Abstract
Background
Jamestown Canyon virus (JCV) and snowshoe hare virus (SSHV) are wide-ranging mosquito-borne arboviruses in the California serogroup viruses (CSGV) that are known to circulate in New Brunswick. Despite potential for debilitating central nervous system manifestations, the prevalence of human exposure to these viruses in New Brunswick is unknown. The goal of this study was to quantify rates of human exposure in New Brunswick to these neglected arboviruses.
Methods
A retrospective, anonymized provincial serosurvey was performed using a stratified random sample of residual sera submitted between May 2015 and August 2016. To determine the seroprevalence of JCV and SSHV, competitive enzyme-linked immunosorbent assay–positive samples were confirmed positive using plaque-reduction neutralization testing (PRNT).
Results
A total of 452 serum samples were screened. The seroprevalence of antibodies against CSGV was estimated to be 31.6% (95% CI 27.4% to 36.1%) with 143 positive samples. PRNT results indicated that most single virus exposures were due to JCV (38 of 143; 26.6%) rather than SSHV (3 of 143; 2.1%). The species of CSGV, to which the remaining 102 seropositive people were exposed, could not be precisely determined.
Conclusions
The prevalence of human exposure to CSGV is high but comparable to rates observed in other Atlantic Canadian jurisdictions. Studies such as this provide important baseline epidemiological data regarding the risk of exposure to these neglected arboviruses. SSHV and JCV should be considered in the differential diagnosis for undiagnosed febrile and neuroinvasive illness during mosquito season, particularly when testing for common aetiologies is negative or inconclusive.
Keywords: arbovirus, Atlantic Canada, bunyavirus, California serogroup, Jamestown Canyon virus, New Brunswick, seroprevalence, snowshoe hare virus
Résumé
Historique
Le virus de Jamestown Canyon (VJC) et le virus du lièvre d’Amérique (VLA) sont des arbovirus à grande portée transmis par des moustiques des virus du sérogroupe Californie (VSGC) qui circulent au Nouveau-Brunswick (NB). Malgré le risque de manifestations débilitantes du système nerveux central, on ne connaît pas la prévalence d’exposition humaine à ces virus au NB. La présente étude visait à quantifier le taux d’exposition humaine à ces arbovirus négligés au NB.
Méthodologie
Les chercheurs ont réalisé une enquête sérologique rétrospective provinciale anonymisée au moyen d’un échantillon randomisé stratifié de sérum résiduel soumis entre mai 2015 et août 2016 au dépistage systématique. Ils ont stratifié le processus de sélection selon l’âge, le sexe et la zone de santé régionale afin de garantir un échantillonnage proportionné. Pour déterminer la séroprévalence du VJC et du VLA, ils ont confirmé la positivité des résultats d’échantillons positifs au test ELISA au moyen de tests de séroneutralisation par réduction des plaques (TSRP).
Résultats
Au total, 452 échantillons de sérum ont fait l’objet d’un dépistage. Au NB, la séroprévalence des anticorps anti-VSGC était évaluée à 31,6 % (IC à 95 %, 27,4 % à 36,1 %), pour 143 échantillons positifs. Selon les résultats du TSRP, la plupart des expositions à un seul virus étaient causées par le VJC (38 cas sur 143, 26,6 %) plutôt qu’au VLA (trois cas sur 143, 2,1 %). Les espèces de VSGC, auxquelles les 102 autres personnes séropositives ont été exposées, n’ont pas pu être établies avec précision.
Conclusions
La prévalence d’exposition humaine au VSGC est élevée, mais comparable aux taux observés dans d’autres régions des provinces de l’Atlantique. Des études comme celle-ci fournissent des données épidémiologiques de référence importantes à l’égard du risque d’exposition humaine à ces arbovirus négligés. Il faut tenir compte du VLA et du VJC dans le diagnostic différentiel de maladie fébrile et neuro-invasive pendant la saison des moustiques, notamment lorsque les tests pour dépister d’autres étiologies courantes sont négatifs ou non concluants.
