Abstract
Background:
The negative appraisal of emotional stimuli is a feature of social anxiety disorder (SAD). People with SAD demonstrate deficits in neurocognitive performance while performing tasks of attention. However, the relationship between attentional control, working memory, and threat perception in SAD has not been studied well. The present study aimed to identify patterns of threat perception in relation to performance on attention and visuospatial working memory tasks in individuals with SAD.
Methods:
Subjects with SAD (n = 27) and a healthy comparative (HC) group (n = 26) completed tasks of sustained and focused attention, visuospatial working memory, computerized emotion identification, and pictorial emotional Stroop.
Results:
The SAD group had decreased performance in the domains of sustained (P = 0.001) and focused attention (P = 0.04). They also had an enhanced threat perception as demonstrated by greater reaction time to anger (P = 0.03), lower emotion recognition accuracy (P = 0.05), and higher over-identification of the threat to neutral and nonthreatening faces. However, the Stroop effect was not demonstrated across the groups. No group difference was seen in the performance on the visuospatial working memory tasks. Lower focused attention was significantly correlated with higher emotional threat perception (ETP; P = 0.001) in the SAD group.
Conclusion:
People with SAD have greater deficits in attention processing and ETP. The attention deficits were associated with enhanced ETP in social anxiety. The link between threat perception and cognitive functions would aid in a better understanding of SAD and in planning appropriate intervention.
Keywords: Social anxiety disorder, Threat perception, Attention, Visuospatial working memory
Key Messages:
Patients with social anxiety have enhanced threat perception and impaired recognition of facial stimuli. These patterns of threat perception are associated with impaired or biased attention.
Social anxiety disorder (SAD) is characterized by persistent fears of social or performance situations in which embarrassment or negative evaluation by others may occur. 1 Individuals with SAD are highly attuned toward negative appraisal, leading to feelings of distress in social situations, and they attempt to avoid social interactions, which often affects their quality of life. 2 Facial emotional expressions reflect internal emotional states, 3 and negative appraisal of these in a social context is a feature of social anxiety (SA). 4 In a social situation, in the presence of competing stimuli, neurocognitive attentional systems preferentially prioritize emotional over nonemotional content, leading to an enhanced threat perception. 5 Cognitive models of the SAD postulate that attention to threatening stimuli is critical in maintaining SA.6, 7
Studies on neurocognition in individuals with SAD indicate a mixed profile in the domains of cognitive functions. Judah et al. 8 indicated impaired efficiency of attentional control (i.e., deficits in inhibition and shifting functions) on neutral material. While comparing the SAD group with controls, overall, different studies reported impairment in different cognitive functions (e.g., visual working memory, visual scanning, visuoconstructional ability, verbal memory, episodic memory, visuospatial working memory, and processing speed).9–12, 13 On the other hand, some studies reported no difference in cognitive functions between the SAD group and the control group.14, 15 However, some methodological limitations across the studies include unmatched comparative groups on relevant variables (e.g., age), disparity in education level between control group and SA group, predominant female representation in the sample, and use of a community sample that may not represent the severity of symptoms seen in clinical populations.10–13
Studies on perceived threat using facial emotional stimuli in individuals with SAD 16 have been varied. Some studies support that socially anxious individuals prioritize threatening stimuli by paying attention to them5, 17 and overattribute the threat or anger to neutral stimuli.4, 18–20 Other studies have reported that there is an attentional bias away from the threat, that is, avoiding the negative emotions reduces the threat for socially anxious individuals,21–23 and no evidence for overattribution of the threat to neutral emotion stimuli was found.24–25 Schofield et al. 26 hypothesized that the symptoms of SA may be associated with difficulty in disengaging attention from threat signals than from neutral signals. However, some limitations of the previous studies question the generalizability of the findings to the clinical population, including recruitment of the participants without a detailed psychiatric evaluation and a sample consisting of nonclinical population and use of single-item measures for anxiety.4, 17, 23
Overall, evidence suggests that people with SAD demonstrate deficits in neurocognitive performance on attention and enhanced threat perception to emotional stimuli. However, there is limited literature examining the relationship between emotional threat perception (ETP) and attentional control and working memory in SAD. Decreased performance in attention and visuospatial working memory could disrupt social performance because of an inability to attend to and simultaneously process and interpret the many nonverbal cues present in any one social exchange. 11 Understanding the role of attentional control and working memory in threat perception to emotional stimuli could have important clinical implications in designing interventions for SAD. In the present study, we aimed to identify the pattern of ETP associated with attention and visuospatial working memory in individuals with SAD compared to a healthy comparative (HC) group. We hypothesized that enhanced ETP would be associated with poor performance on the neurocognitive task of attention and visuospatial working memory in the SAD group compared to the HC group.
Materials and Methods
Participants
This cross-sectional observational study was carried out from December 2011 to May 2012 in Kasturba hospital, Manipal, in South India. The study was approved by the Institutional Ethics Committee. The study sample was collected using a purposive sampling method after obtaining informed consent from the patients and the healthy participants. The sample consisted of 27 patients with SAD recruited from the Outpatient Department of Psychiatry and 26 healthy participants recruited from the relatives of patients with general medical conditions from the hospital’s waiting room. Patients with SAD had to be aged between 15 years and 50 years, and diagnosed with SAD, with or without comorbid anxiety spectrum disorders, except obsessive compulsive disorder, by a consultant psychiatrist, as per the DSM IV-TR criteria. Participants in the HC group were matched with the SAD group on age, gender, and education. Participants with a history of major neurological disorders (seizures, stroke, and head injury), clinically below-average intelligence, and substance abuse (except nicotine) were excluded from both groups. In addition, the HC group was screened for Axis I psychiatric disorders or family history of mental illness and were excluded if present.
