Abstract
Background and Aim:
Ciprofloxacin (CIP) is recommended for salmonellosis treatment as the drug of choice; however, overuse of this drug can cause drug resistance issues and failure to treat diseases. Phage therapy is an alternative approach for combatting CIP-resistant infection. This study aimed to estimate the prevalence of CIP-resistant Salmonella isolated from the broiler production chain and evaluated the lytic ability of novel Salmonella phages isolated from water samples.
Materials and Methods:
Samples were obtained from the broiler production chain and used for Salmonella isolation. serovar and CIP resistance of each isolate were characterized through latex agglutination and agar disk diffusion test, respectively. Water samples from different sources were acquired for phage isolation. The lytic activity of novel-isolated phages was also examined.
Results:
In this study, 51 Salmonella isolates were recovered from the broiler production chain (two commercial farms, one free-range farm, two slaughterhouses, and three stalls from the wet market). Kentucky was the major serovar characterized (16), followed by Typhimurium (9), Agona (5), Corvalis (5), Schwarzengrund (5), Singapore (3), Weltevreden (3), Mbandaka (2), Give (2), and Albany (1). The serovars that exhibited CIP resistance were 14/16 isolates of serovar Kentucky (87.5%) and one isolate of serovar Give (50%), whereas eight other serovars were susceptible to this drug. Overall, the prevalence of CIP-resistant Salmonella recovered from the sources included in this study was 29.4%. This study identified 11 Salmonella phages isolated from wastewater samples derived from broiler farms, wastewater treatment stations, and natural reservoirs. Our phages showed the total percentage of lysis ability ranging from 33.3% to 93.3% against CIP-resistant isolates. However, only one bacterial isolate, namely 210SL, recovered from the food contact surface of a wet market stall and was resistant to all phages.
Conclusion:
Diverse serovars of Salmonella were recovered in the broiler production chain in this study, while the isolates presenting CIP-resistant Salmonella were as high as 29.4%. Overall, Salmonella phages showed high lysis ability against these CIP-resistant Salmonella isolates, suggesting the potential application of phage-based treatments or biocontrol in the broiler production chain.
Keywords: antibiotics, bacteriophage, fluoroquinolone, phage lysis, poultry
Introduction
Antibiotic resistance in food-borne bacterial pathogens is a significant concern for public health safety worldwide through the misuse of antibiotics in food and animal production systems. Antibiotic-resistant bacteria can be spread to humans through the food supply chain [1, 2]. The infection with antibiotic-resistant bacteria causes a serious illness and failure to treat diseases with regular treatment of the usual antibiotics. In the United States, food-borne illnesses caused by antibiotic-resistant bacteria affect 2.8 million cases and 35,000 deaths annually [3].
Salmonella is one of the most reported pathogenic bacteria associated with human illness. The infection can be linked to consuming contaminated food and water. Animals, particularly poultry, are the primary carriers of this bacterium [4], and their products are associated with massive outbreaks [5]. Therefore, antibiotic resistance in Salmonella is a considerable threat to public health and food safety. At present, Salmonella has been reported to be resistant to many antibiotics [1, 6]. Some Salmonella strains showed resistance to at least one drug in three or more antimicrobial categories, showing multidrug-resistant (MDR) strains. The incidence of those is continually increasing worldwide [7]. Ciprofloxacin (CIP) is a member of the fluoroquinolone drug class used to treat numerous bacterial infections, especially Salmonella infection [8]. It inhibits bacterial DNA topoisomerase and DNA-gyrase activities during DNA replication [9]. Ciprofloxacin is classified as critically vital for human medicine by the World Health Organization [10]. This drug exhibits a broad spectrum of activity against Gram-negative bacteria such as Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, and Pseudomonas aeruginosa [11]. Still, it is less effective against Gram-positive bacteria such as Staphylococcus aureus and Streptococcus pneumoniae [9, 12]. Ciprofloxacin is recommended for salmonellosis treatment as a drug of choice; however, overuse of this drug can cause drug resistance issues, failure to treat diseases, and subsequent severe clinical outcomes [13]. Ciprofloxacin resistance in Salmonella has been increasingly reported worldwide because of plasmid-mediated-quinolone resistance in bacterial plasmids or chromosomes [14].
Bacteriophages or phages are viruses that can specifically kill bacteria and are not harmful to human and animal health. Phages are widely used to treat various pathogenic and MDR bacteria with strong bactericidal effects and high specificity [15]. In addition, phages can be used as an alternative approach for combatting CIP-resistant Salmonella [16].
Therefore, this study aimed to estimate the distribution of Salmonella and CIP-resistant Salmonella in the broiler production chain and assess the lytic ability of novel Salmonella phages on CIP-resistant Salmonella recovered from this source. Data from this study will be helpful in designing phage-based therapies for controlling the widespread CIP-resistant Salmonella in the broiler production chain to resolve the harmful effects of antibiotic resistance threats on human and animal health.
Materials and Methods
Ethical approval
The study was not conducted on humans or animals and hence, it does not require ethical approval.
