Abstract
We describe the first documented case of meningitis caused by Lodderomyces elongisporus. Identification of L. elongisporus was made on the basis of an arachnoid biopsy with pathology samples sent for fungal internal transcribed spacer sequencing after multiple central nervous system (CNS) fungal culture specimens were negative. After final diagnosis, treatment was transitioned from amphotericin to fluconazole, which, combined with insertion of lumbar drain followed by a permanent ventriculopleural shunt, resulted in significant clinical improvement. Our report reviews the literature of (1) cases of L. elongisporus, which almost exclusively describe fungemia or endocarditis; (2) CNS infections caused by Candida parapsilosis, an organism with which L. elongisporus was previously conflated; and (3) management of fungal meningitis–associated hydrocephalus.
Keywords: fungal meningitis, ITS sequencing, Lodderomyces elongisporus
Résumé
Les chercheurs décrivent le premier cas répertorié de méningite causée par le Lodderomyces elongisporus. Ils ont dépisté le L. elongisporus après avoir effectué une biopsie de l’arachnoïde et envoyé les prélèvements pathologiques au séquençage de l’espaceur transcrit interne fongique après l’obtention de multiples cultures fongiques négatives. Après le diagnostic définitif, le traitement d’amphotéricine a été remplacé par du fluconazole qui, combiné à l’insertion d’un drain lombaire suivie par l’installation d’une dérivation ventriculopleurale permanente, a favorisé une amélioration clinique évidente. L’analyse bibliographique a permis d’extraire 1) des cas de L. elongisporus, qui ont été observés presque exclusivement dans des cas de fongémie auparavant, 2) des infections du système nerveux central causées par le Candida parapsilosis, un organisme avec lequel le L. elongisporus a déjà été confondu et 3) la prise en charge de l’hydrocéphalie associée à la méningite fongique.
Mots-clés : méningite fongique, séquençage ITS, Lodderomyces elongisporus
Case Presentation
A 62-year-old man presented to a peripheral hospital with a 2-week history of headache and dizziness. His medical history included T3bN+ adenocarcinoma of the rectum, hypertension, hyperlipidemia, and chronic lymphopenia (0.3 × 109 g/L). One year before presentation, he was treated with neoadjuvant capecitabine and pelvic radiation for his rectal adenocarcinoma. This treatment was followed by surgical resection of his malignancy, and finally five cycles of adjuvant oxaliplatin and capecitabine, completed 2 months before his presentation.
On presentation, his initial vital signs were within normal limits. Computed tomography scan of the head showed hydrocephalus. One day after admission, gait ataxia, urinary incontinence, and confusion were noted. Neurology was consulted, and he was subsequently transferred to a larger peripheral hospital for further workup and treatment while awaiting transfer to an inpatient neurology unit at a tertiary referral centre. MRI showed diffuse dilation of the ventricular system, consistent with evidence of worsening communicating hydrocephalus. Lumbar puncture was performed, which showed a neutrophilic-predominant cerebrospinal fluid (CSF) pleocytosis (344 × 106/L; 52% neutrophils, 41% lymphocytes, 7% monocytes), hypoglycorrhacia (1.1 mmol/L), and significantly elevated protein (4.152 g/L). An opening pressure was 28.5 cm H2O (seated position). He was started on empiric coverage for meningitis with ceftriaxone, vancomycin, and acyclovir.
He was subsequently transferred to our institution under the neurology service 4 days later (7 d after initial admission). At the time of transfer, he had a decreased level of consciousness (one-word responses, obeying one-step commands) without focal or lateralizing deficits. The Infectious Disease Service raised concern for fungal meningitis on the basis of his clinical presentation, imaging, and CSF profile, and the patient was started on liposomal amphotericin.
An initial CSF specimen was collected in the absence of administration of antifungal therapy, but the fungal culture was negative. Bacterial, mycobacterial, and fungal culture investigations were negative for multiple CSF specimens collected after the initiation of antibacterial and antifungal therapy. Cryptococcal antigen and enterovirus, herpes simplex virus, and varicella zoster virus polymerase chain reaction (PCR) investigations were also negative. Cytology did not detect any evidence of leptomeningeal carcinomatosis. Repeat MRI showed extensive leptomeningeal enhancement with signal abnormality in the brain, especially at the pontomedullary junction (Figures 1, 2, 3), where a complex septated abscess-like collection was visualized anterior to the brain stem in the prepontine cistern and where there also appeared to be intraparenchymal involvement of the upper medulla. For management of hydrocephalus, the patient required two large-volume lumbar punctures, followed by lumbar drain insertion, and he showed clinical improvement after this intervention (Glasgow Coma Scale improved to 14 from 8 immediately post-insertion on one occasion). Five lumbar drains were sequentially inserted and removed, with revisions needed as a result of frequent drain occlusions that caused a parallel decline in clinical status on each occasion.
