Abstract
There is a very complex interaction between the brain and the cerebral vasculature to meet the metabolic demands of the brain for proper function. Preservation of vascular networks and cerebrovascular function ultimately plays a key role in this intricate communication within the brain in health and disease. Experimental evidence showed that diabetes not only affects the architecture of cerebral blood arteries causing adverse remodeling, pathological neovascularization, and vasoregression, but also alters cerebrovascular function resulting in compromised myogenic reactivity and endothelial dysfunction. Coupled with the disruption of blood brain barrier (BBB) integrity, changes in blood flow and microbleeds into the brain can rapidly occur. When an ischemic insult is superimposed on this pathology, not only is the neurovascular injury greater, but repair mechanisms fail, resulting in greater physical and cognitive deficits. While clinically it is known that women suffer disproportionately from diabetes as well as ischemic stroke and post-stroke cognitive impairment, the cerebrovascular architecture, patho/physiology, as well as cerebrovascular contributions to stroke recovery in female and diabetic animal models are inadequately studied and highlighted in this review.
Keywords: cerebrovasculature, vascularization, stroke, diabetes, sex differences
Introduction
The cerebral circulation has unique features that are critical for brain function. First, the brain is a highly metabolic organ needing about 20% of cardiac output with each systole and as such relies on constant cerebral blood flow (CBF)1. Second, tight autoregulation of CBF achieved by myogenic, metabolic, chemical, and endocrine processes protects the brain from fluctuations in perfusion pressure and provides the blood supply2. Third, cerebral perfusion is fine-tuned by neurovascular coupling to deliver blood where there is a higher need3. Fourth, the blood brain barrier (BBB), formed by the endothelium, basal lamina, astrocytes and pericytes, provides extra protection for the brain. Thus, it is not surprising that an extensive vascular network with specialized architecture exists to achieve such complex functions1. It is also not surprising that brain health is linked to cardiovascular health4. Metabolic diseases such as obesity, insulin resistance, and diabetes are not only major risk factors for cardiovascular disease but also cerebrovascular diseases including stroke and cognitive impairment5. In this context, it has to be recognized that there are sex differences in cerebrovascular function/architecture as well as in the metabolic diseases that have detrimental impact on the cerebrovasculature6,7. A recent comprehensive review on cerebrovascular physiology highlighted that while considerable work has been reported in male animal models under physiological and pathological conditions, our knowledge of cerebrovascular function and structure in females is limited8. Here we will focus on what is known about the sex dependent influences of diabetes on recovery from ischemic stroke.
Sex hormones and their receptors in the cerebrovasculature mediate many of the sex differences in cerebrovascular reactivity and regulation of blood flow6, angiogenesis and cerebrovascular remodeling9, and inflammation and oxidative stress10,11. The cerebrovasculature is an important target of sex hormones, including androgens, estrogens, progestins, and their derivatives, throughout the female lifespan. Steroid hormone receptors ER-alpha, ER-beta, and AR have been identified in human brain microvascular endothelial cells and vascular smooth muscle cells in both males and females12,13. The variations in sex hormone levels throughout a female’s reproductive lifespan have been associated with persistent sex differences in cerebral blood flow6. However, more research is needed to understand how fluctuations in sex hormones spanning from puberty, pregnancy, and menopause contribute to underlying changes in cerebrovascular structure and function.
The purpose of this review is to provide an update on cerebrovascular structure and function in metabolic diseases and stroke from a female perspective. The review is organized into two main sections focusing on cerebrovascular structure/architecture, including BBB, and cerebrovascular function, including vasoreactivity and CBF. Each section starts with what is known under physiological conditions, followed by what is known in diabetes and finally how ischemic stroke affects the cerebrovasculature under these experimental conditions, if known. We also point out specific examples of sex hormonal influence on parts of the cerebrovasculature.
A. Cerebrovascular Networks and Structure
There are three tiers of vasculature that supply blood to the brain14,15. Surface pial arteries and veins forming the top level provide circulation of the surface of the brain2,16. Penetrating arteries originating from this network form the second level and give rise to penetrating arterioles that dive deep into the brain parenchyma17. The third tier is the 3-dimensional capillary network. Intracerebral arterioles and capillaries form the neurovascular unit where there is constant bidirectional communication among neurons, glial cells, and the vasculature to couple neuronal signals to vascular responses17. In population-based studies, microvascular dysfunction manifesting as white matter hyperintensities18,19 or as lacunes20 are associated with increased risk for stroke and global or selective cognitive deficits, changes in mood, decrease motor function21,22. Thus, there is a great interest to better understand the cerebrovascular networks but imaging of the small vessels, especially capillaries, has been challenging. Most of our knowledge of the network topology and angioarchitecture of the brain comes from multiphoton imaging modalities, which have high resolution but are restricted to cortical layers due to penetration limitations, or reconstructed from 3-dimensional confocal imaging of brain sections, mostly from male animals8,23.
A1. Cerebrovascular angioarchitecture in female and male experimental models
There are intrinsic structural differences between sexes in cerebrovascular angioarchitecture in animal models, such as young Sprague-Dawley (SD) rats24. Female middle cerebral arteries (MCAs) have a smaller wall thickness and inner diameter than male MCAs and are associated with diminished myogenic response and CBF autoregulation24. Female MCAs also have less vascular smooth muscle cells and more collagen in the tunica media than males. Although these structural differences do not lead to altered neurovascular coupling and cognition in young SD rats, it is currently unclear if they play a role in the development of CVD in aged animals. We discuss additional sex differences in other experimental models as they relate to diabetes and cerebrovascular angioarchitecture in section A2.