Mots-clés : bunyavirus, Nouveau-Brunswick, provinces de l’Atlantique, sérogroupe Californie, séroprévalence, virus de Jamestown Canyon, virus du lièvre d’Amérique
Introduction
According to the World Health Organization, zoonoses have accounted for 75% of emerging infectious diseases in humans over the past 10 years (1). Across North America, California serogroup viruses (CSGV) are now recognized as the second most common cause of arbovirus-associated neurological disease (2). California serogroup viruses belong to the genus Orthobunyavirus and family Peribunyaviridae and are under-studied arthropod-borne viruses (arboviruses) in North America (3). Jamestown Canyon virus (JCV) and snowshoe hare virus (SSHV) are the most common CSGV found in Canada. Despite literature supporting a high risk of exposure across Canada, clinical disease is infrequently documented. The low number of reported cases is likely the result of both subclinical or asymptomatic presentation and a general lack of awareness among health care providers regarding CSGV epidemiology and clinical manifestations (4).
Increased surveillance has revealed a recent increase in recognition of CSGV and clinical cases in Canada (5). In 2019, the National Microbiology Laboratory (NML) in Winnipeg, Manitoba, reported 19 human cases or past exposures to CSGV in nine provinces (6). Of the 19 reported CSGV cases, 14 were determined to be JCV, 1 was SSHV, and the identity of the remaining 4 could not be definitively established.
A serosurvey conducted on wildlife and livestock populations in New Brunswick between 1976 and 1979 was the first indication of CSGV activity in the province. Tests were conducted on horse and deer blood samples, with SSHV antibodies present in 26.5% and 4.7%, respectively. Blood samples from moose (Alces alces americana Clinton) were also tested, with antibodies against JCV, SSHV, or both viruses in 3.2%, 74.0%, and 13.4%, respectively (7).
Human serosurveys conducted in areas with similar environmental and geographical conditions to New Brunswick demonstrate a notably high seroprevalence for CSGV. In Nova Scotia, 20.6% (95% CI 16.0% to 25.9%) of residual human sera collected from patients for unrelated diagnostic testing had JCV antibodies (8). In Quebec, seroprevalence varied widely from 1% to 42% for SSHV antibodies and from 9% to 24% for JCV antibodies, depending on the community (9). The wide ranges in prevalence were attributed to differences in diagnostic methods used to test sera as well as environmental factors that may influence the abundance of mosquitoes and deer (9). Detection of antibodies to JCV among horse, sheep, and cattle in Newfoundland and Labrador further supports the circulation of CSGV in Atlantic Canada (10).
JCV and SSHV exposures are well documented among New Brunswick residents (5,11). However, estimates of population-level exposure of persons residing in New Brunswick are currently unavailable. Infections with CSGV are often subclinical, not routinely tested for, and not notifiable. As such, it is likely that cases are drastically under-identified. To test this hypothesis, an anonymized provincial serosurvey was undertaken to estimate the seroprevalence of JCV and SSHV virus-specific antibodies among residents of New Brunswick.
Methods
Study design and sampling methodology
For this study, the required sample size was calculated assuming a seroprevalence of antibodies to CSGV of 30% in New Brunswick with a 98% confidence level and an absolute precision of 5% on either side of the prevalence. This resulted in a total required sample size of 452 serum samples. These serum samples were obtained by extracting a random subset from a serosurvey of 1,821 samples collected in New Brunswick between May 2015 and August 2016. Sera specimens tested included those submitted for electrolyte analysis, fasting lipid profile, HIV screening, or routine prenatal screening. A representative sample of the New Brunswick population consistent with the proportional age, sex, and geographic distribution was derived from the original sample sera (see Table 1). Samples were anonymized by the local laboratories and only age, sex, and postal code were retained on the sample. Serum samples were held at –70oC during long-term storage.