Figure 1:
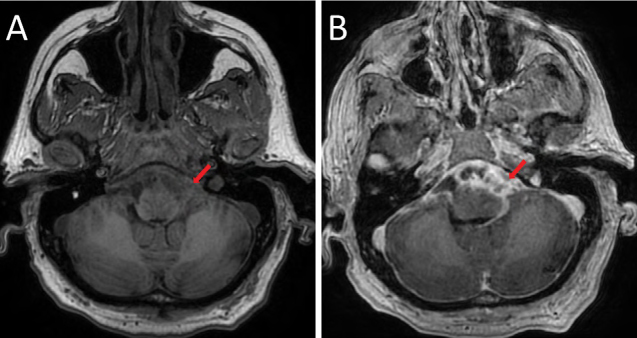
Axial T1-weighted images pre– (A) and post– (B) contrast administration demonstrating extensive, complex, multiseptated enhancement through the pre-pontine cistern and along the leptomeningeal surface of the brainstem. There is mass effect, with effacement of the ventral brain stem
Figure 2:
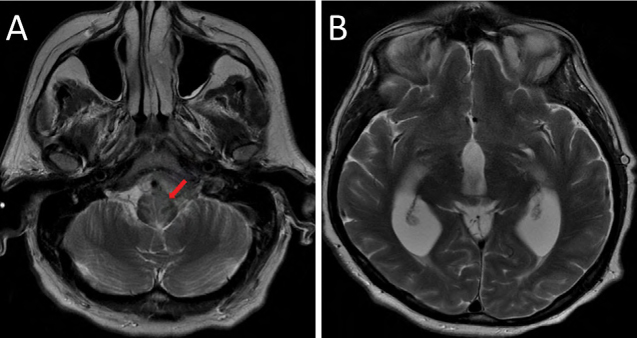
Axial T2-weighted images: (A) shows abnormal signal hyperintensity within the upper medulla, adjacent to the areas of cisternal enhancement; (B) demonstrates hydrocephalus with expansion in the caliber of the third and lateral ventricles
Figure 3:
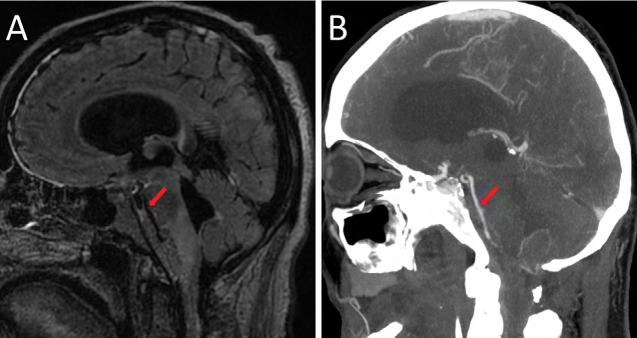
(A) Sagittal fluid-attenuated inversion recovery image demonstrating the full extent of abnormal signal material throughout the pre-pontine cistern, surrounding the expected course of the basilar artery; (B) sagittal reconstruction of a computed tomography angiogram demonstrating multifocal luminal caliber narrowing of the basilar artery
Because of the lack of a diagnosis, a C1 laminectomy and biopsy of arachnoid tissue at the cervicomedullary junction was performed approximately 3 weeks into his admission. Repeat bacterial, fungal, and mycobacterial cultures were negative, and pathology showed a mixed inflammatory infiltrate with some multinucleated giant cells. Argyrophilic particles noted on silver methenamine stain were suspicious for yeast-like fungal forms (Figure 4).
