Abstract
With the modernization and internationalization of traditional Chinese medicine (TCM), the requirement for quality control has increased. The quality marker (Q-marker) is an important standard in this field and has been implemented with remarkable success in recent years. However, the establishment of Q-markers remains fragmented and the process lacks systematicity, resulting in inconsistent quality control and insufficient correlation with clinical efficacy and safety of TCM. This review introduces four multimodal integrated approaches that contribute to the discovery of more comprehensive and accurate Q-markers, thus aiding in the establishment of new quality control patterns based on the characteristics and principles of TCM. These include the whole-process quality control strategy, chemical-activity-based screening method, efficacy, safety, and consistent combination strategy, and TCM theory-guided approach. Furthermore, methodologies and representative examples of these strategies are described, and important future directions and questions in this field are also proposed.
Keywords: Traditional Chinese medicine (TCM), Quality marker (Q-marker), Multimodal integrated strategy, Quality control systems
Graphical abstract
Highlights
-
•
Four multimodal integrated strategies were introduced to establish Q-markers.
-
•
Quality control of TCM should focus on the entire process chain.
-
•
The identification of Q-markers needs to be guided by TCM theory.
-
•
Ensuring efficacy, safety, and consistency is an essential goal of Q-markers.
-
•
Multidisciplinary techniques are the driving force for improving Q-markers.
1. Introduction
Traditional Chinese medicine (TCM) is playing an ever-increasing role in modern medicine and healthcare. The TCM industry has made great strides in implementing modernization strategies over the past two decades. In 2019, the gross value of the TCM industry was 6,520 billion CNY, accounting for 25% of the gross value of the Chinese pharmaceutical industry. TCM is gradually gaining recognition worldwide as several TCM preparations are undergoing clinical trials in the United States and 73 herbal drugs considered as TCM are included in the European Pharmacopoeia [1,2]. However, inheritance and innovation of TCM is a challenge for both the academia and the industry. Lack of standardized quality control restricts the modernization and globalization of TCM. Unlike chemically synthesized drugs, the highly complex chemical nature of the numerous ingredients in TCM preparations and the variability in the pharmaceutical processes negatively affect quality control. Regarding to raw materials, variety, cultivation technique, ecological environment, and harvesting time are factors that can affect TCM quality. Subsequent processing activities, such as fumigation and stir-frying, also affect the quality of TCM slices. TCM slices is the collective term for the common materials used in standard decoctions and TCM preparations. Furthermore, pharmaceutical processes, including extraction, purification, separation, concentration, and drying, affect the quality transfer of TCM. Hence, establishing scientific quality evaluation systems that cover the entire industrial chain and conform to the characteristics and principles of TCM will be a cornerstone for validating the clinical efficacy and safety of TCM.
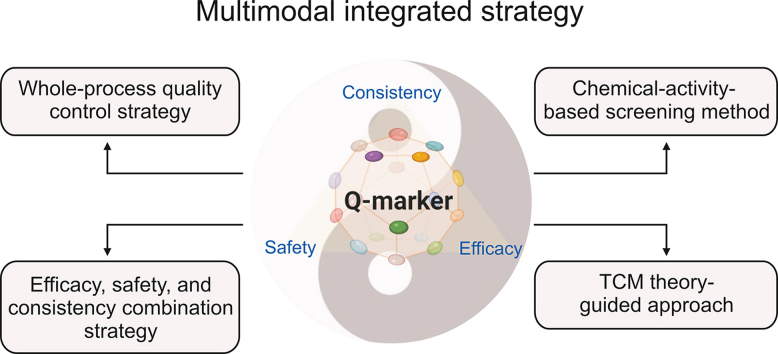
Quality marker (Q-marker), proposed by Liu et al. [3] in 2016, opens a new paradigm for establishing TCM quality control systems. The core elements of the Q-marker are measurability, specificity, transitivity, being effect-related, and relevance to TCM theory [4]. These factors help control the manufacturing process and product quality, guaranteeing the clinical efficacy and safety of TCM. Measurability means that Q-markers should be qualitatively identified and quantitatively determined. Specificity indicates that Q-markers are specific to an individual or group of TCMs and are inherently present in the raw materials and products of said TCMs, or are formed during processing. Transitivity implies that Q-markers can be detected in the whole manufacturing process, i.e., from raw material to final product. Effect-related Q-markers indicate that they should be closely associated with the efficacy and toxicity of the particular TCM. TCM theory relevance suggests that Q-markers are considered from the monarch as well as the minister, assistant, and guide based on the compatibility theory of TCM. In recent years, the discovery and establishment of Q-markers have presented a multidimensional integrated pattern [5]. New strategies integrating multidisciplinary approaches, such as phytochemistry, pharmacokinetics, systems biology, network pharmacology, and computational techniques, have been proposed [[6], [7], [8]]. This review discusses the progress made in the establishment of Q-markers, as well as TCM quality control systems, by highlighting four multimodal integrated approaches: the whole-process quality control strategy; chemical-activity-based screening method; efficacy, safety, and consistency combination strategy; and TCM theory-guided approach. These comprehensive and promising strategies aim to improve TCM quality. The integrated strategy is shown in Fig. 1. Notably, the discovery and establishment of Q-markers for TCM are still in their infancy. Several limitations exist, including the determination of the content range of Q-markers, selection of influencing factors for the discovery and establishment of Q-markers, and fragmentation of quality control systems. Although quality indicators are gradually being discovered, simultaneous control of Q-markers during the manufacturing process remains a rate-limiting step. Therefore, further improvements are required in these areas.
Fig. 1.
Schematic representation of the four multimodal integrated strategies. PK/PD: pharmacokinetics/pharmacodynamics; QbD: quality by design; Q-marker: quality marker; TCM: traditional Chinese medicine.
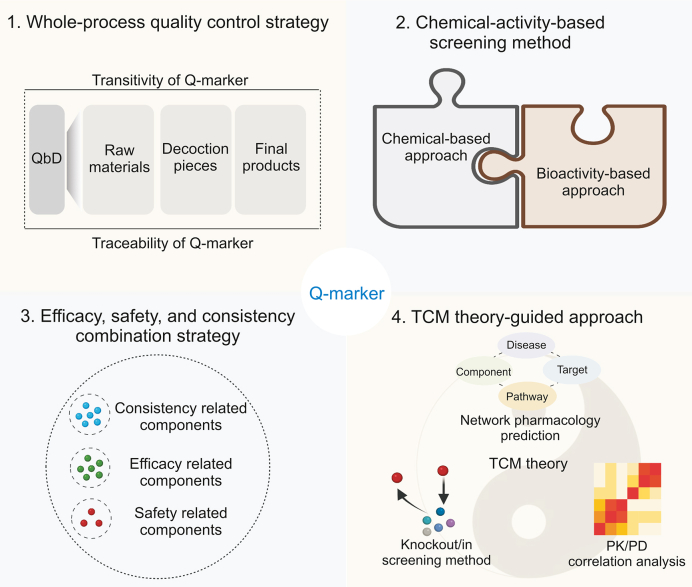
2. Whole-process quality control strategy
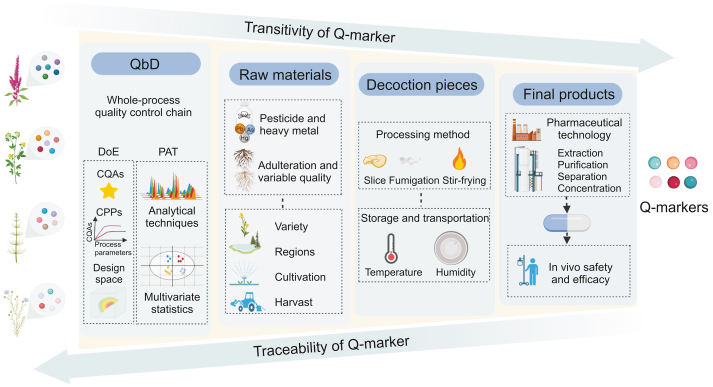
At present, the quality control of TCM mostly adopts various online or offline analysis methods to monitor key pharmaceutical process units or intermediates with easily available or detectable ingredients. This approach does not consider the transitivity law of the components among the production units from the perspective of the entire process chain. In particular, the current Q-markers and standards among TCM raw materials, decoction pieces, and final preparations lack close connection and systematic integration, leading to inconsistencies in the quality control of different processes. Thus, establishing a whole-process quality control system based on the transitivity and traceability of the Q-marker is a significant step toward improving the quality of TCM.
