening. Of children screened, 18.1% (62/343) had abnormal screen results and were more likely to have an elevated body mass index (P < .001), though 30.1% (19/62) had no risk factors. A total of 10.2% (35/343) had dyslipidemia. A total of 77.1% of children with dyslipidemia received nutrition/exercise counseling and 57.1% received dietitian referrals; only 68.6% had a follow-up visit and 31.4% had repeat lipid testing. Pediatricians would benefit from more practical strategies for universal testing such as point-of-care testing and long-term management to ensure ULS is an effective screening tool.
Keywords: cholesterol, universal screening, pediatrics, physician management, long-term follow-up
Background
Cardiovascular disease (CVD), including hypertension, coronary artery disease, heart failure, and stroke, remains the leading cause of death in the United States, and almost half (48.0%) of American adults suffer from CVD.1 There is evidence that atherosclerosis originates in childhood and may be prevented if risk factors are identified and treated during childhood and adolescence.2 Therefore, identification of children at risk for CVD and the development of effective interventions should be a focus for pediatricians to prevent CVD in adulthood and promote lifelong health.
Over the past several years, policy organizations have reviewed the scientific evidence related to hyperlipidemia and CVD and have arrived at differing conclusions about lipid screening in childhood. In 2011, the Expert Panel of Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents published by the National Heart, Lung, and Blood Institute (NHLBI) and endorsed by the American Academy of Pediatrics (AAP) recommended universal lipid screening (ULS) among 9- to 11-year-old children.3 In contrast, the US Preventive Services Task Force concluded in 2016 that there was “insufficient evidence” to assess the benefits and harms of universal screening and recommended against ULS.4 As a result, ULS remains controversial in the pediatric community.5-7 In a nationally representative survey conducted in 2017, only 55% of pediatricians agreed with ULS of children.8 Prior to the NHLBI guidelines, pediatric lipid screening rates in large-scale studies were low at 3.4%9 to 9.8%.10 Following the recommendation for universal screening, studies have continued to demonstrate low but increasing rates at 3.5%11 to 27.5%.12 A study involving a large cohort of 55 610 children from 51 practices in 3 states revealed that lipid screening of children turning 12 years increased from 9% in 2011 to 20% in 2016.13
Despite the controversy in recommendations, several academic and community pediatric practices have implemented projects to promote universal screening.14-16 In 2014, we conducted a clinic-wide quality improvement (QI) project designed to increase ULS among children aged 9 to 11 years old during well-child visits. During the 12-month-long QI project, physician ordering of lipid screening increased from a baseline of 6.2% in 2013 to 84.8% in 2014, and 20.1% of children who completed screening had an abnormal result.17 Abnormal screens were associated with being overweight/obese, Black, or Hispanic. Participating pediatricians raised concerns about the long-term follow-up of children with abnormal screens and the impact of universal screening, and questioned whether more targeted screening could be more effective.
To date, there have been limited studies that have examined the long-term outcomes and follow-up care of children aged 9 to 11 years with an abnormal lipid screen in the context of ULS in the United States. In the United States, a longitudinal study of 1808 nine- to 11-year-old children revealed that universal cholesterol screening itself made no impact on body mass index (BMI) trajectory over a 3-year period when compared with matched controls who did not undergo screening.18
Although no studies have examined specific physician management behaviors in the care of children with dyslipidemia diagnosed during ULS, a recent study revealed that 9- to 11-year-old children with higher cholesterol levels were more likely to be seen in follow-up primary care visits (75% vs 63%) and have specialty referrals (20% vs 14%) but no more likely to undergo subsequent laboratory testing (22% vs 23%).18 According to NHLBI/AAP guidelines, children with an abnormal lipid screen should complete a fasting lipid panel (FLP) twice within 3 months to evaluate the abnormal screen.3 If the FLPs confirm dyslipidemia, the child should be referred to a registered dietitian, managed initially with dietary and lifestyle modifications for 6 months, and then reassessed with a repeat FLP.3 It is unclear whether physicians and patients are engaging in this recommended follow-up care.
Considering the varying recommendations from national organizations and few studies on the long-term management of children with abnormal lipid screens, more data are needed on ULS results, physician management of children with abnormal screening results, and long-term outcomes to inform future recommendations and clinical care. The objectives of this study were the following: (1) to identify the proportion of 9- to 11-year-old patients who had abnormal results on a FLP obtained in the context of ULS and (2) to examine physician management behaviors and follow-up of children with abnormal FLPs over a 3-year period.
