Abstract
Background and Aim:
Raw milk can be a source of food-borne disease transmission and a medium for spreading antibiotic-resistant bacteria. Staphylococcus aureus and Escherichia coli are bacteria that have the pathogenic ability to attack host cells and are capable of harboring antibiotic-resistant genes. This study estimated the prevalence and antibiotic resistance of S. aureus and E. coli isolated from raw milk in East Java, Indonesia.
Materials and Methods:
Two hundred and fifty raw milk samples were collected from five dairy farms in East Java. S. aureus and E. coli were isolated using their respective selective media, whereas antibiotic susceptibility testing was performed using the Kirby–Bauer disk diffusion method. The methicillin-resistant S. aureus (MRSA) was confirmed using the oxacillin resistance screen agar test, and extended-spectrum beta-lactamase (ESBL)-producing E. coli was determined using the double-disk synergy test. The presence of mecA and blaTEM genes were screened by the polymerase chain reaction method.
Results:
Results indicated that the prevalence of S. aureus was 138 (55.2%) and that E. coli was 176 (70.4%). Of the 138 S. aureus isolated, 27 (19.6%) were MRSA, and among the 176 E. coli isolates identified, 3 (1.7%) were ESBL producers. The mecA gene was observed in 2 (7.4%) MRSA and all 3 (100%) ESBL-producing E. coli isolated harbored blaTEM genes.
Conclusion:
The presence of MRSA and ESBL-producing E. coli in raw milk is a serious public health threat, and public awareness should be raised about the dangers posed by these pathogenic organisms.
Keywords: Escherichia coli, extended-spectrum beta-lactamase, methicillin-resistant Staphylococcus aureus, public health, raw milk, Staphylococcus aureus
Introduction
Milk is an excellent medium for bacterial growth and can be a means for spreading bacteria harmful to human health. Besides the benefits and all the nutritional values contained in it, the possibility of using milk as a medium for transmitting disease infections is quite common and often occurs in cases [1, 2]. Microorganism contamination can be found in milk if the handling does not consider hygiene aspects [3]. Efforts to fulfill the availability of milk must be accompanied by enhancing the quality and safety of dairy products because no matter how high the nutritional value of a food ingredient is, it will be useless if the food is harmful to human health [4]. Diseases transmitted from animals to humans through food are generally caused by bacterial contamination. Bacterial contamination in milk can come from poor cage management, maintenance, and unhygienic milking processes. Poor milking can cause milk to be contaminated with environmental microorganisms; thus, milk quality reduces [5]. The process of microbial contamination in milk begins when dairy cattle milk is milked; bacteria in the environment and around the udder can be carried away during the milking process if good sanitation and hygiene practices are not performed. Other contaminating milk sources include cow skins, udders, water, soil, dust, humans, and milking equipment [3].
In dairy farming in East Java, the lack of production quantity is also offset by the potential for low quality, where the feeding system, milking management, high temperature, and humidity contribute significantly to the contamination of pathogenic bacteria, such as Staphylococcus aureus and Escherichia coli [6, 7]. In line with this, Kupradit et al. [8] reported that in milking management, the teats of cows or the Milker’s hands have a significant effect on bacterial milk contamination. Such contamination can also occur with the movement through the intermediaries of workers, water, and production equipment [9, 10].
The milk-borne disease is a fundamental problem in the public health sector. It does affect not only human health but also the economic sector [11]. Cases of the food-borne disease have been found due to raw milk consumption [8], contamination with S. aureus, and E. coli bacteria that can come from raw milk. Thus, this study aimed to estimate the prevalence of S. aureus and E. coli from raw milk and the presence of crucial antimicrobial-resistant gene encodings such as the mecA gene in S. aureus and the blaTEM gene in E. coli are expected to provide a clear picture of the findings of the distribution of antimicrobial resistance (AMR) isolated from raw milk in East Java Province, Indonesia.
Materials and Methods
Ethical approval
Raw milk was used in this study; hence, ethical approval was not necessary. Raw milk samples were collected from five dairy farms in East Java Province, Indonesia.
Study period and location
The study was conducted from December 2019 to March 2020. Samples were collected from 5 dairy farms in East Java Province;, Kertajaya Farm, Argopuro Farm, Suka Makmur Farm, Harapan Jaya Farm, and Semen Farm. Samples were processed at the Laboratory of the Department of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Airlangga.
Sampling
Two hundred and fifty milk samples (25 mL each of raw milk) were obtained and 50 raw milk each from five dairy farms in East Java [12]. The samples were collected in a sterile screw-capped bottle and transported to the laboratory in an icebox within 2 h and analyzed.
Isolation and identification of S. aureus and E. coli
The isolation of S. aureus and E. coli was done through enrichment in buffered peptone water (pH 7.0) and cultured in mannitol salt agar (Merck, Germany) and eosin methylene blue media (Merck), respectively [13, 14]. Distinct colonies of S. aureus were found and verified using Gram staining, catalase, and coagulase test. Distinct colonies of E. coli were identified and verified by growth on triple sugar iron agar and lysine iron agar, fermentative glucose degradation, citrate usage, urease production, indole fermentation, tryptophan degradation, glucose degradation, and motility.