This whole-process quality control chain should be carried out in two dimensions. The first dimension is the transformation from raw materials to the clinical form, which focuses on quality transitivity. The main task here is to systematically identify the chemical composition and transmission law of every production unit in the formation and clinical applications of TCM, respectively. The second dimension is clinical efficacy- and safety-oriented traceability from the clinical form to raw materials. The primary task in this dimension is to guide and optimize the quality control methods and standards of each unit in TCM production based on Q-markers that can reflect clinical efficacy and safety. The quality of the raw materials is a fundamental guarantee of the safety and efficacy of the final TCM preparations. However, owing to the lack of standardized guidance, raw materials of TCMs may have variations in ingredients and their quality. This is caused by differences in harvesting time, geographical regions, pesticide residues, and adulteration, resulting in overall concerns about the quality of raw materials. Therefore, based on the transitivity and traceability of Q-markers, determining and controlling the main factors affecting the quality of raw materials is key to ensuring the safety and efficacy of the final products. Decoction pieces are intermediates between TCM raw materials and final preparations. Processing, storage, and transportation are the main factors affecting their quality. Combining Q-markers with these processes will help build a whole-process quality control system by clarifying the changes in characteristics of the chemical composition and the influence of these alterations on the quality of the final products. Additionally, monitoring the extraction, purification, separation, and concentration using Q-markers is pivotal because the chemical composition of intermediates can be traced back to the raw materials, which ultimately affects the active substances in vivo. It is worth pointing out that some substances that do not have transitivity characteristics, but can guide in-process control and process validation in a specific unit, should also be considered as Q-markers, such as the ingredients indicating appropriate conditions for drying and storage [9].
Quality by design (QbD) is a concept that has been applied to quality control of TCM in recent years, replacing the earlier methods of quality by testing and quality by production. It is a systematic approach that has predetermined objectives and focuses on understanding and controlling the entire product lifecycle based on existing knowledge and quality risk management [10,11]. QbD aims to design effective and efficient manufacturing processes to achieve and ensure product quality and performance. The theories, methods, and goals of QbD are compatible with the requirements for holistic quality control in TCM. QbD is mainly used to identify a quality target product profile, determine critical quality attributes (CQAs), define critical processes and critical process parameters (CPPs), establish quantitative models, develop design space, and continuously improve the control strategy, aiming to guarantee the safety, efficacy, and quality consistency of TCM products [12].
Design of experiment (DoE), together with process analytical technology (PAT), represents two of the main tools in the application of QbD in TCM quality control. DoE involves three steps: 1) fully understanding the inputs and outputs, 2) determining the appropriate measure for the output, and 3) establishing a design matrix for the factors being investigated. In the context of the complete quality control process for TCM, DoE is used to screen CPPs that have significant impacts on CQAs, determine acceptable ranges for individual CPPs, and then optimize the production process by building mathematical models and process design space. PAT, as a method for designing, analyzing, and controlling the production process, ensures the quality of the final product through the online or offline detection of CQAs and CPPs in the production chain. Modern analytical techniques are essential contributors to PAT, including liquid chromatography, gas chromatography, mass spectrometry (MS), nuclear magnetic resonance spectroscopy, near-infrared spectroscopy (NIRS), mid-infrared spectroscopy, and Raman spectroscopy. Among them, NIRS is the most commonly used detection technology, which can perform qualitative and quantitative analyses in a fast and simple manner. It manages this through establishment of a model by spectral data pretreatment and has the advantages of overall analysis and intact sampling. Moreover, chromatography techniques have been frequently applied in the quality control of production processes owing to their easy operability, speed of analysis, and high separation efficiency [13]. Additionally, chromatography coupled with MS is increasingly being used in PAT owing to its sensitivity for detecting compounds at low concentrations with high accuracy [13,14]. The massive amount of data generated during the production process requires a multivariate statistical analysis to extract important information and discover patterns. Principal component analysis (PCA) and partial least squares (PLS) regression are the most commonly used methods. For instance, Wu and colleagues [15] established a principal component analysis-moving block standard deviation (PCA-MBSD) model to realize the online control of boiling time in the extraction process of Phellodendron chinense by NIRS. Jing and co-workers [16] combined the approaches of high-performance liquid chromatography (HPLC), NIRS, and PLS to achieve rapid and simultaneous determination of the main active components in rhubarb and to compare the impact of different geographical regions and processing patterns. Therefore, applying the concept and methods of QbD can enhance the whole-process control of TCM production. Fig. 2 summarizes the overall process of this strategy.
Fig. 2.
The whole-process control strategy based on the transitivity and traceability of the Q-markers. Q-marker: quality marker; QbD: quality by design; CPPs: critical process parameters; CQAs: critical quality attributes; DoE: design of experiment; PAT: process analytical technology.
An example of the entire process control using Q-markers is introduced here in the form of Xuesaitong injection (XST) as a proof-of-concept. XST, a preparation of Panax notoginseng total saponins, is one of the best-selling TCM in China. Considering the potential that every compound can become a Q-marker, comprehensive chemical profiling was conducted using HPLC-MS, which not only focused on the main components but also on the minor and trace constituents of XST. As a result, 97 constituents were characterized or tentatively identified, including the main constituents notoginsenoside R1, ginsenoside Rg1, Re, Rb1, and Rd (their contents accounted for 61.69%–71.39% of the total saponins) as well as minor and trace ingredients ginsenoside Rh4, Rg6, and notoginsenoside T5 [17]. Bioactive equivalent combinatorial components (BECCs) that exhibit pharmacological activities similar to those of XST were then determined using the content-weighted ingredient–target network [18], as well as the adjusted efficacy score [19]. The content-weighted ingredient–target network is a novel approach that was proposed to uncover BECCs, especially because the existing methods only focus on the potential pharmacological effects without considering the contents of the ingredients that determine their efficacy. Notoginsenoside R1, ginsenoside Rg1, Rb1, Rd, and Re were identified as BECCs by network prediction and in vivo validation experiments [18]. Consistent results were obtained using the adjusted efficacy score, which was designed to identify BECCs connected to the diverse activities of XST in cardiovascular and cerebrovascular diseases, without focusing on a single disease. Thus, notoginsenoside R1, ginsenoside Rg1, Rb1, Rd, and Re were identified as Q-markers. Next, the key processes in XST preparation were determined and optimized based on these Q-markers. In particular, extraction and column chromatography processes were identified as key steps. Using these processes, computational models with good prediction ability were built to describe the relationship between CPPs and Q-markers. Optimal CPPs have been obtained to simultaneously ensure intermediate quality and improve process efficiency [20]. Moreover, several online detection methods have been developed to monitor the quality of intermediates using these Q-markers. For instance, a method integrating convolutional neural networks and NIRS was designed to monitor the chromatographic elution process in the production of P. notoginseng total saponins using five Q-markers [21]. A similar approach of combining NIRS and PLS was established for online control of the extraction step [22]. Subsequently, a feedforward control strategy based on the spectra of raw materials was established to optimize the alcohol extraction process of P. notoginseng using these Q-markers, which reduced the effect of fluctuation of raw material variation on product quality [23]. Notably, besides being a bioactive constituent, ginsenoside Rd was also identified as an allergenic ingredient in XST [24]. Thus, based on the theory of QbD, corresponding internal control quality standards were set up to control ginsenoside Rd content within a precise range that neither induced anaphylactoid reactions nor reduced the efficacy of XST [25].