Methods
Sample and Site Characteristics
We conducted a retrospective chart review of the electronic medical record (EMR) of all 9- to 11-year-old children who presented for a well-child visit between January 1, 2014, and December 31, 2016. This study included visits that occurred during a successful year-long QI project designed to increase ULS among children aged 9 to 11 years old during well-child visits in 2014, and the 2 subsequent years 2015 and 2016. These children presented to the University of California, San Diego Pediatrics Clinic, a large academic practice with 2 sites that serve as the main outpatient teaching sites for UC San Diego medical students, pediatric residents, and medicine-pediatric residents. These clinics have 14 full-time and part-time faculty pediatricians who care for a diverse population in San Diego County, averaging more than 18 000 visits annually.
Screening Protocol
The NHLBI/AAP guidelines recommended lipid screening at 2 to 8 years old for children with a family history of CVD or dyslipidemia, or for children with diabetes, hypertension, and/or elevated BMI. However, these guidelines had rarely been followed in our clinic population prior to the study period and almost no children had lipid screening done prior to age 9 to 11 years old. Thus, all children 9 to 11 years old were eligible for inclusion, regardless of whether they were screened at a younger age due to family history or medical conditions. Children aged 9 to 11 years who completed lipid screening during the study period were then excluded if they presented for another well-child visit during the study period.
Point-of-care testing and in-clinic phlebotomy was not available; all children were required to go to an off-site laboratory for lipid screening. Abnormal non-fasting lipid screens and FLP results were determined based on the NHLBI/AAP guidelines3 (Figure 1). In our screening protocol, pediatricians could choose to order an initial lipid screen as either a non-fasting lipid screen (non-fasting total cholesterol (TC) and high-density lipoprotein [HDL]-cholesterol) or a FLP. If the initial non-fasting lipid screen was abnormal (Figure 1), the pediatrician was expected to order a FLP for confirmatory testing. A child was considered to have an abnormal lipid screen if either the initial non-fasting lipid screen or the initial FLP was abnormal. A child was considered to have dyslipidemia if either the confirmatory FLP result or the initial FLP was abnormal. Of note, after initially attempting to follow the NHLBI guidelines to obtain 2 separate FLPs within a 3-month period to confirm an abnormal FLP, pediatricians in this practice found this guideline recommendation to be impractical and instead opted to complete only one FLP to diagnose dyslipidemia.
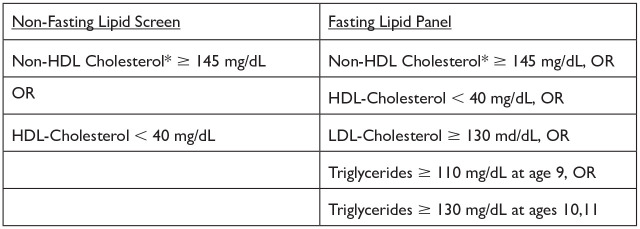
Figure 1.
The National Heart, Lung, and Blood Institute/American Academy of Pediatrics definition of abnormal lipid tests. HDL, high-density lipoprotein; LDL, low-density lipoprotein.
*Non-HDL cholesterol = total cholesterol − HDL-cholesterol.
For the management of children with dyslipidemia, pediatricians were instructed to provide nutrition and exercise counseling, provide a referral to a dietitian (which included a weight management program), and repeat a follow-up FLP 6 months later, as recommended by the NHLBI guidelines.3 Although pediatricians may have provided nutrition and exercise counseling and referral to a dietitian for another indication such as elevated BMI, this management for children with dyslipidemia was emphasized to follow the NHLBI guidelines. Nutrition and exercise counseling was determined to have been provided if specifically documented in the EMR during a primary care clinic visit note or telephone discussion. Physician referrals to a dietitian and other specialists including cardiology and endocrinology were extracted from the referral section of the EMR. All follow-up visits during the study period were reviewed including any dyslipidemia follow-up visits, well-child visits, and/or any other visits because repeat FLP testing may have occurred at any of these visits. Repeat FLP ordering and any repeat FLP results were also extracted from the EMR during the 3-year study period.
Measures
Demographic characteristics (age, sex, insurance status, and parent-reported race/ethnicity), clinical data (month and year of visit, BMI, BMI percentile, and family history of CVD), lipid screening and FLP results, physician management (documentation of nutrition and/or exercise counseling, nutrition referrals), and follow-up indicators (return visits, month and year of return visit, repeat FLP ordering and results) were extracted from the EMR. The physician management actions and follow-up indicators were selected because these are the specific actions recommended by the 2011 NHLBI guidelines. Data were extracted by one author (LE) and reviewed for accuracy by a second author (LK). The study was approved by the UC San Diego Institutional Review Board.
