Abstract
Photoalignment control of hierarchical structures is a key process to enhance the properties of optical and mechanical materials. We developed an in situ molecular alignment method, where photopolymerization with the scanned slit light causes molecular flow, leading to two-dimensional precise alignment of molecules over large areas; however, the alignment control has been explored only on a molecular scale. In this study, we demonstrate this photopolymerization-induced molecular flow, enabling mesoscopic alignment of smectic layer structures composed of anisotropic molecules. Side-chain liquid-crystalline polymers were obtained from two different monomers with or without alkyl spacers by photopolymerization with one-dimensionally scanned slit light. The polymer with an alkyl spacer displayed mesogens aligned parallel to the scanning direction, while the polymer with no alkyl spacer resulted in perpendicular alignment of mesogens to the scanning direction, regulated by the alignment of the polymer main chain along the light scanning direction. Moreover, the polymerization with the scanned light aligned not only the mesogens but also mesoscopic smectic layer structures over large areas, depending on the structure and scanning pattern of light. We envision that such a simple polymerization technique could become a powerful and versatile alignment platform of anisotropic materials in a wide range of scales.
Keywords: liquid crystals, coatings, photopolymerization, molecular alignment, molecular flow, photoalignment
Introduction
Alignment control over anisotropic molecular groups such as liquid crystal (LC) and polymeric materials can provide dramatically enhanced mechanical,1,2 electrical,3 and optical4,5 properties and long-range supramolecular self-organized structures.6,7 Aligned soft materials composed of these anisotropic molecules have been adopted to develop high-performance devices in a variety of areas, including optics,5,8,9 soft robotics,2,10,11 and biomedical applications.12 To date, photochemical alignment control, which utilizes axis-selective photochemical reactions of dye molecules with linearly polarized light, has been well developed. A representative photochemical reaction is the photoisomerization of azobenzene, which was employed by Ichimura, Seki et al. to achieve a successful thin active photoreactive alignment layer, referred to as a “command surface,”13 leading to large-scale photochemical alignment control for improved optical devices. Photoalignment layers are generally composed of light-responsive azobenzene14−16 or cinnamate dyes17,18 to enable the arbitrary and complex two-dimensional (2D) alignment patterns that have been applied for the development of displays, holograms, and actuators.19,20 The light-directed processes with photochromic molecules provide advantages of remote and fine control and thus have been well studied for further development of photoalignment as well as photoinduced motion systems, e.g., of soft actuators.16
On the other hand, as a contrasting method from the above, a shear flow process has also been widely explored. Shear stress has the ability to align nonabsorbing LC mesogens and polymer main chains along the shear direction.21−23 The effect of shear flow on LC alignment has been studied by both comprehensive experiments24 and theoretical studies with a mathematical description rationalized by Leslie–Ericksen theory.25,26 To date, flow alignment of functional LC polymers has been achieved under shear flow in Couette cells,27 by blade coating during solvent evaporation28 and via photopolymerization in a microfluidic setup.29−31 The shear flow process has distinct advantages over photoalignment in that a wider variety of molecules can be aligned; however, a mechanical contact process is, in principle, required.
Recently, we have proposed a photoalignment process, where photopolymerization with a scanned slit light causes molecular flow and aligns LCs along the flow direction.32−38 When photopolymerization is conducted in selected localized regions of slit light, a gradient of chemical potential is generated between irradiated and unirradiated regions, resulting in the flow from molecular diffusion.39−41 This flow aligns LCs at the boundary of both regions. This method enables one to inscribe arbitrary high-resolution 2D alignment patterns over large areas in a single step simply by designing irradiation patterns. However, control of hierarchical mesoscopic structures of LC polymers, which might enhance the optical and mechanical properties of materials, remains unexplored by photopolymerization with the scanned slit light.
In this study, we report mesoscopic alignment of smectic layer nanostructures by photopolymerization with a one-dimensional (1D) scanned slit light. Specifically, we prepared two monomers showing different LC phases of nematic or smectic phases that have the same polymerizable acrylate group and mesogenic unit but structurally differ only in the presence or absence of a flexible alkyl spacer. To achieve the mesoscopic alignment of smectic layer nanostructure, we first investigated how these side-chain LC units are aligned by the photopolymerization process with the scanned slit light because the alignment degree and direction of the LC units are strongly influenced by flexible spacers in the side chain during conventional alignment processes such as elongation, application of electric fields, and photoreactions.42−45 Note that we have found that the polymer main chains are aligned along the light scanning direction regardless of the molecular structure of monomers used and that the aligned polymer main chain governs the mesoscopic alignment of smectic layer nanostructures over large areas.