A recent elegant study provided comparative information with respect to geometry, topology and complexity of the cerebrovasculature of the entire brain as well as brain region specific information in both female and male mice using high resolution micro-CT imaging25. This study used vascular corrosion casts made by transcardial perfusion of a polyurethane resin in 3-month-old mice. Analysis of the whole brain vasculature showed that vessels in the 2–15 μm range formed the largest proportion of vessels in both female and male mice (Figure 1). There were very intriguing sex differences. Total vascular volume was higher in females (2.2 × 1010 ± 4.0 × 109μm3) than in males (1.3 × 1010 ± 1.7 × 109μm3). On the other hand, intervessel distance was higher and revealed a larger number of parenchymal spaces in male mice. Fractal dimension, an index of complexity of structures, was greater in females suggesting a more intricate network. When the authors looked at the primary somatosensory cortex, they noted that 4 and 6 μm capillaries represented approximately 60% of the total population of vessel-vessel connections. Within this group of capillaries, they noted that branch points of 6 μm vessels were greater in female mice. The authors also reported subregion specific differences. For example, the density of capillaries was highest in the primary somatosensory cortex and lowest in the dentate gyrus in both female and male mice. Capillary length in the cingulate gyrus was higher in males, whereas in the medial orbital frontal cortex, females had greater values. In the context of ischemic stroke, the authors suggested that in addition to sex hormone effects, a greater total number of capillaries organized into a more complex network may be an important factor contributing to lower vulnerability to ischemic injury in adult female mice25. It was also noted that rarefaction of the cerebral collateral circulation reported with hormonal decline and/or metabolic risk factors may explain more severe ischemic injury after menopause26.
Figure 1.
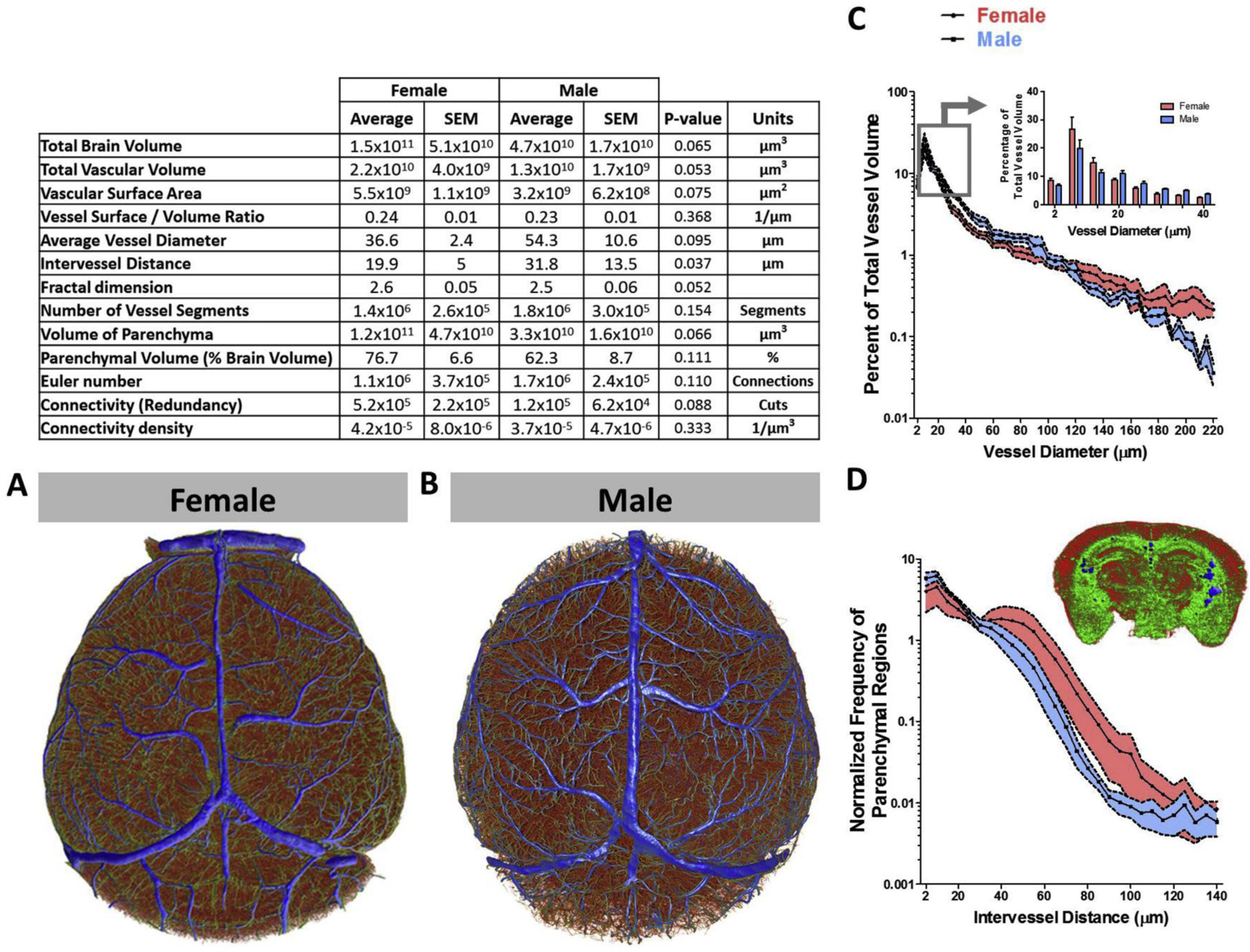
There are sex differences in the angioarchitecture of female and male mice. MicroCT analysis of cerebrovascular corrosion casts revealed greater intervessel distance in males whereas females exhibited greater vascular density. (Reproduced with permission from ref 9).
