Abstract
Astragali Radix (AR) is a clinically used herbal medicine with multiple immunomodulatory activities that can strengthen the activity and cytotoxicity of natural killer (NK) cells. However, owing to the complexity of its composition, the specific active ingredients in AR that act on NK cells are not clear yet. Cell membrane chromatography (CMC) is mainly used to screen the active ingredients in a complex system of herbal medicines. In this study, a new comprehensive two-dimensional (2D) NK-92MI CMC/C18 column/time-of-flight mass spectrometry (TOFMS) system was established to screen for potential NK cell activators. To obtain a higher column efficiency, 3-mercaptopropyltrimethoxysilane-modified silica was synthesized to prepare the NK-92MI CMC column. In total, nine components in AR were screened from this system, which could be washed out from the NK-92MI/CMC column after 10 min, and they showed good affinity for NK-92MI/CMC column. Two representative active compounds of AR, isoastragaloside I and astragaloside IV, promoted the killing effect of NK cells on K562 cells in a dose-dependent manner. It can thus suggest that isoastragaloside I and astragaloside IV are the main immunomodulatory components of AR. This comprehensive 2D NK-92MI CMC analytical system is a practical method for screening immune cell activators from other herbal medicines with immunomodulatory effects.
Keywords: NK cell activators, Cell membrane chromatography, Immunomodulatory herbal medicine, Covalent bonding
Graphical abstract
Highlights
-
•
A comprehensive 2D NK-92MI/CMC system was developed to screen for immune cell activators.
-
•
Nine components of Astragali Radix were screened as potential immune activators.
-
•
Isoastragaloside I and astragaloside IV were first confirmed to have immunomodulatory effects.
1. Introduction
Immunotherapies have become a significant strategy for tumor treatment in clinical practice [1]. Natural killer (NK) cells, which are important innate immune cells [2], have great development prospects in modern immunotherapy [3]. A variety of inhibitory and activating receptors can be expressed by NK cells, and the balance between these two receptors regulates the activation of NK cells [2]. For example, killer inhibitory receptors, including NK group 2 family of receptor A, among others, are NK cell inhibitory receptors called “checkpoint” receptors, which can inhibit NK cells from properly performing antitumor functions in the tumor microenvironment. Hence, activating the natural immune function of NK cells in the tumor microenvironment is emerging as a promising direction for tumor immunotherapy [4].
Astragali Radix (AR) is a Chinese herbal medicine that has multiple pharmacological effects, such as anti-cancer, anti-viral, and immunomodulatory ones [5]. Among them, studies on the immunoregulatory effect of AR have developed rapidly and have made much progress [6]. Research has confirmed that AR regulates NK functions [7]. For example, Han et al. [8] found that the total flavonoids from the seeds of AR could activate NK-92 cells by promoting the expressions of the NK group 2 family of receptor D and natural cytotoxicity triggering receptor 2. Another study suggested that the expression of killer cell lectin-like receptor K1 could be upregulated by AR to improve the killing effect of NK cells [5]. However, owing to the complexity of the composition and target uncertainty of AR, it is a great challenge to explore the interaction between immunoregulatory substances of AR and NK cells [5].
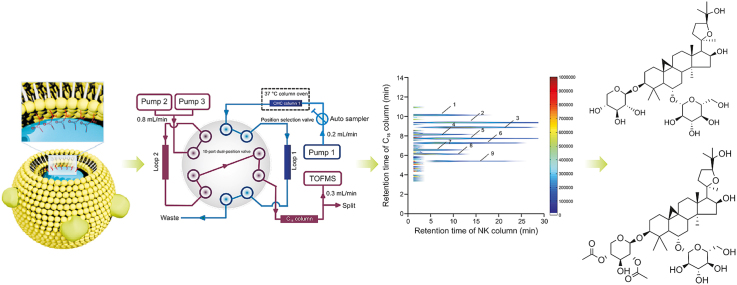
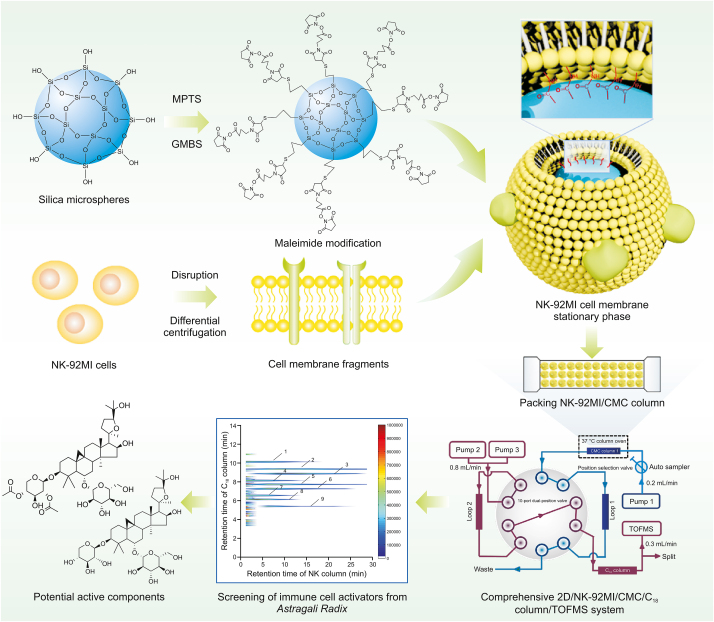