Antibiotic susceptibility testing of isolates
The isolates of S. aureus and E. coli were subjected to antibiotic susceptibility testing using the Kirby–Bauer disk diffusion technique as per the recommendation of the Clinical and Laboratory Standards Institute (CLSI) [15]. Briefly, Mueller-Hinton agar (Merck) was prepared according to the manufacturer’s instructions and allowed to cool to 45–50°C before pouring into plates. After the agar had solidified, plates were allowed to dry before use. An 18–24-h-old broth culture of S. aureus and E. coli isolates was standardized by diluting to 0.5 McFarland’s standard. A sterile swab stick was inserted into the standardized S. aureus and E. coli inoculum, drained to eliminate excess inoculum load, and inoculated by spreading on the surface of prepared Mueller-Hinton agar plates. After this, the inoculated Mueller-Hinton agar (Merck) plate was allowed to dry for a few minutes at room temperature (29°C) with the lid closed. After the agar surface has dried for a few minutes, antibiotic-impregnated disks of known concentrations (Oxoid, UK), oxacillin (30 μg), cefoxitin (30 μg), tetracycline (30 μg), erythromycin (15 μg), and gentamicin (10 μg) for S. aureus, and tetracycline (30 μg), streptomycin (10 μg), chloramphenicol (30 μg), trimethoprim (5 μg), and aztreonam (30 μg) for E. coli, were carefully applied on the inoculated Mueller-Hinton agar (Merck) plates using sterile forceps. The plates were then incubated at 37°C for 18–24 h, and the diameters of the inhibition zones were measured using a ruler to the nearest millimeter. Results were recorded and interpreted according to the CLSI [15].
Confirmation test for methicillin-resistant S. aureus (MRSA), DNA extraction, and mecA gene detection
S. aureus isolates were tested for MRSA using oxacillin resistance screen agar (ORSA) (Merck) [16]. ORSA was inoculated directly with an isolated colony of S. aureus prepared as a liquid suspension approximately equivalent to 0.5 McFarland turbidity standards. The medium was prepared according to the manufacturer’s instructions before inoculation. The inoculated plates were incubated for 18–24 h at 37°C. The colonies showing blue indicators were recorded as MRSA, and colonies with white on the agar were recorded as methicillin-susceptible S. aureus after 24 h of incubation. All the MRSA verified by the ORSA were tested using a polymerase chain reaction (PCR) to detect the presence of the mecA gene [17]. The DNA extraction process was performed according to the QIAamp DNA Mini Kit (Promega, USA) protocol (51304 and 51306) [17]. The PCR method and primers were used as described by Ramandinianto et al. [18], as shown in Table-1 [18]. Positive control was S. aureus ATCC BAA 1026, and negative control was S. aureus ATCC 25923.
Table-1.
Details of primers used in this study.
Confirmation test for extended-spectrum beta-lactamase (ESBL)-producing E. coli, DNA extraction, and blaTEM gene detection
E. coli isolates were studied for the presence of ESBL using the double-disk synergy test (DDST). The antibiotic disks used for DDST were amoxicillin-clavulanate (20/10 μg), cefotaxime (30 μg), and ceftazidime (30 μg) [19]. The ESBL-producing E. coli detected was further examined at a molecular level. Bacterial DNA was extracted using the QIAamp DNA Mini Kit (Promega) protocol according to Kristianingtyas et al. [19], and the blaTEM gene was detected using the PCR method as described by Putra et al. [20] and Ansharieta et al. [21] as indicated in Table-1. After the amplification, products were visualized by exposure of the gel to ultraviolet light and subsequently photographed and documented using agel documentation system (Promega). Positive control was E. coli ATCC 35218, and negative control was E. coli ATCC 25922.
Results
Prevalence and antibiotic resistance of S. aureus
This study indicated that of 250 milk samples taken from five dairy farms in East Java, Indonesia, 138 (55.2%) were positive for S. aureus isolates (Table-2).
Table-2.
Prevalence and antimicrobial resistance profile of S. aureus collected from raw milk in East Java.
| Location | Sample size | Confirmed S. aureus | Resistant to | ORSA test | mecA gene | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| TE | OX | FOX | E | CN | |||||
| Kertajaya Farm | 50 | 20 | 14 | 6 | 4 | 0 | 2 | 6 | 0 |
| Argopuro Farm | 50 | 30 | 25 | 10 | 8 | 5 | 0 | 8 | 0 |
| Suka Makmur Farm | 50 | 24 | 12 | 6 | 3 | 1 | 0 | 1 | 0 |
| Harapan Jaya Farm | 50 | 38 | 11 | 8 | 6 | 3 | 1 | 6 | 2 |
| Semen Farm | 50 | 26 | 8 | 8 | 1 | 6 | 0 | 6 | 0 |
| Total | 250 | 138 | 70 | 38 | 22 | 15 | 3 | 27 | 2 |
| Percentage (%) | 100 | 138/250 (55.2) | 50.7 | 27.5 | 15.9 | 10.9 | 2.2 | 27/138 (19.6) | 2/27 (7.4) |
TE=Tetracycline (30 mg), FOX=Cefoxitin (30 mg), OX=Oxacillin (30 mg), E=Erythromycin (15 mg), CN=Gentamicin
(10 mg), ORSA=Oxacillin resistance screen agar test, S. aureus=Staphylococcus aureus
The results of the antibiotic sensitivity test of S. aureus isolates in Table-2 show that different S. aureus isolates were found to be resistant to all the antibiotics tested. One hundred and thirty-eight S. aureus isolates were detected; 38 (27.5%) S. aureus isolates were oxacillin resistant, whereas 22 (15.9%) S. aureus isolates were cefoxitin resistant. In the test of tetracycline, 70 (50.7%) S. aureus isolates were resistant, 15 (10.9%) isolates of S. aureus were erythromycin resistant, and only 3 (2.2%) were gentamicin resistant. The phenotypic MRSA confirmation test was continued using the ORSA test with a blue culture indicator indicating positive confirmation results. By contrast, the white results were negative confirmation results (Figure-1). ORSA test indicated that 27 (19.6%) S. aureus isolates were positively confirmed MRSA, as shown in Table-2. Isolates verified as MRSA phenotypically using the ORSA method were further tested genotypically using the PCR method to detect the presence of the mecA gene in the isolates. Twenty-seven MRSA isolates verified by ORSA were tested using the PCR method, and two isolates (7.4% of the tested isolates) were detected to harbor the mecA gene (Figure-2).