Table 1:
Serum samples analyzed for CSGV antibodies in the different regional health authorities by age and sex of sample donors
| RHA zone | Age group (in y) and sex
|
Total no. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10–19
|
20–29
|
30–39
|
40–49
|
50–59
|
60–64
|
||||||||
| M | F | M | F | M | F | M | F | M | F | M | F | ||
| 1 | 9 | 9 | 10 | 10 | 11 | 11 | 12 | 13 | 13 | 13 | 6 | 6 | 123 |
| 2 | 9 | 9 | 8 | 8 | 8 | 9 | 10 | 11 | 11 | 12 | 5 | 5 | 105 |
| 3 | 9 | 8 | 9 | 9 | 9 | 9 | 10 | 11 | 10 | 11 | 5 | 5 | 105 |
| 4 | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 3 | 4 | 4 | 2 | 2 | 30 |
| 5 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 1 | 1 | 16 |
| 6 | 3 | 3 | 3 | 3 | 3 | 4 | 5 | 5 | 6 | 6 | 3 | 3 | 47 |
| 7 | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 3 | 3 | 3 | 1 | 1 | 26 |
| Total no. | 35 | 34 | 35 | 35 | 36 | 38 | 45 | 48 | 49 | 51 | 23 | 23 | 452 |
CSGV= California serogroup viruses; RHA = Regional health authority; M = Male; F = Female
Laboratory methodology
The serosurvey was approved by the Horizon Health Network Research Ethics Board, and selected residual sera in pre-labelled cryotubes were shipped (minimum 90 µL) on dry ice to the NML, where they remained frozen before processing.
Detection of antibodies to CSGV was undertaken in a two-step process. Initially, all 452 serum samples were tested using an in-house competitive enzyme-linked immunosorbent assay (cELISA) as described in Patriquin et al (8). The antigen used in this assay was JCV; it is well established that cross-reaction occurs among the members of the CSGV (12). For this study, we note that cELISA detects both JCV and SSHV antibodies with comparable sensitivity. Sera that produced 30% or more inhibition in the cELISA underwent confirmatory plaque reduction neutralization testing (PRNT). Sera that had less than 30% inhibition in the cELISA were considered screen negative. PRNT was performed using JCV and SSHV in an attempt to differentiate to which species of CSGV people had been exposed. Titres of 1:20 or more were considered positive for a given virus, but the identity of specific viruses could only be considered when a four-fold or greater difference in PRNT titres for JCV and SSHV was observed on the virus-specific PRNTs. In instances in which a four-fold or greater difference in the PRNT for JCV and SSHV were not seen, samples were considered positive for the presence of CSGV antibody, but the infecting species could not be precisely established or determined.
Statistical analyses
The provincial antibody seroprevalence for each pathogen under study was calculated with 95% confidence intervals using the Clopper–Pearson exact method. Association of 2 × n tables was analyzed using χ2 or Fisher’s exact significance tests. Individual tests of association used a two-group proportion test.
Results
Among the 452 serum samples analyzed, 143 were found to meet the defined lab criteria for seropositivity for CSGV. As such, the estimated seroprevalence was determined to be 31.6% (95% CI 27.4% to 36.1%) for CSGV in New Brunswick. Of these 143 seropositive samples, distinct exposure to one specific CSGV could be differentiated by PRNT results in only 41 specimens. PRNT results indicated that most of these single virus exposures were due to JCV (38/143 or 26.6%; 95% CI 19.5% to 34.6%), and SSHV accounted for only a small percentage (3/143 or 2.1%; 95% CI 0.4% to 6.0%). The remaining 102 specimens were considered CSGV seropositive, but the infecting species of CSGV could not be determined on the basis of the defined laboratory criteria.
Male sex was significantly associated with an increased seroprevalence of CSGV antibodies, χ2(1) = 20.36, p < 0.001. Prevalence of CSGV antibodies among males was 41.7% (95% CI 35.2% to 48.2%), and seroprevalence among females was 21.9% (95% CI 16.6% to 27.3%).