In terms of BBB, it is well established that female sex and sex hormones confer protective effects. Endothelial cells are the mainstay of BBB. Recent studies showed there are sex specific differences in endothelial cell biology/pathology27,28. For example, brain endothelial cells from female animals are more resistant to cell death28, which may contribute to better BBB integrity in females. Studies, however, suggest that the protective effect of estrogen on BBB integrity declines with age and other disease conditions. Readers are referred to a recent review that summarizes sex differences in BBB permeability, tight junctions, leukocyte migration, water homeostasis and transport6.
A2. Diabetes, stroke and the cerebral vascularization in female and male experimental models
Metabolic diseases have been shown to induce remodeling and alterations of the structure of cerebrovasculature, but most studies were conducted using male animals8,29,30. Leptomeningeal arteries including pial arteries perfuse the superficial surface of the brain and provide collateral circulation. In a comprehensive study, different mouse models of hyperlipidemia, obesity, and diabetes were compared for pial collateral number as well as diameter in response to an ischemic insult in both sexes26. In obese mice (Lepob/ob) pial collateral numbers were equivalent to wild type but collateral diameter was reduced. However, fewer native pial collaterals were observed in mice with hyperlipidemia and moderate obesity (Ldlr−/− fed a Western diet), hyperlipidemic mice (Apoe−/− fed a normal diet), and type 1 diabetic mice (Ins2Akita). Mice with metabolic syndrome (Lepob/ob; Ldlr−/−) that have type 2 diabetes, hypertension, moderate obesity, and hyperlipidemia had pronounced reduction in perfusion as well as number and diameter of pial collaterals26. The authors did not report any sex differences. On the other hand, greater tortuosity as well as collateral numbers and size in the pial vasculature were reported in male Goto Kakizaki (GK) rats, a nonobese model of diabetes31. Diabetes significantly increased intra-tree arteriole-to-arteriole anastomoses between pial anterior and middle cerebral arteries and the BBB permeability through the decrease in tight junction proteins31. Further studies using 3-dimensional reconstruction of the fluorescein isothiocyanate (FITC)-filled vessels showed greater cortical and striatal vascularization as evidenced by greater vascular density, volume, surface area, and branching in the GK model as compared to control Wistar rats32,33. As reported in other species, vascularization was greater in the cortex than striatum and these newly formed/remodeled vessels lacked proper pericyte coverage. Furthermore, colocalization of the FITC signal with isolectin B4, an endothelial cell marker, showed that there was relatively more isolectin staining in diabetic sections suggesting the presence of more non-perfused vessels32. These alterations that occurred early in the disease process did not progress as animals aged34.
A different model of diabetes, the Leprdb/db model, also showed greater branch density and tortuosity with a trend for decreased lumen diameter of the penetrating arterioles. There was an increase in vascular volume and surface area that was more dominant in the microvasculature32,33. Similar cortical and striatal aberrant vascularization patterns were observed in male rats in which diabetes was induced by a high fat diet/low streptozotocin (HFD/STZ) combination35. However, the same brain regions in female rats showed no difference in vascular volume, surface area, or density between control and HFD/STZ diabetic rats (unpublished data). Vascular density appeared to be greater in control and diabetic female rats than male rats as reported in control mice (Figure 2, unpublished results). On the other hand, a direct comparison of the vascularization status of the dentate gyrus, an important domain for learning and memory, between control and diabetic female and male rats suggested sex differences36. While diabetes was associated with higher branch density in both sexes, surface area was lower in diabetic males but higher in diabetic females. Interestingly, male diabetic rats had less neurons, but diabetes did not affect neuronal counts in females36.
Figure 2.
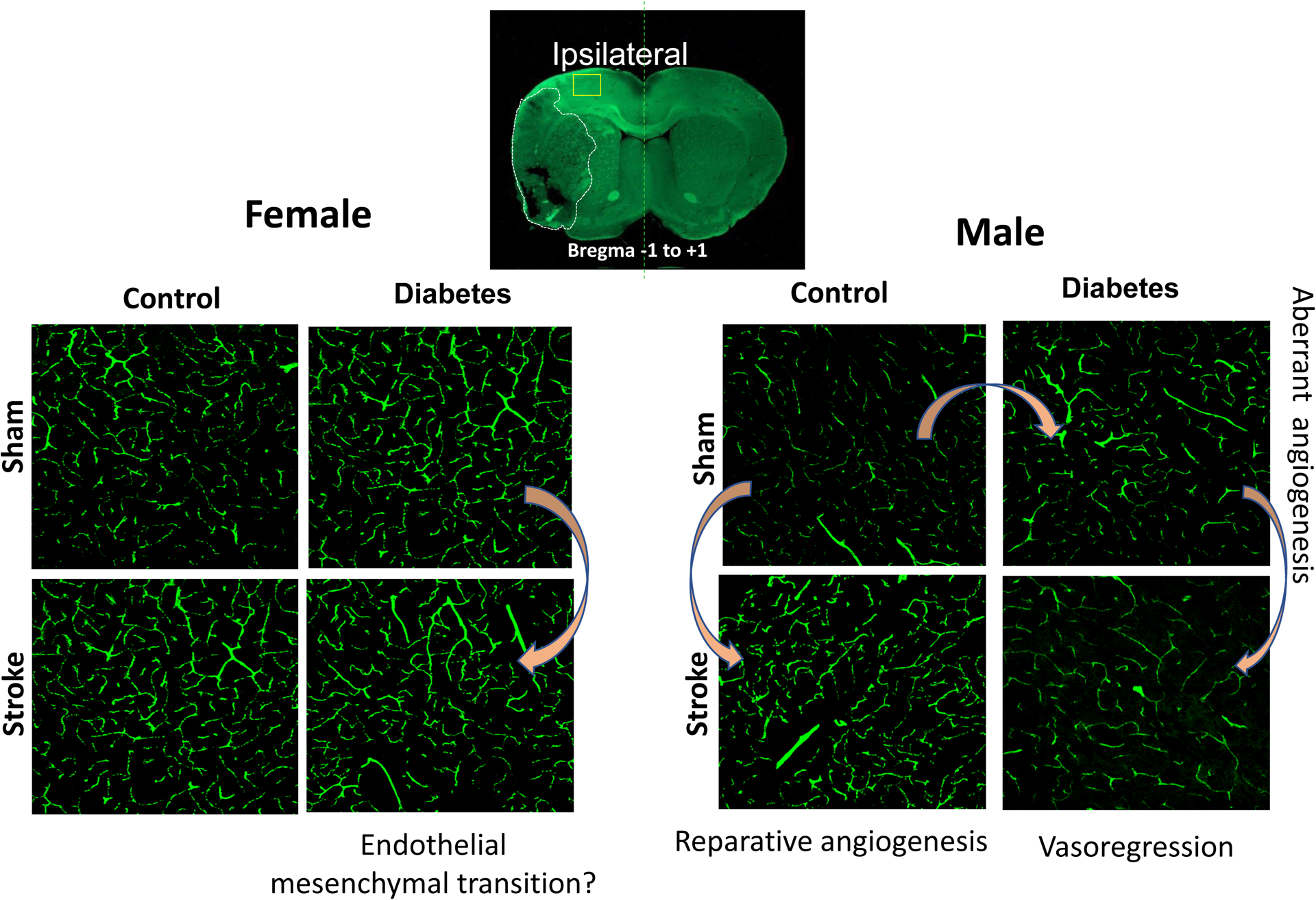
