Abstract
Objective:
The relation between respiratory symptoms and the range of tobacco product use among U.S. adolescents/young adults is not yet clear. This cross-sectional analysis examines tobacco product use and respiratory symptoms in a nationally representative sample of 21,057 adolescents/young adults aged 12-24 years from Wave 4 (2016-17) of the Population Assessment of Tobacco and Health Study.
Methods:
Presence of functionally-important respiratory symptoms was defined by questions regarding wheezing and nighttime cough at a cutoff score associated with poorer functional health status. Past-30-day tobacco use was analyzed two ways: never-tobacco users (reference) vs. combustible users, noncombustible-only users, and former users; or frequency of use of cigarettes and/or e-cigarettes. Weighted Poisson regression adjusted for past-30-day marijuana use, secondhand smoke exposure, and asthma.
Results:
Functionally-important respiratory symptoms were present in 10.0% overall: 13.8% of combustible users, 9.0% of noncombustible users, 8.2% of noncurrent users and 9.7% of never users. Functionally-important respiratory symptoms were associated with combustible tobacco use (relative risk [RR]=1.52[95% CI 1.29, 1.80]), marijuana use (RR=1.54[1.34, 1.77]) and secondhand smoke exposure (RR=1.04[1.03, 1.05]). Higher cigarette smoking frequency was also associated with functionally-important respiratory symptoms for frequency categories >14 days/month (e.g. RR=1.93[1.50, 2.49] for 15-29 days/month). Frequency of e-cigarette use was not associated with functionally-important respiratory symptoms.
Conclusions:
During 2016-17, smoking cigarettes, marijuana use, and secondhand smoke exposure were cross-sectionally associated with functionally-important respiratory symptoms in adolescents/young adults. Risk increased with increased frequency of cigarette use but not e-cigarette use. Given changes to contemporary e-cigarettes and use, findings may not generalize to newer products.
Keywords: Adolescents, Young Adults, Tobacco Use, Respiratory Symptoms
Introduction
Tobacco use causes disease across all ages, with multiple studies demonstrating an association between adolescent cigarette smoking and asthma as well as respiratory symptoms including cough, phlegm, wheeze and shortness of breath.1–3 Less is known about health risks from tobacco products other than cigarettes, and whether there are thresholds of use above which symptoms are experienced. E-cigarettes and other electronic vaping devices (hereafter e-cigarettes) have been readily adopted by adolescents, with prevalence of use surpassing combusted tobacco cigarettes and all other forms of tobacco among high school students. Pre-pandemic, in 2019 27.5% of high school students reported e-cigarette use in the past 30 days, compared to 5.8% reporting cigarette use in this period.4,5 More recent assessments of e-cigarette use among high school students in 2021 have shown substantial decline, with 11.3% reporting current use,6 however these data were collected on-line to allow for remote student participation and are not directly comparable to previous in-person survey data. E-cigarettes represent a diverse product category regarding nicotine content, flavors and aerosol delivery that has only been sold since the mid 2000’s, with substantively different product characteristics (nicotine salt-based pod devices) entering the market in late 2015.7
There is biologic plausibility for respiratory compromise from e-cigarettes because they deliver ultrafine particulates at similar quantities to cigarettes, and these ultrafine particulates are known to cause inflammation and respiratory disease.8 Thus, it is important to try to ascertain e-cigarette-related health risks in population samples.9 Assessing the risk of respiratory impairment associated with e-cigarette use is challenging, however, given that e-cigarette users often also use other tobacco products and marijuana. To date, cross-sectional studies in adolescents have shown an association between e-cigarette use and higher risk of cough/phlegm;10–12 these studies did not measure marijuana use and were inconsistent with regards to secondhand smoke exposure measurement. Another recent study assessed e-cigarette use among 12-17 year olds in the Population Assessment of Tobacco and Health (PATH) Study, finding no longitudinal relation between e-cigarette use or combustible tobacco use and a single item measuring wheeze.13 As wheeze may be due to airway hyperresponsiveness to stimuli, contemporaneous assessment may be more indicative of acute tobacco effects.
In order to better understand the relationships between multiple tobacco product use and respiratory symptoms among adolescents and young adults, data were analyzed from Wave 4 (2016-17) of the PATH Study. The PATH Study is a household-based, nationally representative, longitudinal cohort study of U.S. adults and adolescents on tobacco use patterns and associated health behaviors. Our focus was on the relation between tobacco product use and respiratory symptoms (wheezing/nighttime cough), while adjusting for potential confounders, including marijuana use, secondhand smoke exposure and concurrent diseases (asthma) that could impact tobacco use and respiratory status.
METHODS
Study design, setting and participants
The National Institute on Drug Abuse partners with the U.S. Food and Drug Administration’s Center for Tobacco Products to conduct the PATH Study under a contract with Westat. The PATH Study employed a stratified, address-based, area probability sampling design at Wave 1 (2013-14) that oversampled adult tobacco users, young adults (18-24 years), and African-American adults. A screener was used to select youths aged 12-17 (hereafter referred to as adolescents) and adults (18 years and older) from households for participation in the study, and audio computer-assisted self-interviews collected information on tobacco-use patterns and health behaviors.
Data from Wave 4 (2016-17) Restricted Use File were analyzed,14 and included 25,446 adolescents and young adults from 12-24 years of age without respiratory diseases other than asthma, of whom 21,054 subjects had complete data (see supplemental Figure 1 for a flow diagram) after missing data on sex and race/Hispanic ethnicity were imputed.15 Wave 4 sampling included a probability replenishment sample of adults and adolescents selected from the U.S. civilian noninstitutionalized population. A more detailed description of the sampling procedure for Wave 4 of the PATH Study, along with response rates, can be found in Supplement Section 2.
Full-sample and replicate weights were created that adjust for the complex sample design and nonresponse, enabling the PATH Study to obtain statistically valid estimates of U.S. civilians ages 12 years and older at the time of Wave 4, with associated measures of statistical precision. Further details regarding the PATH Study design and methods are published elsewhere;16 interview procedures, questionnaires, sampling, weighting, response rates, and data access are described in the PATH Study Restricted Use Files User Guide.17
All respondents ages 18 and older provided informed consent, with adolescent respondents ages 12 to 17 providing assent while a parent/legal guardian provided consent. The study was approved by the Westat Institutional Review Board.