The various tools are described in the following sections:
Clinical Assessments
A semi-structured interview was used to collect sociodemographic and clinical details. Patients in the SAD group were assessed on a Mini International Neuropsychiatric Interview (MINI) 5.0.0 27 to confirm the diagnosis of SAD. Similarly, the HC group was screened on the Modified MINI Screen 27 to rule out the presence of any Axis I disorders. All subjects were assessed on the Social Interaction Anxiety Scale (SIAS) 28 to assess the fear of interacting in dyads. This scale contains 20 items, and each item is self-rated on a five-point Likert scale ranging from 0 (not at all characteristic of me) to 4 (extremely characteristic of me). Three items (5, 9, and 11) require reverse scoring. Total scores range 0 to 80, with higher scores indicating higher SA. The scale was translated into the vernacular (Kannada) by a language expert and then translated back by a different person. This was compared with the original scale, and required changes were made before the administration.
Emotional Threat Perception
ETP was assessed in both groups by administering the Tool for Recognition of Emotions in Neuropsychiatric Disorders (TRENDS) and a Pictorial Emotional Stroop Test (PEST) derived from TRENDS as described further.
TRENDS is an emotion recognition assessment tool that measures threat perception and was developed by Behere et al. 29 It is a novel, culturally sensitive tool validated for use on the Indian population, and it captures the full range and nature of emotional expressions akin to real-life situations. The tool consists of 52 static (still) and 28 dynamic (video clip) images (i.e., totally 80 images) of six basic emotions—happiness, sadness, fear, anger, surprise, and disgust—and a neutral expression emoted by four experienced actors (a young man, a young woman, an older man, and an older woman). The emotion recognition paradigm used in this study was derived from the TRENDS image set. Images of the emotions of happiness and surprise (grouped as nonthreatening emotions), anger and fear (grouped as threatening emotions), and neutral stimuli were selected. Grouping of stimuli was considered based on the emotional salience of these stimuli described earlier by Behere et al. 29 Forty-eight stimuli ([2images X 3 groups X 4 actors] X 2 trials) were incorporated into a computer-based task to present the stimuli in a random order and record accurate reaction times. The target stimulus depicting the image of facial expression appeared on the screen for 3000 milliseconds, followed by a blank screen for 1000 milliseconds before the next image. The subjects were instructed to identify the emotion depicted in the image and press one of the three specified buttons of the keyboard corresponding to threatening, nonthreatening, and neutral images. Pressing a button other than the one corresponding to the displayed image was considered a misidentification error. The software recorded display times and response keystroke times automatically with one-millisecond accuracy. The test was administered in a quiet environment with the subjects placed 50 cm away from the computer screen. The performance on TRENDS was assessed by calculating the TRENDS accuracy score and the misidentification score. The TRENDS accuracy score was defined as the total number of correct responses (out of 48) expressed as a percentage. TRENDS misidentification was divided into two parts: over-identification and under-identification. The over-identification score was defined as the total number of nonthreatening or neutral images (out of 32) misidentified as threatening and was expressed in percentage. The under-identification score was defined as the total number of threatening stimuli (out of 32) misidentified as neutral or nonthreatening stimuli and was expressed in percentage. The tool has been validated in the Indian population and is found to have an inter-rater agreement above 80% and internal consistency of 0.80. 29
PEST is a computerized task constructed using emotional stimuli derived from the TRENDS image set as interference. It consists of 18 static images comprising combinations of three background colors (red, white, and green) and three basic emotions (happiness, anger, and a neutral expression), emoted by two experienced actors (an older man and an older woman). The emotional stimuli were presented in the center of the colored background. The stimulus was presented from different image groups in random order. A total of 54 images were presented ([3colors X 3 emotions X 2 actors] X 3 trials). Target stimulus depicting the image of facial expression appeared on screen for 5000 ms, followed by a blank screen with a crosshair for 1000 ms to focus the subject’s attention onto the center of the screen. The subjects were instructed to identify the background color depicted in the stimuli while ignoring the emotional stimuli presented and to provide their responses by pressing one of the three specified buttons of the keyboard corresponding to the color red, white, or green. Pressing a button other than the one corresponding to the displayed image was considered an error. The software recorded display times and response keystrokes times automatically with one-millisecond accuracy. The test was administered in a quiet environment with the subjects placed 50 cm away from the computer screen. The performance on PEST was assessed by comparing the total mean reaction times when angry, neutral or happy faces were presented as the interference stimuli. The PEST was validated on the Indian population in the present study before its administration in the SAD and HC groups.
Neurocognitive Assessments
Neurocognitive tests were administered on both groups to assess sustained attention, focused attention, and visuospatial working memory.
The Digit Vigilance Test 30
The Digit Vigilance Test (DVT) was administered to assess sustained attention. The subject is given a sheet with the numbers one to nine placed on it in a random order. The subject has to scan and cancel the target digits six and nine by marking (/) on them. Sustained attention was assessed in terms of the total time taken by the subject to complete the task and the number of errors made in the task. The test has been standardized on the Indian population. 31 It has sensitivity and specificity values of 64% and 86%, respectively, for reaction time and has a specificity score of 87% for accuracy.