Weight status was determined based on the Centers for Disease Control and Prevention guidelines with BMI <85th percentile characterized as being normal, BMI 85th to 94th percentile as overweight, and BMI ≥95th percentile as obese.19 Family history of CVD was defined as a family history of heart disease, hypertension, dyslipidemia, and/or stroke. These data were extracted from the family history section of the well-child visit note in the EMR, but review of this section was not specifically required to complete the well-child visit.
Analysis
Descriptive statistics (means and frequencies) were utilized to describe patient demographic and clinical characteristics (age, sex, insurance status, race/ethnicity, BMI, BMI percentile, and family history of CVD). Descriptive statistics were also used to assess the frequency of abnormal lipid screens and FLPs, physician management behaviors (nutrition/exercise counseling, dietitian referrals), and follow-up indicators (frequency of return visits and repeat FLP ordering, completion rates, and results) during the 3-year follow-up period. Pearson χ2 test was used to examine the association between abnormal lipid screen results and patient characteristics. Multivariate logistic regression was performed to further assess the association between abnormal lipid screen results and patient characteristics. We originally planned to conduct multivariate analysis of the patient characteristics of those with confirmed dyslipidemia but the number of children was too small to conduct this analysis. Data were analyzed using R.20
Results
Patient Characteristics
Between January 1, 2014, and December 31, 2016, a total of 1039 children were seen for a 9-, 10-, or 11-year-old well-child visit. Patient characteristics are listed in Table 1. Overall, 53.2% were male; 49.5% were White non-Hispanic, 18.7% were Hispanic, 12.0% were Asian, and 6.0% were Black; 79.5% had private insurance; 12.9% had overweight and 10.5% had obesity; and 23.3% reported a family history of CVD.
Table 1.
Patient Characteristics from 2014 to 2016 and Association Between Patient Characteristics and Lipid Screen Results.
| Overall | Lipid screen abnormal | P | ||
|---|---|---|---|---|
| No | Yes | |||
| Gender, n (%) | N = 1044 | N = 281 | N = 62 | 1.000 |
| Female | 489 (46.8%) | 130 (46.3%) | 29 (46.8%) | |
| Male | 555 (53.2%) | 151 (53.7%) | 33 (53.2%) | |
| Insurance, n (%) | N = 1044 | N = 280 | N = 61 | .110 |
| Public | 203 (19.4%) | 54 (19.3%) | 18 (29.5%) | |
| Private | 830 (79.5%) | 226 (80.7%) | 43 (70.5%) | |
| Race/ethnicity, n (%) | N = 1043 | N = 281 | N = 62 | .347 |
| White, non-Hispanic | 516 (47.5%) | 129 (45.9%) | 21 (33.9%) | |
| Asian | 125 (12.0%) | 37 (13.2%) | 8 (12.9%) | |
| Black | 63 (6.0%) | 14 (5.0%) | 5 (8.1%) | |
| Hispanic | 195 (18.7%) | 60 (21.4%) | 19 (30.6%) | |
| Other | 142 (13.6%) | 41 (14.6%) | 9 (14.5%) | |
| Age, n (%) | N = 1044 | N = 281 | N = 62 | .524 |
| 9 years | 423 (40.5%) | 123 (43.8%) | 32 (51.6%) | |
| 10 years | 299 (28.6%) | 82 (29.2%) | 15 (24.2%) | |
| 11 years | 322 (30.8%) | 76 (27.0%) | 15 (24.2%) | |
| BMIa category, n (%) | N = 1022 | N = 276 | N = 62 | <.001 |
| Normal | 783 (76.6%) | 220 (79.7%) | 30 (48.4%) | |
| Overweight | 132 (12.9%) | 30 (10.9%) | 13 (21.0%) | |
| Obese | 107 (10.5%) | 26 (9.4%) | 19 (30.6%) | |
| Family history of CVDb, n (%) | N = 1044 | N = 281 | N = 62 | .141 |
| No | 801 (76.7%) | 218 (77.6%) | 42 (67.7%) | |
| Yes | 243 (23.3%) | 63 (22.4%) | 20 (32.3%) | |
Abbreviations: BMI, body mass index; CVD, cardiovascular disease.