Self-reported outcomes
Respiratory symptoms and functionally-important respiratory symptoms
Respiratory symptom questions included in the Wave 4 PATH Study were from the core wheezing and asthma module from the International Study of Asthma and Allergies in Children (ISAAC),18 which has been used in more than 100 countries and 2 million children to assess respiratory health. Questions included items related to wheezing and nighttime cough indicative of asthma and have high face validity and test-retest reliability.19 Items/responses, weighted prevalence of each item in Wave 4, and prevalence of similar questions from the 2011-12 National Health and Nutrition Examination Survey are all included in Supplemental Table 1; 39.6% of the PATH Study sample reported one or more of these symptoms. Responses from the ISAAC questions were summed to create a respiratory symptom index which ranged from 0 (no symptoms) to 9 (worst). Higher scores on the index were indicative of greater functional limitations (e.g., walking, fatigue) among adults.21 Given the high frequency of any reported respiratory symptoms among this adolescent/young adult sample, we sought to determine a cutoff score associated with impact on health and functioning e.g., self-reported overall health rating or health-related school absenteeism. The threshold validation for adolescents and young adults within this PATH Study is detailed in the Supplement and described briefly below.
Exposures
Adolescents and young adults reported their lifetime and current (i.e. past 30 day) use of cigarettes, traditional cigars, cigarillos, filtered cigars, pipe tobacco, hookah, snus pouches, other smokeless tobacco (loose snus, moist snuff, dip, spit, or chewing tobacco), and e-cigarettes. Pictures and descriptions were displayed for each product to ensure accuracy. Those who had never used any tobacco product and former experimental users (not currently using, never used any product “fairly regularly”, and had not used at least 100 cigarettes in their lifetime) were combined into a “never” reference category.
Tobacco exposure was analyzed in two ways. The first analysis categorized use of tobacco as four mutually exclusive product use groups: never use; noncurrent use (ever used tobacco, but not in the past 30 days); current (past 30-day) noncombustible only use, which included e-cigarettes; and current combustible use which included cigarettes, all types of cigars, pipes and hookah (regardless of noncombustible use). We considered breaking out dual users of cigarettes and e-cigarettes separately, but an unadjusted analysis indicated that the prevalence of functionally-important symptoms in that group was not significantly different from exclusive cigarette users.
The second analytic approach focused on the two most common subcategories of tobacco use, cigarette and e-cigarette use, assessing frequency of use regardless of how much product was used each day. For these analyses, frequency of use was based on number of days used in the past month; categories included never use, ever use but not current use, and current use on 1-14/30 days, on 15-29/30 days and daily.
Covariates
Wave 4 covariates (Supplemental Table 5) included variables that could be associated both with tobacco exposure and functionally-important respiratory symptoms, including age, sex, and race/ethnicity, asthma diagnosis (based on self-report of “have you been told by a doctor, nurse or other health professional that you have asthma”) and obesity (based on body mass index [BMI]). Asthma was further categorized by medication use in the past 12 months: no medications, rescue only medications (e.g. albuterol), and controller medications (e.g. inhaled corticosteroids). Other potentially smoke-related exposures that could confound the association between tobacco use and respiratory symptoms included secondhand smoke exposure and past-month marijuana use. Second-hand smoke (SHS) exposure was a continuous variable, with each 1-point increase indicative of an additional 5 hours of exposure per week; the variable was skewed with a mean of 1.6, a median of 0, 75th percentile = 1, and 95th percentile =15.
Statistical Analysis
Functionally-important respiratory symptoms scale validation
A respiratory symptoms index cut-off score was sought that would serve as a binary indicator of functionally-important respiratory symptoms, validated against functional status (see Supplement). Given limitations of questions spanning youth and adult survey instruments, relevant available measures were used to validate the index (Supplemental Table 2), including global ratings for physical health (assessed in both adolescents/young adults), frequency of missing school due to illness at Wave 2 (adolescent-only measure), and trouble walking 1 mile (young adult-only measure). As shown in Supplemental Figure 2, functional impairment increased as respiratory symptom scores increased. Unweighted receiver-operator curves (ROC) were used to determine a cutoff score while minimizing the false positive rate for its association with functional outcomes (Supplemental Table 3 shows values for several cut-off scores, with the selected cut-off being consistent with prevalence of asthma in the general population.)20 The index was modestly associated with each functional outcome (area under the curve ranged from 0.54 to 0.63). A cut-off score of <3 vs. ≥3 was chosen, consistent with functionally-important respiratory symptoms among older adults in the PATH Study.21
A series of weighted logistic or multinomial regressions was then completed to fit each functional outcome on respiratory symptoms score ≥3, adjusted for age, gender, race/ethnicity, and BMI (Supplemental Table 4). In each regression, a score ≥3 was independently associated with the functional outcome. For example, a respiratory symptom score ≥3 was independently associated with fair/poor (compared to excellent) physical health among adolescents and young adults with combined AOR 3.74 (95% CI 3.13, 4.46) (AOR were similar for young adults (4.16 [3.10, 5.58]) and adolescents (3.17 [2.51, 4.00]) when analyzed separately.) Based on these analyses, a score ≥3 was concluded to indicate “functionally-important respiratory symptoms” among adolescents and young adults.
Analytical approach
All analyses were weighted using the Cohort 4, Wave 4 cross-sectional full-sample and replicate weights, except where noted in footnotes, to adjust for the complex sample design and loss to follow up. Variances were estimated using balanced repeated replication with Fay’s adjustment set to 0.3 to increase estimate stability.22,23
Associations between tobacco product use and covariates were assessed using chi-square, t-tests or ANOVA, as appropriate. Multivariable weighted Poisson regression was used to obtain the adjusted risk of functionally-important respiratory symptoms by tobacco product use and, separately, by cigarette and e-cigarette use.
We also conducted a sensitivity analysis to assess the robustness of the results by repeating the analysis using multivariable linear regression to compare the respiratory symptoms index as a continuous measure.
All analyses were conducted using Stata survey data procedures, version 15.1 (StataCorp LLC, College Station, TX), and using the PATH Study Restricted Use Files.14 A complete set of aims, hypotheses and validation methods may be found in the Supplement.
Results:
Sample Description
Missing data
As shown in Supplemental Table 6, the analytic sample had a higher weighted proportion of 12-14 year olds (24.0%) than the non-analytic sample (18.6%, p<0.0001) and a lower proportion of 18-20 year olds (21.9% vs. 30.4%, p<0.0001). There were more “never” cigarette smokers in the analytic sample (71.6% vs. 60.7%, p<0.0001). The weighted percent of functionally important respiratory symptoms was similar in the analytic vs. non-analytic sample (10.0% vs. 11.2%, p=0.052).