Trail Making Test A and B
The Trail Making Test (TMT) measures focused attention. It was originally developed by Reitan and Wolfson, 32 and the version used in the present study has been standardized on the Indian population. 33 It has two parts, A and B, which are administered in two trials. In the TMT-A, the subject is presented with a sheet of paper in which 25 printed circles are scattered, each enclosing one of the numbers from 1 to 25. The subject is required to join the circles in numerical order as quickly as possible. In TMT-B, the subject is presented with a similar sheet of paper on which 25 circles are scattered, enclosing numbers from 1 to 13 and alphabets from A to L. The subject has to join the circles in an alternating number and alphabet order, for example, 1 A, 2 B, 3 C, and so on, as quickly as possible. In each trial, the time taken to complete the task and the number of errors committed in terms of both commissions and omissions are recorded.
Spatial Span Test
Spatial Span Test (SST), a subtest of the Wechsler Memory Scale-III, 34 assesses visuospatial working memory and taps into an individual’s ability to hold a visuospatial sequence of locations in their working memory and then reproduce the sequence. The subtest has been standardized in the Indian population 35 and had a test-retest reliability of 0.81 at an interval of six weeks. There are two conditions, namely, forward and backward. The SST board consists of ten cubes numbered one to ten randomly. The board is placed such that only the examiner can see the numbers on the cubes and not the subject. In Spatial Span-forward (SSF), there are a total of eight items with two trials each. The sequence length starts with a span of two and increases up to a span of nine. The examiner first taps the cubes, and the subject is asked to tap the same sequence tapped by the examiner. For each trial, a score of 1 is given for the exact reproduction of the sequence. A score of 0 is given if the subject omits a cube or makes an error in the sequence. Spatial Span-backward is similar to SSF in terms of the number of items, number of trials, and sequence length. However, unlike SSF, in this task, the subject is asked to tap cubes in the reverse order after the examiner taps the cubes in a specified sequence. The scoring is the same as SSF.
Procedure
A pilot study was conducted to validate the PEST. The PEST was first administered to 80 healthy consenting participants to assess the Stroop effect. Out of the 80 participants, 13 were excluded because of incomplete data or obtaining an accuracy rate below 96% in correctly identifying the background color. Responses from the remaining 67 individuals were considered for validation. After the pilot exercise, PEST was used in the main study. The subjects were recruited as per the inclusion and exclusion criteria, and the study’s rationale was informed. In the SAD group, patients on benzodiazepines were asked to avoid taking medication 6 h before the assessment. 15 First, sociodemographic and clinical data were collected, and then SIAS was administered in both groups. Then the MINI was administered in the SAD group and the Modified MINI Screen in the HC group. Both groups were assessed on ETP (TRENDS and PEST) and neurocognitive tasks (DVT, TMT-A, and B, and SST).
Analysis
The data were analyzed using the Statistical Package for Social Sciences (SPSS version 16.0) 36 for Windows. Parametric statistical tests were employed for all analyses. Repeated measures ANOVA was used to compare the reaction times between the three emotion groups on the PEST. Student’s independent t-test or chi-square tests were applied to examine the group differences in sociodemographic, neurocognitive, and ETP variables between SAD and HC groups. Pearson product-moment correlation was applied to assess the association of threat perception with performance in the domain of attention and visuospatial working memory in the SAD group.
Results
Analysis of the Pilot Study
Mean (SD) scores for age and years of education in the PEST validation group (N = 67) was 22.5 (3) and 13.8 (2.2), respectively. The subjects’ age ranged from 19 years to 35 years and their education levels ranged from 12 years to 17 years of education.
The emotional Stroop effect was seen in the results, as the mean reaction time for anger (2467.3 [603.7]) was greater than that for happy (2412.7 [573.9]) or neutral (2421.5 [548.3]) emotions, thus indicating the expected trend of greater response latencies toward threatening stimuli. However, the differences were not statistically significant (F = 1.37; df = 2,198; P = 0.25).
Subjects for the main study included 27 patients with SAD and 26 healthy participants (after excluding two and four subjects, respectively, because of incomplete data on computerized tasks). The SAD and HC groups were comparable on sociodemographic variables (Table 1). Both groups had age-, years-of-education-, and gender-matched subjects. The other sociodemographic variables are shown in Table 2. On SIAS, the SAD group had significantly greater anxiety. In the SAD group, 37% had comorbid anxiety disorders along with SAD. The mean duration of illness was 7.74 (5.82) years. Further, 46.7% of participants were on pharmacotherapy alone. The remaining were either on psychotherapy alone or on a combination of both (Table 3).
Table 1.
Comparison of Age, Education, Sex Distribution, and Social Anxiety Scores Between SAD (n = 27) and HC (n = 26) Groups
| Variables | SAD Group | HC Group | t/x2 | P |
| Age | 27.4 (8.6) | 24.4 (7.2) | 1.367 | 0.178 |
| Education | 12.4 (2.4) | 13.4 (2.5) | 1.461 | 0.150 |
| SIAS | 43.9 (13.79) | 19.9 (9.68) | 7.371 | 0.001 |
| Sex Male (%) Female (%) |
16 (59.3) 11 (40.7) |
10 (38.5) 16 (61.5) |
2.292 |
0.173 |
All values are mean (SD); *Significance at P ≤ 0.05, df: (1,51); SIAS: Social Interaction Anxiety Scale, SAD: social anxiety disorder, HC group: healthy comparative group.
Table 2.