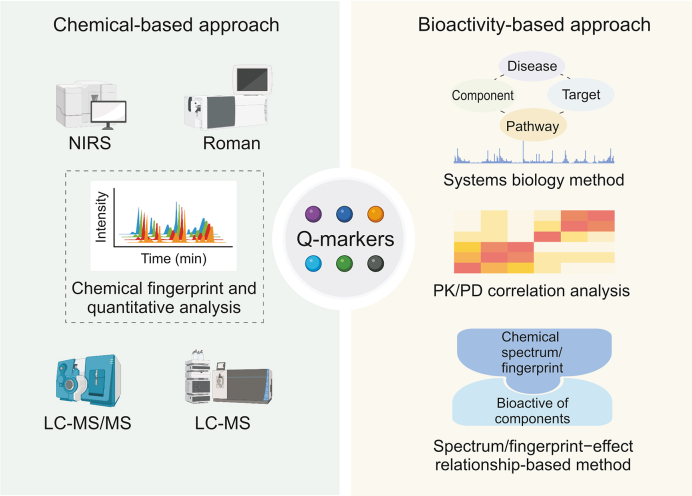
3. Chemical-activity-based screening method
Efficacy and safety are the key requirements for the quality evaluation and quality control of TCM. However, most of the current methods and standards for TCM quality control are chemically oriented, including those in the Chinese Pharmacopoeia, ministerial standards, and local standards. These chemical composition indicators cannot be directly related to their activities, which significantly reduces the actual value of quality control. Moreover, because of the complexity of the synergistic and antagonistic effects between the components, especially after compounding, revealing the mode of action of one or several ingredients cannot fully reflect the efficacy and safety of TCM products. Thus, the chemical-activity-based systematic screening strategy of Q-markers, which aims to comprehensively discover ingredients related to efficacy and safety, has become a general practice (Fig. 3). These include the emerging approaches of “integrative pharmacology-based traditional Chinese medicine” (TCMIP) [26], “effect-compound-target-fingerprint” [27], “network pharmacology and bioactive equivalence assessment integrated strategy” [28], “efficacy-oriented effect-constituent index” [29], “bioassay and spectrum–effect relationship-based strategy” [30], “Spider-web mode-based strategy” [31], and “Q-biomarkers integrated strategy” [32]. Network pharmacology or systems biology-based bioactive equivalence assessment is a main approach in this area, while spectrum/fingerprint-effect relationship-based methods and pharmacokinetic-pharmacodynamic (PK–PD) analysis-based strategies are also important. Additionally, because of the heavy workload and low throughput of the current bioactivity evaluation methods, developing strategies with automatic, rapid, sensitive, and high-throughput characteristics for the simultaneous screening of chemical and active ingredients is an important matter to be considered. In this section, we focused on summarizing and discussing the three integrated strategies mentioned above, as well as the future direction.
Fig. 3.
The chemical-activity-based screening method of Q-markers. Q-marker: quality marker; NIRS: near-infrared spectroscopy; LC-MS/MS: liquid chromatography-tandem mass spectrometry; LC-MS: liquid chromotography-mass spectrometry; PK/PD: pharmacokinetics/pharmacodynamics.
The biological effects of TCM present a “multi-component, multi-target, multi-pathway” mode. Thus, using systematic approaches such as network pharmacology or systems biology to determine which ingredients are responsible for therapeutic efficacy and clinical safety is important for Q-marker screening [33]. Moreover, BECCs with the efficacy of the original TCM have been proposed to improve the quality control of TCM [34]. Thus, a novel strategy combining these two approaches has recently been emerged, which contends that the bioequivalence of components in potentially active compounds detected by network pharmacology should be proven; subsequently, the components can be determined as Q-markers [28]. This strategy not only uses network pharmacology to quickly discover potential active ingredients and corresponding targets but also can identify the ingredients closely related to the overall efficacy of TCM by both in vitro and in vivo bioactive equivalence assessments. Notably, network pharmacology is highly dependent on databases and cannot distinguish whether the corresponding targets are indeed affected by TCM treatment. Therefore, approaches involving systems biology often serve as a supplement to determine compounds related to TCM efficacy and safety. A few studies have adopted this strategy to screen Q-markers. For instance, Xu and co-workers [28] integrated network pharmacology and bioactive equivalence assessment to discover Q-markers for the Da-Cheng-Qi decoction in the treatment of intestinal obstruction. In the decoction, 11 ingredients were finally confirmed in bioequivalence with Da-Cheng-Qi decoction and served as Q-markers. Yang and co-workers [35] used a metabolic exposure-oriented network pharmacology strategy to reveal the effective combination in the Ginkgo biloba extract, where a combination of 12 active compounds was found to be bioequivalent to that of the original herb used in the treatment of ischemic stroke and could act as Q-markers.
Chemical fingerprinting has played an important role in the quality control of TCM over the past two decades, with the aim of monitoring batch-to-batch consistency [36]. This method can reflect the types and quantities of chemical components intuitively contained in TCM and therefore exhibit the overall characteristics of TCM [37]. However, this cannot be correlated with the efficacy and safety of TCM. Thus, a spectrum/fingerprint–effect relationship-based method was proposed to identify Q-markers from the perspective of combining the biological and chemical information of TCM [38]. In other words, this method correlates the chemical spectrum/fingerprint and the biopotency of the components. The general methodology was as follows: 1) a standardized bioassay method was established with appropriate indicators and optimized experimental conditions; 2) the biopotency of TCM was evaluated using the bioassay method; 3) the chemical fingerprints of TCM were collected; and 4) the relationship between the chemical properties and bioactivities was determined through correlation analysis, to discover the spectrum/fingerprint–effect relationship. An example of this method is an effective component fingerprint in which the peak area of the active component in a fingerprint is corrected by its efficacy [39]. Using Scutellaria baicalensis Georgi as an example, when the antibacterial potency of this herbal medicine was first detected, a chemical fingerprint was established. The gray correlation analysis method was used to identify the main antibacterial components, and the peak areas of the corresponding components were corrected based on their antibacterial potency. Compared to the original chemical fingerprint, the effective component fingerprint of S. baicalensis Georgi can reflect the differences between samples more clearly and accurately [39]. Moreover, this approach was also used to identify Q-markers for the antiplatelet aggregation activity of Polygonum multiflorum Thunb, where trans-2,3,5,4′-tetrahydroxy-stilbene-2-O-β-d-glucoside and catechin were identified as the main active constituents and Q-markers by spectrum–effect relationships [30].
Recently, a PK–PD analysis-based strategy has also provided a promising notion for Q-marker identification. The core idea of this strategy is to establish a qualitative and quantitative PK–PD correlation from different in vivo PK–PD processes. The TCMIP concept proposed by Liu and co-workers [26] is a representative example. In this strategy, based on comprehensive characterization of TCM components and PK profiles, as well as PD profiles of TCM in diseases, the qualitative association of PK–PD was first constructed to identify key active constituents (KACs), critical molecular targets (CMTs), and critical pharmacological effects (CPEs) of TCM on diseases by establishing a multidimensional network. Then, quantitative PK–PD correlations, especially between the KACs and CPEs, were established using artificial intelligence-related algorithms and dynamic in vitro PK–PD complex models. Finally, knockout/in of KACs or CMTs was carried out to verify the contribution of KACs to the whole TCM activity and synergetic mechanism of multiple KACs. A comprehensive investigation of the three forms of active substances (absorbed prototypes, absorbed metabolites, and unabsorbed constituents) and the three modes of action of these substances (direct, indirect, and auxiliary effects) is emphasized in this strategy. According to the concept and methodology of TCMIP, the potential Q-markers of Yuanhu Zhitong tablets and Xin-Su-Ning capsules were identified [[39], [40], [41], [42], [43], [44], [45], [46], [47]], and detailed results and information were published [26]. The significance of this PK–PD analysis-based strategy is the ability to discover the relationship between KACs and CPEs through quantitative PK–PD correlation, which is conducive to the identification of Q-markers closely related to the activity of TCM. Therefore, more accurate quality control standards can be established.
Owing to the complexity of TCM, the biological evaluation methods of TCM face the disadvantages of heavy workload and low throughput. Automatic, rapid, sensitive, and high-throughput screening of bioactive components that represent the efficacy of the original TCM is an urgent problem that needs to be solved. Although this field is still in its infancy, several studies have been conducted. Cheng and co-workers [48] developed a microfluidic chip-based multiple-biomarker assay to evaluate the biological consistency of QiShenYiQi pills. They utilized magnetic beads as an efficient surface to immobilize thrombin and angiotensin-converting enzyme and designed a dual-channel microfluidic chip to perform the bioassay, in which the enzyme–magnetic bead complex formation, enzymatic reaction, and biopotency screening were integrated. This chip-based approach showed a better discrimination ability for different batches of QiShenYiQi pills than chemical fingerprinting. Furthermore, this approach was used to identify the bioactive constituents of QiShenYiQi pills in a convenient and high-throughput manner by detecting the degree of nonspecific binding of compounds using LC-MS. Therefore, this chip-based method can be used to screen Q-markers in TCM in a fast and high-throughput manner. Additionally, Qu and co-workers [49] developed a high-throughput chemiluminescence platform that can perform complete analysis in a 96-well plate format to evaluate the antioxidant activity of total flavonoid glycosides from plant extracts.
The inclusion of biological evaluation in the quality control system of TCM is a significant breakthrough in the development of TCM quality control, but it also faces a series of problems. The lack of standardized in vivo and in vitro models and evaluation indicators that fully reflect the clinical efficacy of TCM remains an important issue. Most of the current models and evaluation indicators are only related to a certain clinical effect or the effects on a particular disease, whereas the active ingredients of TCM in the treatment of each disease may differ. Moreover, as network pharmacology, systems biology approaches, and PK–PD correlation analyses are highly database-dependent, comprehensive TCM chemical and biological databases at both the clinical and preclinical levels must be established. Additionally, efficient algorithms and mathematical models that can fully reveal the relationship between chemical composition and bioactivity require further development.
