Abstract
To determine whether pathogenic Acanthamoeba culbertsoni trophozoites and lysate can induce cytopathic changes in primary-culture microglial cells, morphological changes were observed by transmission electron microscopy (TEM). In addition, the secretion of two kinds of cytokines, tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β), from microglial cells was observed. Trophozoites of pathogenic A. culbertsoni made contact with microglial cells and produced digipodia. TEM revealed that microglial cells cocultured with amoebic trophozoites underwent a necrotic process, accompanied by lysis of the cell membrane. TEM of microglial cells cocultured with amoebic lysate showed that the membranes of the small cytoplasmic vacuoles as well as the cell membrane were lysed. The amounts of TNF-α secreted from microglial cells cocultured with A. culbertsoni trophozoites or lysate increased at 6 h of incubation. The amounts of IL-1β secreted from microglial cells cocultured with A. culbertsoni trophozoites at 6 h of incubation was similar to those secreted from the control group, but the amounts decreased during cultivation with A. culbertsoni lysate. These results suggest that pathogenic A. culbertsoni induces the cytopathic effects in primary-culture rat microglial cells, with the effects characterized by necrosis of microglial cells and changes in levels of secretion of TNF-α and IL-1β from microglial cells.
Acanthamoeba spp. are small, free-living limax amoebae which can cause chronic granulomatous amoebic encephalitis (GAE) and acanthamoebic keratitis in humans and experimental animals (7, 18, 21). Generally, a virulent amoeba that causes GAE in mice is cytotoxic to target cells (12, 13). Moore et al. (11) suggested that the cytopathic effects (CPEs) induced by Acanthamoeba castellanii against human corneal epithelial cells was the result of cytolytic enzymes released from trophozoites and subsequent phagocytosis by amoebae. The CPE of A. castellanii trophozoites against target cells involves calcium channels, cytoskeletal elements necessary for phagocytosis, and amoeba motility (17). Alizadeh et al. (1) and Dove Pettit et al. (4) reported that apoptosis was also a mechanism of cytolysis of corneal epithelial cells and murine neuroblastoma cells by pathogenic Acanthamoeba.
Microglial cells, a type of brain macrophage, have been cultured from rats and mice to understand the pathogenesis responsible for infection with various microorganisms (6, 19). Microglial cells occur in three morphological forms following cell differentiation, i.e., an amoeboid form during embryogenesis, a ramified shape in the mature normal brain, and a rod-shaped morphology around inflammatory lesions in the central nervous system (CNS) (6, 15). Moreover, they function as phagocytotic cells and produce cytokines such as interleukin-1 (IL-1), IL-6, and tumor necrosis factor alpha (TNF-α) (3, 16). Thus, it has been suggested that microglial cells play an important role as inflammatory cells or immunoregulatory cells in the protective immune system of the CNS (16). The purpose of present study was to determine whether primary-culture rat microglial cells show cytopathic changes in response to pathogenic Acanthamoeba trophozoites and lysate. Morphological changes of microglial cells were observed by transmission electron microscopy (TEM). In addition, the levels of secretion of TNF-α and IL-1β from microglial cells were determined.
A pathogenic strain of Acanthamoeba culbertsoni (donated by J. B. Jardin in 1977) was axenically cultured in peptone-yeast-glucose medium (20) at 37°C. The pathogenicity of this strain had previously been confirmed, in which 60% mortality occurred when mice were infected with 104 trophozoites (8). To prepare the amoeba lysate, trophozoites (108) of amoeba were harvested and washed with phosphate-buffered saline (PBS; pH 7.4). Trophozoites suspended in 1 ml of PBS were frozen (−70°C) and thawed (37°C) three times and centrifuged at 10,000 × g for 2 h. The supernatant was filtered with a 0.25-μm-pore-size disk filter, and the protein concentration (adjusted to 10 mg/ml) was determined by the assay described by Bradford (2).