Distribution of Other Sociodemographic Variables in SAD (n = 27) and HC (n = 26) Groups
| Sociodemographic Variables | SAD Group n (%) | HC Group n (%) | |
| Occupation | Professional | 1 (3.7%) | 0 |
| White collar | 4 (14.8) | 1 (3.8) | |
| Skilled worker | 4 (14.8) | 0 (0) | |
| Semi-skilled | 2 (7.4) | 1 (3.8) | |
| Unskilled | 2 (7.4) | 2 (7.7) | |
| Housewife | 3 (11.1) | 0 (0) | |
| Student | 9 (33.3) | 22 (84.6) | |
| Unemployed | 2 (7.4) | 0 (0) | |
| Religion | Hindu | 22 (81.5) | 9 (34.6) |
| Muslim | 1 (3.7) | 5 (19.2) | |
| Christian | 4 (14.8) | 12 (46.2) | |
| Residence | Rural | 7 (25.9) | 2 (7.7) |
| Semi-urban | 10 (37) | 16 (61.5) | |
| Urban | 10 (37) | 8 (30.8) | |
| Family type | Nuclear | 23 (85.2) | 21 (80.8) |
| Joint/extended | 4 (14.8) | 5 (19.2) | |
| Income | ≤5,000 | 1(3.7) | 3 (11.5) |
| 5000–10,000 | 8 (29.6) | 1 (3.8) | |
| ≥10,000 | 18 (66.7) | 22 (84.6) | |
SAD: social anxiety disorder, HC group: healthy comparative group.
Table 3.
Distribution of Clinical Variables in SAD (n = 27) Group
| Clinical Variables | n (%) | |
| Diagnosis | SAD | 17 (63.0) |
| SAD & AAPD | 5 (18.5) | |
| SAD & anxiety NOS | 3 (11.1) | |
| SAD & GAD | 1 (3.7) | |
| SAD & specific phobia | 1 (3.7) | |
| Treatment type | Pharmacotherapy | 11 (40.7) |
| Psychotherapy | 6 (22.2) | |
| Combination | 10 (37) | |
| Duration | <5 years | 12 (44.4) |
| 5years–10 years | 10 (37) | |
| >10 years | 5 (18.5) | |
SAD: social anxiety disorder, HC group: healthy comparative group, AAPD: anxious avoidant personality disorder, NOS: not otherwise specified, GAD: generalized anxiety disorder.
The main outcome variables of the study were response time on PEST, TRENDS accuracy score (TRACS), over- and under-identification scores on TRENDS, and neuropsychological test scores (time taken and total errors).
Performance on Emotional Threat Perception and Neurocognitive Test
Table 4 shows the comparison of performance on measures of threat perception in terms of accuracy score and mean reaction time for angry, neutral, and happy stimuli on PEST, and accuracy, under-identification, and over-identification scores on TRENDS between the SAD and HC groups. Independent samples t-test showed that the SAD and HC groups’ mean accuracy scores for color identification on the PEST task were similar, indicating that the reaction time to stimuli conditions was not influenced by accuracy. There were significant differences between the SAD and HC groups in reaction time for happy and angry stimuli, indicating the interference effect of emotion stimuli in people with SA. There was no significant difference in reaction time of neutral stimuli on PEST between the groups. On repeated measures ANOVA, there were no within-group significant differences in reaction times of emotion in both SAD (F = 1.02, P = 0.36) and HC (F = 1.5, P = 0.23) groups.
Table 4.
Comparison of Performance on Measures of Threat Perception
| Variable | SAD Group n = 27 | HC Group n = 26 | t | P | Cohen’s d |
| PEST accuracy (%) | 95.0 (11.4) | 97.6 (2.7) | 1.11 | 0.27 | 0.31 |
| Anger RT (ms) | 2678.9 (866.7) | 2188.8 (670.1) | 2.30 | 0.03 | 0.63 |
| Neutral RT (ms) | 2692.9 (937.5) | 2304.2 (917.2) | 1.53 | 0.13 | 0.41 |
| Happy RT (ms) | 2752.4 (886.2) | 2187.3 (673.4) | 2.61 | 0.01 | 0.71 |
| TRACS (%) | 67.7 (20.8) | 78.5 (17.9) | 2.01 | 0.05 | 0.55 |
| UI (%) | 29.5 (36.6) | 15.9 (25.9) | 1.56 | 0.125 | 0.42 |
| OI (%) | 35.1 (28.9) | 19.8 (9.3) | 2.58 | 0.01 | 0.71 |
All values are mean (SD); P ≤ 0.05 considered significant, df: (1,51), TRACS: TRENDS accuracy score, UI: Under-identification, OI: Over-identification score, SAD: social anxiety disorder, HC group: healthy comparative group. PEST: Pictorial Emotional Stroop Test, RT: reaction time.
There were significant differences in the TRENDS accuracy score and the over-identification score, indicating that the SAD group had decreased accuracy on recognition of facial emotional stimuli and misinterpreted neutral and nonthreatening stimuli as threatening stimuli. No significant difference was observed in the under-identification score.
The two groups differed significantly on sustained attention (DVT) in terms of time taken and number of errors, and on focused attention (TMT-A) in terms of time (Table 5).
Table 5.