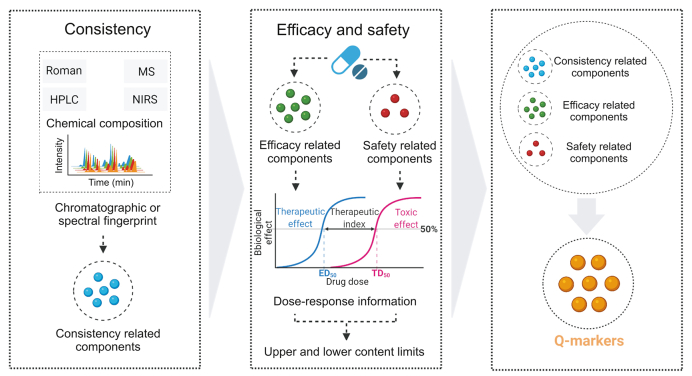
4. Efficacy, safety, and consistency combination strategy
Consistency in quality, including batch-to-batch and manufacturer-to-manufacturer consistency, is the core component of TCM quality control. Initially, the evaluation for consistency in TCM focused on qualitative and quantitative analyses of chemical compositions to ensure the consistency of the main components in different batches or between manufacturers [50]. The fingerprinting method has been widely used to establish quality standards to guarantee the consistency and stability of TCM [51]. However, these chemical-based consistency standards are insufficient to meet the efficacy and safety criteria for the clinical application of TCM. Ensuring the safety and efficacy of TCM is extremely important because these are the essential requirements for drugs. It is difficult to define the activity and toxicity of TCM owing to common problems such as unclear clinical positioning and targets. As a result, the effective and toxic ingredients of TCM are often unclear and not included in the quality evaluation of TCM, falling into the dilemma of disconnection between quality control standards and clinical efficacy and safety. Thus, quality consistency evaluation systems should be updated from two major perspectives: the embedding of safety and efficacy evaluations. The former involves the monitoring and control of pesticide residues, heavy metals, solvent residues, microbial loads, aflatoxins, and toxic ingredients. The latter implicates systematic screening of active ingredients in in vitro and in vivo models. Either constituent that displays sensitivity to changes in product quality at these two perspectives should serve as Q-markers and must ensure consistency between different batches or manufacturers. However, not all pharmacodynamic ingredients are suitable as Q-markers because of the requirements for measurability and transferability. In contrast, Q-markers should include components with important pharmacological effects. Furthermore, similar to the quality control of herbal medicines in the United States and Europe, degradation products produced from active substances or associated with TCM toxicity should also be considered [52,53]. Understanding the significance of these changes and providing adequate control of these degradation products will further improve the quality of TCM. Taken together, this integrated strategy not only is conducive to the improvement of quality standards but also benefits the retrospective investigation of manufacturing processes, aiming to provide a scientific basis for quality control during production, packaging, transportation, storage, and use of TCM with safety and efficacy.
The general methodology of this strategy is as follows: first, chromatographic or spectral fingerprinting is conducted to comprehensively evaluate the quality of TCM, and the components that are closely associated with chemical consistency need to be revealed by analytical techniques; second, the biological activity and toxicity of representing classes or individual constituents should be determined and the dose–response information across various endpoints and dosages needs to be obtained, which are used to determine the upper and lower concentration limits of certain ingredients; third, the components related to TCM efficacy, safety, and consistency are all identified as Q-markers, and all processes that are applied during TCM manufacturing are optimized and monitored through these markers as well as their limits of content ranges. A diagram of this strategy is shown in Fig. 4. Several representative examples of this strategy are introduced here.
Fig. 4.
The efficacy, safety, and consistency combination strategy for Q-marker identification. Q-marker: quality marker; MS: mass spectrometry; HPLC: high performance liquid chromatography; NIRS: near-infrared spectroscopy; ED50: median effective dose; TD50: median toxic dose.
Xiyanping, a TCM injection, is widely used clinically as an anti-inflammatory drug [54]. Currently, the total andrographolide sulfonate content is adopted as the quality standard for the Xiyanping injection [55]. Nevertheless, this marker cannot directly reflect the efficacy and safety of the Xiyanping injection. Thus, establishing a quality control system using Q-markers that are closely related to the consistency, efficacy, and safety is critical for the clinical application of this injection. Zhan [56] found that sodium dehydro-17-hydro-andrographolide-3,19-yl disulfate, sodium dehydro-17-hydro-andrographolide-3-yl sulfate, sodium dehydro-17-hydro-andrographolide-19-yl sulfate, and 9-dehydro-17-hydro-andrographolide were the main constituents of the Xiyanping injection, and all of them exhibited antibacterial activity against Staphylococcus aureus. Based on these results, a simultaneous determination method was established to detect the contents of these four constituents, which is also an approach for the quality control of the Xiyanping injection [57]. However, with extensive application, Xiyanping injection-induced anaphylactoid reactions have frequently been reported in recent years [58]. Thus, it is important to determine potential anaphylactic constituents and include them as safety-related Q-markers. Using in silico molecular docking analysis, Xu and co-workers [59] found that dehydroandrographolide forms hydrogen bonds with the amino acid residue Trp113 on the high-affinity immunoglobulin E receptor, which is a crucial receptor on the surface of mast cells to mediate immediate allergic reactions. This suggests that dehydroandrographolide is a potential anaphylactic agent in the Xiyanping injection. Similarly, Sun and co-workers [60] developed a rapid screening method to identify potentially anaphylactic constituents in Chuanxinlian injection (which is made of Andrographis paniculata Nees, like the Xiyanping injection) using human mast cell line-1 cell membrane chromatography coupled with HPLC-electrospray ionization (ESI)-MS/MS analysis. Dehydroandrographolide was also identified as a potential anaphylactic ingredient. The anti-inflammatory efficacy of dehydroandrographolide has also been reported [61]. Therefore, in addition to the four efficacy-related Q-markers, dehydroandrographolide should be considered as both an efficacy-related Q-marker and a safety-related Q-marker. The content range of dehydroandrographolide in the Xiyanping injection that neither reduces the efficacy of this injection nor causes anaphylactoid reaction needs further exploration and could be used to optimize and monitor the production process.
Tripterygium glycoside tablets are commercial TCM frequently used for the treatment of autoimmune and inflammatory diseases in China [62]. A clinical trial that aimed to evaluate the effects of tripterygium glycoside tablets on rheumatoid arthritis was approved by the U.S. Food and Drug Administration [63,64]. Although tripterygium glycoside tablets are promising drugs, their quality control requires continuous improvement. Hang and co-workers [65] established a rapid and sensitive HPLC time-of-flight (TOF)-MS method for the quantitative analysis of 11 bioactive constituents (triptolide, triptriolide, epitriptriolide, triptonide, tripterine, wilforlide A, wilforlide B, wilforine, wilforgine, wilfordine, and wilfortrine) in tripterygium glycoside tablets. Using this method, they found that the contents of these 11 constituents varied significantly among different manufacturers, as well as among different batches from the same manufacturer. Moreover, differences in anti-inflammatory activity and hepatotoxicity were also detected in the tripterygium glycoside tablets produced by various manufacturers, which is probably due to the differences in the chemical constituents [62]. Therefore, it is necessary to control the consistency of these components. He and co-workers [66] determined the cytotoxicity and pharmacokinetic characteristics of four alkaloids in tripterygium glycoside tablets. They found that wilforine could serve as a safety-related Q-marker as well as a PK-marker. It induced cytotoxicity in HepG2 cells and exhibited dose- and time-dependent characteristics in PK analysis. Since these components not only exhibit pharmacological effects but also show serious adverse reactions, including multisystem damage, it is important to explore the content range of these components that will neither reduce drug efficacy nor cause adverse reactions in tripterygium glycoside tablets in the future.