Cerebral vascularization in the frontal cortex of female and male rats exhibit sex differences in diabetes and after stroke. Diabetes promotes pathological angiogenesis in male rats whereas there are no changes in vascularization patterns in diabetic female rats as compared to controls. Ischemic stroke triggers reparative angiogenesis in control male rats whereas diabetic male rats develop vasoregression characterized by regulated endothelial cell death by Day 14 after stroke. In female control rats, stroke does not affect vascularization status, but diabetic female rats show phenotypic changes characteristic of endothelial mesenchymal transition.
As described above, diabetes alters both the structure and function of the cerebrovasculature. These changes may not only increase the risk but may also worsen the recovery and functional outcome when the brain is challenged with a second insult such as stroke37–39. It is now well accepted that protection of neuronal death is not sufficient for a recovery from stroke and neuro-restoration should be accompanied and supported by vascular restoration. It has been shown that male GK rats that exhibit pathological excessive vascularization as described above develop hemorrhagic transformation and profound loss of vasculature 14 days after stroke in peri-infarct area, cortex and striatum relatively distal to the injured zone38,40. This loss is associated with increased endothelial apoptosis driven by peroxynitrite-mediated switch of VEGF-akt1-cell survival pathway to VEGF-MAPK-cell death pathway41. Treatment with metformin during the 14 day recovery period following stroke prevents this dramatic loss of vasculature resulting in better functional outcomes.
On the other hand, in type 1 diabetes, vascular density was reported to be increased in the peri-infarct area 14 days after stroke42. Another study measured vessel density and sprouting after photothrombotic stroke in a model of type 1 diabetes using elegant histological and in vivo time lapse imaging43. Stroke injury increased vascular density in both control and diabetic animals, and this was postulated to be a result of the robust dilation rather than the sprouting of vessels. Interestingly, this study also reported lesser branch points indicative of increased pruning after stroke as seen in GK rats but to a lesser extent43.
Studies with the HFD/STZ model of diabetes suggested that the loss of cerebrovasculature after stroke may be dependent on the extent of vascular injury and presence of hemorrhagic transformation induced by ischemia35. Indeed, a recent follow-up study showed that iron chelation by deferoxamine after thromboembolic stroke prevented the cerebral vasoregression and this was associated with better functional outcomes 14 days after stroke in male diabetic rats44. In diabetic female rats, thromboembolic stroke caused severe hemorrhagic transformation45 but in contrast to males, there was no obvious vasoregression (Figure 2, unpublished results). It is recognized differences between animal strains, experimental models, duration of insult and the number of metabolic risk factors present within each of these models may contribute to these differences but at least in the HFD/TZ model, same strain of animals were matched for age and duration/severity of diabetes were similar. In vitro studies utilizing male and female brain microvascular endothelial cell lines suggest that male cells may be more susceptible to ferroptosis, an iron dependent form of cell death, when challenged with iron or hypoxia44, whereas, female cells survive and undergo phenotypic changes such as endothelial mesenchymal transition under similar conditions (unpublished results). These differences in endothelial cell responses and vascular restoration patterns may be critical for developing sex specific therapies after stroke and warrant further investigations.
B. Cerebrovascular Function
Diabetes has been associated with accelerated impairment of neurovascular coupling16. The cerebrovasculature relies on neurovascular coupling to maintain homeostasis of the cerebral microenvironment. Impairments in neurovascular coupling can lead to poor delivery of energy substrates necessary for neuronal activity and build-up of potentially harmful toxic by-products of brain metabolism3. Iadecola has provided two excellent reviews that provide an extensive overview of the potential mediators of neurovascular coupling, including the role of each cell type within the neurovascular unit3,46. In the following sections, we will discuss how some of these mediators are affected by sex and disease, leading to alterations in cerebrovascular function.