Figure 4:
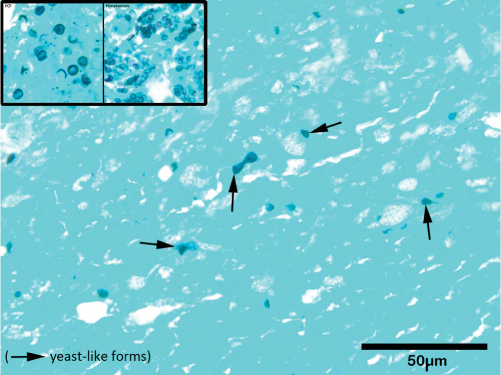
Silver methenamine stain; fungal fragments seen (marked with arrows)
Pneumocystis jirovecii and histoplasmosis control images are provided in left corner
Internal transcribed spacer (ITS) PCR and sequencing performed on the tissue biopsy specimen identified the presence of Lodderomyces elongisporus. After identification of the argyrophilic particles, voriconazole was added to the patient’s regimen. Once L. elongisporus was identified, the patient was transitioned to intravenous fluconazole for 2 weeks, followed by an indefinite course of oral fluconazole. During his stay, complications included a left hemispheric convexity subdural collection likely secondary to anticoagulation needed for a line-associated upper limb deep vein thrombosis and a small medullary stroke secondary to vertebrobasilar arterial narrowing arising from adjacent effect of the fungal abscess (Figure 3), with resultant mild left hemiparesis.
Clinical Outcome
Four months after admission, the patient was transferred to a rehabilitation facility on oral fluconazole. At the time, he was alert and conversational but non-ambulatory and dependent on all activities of daily living owing to physical and cognitive deficits. In the following 3 months, his recovery plateaued; he remained unable to sit up or walk unassisted despite near-full recovery from his stroke-related hemiparesis and absence of any limb ataxia. Follow-up MRI at 6 months after initial presentation showed similar appearance of the prepontine collection, a slight increase in hydrocephalus, and resolution of the subdural collection. Repeat lumbar puncture showed near normalization to a nucleated cell count of 6 × 106/L (97% lymphocytes), protein of 0.84 g/L, and glucose of 2.7 mmol/L. Because the persistent hydrocephalus was suspected to be causing the plateau in recovery and sufficient time had elapsed that shunt infection risk was adjudged by neurosurgery to be acceptably reduced, a permanent shunt was inserted at 10 months after presentation. A ventriculo-pleural shunt was preferred to a ventriculo- (or lumbo-) peritoneal shunt given his rectal cancer history. Despite a postoperative complication of right-sided subdural hematoma that required craniotomy and subdural drain, he has resumed ambulatory status with a cane and described subjective memory improvement 1 month after drain insertion. He has rejoined his wife at home.
Discussion
L. elongisporus was revealed as a distinct fungal species via sequencing of the ribosomal RNA gene in 1994 (1). Before 1994, it was believed to be the teleomorph of Candida parapsilosis (2). Its role in human infection and disease has been reported only in case studies. Previous reports have documented six cases of L. elongisporus fungemia (3–8), one case of a catheter-tip infection in a patient with fungemia (9), and two cases of endocarditis (10,11). Early case studies share a common theme, with authors describing a delay in the identification of the fungal species as a result of limitations in the traditional fungal identification system (3,5,9). It is now recognized that cases of L. elongisporus may previously have been under-reported, because a molecular study characterizing isolates phenotypically identified as C. parapsilosis from a worldwide collection revealed that 10 isolates were actually L. elongisporus (12). Conventional biochemical methods are not able to reliably distinguish the four species of the C. parapsilosis complex, including L. elongisporus. However, recent reports demonstrate the accuracy of matrix-assisted laser desorption ionization–time of flight for the identification of L. elongisporus from cultured isolates (8,11).
In the case we have described, the arachnoid tissue biopsy was culture-negative, likely as a result of prolonged antifungal therapy at the time of biopsy, and ITS sequencing was required to identify L. elongisporus. Fungal PCR was performed using pan-fungus primers targeting ribosomal RNA genes in the ITS region followed by DNA sequence analysis. Testing was performed by the Department of Paediatric Laboratory Medicine at the Hospital for Sick Children in Toronto.
Previous case reports have documented infection with L. elongisporus in a variety of geographic settings (Table 1). Most cases identified clear risk factors for fungal infection, including the presence of central lines (4–6,9,10). In our case, the patient described had a history of chemotherapy on two occasions in the year before presentation and frequent contact with the health care system; however, he did not have a central line. In addition, he had chronic lymphopenia and prior gastrointestinal surgery. No other risk factors were identified. HIV serology, immunoglobulins, and autoimmune workup were negative. L. elongisporus was identified from ITS sequencing, and therefore susceptibility testing was not possible.