Defining the acceptable range or limits of Q-markers is the main purpose of TCM quality research and can provide a basis for controlling the production process and establishing quality standards. In addition to quantitative analytical methods and dose–response analyses [67,68], several statistical techniques have been adopted to define the acceptable range of Q-markers [69,70]. For instance, Xia and co-workers [69] used linear regression analysis or a weighted least squares model to predict the effective content of chemical markers based on quantitative information between validated batches and other batches of Chaiqin chengqi decoction, while in vitro and in vivo anti-pancreatic activity evaluation was also performed. As a result, effective and antagonistic constituents were identified, along with their lower and upper limits. Li and co-workers [70] integrated the active ingredients, components transfer process analysis, and comprehensive evaluation by “Spider-web” mode to select the Q-markers and in effect, quality control of the Shenmai injection (SMI). Specifically, the “Spider-web” mode was used to comprehensively evaluate representative constituents by the dimensions of content level, content consistency in the intermediate extract, and the finished product. As a result, six constituents were selected as Q-markers, and quality control methods were developed by ultra-performance liquid chromatography coupled with diode array detection and HPLC coupled with an evaporative light scattering detector. Furthermore, the total content range of these six Q-markers was determined to be 13.844–22.557 mg/mL in the different batches of SMI.
Although the quality control method has been transformed from the initial chemical consistency evaluation to the consistency evaluation of drug efficacy and safety, the current research on the PK–PD and toxic material basis of TCM is insufficient, which severely limits the development of this integrated strategy. This strategy also relies on the development of analytical techniques as well as PK–PD analysis methods, which require sensitive and rapid detection techniques for the qualitative and quantitative analysis of components in TCM and ideally achieve simultaneous determination of bioactivity and high-risk substances. More importantly, further studies are warranted to determine the content range of these constituents that act as both efficacy- and safety-related Q-markers and to control their contents through process optimization.
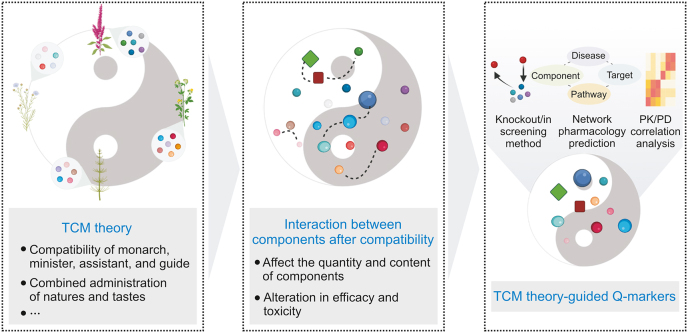
5. TCM theory-guided approach
Formulas containing several medicinal materials are the main form of clinical application in TCM. Their constitution is strictly guided by TCM theory, such as the “compatibility of monarch, minister, assistant, and guide” and “combined administration of natures and tastes”. Based on these theories, TCM formulas can increase efficiency and reduce toxicity through interactions between components. More importantly, in different compatibility environments, the same medicinal materials may play different roles, and their effective and toxic constituents may also be different [71]. In the same recipe of composed medicinal materials, the Q-markers of the TCM formula may be different due to the divergence in the composition ratio and disease targeted. Therefore, Q-marker identification should be organically combined with TCM theory using modern science and technology. However, to date, few studies have incorporated this concept into Q-marker identification. The main reason for this phenomenon is that the scientific principles and material basis of TCM theories have not yet been fully elucidated. Thus, the TCM theory-guided approach for Q-marker identification should take its roots in the compatibility environment and first determine the scientific principles and material basis of the TCM formula by knockout/in-screening methods, network pharmacology prediction, and PK/PD analysis. The Q-markers could later be identified from the material basis of the formula (Fig. 5). Several representative examples and methodologies for this strategy are presented herein.
Fig. 5.
The TCM theory-guided approach for Q-marker identification. The dotted lines indicate the interactions between components. Q-marker: quality marker; TCM: traditional Chinese medicine; PK/PD: pharmacokinetics/pharmacodynamics.
Danggui-Shaoyao San (DSS) is a well-known formula that has been widely used in Asia to treat various gynecological diseases. DSS mainly activates blood circulation and promotes urination [72]. Xu and co-workers [73] used serum pharmacochemistry techniques to investigate the compatibility mechanisms and material basis of DSS by dividing this formula into two groups, a blood-associated herb group and a diuretic-associated herb group, according to TCM theory. Differences in the main components between the co-decoction and individual decoction were the focus of this study. Based on the different pharmacological activities and TCM theory, the six DSS herbs were separated into blood-associated herbs (Angelica sinensis, Paeonia lactiflora, and Ligusticum chuanxiong) and water-associated herbs (Atractylodes macrocephala, Alisma orientale, and Poria cocos). While blood-associated herbs focus on promoting blood circulation, water-associated herbs concentrate on invigorating the spleen to eliminate dampness. The authors demonstrated that the contents of four bioactive constituents (paeoniflorin, albiflorin, ferulic acid, and alisol B 23-acetate) in DSS were significantly higher than those in the blood-associated herb group, water-associated herb group, and individual decoction, uncovering the synergistic mechanism of DSS. Additionally, transitional blood components related to both water- and blood-associated herbs were identified using serum pharmacochemical analysis. Thus, the four bioactive constituents related to the compatibility mechanisms, as well as the metabolites and prototype components detected from the two herb groups, could serve as Q-markers to monitor the whole production process.
Licorice is an essential herbal medicine that has been frequently used to reduce toxicity and/or increase the efficacy of other herbal medicines. The underlying synergistic mechanisms and material basis of these combinations are research hotspots in TCM theory. It has been demonstrated that the interactions between the chemical constituents of licorice and other herbal medicines, variation in chemical composition following compounding, and resulting changes in PK/PD are the main mechanisms of these combinations [74]. Many studies have shown that licorice can reduce the solubility of other toxic ingredients to produce precipitation in the decoction and cause fewer toxic ingredients to enter the body [74,75]. This may be related to glycyrrhiza saponins, which act as excellent surfactants in licorice [76]. Moreover, the influence of licorice on the PK/PD of other herbal medicines has been revealed in some combination preparations [74,77]. Di and co-workers [77] reported that the combination of licorice and Platycodi Radix (Jiegeng in Chinese) can significantly increase the peak concentration and area under the curve of platycodin D compared with that in rats treated with Platycodi Radix alone, while the elimination half-life and time to maximum plasma concentration of platycodin D and deapio-platycodin D were prolonged after the combination of these two herbal medicines. Thus, the Q-markers of licorice are not rigid and need to be changed when licorice is combined with other herbal medicines. This is because the key constituents of licorice may react with other herbal medicines to produce precipitants, and the active ingredients of both licorice and the other herbal medicines may change after interactions.
Based on the nature and taste, the compatibility mechanisms and material basis of Yuanhu Zhitong (YHZT) dropping pills were also revealed. YHZT is composed of Corydalis rhizoma and Angelicae dahuricae radix. While Corydalis rhizoma tastes bitter and pungent, Angelicae dahuricae radix tastes pungent only. Liu and co-workers [78] characterized the main chemical components of YHZT in vitro and identified absorbed prototype constituents and their metabolites in the plasma and brain tissues of rats following oral administration of YHZT. They found that both Corydalis rhizoma and Angelicae dahuricae radix could act on multiple G-protein-coupled receptors (GPCR), and the combination of these two herbs had a better effect than that of a single herb. Tetrahydropalmatine and protopine were detected as the material basis of pungent and bitter tastes, and imperatorin was observed as the material basis of pungent taste using olfactory and taste biomimetic models, molecular docking, and GPCR experiments. More importantly, an obvious in vivo pharmacokinetic interaction between the constituents of Corydalis rhizoma (corydaline, tetrahydropalmatine and protopine) and Angelicae dahuricae radix (imperatorin and isoimperatorin) was identified, further revealing the material basis of the compatibility of this formula. Finally, combined with the results of the PD/PK and network pharmacology analyses, corydaline, tetrahydropalmatine, protopine, imperatorin, and isoimperatorin were identified as the Q-markers of YHZT.
The knockout/in-screening method combined with TCM theory is a useful technique for Q-marker identification. Kong and co-workers [79] adopted a knockout method to investigate the influence of the “monarch, minister, assistant, and guide” compatibility of the Banxiahoupo decoction on the contents of the antidepressant ingredients honokiol and magnolol. They found that the assistant P. cocos (Fuling in Chinese) increased the honokiol and magnolol contents when combined with Pinellia ternata (monarch) and Magnolia bark (minister). They also discovered that the other assistant, fresh ginger, decreased the contents of these two ingredients and that the guide, Perillae folium, had no effect when combined with the monarch and the minister. More interestingly, the honokiol and magnolol contents significantly decreased when either the assistant or the guide was withdrawn from the whole formula, indicating an internal relationship between the compatibility of the monarch, the minister, the assistant, and the guide.