B1. Cerebrovascular physiology and CBF in female and male experimental models
CBF is tightly regulated by mechanisms intrinsic to the vascular wall, i.e. myogenic autoregulation, as well as responses to vasoactive factors. An earlier study compared myogenic tone in middle cerebral arteries (MCA) from male and female mice as well as in ovariectomized (OVX) and estrogen supplemented female mice. The authors provided evidence that sex and estrogen status affect myogenic reactivity of cerebral arteries and that inhibition of nitric oxide synthase (NOS) mediated greater myogenic tone in arteries from male mice47. Another study reported that MCA myogenic and distensibility characteristics exhibit significant sex- and strain-dependent differences48. For example, myogenic reactivity was impaired in male Wistar Kyoto (WKY) rats as well as in stroke-prone spontaneously hypertensive rats (SHRSP) when compared to females in each strain. Comparison of SHRSP with WKY in males revealed similar myogenic reactivity, but in females SHRSP exhibited augmented myogenic reactivity. Stress-strain relationships in WKY rats showed stiffer vessels in females than in males. Conversely, in SHRSP, male vessels were stiffer than female vessels. A recent comparative study showed that aging augmented the myogenic reactivity of MCAs in female rats, but the effect was attenuated in males. The authors also reported that this effect in females was due to downregulation of large conductance potassium (BK) channels49. These differences in myogenic reactivity and tone may contribute to the differences in stroke-related cerebrovascular events in female and male animals.
Vasoactive substances may also exert sexually dimorphic contractile and/or dilatory responses. MCAs from female rodents display greater NO-dependent vasodilatation50,51. In general, estrogen promotes the production of vasodilatory factors such as NO and prostacyclin. However, as recently reviewed, estrogen-mediated vasoreactivity shows sex differences under pathological conditions6. It is interesting to note that contractile responses to endothelin-1 (ET-1) and angiotensin II are attenuated in human cerebral arteries isolated from women52. While regulation of cerebral vasoreactivity is multifactorial, given that ET-1 is the most potent vasoconstrictor with proliferative, proinflammatory and prosclerotic properties that has been shown to contribute to cerebrovascular dysfunction/remodeling associated with diabetes as discussed below, it is important to summarize the brain ET system with respect to sex differences for the purpose of this review53.
The family of endothelins, consisting of three related vasoactive peptides, ET-1, ET-2, and ET-3, plays important roles in cardiovascular (patho)physiology and embryonic development54,55. While ET-1 was the first isoform to be isolated from endothelial cells as the most potent vasoconstrictor peptide, ETs mediate a wide spectrum of physiological functions via two distinct G protein-coupled receptor subtypes, ETA and ETB. The ETA receptor, localized mainly on vascular smooth muscle cells (VSMCs) of arteries and arterioles as well as on pericytes surrounding capillaries, is responsible for the contractile and proliferative responses to ET-1. It was recently shown that this receptor could be found on brain microvascular endothelial cells (BMVECs), which were previously thought to have exclusively ETB receptors56. The role of ETB in vasoreactivity is more complex. For instance, ETB receptor located on endothelial cells mediates vasodilation via the release of relaxing factors such as NO. This receptor subtype can also lead to vasoconstriction when the receptors are expressed on VSMCs, as occurs in disease conditions57. Sex differences with respect to expression and biological effects of ET-1 and its receptors have been reported in the kidney58. In the brain, a recent study showed that plasma and brain ET-1 levels were similar in both female and male Wistar rats under physiological conditions59. The same study also reported that a human BMVEC line established from a male patient (hBEC5i) secretes 10-fold higher levels of ET-1 than female BMVECs (hCMEC/D3) do. Both female and male BMVECs were found to express the ETA receptor which was previously reported in primary BMVECs isolated from male rats. Whether these interesting in vitro findings hold in vivo remain to be demonstrated.
With respect to CBF, it is known that CBF is higher in women than in men across the lifespan60–62. Given that men have a thicker cortex and higher synaptic density as compared to women, this has been puzzling. As discussed earlier, comparative analysis of the cerebrovascular angioarchitecture in male and female mice showed greater microvascular density and complexity in females. This finding may explain sex differences in CBF and deserves further investigation to determine the physiological significance25. In this regard, it is interesting to note that an early study reported that ET-1 secreted from brain capillary endothelial cells causes actin cytoskeleton reorganization and morphological changes in pericytes in a paracrine manner via ETA-induced increase in intracellular calcium levels63. While in vivo evidence is lacking, in light of the fact that capillaries constitute about 85% of the cerebrovasculature, capillary endothelial cells and pericytes may have a profound effect on regulation of CBF, and ET-1 is likely to be an important contributor64,65.
B2. Diabetes, stroke, and cerebrovascular dysfunction in female and male experimental models
Diabetes is associated with endothelial dysfunction and morphological changes in the cerebral arteries, which collectively set a platform for impaired myogenic response. Various experimental studies, mostly using male animals, have demonstrated that arteries isolated from diabetic animals tend to develop more tone than control animals in response to increases in pressure in both type 1 diabetes and type 2 models (please see8 for complete review). Zimmermann et al reported that diabetes increases myogenic tone in MCAs isolated from female Sprague-Dawley rats66. MCAs isolated from GK rats rapidly lose their myogenic tone after ischemic injury30 which may be in part responsible for increasing the incidence of hemorrhagic transformation in diabetes67. ET-1 increases myogenic tone of isolated pial arteries and relatively smaller parenchymal arterioles in rodents68. VSMC ETB receptors are upregulated in basilar and MCAs in diabetes and after ischemic events53,57. Treatment with a dual antagonist that blocks both ET receptor subtypes prevents this increase in myogenic tone of small arteries isolated from male rats69. ET antagonism also reverses the myogenic dysfunction when treatment is given after vascular disease is established in diabetes57. Unfortunately, comparative studies on cerebrovascular function in female animals under physiological, diabetes and/or stroke conditions are lacking. Coming back to the ET system, one recent study reported higher plasma ET-1 levels in both female and male diabetic rats as compared to controls. Comparison of plasma ET-1 in diabetic female and male animals subjected to sham or stroke surgery also revealed higher levels in male rats, especially after stroke59. At the cellular level, hypoxia further increased ET-1 secretion in male BMVECs but lowered it in female cells. In addition, male BMVECs expressed more ETA receptors than female cells59. Both hypoxia and diabetes-mimicking growth conditions increased the expression of this receptor subtype. Although group size is small, these intriguing findings need to be further explored.