Across age groups, CSGV antibody prevalence differed significantly, χ2(5) = 23.47, p < 0.001. Among the group aged 10–19 years, significantly lower CSGV antibody seroprevalence was noted in comparison with all other age groups except for those aged 20–29 years. The group aged 20–29 years had significantly lower prevalence when individually compared with only the groups aged 50–59 years and 60–64 years. The prevalence for the group aged 40–49 years was significantly lower than that for the group aged 50–59 years. No other age group comparisons were statistically significant (see Figure 1).
Figure 1:
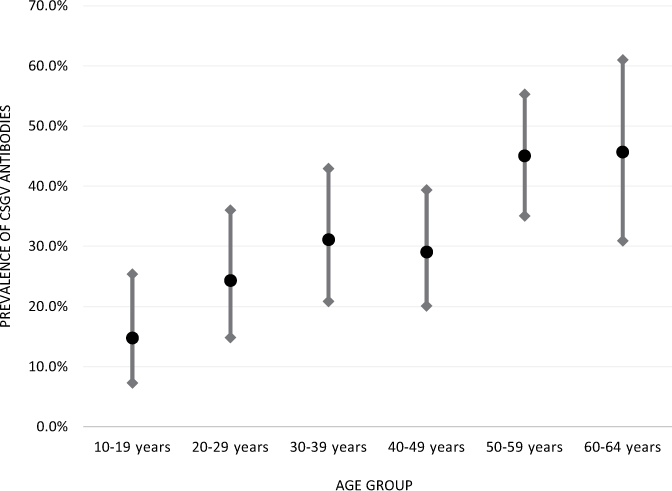
CSGV antibody prevalence by age group with 95% confidence intervals in New Brunswick, Canada
CSGV = California serogroup viruses
Overall, CSGV antibody prevalence did not differ significantly across the seven health zones in New Brunswick, χ2(6) = 8.57, p = 0.199 (see Figure 2). However, testing individual zones in comparison with others using two-group proportion tests showed significantly lower prevalence of CSGV antibodies in zone 2 over zone 1 (p = 0.0157) and in zone 2 over zone 3 (p = 0.0325). These three zones are the most heavily populated zones of the province.
Figure 2:
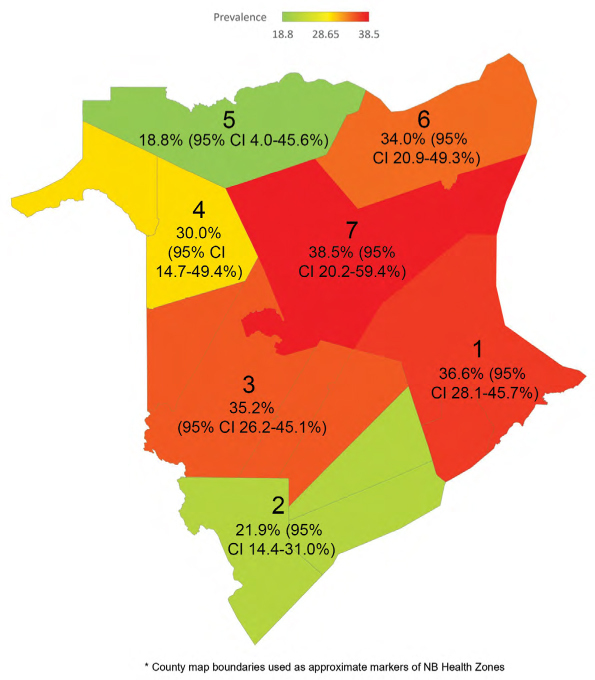
Seroprevalence of CSGV antibodies in New Brunswick by regional health zone (1–7)
CSGV = California serogroup viruses
Discussion
CSGV have been known to be transmitted by mosquitoes for many decades. JCV was named for the Jamestown Canyon locality in Colorado where the virus was first isolated from a pool of Culiseta inornata mosquitoes in 1961. Clinical disease from exposure to JCV is most commonly reported among adults. A review of JCV testing in the United States from 2000 to 2013 found a male predominance with peak presentation at ages 40–60 years (13). Symptomatic infection can present with non-specific febrile, neuroinvasive disease (eg, meningitis, encephalitis), respiratory involvement (4,5), or both. A recent case study in Manitoba described a JCV infection misdiagnosed as a complex migraine (4). Unlike SSHV, the majority of JCV cases appear to be asymptomatic, with the ratio of asymptomatic to symptomatic JCV infections estimated to range from 100:1 to 1,500:1 (5). Death from JCV infection is uncommon; however, neuroinvasive infections may lead to long-lasting cognitive deficits (5).