Microglial cells were prepared by the method of Giulian and Baker (6), with some modifications (14). Briefly, the cortex of the brain was obtained from a newborn Sprague-Dawley rat and homogenized with a 21-gauge syringe. The mixture was centrifuged at 300 × g for 10 min and resuspended in Eagle's minimal essential medium (EMEM) with 10% fetal bovine serum (FBS). The suspension was put onto 75-cm2 tissue culture flasks pretreated with polylysine (Sigma Chemical Co., St. Louis, Mo.), in order to increase the level of adherence of the cells, and the flasks were incubated at 37°C in a 5% CO2 atmosphere for 1 week. Microglial cells were harvested by vigorously shaking each culture flask and were then filtered with nylon wool to remove any remaining astrocytes and centrifuged at 300 × g for 10 min. The pellet was resuspended in EMEM with 10% FBS, and the mixture was incubated at 37°C for 2 h. The attached microglial cells were harvested and adjusted to a concentration of 105 cells per well in a 24-well culture plate for the treatment of Acanthamoeba trophozoites (1:1) or lysate.
Following coincubation of the microglial cells with trophozoites or amoeba lysate, the culture was fixed in modified Karnovsky's fixative solution in cacodylate buffer (pH 7.4) and postfixed in 1% osmium tetroxide–1.5% potassium ferrocyanide. The cells were stained en bloc in 0.5% uranyl acetate, dehydrated through a graded ethanol series, and embedded in resin (Polyscience, Warrington, Pa.). Then, the blocks were sectioned with a Reichert-Jung Ultracut S ultramicrotome and stained with Ultrostain 1H and 2 (Leica, Vienna, Austria). The specimens were observed and photographed with a Zeiss EM 902 A electron microscope (Leo, Oberkohen, Germany).
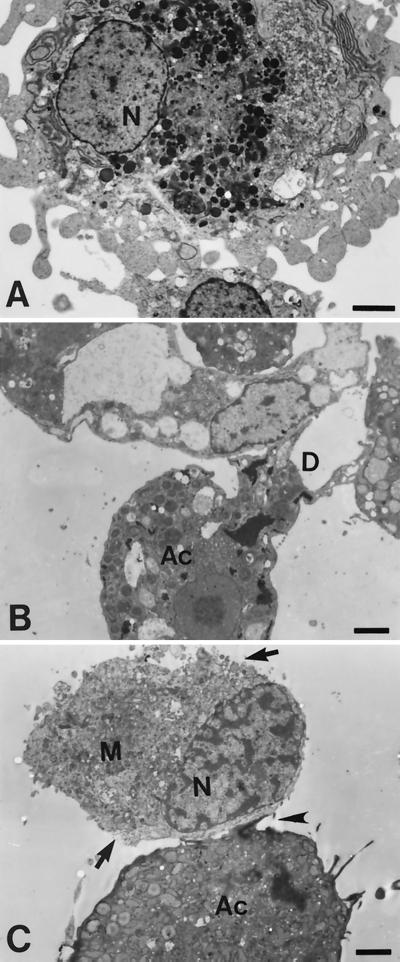
Observations by TEM revealed that trophozoites of pathogenic A. culbertsoni characterized by a large karyosome in the nucleus made contact with microglial cells and produced a digipodium (Fig. 1B). TEM showed that microglial cells underwent necrotic processes which were accompanied by clumping of chromatin materials in the nucleus and lysis of the cell membrane (Fig. 1C).
FIG. 1.
Findings for microglial cells and Acanthamoeba spp. obtained by TEM (A) A primary-culture microglial cell shows numerous pseudopodia and a large nucleus (N) containing marginally scattered chromatin materials. (B and C) Coculture of microglial cells (M) with A. culbertsoni trophozoites (Ac) for 6 h. An amoeba is attached on the surface of a microglial cell (arrowhead). A trophozoite of A. culbertsoni produced a digipodium (D) on a microglial cell. Lysis of the cell membrane of a microglial cell was also apparent (arrow). Bars, 2.5 μm.