Study period and location
The study was conducted from October 2021 to March 2022. Samples were collected from commercial broiler farms, free-range farms, slaughterhouses, and retail markets in Hat Yai city, Songkhla province, Thailand.
Sampling location and collection of samples
This study included two commercial broiler farms, one free-range farm, two slaughterhouses, and three stalls from a wet market in southern Thailand. Two commercial farms are enclosed using an evaporative cooling system for animal cultivation (>24,000 broilers/cultivation cycle). Both farms are far from main roads, natural reservoirs, and human communities. These farms have adequate biosecurity measures for controlling straying insects and animals and highly hygienic conditions. These farms have their wastewater treatment ponds, and all dried waste was eliminated by incinerating or discarding it in a waste dump. Bedding material (rice husk) was packaged in the animal houses before being sold as manure. The free-range farm is not enclosed but far away from main roads and natural reservoirs. This farm contains about 500 birds/cultivation cycle. Two slaughterhouses are situated near main roads with high infrastructure and biosecurity measures to control straying insects and animals and are located far away from natural reservoirs. Waste was eliminated by incineration, and the wastewater was previously treated in its own small wastewater treatment ponds before being released into the environment. Three chicken meat stalls from a big wet market in Hat Yai city are not enclosed and are located near main roads and human communities. Wastewater and sewage were drained using the market drainpipe before integrating with other wastewater and sewage in the wastewater treatment canal of the city.
In commercial and free-range broiler farms, animal feed, boot cover swabs, cooling pad water, drinking water, soil, and bedding material (rice husk) were collected and acquired for Salmonella isolation. In slaughterhouses, food contact surfaces (working table, knife, cutting board, basket, and bucket), nonfood contact surfaces (wall and floor), and equipment (conveyor) surfaces were obtained through swabbing using a sterile cotton swab and kept in transported a liquid medium. For stalls at the wet market, chicken meat (about 200 g) was collected in sterile plastic bags. Food contact surfaces (stall surface, cutting board, knife, and ice bucket) and nonfood contact surfaces (wall and floor) were also obtained through swabbing, as previously described by Sripaurya et al. [17]. All samples were kept in an ice box (4°C) during delivery to the laboratory for analysis.
Isolation and confirmation of Salmonella from samples
For Salmonella isolation, obtained samples were handled according to the protocol provided by the Biomérieux company (modified ISO 6579:2002). Approximately 25 g or 25 mL of animal feed, cooling pad water, drinking water, soil, bedding material, and chicken meat were mixed with 225 mL buffered peptone water (BPW, (Biomérieux, Marcy l’Étoile, France), and one tablet of Salmonella Supplement (Biomérieux) was added to inhibit undesired microorganisms. In addition, a 90 mL BPW and Salmonella Supplement Tablet was added to the boot cover and cotton swab samples. Then, all samples were incubated at 41.5°C for 18 h. Salmonella presented in the enriched samples was taken using streaking on a SALMA® plate (Biomérieux) and incubated at 37°C for 24 h. A suspected colony (typical pink to purple) presented on the plate was further selected and restreaked on a tryptic soy agar plate to get a single colony. The stocks of each isolate were kept at −20°C in a 15% glycerol solution for further investigation. Then, single colonies were submitted for serotyping by a commercial service company (S&A Reagents Lab. Ltd., Part., Bangkok, Thailand) using a latex agglutination test.
Characterization of CIP resistance profile in Salmonella
The CIP resistance test was examined with Salmonella isolated from positive samples (51 isolates). A standard agar diffusion test was conducted following the Clinical and Laboratory Standard Institute guidelines [18]. Isolates were propagated in tryptic soy broth (TSB) (HiMedia Laboratories, India) at 37°C overnight. The cell density of each culture was adjusted using TSB to attain an optical density (OD625) between 0.08 and 0.1. The diluted culture was swabbed onto Mueller-Hinton agar (MHA; Oxoid) using a sterile cotton swab. The CIP disk (5 mg, Oxoid Ltd., PW, UK) was placed on the center surface of the MHA plate. All plates were incubated at 37°C for 18 h to observe the inhibitory zone. Results were interpreted and recorded as sensitive, intermediate, and resistant according to the standard guideline. Two independent replicates were conducted [17, 18].
Collection of water samples
Five wastewater samples from a broiler farm located in Bang Klam city, Songkhla Province, Thailand (W1), a wastewater treatment pond of the student dormitory of Prince of Songkla University (PSU) (W2 and W3), and a wastewater treatment pond at PSU hospital (W4 and W5) were obtained and used for phage isolation. One water sample from a natural reservoir (NR) in Hat Yai city, Songkhla, Thailand was also obtained. Approximately 500 mL of each sample was collected in a 1 L sterile white bottle and kept in an icebox (4°C) during delivery to the laboratory for further analysis.