Cell membrane chromatography (CMC) is a biochromatographic analysis method based on the selective interactions between membrane receptors and ligands [9,10]. It combines activity characterization with chromatographic separation and is mainly used for the high-throughput screening of active substances that target membrane receptors. In recent years, other affinity analysis technologies based on CMC have been developed [11,12]. After discovery and practice, a comprehensive two-dimensional (2D) CMC system was built and applied to screen active substances from different herbal medicines [13,14]. Similarly, this method can also simulate the cell membrane state of NK cells in vitro to screen active compounds in herbal medicines. Furthermore, in a previous study, 3-mercaptopropyltrimethoxysilane (MPTS)-modified silica gel was used to prepare CMC columns, which could improve the lifespan and stability of CMC columns. Fortunately, this method solves the problems of the difficult acquisition and slow proliferation of NK cells [15]. In this study, a comprehensive 2D NK-92MI CMC/C18 column/time-of-flight mass spectrometry (TOFMS) system was developed to screen for potential NK cell activators from AR for the first time. This analysis model can help us focus on the active components in AR, which have the potential to enhance NK cell cytotoxicity by targeting NK cell membrane receptors. A general flowchart of the experiments is shown in Fig. 1. To further support the results of the screening, the effects of the active substances on NK cells were confirmed by detecting the cytotoxicity of NK cells. The establishment of this analysis system provides us with a new idea for the rapid screening of immune cell activators from complex components.
Fig. 1.
General flow chart of experiments. MPTS: 3-mercaptopropyltrimethoxysilane; GMBS: N-γ-maleimidobutyryl-oxysuccinimide ester; NK: natural killer; CMC: cell membrane chromatography; TOFMS: time-of-flight mass spectrometry; 2D: two-dimensional.
2. Experimental
2.1. Materials and reagents
AR was obtained from Shanghai Guoda Drugstore Co., Ltd. (Shanghai, China). Isoastragaloside I and astragaloside IV were obtained from Shanghai Yuanye Biotech Co., Ltd. (Shanghai, China). Istradefylline was purchased from MedChemExpress Co., Ltd. (Princeton, NJ, USA). Ebselen was purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). The NK-92MI and K562 cell lines were purchased from the Cell Bank of the Shanghai Branch of the Chinese Academy of Sciences (Shanghai, China). Minimum essential medium α (α-MEM), fetal bovine serum (FBS), and horse serum were purchased from Gibco Life Technology Co., Ltd. (Logan, UT, USA). 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) was purchased from BioGems International, Inc. (Rocky Hill, NJ, USA). 7-aminoactinomycin D (7-AAD) was purchased from Abcam Co., Ltd. (Cambridge, UK). The lactate dehydrogenase (LDH) assay kit was purchased from Dojindo Chemical Technology Co., Ltd. (Shanghai, China). Phosphate buffered saline (PBS) was purchased from Corning Inc. (Corning, NY, USA). Silica gel (5 μm, 200 Å) was purchased from Meigao Chemical Co., Ltd. (Qingdao, China). High performance liquid chromatography (HPLC)-grade acetonitrile and mass spectrometry (MS)-grade ammonia acetate were obtained from Sigma-Aldrich Co., Ltd. (St. Louis, MO, USA).
2.2. Preparation of samples and standard solutions
First, AR (50 g) was broken in a grinder and immersed in 50% ethanol (0.5 L). The immersed samples were heated and refluxed for 1.5 h. The filtrate was recycled, and the filter residue was treated again as described previously herein. Finally, the filtrates were mixed well and condensed to a final concentration of 1.0 g/mL. Istradefylline and ebselen (1 mM each) were dissolved in dimethyl sulfoxide (DMSO). Standard compounds were also dissolved in DMSO.
2.3. Cell culture
NK-92MI cells were cultured in α-MEM supplemented with 12.5% (V/V) FBS and 12.5% (V/V) horse serum. K562 cells were cultured in Roswell Park Memorial Institute-1640 medium supplemented with 10% (V/V) FBS, 100 U/mL benzylpenicillin, and 100 g/mL streptomycin. NK-92MI and K562 cells were grown at 37 °C with 5% CO2.
2.4. Preparation of the NK-92MI/CMC column
According to previous studies [16,17], three key factors in the preparation of CMC columns, namely, cell load, cell disruption, and the column loading process, have been explored and optimized systematically. Simultaneously, MPTS-modified silica gels were used to prepare NK-92MI/CMC columns to improve the life span of the CMC column and reduce the cell dosage [15]. In this experiment, NK-92MI/CMC columns were prepared using a traditional approach.