These examples indicate that Q-marker identification needs to embrace the TCM theory, which is conducive to discovering the ingredients directly related to clinical efficacy, revealing the unique components of the same medicinal material in different formulas, as well as in similar formulas with different composition ratios, uncovering the representative ingredients of the same formula used for different diseases and ultimately improving the quality control of TCM. Nevertheless, further efforts are required to elucidate the scientific principles and material basis of TCM theories.
6. Conclusion
Product quality is essential for the sustainable development of TCM. Academia and industry must endeavor to ensure that TCM is safe, effective, stable, and quality controllable. Thus, establishing a quality control system that not only meets the characteristics and principles of TCM but also is recognized by both domestic and foreign pharmaceutical industries is of urgent importance. The concept of quality by testing is outdated because of hysteresis, whereas the proposal of Q-markers with the core features of transitivity and traceability will modernize the quality control of TCM. Four multimodal integrated strategies are reviewed here. They point out important future directions for the establishment of Q-markers and quality control systems. Although the last three strategies have a distinct focus, they are interconnected and their roles may overlap. The TCM theory-guided approach is central and should be embedded within the chemical-activity-based screening and the efficacy, safety, and consistency-dependent identification of Q-markers. Simultaneously, TCM theory-guided and chemical-activity-based Q-markers should be closely linked to clinical efficacy, safety, and quality consistency. Therefore, from an interdisciplinary perspective, the interconnection of these strategies may further enrich TCM quality control. Overall, to achieve comprehensive control, the establishment of Q-markers needs to focus on the entire production chain from raw materials to useable products, whereas the corresponding quality control system should be established by QbD and applied during the entire workflow path. More importantly, the identification of Q-markers should not only be concerned with bioactivity but should also include essential considerations that are guided by TCM theories and are directly related to clinical efficacy and safety. In other words, Q-markers need to reflect the efficacy, safety, and consistent quality of TCM as much as possible and can then be used to control the entire production process. It is worth noting that the Q-markers are not the sum of the quality indicators of each herb in a formula and that not all active components are suitable as Q-markers. Measurability and transferability are essential elements. Moreover, convenience and economic costs need to be considered as well, where instead of monitoring the largest number of ingredients, it is necessary to understand the balance between the variety and quantity of Q-markers and the cost and convenience of detection. This will come in handy in the green manufacturing of TCM. Additionally, several challenges still need to be overcome: 1) the chemical composition of TCM needs to be fully revealed; 2) the scientific principles and material basis behind TCM theories that guide the identification of Q-markers need to be elucidated; 3) the effective and toxic substances of the same medicinal material in different diseases as well as in different formulas need further identification; and 4) the content range of the constituents that belong to both efficacy- and safety-related Q-markers should be determined, and their contents should be monitored and controlled through process optimization. We believe that accelerating the application of multidisciplinary techniques, such as high-throughput and high-content technology, is the driving force in overcoming these challenges.
CRediT author statement
Xiaoyan Lu: Investigation, Writing - Original draft preparation, Reviewing and Editing; Yanyan Jin: Investigation, Writing - Original draft preparation, Reviewing and Editing; Yuzhen Wang: Investigation; Yunlong Chen: Methodology, Supervision, Writing - Reviewing and Editing; Xiaohui Fan: Methodology, Project administration, Funding acquisition, Supervision, Writing - Reviewing and Editing.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No.: 81973701), Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (Grant No.: ZYYCXTD-D-202002), and Westlake Laboratory (Westlake Laboratory of Life Sciences and Biomedicine).
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Contributor Information
Yunlong Chen, Email: chenyl2007@163.com.
Xiaohui Fan, Email: fanxh@zju.edu.cn.
References
- 1.Li Y., Xie Y., He Y., et al. Quality markers of traditional Chinese medicine: Concept, progress, and perspective. Engineering. 2019;5:888–894. [Google Scholar]
- 2.Leong F., Hua X., Wang M., et al. Quality standard of traditional Chinese medicines: Comparison between European pharmacopoeia and Chinese pharmacopoeia and recent advances. Chin. Med. 2020;15 doi: 10.1186/s13020-020-00357-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu C.-X., Chen S.-L., Xiao X.-H., et al. A new concept on quality marker of Chinese materia medica: Quality control for Chinese medicinal products. Chin. Tradit. Herb. Drugs. 2016;47:1443–1457. [Google Scholar]
- 4.Liu C.-X., Cheng Y.-Y., Guo D.-A., et al. A new concept on quality marker for quality assessment and process control of Chinese medicines. Chin. Herb. Med. 2017;9:3–13. [Google Scholar]
- 5.Zhang T.-J., Bai G., Chen C.-Q., et al. Research approaches of quality marker (Q-marker) of Chinese materia medica formula based on “five principles”. Chin. Tradit. Herb. Drugs. 2018;49:1–13. [Google Scholar]
- 6.Li Z.-T., Zhang F.-X., Fan C.-L., et al. Discovery of potential Q-marker of traditional Chinese medicine based on plant metabolomics and network pharmacology: Periplocae Cortex as an example. Phytomedicine. 2021;85 doi: 10.1016/j.phymed.2021.153535. [DOI] [PubMed] [Google Scholar]
- 7.Bai G., Zhang T., Hou Y., et al. From quality markers to data mining and intelligence assessment: A smart quality-evaluation strategy for traditional Chinese medicine based on quality markers. Phytomedicine. 2018;44:109–116. doi: 10.1016/j.phymed.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Sun K., Su C., Li W., et al. Quality markers based on phytochemical analysis and anti-inflammatory screening: An integrated strategy for the quality control of Dalbergia odorifera by UHPLC-Q-Orbitrap HRMS. Phytomedicine. 2021;84 doi: 10.1016/j.phymed.2021.153511. [DOI] [PubMed] [Google Scholar]
- 9.Länger R., Stöger E., Kubelka W., et al. Quality standards for herbal drugs and herbal drug preparations-appropriate or improvements necessary? Planta Med. 2018;84:350–360. doi: 10.1055/s-0043-118534. [DOI] [PubMed] [Google Scholar]
- 10.International Conference on Harmonisation . 2009. ICH Harmonised Tripartite Guideline: Pharmaceutical Development Q8 (R2)https://database.ich.org/sites/default/files/Q8_R2_Guideline.pdf [Google Scholar]
- 11.International Conference on Harmonisation . 2008. ICH Harmonised Tripartite Guideline: Pharmaceutical Quality System Q10.https://database.ich.org/sites/default/files/Q10_Guideline.pdf [Google Scholar]
- 12.Gong X.-C., Chen T., Qu H.-B. Research advances in secondary development of Chinese patent medicines based on quality by design concept. China J. Chin. Mater. Med. 2017;42:1031–1036. doi: 10.19540/j.cnki.cjcmm.20170223.020. [DOI] [PubMed] [Google Scholar]
- 13.Liu X., Jiang W., Su M., et al. Quality evaluation of traditional Chinese medicines based on fingerprinting. J. Separ. Sci. 2020;43:6–17. doi: 10.1002/jssc.201900365. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y.-H., Bi J.-H., Xie M., et al. Classification-based strategies to simplify complex traditional Chinese medicine (TCM) researches through liquid chromatography-mass spectrometry in the last decade (2011-2020): Theory, technical route and difficulty. J. Chromatogr. A. 2021;1651:462307. doi: 10.1016/j.chroma.2021.462307. [DOI] [PubMed] [Google Scholar]
- 15.Zeng J.-Q., Zhang J., Zhang F.-Y., et al. Critical quality attribute assessment of big brand traditional Chinese medicine: Online NIR quality control research on boiling time during extraction process. China J. Chin. Mater. Med. 2021;46:1644–1650. doi: 10.19540/j.cnki.cjcmm.20210205.304. [DOI] [PubMed] [Google Scholar]
- 16.Xue J., Shi Y., Ye L., et al. Near-infrared spectroscopy for rapid and simultaneous determination of five main active components in rhubarb of different geographical origins and processing. Spectrochim. Acta Mol. Biomol. Spectrosc. 2018;205:419–427. doi: 10.1016/j.saa.2018.07.055. [DOI] [PubMed] [Google Scholar]
- 17.Yao H., Shi P., Shao Q., et al. Chemical fingerprinting and quantitative analysis of a Panax notoginseng preparation using HPLC-UV and HPLC-MS. Chin. Med. 2011;6:9. doi: 10.1186/1749-8546-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L., Li Z., Shao Q., et al. Dissecting active ingredients of Chinese medicine by content-weighted ingredient-target network. Mol. Biosyst. 2014;10:1905–1911. doi: 10.1039/c3mb70581a. [DOI] [PubMed] [Google Scholar]