Isolation, purification, and preparation of Salmonella phages
The enrichment isolation method was used for Salmonella phage isolation utilizing the mixture of three Salmonella strains in our laboratory culture collection (Salmonella Agona H2-016, Salmonella Anatum A4-525, and Salmonella Kentucky S1H28). Enrichment and isolation steps were conducted following a principal protocol from our laboratory [15]. Plaques were evaluated on the bacterial host lawn, and the plaque morphotype was measured using a Vernier caliper. Every single plaque was selected for further purification thrice with the given host using a double-layer agar method. Purified plaque in salt magnesium (SM) buffer was ten-fold diluted to manufacture the semiconfluent lysis plates. A 5 mL SM buffer was added to each plate. All plates were orbitally shaken at 50 rpm at 25 °C for 2 h and then the buffer was obtained, and a top layer of agar was scrapped into 15 mL conical tubes. The supernatant was obtained by centrifugation at 6000 rpm for 15 min at 4°C, followed by filtration through 0.20 mm syringe filters before storing at 4°C. The titer of phages was counted using the double overlay technique [15].
Determination of phage lytic ability
The lytic ability for each isolated phage was performed using a spot test on the given CIP-resistant Salmonella isolates. Briefly, 20 mL of each phage lysate was dropped on the bacterial host lawn. The lytic patterns were observed after 24 h of incubation at 37°C [19].
Results
Prevalence of Salmonella from different sources
Fifty-one Salmonella isolates were recovered from positive samples from all sources included in this study. Samples from slaughterhouse A presented the highest Salmonella prevalence (27.5%), followed by those from stall A (17.6%), commercial farm B (15.7%), and stall C (13.7%). However, samples from commercial farm A indicated a relatively low Salmonella prevalence (1.9%) (Table-1). The highest prevalence of Salmonella was associated with serovar Kentucky (31.4%), followed by Typhimurium (17.6%), Agona, Corvalis, and Schwarzengrund. Serovars including Albany, Give, Mbandaka, Singapore, and Weltevreden indicated a lower prevalence than the above serovars (Table-1).
Table-1.
Prevalence of major Salmonella serovars in the broiler production chain.
| Serovars | Sources | No. of isolates (% prevalence)a | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Commercial farm | Free- range farm | Slaughterhouse | Stall | ||||||
|
|
|
|
|||||||
| A | B | A | B | A | B | C | |||
| Agona | - | - | - | - | 1 | 2 | 1 | 1 | 5/51 (9.8) |
| Albany | - | - | - | - | - | 1 | - | - | 1/51 (1.9) |
| Corvalis | - | - | - | 1 | - | 1 | 1 | 2 | 5/51 (9.8) |
| Give | - | - | - | 1 | - | - | - | 1 | 2/51 (3.9) |
| Kentucky | - | - | 3 | 7 | 1 | 2 | 1 | 2 | 16/51 (31.4) |
| Mbandaka | - | 2 | - | - | - | - | - | - | 2/51 (3.9) |
| Schwarzengrund | - | - | - | 2 | 2 | - | - | 1 | 5/51 (9.8) |
| Singapore | - | - | - | 3 | - | - | - | - | 3/51 (5.9) |
| Typhimurium | - | 4 | - | - | - | 3 | 2 | - | 9/51 (17.6) |
| Weltevreden | 1 | 2 | - | - | - | - | - | - | 3/51 (5.9) |
| No. of isolates (% prevalence)b | 1/51 (1.9) | 8/51 (15.7) | 3/51 (5.9) | 14/51 (27.5) | 4/51 (7.8) | 9/51 (17.6) | 5/51 (9.8) | 7/51 (13.7) | |
The prevalence of each serovar found in all sources.
The prevalence of Salmonella found in each source
Prevalence of CIP-resistant Salmonella
CIP-resistant profiles were obtained from screening 51 Salmonella isolates (Table-2). Of these tested, 14/16 isolates (87.5%) of Kentucky and one isolate (50%) of Give showed to be CIP-resistant, whereas the other eight serovars were susceptible. Overall, the prevalence of CIP-resistance in Salmonella recovered from all sources included in this study was 29.4%. Overall, free-range farms, slaughterhouse A, and stall C were the predominant sources associated with the distribution of CIP-resistant Salmonella. Interestingly, no resistance was found in isolates collected from both commercial farms.
Table-2.
Prevalence of ciprofloxacin-resistant Salmonella distributed in the broiler production chain.
| Serovars | Sources | No. of resistant isolates (% prevalence)a | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Commercial farm | Free- range farm | Slaughter house | Stall | ||||||
|
|
|
|
|||||||
| A | B | A | B | A | B | C | |||
| Agona | - | - | - | - | - | - | - | - | 0/5 |
| Albany | - | - | - | - | - | - | - | - | 0/1 |
| Corvalis | - | - | - | - | - | - | - | - | 0/5 |
| Give | - | - | - | - | - | - | - | 1 | 1/2 (50.0) |
| Kentucky | - | - | 3 | 6 | 1 | 1 | 1 | 2 | 14/16 (87.5) |
| Mbandaka | - | - | - | - | - | - | - | - | 0/2 |
| Schwarzengrund | - | - | - | - | - | - | - | - | 0/5 |
| Singapore | - | - | - | - | - | - | - | - | 0/3 |
| Typhimurium | - | - | - | - | - | - | - | - | 0/9 |
| Weltevreden | - | - | - | - | - | - | - | - | 0/3 |
| No. of resistant isolates (% prevalence)b | 0/51 | 0/51 | 3/51 (5.9) | 6/51 (11.8) | 1/51 (2.0) | 1/51 (2.0) | 1/51 (2.0) | 3/51 (5.9) | 15/51 (29.4) |
The prevalence of ciprofloxacin resistance Salmonella found in all sources.