Figure-1.
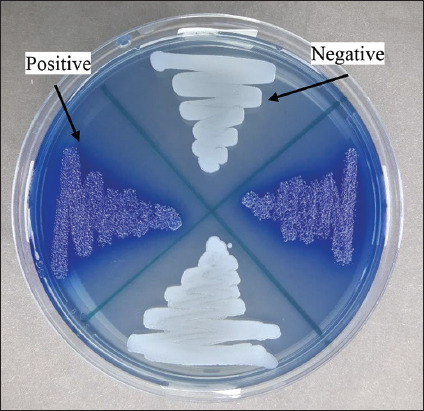
Results of oxacillin resistance screen agar test on methicillin-resistant Staphylococcus aureus (MRSA) isolates. Note: Positive results of MRSA are indicated by a blue indicator (aniline blue), while negative results are indicated by white/pale color indicators.
Figure-2.
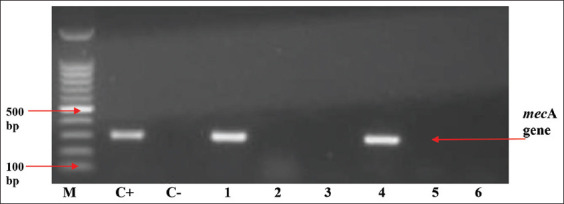
mecA gene on polymerase chain reaction results with positive bands at 310 bp from Harapan Jaya Farm. M line: 100 bp molecular weight markers, line C+: methicillin-resistant Staphylococcus aureus ATCC BAA 1026 (positive control), line C-: Staphylococcus aureus ATCC 25923 (negative control), lines 1 and 4: Positive isolate for mecA from Harapan Jaya Farm, and lines 2, 3, 5, and 6: Negative isolate for mecA gene.
Prevalence and antibiotic resistance of E. coli
Of the 250 raw milk samples collected from different dairy farms, 176 samples (70.4%) were positive for E. coli. E. coli isolates were found to exhibit resistance to antibiotics such as tetracycline 30 (17.05%), streptomycin 25 (14.2%), trimethoprim 17 (9.7%), chloramphenicol 14 (7.9%), and aztreonam 3 (1.7%) isolates. The AMR profiles of the bacterial isolates are summarized in Table-3.
Table-3.
Prevalence and antimicrobial resistance profile of E. coli collected from raw milk in East Java.
| Location | Sample size | Confirmed E. coli | Resistant to | DDST | blaTEM gene | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| TE | S | W | C | ATM | |||||
| Kertajaya Farm | 50 | 35 | 7 | 5 | 1 | 4 | 0 | 0 | 0 |
| Argopuro Farm | 50 | 36 | 3 | 2 | 3 | 2 | 0 | 0 | 0 |
| Suka Makmur Farm | 50 | 30 | 7 | 5 | 8 | 1 | 1 | 1 | 1 |
| Harapan Jaya Farm | 50 | 37 | 9 | 5 | 1 | 1 | 0 | 0 | 0 |
| Semen Farm | 50 | 38 | 4 | 8 | 4 | 6 | 2 | 2 | 2 |
| Total | 250 | 176 | 30 | 25 | 17 | 14 | 3 | 3 | 3 |
| Percentage (%) | 100 | 176/250 (70.4) | 17.0 | 14.2 | 9.7 | 7.9 | 1.7 | 3/176 (1.7) | 3/3 (100) |
TE=Tetracycline, S=Streptomycin, W=Trimethoprim, C=Chloramphenicol, ATM=Aztreonam, DDST=Double disk synergy test, E. coli=Escherichia coli
Three (1.7%) ESBL-producing E. coli isolates were found among 176 (70.4%) E. coli isolated from raw milk, and the “keyhole” effect in DDST testing is shown in Figure-3. The three isolates were tested using the PCR method to discover the encoded ESBL gene. The three positive ESBL-producing E. coli was observed to harbor the blaTEM gene (Figure-4).
Figure-3.
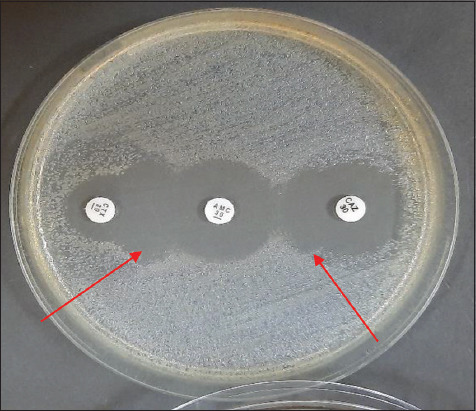
Extended-spectrum beta-lactamase-producing E. coli by double-disk synergy test (DDST)-positive result (red arrows showed positive synergy or keyhole effect). Note: Antibiotics disks used for DDST were amoxicillin-clavulanate (20/10 μg), cefotaxime (30 μg), and ceftazidime (30 μg).