First, 2 × 107 NK-92MI cells were harvested and disrupted at 400 W for seven cycles (lasting for 2 s with an interval of 20 s). The suspension was centrifuged at 1,000 g for 10 min, and the supernatant was collected and centrifuged at 12,000 g for 20 min to obtain the NK-92MI cell membrane. Subsequently, the NK-92MI cell membrane stationary phase (CMSP) was prepared via covalent binding between the cell membrane suspension and 0.04 g of MPTS-modified silica gels (pre-dried and activated). This experiment was performed under a vacuum at 4 °C. The samples were then mixed in a rotating manner overnight. Finally, the NK-92MI/CMSP was washed and resuspended in PBS. The NK-92MI/CMSP suspension was packed into a column (10 mm × 2 mm i.d., 5 μm; Agilent Technologies, Santa Clara, CA, USA) using a wet packing procedure, which was improved by our group [16,17].
2.5. Comprehensive 2D NK-92MI/CMC system analysis
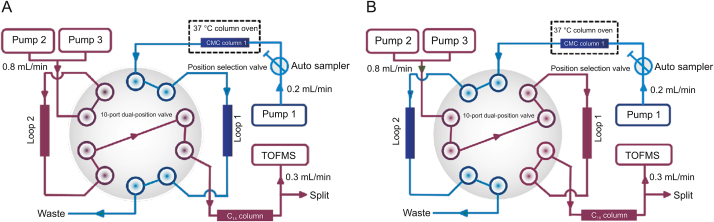
Fig. 2 shows that the NK-92MI column/C18 column/TOFMS system was mainly composed of an Agilent 1,200 series HPLC system and an Agilent 6,220 TOF mass spectrometer. The equipment was controlled using an Agilent MassHunter workstation. The first-dimensional column was an NK-92MI/CMC column. The mobile phase was ammonia acetate (10 mM) and the flow was 0.2 mL/min. The second-dimensional column was an XBridge TM C18 column (100 mm × 3.0 mm i.d., 3.5 μm; Waters, Wexford, Ireland). Using a linear elution procedure, a mixture of 0.1% (V/V) formic acid and acetonitrile was used as the mobile phase [15]. The flow velocity was set to 0.8 mL/min. More detailed information about the comprehensive 2D system is available in previous reports [18,19].
Fig. 2.
Brief scheme of the comprehensive two-dimensional natural killer (NK)-92MI/cell membrane chromatography (CMC)/CMC column/C18 column/time-of-flight mass spectrometry (TOFMS) system: (A) position 1 and (B) position 2.
2.6. Detection of NK cell toxicity by flow cytometry
Each sample was assayed in triplicate. K562 cells with a good growth status were harvested and washed twice. The cleaned K562 cells were resuspended to 5 × 106 cells/mL in PBS, and CFSE was added at a final concentration of 2 μM. The sample was incubated for 20 min and mixed well once. Staining was terminated by adding cold complete medium (pre-placed in ice water). The stained K562 cell suspension was washed once with complete medium and resuspended at a density of 4 × 105 cells/mL.
NK-92MI cells with good growth status were harvested. The NK-92MI cells were washed with the corresponding medium after cell counting. After 1 × 106 NK-92MI cells and 2 × 105 K 562 cells were added to the flow tube, a specified concentration of the candidate drug was added to a final volume of 1 mL. The samples were then incubated for 5 h. The cells were then collected, washed twice, resuspended in 100 μL of PBS with 1 μL of 7-AAD fluorescent dye, and incubated at room temperature for 15 min in the dark. After incubation, the samples were washed and resuspended in PBS. The suspension was analyzed using flow cytometry.
2.7. LDH release cytotoxic assay
NK-92MI cells were added to 96-well microplates at a density of 5 × 105 cells/well and incubated with the candidate drugs according to the concentration gradient. The prepared NK-92MI and K562 cells were mixed at a ratio of 5:1. The samples were incubated for 5 h, and the absorbance was recorded using a microplate reader at 490 nm. According to directions of LDH assay kit [20], the cytotoxicity was calculated using the following formula:
| Cytotoxicity (%) = [(Asample – Alow control)/(Ahigh control – Alow control)] × 100 |
where Asample represents the absorbance of sample (sample well−sample blank well), Ahigh control represents the absorbance of high control (high control wells−high control blank wells), and Alow control represents the absorbance of low control (low control well−background blank well).
2.8. Statistical analysis
The following software was used to analyze the obtained data: Microsoft Excel 2019 and GraphPad Prism 6.0. Statistical analysis of the results was performed using the t-test. Statistical significance was set at P < 0.05.
3. Results and discussion
3.1. Suitability of the comprehensive 2D CMC/TOFMS system
First, scanning electron microscopy was selected as the main method to characterize the surface modification of thee silica gel. Detailed information is shown in Fig. S1. The cell membrane was successfully bound to the surface of the MPTS-modified silica gel.