The prevalence of ciprofloxacin resistance Salmonella found in each source
Isolation and lysis profiles of Salmonella phages
Eleven Salmonella phages were recovered from different wastewater and water samples. One phage (WPX4) was isolated from wastewater collected from the broiler farm. Two phages (WPX6 and WPX13) and one phage (WPX12) were recovered from the wastewater sediment and aerated treatment pond of the Prince of Songkhla University student dormitory wastewater treatment station, respectively. Three phages (WPX5, WPX10, and WPX11) and two phages (WPX8 and WPX9) from the sediment and aerated treatment pond of the wastewater treatment station of Prince of Songkhla University hospital, respectively. In addition, two phages were isolated from water samples of a natural reservoir (Table-3).
Table-3.
Characteristics of Salmonella phages isolated in this study.
| Properties | Salmonella phages | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| WPX4 | WPX5 | WPX6 | WPX7 | WPX8 | WPX9 | WPX10 | WPX11 | WPX12 | WPX13 | WPX14 | |
| Host of isolation | A4-525 | S1H28 | A4-525 | A4-525 | H2-016 | H2-016 | H2-016 | H2-016 | H2-016 | H2-016 | H2-016 |
| Source of isolation | W1 | W4 | W2 | NR | W5 | W5 | W4 | W4 | W3 | W2 | NR |
| Plaque morphotype (mm) | 0.5 | 1.0 | 8.0 | 0.1 | 3.0 | 0.5 | 2.5 | 0.5 | 1.0 | 0.2 | 1.0 |
All selected phages showed different plaque morphotypes, ranging from a tiny 0.1 mm to a large plaque size of 8.0 mm (Table-3). Our phages exhibited a percentage lysis ability ranging from 33.3% to 93.3% in 15 CIP-resistant isolates (Figure-1). Most phages (8 of 11 phages) showed high lysis ability (93.3%), followed by two phages, including WPX4 and WPX10, which indicated a lysis ability of 87.6%. Only one phage (WPX13) exhibited the lowest lysis (33.3%). In this study, only one Salmonella isolates, namely 210SL, previously isolated from stall C, showed resistance to all phages tested.
Figure-1.
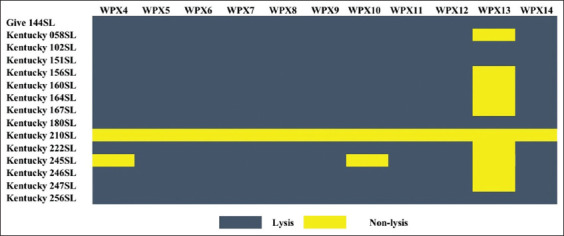
Lysis profiles of Salmonella phages on 15 ciprofloxacin-resistant Salmonella recovered from broiler production chain (blue area indicates lysis and yellow area indicates no lysis).
Discussion
Different samples from poultry farms, including bedding materials, feed, water, cloacal swab, boot cover swab, dust, and carcasses, have been reported as common sources of Salmonella [20]. In addition, poultry processing environments such as food contact surface, nonfood-contact surface, or equipment have been indicated to have Salmonella contamination [18]. Overall, Salmonella found in both the preharvest level (farm) and postharvest level (mainly slaughterhouse and processing plant) could be linked to the contamination of poultry meat products [21, 22]. Ziyate et al. [23] reported that Salmonella serovars, including Agona, Amsterdam, Enteritidis, Infantis, Kentucky, Thompson, and Typhimurium, were the significant serovars distributed in a poultry farm in Morocco, whereas S. Kentucky was frequently isolated from broiler farms located in Bangladesh [24] and Nigeria [25]. Other serovars, including Haifa, Kentucky, Saintpual, and Typhimurium, were found on broiler farms in Ethiopia [26]. In Thailand, common serovars including Albany, Agona, Corvalis, Enteritidis, Give, Kentucky, Mbandaka, Schwarzengrund, and Typhimurium were predominant serovars found in broiler farms, free-range farms, slaughterhouses, and stalls from the wet market [18, 19, 27, 28]. Similar to a previous report, S. Kentucky was the primary serovar contaminated in all sources included in our study (farm, slaughterhouse, and wet market). On a wet market, S. Agona, S. Corvalis, and S. Typhimurium were the predominant serovars found, whereas S. Mbandaka, S. Typhimurium, and S. Weltevreden were the predominant serovars detected in commercial and free-range farms. For the slaughterhouses, S. Schwarzengrund and S. Singapore were the most commonly detected.