SSHV was first isolated and identified in Montana in 1958 from the serum of a snowshoe hare (Lepus americanus). SSHV infection can cause neuroinvasive disease, including encephalitis and meningitis. Documented SSHV cases tend to predominantly involve children, an epidemiological finding also noted with the closely related La Crosse virus (5,14). The first documented cases in Canada occurred in 1978 among three young boys in Quebec (ages 6–10 y) and a 30 year-old man in Ontario (15). Clinical manifestations including fever, nausea, vomiting, headache, confusion, and agitation have been described (5). However, clinical presentation varies widely from mild febrile illness to acute flu-like symptoms or neuroinvasive involvement (15).
The enzootic transmission cycle of CSGV involves mosquitoes as vectors with various mammal species serving as amplifying hosts or reservoirs. More than 20 species of mosquitoes, mainly non-Culex species, can transmit CSGV. Mosquitoes transmit viruses to hosts through infectious bites, and small mammals such as squirrels, chipmunks, hares, and other rodents are the most common amplifying hosts for SSHV, whereas JCV is amplified in larger mammals such as white-tailed deer and elk (5). Because many different mosquito species are involved in transmission, infections with JCV or SSHV can occur throughout the mosquito season, which is typically from May to October in much of Canada, including New Brunswick, with peak intensity in the late summer months (July to early September) (4). Transovarial transmission is the primary mechanism for overwintering of CSGV, and as a result, overwintered infected adult female mosquitoes may transmit viruses to people in the early part of the mosquito season (May and June) (5).
In this study, JCV contributed to the majority of CSGV infections among the sera analyzed. Since 2006, more than 200 cases of SSHV and JCV have been documented across Canada, 70% of which were JCV infections. These findings contrast with those of the 1970s and 1980s, when SSHV made up the majority of Canadian CSGV cases (5). Whether this difference is the result of changes in the dynamics of virus circulation or abundance or whether it is due to changes in sensitivity and specificity of serological testing is still unknown (5). A previous review article summarizing human JCV cases in the United States between 2000 and 2013 found that more than 50% of cases were identified the first year in which the Centers for Disease Control and Prevention implemented routine JCV immunoglobulin M antibody testing (13). This finding supports our claim that JCV disease has been historically under-reported. In this study, we noted that many of the CSGV exposures could not be specifically identified as JCV or SSHV. The precise reasons for this are unknown, but it is possible that these people were exposed to more than one species of CSGV or experienced secondary exposures to different species of CSGV, which could have resulted in static PRNT titres between JCV and SSHV. Although it seems less likely, it is also possible that infections were due to viruses other than JCV or SSHV, such as LaCrosse virus, which was not included in the panel of viruses tested in the PRNT assay.
The findings of this study add to the existing knowledge that CSGV are widespread in northeastern North America and potentially far more prevalent than was once thought. The reported seroprevalence rates in New Brunswick are comparable to those reported in neighbouring provinces (8,9). The association found between CSGV seropositivity and male sex in this study also reflects past findings reported in neighbouring Nova Scotia (8). Increasing age was a significant predictive variable for CSGV exposure in New Brunswick. This finding again supports data collected in Nova Scotia, which also identified increasing age as a predictor of antibodies against JCV (8). As people accumulate years of life, exposure to mosquitoes infected with CSGV increases, likely explaining higher prevalence of CSGV antibodies in older cohorts.