Comparison of Performance on Measures of Attention and Visuospatial Working Memory
| Variables | SAD Group n = 27 | HC Group n = 26 | t | P | Cohen’s d |
| DVT-TT in seconds | 529.5 (137.1) | 456 (115.7) | 2.11 | 0.04 | 0.57 |
| DVT-E | 9.07 (5.9) | 3.6 (3.1) | 4.23 | 0.001 | 1.16 |
| TMT A-TT in seconds | 60.6 (35.4) | 43.8 (19.0) | 2.15 | 0.04 | 0.59 |
| TMT A-E | 0.11 (0.42) | 0.04 (0.19) | 0.80 | 0.43 | 0.21 |
| TMT B- TT in seconds | 116.2 (66.7) | 91.3 (69.5) | 1.33 | 0.19 | 0.36 |
| TMT B- E | 0.81 (2.3) | 0.08 (0.27) | 1.61 | 0.11 | 0.44 |
| SS | 14.41 (3.1) | 15.31 (2.8) | 1.10 | 0.28 | 0.30 |
All values are mean (SD); P ≤ 0.05 considered significant, df: (1,51), DVT-TT: Digit vigilance test time taken, DVT-E: Digit vigilance test errors, TMT A-TT: Trail making test-A time taken, TMT-A-E: Trail making test-A errors, TMT-B-TT: Trail making test-B time taken, TMT-B- E: trail making test B errors, SS: spatial span, SAD: social anxiety disorder, HC group: healthy comparative group.
On correlation analysis, in the SAD group, TRENDS accuracy score was negatively correlated with error score (r = –0.43) and time taken (r = 0.60) on TMT-A. Time taken on TMT-A was also significantly correlated with over-identification scores (r = 0.43), indicating that decreased recognition of facial emotions and a misinterpretation of nonthreatening and neutral stimuli as threatening were associated with reduced focused attention in SAD.
Error scores on TMT-A and B were found to have a significant positive correlation with reaction time on PEST anger condition (TMT-A r = 0.42, TMT-B r = 0.40), neutral condition (TMT-A r = 0.42, TMT-B r = 0.45), and happy condition (only with TMT-A errors r = 0.46), indicating that decreased focused attention (increased number of errors) was associated with interference by emotional stimuli on the Stroop task.
Further, post hoc estimation showed that a sample size of 53 (27 patients) had a power of 73% to detect the observed effect size of 0.63 (Primary outcome of emotion threat perception—reaction time for the emotion of anger) with a probability of alpha error of 0.05.
Discussion
Participants with SAD showed greater emotional threat perception (on a facial emotion recognition task and an emotional Stroop task), which was associated with poorer focused attention.
Pilot Study on PEST
Healthy participants’ performance on PEST showed a trend of higher response latencies toward anger stimuli, followed by happy stimuli. As facial emotional information plays a major role in nonverbal communication, 3 the longer response latencies in responding to color in an angry and happy condition suggest that facial emotion stimuli automatically capture attention and function as a task-irrelevant distracter. Thus, the results indicate the presence of attentional interference and bias, where emotional stimuli attract greater processing resources and the increased latencies result from task-irrelevant stimuli acting as a distracter and consuming attentional capacity.
Threat Perception in the SAD Group Compared to the HC Group
On PEST, the SAD group had higher response latencies to angry and happy stimuli compared to the HC group, suggesting greater interference in task performance in the presence of competing emotional stimuli. Studies have found similar results 26, 37–43 using social-threat-related verbal stimuli and dot-probe task with emotional faces. As socially anxious individuals are hypothesized to have enhanced selective attention to threat, 7 the higher response latencies may have resulted from the SAD group paying more attention to the task-irrelevant, nevertheless socially meaningful, facial stimuli, supporting the hypothesis that such individuals detect potentially threatening signs rapidly and have difficulty in disengaging attention from them.
The SAD group showed a decreased ability to recognize facial emotional stimuli accurately and an increased tendency to identify a greater number of neutral and nonthreatening stimuli as threatening on TRENDS than the HC group. The findings are supported by similar observations made previously.4, 18, 25, 44–46 According to Mathew and Mackintosh, 47 interpretive biases arise from selective attention to threat in their model of the threat evaluation system, leading to enhanced attribution of the threat to ambiguous stimuli. Thus, when presented with socially relevant ambiguous stimuli, an individual may be more likely to interpret them negatively compared to other individuals who are not socially anxious.
The findings can also be explained through the vigilance-avoidance hypothesis6, 7, 23, 26 of selective attention, which claims that a socially anxious person may pay exaggerated attention to the interpretation of emotions (negative) in a social situation and then avoid these cues to regulate their anxiety. Similarly, in our study, being hyper-vigilant toward emotional cues and eventually avoiding them could have contributed to perceiving nonthreatening stimuli as threatening and to the low accuracy in emotion recognition.
Neurocognitive Functions in the SAD Group Compared to HC Group
The SAD group showed lower sustained and focused attention on DVT and TMT-A and B, respectively, compared to the HC group. The findings align with previous studies13, 48–50 that indicated decreased sustained and focused attention in patients with SA.
Some studies9, 10 did not find impairment in attention tasks, but deficits were present on tasks of visual scanning, visuospatial constructional ability, and visuospatial working memory, indicating the presence of attentional impairment.
Top-down processing deficits may play a role in the observed pattern of decreased performance in sustained and focused attention. According to Kastner and Ungerleider, 51 top-down processing is a knowledge-driven mechanism designed to enhance the neuronal processing of relevant sensory input by facilitating the discrimination between target and distracters and by biasing the subject toward particular locations where the target may appear. 52 The longer time taken in performing sustained attention and focused attention tasks suggests that the SAD group had difficulty discriminating between the target stimuli and the distracter stimuli. Hence, decreased performance in DVT and TMT-A and B can be attributed to faulty top-down processing in the SAD group.