- 19.Yang Z., Shao Q., Ge Z., et al. A bioactive chemical markers based strategy for quality assessment of botanical drugs: Xuesaitong injection as a case study. Sci. Rep. 2017;7:2410. doi: 10.1038/s41598-017-02305-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong Y., Zhu J., Yang Z., et al. Q-marker based strategy for CMC research of Chinese medicine: A case study of Panax notoginseng saponins. Phytomedicine. 2018;44:129–137. doi: 10.1016/j.phymed.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 21.Yan X., Fu H., Zhang S., et al. Combining convolutional neural networks and in-line near-infrared spectroscopy for real-time monitoring of the chromatographic elution process in commercial production of notoginseng total saponins. J. Separ. Sci. 2020;43:663–670. doi: 10.1002/jssc.201900874. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Y.F., Zhou L.H., Zhang F.L., et al. The online control in the extraction of Panax notoginseng by using near infrared spectroscopy. Chin. Med. Mat. 2019;42:2367–2370. [Google Scholar]
- 23.Wang X.-Y., Li W.-L., Qu H.-B. Application of feedforward control strategy based on spectra of raw materials to optimize alcohol extraction process of Panax notoginseng. China J. Chin. Mater. Med. 2018;43:3127–3134. doi: 10.19540/j.cnki.cjcmm.20180611.007. [DOI] [PubMed] [Google Scholar]
- 24.Lu X., Lian X., Zheng J., et al. LC-ESI-TOF-MS-based metabolomic analysis of ginsenoside Rd-induced anaphylactoid reaction in mice. RSC Adv. 2016;6:19545–19554. [Google Scholar]
- 25.Cheng Y.-Y., Zhang B.-L., Fang T.-H., et al. Intelligent and lean manufacturing for Chinese medicine: Theory and its applications, Chin. J. Chin. Mater. Med. 2019;44:5017–5021. doi: 10.19540/j.cnki.cjcmm.20191203.301. [DOI] [PubMed] [Google Scholar]
- 26.Xu H., Zhang Y., Wang P., et al. A comprehensive review of integrative pharmacology-based investigation: A paradigm shift in traditional Chinese medicine. Acta Pharm. Sin. B. 2021;11:1379–1399. doi: 10.1016/j.apsb.2021.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao M., Shang H., Li Y., et al. An integrated approach to uncover quality marker underlying the effects of Alisma orientale on lipid metabolism, using chemical analysis and network pharmacology. Phytomedicine. 2018;45:93–104. doi: 10.1016/j.phymed.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Li D., Lv B., Wang D., et al. Network pharmacology and bioactive equivalence assessment integrated strategy driven Q-markers discovery for Da-Cheng-Qi decoction to attenuate intestinal obstruction. Phytomedicine. 2020;72:153236. doi: 10.1016/j.phymed.2020.153236. [DOI] [PubMed] [Google Scholar]
- 29.Chen Z., Wang M., Yang Y., et al. Promotion of a quality standard for Porana sinensis Hemsl. based on the efficacy-oriented Effect-Constituent Index. Biomed. Chromatogr. 2020;34 doi: 10.1002/bmc.4726. [DOI] [PubMed] [Google Scholar]
- 30.Li C., Tu C., Che Y., et al. Bioassay based screening for the antiplatelet aggregation quality markers of Polygonum multiflorum with UPLC and chemometrics. J. Pharm. Biomed. Anal. 2019;166:264–272. doi: 10.1016/j.jpba.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Jiang Z., Yang J., Wang Y. Discrimination and identification of Q-markers based on ‘Spider-web’ mode for quality control of traditional Chinese medicine. Phytomedicine. 2018;44:98–102. doi: 10.1016/j.phymed.2017.12.034. [DOI] [PubMed] [Google Scholar]
- 32.Wu X., Zhang H., Fan S., et al. Quality markers based on biological activity: A new strategy for the quality control of traditional Chinese medicine. Phytomedicine. 2018;44:103–108. doi: 10.1016/j.phymed.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 33.Huo M.-Q., Peng S., Ren Y., et al. Discovery and application of traditional Chinese medicine efficacy markers based on systematic traditional Chinese medicine. China J. Chin. Mater. Med. 2020;45:3245–3250. doi: 10.19540/j.cnki.cjcmm.20200210.402. [DOI] [PubMed] [Google Scholar]
- 34.Liu P., Yang H., Long F., et al. Bioactive equivalence of combinatorial components identified in screening of an herbal medicine. Pharm. Res. 2014;31:1788–1800. doi: 10.1007/s11095-013-1283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X.-G., Cheng C.-Y., Wang J.-X., et al. A metabolic exposure-oriented network regulation strategy for the identification of effective combination in the extract of Ginkgo biloba L. J. Pharm. Biomed. Anal. 2018;149:151–159. doi: 10.1016/j.jpba.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Gong L., Xu H., Wang L., et al. Identification and evaluation of the chemical similarity of Yindan xinnaotong samples by ultra high performance liquid chromatography with quadrupole time-of-flight mass spectrometry fingerprinting. J. Separ. Sci. 2016;39:611–622. doi: 10.1002/jssc.201500836. [DOI] [PubMed] [Google Scholar]
- 37.Liang Y.-Z., Xie P.-S., Chan K. Perspective of chemical fingerprinting of Chinese herbs. Planta Med. 2010;76:1997–2003. doi: 10.1055/s-0030-1250541. [DOI] [PubMed] [Google Scholar]
- 38.Xu G.-L., Xie M., Yang X.-Y., et al. Spectrum-effect relationships as a systematic approach to traditional Chinese medicine research: Current status and future perspectives. Molecules. 2014;19:17897–17925. doi: 10.3390/molecules191117897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H.-B., Wu S.-H., Tang J.-F., et al. Research progress on Q-biomarker of Chinese materia medica. Chin. Tradit. Herb. Drugs. 2019;50:4556–4561. [Google Scholar]
- 40.Li K., Li J., Su J., et al. Identification of quality markers of Yuanhu Zhitong tablets based on integrative pharmacology and data mining. Phytomedicine. 2018;44:212–219. doi: 10.1016/j.phymed.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Guo R., Zhang X., Su J., et al. Identifying potential quality markers of Xin-Su-Ning capsules acting on arrhythmia by integrating UHPLC-LTQ-Orbitrap, ADME prediction and network target analysis. Phytomedicine. 2018;44:117–128. doi: 10.1016/j.phymed.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 42.Wang H., Sun H., Zhang A., et al. Rapid identification and comparative analysis of the chemical constituents and metabolites of Phellodendri amurensis cortex and Zhibai dihuang pill by ultra-performance liquid chromatography with quadrupole TOF-MS. J. Separ. Sci. 2013;36:3874–3882. doi: 10.1002/jssc.201300794. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y., Xu H., Chen X., et al. Study on the application of intestinal absorption in vitro coupled with bioactivity assessment in Yuanhu Zhitong preparation. J. Med. Plants Res. 2012;6:1941–1947. [Google Scholar]
- 44.Zhang Y., Xu H., Chen X., et al. Simultaneous quantification of 17 constituents from Yuanhu Zhitong tablet using rapid resolution liquid chromatography coupled with a triple quadrupole electrospray tandem mass spectrometry. J. Pharm. Biomed. Anal. 2011;56:497–504. doi: 10.1016/j.jpba.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 45.Tao Y., Xu H., Wang S., et al. Identification of the absorbed constituents after oral administration of Yuanhu Zhitong prescription extract and its pharmacokinetic study by rapid resolution liquid chromatography/quadrupole time-of-flight. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013;935:1–9. doi: 10.1016/j.jchromb.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 46.Wang P., Zhang T., Yu G., et al. Poly-pharmacokinetic strategy-delineated metabolic fate of bioactive compounds in a traditional Chinese medicine formula, Yuanhu Zhitong tablets, using parallel reaction monitoring mode. Phytomedicine. 2019;53:53–61. doi: 10.1016/j.phymed.2018.09.026. [DOI] [PubMed] [Google Scholar]
- 47.Xu H., Tao Y., Lu P., et al. A computational drug-target network for Yuanhu Zhitong prescription. Evid. Based Complement Alternative Med. 2013;2013:658531. doi: 10.1155/2013/658531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Z.-H., Ai N., Yu L., et al. A multiple biomarker assay for quality assessment of botanical drugs using a versatile microfluidic chip. Sci. Rep. 2017;7:12243. doi: 10.1038/s41598-017-12453-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao H., Wu B., Cheng Y., et al. High throughput chemiluminescence platform for evaluating antioxidative activity of total flavonoid glycosides from plant extracts. Food Chem. 2009;115:380–386. [Google Scholar]
- 50.Liu E.-H., Qi L.-W., Li K., et al. Recent advances in quality control of traditional Chinese medicines. Comb. Chem. High Throughput Screen. 2010;13:869–884. doi: 10.2174/138620710793360301. [DOI] [PubMed] [Google Scholar]
- 51.Shen M.-R., He Y., Shi S.-M. Development of chromatographic technologies for the quality control of traditional Chinese medicine in the Chinese Pharmacopoeia. J. Pharm. Anal. 2021;11:155–162. doi: 10.1016/j.jpha.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.U.S. Food and Drug Administration, Botanical Drug Development: Guidance for Industry. https://www.fda.gov/media/93113/download. (Accessed 28 February 2022).