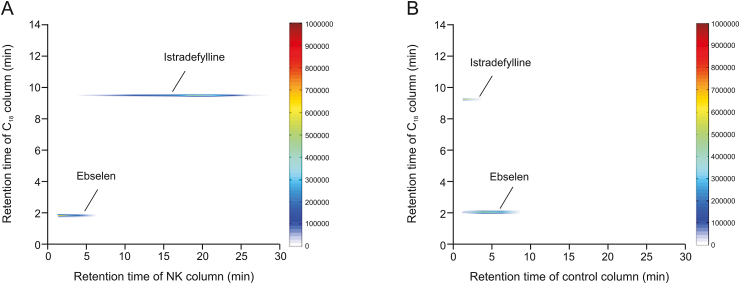
A positive drug and a negative drug were selected for the experiments to confirm the selectivity and reproducibility of the comprehensive 2D CMC screening system. A2A adenosine receptor (A2AR), which is a checkpoint molecule on NK cells, can bind adenosine to restrain the maturation of NK cells and impair anti-tumor effector functions [21,22]. Istradefylline was selected as the positive control because it is a very potent and selective A2AR antagonist [23]. Ebselen, an inhibitor of 3C-like proteinase, was selected as a negative control [24]. Istradefylline and ebselen (1 mM each) were mixed as standard solutions for the experiment. As shown in Fig. 3A, istradefylline was washed out from the NK-92MI/CMC column after 5 min, and the peak time was approximately 20 min, indicating high affinity for the NK-92MI/CMC column. However, as shown in Fig. 3B, in terms of retention time, istradefylline was eluted before 5 min in the control/CMC column. The control/CMC column was packed with only MPTS-modified silica gels. There was almost no affinity for the control/CMC columns. This indicated that the NK-92MI cell membrane with active proteins was successfully bound to MPTS-modified silica, such that the prepared NK-92MI/CMC columns were able to screen potential active compounds from AR.
Fig. 3.
Typical two-dimensional (2D) plots of istradefylline and ebselen mixed standards obtained by the comprehensive 2D (A) natural killer (NK)-92MI/cell membrane chromatography (CMC) column, and (B) control/CMC column.
In contrast, ebselen had almost no affinity for the NK-92MI or control/CMC columns, which were deemed negative results. These results indicate that the established screening system is suitable for identifying active components bound to membrane receptors. To test the reproducibility of this system, the retention time of istradefylline was selected as a marker. Three NK-92MI/CMC columns were prepared separately, and the retention time of istradefylline was electronically archived. The relative standard deviations should be less than 10%.
3.2. Practical applications
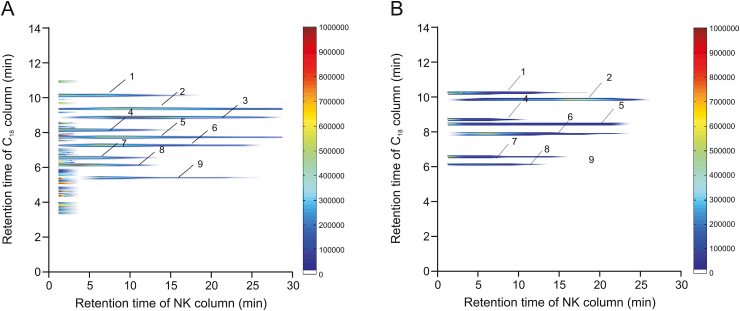
A comprehensive 2D NK-92MI/CMC system was used to screen potential active substances from the AR extraction. According to the C18 column TOFMS analysis, the results of AR compound identification are shown in Table 1. In this table, k is retention factor, k = (tR − t0)/t0, where tR is the retention time of retention component and t0 is the dead time determined by the non-retained compound [19]. The 2D contour spectra of the AR drawn from NK-92MI/CMC are shown in Fig. 4A. In total, nine compounds showed retention behavior in the NK-92MI/CMC models by matching with the compound database of AR. Next, standard solutions of the seven compounds were used to confirm their retention behavior. As shown in Fig. 4B, the seven compounds were retained on the NK-92MI/CMC column, indicating their affinity for NK-92MI cell membrane receptors.
Table 1.
Nine potentially active components in Astragali Radix characterized by the comprehensive 2D natural killer (NK)-92MI/cell membrane chromatography (CMC) analysis system.
| No. | Identification | Retention time (min)/k | [M+H]+ (m/z) |
Molecular ions (m/z) | Formula | ||
|---|---|---|---|---|---|---|---|
| Exact | Accurate | Error (ppm) | |||||
| 1 | Isomucronulatol | 0.5–17.5/10.2 | 302.1162 | 302.1154 | 2.66 | 303.1227, 320.1492 | C17H18O5 |
| 2 | Isoastragaloside I | 0.5–27.5/16.8 | 868.4819 | 868.4820 | −0.11 | 869.4893, 870.4927, 886.5159, 887.5192 | C45H72O16 |
| 3 | Astragaloside VIII | 2.5–27.5/15.4 | 912.5070 | 912.5083 | −1.38 | 913.5155, 914.5189, 930.5421, 931.5454 | C47H76O17 |
| 4 | Calycosin | 0.5–15.0/8.5 | 284.0705 | 284.0685 | 7.09 | 285.0757, 302.1023 | C16H12O5 |
| 5 | Isoastragaloside II | 0.5–27.5/16.8 | 826.4715 | 826.4715 | 0.07 | 827.4787, 828.4822, 844.5053, 845.5087 | C43H70O15 |
| 6 | Astragaloside IV, astragaloside A, and 3-O-β-d-xylopyranosyl-25-O-β-d-glucopyranosylcycloastragenol |
0.5–25.0/15.1 | 784.4608 | 784.4609 | −0.17 | 785.4682, 786.4716, 802.4947, 803.4981 |
C41H68O14 |
| 7 | 7,2′-dihydroxy-3′,4′-dimethoxyisoflavane-7-O-glucoside | 0.5–12.5/6.9 | 464.1693 | 464.1682 | 2.36 | 465.1755, 482.2021 | C23H28O10 |
| 8 | Methylnissolin-3-O-glucoside | 0.5–12.5/6.9 | 462.1538 | 462.1526 | 2.52 | 463.1599, 480.1864 | C23H26O10 |
| 9 | Astragaloside V | 5.0−25.0/12.2 | 946.5127 | 946.5137 | −1.07 | 947.5210, 948.5244, 964.5476, 965.5509 | C47H78O19 |
k: retention factor.