Figure-4.
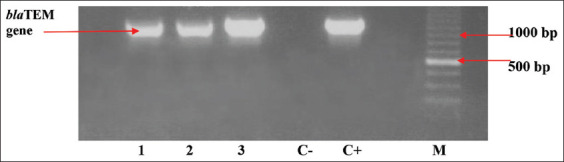
blaTEM gene on polymerase chain reaction results with positive bands at 1086 bp. Lane 1: Suka Makmur Farm, 2: Semen Farm, 3: Semen Farm, C-: Negative control (ATCC 25922), C+: Positive control for blaTEM gene (ATCC 35218), and M: Marker.
Discussion
In this study, 250 samples of raw milk were assessed; 138 (55.2%) were contaminated by S. aureus and 176 (70.4%) by E. coli isolates. The presence of bacterial contaminants in raw milk as found in this study is almost similar to a study in North India, which stated that differences could influence the differences in the number of isolates found in the study design, such as population and geographic distribution of samples, types of antibiotics used, and infection control practices [22–24]. The high level of S. aureus contamination of raw milk found conforms to the observation of Swetha et al. [25], who isolated 57.0% of staphylococci strains, of which 73.6% were S. aureus in dairy farms that have low milking hygiene.
In this study, S. aureus and E. coli recorded the highest antibiotic resistance to tetracycline (50.7% and 17.05%), respectively. Tetracyclines have the highest antibiotic resistance because they are often used in veterinary medicine, and other antibiotics used in this study such as beta-lactams such as oxacillin (27.5%) and cefoxitin (15.9%), macrolides such as erythromycin (10.9%), and aminoglycosides such as gentamicin (2.2%). The use of broad-spectrum antibiotics such as tetracyclines and beta-lactams is more common in cases of clinical mastitis in dairy cattle because of their effective treatment results. Twenty-seven (19.6%) MRSA isolates were validated using ORSA, and the highest percentage was detected in Argopuro farms, as indicated in Table-2. The presence and detection of MRSA in raw milk, as observed using the ORSA test, is in agreement with the study by Ramandinianto et al. [18] and Yunita et al. [26], where the presence of MRSA was observed by the ORSA test. It was also deduced that the blue culture indicator showed positive confirmed results, whereas white is negative confirmed results [26].
Handling unclean and unhygienic food during the production process, packaging, and distribution plays an important role in food poisoning [27]. Other researchers have stated that cow milk can transmit different pathogens, including strains of staphylococci [28]. Research on antimicrobial drug resistance of S. aureus reports that dairy product-related contamination is widespread globally. Some researchers report that bacterial outbreaks in milk and dairy products in countries are approximately 2–6% [29]. MRSA is resistant to all beta-lactam antibiotics, including cephalosporins and monobactams, an essential group of antibiotics for treating staphylococcal infections [22] and agreed with the results of this study. MRSA infection causes therapeutic problems and facilitates its spread, necessitating rapid and early diagnosis and accurate MRSA identification [30]. In this study, of S. aureus isolates, 27.5% were found to be resistant to oxacillin and 15.9% to cefoxitin in the disk diffusion method.
Presumptive MRSA can be made using oxacillin and cefoxitin. Brown and Walpole [31] stated that MRSA detection using phenotypic methods still does not indicate optimal results, and mecA genotype testing remains the major recommendation even though it cannot be applied to routine testing. To identify accurate MRSA, fast and cost-effective, a phenotypic technique with the ORSA test can be used [32]. Cefoxitin and oxacillin disk diffusion have the same sensitivity level of 100%. In specificity, cefoxitin disk diffusion was 92.59%, whereas oxacillin disk diffusion was 74.07% [22]. A study conducted by Boubaker et al. [33] indicated that the cefoxitin disk method has a better sensitivity level than the oxacillin disk technique in detecting MRSA. Therefore, the oxacillin disk technique still has a false-positive rate.
All ORSA-positive isolates were genotypically tested using PCR to detect the presence of the mecA gene, the gold standard for detecting MRSA. Two (7.4%) S. aureus isolates from the Harapan Jaya Farm were discovered to have the mecA gene. Cefoxitin is an excellent inducer to express the presence of the mecA gene because it can increase the expression of penicillin-binding protein 2a, encoded by the mecA gene [18]. The results of this study show that milk contamination by MRSA can be caused by different factors, one of which is low milking hygiene. MRSA contamination is hazardous to public health; it increases the potential for the spread of difficult-to-treat staphylococcal infections. It needs the ability to accurately, quickly, and cost-effectively identify MRSA contamination in transmission media such as food of animal origin. Genotypic detection using PCR to detect the presence of the mecA gene is the gold standard for MRSA detection; however, there are still numerous laboratories that cannot conduct molecular testing; cefoxitin diffusion can be used as a marker for MRSA detection. This is based on the cefoxitin disk diffusion test’s ability to detect the expression of the mecA gene so that it can be a solution as a more effective and efficient MRSA screening instrument in terms of cost and technical applications.