From a health care perspective, the province of New Brunswick is separated into seven health zones (16). The three major cities of the province include Moncton in zone 1, Saint John in zone 2, and Fredericton in zone 3. The lowest CSGV seroprevalence rates were detected in zones 2 and 5 at 21.9% (95% CI 14.4% to 31.0%) and 18.8% (95% CI 4.0% to 45.6%), respectively. Zone 2 encompasses the southern region of the province and is a largely coastal zone with generally cooler temperatures in the summer months as a result of its proximity to the Bay of Fundy. It is possible that climate and local ecology may influence mosquito abundance, the dynamics of transmission cycles, or both in zone 2. The low seroprevalence in zone 5 is also notable, but it is not statistically significant. Nonetheless, this northern zone is predominantly populated in coastal settings, has areas of relatively high wind, and is noted to have low density of white-tailed deer (17,18). These factors could reduce mosquito exposure and CSGV amplification, resulting in lower rates of local infection, although further research is required to support this speculation.
Implications
Clinical cases of CSGV infection have been described in Nova Scotia, New Brunswick, Quebec, Ontario, and Manitoba, as well as in the northeastern United States (4,8,9,11,13,15). Given their widespread distribution, arboviruses, such as those described here, likely account for a significant proportion of undiagnosed febrile and neuroinvasive disease across Canada (5,19).
In Canada, the diagnosis of CSGV infection is supported by in-house serological assays, available at the NML. Treatment of CSGV infection is supportive and focuses on management of symptoms and potential complications, including increased intracranial pressure (5). There are currently no available vaccines for CSGV; therefore, disease prevention is centred around public education and risk reduction measures. Clinicians and public health officials should increase awareness of mosquito-borne diseases and encourage the public to reduce their risk of exposure to mosquito bites by wearing protective clothing and using insect repellant when outdoors, as well as using screens and air conditioning when indoors (5,13). Raising awareness of the epidemiology and manifestations of CSGV infections is critical for improving detection. The non-specific clinical presentation of these viruses can make early detection difficult. Thus, educating health care professionals with regard to JCV and SSHV will help to place these viral infections in the differential diagnosis of at-risk patients (5,11).
This study’s findings of high seroprevalence of antibodies to JCV, and to a much lesser extent SSHV, among New Brunswick residents can assist clinical assessment. CSGV infection should be considered among patients who present with acute febrile illness or neurological disease during mosquito season, particularly when more common aetiologies cannot be identified (eg, herpes simplex virus and enterovirus) (4,13). A wide variation of clinical manifestations are associated with CSGV infection, ranging from mild febrile illness to severe neuroinvasive complications, including meningitis, encephalitis, or meningoencephalitis (12). A case report from 2015 described a New Brunswick resident infected with CSGV (11). This individual suffered post-encephalitic dementia, which highlights the severe and debilitating complications that can result from CSGV infection.
Limitations
It is important to recognize that this study has potential limitations. First, although residual sera sampling has been documented as an appropriate method for establishing population immunity data (20), its inherent convenience sampling has limitations. For instance, residual sera sampling may bias toward populations with medical comorbidities or health-seeking behaviours. In attempts to mitigate this potential bias, our study relied on residual sera that were collected for routine diagnostic testing. However, there is still a risk that our sample may not be representative of the New Brunswick population because of healthy person bias, exclusion of residents who do not access regular medical care, or both (21).
Second, using an anonymized sampling process, we were unable to collect any exposure or travel histories. It is possible that exposure to CSGV occurred outside of the respective health zones or outside of New Brunswick altogether. The anonymity of our sample also limited our ability to correlate findings with clinical cases, limiting our conclusions about overall disease risk.
Third, the possibility of false-negative cELISA screen among samples with less than 30% inhibition is noted. Sensitivity of the cELISA is not 100%, so it is possible that some excluded samples may have represented true exposures to CSGV. Thus, the findings of this study may under-represent the true rate of human exposure to CSGV in the province.