Fig. 4.
(A) Comprehensive two-dimensional (2D) plots of Astragali Radix extracts. (B) 2D plots of standard solutions obtained by the comprehensive 2D natural killer (NK)-92MI/cell membrane chromatography (CMC) column/C18 column/time-of-flight mass spectrometry (TOFMS) system.
Among them, two highly abundant compounds, isoastragaloside I and astragaloside IV, showed the significant retention on the NK-92MI/CMC column. They are typical cycloartane triterpene glycoside structures with the same parent nucleus as cycloastragalus alcohol (tetracyclic triterpenoids) and several glycoside groups [25]. It has been reported that tetracyclic triterpenoids have pharmacological activity by enhancing nonspecific immune responses and reversing the immunosuppressive effect of chemotherapeutic drugs to achieve antitumor effects [26]. Astragaloside IV, a representative component of cycloastragalus alcohol saponins in AR, has dual activities as astragalus polysaccharides and terpenes [27,28]. Based on the results, isoastragaloside I and astragaloside IV are worthy of further exploration into the effect of these two drugs on NK cell function. Thus, isoastragaloside I and astragaloside IV were selected to carry out in-depth research on the effects of NK cell killing activity, further verifying the accuracy of our screening system.
In this study, the chemical modification strategy of the MPTS-modified stationary phase was successfully applied to screen membrane protein affinity components of NK-92MI cells for the first time. Compared to that with the traditional CMSP, which is mainly prepared using non-modified or 3-aminopropyltriethoxysilane-modified silica gel [16,18], the use of MPTS-modified silica gel could provide mercapto-maleimide with higher reactivity by reacting with amino groups, bonding with more cell membranes, and obtaining higher column efficiency under mild and fast reaction conditions [15]. However, there are some limitations to this screening system. This system directly uses the extracted cell membrane as the stationary phase material. There are many types of membrane proteins on the cell membrane; therefore, it is impossible to screen the active components of a specific membrane receptor, and the specificity of the screening system is limited.
3.3. Effects of the potential selective substances on NK-92MI cell toxicity
To further study the influence of isoastragaloside I and astragaloside IV on the cytotoxicity of NK-92MI cells, flow cytometry and LDH assays were selected as the main methods. Each drug was administered as a series of gradients. The cytotoxicity of NK-92MI cells was evaluated by measuring the percentage of NK-92MI cell killing using K562 cells at an effector-to-target ratio of 5:1.
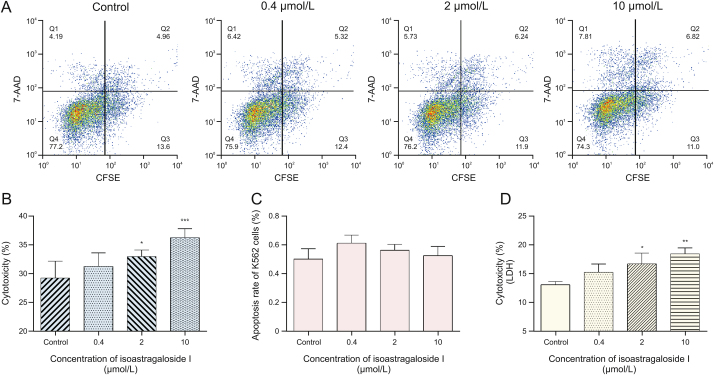
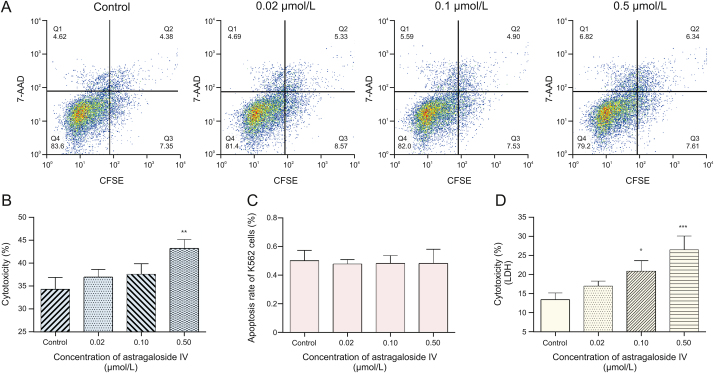
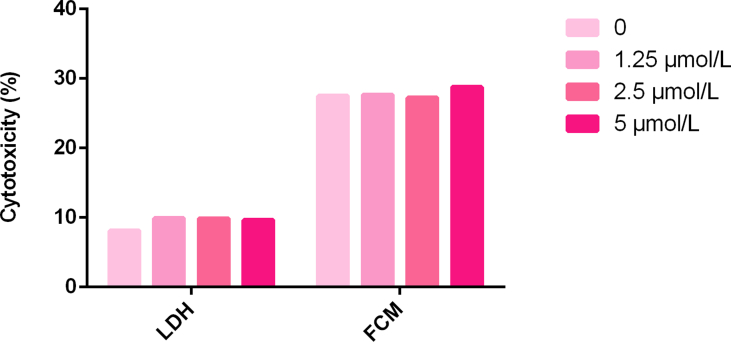
First, CFSE/7-AAD staining and flow cytometry were used to detect cytotoxicity in NK-92MI cells. For isoastragaloside I, the results are shown in Figs. 5A and B. Cytotoxicity of NK-92MI cells was 26.72% in the control group, 30.02% with 0.4 μM, 34.40% with 2 μM (P < 0.05), and 38.27% with 10 μM isoastragaloside I (P < 0.001). At concentrations of isoastragaloside I up to 10 μM, the cytotoxicity of NK-92MI cells significantly increased in a dose-dependent manner. At the same time, for astragaloside IV, the results are shown in Figs. 6A and B. Cytotoxicity of NK-92MI cells was 37.34% in controls, 38.35% with 0.02 μM, 39.42% with 0.1 μM, and 45.45% with 0.5 μM astragaloside IV (P < 0.01). When the concentration of astragaloside IV was up to 0.5 μM, there was a significant difference between the sample and control groups.