Association of Threat Perception with Neurocognition in the SAD Group
Response latencies to angry, neutral, and happy stimuli on PEST positively correlated with focused attention. This shows that reduced focused attention leads to an increase in attentional interference and bias toward the threat. On the other hand, the ability to recognize emotions on TRENDS was negatively correlated with focused attention. Focused attention was also positively correlated with errors of misidentification of neutral and nonthreatening stimuli as threatening and vice versa. Eysenck et al. 53 suggested that anxiety impairs inhibition by weakening top-down regulatory control, thus making it difficult to inhibit automatic responses, leading to difficulty in disengaging attention from distracting threat stimuli. As mentioned earlier, weak top-down processing leads to a decreased focused attention and, consequently, more focus on irrelevant stimuli, that is, emotional expressions and misinterpretation of the same in a social situation. 5 According to Mathew and Mackintosh’s 47 Threat Evaluation System model, threatening stimuli actively compete for attention, and higher levels of anxiety lower the threshold at which stimuli would be considered threatening enough to actively compete. In the SAD group, faulty top-down processing can lead to a weaker degree of control being exercised over competing threatening stimuli, leading to greater over-identification errors.
Although no significant correlations were observed between visuospatial working memory and threat perception, significant correlations were seen between focused attention and threat perception. Thus, the results partially support the hypothesis that there would be a significant association between performance on attention tasks and visuospatial working memory and threat perception in the SAD group.
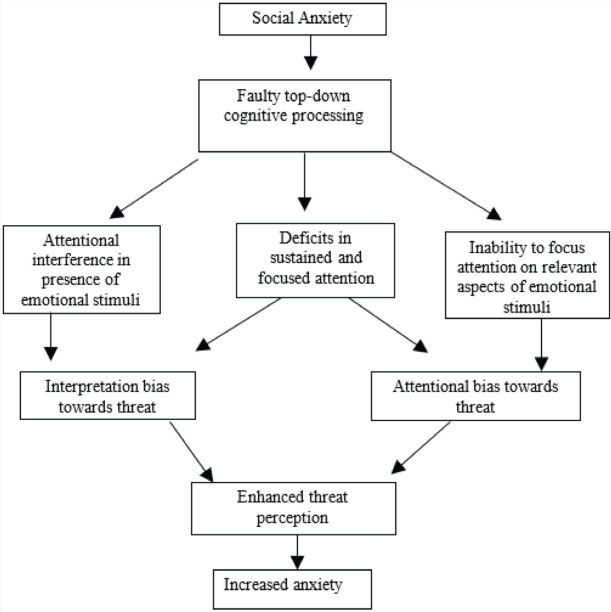
Based on the present findings, Figure 1 proposes a model of the role of attention in enhanced threat perception in the genesis of SA.
Figure 1. Model of Attentional Correlates in Enhanced Threat Perception.
The findings have implications for understanding the link between threat perception and cognitive processing in the symptomatology of SAD. Conventional cognitive-behavioral techniques focus on top-down cognitive processing. Cognitive remediation to enhance attention can improve aberrant threat perception and potentially improve SA.
The study’s strengths are that it comprehensively assessed neurocognitive functions and emotional threat perceptions (on emotional Stroop task and emotion recognition task) to understand the association between neurocognitive performance and emotional threat perception. Some of the limitations include that the threat may be attributed to many aspects within a face, besides emotions, which were not controlled for in the study. We used similar faces in both the Stroop and the emotion identification tasks, leading to a practice effect. Many participants with SAD were on treatment with benzodiazepines, which may have affected the task performance. However, all tests were done at least 6 h after the last dose to minimize the effect of benzodiazepines, if any. 15 The mean reaction time among the emotions was not significantly different in the pilot study on PEST. However, the reaction time was significantly different between the SAD and HC groups in the main study. Further, the Stroop effect was not demonstrated across the groups, which may be because of the small sample size. The potential moderating effect of ongoing treatment in the SAD group was not considered. Lastly, the small sample size of the study yielded 73% power at the probability of alpha error at 5%, which could have influenced the findings.