Conclusions
It is well established that diabetes increases the risk and severity of stroke as well as impairing physical and cognitive recovery after stroke. Given that 1) constant CBF and BBB integrity are essential for brain homeostasis, and 2) cerebral circulation is an early target in diabetes, it is highly likely that changes in cerebrovascular structure and function play an important role in the detrimental effects of diabetes on stroke injury and repair. Sex differences in the overall incidence and mortality associated with stroke have been well-documented70,71. Interestingly, diabetes appears to confer a greater risk of vascular dementia and stroke in women compared to men72,73. However, we would like to emphasize that with the exception of a few studies, most of the preclinical studies referenced in this review studied cerebrovasculature only in male animals. There is an urgent need to incorporate age and biological sex into experimental approaches to investigate cerebral circulation in health and disease. In this regard, diabetes, which promotes premature vascular aging, also serves as a model of metabolic aging to identify cerebrovascular protection and restoration strategies for brain health in both sexes.
Acknowledgements
This study was supported by Veterans Affairs (VA) Merit Review (BX000347), VA Senior Research Career Scientist Award (IK6 BX004471), National Institute of Health (NIH) RF1 NS083559 (formerly RO1 NS083559) and R01 NS104573 (multi-PI, Susan C. Fagan as co-PI) to Adviye Ergul; Diabetic Complications Research Consortium DiaComp awards 17AU3831/18AU3903 (DK076169/115255) to Weiguo Li; and NRSA Individual Postdoctoral Fellowship to Victoria Wolf (F32 HL158011-01).
References
- 1.Cipolla MJ. Integrated Systems Physiology: From Molecule to Function. In: The Cerebral Circulation. San Rafael (CA): Morgan & Claypool Life Sciences Copyright © 2010 by Morgan & Claypool Life Sciences.; 2009. [PubMed] [Google Scholar]
- 2.Cipolla MJ. In: The Cerebral Circulation. San Rafael (CA) 2009. [Google Scholar]
- 3.Iadecola C The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron. 2017;96(1):17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorelick PB, Furie KL, Iadecola C, et al. Defining Optimal Brain Health in Adults: A Presidential Advisory From the American Heart Association/American Stroke Association. Stroke. 2017;48(10):e284–e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141(9):e139–e596. [DOI] [PubMed] [Google Scholar]
- 6.Robison LS, Gannon OJ, Salinero AE, Zuloaga KL. Contributions of sex to cerebrovascular function and pathology. Brain Res. 2019;1710:43–60. [DOI] [PubMed] [Google Scholar]
- 7.Gannon OJ, Robison LS, Custozzo AJ, Zuloaga KL. Sex differences in risk factors for vascular contributions to cognitive impairment & dementia. Neurochem Int. 2019;127:38–55. [DOI] [PubMed] [Google Scholar]
- 8.Coucha M, Abdelsaid M, Ward R, Abdul Y, Ergul A. Impact of Metabolic Diseases on Cerebral Circulation: Structural and Functional Consequences. Compr Physiol. 2018;8(2):773–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strehlow K, Werner N, Berweiler J, et al. Estrogen increases bone marrow-derived endothelial progenitor cell production and diminishes neointima formation. Circulation. 2003;107(24):3059–3065. [DOI] [PubMed] [Google Scholar]
- 10.Sunday L, Tran MM, Krause DN, Duckles SP. Estrogen and progestagens differentially modulate vascular proinflammatory factors. Am J Physiol Endocrinol Metab. 2006;291(2):E261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park EM, Cho S, Frys KA, et al. Inducible nitric oxide synthase contributes to gender differences in ischemic brain injury. J Cereb Blood Flow Metab. 2006;26(3):392–401. [DOI] [PubMed] [Google Scholar]
- 12.Zuloaga KL, Swift SN, Gonzales RJ, Wu TJ, Handa RJ. The androgen metabolite, 5α-androstane-3β,17β-diol, decreases cytokine-induced cyclooxygenase-2, vascular cell adhesion molecule-1 expression, and P-glycoprotein expression in male human brain microvascular endothelial cells. Endocrinology. 2012;153(12):5949–5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuloaga KL, O’Connor DT, Handa RJ, Gonzales RJ. Estrogen receptor beta dependent attenuation of cytokine-induced cyclooxygenase-2 by androgens in human brain vascular smooth muscle cells and rat mesenteric arteries. Steroids. 2012;77(8–9):835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shih AY, Ruhlmann C, Blinder P, et al. Robust and fragile aspects of cortical blood flow in relation to the underlying angioarchitecture. Microcirculation. 2015;22(3):204–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filosa JA, Morrison HW, Iddings JA, Du W, Kim KJ. Beyond neurovascular coupling, role of astrocytes in the regulation of vascular tone. Neuroscience. 2016;323:96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Zhang X, Ma G, et al. Neurovascular coupling alterations in type 2 diabetes: a 5-year longitudinal MRI study. BMJ Open Diabetes Research & Care. 2021;9(1):e001433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahl E The fine structure of intracerebral vessels. Z Zellforsch Mikrosk Anat. 1973;145(4):577–586. [DOI] [PubMed] [Google Scholar]
- 18.Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34(5):1126–1129. [DOI] [PubMed] [Google Scholar]
- 19.Kuller LH, Longstreth WT Jr., Arnold AM, Bernick C, Bryan RN, Beauchamp NJ Jr. White matter hyperintensity on cranial magnetic resonance imaging: a predictor of stroke. Stroke. 2004;35(8):1821–1825. [DOI] [PubMed] [Google Scholar]
- 20.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348(13):1215–1222. [DOI] [PubMed] [Google Scholar]
- 21.Pantoni L, Basile AM, Pracucci G, et al. Impact of age-related cerebral white matter changes on the transition to disability -- the LADIS study: rationale, design and methodology. Neuroepidemiology. 2005;24(1–2):51–62. [DOI] [PubMed] [Google Scholar]