Under-identification of CSGV infection, in general, may also be attributed to our limited capacity for testing, with the NML serving as Canada’s only site for this specialized testing. The only existing commercial serological assay for CSGV antibody uses the cross-reactivity properties of La Crosse virus antigen (Focus Diagnostics Arbo IFA; https://www.focusdx.com/product-catalog/dxselect/ifa-iha-kits/ous). Serological cross-reactivity between CSGV allows for detection of SSHV- and JCV-specific antibodies, but there is evidence to suggest that some cases may go undetected (22). There is a need for development of additional commercially available diagnostic assays with improved specificity for a wider variety of orthobunyaviruses (5).
Conclusion
Overall, the provincial CSGV seroprevalence in New Brunswick was 31.6% (95% CI 27.4% to 36.1%), and most single virus exposures were due to JCV. This study is the first to establish seroprevalence rates for CSGV among New Brunswick residents. Despite the widespread presence of these viruses across the region, under-identification of CSGV infections in clinical settings highlights the need for improved awareness of mosquito-borne bunyaviruses. JCV and SSHV should be considered in the differential diagnosis for undiagnosed febrile and neuroinvasive illness during mosquito season, particularly when testing for other common aetiologies is negative or inconclusive.
Acknowledgements:
The authors thank the Saint John Regional Hospital Microbiology Laboratory staff, specifically Tammy Mahaney, who coordinated collection of sera throughout the province and maintained the sera at the Saint John Regional Hospital Microbiology Laboratory. The authors also acknowledge Brooks Waitt at the National Microbiology Laboratory in Winnipeg, Manitoba, for technical assistance with this study. This work originated from Dalhousie Medicine New Brunswick, Saint John, New Brunswick, and Saint John Regional Hospital, Saint John, New Brunswick
Funding Statement
Funding was provided by New Brunswick Public Health, a Fund for Innovative Research Excellence grant, and a Dalhousie Medicine New Brunswick Summer Studentship grant.
Ethics Approval:
The Horizon Health Network Institutional Review Board (Saint John, New Brunswick, Canada) approved this study.
Informed Consent:
N/A
Funding:
Funding was provided by New Brunswick Public Health, a Fund for Innovative Research Excellence grant, and a Dalhousie Medicine New Brunswick Summer Studentship grant.
Disclosures:
The authors have nothing to disclose.
Peer Review:
This manuscript has been peer reviewed.
Animal Studies:
N/A
References
- 1.World Health Organization. Neglected tropical diseases: Neglected zoonotic diseases. https://www.who.int/teams/control-of-neglected-tropical-diseases/neglected-zoonotic-diseases (April 26, 2020).
- 2.Meier-Stephenson V, Langley JM, Drebot M, Artsob H. Encephalitis in the summer: a case of snowshoe hare (California serogroup) virus infection in Nova Scotia. Can Commun Dis Rep. 2007;33(11):23–26. [PubMed] [Google Scholar]
- 3.Lindsey NP, Lehman JA, Staples JE, Fischer M. West Nile virus and other arboviral diseases—United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(25): 513–517. [PMC free article] [PubMed] [Google Scholar]
- 4.Vosoughi R, Walkty A, Drebot MA, Kadkhoda K. Jamestown Canyon virus meningoencephalitis mimicking migraine with aura in a resident of Manitoba. CMAJ. 2018;190(9):E262–4. 10.1503/cmaj.170940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drebot M. Emerging mosquito-borne bunyaviruses in Canada. Can Commun Dis Rep. 2015;41(6):117–123. 10.14745/ccdr.v41i06a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Public Health Agency of Canada. Mosquito-borne disease surveillance report: September 29 to October 12, 2019 (Week 40 & 41). 2019. https://www.canada.ca/en/public-health/services/publications/diseases-conditions/west-nile-virus-surveillance/2019/week-40-41.html (July 2, 2020).