The result of CIP resistance in Salmonella in our study is consistent with that of a previous report, which showed a lower frequency in commercial broiler farms (19%; interquartile range [IQR] 0.6%–40.1%) than other fluoroquinolone drugs (nalidixic acid, 80.3%; IQR 43.6%–97.6%) and ampicillin (64.8%, IQR 17.7%–92.1%). However, CIP resistance was highly observed in typical Salmonella serovars related to food contamination and human illnesses, including Enteritidis, Kentucky, Infantis, Typhi, Typhimurium, and Virchow [29–34]. Similarly, Xiong et al. [35] reported that up to 60.3% of Salmonella Kentucky isolated from the broiler production chain and patient specimens in China between 2010 and 2016 were identified as CIP-resistant because of the mutations in the quinolone resistance-determining regions and the presence of Salmonella genomic island 1. The wide spread of CIP resistance in S. Kentucky might be linked to the high prevalence of this serovar most commonly distributed in the broiler production chain worldwide, wherein this serovar has conferred multidrug resistance under antibiotic stresses [35–37]. To the best of our knowledge, there are no reports of CIP resistance in S. Give.
Phages are the most abundant biological entities on earth [38]. They have been commonly isolated from different types of water samples, especially wastewater and sewage [39]. Most phages also show diverse bacteriolytic patterns against many pathogenic bacteria [39–44]. Salmonella phages have also been recovered from wastewater and sewage [15, 19, 45]. The diversity of phage lytic activity depends on the source of phage origin. Some Salmonella phages exhibited a narrow host range or serovar-specific [46, 47], whereas others exhibited a diverse pattern of lytic ability [15, 19]. However, phages with a broad lytic activity are preferred to be used as a biocontrol to kill Salmonella. For example, the SEG5 phage could infect 16 of 22 (73%) Salmonella serovars tested [48]. Salmonella phages STm101 and STm118 isolated from Thai poultry farms could infect eight different Salmonella serovars commonly present in the poultry production chain [49]. In our previous study, vB_SenP_P32 phage isolated from a wastewater treatment pond exhibited the strongest ability to lyse up to 29 serovars of Salmonella (87.8%) [15]. Lytic phages induce bacterial cell lysis on endolysin action by destroying and breaking the peptidoglycan layer on the bacterial cell wall. Phages are continually reproduced after infecting the neighboring cells [38, 50]. Hence, the phage application in the food production chain is an attractive intervention because of the phage’s specificity, easy administration, and no harmful effect on human and animal health [51]. In this study, our isolated phages showed strong lytic activity against CIP-resistant Salmonella recovered from the broiler production chain included in this study. This suggests that their lytic activities are advantageous in combating CIP-resistant Salmonella strains in the broiler production chain and preventing their spread through the food supply chain. However, only one CIP-resistant isolate (SL210) was resistant to all phages tested. This might be because of the modification of phage receptors on the bacterial surface, restriction–modification systems, and/or adaptive immunity through the CRISPR-Cas system against phages [52–55].
Conclusion
Salmonella phages obtained here provide useful information for designing the phage-based therapy for combatting CIP-resistant Salmonella in the poultry production chain. These phages can also be further applied to minimize the use of antibiotics in animal farming to reduce the emergence of CIP-resistant bacteria. Overall, phages are a potential alternative for controlling the spread of CIP-resistant Salmonella in broiler and other food production chains.
Authors’ Contributions
WP: Designed the study, conducted the experiments, data analysis, discussion, and wrote the manuscript. AS, AK, and KV: Designed the study, participated in experiments, contributed to the conception, and funding. All authors have read and approved the final manuscript.
Acknowledgments
The study was funded by the Faculty of Veterinary Medicine, Kasetsart University (FFK), Thailand. The authors acknowledged the Department of Biotechnology, Faculty of Agro-Industry, Kasetsart University, Bangkok, Thailand, for providing facilities and instruments to conduct the study.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published institutional affiliation.