The results also indicated that the prevalence of E. coli found in milk was 70.4%. These data show the poor sanitation practices of farmers during the milking process [34]. This figure is similar to that reported by Chey et al. [35], stating that the prevalence of E. coli was highest (72.2%) in raw milk. In line with other developing countries, namely Bangladesh, as much as 75% of the milk samples studied contained E. coli [36]. Tetracyclines have the highest antibiotic resistance of 17.0% because they are commonly used in veterinary medicine, and other antibiotics used in this study, such as aminoglycosides such as streptomycin (14.2%), sulfonamides such as trimethoprim (9.6%), and macrolides such as chloramphenicol (7.9%). Broad-spectrum antibiotics such as tetracyclines and beta-lactams are more common in cases of clinical mastitis in dairy cattle in Indonesia because of their effective treatment results. The tetracycline and aminoglycoside groups are the first-choice antibiotics for respiratory and digestive tract problems. By contrast, the second choice is the macrolide and sulfonamide-trimethoprim drug combinations, which significantly affect rumen microbial activity. The last choice is the third- and fourth-generation antibiotics from cephalosporins. By contrast, the combination of sulfonamide-trimethoprim drugs significantly affects the rumen microbial activity, and the last resort is the third-generation cephalosporins [37]. Three ESBL-producing E. coli (1.7%) isolates were identified from raw milk. The discovery of ESBL Enterobacteriaceae (E. coli) originating from milk shows the presence of environmental pollution and a lack of environmental sanitation when milking is performed [38]. E. coli is a bacterium that can be a reservoir of different antibiotic resistance genes [39], including beta-lactam antibiotic resistance genes, which make E. coli capable of producing beta-lactamase enzymes [40]. ESBL enzymes are produced by many strains belonging to the Enterobacteriaceae family. These bacteria can hydrolyze penicillins and third-generation cephalosporins, monobactam, and other antibiotics, except for carbapenems [41]. These enzymes are mainly encoded by many specific genes, namely, the blaSHV, blaCTX-M, and blaTEM genes [42]. Sanitation of the cage, the bottom of the cage, and the drainage of the cage need to be considered by farmers to prevent contamination of milk by suspected ESBL-producing bacteria. The occurrence of antibiotic resistance originates from bacterial plasmids that can accommodate resistance genes and spread them to other bacteria [43]. Different resistance genes can accumulate in bacterial plasmids, usually in the R (resistance) plasmid, which is the reason for finding bacterial isolates that are resistant to different antibiotics and can create new gene sequences [44].
The prevalence of the blaTEM genes in ESBL-producing E. coli was 3 (1.7%). This finding is in line with the research conducted by Ansharieta et al. [21], who stated that E. coli contamination found in milk from dairy farms tends to encode the blaTEM gene in ESBL-producing E. coli bacteria. These results show that pathogenic E. coli originating from food of animal origin are also exposed to antibiotics and can transfer these genes to other pathogenic bacteria under certain conditions [45]. Therefore, the presence of ESBL bacteria in raw milk is quite dangerous. ESBL-producing E. coli strains obtained from raw milk samples are of particular concern because these pathogens can affect human and calf consumers and cause the spread of this antibiotic-resistant pathogen to humans and animals [46]. During lactation, ESBL-producing E. coli can also be found in raw milk with or without mastitis symptoms. This shows that the cleanliness of the cage that contaminates the milk cage is also a risk factor for ESBL-producing organisms, which can contaminate raw milk products [47, 48].
Therefore, genetic evidence encoding MRSA and ESBL-producing E. coli can be used to confirm interactions at the microbial level in humans and animals, especially between commensal and pathogenic bacteria, facultative and obligate bacteria in the same environment, and horizontal gene transfer of the bacteria making the distribution. An integrative approach such as “One Health” is needed to understand and identify the possibility of preventing the spread of MRSA and ESBL-coding genes and infection in humans [49]. The application of the concept of One Health integration is assumed to accelerate disease prevention and prediction to control these bacteria [50].
Food-borne disease is a significant concern worldwide. This is a leading problem in developing countries that lack high sanitation management during collecting and processing cow’s milk. As seen in this study, S. aureus and E. coli contamination found in raw milk can be caused by cross-contamination of milk with feces or by a lack of hygienic measures during milk collection and processing [9]. According to Ukah et al. [51], a factor causing antibiotic resistance in humans is consuming food of animal origin in raw or undercooked form. A multisectoral approach to medical treatment in veterinary medicine and animal food production can realize global cooperation in controlling the ecological development of antibiotic resistance for public health [52].
Conclusion
The presence of MRSA and ESBL-producing E. coli in raw milk is a serious public health threat, and public awareness should be raised about the dangers posed by these pathogenic organisms. Evidence by molecular identification indicated the presence of mecA and blaTEM genes in S. aureus and E. coli found in raw milk obtained from five dairy farms in East Java, Indonesia. Although the results indicated that MRSA and ESBL-producing E. coli from raw milk had a relatively low prevalence at the molecular level, MRSA and ESBL-producing E. coli in the food chain is a potential threat if not controlled since it can spread from animals to humans.
Authors’ Contributions
MHE and WT: Conceptualization and supervision of the study and drafted the manuscript. MHE, SCR, and RA: Data curation. WT and AMW: Formal analysis. AMW, DAP, and DKW: Investigation. MHE and AMW: Methodology. DAP, DKW, and AMW: Project administration. MHE, SCR, and RA: Resources. MHE, WT, and ENU: Validation. SCR, RA, and AMW: Visualization. MHE and ENU: Review and editing. All authors have read and approved the final manuscript.
Acknowledgments
This study was supported in part with the Penelitian Hibah Mandat funding from Universitas Airlangga, Indonesia, in the fiscal year 2020, with grant number 368/UN3.14/PT/2020.
Footnotes
This study was supported in part with the Penelitian Hibah Mandat funding from Universitas Airlangga, Indonesia, in the fiscal year 2020, with grant number 368/UN3.14/PT/2020.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published institutional affiliation.