- 22.Sachdev PS, Wen W, Christensen H, Jorm AF. White matter hyperintensities are related to physical disability and poor motor function. J Neurol Neurosurg Psychiatry. 2005;76(3):362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Driscoll JD, Shih AY, Drew PJ, Cauwenberghs G, Kleinfeld D. Two-photon imaging of blood flow in the rat cortex. Cold Spring Harbor protocols. 2013;2013(8):759–767. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Zhang H, Liu Y, et al. Sex differences in the structure and function of rat middle cerebral arteries. American journal of physiology Heart and circulatory physiology. 2020;318(5):H1219–h1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quintana DD, Lewis SE, Anantula Y, et al. The cerebral angiome: High resolution MicroCT imaging of the whole brain cerebrovasculature in female and male mice. Neuroimage. 2019;202:116109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore SM, Zhang H, Maeda N, Doerschuk CM, Faber JE. Cardiovascular risk factors cause premature rarefaction of the collateral circulation and greater ischemic tissue injury. Angiogenesis. 2015;18(3):265–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huxley VH, Kemp SS, Schramm C, et al. Sex differences influencing micro- and macrovascular endothelial phenotype in vitro. The Journal of physiology. 2018;596(17):3929–3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta NC, Davis CM, Nelson JW, Young JM, Alkayed NJ. Soluble epoxide hydrolase: sex differences and role in endothelial cell survival. Arteriosclerosis, thrombosis, and vascular biology. 2012;32(8):1936–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ergul A, Kelly-Cobbs A, Abdalla M, Fagan SC. Cerebrovascular complications of diabetes: focus on stroke. Endocrine, metabolic & immune disorders drug targets. 2012;12(2):148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly-Cobbs AI, Prakash R, Coucha M, et al. Cerebral myogenic reactivity and blood flow in type 2 diabetic rats: role of peroxynitrite in hypoxia-mediated loss of myogenic tone. The Journal of pharmacology and experimental therapeutics. 2012;342(2):407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li W, Prakash R, Kelly-Cobbs AI, et al. Adaptive cerebral neovascularization in a model of type 2 diabetes: relevance to focal cerebral ischemia. Diabetes. 2010;59(1):228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prakash R, Somanath PR, El-Remessy AB, et al. Enhanced cerebral but not peripheral angiogenesis in the Goto-Kakizaki model of type 2 diabetes involves VEGF and peroxynitrite signaling. Diabetes. 2012;61(6):1533–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prakash R, Johnson M, Fagan SC, Ergul A. Cerebral neovascularization and remodeling patterns in two different models of type 2 diabetes. PloS one. 2013;8(2):e56264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdelsaid M, Kaczmarek J, Coucha M, Ergul A. Dual endothelin receptor antagonism with bosentan reverses established vascular remodeling and dysfunctional angiogenesis in diabetic rats: relevance to glycemic control. Life sciences. 2014;118(2):268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li W, Valenzuela JP, Ward R, et al. Post-stroke neovascularization and functional outcomes differ in diabetes depending on severity of injury and sex: Potential link to hemorrhagic transformation. Experimental neurology. 2019;311:106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ward R, Valenzuela JP, Li W, Dong G, Fagan SC, Ergul A. Poststroke cognitive impairment and hippocampal neurovascular remodeling: the impact of diabetes and sex. American journal of physiology Heart and circulatory physiology. 2018;315(5):H1402–H1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sweetnam D, Holmes A, Tennant KA, et al. Diabetes impairs cortical plasticity and functional recovery following ischemic stroke. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(15):5132–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ergul A, Abdelsaid M, Fouda AY, Fagan SC. Cerebral neovascularization in diabetes: implications for stroke recovery and beyond. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014;34(4):553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ergul A, Valenzuela JP, Fouda AY, Fagan SC. Cellular connections, microenvironment and brain angiogenesis in diabetes: Lost communication signals in the post-stroke period. Brain research. 2015;1623:81–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prakash R, Li W, Qu Z, Johnson MA, Fagan SC, Ergul A. Vascularization pattern after ischemic stroke is different in control versus diabetic rats: relevance to stroke recovery. Stroke. 2013;44(10):2875–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abdelsaid M, Prakash R, Li W, et al. Metformin treatment in the period after stroke prevents nitrative stress and restores angiogenic signaling in the brain in diabetes. Diabetes. 2015;64(5):1804–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye X, Chopp M, Cui X, et al. Niaspan enhances vascular remodeling after stroke in type 1 diabetic rats. Experimental neurology. 2011;232(2):299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tennant KA, Brown CE. Diabetes augments in vivo microvascular blood flow dynamics after stroke. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(49):19194–19204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdul Y, Li W, Ward R, et al. Deferoxamine Treatment Prevents Post-Stroke Vasoregression and Neurovascular Unit Remodeling Leading to Improved Functional Outcomes in Type 2 Male Diabetic Rats: Role of Endothelial Ferroptosis. Translational stroke research. 2021;12(4):615–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li W, Ward R, Valenzuela JP, Dong G, Fagan SC, Ergul A. Diabetes Worsens Functional Outcomes in Young Female Rats: Comparison of Stroke Models, Tissue Plasminogen Activator Effects, and Sexes. Translational stroke research. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaeffer S, Iadecola C. Revisiting the neurovascular unit. Nat Neurosci. 2021;24(9):1198–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geary GG, Krause DN, Duckles SP. Estrogen reduces mouse cerebral artery tone through endothelial NOS- and cyclooxygenase-dependent mechanisms. American journal of physiology Heart and circulatory physiology. 2000;279(2):H511–519. [DOI] [PubMed] [Google Scholar]