BMI with overweight defined as BMI from 85th to 94th percentile for age and obese defined as BMI ≥95th percentile for age.
CVD defined as any family history of heart disease, hypertension, dyslipidemia, and/or stroke.
Lipid Screening and Fasting Lipid Panel Results
Although pediatricians ordered ULS for nearly three quarters (69.2%, 719/1039) of children seen for a 9- to 11-year-old well-child visit during the 3-year study period, only one third of all children (33.0%, 343/1039; or about half of those for whom a screening test was ordered, 47.7%, 343/719) completed the lipid screening test (Figure 2). In our study, Asian children and Hispanic children were more likely to complete the lipid screening test compared with children of other race/ethnicity (P = .04), but lipid screening completion rates did not differ based on gender, insurance type, BMI status, and family history of CVD. Of the 343 children who completed ULS, 80.2% (275/343) completed an initial non-fasting lipid screen and the other 19.8% (68/343) completed an initial FLP. Among those who completed screening, 18.1% (62/343) had abnormal screening results (either non-fasting lipid screen or FLP). Of note, 39.7% (27/68) of those who completed an initial FLP had an abnormal result, while only 12.7% (35/275) of children who completed an initial non-fasting lipid screen had an abnormal result. Ultimately, 10.2% (35/343) of all children who completed screening were confirmed to have dyslipidemia with an abnormal FLP result (Figure 2). For the 35 children with dyslipidemia, 34.3% (12/35) had an abnormal TC, 42.9% (15/35) had an abnormal non-HDL cholesterol, 34.3% (12/35) had an abnormal HDL-cholesterol, 22.9% (8/35) had an abnormal LDL-cholesterol, and 60% (21/35) had abnormal triglycerides.
Figure 2.
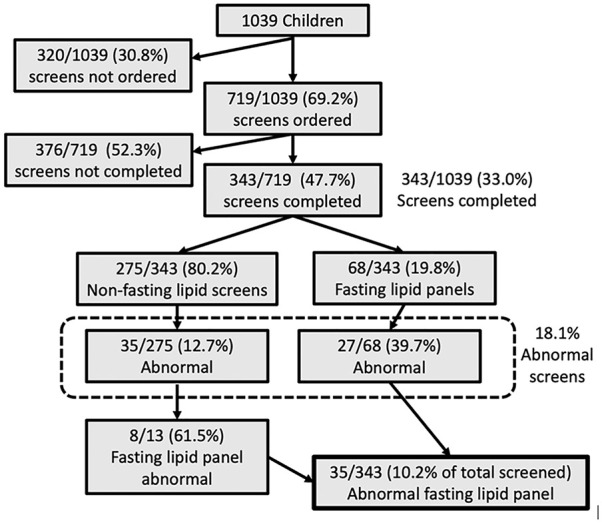
Lipid screen and fasting lipid panel results for 1039 nine- to 11-year-old children.
Associations Between Patient Characteristics and Lipid Screening Results
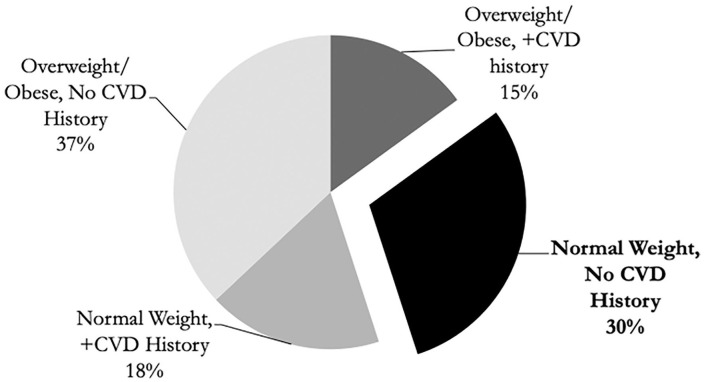
Table 1 shows the associations between patient characteristics and lipid screening results. Children with overweight and those with obesity were more likely to have an abnormal lipid screen (P < .001). In contrast, the child’s gender, age, insurance status, race/ethnicity, and family history of CVD were not associated with having an abnormal lipid screen. In a multivariate analysis, the odds of an abnormal screen for children with overweight/obesity was 4.19 times greater than children with normal BMI for age percentile (confidence interval = 2.3-7.5, P < .001). Figure 3 demonstrates the association between an elevated BMI and/or a family history of CVD with an abnormal lipid screen. Of note, 30.1% (19/62) of children with an abnormal lipid screen had neither an elevated BMI nor a family history of CVD.