References
- 1.Khairullah A.R, Sudjarwo S.A, Effendi M.H, Harijani N, Tyasningsih W, Rahmahani J, Permatasari D.A, Ramandinianto S.C, Widodo A, Riwu K.H.P. A review of methicillin-resistant Staphylococcus aureus (MRSA) on milk and milk products:Public health importance. Syst. Rev. Pharm. 2020;11(8):59–69. [Google Scholar]
- 2.Widodo A, Effendi M.H, Khairullah A.R. Extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli from livestock. Syst. Rev. Pharm. 2020;11(7):382–392. [Google Scholar]
- 3.Oliver S.P, Jayarao B.M, Almeida R.A. Foodborne pathogens in milk and the dairy farm environment:Food safety and public health implications. Foodborne Pathog. Dis. 2005;2(2):115–129. doi: 10.1089/fpd.2005.2.115. [DOI] [PubMed] [Google Scholar]
- 4.LeJeune J.T, Schultz P.J.R. Unpasteurized milk:A continued public health threat. Clin. Infect. Dis. 2009;48(1):93–100. doi: 10.1086/595007. [DOI] [PubMed] [Google Scholar]
- 5.Dhanashekar R, Akkinepalli S, Nellutla A. Milk-borne infections. An analysis of their potential effect on the milk industry. Germs. 2012;2(3):101–109. doi: 10.11599/germs.2012.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harijani N, Wandari A, Effendi M.H, Tyasningsih W. Molecular detection of encoding enterotoxin c gene and profile of antibiotic-resistant on Staphylococcus aureus isolated from several dairy farms in East Java, Indonesia. Biochem. Cell. Arch. 2020;20(1):3081–3085. [Google Scholar]
- 7.Effendi M.H, Harijani N, Yanestria S.M, Hastutiek P. Identification of Shiga toxin-producing Escherichia coli in raw milk samples from dairy cows in Surabaya, Indonesia. Philipp. J. Vet. Med. 2018;55(SI):109–114. [Google Scholar]
- 8.Kupradit C, Innok S, Woraratphoka J, Ketudat-Cairns M. Prevalence and characterization of pathogenic bacteria in Bulk Tank Raw Milk, Thailand. Walailak J. Sci. Technol. 2020;17(6):588–599. [Google Scholar]
- 9.Tanzin T, Nazir K.H.M, Zahan M.N, Parvej S.M, Zesmin K, Rahman M.T. Antibiotic resistance profile of bacteria isolated from raw milk samples of cattle and buffaloes. J. Adv. Vet. Anim. Res. 2016;3(1):62–67. [Google Scholar]
- 10.Tyasningsih W, Effendi M.H, Budiarto B, Syahputra I.R. Antibiotic resistance to Staphylococcus aureus and methicillin-resistant Staphylococcus aureus (MRSA) isolated from dairy farms in Surabaya, Indonesia. Indian Vet. J. 2019;96(11):27–31. [Google Scholar]
- 11.Ranjbar R, Dehkordi F.S, Shahreza M.H.S, Rahimi E. Prevalence, identification of virulence factors, O-serogroups and antibiotic resistance properties of Shiga-toxin producing Escherichia coli strains isolated from raw milk and traditional dairy products. Antimicrob. Resist. Infect. Control. 2018;7(1):53. doi: 10.1186/s13756-018-0345-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Regasa S, Mengistu S, Abraha A. Milk safety assessment, isolation, and antimicrobial susceptibility profile of Staphylococcus aureus in selected dairy farms of Mukaturi and Sululta Town, Oromia Region, Ethiopia. Vet. Med. Int. 2019;2019(1):3063185. doi: 10.1155/2019/3063185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Effendi M.H, Hisyam M.A.M, Hastutiek P, Tyasningsih W. Detection of coagulase gene in Staphylococcus aureus from several dairy farms in East Java, Indonesia, by polymerase chain reaction. Vet. World. 2019;12(1):68–71. doi: 10.14202/vetworld.2019.68-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wibisono F.J, Sumiarto B, Untari T, Effendi M.H, Permatasari D.A, Witaningrum A.M. CTX gene of extended-spectrum beta-lactamase (ESBL) producing Escherichia coli on broilers in Blitar, Indonesia. Syst. Rev. Pharm. 2020;11(7):396–403. [Google Scholar]
- 15.Clinical and Laboratory Standards Institute (2018) M100 Performance Standards for Antimicrobial Susceptibility Testing. 28th ed. USA: Clinical and Laboratory Standards Institute; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Decline V, Effendi M.H, Rahmaniar R.P, Yanestria S.M, Harijani N. Profile of antibiotic-resistant and presence of methicillin-resistant Staphylococcus aureus from nasal swab of dogs from several animal clinics in Surabaya, Indonesia. Int. J. One Health. 2020;6(1):90–94. [Google Scholar]
- 17.Rahmaniar R.P, Yunita M.N, Effendi M.H, Yanestria S.M. Encoding gene for methicillin-resistant Staphylococcus aureus (MRSA) isolated from nasal swab of dogs. Indian Vet. J. 2020;97(2):37–40. [Google Scholar]
- 18.Ramandinianto S.C, Khairullah A.R, Effendi M.H. MecA gene and methicillin-resistant Staphylococcus aureus (MRSA) isolated from dairy farms in East Java, Indonesia. Biodiversitas. 2020;21(8):3562–3568. [Google Scholar]