To conclude, we demonstrated that participants with SAD have greater deficits in attention processing and emotional threat perception than healthy participants. The enhanced emotional threat perception was associated with attention deficits in SA. Cognitive remediation to improve attention processing in addition to conventional cognitive-behavioral techniques may help reduce emotional threat perception and improve the symptoms in SAD.54–55
Acknowledgments
We would like to thank Ganesan Venkatasubramanian and Sunil Kalmady for his help in designing the PEST.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. (text revised). Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
- 2.Barrera TL and Norton PJ. Quality of life impairment in generalized anxiety disorder, social phobia, and panic disorder. J Anxiety Disord, 2009; 23 1086–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blair RJR. Facial expressions, their communicatory functions and neuro-cognitive substrates. Philos Trans R Soc Lond B Biol Sci, 2003; 358 561–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoon KL and Zinbarg RE. Interpreting neutral faces as threatening is a default mode for socially anxious individuals. J Abnorm Psychol, 2008; 117 680–685. [DOI] [PubMed] [Google Scholar]
- 5.Sussman TJ, Jin J, and Mohanty A. Top-down and bottom-up factors in threat-related perception and attention in anxiety. Biol Psychol, 2016; 121(Pt B): 160–172. [DOI] [PubMed] [Google Scholar]
- 6.Clark DM and Wells A. A cognitive model of social phobia. In: Liebowitz MR. (ed) Social phobia: Diagnosis, assessment, and treatment. New York: Guilford Press, 1995, pp. 69–93. [Google Scholar]
- 7.Rapee RM and Heimberg RG. A cognitive–behavioral model of anxiety in social phobia. Behav Res Ther, 1997; 35 741–756. [DOI] [PubMed] [Google Scholar]
- 8.Judah MR, Grant DM, Mills AC, et al. The neural correlates of impaired attentional control in social anxiety: An ERP study of inhibition and shifting. Emotion (Washington, DC) 2013; 13: 1096–1106. [DOI] [PubMed] [Google Scholar]
- 9.O’Toole MS and Pedersen AD. A systematic review of neuropsychological performance in social anxiety disorder. Nord J Psychiatry, 2011; 65 147–161. [DOI] [PubMed] [Google Scholar]
- 10.O’Toole MS, Pedersen AD, Hougaard E, et al. Neuropsychological test performance in social anxiety disorder. Nord J Psychiatry, 2015; 69 1726–1734. [DOI] [PubMed] [Google Scholar]
- 11.Sutterby SR. Neuropsychological functioning in social phobia (master’s thesis), 2009. https://stars.library.ucf.edu/cgi/viewcontent.cgi?referer=https://www.google.com/&httpsredir=1&article=5126&context=etd
- 12.Topçuoğlu V, Fistikci N, Ekinci O, et al. Assessment of executive functions in social phobia patients using the Wisconsin card sorting test. Turk Psikiyatri Derg, 2009; 20 322–331. [PubMed] [Google Scholar]
- 13.Graver CJ and White PM. Neuropsychological effects of stress on social phobia with and without comorbid depression. Behav Res Ther, 2007; 45 1193–1206. [DOI] [PubMed] [Google Scholar]
- 14.Airaksinen E, Larsson M, and Forsell Y. Neuropsychological functions in anxiety disorders in population-based samples: Evidence of episodic memory dysfunction. J Psychiatr Res, 2005; 39 207–214. [DOI] [PubMed] [Google Scholar]
- 15.Sutterby SR and Bedwell JS. Lack of neuropsychological deficits in generalized social phobia. PLoS One, 2012; 7: e42675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Machado-de-Sousa JP, Arraisa KC, Alvesb NT, et al. Facial affect processing in social anxiety: Tasks and stimuli. J Neurosci Methods, 2010; 193 1–6. [DOI] [PubMed] [Google Scholar]
- 17.Eldar S, Yankelevitch R, Lamy D, et al. Enhanced neural reactivity and selective attention to threat in anxiety. Biol Psychol, 2010; 85 252–257. [DOI] [PubMed] [Google Scholar]
- 18.Gutiérrez-García A and Calvo M. Social anxiety and threat-related interpretation of dynamic facial expressions: Sensitivity and response bias. Pers Individ Differ, 2017; 107 10–16. [Google Scholar]
- 19.Moser JS, Hajcak G, and Simons RF. The temporal dynamics of facial processing in social anxiety: Evidence from a flankers task. Lisbon, Portugal: Poster presented at the 45th Annual Convention of the Society for Psychophysiological Research, 2015 September. https://cdn.ymaws.com/sprweb.org/resource/resmgr/poster_award_winners/2005/jasonmoser.pdf [Google Scholar]
- 20.Tsuji Y and Shimada S. Socially anxious tendencies affect impressions of others’ positive and negative emotional gazes. Front Psychol, 2018; 9: 2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heur K, Rinck M, and Becker ES. Avoidance of emotional facial expressions in social anxiety: The approach–avoidance task. Behav Res Ther, 2007; 45 2990–3001. [DOI] [PubMed] [Google Scholar]
- 22.Moukheiber A, Rautureau G, Perez-Diaz F, et al. Gaze avoidance in social phobia: Objective measure and correlates. Behav Res Ther, 2010; 48 147–151. [DOI] [PubMed] [Google Scholar]
- 23.Reichenberger J, Pfaller M, and Mühlberger A. Gaze behavior in social fear conditioning: An eye-tracking study in virtual reality. Front Psychol, 2020; 11: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper RM, Rowe AC, and Penton-Voak IS. The role of trait anxiety in the recognition of emotional facial expressions. J Anxiety Disord, 2008; 22 1120–1127. [DOI] [PubMed] [Google Scholar]
- 25.de Jong PJ, Koster EHW, van Wees R, et al. Angry facial expressions hamper subsequent target identification. Emotion, 2010; 10 727–732. [DOI] [PubMed] [Google Scholar]