References
- 1.Walsh C, Fanning S. Antimicrobial resistance in foodborne pathogens-a cause for concern? Curr. Drug Targets. 2008;9(9):808–815. doi: 10.2174/138945008785747761. [DOI] [PubMed] [Google Scholar]
- 2.Nair D.V.T, Venkitanarayanan K, Kollanoor J.A. Antibiotic-resistant Salmonella in the food supply and the potential role of antibiotic alternatives for control. Foods. 2018;7(10):167. doi: 10.3390/foods7100167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2019. US Department of Health and Human Services. Atlanta, GA, USA: Centers for Disease Control and Prevention; 2019. [Google Scholar]
- 4.Obukhovska O. The natural reservoirs of Salmonella Enteritidis in populations of wild birds. Online J. Public Health Inform. 2013;5(1):e171. [Google Scholar]
- 5.Kalaba V, Golić B, Sladojević Ž, Kalaba D. Proceedings of 59th International Meat Industry Conference, IOP Conference Series:Earth and Environmental Science. Vol. 85. United Kingdom: IOP Publishing; 2017. Incidence of Salmonella Infantis in poultry meat and products and the resistance of isolates to antimicrobials; p. 012082. [Google Scholar]
- 6.Wang X, Biswas S, Paudyal N, Pan H, Li X, Fang W, Yue M. Antibiotic resistance in Salmonella typhimurium isolates recovered from the food chain through national antimicrobial resistance monitoring system between 1996 and 2016. Front. Microbiol. 2019;10:985. doi: 10.3389/fmicb.2019.00985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magiorakos A.P, Srinivasan A, Carey R.B, Carmeli Y, Falagas M.E, Giske C.G, Harbarth S, Hindler J.F, Kahlmeter G, Olsson-Liljequist B, Paterson D.L, Rice L.B, Stelling J, Struelens M.J, Vatopoulos A, Weber J.T, Monnet D.L. Multidrug-resistant, extensively drug-resistant and pan drug-resistant bacteria:An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 8.Brunner H, Zeiler H.J. Oral ciprofloxacin treatment for Salmonella typhimurium infection of normal and immunocompromised mice. Antimicrob. Agents Chemother. 1988;32(1):57–62. doi: 10.1128/aac.32.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis R, Markham A, Balfour J.A. Ciprofloxacin. Drugs. 1996;51(6):1019–1074. doi: 10.2165/00003495-199651060-00010. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Critically Important Antimicrobials for Human Medicine, 6th Revision. Geneva, Switzerland: World Health Organization; 2019. [Google Scholar]
- 11.Troughton J.A, Millar G, Smyth E.T, Doherty L, McMullan R. Ciprofloxacin use and susceptibility of Gram-negative organisms to quinolone and non-quinolone antibiotics. J. Antimicrob. Chemother. 2011;66(9):2152–2158. doi: 10.1093/jac/dkr264. [DOI] [PubMed] [Google Scholar]
- 12.Righter J. Ciprofloxacin treatment of Staphylococcus aureus infections. J. Antimicrob. Chemother. 1987;20(4):595–597. doi: 10.1093/jac/20.4.595. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Advice to Clinicians. 2019b. [Retrieved on 25-12-2021]. Available from: https://www.cdc.gov/salmonella/infantis-10-18/advice.html .
- 14.Chen K, Dong N, Chan E.W.C, Chen S. Transmission of ciprofloxacin resistance in Salmonella mediated by a novel type of conjugative helper plasmids. Emerg. Microbes Infect. 2019;8(1):857–865. doi: 10.1080/22221751.2019.1626197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pelyuntha W, Vongkamjan K. Combined effects of Salmonella phage cocktail and organic acid for controlling Salmonella enteritidis in chicken meat. Food Control. 2022;133(B):108653. [Google Scholar]
- 16.Jeon G, Ahn J. Assessment of phage-mediated inhibition of Salmonella typhimurium treated with sublethal concentrations of ceftriaxone and ciprofloxacin. FEMS Microbiol. Lett. 2020;367(19):fnaa159. doi: 10.1093/femsle/fnaa159. [DOI] [PubMed] [Google Scholar]
- 17.Sripaurya B, Ngasaman R, Benjakul S, Vongkamjan K. Virulence genes and antibiotic resistance of Salmonella recovered from a wet market in Thailand. J. Food Saf. 2019;39(2):e12601. [Google Scholar]
- 18.Clinical and Laboratory Standards Institute. CLSI Document M100-S25. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2015. Performance Standards for Antimicrobial Susceptibility Testing:25th Informational Supplement. [Google Scholar]
- 19.Pelyuntha W, Ngasaman R, Yingkajorn M, Chukiatsiri K, Benjakul S, Vongkamjan K. Isolation and characterization of potential Salmonella phages targeting multidrug-resistant and major serovars of Salmonella derived from broiler production chain in Thailand. Front. Microbiol. 2021;12:662461. doi: 10.3389/fmicb.2021.662461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mueller-Doblies D, Sayers A.R, Carrique-Mas J.J, Davies R.H. Comparison of sampling methods to detect Salmonella infection of turkey flocks. J. Appl. Microbiol. 2009;107(2):635–645. doi: 10.1111/j.1365-2672.2009.04230.x. [DOI] [PubMed] [Google Scholar]