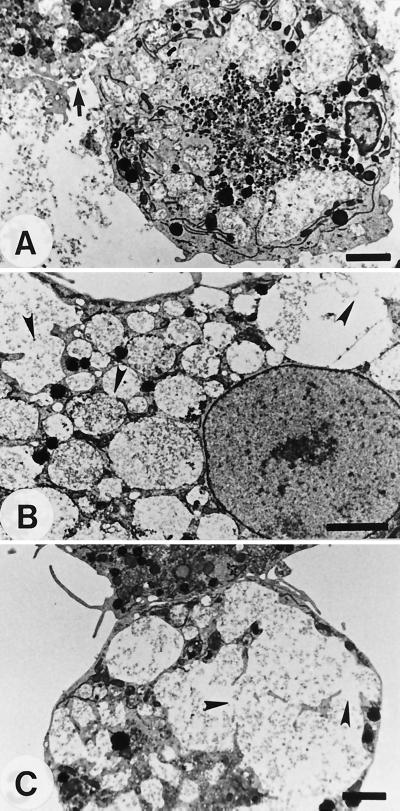
When microglial cells were cultured with amoeba lysate at concentrations from 1 to 0.25 mg/ml, lysis of the cell membrane was detected (Fig. 2A). In addition, many food vacuoles containing pathogenic A. culbertsoni lysate were detected in the cytoplasm, and the membranes of these food vacuoles were lysed (Fig. 2B); in contrast, food vacuoles were not detected in untreated microglial cells (Fig. 1A). In some microglial cells, small vacuoles condensed into larger vesicles (Fig. 2C).
FIG. 2.
TEM studies of microglial cells cocultured with 1 mg of a lysate of A. culbertsoni per ml for 6 h. (A) A microglial cell showed numerous food vacuoles containing amoeba lysate and a lysed cell membrane (arrow). (B) The membranes of cytoplasmic vacuoles were lysed (arrowheads). (C) The lysed membranes (arrowheads) were integrated into a large one. Bars, 2.5 μm.
To determine whether microglial cells showed any change in cytokine release as a result of a CPE induced by pathogenic Acanthamoeba trophozoites and lysate, assays for cytokines such as TNF-α and IL-1β were performed with culture supernatants with enzyme-linked immunosorbent assay kits (Endogen, Woburn, Mass.). The amounts of TNF-α secreted from microglial cells cultured in EMEM and PBS, used as volume controls, were 32.5 and 40.5 pg, respectively, at 6 h of incubation (Table 1). In microglial cells cocultured with A. culbertsoni trophozoites for 6 h, the amount of TNF-α secreted significantly increased to 114.3 pg in comparison with the amount secreted by the control group (by Student's t test, P < 0.01). In comparison with the amount secreted by the control group, the amount of TNF-α secreted from microglial cells cocultured with a lysate (1 mg/ml) of A. culbertsoni increased to 95.6 pg at 6 h (P < 0.01). In addition, the degree of increase revealed the same patterns by treatment with 0.5 mg of amoeba lysate per ml (Table 1). In microglial cells cocultured with trophozoites of A. culbertsoni for 6 h, the amount of IL-1β secreted was 129.6 pg, which was similar to the amounts secreted by the control groups (Table 1). By contrast, the amounts of IL-1β secreted from microglial cells cocultured with 1 and 0.5 mg of A. culbertsoni lysate per ml for 6 h decreased to 18.2 and 20.6 pg, respectively (P < 0.01).
TABLE 1.
Amounts of TNF-α and IL-1β secreted from microglial cells cocultured with A. culbertsoni trophozoites or lysate for 6 h
| Culture conditions | Amt (pg/ml) secreted
|
|
|---|---|---|
| TNF-α | IL-1β | |
| Microglial cells: | ||
| In EMEM medium | 32.5 ± 2.97a | 131.4 ± 43.91 |
| With PBS | 40.5 ± 4.38 | 150.4 ± 13.44 |
| Microglial cells with: | ||
| A. culbertsoni trophozoites (1:1)b | 114.3 ± 7.99 | 129.6 ± 19.30 |
| 1 mg of lysate per ml | 95.6 ± 0.71 | 18.2 ± 6.05 |
| 0.5 mg of lysate per ml | 119.9 ± 1.41 | 20.6 ± 1.69 |
Values are means ± standard deviations.
The ratio is the number of microglial cells to the number of trophozoites.
Pathogenic Acanthamoeba produces a CPE on various cells both in vitro and in vivo. Dove Pettit et al. (4) demonstrated that a digipodium of pathogenic Acanthamoeba made contact with rat neuroblastoma cells, similar to the CPE of Naegleria fowleri, a kind of contact-dependent cell lysis referred to as troglocytosis (10). In the present study, microglial cells cultured from newborn rat brain were used as target cells because these cells could play a protective role during Acanthamoeba infection in the CNS. When amoebae were cocultured with microglial cells, digipodia were shown on pathogenic A. culbertsoni trophozoites. Thus, this result could be considered apparent evidence of the CPE induced by pathogenic Acanthamoeba.