Fig. 5.
(A) Flow cytometric analysis of the effect of different concentrations of isoastragaloside I on the cytotoxicity of natural killer (NK)-92MI cells. (B) Flow cytometry: effects of isoastragaloside I on NK-92MI cell-induced cytotoxicity towards K562 cells. (C) Effects of isoastragaloside I on the apoptosis of K562 cells. (D) Lactate dehydrogenase (LDH) assay: effects of isoastragaloside I on NK-92MI cell-induced cytotoxicity towards K562 cells. Compared with control group, ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. 7-AAD: 7-aminoactinomycin D; CFSE: 5,6-carboxyfluorescein diacetate succinimidyl ester.
Fig. 6.
(A) Flow cytometric analysis of the effect of different concentrations of astragaloside IV on cytotoxicity of natural killer (NK)-92MI cells. (B) Flow cytometry: effects of astragaloside IV on NK-92MI cell-induced cytotoxicity towards K562 cells. (C) Effects of astragaloside IV on the apoptosis of K562 cells. (D) Lactate dehydrogenase (LDH) assay: effects of astragaloside IV on NK-92MI cell-induced cytotoxicity towards K562 cells. Compared with control group, ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. 7-AAD: 7-aminoactinomycin D; CFSE: 5,6-carboxyfluorescein diacetate succinimidyl ester.
To eliminate the inhibitory effect of the drugs on K562 cells, the same experiments were performed without adding NK-92MI cells. The results are shown in Fig. 5, Fig. 6C. The pictures illustrate that these two drugs had little effect on K562 cells in the corresponding concentration range. Based on the results of these two experiments, it is clear that isoastragaloside I and astragaloside IV can promote cytotoxicity in NK-92MI cells within a certain concentration range.
Further investigation based on the LDH assay revealed similar results. LDH is an enzyme present in the cytoplasm of living K562 cells [29]. Under normal circumstances, it cannot penetrate the cell membrane. When K562 cells are damaged by the cytotoxicity of NK-92MI cells, the permeability of the cell membrane changes and LDH can be released outside the cells. The number of dead K562 cells was directly proportional to the activity of LDH in the cell culture supernatant. As the drug concentration increased, the death rate of K562 cells also increased. As shown in Fig. 5D, for isoastragaloside I, when the concentration reached 10 μM, the cytotoxicity of NK-92MI towards K562 cells was the highest (P < 0.01). The rate of cytotoxicity of K562 cells was 18.6%. In Fig. 6D, for astragaloside IV, the concentration with the highest killing efficiency was 0.5 μM (P < 0.001). The rate of cytotoxicity of K562 cells was 27%. In contrast, the control group was less than 15%, which was approximately half the highest value. These results demonstrated that isoastragaloside I and astragaloside IV have immunomodulatory properties that can upregulate the cytotoxicity of NK-92MI cells.
Finally, to exclude some drugs that were found to have weak affinity with the NK-92MI/column, taking methylnissolin-3-O-glucoside as an example, LDH and flow cytometry were used to verify its pharmacological effect. These results showed that it did not promote NK cell cytotoxicity. Detailed information is shown in Fig. S2.