- 26.Schofield CA, Johnson AL, Inhoff AW, et al. Social anxiety and difficulty disengaging threat: Evidence from eye-tracking. Cogn Emotion, 2012; 26 300–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini International Neuropsychiatric Interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry, 1998; 59 22–57. [PubMed] [Google Scholar]
- 28.Mattick RP and Clarke JC. Development and validation of measures of social phobia scrutiny fear and social interaction anxiety. Behav Res Ther, 1998; 36 455–470. [DOI] [PubMed] [Google Scholar]
- 29.Behere RV, Raghunandan VNGP, Venkatasubramanium G, et al. TRENDS- A tool for recognition of emotions in neuropsychiatric disorders. Indian J Psychol Med, 2008; 30 32–38. [Google Scholar]
- 30.Lezak MD. Neuropsychological assessment. 3rd ed. New York: Oxford University Press, 1995. [Google Scholar]
- 31.Rao SL, Subbakrishna DK, and Gopukumar K. NIMHANS neuropsychology battery manual. Bangalore, India: NIMHANS publications, 2004. [Google Scholar]
- 32.Reitan RM and Wolfson D. The Halsted-Reitan Neuropsychological Test Battery: Theory and clinical interpretation. Tucson, AZ: Tucson Neuropsychological Press, 1985. [Google Scholar]
- 33.Mukundan CR. NIMHANS neuropsychological battery: Test descriptions, instructions, clinical data and interpretation. In proceedings of the National Workshop in Clinical Neuropsychology, 24–29th October, NIMHANS, Bangalore, India: NIMHANS publications, 1996. [Google Scholar]
- 34.Wechsler D. Wechsler Memory Scale (WMS III): Administration and scoring manual. 3rd ed. San Antonio, TX: The Psychological Corporation, 2010. [Google Scholar]
- 35.Gurappa P. Wechsler Memory Scale. 3rd ed., India: Adaptation and standardization project. India: Pearson, 2008. https://pearsonclinical.in/solutions/wms-iii-india-complete-kit/ [Google Scholar]
- 36.SPSS Inc. Statistical Software for Social Sciences (SPSS) version 16.0. SPSS Inc, Chicago. [Google Scholar]
- 37.Amir N, Freshman M, and Foa E. Enhanced Stroop interference for threat in social phobia. J Anxiety Disord, 2002; 16 1–9. [DOI] [PubMed] [Google Scholar]
- 38.Andersson G, Westöö J, Johansson L, et al. Cognitive bias via the internet: A comparison of web-based and standard emotional Stroop tasks in social phobia. Cogn Behav Ther, 2006; 35 55–62. [DOI] [PubMed] [Google Scholar]
- 39.Becker ES, Rinck M, Margraf J, et al. The emotional Stroop effect in anxiety disorders: General emotional or disorder specificity? J Anxiety Disord, 2001; 15 147–159. [DOI] [PubMed] [Google Scholar]
- 40.Lundh LG and Öst LG. Stroop interference, self-focus and perfectionism in social phobics. Pers Individ Differ, 1996; 20 725–731. [Google Scholar]
- 41.Maidenberg E, Chen E, Craske M, et al. Specificity of attentional bias in panic disorder and social phobia. J Anxiety Disord, 1996; 10 529–541. [Google Scholar]
- 42.Mattia JI, Heimberg RG, and Hope DA. The revised Stroop colornaming task in social phobics. Behav Res Ther, 1993; 31 305–313. [DOI] [PubMed] [Google Scholar]
- 43.Spector I, Pecknold JC, and Libman E. Selective attentional bias related to the noticeability aspect of anxiety symptoms in generalized social phobia. J Anxiety Disord, 2003; 17 517–531. [DOI] [PubMed] [Google Scholar]
- 44.Gutiérrez-García A and Calvo MG. Social anxiety and interpretation of ambiguous smiles. Anxiety Stress Coping, 2014; 27 74–89. [DOI] [PubMed] [Google Scholar]
- 45.Gutiérrez-García A and Calvo M. Social anxiety and trustworthiness judgments of dynamic facial expressions of emotion. J Behav Ther Exp Psychiatry, 2016; 52 119–127. [DOI] [PubMed] [Google Scholar]
- 46.Winton EC, Clark DM, and Edelmann RJ. Social anxiety, fear of negative evaluation and the detection of negative emotion in others. Behav Res Ther, 1995; 33 193–196. [DOI] [PubMed] [Google Scholar]
- 47.Mathews A and Mackintosh B. A cognitive model of selective processing in anxiety. Cognit Ther Res, 1998; 22 539–560. [Google Scholar]
- 48.Asmundson GJ, Stein MB, Larsen DK, et al. Neurocognitive function in panic disorder and social phobia patients. Anxiety, 1994; 1 201–207. [PubMed] [Google Scholar]
- 49.Cohen LJ, Hollander E, DeCaria CM, et al. Specificity of neuropsychological impairment in obsessive-compulsive disorder: A comparison with social phobic and normal control subjects. J Neuropsychiatry Clin Neurosci, 1996; 8 82–85. [DOI] [PubMed] [Google Scholar]
- 50.Sachs G, Anderer P, Margreiter N, et al. P300 event-related potentials and cognitive function in social phobia. Psychiatry Res, 2004; 131 249–261. [DOI] [PubMed] [Google Scholar]
- 51.Kastner S and Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci, 2000; 23 315–341. [DOI] [PubMed] [Google Scholar]
- 52.Sarter M, Givens B, and Bruno JP. The cognitive neuroscience of sustained attention: Where top-down meets bottom-up. Brain Res Rev, 2001; 35 146–160. [DOI] [PubMed] [Google Scholar]
- 53.Eysenck MW, Derakshan N, Santos R, et al. Anxiety and cognitive performance: Attentional control theory. Emotion (Washington, DC), 2007; 7 336–353. [DOI] [PubMed] [Google Scholar]
- 54.Heeren A, Mogoașe C, Philippot P, et al. Attention bias modification for social anxiety: A systematic review and meta-analysis. Clin Psychol Rev, 2015; 40: 76–90 [DOI] [PubMed] [Google Scholar]
- 55.Kim EJ, Bahk YC, Oh H, et al. Current status of cognitive remediation for psychiatric disorders: A review. Front Psychiatry 2018; 9: 461. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6178894/ [DOI] [PMC free article] [PubMed] [Google Scholar]