Figure 3.
Abnormal lipid screens by BMI and CVD family history (n = 62). BMI, body mass index; CVD, cardiovascular disease.
*Overweight/obese defined as BMI ≥85th percentile for age.
**CVD history = Cardiovascular disease defined as any family history of heart disease, hypertension, dyslipidemia, and/or stroke.
Pediatrician Management and Follow-up of Children With Dyslipidemia
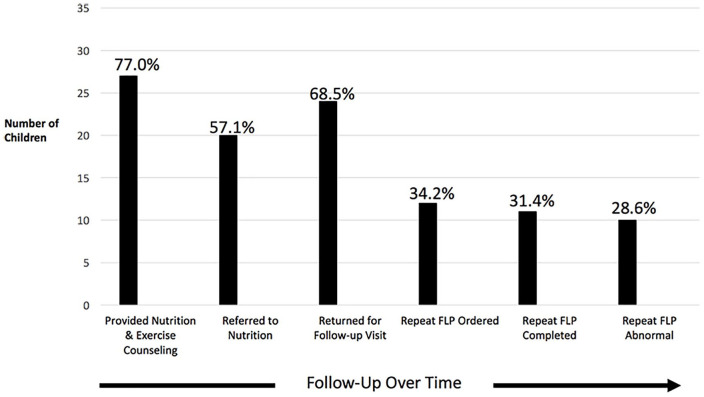
Figure 4 illustrates the long-term physician management of those children with dyslipidemia from 2014 to 2016. The majority (77.1%, 27/35) of children received nutrition and exercise counseling, and 57.1% (20/35) were referred to a dietitian (which included a weight management program). While 68.6% (24/35) returned for any follow-up visit (including dyslipidemia follow-up visits, well-child visits, and/or any other visits) in the 3-year period, only 34.2% (12/35) had a repeat FLP ordered by their pediatrician and 91.7% (11/12) of these children completed a repeat FLP during the 3-year follow-up period. Of note, most of those who completed a repeat FLP (90.9%, 10/11) had persistently abnormal results. Few children were referred to cardiologists or other dyslipidemia specialists, and none started pharmacologic interventions during the study period.
Figure 4.
Follow-up of children with abnormal fasting lipid panels (FLPs; N = 35).
Discussion
During a 3-year period, ~70% of 9- to 11-year-old children had a lipid screening test ordered by their pediatrician and slightly less than half of 9- to 11-year-old children actually completed the screening test (equating to ~33% of all 9- to 11-year-old children screened during the 3-year period). This relatively low screening completion rate is consistent with other intervention studies that have sought to improve rates of ULS in pediatric practices. Despite an increase in pediatrician ordering of ULS, these other studies had lipid screening completion rates ranging from 20.1%14 to 21.4%.15 Taken together, these results indicate that lipid screening tests are not being completed by the majority of children and it may be inferred that lipid screening blood tests may not be acceptable to the majority of 9- to 11-year-old children and their parents. The results also indicate that we are not achieving ULS and that further research is needed on how to improve lipid screening test completion rates. A recent survey found that parental support for ULS is significantly associated with their attitudes about universal screening rather than their knowledge about cholesterol, family history of CVD, or demographics.21 Future research could further examine parental attitudes about ULS, pediatrician communication about ULS with patients/families, the barriers to and motivators for completing lipid screening, and the effectiveness of in-clinic phlebotomy and/or point-of-care testing for increasing screening rates.
Our study illustrates that pediatricians did not always strictly follow the suggested NHLBI/AAP guidelines for initial ULS. While pediatricians initially ordered a non-fasting lipid screen for over 75% of children, pediatricians ordered a FLP as the initial screening test instead for ~20% of children. Some pediatricians may have suspected that certain children were more likely to have an abnormal FLP based on a positive family history of CVD or an elevated BMI, and thus opted to skip the initial non-fasting screen. This appears to be true, as 36.8% of those who had a FLP as an initial screen had an abnormal result, while only 13.5% of those with an initial non-fasting lipid screen had an abnormal result. Our practice found that the NHLBI/AAP guideline recommendation to order 2 FLPs in a 3-month period to diagnose dyslipidemia was impractical. Given the difficulty of getting families to complete one, let alone 2 blood tests, consideration should be made to recommending a FLP in certain populations.