- 19.Kristianingtyas L, Effendi M.H, Tyasningsih W, Kurniawan F. Genetic identification of blaCTX-M Gene and blaTEM gene on extended-spectrum (ESBL) producing Escherichia coli from dogs. Indian Vet. J. 2020;97(1):17–21. [Google Scholar]
- 20.Putra A.R.S, Effendi M.H, Koesdarto S, Tyasningsih W. Molecular identification of extended-spectrum beta-lactamase (ESBL) producing Escherichia coli isolated from dairy cows in East Java Province, Indonesia. Indian Vet. J. 2019;96(10):26–30. [Google Scholar]
- 21.Ansharieta R, Effendi M.H, Ramandinianto S.C, Plumeriastuti H. Molecular identification of blaCTX-M and blaTEM genes encoding extended-spectrum ß-lactamase (ESBL) producing Escherichia coli isolated from raw Cow's milk in East Java, Indonesia. Biodiversitas. 2021;22(4):1600–1605. [Google Scholar]
- 22.Vyas A, Sharma M, Kumar S, Kumar M, Mehra S.K. A comparative study of oxacillin screen agar, oxacillin disc diffusion and cefoxitin disc diffusion, oxacillin E-test method for routine screening of methicillin-resistant Staphylococcus aureus. Int. J. Curr. Res. Rev. 2015;7(10):55–60. [Google Scholar]
- 23.Jamali H, Paydar M, Radmehr B, Ismail S, Dadrasnia A. Prevalence and antimicrobial resistance of Staphylococcus aureus isolated from raw milk and dairy products. Food Control. 2015;54(1):383–388. [Google Scholar]
- 24.Oberoi L, Kaur K, Aggarwal A. Prevalence and antimicrobial susceptibility pattern of methicillin-resistant Staphylococcus aureus (MRSA) in a rural tertiary care hospital in North India. Int. J. Appl. Biol. Pharm. Technol. 2012;3(1):200–205. [Google Scholar]
- 25.Swetha C.S, Supriya R.A, Goud S.S, Babu A.J, Rao T.M. A study on the prevalence of zoonotic important methicillin-resistant and vancomycin-resistant Staphylococcus aureus (MRSA and VRSA) and coagulase-negative staphylococci (MR-CNS and VR-CNS) in raw milk samples of Tirupati, Andhra Pradesh. Pharm. Innov. Int. J. 2017;6(9):17–24. [Google Scholar]
- 26.Yunita M.N, Effendi M.H, Rahmaniar R.P, Arifah S, Yanestria S.M. identification of spa gene for strain typing of methicillin-resistant Staphylococcus aureus (MRSA) isolated from nasal swab of dogs. Biochem. Cell. Arch. 2020;20(1):2999–3004. [Google Scholar]
- 27.De Buyser M.L, Dufour B, Maire M, Lafarge V. Implication of milk and milk products in food-borne diseases in France and in different industrialized countries. Int. J. Food Microbiol. 2001;67(1–2):1–17. doi: 10.1016/s0168-1605(01)00443-3. [DOI] [PubMed] [Google Scholar]
- 28.Angelillo I.F, Viggiani N.M.A, Rizzo L, Bianco A. Food handlers and foodborne diseases:Knowledge attitudes and reported behavior in Italy. J. Food Prot. 2000;63(3):381–385. doi: 10.4315/0362-028x-63.3.381. [DOI] [PubMed] [Google Scholar]
- 29.Sasidharan S, Prema B, Latha L.L.Y. Antimicrobial drug resistance of Staphylococcus aureus in dairy products. Asian Pac. J. Trop. Biomed. 2011;1(2):130–132. doi: 10.1016/S2221-1691(11)60010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamazumi T, Marshall S.A, Wilke W.W, Diekema D.J, Pfaller M.A, Jones R.N. Comparison of the Vitek gram-positive susceptibility 106 cards and MRSA screen latex agglutination test for determining oxacillin resistance in clinical bloodstream isolates of Staphylococcus aureus. J. Clin. Microbiol. 2001;39(1):53–56. doi: 10.1128/JCM.39.1.53-56.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown D.F.J, Walpole E. Evaluation of the Mastalex latex agglutination test for methicillin resistance in Staphylococcus aureus grown on different screening media. J Antimicrob Chemother. 2001;47(2):187–189. doi: 10.1093/jac/47.2.187. [DOI] [PubMed] [Google Scholar]
- 32.Krishnan P.U, Miles K, Shetty N. Detection of methicillin and mupirocin resistance in Staphylococcus aureus isolates using conventional and molecular methods:a descriptive study from a burns unit with a high prevalence of MRSA. J. Clin. Pathol. 2002;55(10):745–748. doi: 10.1136/jcp.55.10.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boubaker I.B.B, Ben Abbes R, Ben Abdallah H, Mamlouk K, Mahjoubi F, Kammoun A, Hammami A, Ben Redjeb S. Evaluation of a cefoxitin disk diffusion test for the routine detection of methicillin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 2004;10(8):762–765. doi: 10.1111/j.1469-0691.2004.00919.x. [DOI] [PubMed] [Google Scholar]
- 34.Munera-Bedoya O.D, Cassoli L.D, Machado P.F, Cerón-Muñoz M.F. Influence of attitudes and behavior of milkers on the hygienic and sanitary quality of milk. PLoS One. 2017;12(9):1–13. doi: 10.1371/journal.pone.0184640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chey F.Y, Abdullah A, Ayobb M.K. Bacteriological quality and safety of raw milk in Malaysia. Food Microbiol. 2004;21(5):535–541. [Google Scholar]