The two fundamental mechanisms in the cytolysis of target cells by pathogenic free-living amoebae are the disruption of cell membrane integrity by necrosis via pore-forming lytic molecules or the disruption of cell membrane integrity by apoptosis, or both (1, 4). In the present study, microglial cells cocultured with amoeba trophozoites died mostly as a result of the necrotic process that accompanied the clumping of chromatin materials in the nucleus and lysis of the cell membranes. In addition, in microglial cells cocultured with pathogenic A. culbertsoni lysate, the membranes of small food vacuoles were lysed and integrated. This membrane lysis seems to be affected by lytic molecules released from Acanthamoeba, but further study of that mechanism is needed. In a previous study, it was reported that Naegleria lysate contained pore-forming lytic molecules which affected the lysis of target cells (9).
Apoptosis was characterized by various morphological features, such as cell shrinkage, cell membrane blebbing, the formation of apoptotic bodies, nuclear chromatin condensation, and DNA fragmentation, as determined by electrophoresis and flow cytometry (1, 4). In the present study, cell membrane blebbing and nuclear condensation were observed for less than 10% of microglial cells cocultured with pathogenic A. culbertsoni (14).
Lipopolysaccharide (LPS) stimulated the release of TNF-α and IL-1 from primary murine microglial cell cultures, whereas inhibitors such as pentoxifylline and dexamethasone blocked their release (3). In microglial cells infected with Toxoplasma gondii, the secretion of IL-1 was triggered by bradyzoites and tachyzoites in a time- and a dose-dependent manner and depended on an LPS stimulus (5). In the present study, the level of secretion of TNF-α from microglial cells cocultured with pathogenic A. culbertsoni trophozoites or lysates was increased at 6 h of incubation. The level of secretion of IL-1β from microglial cells cocultured with lysate for 6 h decreased. More extensive studies on the cytokine responses of microglial cells due to pathogenic Acanthamoeba are necessary.
In conclusion, our results demonstrate that pathogenic A. culbertsoni trophozoites make contact with microglial cells and produce digipodia. The primary-culture rat microglial cells cocultured with amoeba lysate undergo a necrotic process following the lysis of the cell membrane and the membranes of the inner vacuoles. The level of secretion of TNF-α from microglial cells increased at 6 h postincubation, whereas the level of secretion of IL-1β decreased. These findings are regarded as the CPE induced by pathogenic Acanthamoeba against microglial cells and may be partly important for understanding of the interaction of pathogenic Acanthamoeba with microglial cells in the development of GAE.
Acknowledgments
This study was supported by a research grant of the Korea Science and Engineering Foundation (grant 981-0701-001-1) and in part by a research grant from Shinheung College (in 1999).
REFERENCES
- 1.Alizadeh H, Pidherney M S, McCulley J P, Niederkorn J Y. Apoptosis as a mechanism of cytolysis of tumor cells by a pathogenic free-living amoeba. Infect Immun. 1994;62:1298–1303. doi: 10.1128/iai.62.4.1298-1303.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Ann Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Chao C C, Hu S, Close K, Choi C S, Molitor T W, Novick W J, Peterson P K. Cytokine release from microglia: differential inhibition by pentoxifylline and dexamethasone. J Infect Dis. 1992;166:847–853. doi: 10.1093/infdis/166.4.847. [DOI] [PubMed] [Google Scholar]
- 4.Dove Pettit D A, Williamson J, Cabral G A, Marciano-Cabral F. In vitro destruction of nerve cell cultures by Acanthamoeba spp.: a transmission and scanning electron microscopy study. J Parasitol. 1996;82:769–777. [PubMed] [Google Scholar]