4. Conclusions
In this study, to identify compounds that can improve NK cell function and activity, we established a comprehensive 2D NK-92MI/CMC system to screen for components with affinity for NK-92MI cell membrane receptors from AR. According to the experiments, nine active substances in AR were screened from the system. Meanwhile, based on the verification of standard solutions, it was proven that isoastragaloside I and astragaloside IV had stronger affinity for NK-92MI/CMC columns. The results of flow cytometry and LDH assays showed that these two compounds improved the cytotoxicity of NK-92MI cells. In summary, isoastragaloside I and astragaloside IV could be considered as representative immune activators of AR and deserve further research. The successful establishment of a comprehensive 2D NK-92MI/CMC system provides a powerful and efficient technique for screening immunomodulatory active substances from herbal medicines. It also represents a new idea for the discovery of new NK cell activators.
CRediT author statement
Xinyi Chai: Validation, Formal analysis, Writing - Original draft preparation, Visualization; Yanqiu Gu: Validation, Visualization; Lei Lv: Resources; Chun Chen: Conceptualization, Methodology; Fei Feng: Validation; Yan Cao: Methodology; Yue Liu: Formal analysis; Zhenyu Zhu: Writing - Reviewing and Editing; Zhanying Hong: Supervision; Yifeng Chai: Conceptualization, Methodology, Formal analysis, Writing - Original draft preparation, Reviewing and Editing, Supervision, Funding acquisition; Xiaofei Chen: Conceptualization, Methodology, Formal analysis, Writing - Original draft preparation, Reviewing and Editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos.: 82073814, 81973291, 82122066, and 82003909) and the Rising-Star Program of Shanghai Science and Technology Committee (Grant No.: 19QA1411500).
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2022.05.006.
Contributor Information
Yifeng Chai, Email: yfchai@smmu.edu.cn.
Xiaofei Chen, Email: xfchen2010@163.com.
Appendix A. Supplementary data
The following are the supplementary data to this article:
figs1.
figs2.
References
- 1.Kennedy L.B., Salama A.K.S. A review of cancer immunotherapy toxicity. CA A Cancer J. Clin. 2020;70:86–104. doi: 10.3322/caac.21596. [DOI] [PubMed] [Google Scholar]
- 2.Vivier E., Tomasello E., Baratin M., et al. Functions of natural killer cells. Nat. Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 3.Kwaśnik P., Lemieszek M.K., Rzeski W. Impact of phytochemicals and plant extracts on viability and proliferation of NK cell line NK-92 - a closer look at immunomodulatory properties of goji berries extract in human colon cancer cells. Ann. Agric. Environ. Med. 2021;28:291–299. doi: 10.26444/aaem/133801. [DOI] [PubMed] [Google Scholar]
- 4.Myers J.A., Miller J.S. Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2021;18:85–100. doi: 10.1038/s41571-020-0426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Z., Liu L., Gao C., et al. Astragali Radix (Huangqi): A promising edible immunomodulatory herbal medicine. J. Ethnopharmacol. 2020;258 doi: 10.1016/j.jep.2020.112895. [DOI] [PubMed] [Google Scholar]
- 6.Li K., Cao Y.X., Jiao S.M., et al. Structural characterization and immune activity screening of polysaccharides with different molecular weights from Astragali Radix. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.582091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu H., Wang Z.-Y., Zhou Y.-C., et al. Immunomodulation of Chinese Herbal Medicines on NK cell populations for cancer therapy: A systematic review. J. Ethnopharmacol. 2021;268 doi: 10.1016/j.jep.2020.113561. [DOI] [PubMed] [Google Scholar]
- 8.Han R., Wu W.-Q., Wu X.-P., et al. Effect of total flavonoids from the seeds of Astragali complanation natural killer cell function. J. Ethnopharmacol. 2015;173:157–165. doi: 10.1016/j.jep.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Ma W., Wang C., Liu R., et al. Advances in cell membrane chromatography. J. Chromatogr. A. 2021;1639 doi: 10.1016/j.chroma.2021.461916. [DOI] [PubMed] [Google Scholar]
- 10.Chen X., Wu Y., Chen C., et al. Identifying potential anti-COVID-19 pharmacological components of traditional Chinese medicine Lianhuaqingwen capsule based on human exposure and ACE2 biochromatography screening. Acta Pharm. Sin. B. 2021;11:222–236. doi: 10.1016/j.apsb.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Q., Bu Y., Cao R., et al. Stability designs of cell membrane cloaked magnetic carbon nanotubes for improved life span in screening drug leads. Anal. Chem. 2019;91:13062–13070. doi: 10.1021/acs.analchem.9b03268. [DOI] [PubMed] [Google Scholar]