Description of the sample
We use weighted percents to describe the sample (Table 2). The youths ranged in age from 12 to 24 years (24.0% 12-14, 23.6% 15-17, 21.9% 18-20, and 30.5% 21-24); 50.0% identified as female. The race/ethnicity categories were broadly representative of the US population: 54.2% White, 13.4% Black, and 22.1% Hispanic. Asthma diagnosis was reported in 18.3%, of whom 23.5% reported medication use. Functionally-important respiratory symptoms were present in 5.3% of youths without asthma, 21.8% of those with asthma but on no medications, 55.2% of those on rescue inhalers only, and 63.5% of those on controller medications.
Table 2.
Weighted cross-sectional associations between tobacco use and selected constructs and functionally-important respiratory symptoms for adolescents (age 12-17) and young adults (age 18-24) at Wave 4 of the Population Assessment of Tobacco and Health Study.a
| Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|
| Risk factor | Nb | %c | % with Functionally-Important Respiratory Symptomsc | Relative Riskd | 95% CI | Relative Riskd | 95% CI |
| Tobacco use statuse (Model 1 only) | |||||||
| Never use | 12,864 | 56.5 | 9.7 | Ref | Ref | ||
| Noncurrent use | 4,413 | 23.5 | 8.2 | 0.96 | [0.85,1.08] | ||
| Current noncombustible use only | 787 | 3.9 | 9.0 | 0.87 | [0.67,1.13] | ||
| Current combustible use | 2,990 | 16.1 | 13.8 | 1.52 | [1.29,1.80] | ||
| Cigarette use frequencyf (Model 2 only) | |||||||
| Never use | 15,827 | 71.6 | 9.2 | Ref | Ref | ||
| Noncurrent use | 3,083 | 16.8 | 9.1 | 1.14 | [0.98,1.32] | ||
| 1-14 days | 1,033 | 5.3 | 9.0 | 1.15 | [0.91,1.46] | ||
| 15-29 days | 273 | 1.4 | 17.2 | 1.93 | [1.50,2.49] | ||
| Daily | 883 | 4.9 | 23.2 | 2.80 | [2.25,3.47] | ||
| E-cigaretteg use frequencyh (Model 2 only) | |||||||
| Never use | 14,943 | 68.2 | 9.3 | Ref | Ref | ||
| Noncurrent use | 4,596 | 24.0 | 11.1 | 0.99 | [0.85,1.16] | ||
| 1-14 days | 1,019 | 5.0 | 13.2 | 1.02 | [0.82,1.27] | ||
| 15-29 days | 195 | 1.0 | 10.7 | 0.73 | [0.42,1.25] | ||
| Daily | 301 | 1.8 | 13.1 | 1.25 | [0.80,1.96] | ||
| Covariates | |||||||
| Age group | |||||||
| 12-14 | 6,315 | 24.0 | 11.8 | Ref | Ref | Ref | Ref |
| 15-17 | 6,246 | 23.6 | 11.7 | 0.87 | [0.79,0.96] | 0.86 | [0.78,0.95] |
| 18-20 | 4,574 | 21.9 | 9.3 | 0.66 | [0.57,0.76] | 0.63 | [0.55,0.73] |
| 21-24 | 3,919 | 30.5 | 7.7 | 0.57 | [0.50,0.65] | 0.50 | [0.43,0.59] |
| Sex | |||||||
| Female | 10,523 | 50.0 | 10.8 | Ref | Ref | Ref | Ref |
| Male | 10,531 | 50.0 | 9.2 | 0.82 | [0.75,0.90] | 0.82 | [0.75,0.90] |
| Race/Ethnicity | |||||||
| Whitei | 10,054 | 54.2 | 10.7 | Ref | Ref | Ref | Ref |
| Blacki | 2,954 | 13.4 | 10.6 | 0.86 | [0.76,0.96] | 0.90 | [0.81,1.01] |
| Otheri,j | 2,027 | 10.3 | 9.6 | 0.89 | [0.77,1.04] | 0.91 | [0.78,1.07] |
| Hispanic | 6,019 | 22.1 | 7.8 | 0.74 | [0.66,0.84] | 0.77 | [0.68,0.87] |
| Asthma status | |||||||
| No asthma | 17,062 | 81.7 | 5.3 | Ref | Ref | Ref | Ref |
| Asthma no meds | 3,017 | 14.0 | 21.8 | 4.05 | [3.65,4.49] | 4.04 | [3.65,4.48] |
| Rescue inhaler only | 325 | 1.5 | 55.2 | 10.05 | [8.86,11.41] | 9.98 | [8.81,11.32] |
| Controller meds | 650 | 2.8 | 63.5 | 11.76 | [10.48,13.19] | 11.47 | [10.19,12.91] |
| BMI | |||||||
| Underweight | 882 | 4.4 | 8.6 | 1.01 | [0.81,1.25] | 0.99 | [0.79,1.23] |
| Normal/Healthy | 11,955 | 56.2 | 9.6 | Ref | Ref | Ref | Ref |
| Overweight | 4,176 | 20.5 | 9.9 | 1.02 | [0.92,1.15] | 1.02 | [0.91,1.14] |
| Obese | 4,041 | 18.9 | 11.6 | 1.07 | [0.96,1.20] | 1.05 | [0.94,1.17] |
| Past-month marijuana usek | |||||||
| No | 18,172 | 85.1 | 9.2 | Ref | Ref | Ref | Ref |
| Yes | 2,882 | 14.9 | 14.4 | 1.54 | [1.34,1.77] | 1.48 | [1.30,1.69] |
| Secondhand smoke exposure (per each additional 5 hours/week) | 21,054 | n/a | n/a | 1.04 | [1.03,1.05] | 1.03 | [1.01,1.04] |
N= 21,054 respondents with complete data at Wave 4.
Unweighted
Weighted to reflect the percent in the United States civilian noninstitutionalized population. Functionally-important respiratory symptoms were present in 10.0% (SE=0.2) overall.
All relative risks adjust for the variables in the table.
Never use = never used tobacco; Noncurrent use = ever used tobacco, but not in the past 30 days; Noncombustible use only = exclusive noncombustible (e-cigarette, smokeless tobacco, or snus) use in the past 30 days; Combustible use = combustible (cigarette, pipe, hookah, or cigar) use in the past 30 days regardless of noncombustible use.
Cigarette use frequency refers to the number of days of cigarette use within the past 30 days, regardless of other tobacco products used. Never = never used cigarettes; noncurrent = ever use of cigarettes, but no cigarette use within the past 30 days; other categories refer to the ranges of days cigarettes were used within the past 30 days.
At W4, participants were asked about use of any e-products (e-cigarettes, e-cigars, e-pipes, and e-hookah), all of which are referred to as e-cigarettes here.