- 5.Fischer H G, Nitzgen B, Reichmann G, Hadding U. Cytokine responses induced by Toxoplasma gondii in astrocytes and microglial cells. Eur J Immunol. 1997;27:1539–1548. doi: 10.1002/eji.1830270633. [DOI] [PubMed] [Google Scholar]
- 6.Giulian D, Baker T J. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:163–178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Im K I, Kim D S. Acanthamoebiasis in Korea: two new cases with clinical cases review. Yonsei Med J. 1998;39:478–484. doi: 10.3349/ymj.1998.39.5.478. [DOI] [PubMed] [Google Scholar]
- 8.Im K I, Shin H J, Seo D W, Jeon S H, Kim T E. Pathogenicity of Korean isolates of Acanthamoeba by observing the experimental infection and zymodemes of five isoenzymes. Korean J Parasitol. 1999;37:85–92. doi: 10.3347/kjp.1999.37.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowrey D M, McLaughlin J. Activation of a heat-stable cytolytic protein associated with the surface membrane of Naegleria fowleri. Infect Immun. 1985;50:478–482. doi: 10.1128/iai.50.2.478-482.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marciano-Cabral F M, Zoghby K L, Bradley S G. Cytopathic action of Naegleria fowleri on rat neuroblastoma target cells. J Protozool. 1990;37:138–144. doi: 10.1111/j.1550-7408.1990.tb05884.x. [DOI] [PubMed] [Google Scholar]
- 11.Moore M B, Ubelaker J E, Martin J H, Silvany R, Dougherty J M, Meyer D R, McCulley J P. In vitro penetration of human corneal pithelium by Acanthameoba castellanii: a scanning and transmission electron microscopy study. Cornea. 1991;10:291–298. doi: 10.1097/00003226-199107000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Pidherney M S, Alizadeh H, Stewart G L, McCulley J P, Niederkorn J Y. In vitro and in vivo tumoricidal properties of a pathogenic/free-living amoeba. Cancer Lett. 1993;72:91–98. doi: 10.1016/0304-3835(93)90016-3. [DOI] [PubMed] [Google Scholar]
- 13.Shin H J, Ra M S, Im K I. Cytotoxicity of Acanthamoeba sp. YM-4 (Korean isolate) Yonsei R Trop Med. 1993;24:31–38. [Google Scholar]
- 14.Shin H J, Cho M S, Kim H I, Lee M, Park S, Sohn S, Im K I. Apoptosis of primary-culture rat microglial cells induced by pathogenic Acanthamoeba spp. Clin Diagn Lab Immunol. 2000;7:510–514. doi: 10.1128/cdli.7.3.510-514.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzumura A, Marrunouchi T, Yamamoto H. Morphological transformation of microglia in vitro. Brain Res. 1991;545:301–306. doi: 10.1016/0006-8993(91)91302-h. [DOI] [PubMed] [Google Scholar]
- 16.Suzumura A, Sawada M, Yamamoto H, Marrunouchi T. Transforming growth factor-β suppresses activation and proliferation of microglia in vitro. J Immunol. 1993;151:2150–2158. [PubMed] [Google Scholar]
- 17.Taylor W M, Pidherney M S, Alizadeh H, Niederkorn Y J. In vitro characterization of Acanthamoeba castellanii cytopathic effect. J Parasitol. 1995;81:603–609. [PubMed] [Google Scholar]
- 18.van Klink F, Alizadeh H, Stewart G L, Pidherney M S, Silvany R E, He Y G, McCulley J P, Niederkorn J Y. Characterization and pathogenic potential of a soil isolate and an ocular isolate of Acanthamoeba castellanii in relation to Acanthamoeba keratitis. Curr Eye Res. 1992;12:1207–1220. doi: 10.3109/02713689208999546. [DOI] [PubMed] [Google Scholar]
- 19.Vela J M, Dalmau I, González B, Castellano B. Morphological and distribution of microglia cells in the young and adult mouse cerebellum. J Comp Neurol. 1995;361:602–616. doi: 10.1002/cne.903610405. [DOI] [PubMed] [Google Scholar]
- 20.Visvesvara G S, Balamuth W. Comparative studies on related free-living and pathogenic amebae with special reference to Acanthamoeba. J Protozool. 1975;22:245–256. doi: 10.1111/j.1550-7408.1975.tb05860.x. [DOI] [PubMed] [Google Scholar]
- 21.Visvesvara G S, Stehr-Green J K. Epidemiology of free-living amoeba infections. J Protozool. 1990;37:25s–33s. doi: 10.1111/j.1550-7408.1990.tb01142.x. [DOI] [PubMed] [Google Scholar]