- 21.Morris G.K, Wells J.G. Salmonella contamination in a poultry-processing plant. Appl. Microbiol. 1970;19(5):795–799. doi: 10.1128/am.19.5.795-799.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rivera-Pérez W, Barquero-Calvo E, Zamora-Sanabria R. Salmonella contamination risk points in broiler carcasses during slaughter line processing. J. Food Prot. 2014;77(12):2031–2034. doi: 10.4315/0362-028X.JFP-14-052. [DOI] [PubMed] [Google Scholar]
- 23.Ziyate N, Karraouan B, Kadiri A, Darkaoui S, Soulaymani A, Bouchrif B. Prevalence and antimicrobial resistance of Salmonella isolates in Moroccan laying hens farms. J. Appl. Poult. Res. 2016;25(4):539–546. [Google Scholar]
- 24.Barua H, Biswas P.K, Olsen K.E, Christensen J.P. Prevalence and characterization of motile Salmonella in commercial layer poultry farms in Bangladesh. PLoS One. 2012;7(4):e35914. doi: 10.1371/journal.pone.0035914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fagbamila I.O, Barco L, Mancin M, Kwaga J, Ngulukun S.S, Zavagnin P, Lettini A.A, Lorenzetto M, Abdu P.A, Kabir J, Umoh J, Ricci A, Muhammad M. Salmonella serovars and their distribution in Nigerian commercial chicken layer farms. PLoS One. 2017;12(3):e0173097. doi: 10.1371/journal.pone.0173097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eguale T. Non-typhoidal Salmonella serovars in poultry farms in central Ethiopia:Prevalence and antimicrobial resistance. BMC Vet. Res. 2018;14:217. doi: 10.1186/s12917-018-1539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lampang K.N, Chailangkarn S, Padungtod P. Prevalence and antimicrobial resistance of Salmonella serovars in chicken farm, Chiang Mai and Lamphun province, Northern of Thailand. Chiang Mai Vet. J. 2014;12(2):85–93. [Google Scholar]
- 28.Phongaran D, Khang-Air S, Angkititrakul S. Molecular epidemiology and antimicrobial resistance of Salmonella isolates from broilers and pigs in Thailand. Vet. World. 2019;12(8):1311–1318. doi: 10.14202/vetworld.2019.1311-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singer R.S, Hofacre C.L. Potential impacts of antibiotic use in poultry production. Avian Dis. 2006;50(2):161–172. doi: 10.1637/7569-033106R.1. [DOI] [PubMed] [Google Scholar]
- 30.Castro-Vargas R.E, Herrera-Sánchez M.P, Rodríguez-Hernández R, Rondón-Barragán I.S. Antibiotic resistance in Salmonella spp. isolated from poultry:A global overview. Vet. World. 2020;13(10):2070–2084. doi: 10.14202/vetworld.2020.2070-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piddock L.J, Griggs D.J, Hall M.C, Jin Y.F. Ciprofloxacin resistance in clinical isolates of Salmonella typhimurium obtained from two patients. Antimicrob. Agents Chemother. 1993;37(4):662–666. doi: 10.1128/aac.37.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Threlfall E.J, Ward L.R, Rowe B. Resistance to ciprofloxacin in non-typhoidal salmonellas from humans in England and Wales-the current situation. Clin Microbiol. Infect. 1999;5(3):130–134. doi: 10.1111/j.1469-0691.1999.tb00525.x. [DOI] [PubMed] [Google Scholar]
- 33.Raveendran R, Wattal C, Sharma A, Oberoi J.K, Prasad K.J, Datta S. High level ciprofloxacin resistance in Salmonella enterica isolated from blood. Indian J. Med. Microbiol. 2008;26(1):50–53. doi: 10.4103/0255-0857.38858. [DOI] [PubMed] [Google Scholar]
- 34.Lin D, Chen K, Chan E.W.C, Chen S. Increasing prevalence of ciprofloxacin-resistant food-borne Salmonella strains harboring multiple PMQR elements but not target gene mutations. Sci. Rep. 2015;5(1):1–8. doi: 10.1038/srep14754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiong Z, Wang S, Huang Y, Gao Y, Shen H, Chen Z, Bai J, Zhan Z, Wen J, Liao M, Zhang J. Ciprofloxacin-resistant Salmonella enterica serovar Kentucky ST198 in broiler chicken supply chain and patients, China, 2010–2016. Microorganisms. 2020;8(1):140. doi: 10.3390/microorganisms8010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haley B.J, Kim S.W, Haendiges J, Keller E, Torpey D, Kim A, Crocker K, Myers R.A, Van Kessel J.A.S. Salmonella enterica serovar Kentucky recovered from human clinical cases in Maryland, USA (2011–2015) Zoonoses Public Health. 2019;66(4):382–392. doi: 10.1111/zph.12571. [DOI] [PubMed] [Google Scholar]