E-cigarette use frequency refers to the number of days of e-cigarette use within the past 30 days, regardless of other tobacco products used. Never = never used e-cigarettes; noncurrent = ever use of e-cigarettes, but no e-cigarette use within the past 30 days; other categories refer to the ranges of days e-cigarettes were used within the past 30 days.
Non-Hispanic
Includes American Indian or Alaska Native, Asian Indian, Chinese, Filipino, Japanese, Korean, Vietnamese, Other Asian, Native Hawaiian, Guamanian or Chamorro, Samoan, Other Pacific Islander, and multiracial
Marijuana use variable does not distinguish between combustible and noncombustible use.
Supplemental Table 10 reports standard errors for all the weighted estimates presented in this table and adjusted RRs for all the variables in the model.
Tobacco use and age
Figure 1 shows unadjusted and weighted tobacco product use by age, demonstrating much higher rates of current combustible use among young adults. For example, the rate of current combustible use among those 15-17 years was 7.1% compared to 28.8% for 21-24 years. Cigarettes were the predominant combustible product used, comprising 78.6% of combustible use among 15-17 year olds and 74.1% in 21-24 year olds. The prevalence of noncombustible use was roughly the same among young adults and 15-17 year olds.
Figure 1.
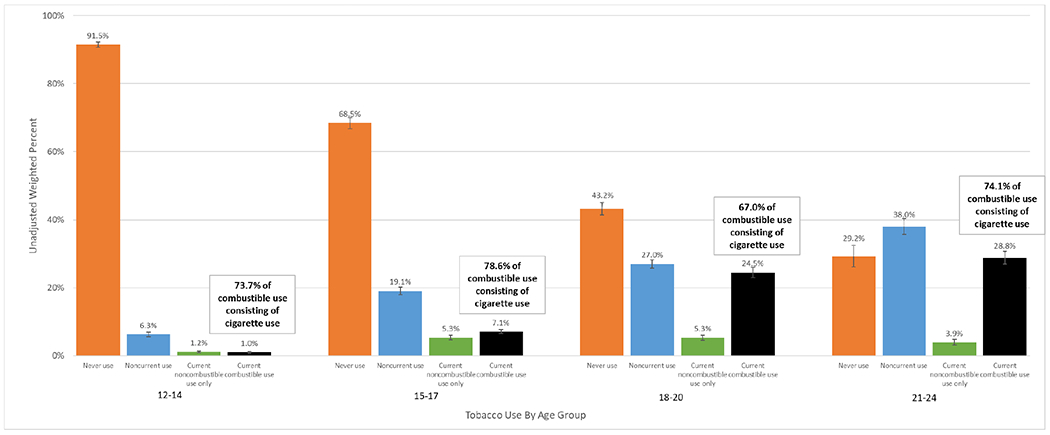
Unadjusted weighted percent of tobacco usea among four age groups of Population Assessment of Tobacco and Health Study Wave 4 adolescents (age 12-17) and young adults (age 18-24).b
notes:
a Never use = never used tobacco; Noncurrent use = ever used tobacco, but not in the past 30 days; Current noncombustible use only = exclusive noncombustible (e-cigarettec, smokeless tobacco, or snus) use in the past 30 days; Current combustible use = combustible (cigarette, pipe, hookah, or cigar) use in the past 30 days regardless of noncombustible use. Error bars represent 95% confidence interval.
b N = 21,054 adolescents and young adult respondents: 12-14 N = 6,315; 15-17 N = 6,246; 18-20 N = 4,574; 21-24 N = 3,919.
c At W4, participants were asked about use of any e-products (e-cigarettes, e-cigars, e-pipes, and e-hookah), all of which are referred to as e-cigarettes here.
Tobacco use and covariates
Table 1 shows the relationships between sociodemographic, medical, and exposure characteristics and the four tobacco use categories. Males and whites were more likely to be current tobacco users than females and minorities. Combustible tobacco users had higher secondhand smoke exposure than any other category. Marijuana use was also more prevalent among past 30-day combustible users (48.6%) than current noncombustible (32.0%), noncurrent (19.4%), and never tobacco users (2.2%).
Table 1 –
Characteristics for adolescents (age 12-17) and young adults (age 18-24) at Wave 4 of the Population Assessment of Tobacco and Health Study,a by tobacco use statusb (percentages and means are weighted to reflect U.S. population).
| Tobacco Use Status | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Current (past 30-day) | |||||||||
|
|
|||||||||
| Never use (N = 12,864) | Noncurrent use (N = 4,413) | Noncombustible use only (N = 787) | Combustible use (N = 2,990) | ||||||
| Nc | % | Nc | % | Nc | % | Nc | % | p-valued | |
| Sociodemographics | |||||||||
| Age | |||||||||
| 12-14 | 5,756 | 38.9 | 415 | 6.4 | 72 | 7.2 | 72 | 1.5 | <0.0001 |
| 15-17 | 4,282 | 28.6 | 1,213 | 19.2 | 306 | 32.4 | 445 | 10.4 | |
| 18-20 | 1,959 | 16.8 | 1,257 | 25.2 | 242 | 29.7 | 1,116 | 33.4 | |
| 21-24 | 867 | 15.7 | 1,528 | 49.3 | 167 | 30.7 | 1,357 | 54.7 | |
| Sex | |||||||||
| Female | 6,565 | 52.0 | 2,346 | 52.9 | 280 | 34.9 | 1,332 | 42.3 | <0.0001 |
| Male | 6,299 | 48.0 | 2,067 | 47.1 | 507 | 65.1 | 1,658 | 57.7 | |
| Race/Ethnicity | |||||||||
| Whitee | 5,939 | 52.4 | 2,028 | 52.6 | 517 | 71.2 | 1,570 | 58.6 | <0.0001 |
| Blacke | 1,893 | 14.1 | 580 | 12.5 | 43 | 6.2 | 438 | 13.7 | |
| Othere,f | 1,269 | 11.2 | 402 | 9.9 | 72 | 8.3 | 284 | 8.2 | |
| Hispanic | 3,763 | 22.2 | 1,403 | 25.1 | 155 | 14.3 | 698 | 19.5 | |
| Relevant medical history | |||||||||
| Asthma status | |||||||||
| No asthma | 10,411 | 81.4 | 3,579 | 82.0 | 627 | 80.0 | 2,445 | 82.7 | 0.001 |
| Asthma no meds | 1,787 | 13.7 | 648 | 13.9 | 126 | 16.0 | 456 | 14.5 | |
| Rescue inhaler only | 203 | 1.5 | 78 | 1.8 | 9 | 1.2 | 35 | 1.1 | |
| Controller meds | 463 | 3.3 | 108 | 2.3 | 25 | 2.8† | 54 | 1.7 | |
| BMI | |||||||||
| Underweight | 599 | 5.0 | 137 | 3.1 | 26 | 4.7† | 120 | 4.0 | <0.0001 |
| Normal | 7,728 | 60.1 | 2,332 | 52.3 | 443 | 53.3 | 1,452 | 48.8 | |
| Overweight | 2,325 | 18.2 | 967 | 23.0 | 160 | 21.6 | 724 | 24.7 | |
| Obese | 2,212 | 16.6 | 977 | 21.6 | 158 | 20.4 | 694 | 22.5 | |
| Other likely smoke exposures | |||||||||
| Past month marijuana useg | |||||||||
| No | 12,593 | 97.8 | 3,536 | 80.6 | 528 | 68.0 | 1,515 | 51.4 | <0.0001 |
| Yes | 271 | 2.2 | 877 | 19.4 | 259 | 32.0 | 1,475 | 48.6 | |
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | ||
| Past week SHSh exposure | 1.6 | 0.1 | 3.1 | 0.2 | 4.7 | 0.4 | 9.2 | 0.5 | <0.0001 |
N = 21,054 adolescents and young adult respondents with complete data on all variables
Never use = never used tobacco; Noncurrent use = ever used tobacco, but not in the past 30 days; Noncombustible use only = exclusive noncombustible (e-cigarettei, smokeless tobacco, or snus) use in the past 30 days; Combustible use = combustible (cigarette, pipe, hookah, or cigar) use in the past 30 days regardless of noncombustible use.