Similar to other studies, 18.1% of the 9- to 11-year-old children who completed screening in our study had an abnormal lipid screening test. Data from the National Health and Nutrition Examination Survey (NHANES) obtained from 2007 to 2010 demonstrated that 22.2% of children aged 9 to 11 years had either low HDL-C or high non-HDL-C.22 Similarly, more recent NHANES data sampled from 2011 to 2014 found that 21% of youths aged 6 to 19 years had elevated TC, low HDL-cholesterol, and/or elevated non-HDL-cholesterol.23 Our study also revealed about one third of these children with an abnormal lipid screening test did not possess an identifiable risk factor, such as a positive family history of CVD or an elevated BMI. This is consistent with other studies such as the CARDIAC Project where 28.6% of 20 266 fifth-grade children in West Virginia found to have dyslipidemia did not have a positive family history,24 and a study in Lebanon where 37.7% of children aged 2 to 10 years with dyslipidemia did not possess any risk factor.25 The evidence review of the 2011 NHLBI/AAP guidelines concluded that 30% to 60% of children with dyslipidemia would be missed if lipid screening were not universal.3 Thus, our results lend further support for the importance of universal rather than targeted lipid screening for the identification of children with dyslipidemia who may not otherwise be recognized.
This study also examined physician management behaviors and long-term outcomes of children found to have dyslipidemia in the context of ULS, an area that has not been examined extensively. Over a 3-year period, our study demonstrates that a majority of children found to have dyslipidemia received exercise/nutrition counseling and a referral to a dietitian, as recommended by the NHLBI/AAP guidelines,3 while few were referred to a pediatric cardiologist or endocrinologist. Counseling and referrals may not have long-term effects, as one systematic review of ULS studies among children found that diet and/or exercise changes occurred 29% to 92% of the time.26 A recent article indicates that ULS may not have long-term impact. A longitudinal study of 1808 nine- to 11-year-old children in the United States revealed that universal cholesterol screening itself made no impact on BMI trajectory over a 3-year period when compared with matched controls who did not undergo screening.18 In our study, the long-term follow-up and impact on dyslipidemia rates was limited. While only 34.2% (12/35) of children with dyslipidemia had a repeat FLP ordered and 31.4% (11/12) completed it in the 3-year period, 90.9% (10/11) of those children demonstrated persistent dyslipidemia. This contrasts with results from a study conducted in Lebanon, where a high proportion of school-age children (53.6%, 52/97 children) were found to normalize their abnormal lipid profile after 3 years.27 However, it is important to note that these 97 Lebanese children who had a follow-up lipid profile after 3 years were from an original study group of 339 children with an abnormal lipid profile, meaning that the longitudinal group represented only 28.6% of those with dyslipidemia.
One of the hallmarks of an effective screening program is that physicians can offer interventions or treatments that make a positive impact on the disease outcome over time. Our results indicate that, although ULS at 9 to 11 years old identifies children with dyslipidemia who might not otherwise be recognized, the challenge lies in the long-term management of these children. This includes challenges providing effective nutrition and exercise interventions, proactively following children with dyslipidemia at subsequent clinic visits, ensuring the completion of repeat FLPs, and, ultimately, positively affecting their lipid profile over time. Our results suggest that, unless more effective and practical protocols for long-term management of children with dyslipidemia are developed, ULS may not have as much of an impact on long-term CVD risk in children as expected.
Our study had several limitations. First, our method of retrospective chart review may have biased our results, as it relied on EMR documentation for accuracy. Not every patient may have had an accurate family history of CVD documented. Second, our results may not be generalizable, as our patient population and physician management may differ from others in different areas of the country. Third, our results may be biased toward a higher abnormal lipid screening rate. It is possible that the children who did not complete ULS may be more likely to have normal lipid levels; perhaps the pediatrician and/or parents were less concerned that the result would be abnormal and therefore did not proceed with screening. Thus, the abnormal lipid screening rate may be falsely inflated, overemphasizing the importance of universal rather than targeted lipid screening. However, our results mirror those of other national studies suggesting that these rates may be accurate, and our ULS rate completion did not differ by BMI status or family history of CVD. Additionally, the decision to use 1 rather than 2 abnormal FLP tests to diagnose dyslipidemia may have led to overdiagnosis. Unfortunately, our study is also limited by a very steep drop off rate at each step involved in ULS. Of the 1039 nine- to 11-year-old children eligible for lipid screening, only 343 (33%) completed screening. Of the 62 children with an abnormal screen, only 35 were confirmed to have dyslipidemia with an abnormal FLP and followed by pediatricians. The majority of children did not complete screening, so we do not know the true number and outcomes of children with dyslipidemia in the context of ULS. Last, our study only spans a 3-year timeframe. Physician management of children with dyslipidemia may have continued past the 3-year period and may not have been captured in this study.