Table 1:
Review of cases of Lodderomyces elongisporus infection
| Case report | Demographics | Location | Comorbid conditions | Site of isolate | Method of identification | Presence of central catheter | Management | Clinical outcome |
|---|---|---|---|---|---|---|---|---|
| Present case | Male, aged 62 y | Canada |
Rectal adenocarcinoma Lymphopenia |
Arachnoid biopsy | Fungal ITS sequencing | No |
Lumbar drain insertion Liposomal amphotericin + voriconazole followed by indefinite fluconazole |
Survived |
| Al-Obaid et al (3) | Female, aged 71 y | Kuwait |
Hypertension Coronary artery disease Peripheral vascular disease Stroke |
Blood | Fungal ITS sequencing | No | Caspofungin | Died |
| Lee et al (6) | Female, aged 56 y | Korea |
Lung cancer MRSA Bacteremia |
Blood |
MALDI-TOF Fungal ITS sequencing |
Yes | None | Died |
| Fernandez-Ruiz et al (4) | Male, aged 79 y | Spain |
Chronic obstructive pulmonary disease Diabetes End-stage renal disease |
Blood | Fungal ITS sequencing | Yes | Caspofungin | Died |
| Hatanaka et al (5) | Male, aged 39 y | Japan | Thoracoabdominal aortic replacement complicated by aorto-esophageal fistula | Blood + catheter tip | Fungal ITS sequencing | Yes | Micafungin | Survived |
| Taj-Aldeen et al (7) | Male, aged 22 y | Qatar | Trauma | Blood |
MALDI-TOF Fungal ITS sequencing |
Unknown |
Caspofungin Fluconazole |
Died |
| Ahmad et al (9) | Male, aged 63 y | Kuwait |
Cardiovascular attack Schizophrenic-like condition |
Central catheter tip | Fungal ITS sequencing | Yes | Fluconazole | Survived |
| Daveson & Woods (10) | Male, aged 30 y | Australia |
Endocarditis Embolic stroke Osteomyelitis Injection drug use |
Aortic valve + blood | Fungal ITS sequencing | Yes |
Aortic valve replacement Caspofungin x 2 wk pre-operatively Liposomal amphotericin + 5-flucytosine x 6 wk, then voriconazole x 7.5 mo |
Survived |
| Thompson et al (11) | Male, aged 46 y | United States |
Mechanical aortic valve Hepatitis C Injection drug use |
Blood |
MALDI-TOF Fungal ITS sequencing |
No |
Cefepime + vancomycin + micafungin Switched to liposomal amphotericin + flucytosine x 6 wk |
Survived |
| Koh et al (8) | Female, aged 54 y | Australia |
Total colectomy + ileostomy Short gut syndrome Total parenteral nutrition |
Blood | MALDI-TOF | Yes |
Anidulafungin x 20 d (14 d after line removal) Central catheter removal |
Survived |
ITS = Internal transcribed spacer; MRSA = Methicillin-resistant Streptococcus aureus; MALDI-TOF = Matrix-assisted laser desorption ionization-time of flight
On the basis of previous case reports, isolated strains have shown similar susceptibility profiles, typically with low minimum inhibitory concentrations (MICs) to azoles (ie, fluconazole MIC 0.25–0.32 µg/mL), echinocandins (ie, caspofungin MIC 0.015–0.5 µg/mL), and amphotericin (MIC ≤0.12–0.5 µg/ml) (7, 9,12,13). There are no established break points for antifungal susceptibility testing for L. elongisporus, but those for C. parapsilosis are often applied as a surrogate (9). Our choice of antifungal therapy was influenced by these previous reports and the need for appropriate central nervous system (CNS) penetration (7,9,12,13).
C. parapsilosis CNS infection and risk factors for clinical disease
Although this is the first documented case of L. elongisporus meningitis, a review of the literature shows documented cases of meningitis (14–16) and device-associated CNS infections (17–20) caused by C. parapsilosis. This clinical entity has been described primarily among neonates, as well as among immunocompromised patients and those with indwelling devices (17,21). In contrast to other species of Candida, invasive C. parapsilosis infection can occur without previous colonization (21). It can be transmitted through contaminated external sources such as medical devices, fluids, parenteral nutrition, prosthetic devices, and health care workers’ hands. Additional described risk factors include a central venous catheter, prolonged antibiotic use, immunosuppressive therapy, and malignancy (21). In a review on C. parapsilosis, Trofa et al suggest that immunocompromised individuals and surgical patients, particularly those undergoing surgery on the gastrointestinal tract, are at highest risk of infection (21). Although this clinical entity appears different than our case of L. elongisporus meningitis, there is overlap of risk factors, including frequent health care exposure, chemotherapy treatment, and surgery on the gastrointestinal tract 6 months before presentation.