Unweighted
Chi-square test was used for categorical variables and ANOVA was used for continuous variables.
Non-Hispanic
Includes American Indian or Alaska Native, Asian Indian, Chinese, Filipino, Japanese, Korean, Vietnamese, Other Asian, Native Hawaiian, Guamanian or Chamorro, Samoan, Other Pacific Islander, and multiracial
Marijuana use variable does not distinguish between combustible and noncombustible use.
SHS = secondhand smoke
At W4, participants were asked about use of any e-products (e-cigarettes, e-cigars, e-pipes, and e-hookah), all of which are referred to as e-cigarettes here.
Estimate should be interpreted with caution because it has low statistical precision. It is based on a denominator sample size of less than 50, or the coefficient of variation of the estimate or its complement is larger than 30%.
Supplemental Table 7 reports standard errors for all the weighted estimates presented in this table.
Association between tobacco use and functionally-important respiratory symptoms
Functionally-important respiratory symptoms were present in 10.0% overall, 13.8% of current combustible users, 9% of current noncombustible users, 8.2% of noncurrent users and 9.7% of never users (p<0.0001). As shown in Table 2, functionally-important respiratory symptoms were more prevalent among females, younger age groups, those with higher BMI, those with past month marijuana use (14.4%), and those with asthma.
Model 1—categorical variables describing combustible and noncombustible use
Table 2 includes the two adjusted multivariable regression models assessing the associations between tobacco use and functionally-important respiratory symptoms. With respect to the four categories of use, Model 1 demonstrated that only current combustible use was independently associated with functionally-important respiratory symptoms (relative risk [RR] =1.52, [95% CI 1.29, 1.80]) compared to never users. Males, older age groups, and Hispanics were less likely to experience functionally-important respiratory symptoms. Asthmatics, those with past-month marijuana use (1.54 [1.34, 1.77]) and those with secondhand smoke exposure (1.04 [1.03, 1.05] for each additional 5 hours/week) were more likely to experience functionally-important respiratory symptoms.
Model 2: cigarette and e-cigarette frequency
With respect to cigarette and e-cigarette frequency, the prevalence of past 30-day cigarette and e-cigarette use was 11.6% and 7.8%, respectively. Figure 2 shows the unadjusted relationships, illustrating a dose-response between higher frequency of cigarette use and functionally-important respiratory symptoms (with prevalence increasing from 9.2% among never users to 23.2% among daily users), but little change with higher frequency of e-cigarette use. This also suggests an apparent threshold for the association between higher frequency of cigarette use and functionally-important symptoms, with little response up to 1-14 days per month of cigarette use. The linear trend for cigarettes was statistically significant (p<0.0001) whereas the trend for e-cigarettes was not (p >0.1).
Figure 2.
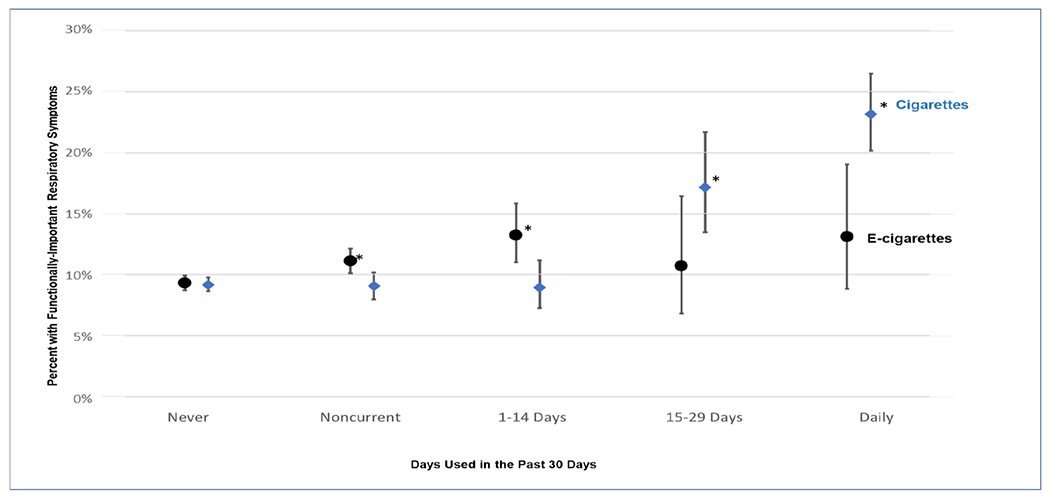
Relationa between the frequency of cigaretteb and e-cigarettec,d use and weighted percentage with functionally-important respiratory symptoms for adolescents (age 12-17) and young adults (age 18-24), unadjusted for covariates, at Wave 4 of the Population Assessment of Tobacco and Health (PATH) Study.
notes:
a The * indicates that these weighted prevalence estimates are significantly different (p<0.05) from never users of that product. The linear trend for cigarettes was statistically significant (p<0.0001) whereas the trend for e-cigarettes was not (p >0.1)
b Cigarette use frequency refers to cigarette use within the past 30 days, regardless of other tobacco products used. Never = never used cigarettes (N=15,827); Noncurrent = ever use of cigarettes, but no cigarette use within the past 30 days (N=3,038); Other categories refer to the ranges of days cigarettes were used within the past 30 days: 1-14 days (N=1,033); 15-29 days (N=273); Daily (N=883). Overall weighted P30D cigarette prevalence was 11.6% (SE=0.3)
c At W4, participants were asked about use of any e-products (e-cigarettes, e-cigars, e-pipes, and e-hookah), all of which will be referred to as e-cigarettes here.
d E-cigarette use frequency refers to e-cigarette use within the past 30 days, regardless of other tobacco products used. Never = never used e-cigarettes (N=14,943); noncurrent = ever use of e-cigarettes, but no e-cigarette use within the past 30 days (N=4,596); other categories refer to the ranges of days e-cigarettes were used within the past 30 days: 1-14 days (N=1,019); 15-29 days (N=195); Daily (N=301). Overall weighted P30D e-cigarette prevalence was 7.8 (SE=0.2).