Results and Discussion
We prepared two photopolymerizable samples with two monoacrylates that differ only in the spacer structure. One monomer was 4′-[6-(acryloyloxy)hexyloxy]-4-cyanobiphenyl (A6CB) with a flexible alkyl spacer. The other was 4-(acryloyloxy)-4′-cyanobiphenyl (A0CB), which has a rigid structure with no alkyl spacer (Figure 1a). A small amount of a hexanediol dimethacrylate (HDDMA) crosslinker was added to each monomer to enhance the degree of molecular alignment due to an increased polymerization rate and, as a result, an increased chemical potential gradient. An Irgacure 651 photoinitiator was added to each monomer to prepare photopolymerizable mixtures. Detailed compositions and preparation procedures are described in the section of Materials and Methods.
Figure 1.
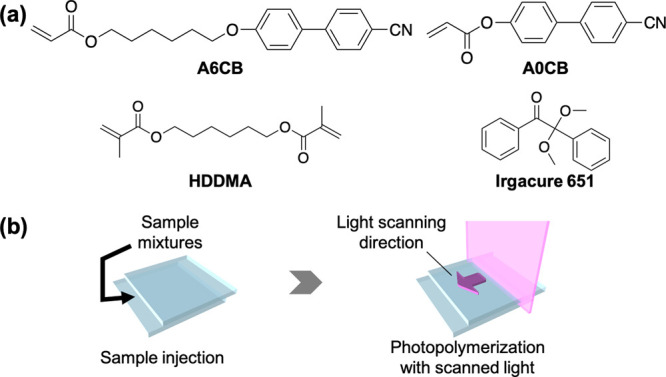
(a) Chemical structures of photopolymerizable samples used in this study. (b) Schematic illustrations of photopolymerization process with the 1D scanned slit light.
To conduct photopolymerization with the 1D scanned slit light, the mixtures were melted at an isotropic temperature of 150 °C and injected into a handmade glass cell (cell gap of 2–5 μm; for preparation details, see the Supporting Information, Text S1). Before photopolymerization, the cell was cooled to 100 °C for the A6CB mixture and heated to 160 °C for the A0CB mixture, respectively. At these temperatures, the isotropic monomers were converted into resultant LC polymers, showing a nematic phase and a smectic phase, respectively (for measured thermodynamic properties, see the Supporting Information, Text S2). Subsequently, to induce 1D molecular alignment by photopolymerization, the sample mixture in the cell was irradiated with 365 nm ultraviolet (UV) light normal to the surface through a slit mask (slit width, 250 μm) 1D scanned at a scanning rate of 20 μm/s (Figure 1b). We optimized the light intensity and set it at 1.2 mW/cm2 for A6CB and 4.2 mW/cm2 for A0CB, respectively, by selecting a different combination of optical filters to maximize the degree of molecular alignment (Supporting Information, Text S3). Then, the slit mask was removed to irradiate throughout the cell for 5 min to fix the generated alignment. After irradiation, the cell was rapidly cooled down to room temperature by immersing it in liquid nitrogen. The molecular alignment of the obtained polymer films was evaluated by optical measurements (for details of characterization methods, see the Supporting Information, Text S4).