Management of Fungal Meningitis–Associated Hydrocephalus
In this case, a lumbar drain was inserted for management of communicating hydrocephalus. The hydrocephalus likely arose from the extremely elevated CSF protein, which can obstruct the arachnoid granulations and reduce resorption of CSF. Intracranial hypertension can be seen in fungal meningitis and is particularly common in cryptococcal meningitis. Clinical practice guidelines of the Infectious Diseases Society of America strongly recommend management of intracranial hypertension with repeated lumbar puncture, lumbar drain insertion, ventriculoperitoneal shunt, or pharmacological approaches (22). Cryptococcal meningitis is most prevalent among immunosuppressed patients and is often seen in those with HIV. Most studies on the use of a lumbar drain for management of intracranial hypertension have involved patients with HIV (23,24); however, Zhang et al show that lumbar drain insertion is an effective and safe alternative to repeated lumbar punctures in non-HIV cryptococcal meningitis (25). In the cases of C. parapsilosis meningitis described earlier, management of intracranial hypertension with repeated lumbar punctures or lumbar drain was not described.
Acknowledgments:
Dr David Ramsay prepared pathology images for presentation in this article.
Ethics Approval:
N/A
Informed Consent:
The authors obtained patient consent for publication of this report.
Funding:
No funding was received for this work.
Disclosures:
The authors have nothing to disclose.
Peer Review:
This manuscript has been peer reviewed.
Animal Studies:
N/A
References
- 1.James SA, Collins MD, Roberts IN. The genetic relationship of Lodderomyces elongisporus to other ascomycete yeast species as revealed by small-subunit rRNA gene sequences. Lett Appl Microbiol. 1994;19(5): 308–11. 10.1111/j.1472-765X.1994.tb00462.x. Medline: [DOI] [PubMed] [Google Scholar]
- 2.Hamajika K, Nishikawa A, Shinoda T, Fukazawa Y. Deoxyribonucleic acid base composition and its homology between two forms of candida parapsilosis and lodderomyces elongisporus. J Gen Appl Microbiol. 1987;33(3):299–302. 10.2323/jgam.33.299. [DOI] [Google Scholar]
- 3.Al-Obaid K, Ahmad S, Joseph L, Khan Z. Lodderomyces elongisporus: a bloodstream pathogen of greater clinical significance. New Microbes New Infect. 2018;26:20–4. 10.1016/j.nmni.2018.07.004. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez-Ruiz M, Guinea J, Puig-Asensio M, et al. Fungemia due to rare opportunistic yeasts: data from a population-based surveillance in Spain. Med Mycol. 2017;55(2):125–36. 10.1093/mmy/myw055. Medline: [DOI] [PubMed] [Google Scholar]
- 5.Hatanaka S, Nakamura I, Fukushima S, Ohkusu K, Matsumoto T. Catheter-related bloodstream infection due to Lodderomyces elongisporus. Jpn J Infect Dis. 2016;69(6):520–2. 10.7883/yoken.JJID.2015.307. Medline: [DOI] [PubMed] [Google Scholar]
- 6.Lee HY, Kim SJ, Kim D, et al. Catheter-related bloodstream infection due to Lodderomyces elongisporus in a patient with lung cancer. Ann Lab Med. 2018;38(2):182–4. 10.3343/alm.2018.38.2.182. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taj-Aldeen SJ, AbdulWahab A, Kolecka A, et al. Uncommon opportunistic yeast bloodstream infections from Qatar. Med Mycol. 2014;52(5): 552–6. 10.1093/mmycol/myu016. Medline: [DOI] [PubMed] [Google Scholar]
- 8.Koh B, Halliday C, Chan R. Concurrent bloodstream infection with Lodderomyces elongisporus and Candida parapsilosis. Med Mycol Case Rep. 2020;28:23–5. 10.1016/j.mmcr.2020.03.007. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmad S, Khan ZU, Johny M, et al. Isolation of Lodderomyces elongisporus from the catheter tip of a fungemia patient in the Middle East. Case Rep Med. 2013;2013: 560406. 10.1155/2013/560406. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daveson KL, Woods ML. Lodderomyces elongisporus endocarditis in an intravenous drug user: a new entity in fungal endocarditis. J Med Microbiol. 2012;61(Pt 9): 1338–40. 10.1099/jmm.0.047548-0. Medline: [DOI] [PubMed] [Google Scholar]