In the second multivariable regression (Model 2, Table 2), cigarette frequency categories above 14 days/month were significantly associated with functionally-important respiratory symptoms in a dose-dependent manner: RR=1.15 [0.91, 1.46] for 1-14 days of cigarette use, 1.93 [1.50, 2.49] for 15-29 days of cigarette use, and 2.80 [2.25, 3.47] for daily cigarette use. As with the previous model, frequency of e-cigarette use was not associated with functionally-important respiratory symptoms.
Sensitivity analysis
The findings in Models 1 and 2 described above were unchanged when all analyses were rerun using the respiratory symptoms index as a continuous dependent variable and multivariable linear regression.
Discussion
In this population-based study of adolescents and young adults, use of combusted tobacco products was independently associated with functionally-important respiratory symptoms, with higher frequency of cigarette smoking associated with higher prevalence of these symptoms. In contrast, we did not find evidence associating any measure of e-cigarette use and functionally-important respiratory symptoms after accounting for cigarette and marijuana use, secondhand smoke exposure and asthma. These findings are similar to that of McConnell et al.,12 who found no relation between e-cigarette use in adolescents and wheezing, although they did find increased risk of cough/phlegm. Unsurprisingly our findings mirror those assessing wheeze in an adolescent-only PATH sample; however, that study did not include cough or look at comparative effects of cigarette use or marijuana on respiratory symptoms.13 Our findings differ from others in the adult literature, including Li et al,24 who found increased risk of wheezing and related respiratory symptoms in current e-cigarette users compared to never users in in the PATH Study adult sample; however, they did not account for past cigarette use or marijuana smoking.
In adolescents/young adults, the tobacco use history is limited. Moreover, cigarette smoking was uncommon among adolescents but increased four-fold in young adults; in contrast, e-cigarette use prevalence was similar among adolescents and young adults. More research can examine the role of early-onset e-cigarette use in young adult transitions to cigarette smoking, given strong evidence from longitudinal studies of adolescents associating e-cigarette use and subsequent onset of cigarette use.25
With regard to marijuana use, there is high plausibility of marijuana contributing to respiratory symptoms given similarities in particulates and tar constituents.26,27 Furthermore, numerous studies to date suggest a link between marijuana use and respiratory disorders.28–30 Although there is no detail for marijuana exposure mode (ingested, smoked, vaped, etc.) in the PATH Study, smoking is the predominant exposure route by frequency in adolescent/young adult users.31 In spite of this measurement limitation, there was a clinically important association between marijuana use and functionally-important respiratory symptoms that clinicians and users should be aware of with legalization and widespread use of recreational marijuana across the U.S.
It is unsurprising that subjects with asthma are more likely to experience functionally-important respiratory symptoms, as wheezing is the cardinal symptom of this disease. Similarly, the link between secondhand smoke exposure and respiratory symptoms is well known.1 Although high exposure to SHS was relatively rare, some 5% of respondents reported 75 hours or more per week of SHS exposure. Those at higher exposure had increased risk for reporting functionally-important respiratory symptoms compared to those not exposed to SHS (a relative risk of 1.04^15 or 1.8). Similar to other national studies that have found reduced odds of asthma in Hispanic adolescents,30 this study found a lower prevalence of functionally-important respiratory symptoms among Hispanic adolescents. The prevalence of asthma within this cohort was higher than expected based on US prevalence data, likely due to self-reporting. This conjecture is supported by the low rate of medication use for asthma among this group. Subjects with asthma not on medications, however, did have markedly increased risk of functionally-important respiratory symptoms.
Limitations
These data are cross-sectional and thus temporal relationships cannot be determined. In addition, these data predate the significant increases in e-cigarette use from 2017, when 11.3% of high school students reported e-cigarette use to the peak seen in 2019 and subsequent decline.4,5 Although lower rates were found in 2021 (11.3%), intensity of use was increased; 43.6% of e-cigarette users report using on 20 or more days/month.6 As previously mentioned, due to changes in the survey collection methodology, no direct comparison can be made of 2021 data with that of previous years. These data also predate the popularity of nicotine-salt pod-based devices such as JUUL that occurred among young people in 2018 to 2019, although these products were on the market during the time of this survey (2016-17). While a combusted tobacco cigarette is a relatively standardized product, there is substantial heterogeneity in the product characteristics of e-cigarette devices (e.g., temperature, voltage, vapor production, refill capacity), as well as in the characteristics of the solutions used within the devices (e.g., humectant type, flavors and nicotine levels). Of concern, studies have found that glycerin and propylene glycol aerosol alter inflammatory pathways in the lung,32 and direct cytotoxicity has been shown in vitro from flavoring chemicals.33 These flavor chemicals are major ingredients of many e-liquids and may have higher concentrations than that of nicotine.33 Further, while toxicologically-relevant constituents have been identified in sufficient quantities within e-cigarette products to cause disease, including increased susceptibility to pneumococcal 34and viral35,36 infections, there have been few human studies.
The outbreak of serious lung injury and respiratory compromise associated with e-cigarette use in the U.S. that was recognized in 2019 is cause for concern.37,38 Recognizing that products in common use in 2016-17 differ from the product and e-juice characteristics in 2019, the present findings regarding e-cigarettes and respiratory symptoms may not be generalizable to newer products. Additional limitations include that all data are self-reported and may include recall bias. Further, tobacco use prevalence was quite low in adolescents under the age of 15, thus caution is warranted in extending these findings to younger adolescents. Asthma diagnosis is reported based on youth’s recollection that a health professional told them that they have asthma, which may be subject to recall bias or misclassification. There may also be other aspects of cognitive bias that may have influenced reporting, such as desire to alter symptom reporting they may attribute to product use or change in use, however this cannot be controlled. The significant strengths of this current study include a large, nationally representative sample enabling population estimates. In addition, survey items have been adapted from national and international surveys with high validity and reliability.