The results of our study indicate that ULS among 9- to 11-year-old children has the potential to identify a significant number of children with dyslipidemia, especially children who might not otherwise be identified with targeted screening. These results suggest that more effective strategies for ULS test completion and the long-term management of identified children are needed to ensure that prevention and treatment efforts can be initiated in childhood rather than adulthood. Future research may attempt to identify strategies to increase ULS test completion rates, including improved parental education and communication about ULS and/or removal of potential barriers of ULS such as providing in-clinic phlebotomy or point-of-care testing. Future research could also focus on protocols to improve long-term physician management of children with dyslipidemia, such as implementing more effective nutrition and exercise interventions, EMR prompts to improve repeat FLP ordering rates, and/or phone/text reminders to ensure adequate follow-up. Most important, we recommend longitudinal, diverse, and large-scale studies of ULS to better assess its impact on the early identification of dyslipidemia, the prevention of CVD, and the promotion of lifelong health of children and young adults.
Author Contributions
LE: Contributed to conception and design; contributed to acquisition, analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
LK: Contributed to conception and design; contributed to acquisition, analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
HW: Contributed to conception and design; contributed to interpretation; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
JC: Contributed to conception and design; contributed to interpretation; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
KER: Contributed to conception and design; contributed to interpretation; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Acknowledgments
We would like to acknowledge statistician James Proudfoot of UC San Diego, and Dr Cindy Reynolds, assistant physician and post-doctoral fellow in Biomedical Informatics.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by the Academy of Clinician Scholars, University of California, San Diego. Supported by the UC San Diego Center for Translational Research: The project was partially supported by the National Institutes of Health, Grant UL1TR001442 of CTSA funding. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ORCID iDs: Leah Kern  https://orcid.org/0000-0003-2116-0129
https://orcid.org/0000-0003-2116-0129
Janet Crow  https://orcid.org/0000-0003-1528-1450
https://orcid.org/0000-0003-1528-1450
References
- 1. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139:e56-e528. doi: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 2. Halfon N, Verhoef PA, Kuo AA. Childhood antecedents to adult cardiovascular disease. Pediatr Rev. 2012;33:51-60. doi: 10.1542/pir.33-2-51 [DOI] [PubMed] [Google Scholar]
- 3. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(suppl 5):S213-S256. doi: 10.1542/peds.2009-2107C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, et al. Screening for lipid disorders in children and adolescents: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:625-633. doi: 10.1001/jama.2016.9852 [DOI] [PubMed] [Google Scholar]
- 5. Newman TB, Pletcher MJ, Hulley SB. Overly aggressive new guidelines for lipid screening in children: evidence of a broken process. Pediatrics. 2012;130:349-352. doi: 10.1542/peds.2012-0481 [DOI] [PubMed] [Google Scholar]
- 6. Schroeder AR, Redberg RF. Cholesterol screening and management in children and young adults should start early—No! Clin Cardiol. 2012;35:665-668. doi: 10.1002/clc.22075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Ferranti SD, Daniels SR, Gillman M, Vernacchio L, Plutzky J, Baker AL. NHLBI integrated guidelines on cardiovascular disease risk reduction: can we clarify the controversy about cholesterol screening and treatment in childhood? Clin Chem. 2012;58:1626-1630. doi: 10.1373/clinchem.2012.182089 [DOI] [PubMed] [Google Scholar]
- 8. de Ferranti SD, Rodday AM, Parsons SK, et al. Cholesterol screening and treatment practices and preferences: a survey of United States pediatricians. J Pediatr. 2017;185:99-105. doi: 10.1016/j.jpeds.2016.12.078 [DOI] [PubMed] [Google Scholar]