- 37.Tay M.Y, Pathirage S, Chandrasekaran L, Wickramasuriya U, Sadeepanie N, Waidyarathna K.D.K, Liyanage L.D.C, Seow K.L.G, Hendriksen R.S, Takeuchi M.T, Schlundt J. Whole-genome sequencing analysis of nontyphoidal Salmonella enterica of chicken meat and human origin under surveillance in Sri Lanka. Foodborne Pathog. Dis. 2019;16(7):531–537. doi: 10.1089/fpd.2018.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clokie M.R, Millard A.D, Letarov A.V, Heaphy S. Phages in nature. Bacteriophage. 2011;1(1):31–45. doi: 10.4161/bact.1.1.14942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Runa V, Wenk J, Bengtsson S, Jones B.V, Lanham A.B. Bacteriophages in biological wastewater treatment systems:Occurrence, characterization, and function. Front. Microbiol. 2021;12:730071. doi: 10.3389/fmicb.2021.730071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khairnar K, Pal P, Chandekar R.H, Paunikar W.N. Isolation and characterization of bacteriophages infecting nocardioforms in wastewater treatment plant. Biotechnol. Res. Int. 2014;2014:151952. doi: 10.1155/2014/151952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jurczak-Kurek A, Gąsior T, Nejman-Faleńczyk B, Bloch S, Dydecka A, Topka G, Necel A, Jakubowska-Deredas M, Narajczyk M, Richert M, Mieszkowska A, Wrǫbel B, W?grzyn G, Węgrzyn A. Biodiversity of bacteriophages:Morphological and biological properties of a large group of phages isolated from urban sewage. Sci. Rep. 2016;6(1):1–17. doi: 10.1038/srep34338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klai N, Sellamuthu B. Bacteriophages isolated from hospital wastewater and its role in controlling drug-resistant pathogens. In: Tyagi R.D, Tiwari B, Drogui P, Pandey A, editors. Current Developments in Biotechnology and Bioengineering:Environmental and Health Impact of Hospital Wastewater. Amsterdam: Elsevier; 2020. pp. 327–376. [Google Scholar]
- 43.Menon N.D, Kumar M.S, Satheesh Babu T.G, Bose S, Vijayakumar G, Baswe M, Chatterjee M, D'Silva J.R, Shetty K, Haripriyan J, Kumar A, Nair S, Somanath P, Nair B.G, Nizet V, Kumar G.B. A novel N4-like bacteriophage isolated from a wastewater source in south India with activity against several multidrug-resistant clinical Pseudomonas aeruginosa isolates. mSphere. 2021;6(1):e01215–e01220. doi: 10.1128/mSphere.01215-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aghaee B.L, Mirzaei M.K, Alikhani M.Y, Mojtahedi A. Sewage and sewage-contaminated environments are the most prominent sources to isolate phages against Pseudomonas aeruginosa. BMC Microbiol. 2021;21(1):1–8. doi: 10.1186/s12866-021-02197-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petsong K, Benjakul S, Chaturongakul S, Switt A.I.M, Vongkamjan K. Lysis profiles of Salmonella phages on Salmonella isolates from various sources and efficiency of a phage cocktail against S. enteritidis and S. Typhimurium. Microorganisms. 2019;7(4):100. doi: 10.3390/microorganisms7040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bao H, Zhang H, Wang R. Isolation and characterization of bacteriophages of Salmonella enterica serovar Pullorum. Poult. Sci. 2011;90(10):2370–2377. doi: 10.3382/ps.2011-01496. [DOI] [PubMed] [Google Scholar]
- 47.Kropinski A.M, Kovalyova I.V, Billington S.J, Patrick A.N, Butts B.D, Guichard J. A, Pitcher T.J, Guthrie C.C, Sydlaske A.D, Barnhill L.M, Havens K.A, Day K.R, Falk D.R, McConnell M.R. The genome of e15, a serotype-converting, Group E1 Salmonella enterica-specific bacteriophage. Virology. 2007;369(2):234–244. doi: 10.1016/j.virol.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duc H.M, Son H.M, Honjoh K.I, Miyamoto T. Isolation and application of bacteriophages to reduce Salmonella contamination in raw chicken meat. LWT. 2018;91:353–360. [Google Scholar]
- 49.Phothaworn P, Dunne M, Supokaivanich R, Ong C, Lim J, Taharnklaew R, Vesaratchavest M, Khumthong R, Pringsulaka O, Ajawatanawong P, Klumpp J, Brown N, Imam M, Clokie M.R.J, Galyov E.E, Korbsrisate S. Characterization of flagellotropic, Chi-Like Salmonella phages isolated from Thai poultry farms. Viruses. 2019;11(6):520. doi: 10.3390/v11060520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo Z, Lin H, Ji X, Yan G, Lei L, Han W, Gu J, Huang J. Therapeutic applications of lytic phages in human medicine. Microb. Pathog. 2020;142:104048. doi: 10.1016/j.micpath.2020.104048. [DOI] [PubMed] [Google Scholar]
- 51.Wang C, Nie T, Lin F, Connerton I.F, Lu Z, Zhou S, Hang H. Resistance mechanisms adopted by a Salmonella typhimurium mutant against bacteriophage. Virus Res. 2019;273:197759. doi: 10.1016/j.virusres.2019.197759. [DOI] [PubMed] [Google Scholar]
- 52.Labrie S.J, Samson J.E, Moineau S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 2010;8(5):317–327. doi: 10.1038/nrmicro2315. [DOI] [PubMed] [Google Scholar]
- 53.Koskella B, Brockhurst M.A. Bacteria-phage coevolution as a driver of ecological and evolutionary processes in microbial communities. FEMS Microbiol. Rev. 2014;38(5):916–931. doi: 10.1111/1574-6976.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bertozzi S.J, Storms Z, Sauvageau D. Host receptors for bacteriophage adsorption. FEMS Microbiol. Lett. 2016;363(4):fnw002. doi: 10.1093/femsle/fnw002. [DOI] [PubMed] [Google Scholar]
- 55.Oechslin F. Resistance development to bacteriophages occurring during bacteriophage therapy. Viruses. 2018;10(7):351. doi: 10.3390/v10070351. [DOI] [PMC free article] [PubMed] [Google Scholar]