In summary, adolescents and young adults who use combustible tobacco, use marijuana or have secondhand smoke exposure were more likely to experience functionally-important respiratory symptoms, with risks increasing further with more frequent cigarette use. These modifiable exposures underscore the roles that tobacco and marijuana may play with respect to asthma symptoms in young people. We also emphasize concern about young adult transitions to cigarette smoking and the role e-cigarette use among adolescents may play in this transition. While there was no independent association between e-cigarette use and functionally-important respiratory symptoms, there are important limitations to this finding, with substantial product heterogeneity and timing prior to the marked increase in e-cigarette use from pod-based systems being introduced to the market. Additional epidemiologic and toxicologic studies assessing e-cigarette exposure and respiratory symptoms will be of high importance, along with longitudinal studies like the PATH Study that carefully assess tobacco use and health outcomes over time.
Supplementary Material
Article Summary:
In this study cigarette use, second-hand smoke exposure and marijuana use were associated with respiratory symptoms among youth, but e-cigarette use was not.
What’s Known on This Subject:
Tobacco use causes disease across all age groups, with studies linking adolescent cigarette smoking and respiratory symptoms including cough and wheeze. Less is known about health risks from other tobacco products and whether there are thresholds of use for symptoms.
What’s New:
In this nationally-representative study of 21,057 12-24 year olds, those who smoke cigarettes, use marijuana, and have secondhand smoke exposure were more likely to report functionally-important respiratory symptoms. Risk increased with increased frequency of cigarette use but not e-cigarette use.
Acknowledgements:
Tragically, co-author Lisa Schwartz, MD, MS, our extraordinary friend and colleague, died before publication of this article.
We would like to acknowledge the contributions Jennifer J. Hebb, BSN (Geisel School of Medicine at Dartmouth, The C. Everett Koop Institute at Dartmouth) who assisted with the many research and administrative tasks required for this protocol.
Conflict of Interest Disclosures:
K. Michael Cummings provides expert testimony on the health effects of smoking and tobacco industry tactics in lawsuits filed against the tobacco industry. He has also received payment as a consultant to Pfizer, Inc., for services on an external advisory panel to assess ways to improve smoking cessation delivery in health care settings.
Maciej Goniewicz has received a research grant from Pfizer and served as a member of scientific advisory board to Johnson & Johnson, pharmaceutical companies that manufacture smoking cessation medications.
Raymond Niaura receives funding from the Food and Drug Administration Center for Tobacco Products via contractual mechanisms with Westat and the National Institutes of Health. Within the past 3 years, he has served as a paid consultant to the Government of Canada via a contract with Industrial Economics Inc. and has received an honorarium for a virtual meeting from Pfizer Inc. Dr. Niaura was an unpaid grant reviewer for the Foundation for a Smoke Free World.
Funding/support:
This manuscript is supported with Federal funds from the National Institute on Drug Abuse, National Institutes of Health, and the Center for Tobacco Products, Food and Drug Administration, Department of Health and Human Services, under contract to Westat (Contract No. HHSN271201100027C).
Role of Funder:
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Department of Health and Human Services or any of its affiliated institutions or agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The other authors have no conflicts of interest to disclose.
Contributor Information
Susanne Tanski, Geisel School of Medicine, Hanover, NH; The C. Everett Koop Institute at Dartmouth, Lebanon, NH.
Michael J Halenar, Westat, Rockville, MD.
Kathryn C Edwards, Westat, Rockville, MD.
Jennifer Emond, Geisel School of Medicine, Hanover, NH; The C. Everett Koop Institute at Dartmouth, Lebanon, NH.
Steven Woloshin, Dartmouth Institute for Health Policy and Clinical Practice, Lebanon, NH; The C. Everett Koop Institute at Dartmouth, Lebanon, NH; The Lisa Schwartz Foundation, Hanover, NH.
Mary Brunette, Geisel School of Medicine, Hanover, NH; The C. Everett Koop Institute at Dartmouth, Lebanon, NH.
Lisa Schwartz, Dartmouth Institute for Health Policy and Clinical Practice, Lebanon, NH; The C. Everett Koop Institute at Dartmouth, Lebanon, NH; The Lisa Schwartz Foundation, Hanover, NH.
Kristie A Taylor, Westat, Rockville, MD.
Maciej L. Goniewicz, Roswell Park Comprehensive Cancer Center, Buffalo, NY.
Ray Niaura, New York University, New York, NY.
Gabriella Anic, U.S. Food and Drug Administration, Center for Tobacco Products, Beltsville, MD.
Yanling Chen, U.S. Food and Drug Administration, Center for Tobacco Products, Beltsville, MD.
Priscilla Callahan-Lyon, U.S. Food and Drug Administration, Center for Tobacco Products, Beltsville, MD.
Lisa D Gardner, U.S. Food and Drug Administration, Center for Tobacco Products, Beltsville, MD.
Theresa Thekkudan, U.S. Food and Drug Administration, Center for Tobacco Products, Beltsville, MD.
Nicolette Borek, U.S. Food and Drug Administration, Center for Tobacco Products, Beltsville, MD.
Heather L Kimmel, National Institute on Drug Abuse, National Institutes of Health, Bethesda, MD.
K Michael Cummings, Medical University of South Carolina, Charleston, SC.
Andrew Hyland, Roswell Park Comprehensive Cancer Center, Buffalo, NY.
James Sargent, Geisel School of Medicine, Hanover, NH; The C. Everett Koop Institute at Dartmouth, Lebanon, NH.