- 36.Islam M.A, Kabir S.M.L, Seel S.K. Molecular detection and characterization of Escherichia coli isolated from raw milk sold in different markets of Bangladesh. Bangladesh J. Vet. Med. 2016;14(2):271–275. [Google Scholar]
- 37.Economou V, Gousia P. Agriculture and food animals are sources of antimicrobial-resistant bacteria. Infect. Drug Resist. 2015;8(1):49–61. doi: 10.2147/IDR.S55778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okeke I.N, Laxminarayan R, Bhutta Z.A, Duse A.G, Jenkins P, O'Brien T.F, Pablos-Mendez A, Klugman K.P. Antimicrobial resistance in developing countries. Part I:Recent trends and current status. Lancet. 2015;5(8):481–493. doi: 10.1016/S1473-3099(05)70189-4. [DOI] [PubMed] [Google Scholar]
- 39.Effendi M.H, Tyasningsih W, Yurianti Y.A, Rahmahani J, Harijani N, Plumeriastuti H. Presence of multidrug resistance (MDR) and extended-spectrum beta-lactamase (ESBL) of Escherichia coli isolated from cloacal swabs of broilers in several wet markets in Surabaya, Indonesia. Biodiversitas. 2021;22(1):304–310. [Google Scholar]
- 40.Putra A.R, Effendi M.H, Koesdarto S, Suwarno S, Tyasningsih W, Estoepangestie A.T. Detection of the extended-spectrum β-lactamase produced by Escherichia coli from dairy cows by using the Vitek-2 method in Tulungagung regency, Indonesia. Iraqi J. Vet. Sci. 2020;34(1):203–207. [Google Scholar]
- 41.Effendi M.H, Bintari I.G, Aksoro E.B, Hermawan I.P. Detection of blaTEM Gene of Klebsiella pneumoniae Isolated from swab of food-producing animals in East Java. Trop. Anim. Sci. J. 2018;41(3):174–178. [Google Scholar]
- 42.Wibisono F.J, Sumiarto B, Untari T, Effendi M.H, Permatasari D.A, Witaningrum A.M. Short communication:Pattern of antibiotic resistance on extended-spectrum beta-lactamases genes producing Escherichia coli on laying hens in Blitar, Indonesia. Biodiversitas. 2020;21(10):4631–4635. [Google Scholar]
- 43.Ramírez-Castillo F.Y, Moreno-Flores A.C, Avelar-González F.J, Márquez-Díaz F, Harel J, Guerrero-Barrera A.L. An evaluation of multidrug-resistant Escherichia coli isolates in urinary tract infections from Aguascalientes, Mexico:Cross-sectional study. Ann. Clin. Microbiol. Antimicrob. 2018;17(1):1–13. doi: 10.1186/s12941-018-0286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nikaido H. Multidrug resistance in bacteria. Annu. Rev. Biochem. 2009;78(1):119–146. doi: 10.1146/annurev.biochem.78.082907.145923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rahmahani J, Salamah Mufasirin, Tyasningsih W, Effendi M.H. Antimicrobial resistance profile of Escherichia coli from a cloacal swab of domestic chicken in Surabaya traditional market. Biochem. Cell Arch. 2020;20(1):2993–2997. [Google Scholar]
- 46.Batabyal K, Banerjee A, Pal S, Dey S, Joardar S.N, Samanta I, Isore D.P, Singh A.D. Detection, characterization, and antibiogram of extended-spectrum beta-lactamase Escherichia coli isolated from bovine milk samples in West Bengal, India. Vet. World. 2018;11(10):1423–1427. doi: 10.14202/vetworld.2018.1423-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Su Y, Yu C.Y, Tsai Y, Wang S.H, Lee C, Chu C. Fluoroquinolone resistant and extended-spectrum β-lactamase (ESBL)-producing Escherichia coli from milk of cow with clinic mastitis in Southern Taiwan. J. Microbiol. Immunol. Infect. 2016;49(6):892–901. doi: 10.1016/j.jmii.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Ali T, Rahman S, Zhang L, Shahid M, Zhang S, Liu G, Gao J, Han B. ESBL producing Escherichia coli from cows suffering mastitis in China contain clinical class 1 integrons with CTX-M linked to ISCR1. Front. Microbiol. 2016;7(1):1931. doi: 10.3389/fmicb.2016.01931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calistri P, Iannetti S, Danzetta L, Narcisi M, Cito V, Di Sabatino F. The components of “one world-one health”approach. Transbound. Emerg. Dis. 2013;60(2):4–13. doi: 10.1111/tbed.12145. [DOI] [PubMed] [Google Scholar]
- 50.Wendt A, Kreienbrock L, Campe A. Zoonotic disease surveillance-inventory of systems integrating human and animal disease information. Zoonoses Public Health. 2015;62(1):61–74. doi: 10.1111/zph.12120. [DOI] [PubMed] [Google Scholar]
- 51.Ukah U.V, Glass M, Avery B, Daignault D, Mulvey M.R, Reid-Smith R.J, Parmley E.J, Portt A, Boerlin P, Manges A.R. Risk factors for acquisition of multidrug-resistant Escherichia coli and development of community-acquired urinary tract infections. Epidemiol. Infect. 2017;146(1):46–57. doi: 10.1017/S0950268817002680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Landers T.F, Cohen B, Wittum T.E, Larson E.L. A review of antibiotic use in food animals:Perspective, policy, and potential. Public Health Rep. 2012;127(1):4–22. doi: 10.1177/003335491212700103. [DOI] [PMC free article] [PubMed] [Google Scholar]