- 48.Ibrahim J, McGee A, Graham D, McGrath JC, Dominiczak AF. Sex-specific differences in cerebral arterial myogenic tone in hypertensive and normotensive rats. American journal of physiology Heart and circulatory physiology. 2006;290(3):H1081–1089. [DOI] [PubMed] [Google Scholar]
- 49.Reed JT, Pareek T, Sriramula S, Pabbidi MR. Aging influences cerebrovascular myogenic reactivity and BK channel function in a sex-specific manner. Cardiovasc Res. 2020;116(7):1372–1385. [DOI] [PubMed] [Google Scholar]
- 50.Geary GG, Krause DN, Duckles SP. Estrogen reduces myogenic tone through a nitric oxide-dependent mechanism in rat cerebral arteries. The American journal of physiology. 1998;275(1):H292–300. [DOI] [PubMed] [Google Scholar]
- 51.Zuloaga KL, Zhang W, Roese NE, Alkayed NJ. Soluble epoxide hydrolase gene deletion improves blood flow and reduces infarct size after cerebral ischemia in reproductively senescent female mice. Front Pharmacol. 2014;5:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahnstedt H, Cao L, Krause DN, et al. Male-female differences in upregulation of vasoconstrictor responses in human cerebral arteries. PloS one. 2013;8(4):e62698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fouda AY, Fagan SC, Ergul A. Brain Vasculature and Cognition. Arteriosclerosis, thrombosis, and vascular biology. 2019;39(4):593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yanagisawa M, Kurihara H, Kimura S, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. [DOI] [PubMed] [Google Scholar]
- 55.Davenport AP, Hyndman KA, Dhaun N, et al. Endothelin. Pharmacol Rev. 2016;68(2):357–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abdul Y, Ward R, Dong G, Ergul A. Lipopolysaccharide-induced necroptosis of brain microvascular endothelial cells can be prevented by inhibition of endothelin receptors. Physiological research. 2018;67(Suppl 1):S227–S236. [DOI] [PubMed] [Google Scholar]
- 57.Li W, Abdul Y, Ward R, Ergul A. Endothelin and diabetic complications: a brain-centric view. Physiological research. 2018;67(Supplementum 1):S83–S94. [DOI] [PubMed] [Google Scholar]
- 58.Mitchell T, De Miguel C, Gohar EY. Sex differences in redox homeostasis in renal disease. Redox Biol. 2020;31:101489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abdul Y, Li W, Vargas JD, et al. Diabetes-related sex differences in the brain endothelin system following ischemia in vivo and in human brain endothelial cells in vitro. Canadian journal of physiology and pharmacology. 2020;98(9):587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aanerud J, Borghammer P, Rodell A, Jonsdottir KY, Gjedde A. Sex differences of human cortical blood flow and energy metabolism. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2017;37(7):2433–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rodriguez G, Warkentin S, Risberg J, Rosadini G. Sex differences in regional cerebral blood flow. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1988;8(6):783–789. [DOI] [PubMed] [Google Scholar]
- 62.Gur RC, Gur RE, Obrist WD, et al. Sex and handedness differences in cerebral blood flow during rest and cognitive activity. Science. 1982;217(4560):659–661. [DOI] [PubMed] [Google Scholar]
- 63.Dehouck MP, Vigne P, Torpier G, Breittmayer JP, Cecchelli R, Frelin C. Endothelin-1 as a mediator of endothelial cell-pericyte interactions in bovine brain capillaries. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1997;17(4):464–469. [DOI] [PubMed] [Google Scholar]
- 64.Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV. The role of brain vasculature in neurodegenerative disorders. Nature neuroscience. 2018;21(10):1318–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-Brain Barrier: From Physiology to Disease and Back. Physiological reviews. 2019;99(1):21–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zimmermann PA, Knot HJ, Stevenson AS, Nelson MT. Increased myogenic tone and diminished responsiveness to ATP-sensitive K+ channel openers in cerebral arteries from diabetic rats. Circ Res. 1997;81(6):996–1004. [DOI] [PubMed] [Google Scholar]
- 67.Elgebaly MM, Prakash R, Li W, et al. Vascular protection in diabetic stroke: role of matrix metalloprotease-dependent vascular remodeling. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30(12):1928–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cipolla MJ, Sweet JG, Gokina NI, White SL, Nelson MT. Mechanisms of enhanced basal tone of brain parenchymal arterioles during early postischemic reperfusion: role of ET-1-induced peroxynitrite generation. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33(10):1486–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dumont AS, Dumont RJ, McNeill JH, Kassell NF, Sutherland GR, Verma S. Chronic endothelin antagonism restores cerebrovascular function in diabetes. Neurosurgery. 2003;52(3):653–660; discussion 659–660. [DOI] [PubMed] [Google Scholar]
- 70.Appelros P, Stegmayr B, Terént A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40(4):1082–1090. [DOI] [PubMed] [Google Scholar]
- 71.Liu F, McCullough LD. Interactions between age, sex, and hormones in experimental ischemic stroke. Neurochem Int. 2012;61(8):1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chatterjee S, Peters SA, Woodward M, et al. Type 2 Diabetes as a Risk Factor for Dementia in Women Compared With Men: A Pooled Analysis of 2.3 Million People Comprising More Than 100,000 Cases of Dementia. Diabetes Care. 2016;39(2):300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383(9933):1973–1980. [DOI] [PubMed] [Google Scholar]