- 11.Thompson CM, Warner N, Hurt CB, Alby K, Miller MB. Closing the brief case: a case of prosthetic valve endocarditis due to Lodderomyces elongisporus. J Clin Microbiol. 2021; 59(2). 10.1128/JCM.01227-20. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lockhart SR, Messer SA, Pfaller MA, Diekema DJ. Lodderomyces elongisporus masquerading as Candida parapsilosis as a cause of bloodstream infections. J Clin Microbiol. 2008;46(1):374–6. 10.1128/JCM.01790-07. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dogen A, Metin B, Ilkit M, de Hoog GS, Heitman J. MTL genotypes, phenotypic switching, and susceptibility profiles of Candida parapsilosis species group compared to Lodderomyces elongisporus. PLoS One. 2017;12(8):e0182653. 10.1371/journal.pone.0182653. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corti M, Solari R, De Carolis L, et al. Candida parapsilosis meningitis in a patient with AIDS. Report of a case and review of the literature. Rev Iberoam Micol. 2013;30(2):122–4. 10.1016/j.riam.2012.11.003. Medline: [DOI] [PubMed] [Google Scholar]
- 15.Chesney PJ, Justman RA, Bogdanowicz WM. Candida meningitis in newborn infants: a review and report of combined amphotericin B--flucytosine therapy. Johns Hopkins Med J. 1978;142(5):155–60. [PubMed] [Google Scholar]
- 16.Fernandez M, Moylett EH, Noyola DE, Baker CJ. Candidal meningitis in neonates: a 10-year review. Clin Infect Dis. 2000;31(2):458–63. 10.1086/313973. Medline: [DOI] [PubMed] [Google Scholar]
- 17.Bagheri F, Cervellione KL, Maruf M, Marino W, Santucci Jr T. Candida parapsilosis meningitis associated with shunt infection in an adult male. Clin Neurol Neurosurg. 2010;112(3):248–51. 10.1016/j.clineuro.2009.11.011. Medline: [DOI] [PubMed] [Google Scholar]
- 18.Bhalla GS, Malik M, Sarao MS, et al. Device-associated central nervous system infection caused by Candida parapsilosis. Cureus. 2018;10(8):e3140. 10.7759/cureus.3140. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fadel H, Moon SJ, Klinger NV, et al. Candida parapsilosis infection of ventriculoperitoneal shunt in adult: case report and literature review. World Neurosurg. 2018;119:290–3. 10.1016/j.wneu.2018.08.023. Medline: [DOI] [PubMed] [Google Scholar]
- 20.Jimenez-Mejias ME, Moreno-Maqueda I, Regordan C, Artola-Igarza JL. [External cerebrospinal fluid diversion and Candida parapsilosis meningitis. Treatment with fluconazole]. Med Clin (Barc). 1993;100(4):156. [PubMed] [Google Scholar]
- 21.Trofa D, Gacser A, Nosanchuk JD. Candida parapsilosis, an emerging fungal pathogen. Clin Microbiol Rev. 2008;21(4):606–25. 10.1128/CMR.00013-08. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(3):291–322. 10.1086/649858. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macsween KF, Bicanic T, Brouwer AE, et al. Lumbar drainage for control of raised cerebrospinal fluid pressure in cryptococcal meningitis: case report and review. J Infect. 2005;51(4):e221–4. 10.1016/j.jinf.2005.02.010. Medline: [DOI] [PubMed] [Google Scholar]
- 24.Manosuthi W, Sungkanuparph S, Chottanapund S, et al. Temporary external lumbar drainage for reducing elevated intracranial pressure in HIV-infected patients with cryptococcal meningitis. Int J STD AIDS. 2008;19(4):268–71. 10.1258/ijsa.2007.007286. Medline: [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q, Li H, Zhang K, et al. Lumbar drainage for the treatment of refractory intracranial hypertension in HIV-negative cryptococcal meningitis. Future Microbiol. 2019;14(10):859–66. 10.2217/fmb-2019-0084. Medline: [DOI] [PubMed] [Google Scholar]