- 9. Vinci SR, Rifas-Shiman SL, Cheng JK, Mannic RC, Gillman MW, de Ferranti SD. Cholesterol testing among children and adolescents during health visits. JAMA. 2014;311:1804-1807. doi: 10.1001/jama.2014.2410 [DOI] [PubMed] [Google Scholar]
- 10. Margolis KL, Greenspan LC, Trower NK, et al. Lipid screening in children and adolescents in community practice: 2007-2010. Circ Cardiovasc Qual Outcomes. 2014;7:718-726. doi: 10.1161/CIRCOUTCOMES.114.000842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mihalopoulos NL, Sitpelman C, Hemond J, Brown LL, Young PC. Universal lipid screening in 9- to 11-year-olds before and after 2011 guidelines. Acad Pediatr. 2018;18:196-199. doi: 10.106/j.acap.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 12. Valle CW, Binns HJ, Quadri-Sheriff M, Benuck I, Patel A. Physicians’ lack of adherence to National Heart, Lungs, and Blood Institute guidelines for pediatric lipid screening. Clin Pediatr (Phila). 2015;54:1200-1205. doi:10.1177;0009922815576885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gregory EF, Miller JM, Wasserman RC, et al. Adherence to pediatric universal cholesterol testing guidelines across body mass index categories: a CER2 cohort study. Circ Cardiovasc Qual Outcomes. 2020;13:e006519. doi: 10.1161/CIRCOUTCOMES.119.006519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wilson DP, Davis S, Matches S, et al. Universal cholesterol screening in community-based ambulatory pediatric clinics. J Clin Lipidol. 2015;9(5 suppl):S88-S92. doi:10.106;j.jacl.2015.05.006 [DOI] [PubMed] [Google Scholar]
- 15. DeSantes K, Dodge A, Eickhoff J, Peterson AL. Improved universal pediatric lipid screening. J Pediatr. 2017;188:87-90. doi: 10.106/j.jpeds.2017.05.030 [DOI] [PubMed] [Google Scholar]
- 16. Stipelman CH, Stoddard GJ, Smith ER, et al. Quality improvement intervention for universal lipid screening in children aged 9 to 11 years. Clin Pediatr (Phila). 2019;58:1528-1533. doi: 10.1177/0009922819884403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kern L, Crow J, Williams CB, Boies E, Gahagan S, Rhee KE. Increasing universal lipid screening among 9- to 11-year-old children through a quality improvement initiative. Clin Pediatr (Phila). 2017;56:640-647. doi: 10.1177/0009922816670979 [DOI] [PubMed] [Google Scholar]
- 18. Gregory EF, Miller JM, Wasserman RC, Seshadri R, Rubin DM, Fiks AG. Routine cholesterol tests and subsequent change in BMI among overweight and obese children. Acad Pediatr. 2019;19:773-779. doi: 10.1016/j.acap.2019.05.131 [DOI] [PubMed] [Google Scholar]
- 19. Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002;(246):1-190. [PubMed] [Google Scholar]
- 20. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2019. Accessed January 12, 2022. https://www.R-project.org/ [Google Scholar]
- 21. Kern L, Eichberger L, Wang H, Lin T, Rhee KE. Parental knowledge and attitudes about universal lipid screening among children aged 9 to 11 years. Clin Pediatr (Phila). 2020;59:439-444. doi: 10.1177/0009922820903043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kit BK, Carroll MD, Lacher DA, Sorlie PD, DeJesus JM, Ogden C. Trends in serum lipids among US youths aged 6 to 19 years, 1988-2010. JAMA. 2012;308:591-600. doi: 10.1001/jama.2012.9136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nguyen D, Kit B, Carroll M. Abnormal cholesterol among children and adolescents in the United States, 2011-2014. NCHS Data Brief. 2015;(228):1-8. [PubMed] [Google Scholar]
- 24. Ritchie SK, Murphy EC, Ice C, et al. Universal versus targeted blood cholesterol screening among youth: the CARDIAC project. Pediatrics. 2010;126:260-265. doi: 10.1542/peds.2009-2546 [DOI] [PubMed] [Google Scholar]
- 25. Georges N, Simon A, Nalm B, Georges N, Georges AF, Tanios A. Universal screening program for lipid disorders in 2-10 years old Lebanese children: a new approach. Int J Pediatr Adolesc Med. 2019;6:101-108. doi:10.106.j.ijpam.2019.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith AJ, Turner EL, Kinra S. Universal cholesterol screening in childhood: a systematic review. Acad Pediatr. 2016;16:716-725. doi: 10.1016/j.acap.2016.06.005 [DOI] [PubMed] [Google Scholar]
- 27. Saadé C, Sleilaty G, Gannagé-Yared M. Longitudinal changes of lipid profile in the Lebanese pediatric population. Lipids Health Dis. 2019;18:48. doi: 10.1186/s12944-019-0991-x [DOI] [PMC free article] [PubMed] [Google Scholar]