References
- 1.U.S. Department of Health and Human Services. The health consequences of smoking-50 years of progress: A report of the surgeon general, 2014. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health;2014. [Google Scholar]
- 2.Hedman L, Bjerg A, Sundberg S, Forsberg B, Ronmark E. Both environmental tobacco smoke and personal smoking is related to asthma and wheeze in teenagers. Thorax. 2011;66(1):20–25. [DOI] [PubMed] [Google Scholar]
- 3.Gomez M, Vollmer WM, Caceres ME, Jossen R, Baena-Cagnani CE. Adolescent smokers are at greater risk for current asthma and rhinitis. Int J Tuberc Lung Dis. 2009;13(8):1023–1028. [PubMed] [Google Scholar]
- 4.Cullen KA, Gentzke AS, Sawdey MD, et al. e-Cigarette Use Among Youth in the United States, 2019. JAMA. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gentzke AS, Creamer M, Cullen KA, et al. Vital Signs: Tobacco Product Use Among Middle and High School Students - United States, 2011-2018. MMWR Morb Mortal Wkly Rep. 2019;68(6):157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park-Lee E, Ren C, Sawdey MD, et al. Notes from the Field: E-Cigarette Use Among Middle and High School Students - National Youth Tobacco Survey, United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(39):1387–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowen A, Xing C, Inventors; PAX Labs, assignee. Nicotine salt formulations for aerosol devices and methods thereof. 2015. [Google Scholar]
- 8.Protano C, Avino P, Manigrasso M, et al. Environmental Electronic Vape Exposure from Four Different Generations of Electronic Cigarettes: Airborne Particulate Matter Levels. Int J Environ Res Public Health. 2018;15(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Academies of Sciences EaM. Public health consequences of e-cigarettes. 2018. [PubMed] [Google Scholar]
- 10.Schweitzer RJ, Wills TA, Tam E, Pagano I, Choi K. E-cigarette use and asthma in a multiethnic sample of adolescents. Prev Med. 2017;105:226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang MP, Ho SY, Leung LT, Lam TH. Electronic Cigarette Use and Respiratory Symptoms in Chinese Adolescents in Hong Kong. JAMA Pediatrics. 2016;170(1):89–91. [DOI] [PubMed] [Google Scholar]
- 12.McConnell R, Barrington-Trimis JL, Wang K, et al. Electronic Cigarette Use and Respiratory Symptoms in Adolescents. Am J Respir Crit Care Med. 2017;195(8):1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tackett AP, Keller-Hamilton B, Smith CE, et al. Evaluation of Respiratory Symptoms Among Youth e-Cigarette Users. JAMA Netw Open. 2020;3(10):e2020671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services. National Institutes of Health. National Institute on Drug Abuse, and US Department of Health and Human Services. Food and Drug Administration. Center for Tobacco Products. Population Assessment of Tobacco and Health (PATH) Study [United States] Restricted-Use Files. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor]; 2019-November-21. 10.3886/ICPSR36231.v21. [DOI] [Google Scholar]
- 15.Population Assessment of Tobacco and Health (PATH) Study [United States] Restricted-Use Files. Inter-university Consortium for Political and Social Research [distributor]. [Google Scholar]
- 16.Hyland A, Ambrose BK, Conway KP, et al. Design and methods of the Population Assessment of Tobacco and Health (PATH) Study. Tob Control. 2017;26(4):371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Population Assessment of Tobacco and Health (PATH) Study Series. https://www.icpsr.umich.edu/icpsrweb/NAHDAP/series/606, 2019. [Google Scholar]
- 18.Asher M, Keil U, Anderson H, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. European respiratory journal. 1995;8(3):483–491. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida K, Adachi Y, Sasaki M, et al. Test-Retest Reliability Of The ISAAC Questionnaire For a Web-Based Study. Journal of Allergy and Clinical Immunology. 2014;133(2):AB6. [DOI] [PubMed] [Google Scholar]
- 20.Mannino DM, Gagnon RC, Petty TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Archives of internal medicine. 2000;160(11):1683–1689. [DOI] [PubMed] [Google Scholar]
- 21.Halenar MJ, Sargent JD, Edwards KC, et al. Validation of an index for functionally-important respiratory symptoms among adults in the nationally representative Population Assessment of Tobacco and Health Study, 2014-2016. International Journal of Envrionmental Research and Public Health. 2021;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Judkins DR. Fay’s method for variance estimation. Journal of Official Statistics. 1990;6(3):223–239. [Google Scholar]
- 23.McCarthy PJ. Pseudoreplication: further evaluation and applications of the balanced half-sample technique. Vital Health Statistics 2. Jan(31):1–24. [PubMed] [Google Scholar]
- 24.Li D, Sundar IK, McIntosh S, et al. Association of smoking and electronic cigarette use with wheezing and related respiratory symptoms in adults: cross-sectional results from the Population Assessment of Tobacco and Health (PATH) study, wave 2. Tob Control. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soneji S, Barrington-Trimis JL, Wills TA, et al. Association Between Initial Use of e-Cigarettes and Subsequent Cigarette Smoking Among Adolescents and Young Adults: A Systematic Review and Meta-analysis. JAMA Pediatrics. 2017;171(8):788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huber GL, First MW, Grubner O. Marijuana and tobacco smoke gas-phase cytotoxins. Pharmacol Biochem Behav. 1991;40(3):629–636. [DOI] [PubMed] [Google Scholar]
- 27.Moir D, Rickert WS, Levasseur G, et al. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem Res Toxicol. 2008;21(2):494–502. [DOI] [PubMed] [Google Scholar]
- 28.Owen KP, Sutter ME, Albertson TE. Marijuana: respiratory tract effects. Clin Rev Allergy Immunol. 2014;46( 1):65–81. [DOI] [PubMed] [Google Scholar]
- 29.Chatkin JM, Zani-Silva L, Ferreira I, Zamel N. Cannabis-Associated Asthma and Allergies. Clin Rev Allergy Immunol. 2019;56(2):196–206. [DOI] [PubMed] [Google Scholar]
- 30.Han YY, Forno E, Celedon JC. Health risk behaviors, violence exposure, and current asthma among adolescents in the United States. Pediatr Pulmonol. 2019;54(3):237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knapp AA, Lee DC, Borodovsky JT, Auty SG, Gabrielli J, Budney AJ. Emerging Trends in Cannabis Administration Among Adolescent Cannabis Users. J Adolesc Health. 2019;64(4):487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott A, Lugg ST, Aldridge K, et al. Pro-inflammatory effects of e-cigarette vapour condensate on human alveolar macrophages. Thorax. 2018;73(12):1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hua M, Omaiye EE, Luo W, McWhirter KJ, Pankow JF, Talbot P. Identification of Cytotoxic Flavor Chemicals in Top-Selling Electronic Cigarette Refill Fluids. Sci Rep. 2019;9(1):2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyashita L, Suri R, Dearing E, et al. E-cigarette vapour enhances pneumococcal adherence to airway epithelial cells. Eur Respir J. 2018;51(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Q, Jiang D, Minor M, Chu HW. Electronic cigarette liquid increases inflammation and virus infection in primary human airway epithelial cells. PLoS One. 2014;9(9):e108342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madison MC, Landers CT, Gu BH, et al. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J Clin Invest. 2019;129(10):4290–4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perrine CG, Pickens CM, Boehmer TK, et al. Characteristics of a Multistate Outbreak of Lung Injury Associated with E-cigarette Use, or Vaping — United States, 2019. MMWR MOrb Mortal Wkly Rep. 2019;68:860–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention. Outbreak of Lung Injury Associated with the Use of E-Cigarette, or Vaping, Products. 2020; https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html. Accessed January 10, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