- 12.Bu Y., Zhang X., Zhu A., et al. Inside-out-oriented cell membrane biomimetic magnetic nanoparticles for high-performance drug lead discovery. Anal. Chem. 2021;93:7898–7907. doi: 10.1021/acs.analchem.1c00567. [DOI] [PubMed] [Google Scholar]
- 13.Pan P., Cheng J., Si Y., et al. A stop-flow comprehensive two-dimensional HK-2 and HK-2/CIKI cell membrane chromatography comparative analysis system for screening the active ingredients from Pyrrosia calvata (Bak.) Ching against crystal-induced kidney injury. J. Pharm. Biomed. Anal. 2021;195 doi: 10.1016/j.jpba.2020.113825. [DOI] [PubMed] [Google Scholar]
- 14.Gu Y., Chen X., Wang R., et al. Comparative two-dimensional HepG2 and L02/cell membrane chromatography/C18/time-of-flight mass spectrometry for screening selective anti-hepatoma components from Scutellariae Radix. J. Pharm. Biomed. Anal. 2019;164:550–556. doi: 10.1016/j.jpba.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 15.Gu Y., Chen X., Wang Y., et al. Development of 3-mercaptopropyltrimethoxysilane (MPTS)-modified bone marrow mononuclear cell membrane chromatography for screening anti-osteoporosis components from Scutellariae Radix. Acta Pharm. Sin. B. 2020;10:1856–1865. doi: 10.1016/j.apsb.2020.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding X., Cao Y., Yuan Y., et al. Development of APTES-decorated HepG2 cancer stem cell membrane chromatography for screening active components from Salvia miltiorrhiza. Anal. Chem. 2016;88:12081–12089. doi: 10.1021/acs.analchem.6b02709. [DOI] [PubMed] [Google Scholar]
- 17.Ding X., Chen X., Cao Y., et al. Quality improvements of cell membrane chromatographic column. J. Chromatogr. A. 2014;1359:330–335. doi: 10.1016/j.chroma.2014.07.071. [DOI] [PubMed] [Google Scholar]
- 18.Chen X., Cao Y., Lv D., et al. Comprehensive two-dimensional HepG2/cell membrane chromatography/monolithic column/time-of-flight mass spectrometry system for screening anti-tumor components from herbal medicines. J. Chromatogr. A. 2012;1242:67–74. doi: 10.1016/j.chroma.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 19.Chen X., Cao Y., Zhang H., et al. Comparative normal/failing rat myocardium cell membrane chromatographic analysis system for screening specific components that counteract doxorubicin-induced heart failure from Acontium carmichaeli. Anal. Chem. 2014;86:4748–4757. doi: 10.1021/ac500287e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao H., Wu C., Gao D., et al. Antitumor effect by hydroxyapatite nanospheres: Activation of mitochondria-dependent apoptosis and negative regulation of phosphatidylinositol-3-kinase/protein kinase B pathway. ACS Nano. 2018;12:7838–7854. doi: 10.1021/acsnano.8b01996. [DOI] [PubMed] [Google Scholar]
- 21.Young A., Ngiow S.F., Gao Y., et al. A2AR adenosine signaling suppresses natural killer cell maturation in the tumor microenvironment. Cancer Res. 2018;78:1003–1016. doi: 10.1158/0008-5472.CAN-17-2826. [DOI] [PubMed] [Google Scholar]
- 22.Raskovalova T., Huang X., Sitkovsky M., et al. Gs protein-coupled adenosine receptor signaling and lytic function of activated NK cells. J. Immunol. 2005;175:4383–4391. doi: 10.4049/jimmunol.175.7.4383. [DOI] [PubMed] [Google Scholar]
- 23.Beltran-Beltran V., Benetó N., Lapeña-Luzón T., et al. Role of adenosine receptors in rare neurodegenerative diseases with motor symptoms. Curr. Protein Pept. Sci. 2021;22:675–694. doi: 10.2174/1389203722666210910110126. [DOI] [PubMed] [Google Scholar]
- 24.Tripathi N., Tripathi N., Goshisht M.K. COVID-19: Inflammatory responses, structure-based drug design and potential therapeutics. Mol. Divers. 2022;26:629–645. doi: 10.1007/s11030-020-10176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji C., Luo Y., Zou C., et al. Effect of astragaloside IV on indoxyl sulfate-induced kidney injury in mice via attenuation of oxidative stress. BMC Pharmacol. Toxicol. 2018;19 doi: 10.1186/s40360-018-0241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu H., Huang F., Wu H., et al. Isoastragaloside I inhibits NF-κB activation and inflammatory responses in BV-2 microglial cells stimulated with lipopolysaccharide. Int. J. Mol. Med. 2017;40:1270–1276. doi: 10.3892/ijmm.2017.3114. [DOI] [PubMed] [Google Scholar]
- 27.Hirotani M., Zhou Y., Rui H., et al. Cycloartane triterpene glycosides from the hairy root cultures of Astragalus membranaceus. Phytochemistry. 1994;37:1403–1407. doi: 10.1016/s0031-9422(00)90420-5. [DOI] [PubMed] [Google Scholar]
- 28.Qi Y., Gao F., Hou L., et al. Anti-inflammatory and immunostimulatory activities of astragalosides. Am. J. Chin. Med. 2017;45:1157–1167. doi: 10.1142/S0192415X1750063X. [DOI] [PubMed] [Google Scholar]
- 29.Fotakis G., Timbrell J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006;160:171–177. doi: 10.1016/j.toxlet.2005.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.