- 7.McFarlane BL, Embree JE, Embil JA, Rozee KR, Artsob A. Antibodies to the California group of arboviruses in animal populations of New Brunswick. Can J Microbiol. 1982;28(2):200–4. 10.1139/m82-026. [DOI] [PubMed] [Google Scholar]
- 8.Patriquin G, Drebot M, Cole T, et al. High seroprevalence of Jamestown Canyon virus among deer and humans, Nova Scotia, Canada. Emerg Infect Dis. 2018;24(1): 118–21. 10.3201/eid2401.170484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sampasa-Kanyinga H, Levesque B, Anassour-Laouan-Sidi E, et al. Zoonotic infections in communities of the James Bay Cree territory: an overview of seroprevalence. Can J Infect Dis Med Microbiol J. 2013;24(2): 79–84. 10.1155/2013/370321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goff G, Whitney H, Drebot MA. Roles of host species, geographic separation, and isolation in the seroprevalence of Jamestown Canyon and snowshoe hare viruses in Newfoundland. Appl Environ Microbiol. 2012;78(18):6734–40. 10.1128/AEM.01351-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webster D, Dimitrova K, Holloway K, Makowski K, Safronetz D, Drebot MA. California serogroup virus infection associated with encephalitis and cognitive decline, Canada, 2015. Emerg Infect Dis. 2017;23(8): 1423–4. 10.3201/eid2308.170239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson AJ, Martin DA, Karabatsos N, Roehrig JT. Detection of anti-arboviral immunoglobulin G by using a monoclonal antibody-based capture enzyme-linked immunosorbent assay. J Clin Microbiol. 2000. 38(5):1827–31. 10.1128/JCM.38.5.1827-1831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pastula DM, Hoang Johnson DK, Fischer M, White JL, Staples JE, Dupuis AP. Jamestown Canyon virus disease in the United States—2000–2013. Am J Trop Med Hyg. 2015;93(2):384–9. 10.4269/ajtmh.15-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haddow AD, Odoi A. The incidence risk, clustering, and clinical presentation of La Crosse virus infections in the eastern United States, 2003-2007. PloS One. 2009;4(7):e6145. 10.1371/journal.pone.0006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fauvel M, Artsob H, Calisher CH, et al. California group virus encephalitis in three children from Quebec: clinical and serologic findings. CMAJ. 1980;122(1):60–4. [PMC free article] [PubMed] [Google Scholar]
- 16.Government of New Brunswick. Regional health authorities. https://www2.gnb.ca/content/gnb/en/services/services_renderer.9435.Regional_Health_Authorities.html (December 31, 2020).
- 17.Government of New Brunswick. Natural resources and energy development: the New Brunswick wind atlas. https://www2.gnb.ca/content/gnb/en/departments/erd/energy/content/resource_maps/wind.html (February 28, 2021).
- 18.Gabriele-Rivet V, Arsenault J, Badcock J, et al. Different ecological niches for ticks of public health significance in Canada. PLoS One. 2015;10(7):e0131282. 10.1371/journal.pone.0131282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulkarni MA, Lecocq AC, Artsob H, Drebot MA, Ogden NH.Epidemiology and aetiology of encephalitis in Canada, 1994–2008: A case for undiagnosed arboviral agents? Epidemiol Infect. 2013;141(11):2243–55. 10.1017/S095026881200252X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly H, Riddell MA, Gidding HF, Nolan T, Gilbert GL. A random cluster survey and a convenience sample give comparable estimates of immunity to vaccine preventable diseases in children of school age in Victoria, Australia. Vaccine. 2002;20(25):3130–6. 10.1016/S0264-410X(02)00255-4 [DOI] [PubMed] [Google Scholar]
- 21.Wilson SE, Deeks SL, Hatchette TF, Crowcroft NS. The role of seroepidemiology in the comprehensive surveillance of vaccine-preventable diseases. CMAJ. 2012;184(1):e70–6. 10.1503/cmaj.110506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makowski K, Dimitrova K, Andonova M, Drebot M. O53 An overview of California serogroup virus diagnostics & surveillance in Canada in 2008. Int J Antimicrob Agents. 2009; 34(Supplement 2):S19. 10.1016/S0924-8579(09)70200-6. [DOI] [Google Scholar]