- 53.European Medicines Agency, Guideline on Specification: Test Procedures and Acceptance Criteria for Herbal Substances, Herbal Preparations and Herbal Medicinal Products/traditional Herbal Medicinal Products. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-specifications-test-procedures-acceptance-criteria-herbal-substances-herbal-preparations_en.pdf. (Accessed 28 February 2022).
- 54.Li Q., Li Z.-Y., Zhang J., et al. Xiyanping plus azithromycin chemotherapy in pediatric patients with Mycoplasma pneumoniae pneumonia: A systematic review and meta-analysis of efficacy and safety. Evid. Based Complement Alternative Med. 2019;2019:2346583. doi: 10.1155/2019/2346583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang G.-F., Xu H., Liu K., et al. Determination of total sulfonate of andrographolide in Xiyanping injection by differential spectrophotometry. Chin. J. Pharm. Anal. 2008;28:1680–1682. [Google Scholar]
- 56.Zhan H.Z. Nanchang: Nanchang University; 2012. Study on improving quality standards of Xiyanping injection [master’s Thesis] [Google Scholar]
- 57.Zhan H.-Z., Chen W.-K., Xiao X.-W., et al. HPLC simultaneous determination of four effective ingredients in Xiyanping injection. Chin. J. Pharm. Anal. 2012;32:140–143. [Google Scholar]
- 58.Chen S., Kwong J.S.W., Zheng R., et al. Normative application of Xiyanping injection: A systematic review of adverse case reports. Evid. Based Complement. Alternat. Med. 2018;2018:4013912. doi: 10.1155/2018/4013912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Q.-W., Li Q., Zhang J., et al. Crystal structure and anti-inflammatory and anaphylactic effects of andrographlide sulphonate E in Xiyanping, a traditional Chinese medicine injection. J. Pharm. Pharmacol. 2019;71:251–259. doi: 10.1111/jphp.13028. [DOI] [PubMed] [Google Scholar]
- 60.Lin Y., Wang C., Hou Y., et al. The human mast cell line-1 cell membrane chromatography coupled with HPLC-ESI-MS/MS method for screening potentical anaphylactic components from Chuanxinlian injection. Biomed. Chromatogr. 2017;31 doi: 10.1002/bmc.4015. [DOI] [PubMed] [Google Scholar]
- 61.Chen W.-Y., Wang Y.-S., Liu C.-Y., et al. Comparison of pulmonary availability and anti-inflammatory effect of dehydroandrographolide succinate via intratracheal and intravenous administration. Eur. J. Pharmaceut. Sci. 2020;147:105290. doi: 10.1016/j.ejps.2020.105290. [DOI] [PubMed] [Google Scholar]
- 62.Lin B., Yang H., Liu Z., et al. Comparative study on fingerprint, anti-inflammatory effect and toxicity in vivo of Tripterygium polyglycosides tablets from various manufacturers. Chin. Pharmacist. 2019;22:206–210+230. [Google Scholar]
- 63.Goldbach-Mansky R., Wilson M., Fleischmann R., et al. Comparison of Tripterygium wilfordii Hook F versus sulfasalazine in the treatment of rheumatoid arthritis: A randomized trial. Ann. Intern. Med. 2009;151:229–240. doi: 10.7326/0003-4819-151-4-200908180-00005. W49−51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Z., Ma L., Zhou G.-B. The main anticancer bullets of the Chinese medicinal herb, thunder god vine. Molecules. 2011;16:5283–5297. doi: 10.3390/molecules16065283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Su M.-X., Zhou W.-D., Lan J., et al. Rapid and sensitive analysis of multiple bioactive constituents in Tripterygium glycosides tablets using liquid chromatography coupled with time-of-flight mass spectrometry. J. Separ. Sci. 2015;38:804–812. doi: 10.1002/jssc.201400946. [DOI] [PubMed] [Google Scholar]
- 66.Gao X., Du X., An L., et al. Wilforine, the Q-marker and PK-maker of Tripterygium glycosides tablet: Based on preparation quantitative analysis and PK–PD study. Phytomedicine. 2019;54:357–364. doi: 10.1016/j.phymed.2018.03.031. [DOI] [PubMed] [Google Scholar]
- 67.Luo X., Chen X., Shen X., et al. Rapid identification and analysis of the active components of traditional Chinese medicine Xiaoxuming decoction for ischemic stroke treatment by integrating UPLC-Q-TOF/MS and RRLC-QTRAP MSn method. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019;1124:313–322. doi: 10.1016/j.jchromb.2019.06.023. [DOI] [PubMed] [Google Scholar]
- 68.Lin Y., Lv Y., Fu J., et al. A high expression Mas-related G protein coupled receptor X2 cell membrane chromatography coupled with liquid chromatography and mass spectrometry method for screening potential anaphylactoid components in Kudiezi injection. J. Pharm. Biomed. Anal. 2018;159:483–489. doi: 10.1016/j.jpba.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 69.Liang G., Yang J., Liu T., et al. A multi-strategy platform for quality control and Q-markers screen of Chaiqin chengqi decoction. Phytomedicine. 2021;85:153525. doi: 10.1016/j.phymed.2021.153525. [DOI] [PubMed] [Google Scholar]
- 70.Zhao C., Liu H., Miao P., et al. A strategy for selecting “Q-markers” of Chinese medical preparation via components transfer process analysis with application to the quality control of Shengmai injection. Molecules. 2019;24:1811. doi: 10.3390/molecules24091811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou M., Hong Y., Lin X., et al. Recent pharmaceutical evidence on the compatibility rationality of traditional Chinese medicine. J. Ethnopharmacol. 2017;206:363–375. doi: 10.1016/j.jep.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 72.Fu X., Wang Q., Wang Z., et al. Danggui-Shaoyao-San: New hope for Alzheimer's disease. Aging Dis. 2015;7:502–513. doi: 10.14336/AD.2015.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Y., Li G., Zhou Y., et al. The difference between blood-associated and water-associated herbs of Danggui-Shaoyao San in theory of TCM, based on serum pharmacochemistry. Biomed. Chromatogr. 2016;30:579–587. doi: 10.1002/bmc.3586. [DOI] [PubMed] [Google Scholar]
- 74.Jiang M., Zhao S., Yang S., et al. An “essential herbal medicine”—licorice: A review of phytochemicals and its effects in combination preparations. J. Ethnopharmacol. 2020;249:112439. doi: 10.1016/j.jep.2019.112439. [DOI] [PubMed] [Google Scholar]
- 75.Wang M., Liu B., Qi Y., et al. Effect of Glycyrrhiza uralensis saponins on acute toxicity of platycodins. Chin. J. Exp. Tradit. Med. Form. 2008;14:59–61. [Google Scholar]
- 76.Cai S.-Y., Lv S.-W., Wang Y.-H., et al. Solubility and apparent oil/water partition coefficient of glycyrrhizin and pachymic acid. Inform. Tradit. Chin. Med. 2012;29:118–121. [Google Scholar]
- 77.Shan J.-J., Zou J.-S., Xie T., et al. Effects of Gancao on pharmacokinetic profiles of platycodin D and deapio-platycodin D in Jiegeng. J. Ethnopharmacol. 2015;170:50–56. doi: 10.1016/j.jep.2015.04.056. [DOI] [PubMed] [Google Scholar]
- 78.Zhang T.-J., Xu J., Shen X.-P., et al. Relation of “property-response-component” and action mechanism of Yuanhu zhitong dropping pills based on quality marker (Q-marker) Chin. Tradit. Herb. Drugs. 2016;47:2199–2211. [Google Scholar]
- 79.Xu Q., Ouyang Z., Chang Y., et al. Principal, assistant, compleaent, guide compatibility of Banxiahoupo decoction on contents of honokiol and magnolol, chin. J. Exp. Tradit. Med. Form. 2008;14:1–3. [Google Scholar]