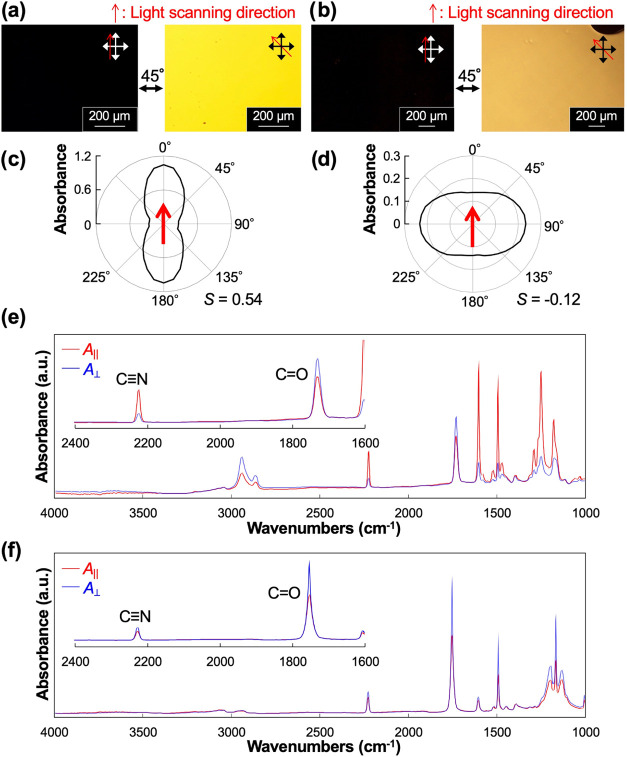
First, the molecular alignment of photopolymerized A6CB and A0CB films was observed in terms of optical anisotropy with a polarized optical microscope (POM). Both films displayed uniform optical anisotropy, indicating that unidirectional molecular alignment was obtained from both A6CB and A0CB monomeric mixtures (Figure 2a,b). Alignment of mesogenic cyanobiphenyl moieties was evaluated by polarized UV–visible (UV–vis) spectroscopy by means of orientational order parameter S [=(A|| – A⊥)/(A|| + 2A⊥)],46 where A|| and A⊥ represent the absorbances parallel and perpendicular to the light scanning direction, respectively, and polar plots from the averaged absorbance of the cyanobiphenyl moiety (333–337 nm). In the polymerized A6CB film, S was found to be 0.54 (absorption spectra are available in the Supporting Information, Text S5), which is as high as that of the previous study,33 and a polar plot of polarized UV–vis absorbance showed its maximum along the light scanning direction (Figure 2c). These results indicate that cyanobiphenyl moieties in the polymerized A6CB film are aligned parallel to the scanning direction. On the other hand, in the case of the polymerized A0CB film, the measured S took a minus value (−0.12) (Supporting Information, Text S5), and a polar plot of the absorbance displayed its maximum at 90° from the scanning direction (Figure 2d). This is the opposite result to that of A6CB, showing that cyanobiphenyl moieties of A0CB are aligned perpendicular to the scanning direction.
Figure 2.
Polarized micrographs of the films obtained by photopolymerization of A6CB (a) and A0CB (b) with the 1D scanned slit light. Polar plots of polarized UV–vis absorbances averaged from 333 to 337 nm for the films photopolymerized with A6CB (c) and A0CB (d). The azimuthal angle was defined as 0° for A|| and 90° for A⊥, where the polarization directions of the incident light are parallel and perpendicular to the light scanning direction, respectively. Polarized IR absorption spectra of the resultant films photopolymerized with A6CB (e) and A0CB (f).
For a further detailed examination, alignment of the side-chain mesogens and main chains were evaluated by polarized Fourier transform infrared (FTIR) spectroscopy. As seen from the IR spectra for the polymerized A6CB film (Figure 2e), a characteristic absorption band was observed near 2230 cm–1, attributed to the C≡N stretching vibration in the mesogenic group. This C≡N band displayed parallel dichroism to the scanning direction, meaning that cyano groups in the mesogens were aligned parallel to the scanning direction, which was consistent with the UV–vis result depicted in Figure 2c. Conversely, the characteristic absorption band near 1730 cm–1, assigned to C=O stretching vibration, showed perpendicular dichroism. As noted in the literature,47,48 the C=O group adjacent to the main chain lies almost perpendicular to the main chain; thus, the perpendicular dichroism of the C=O band reflects the parallel alignment of the main chains in the scanning direction. Hence, IR measurements found a parallel alignment of both mesogens and main chains of the polymerized A6CB film along the scanning direction.
Next, the molecular orientation of the polymerized A0CB film was also investigated with the dichroism of the C≡N and C=O bands (Figure 2f). The dichroism of the C≡N band for the polymerized A0CB film reversed to that of A6CB, indicating a perpendicular alignment of mesogens, as observed similarly in the UV–vis spectra (Figure 2d). Interestingly, the C=O band also showed perpendicular dichroism in the same manner as A6CB, indicating that in both polymerized A6CB and A0CB films, the polymer main chains are aligned parallel to the light scanning direction.
To exclude the influence of other possible factors such as confinement by the glass cell and the effect of the light source, we conducted the control experiment using photopolymerization of A6CB and A0CB mixtures with flood illumination. Contrary to the above, the obtained films did not show any uniform optical anisotropy (Figure S4a,b). Polarized IR spectra also revealed that these films have no dichroism of both the C≡N band and the C=O band (Figure S4c,d). Therefore, we have concluded that the key to inducing uniform molecular alignment during photopolymerization is the selected localized illumination of scanned slit light.
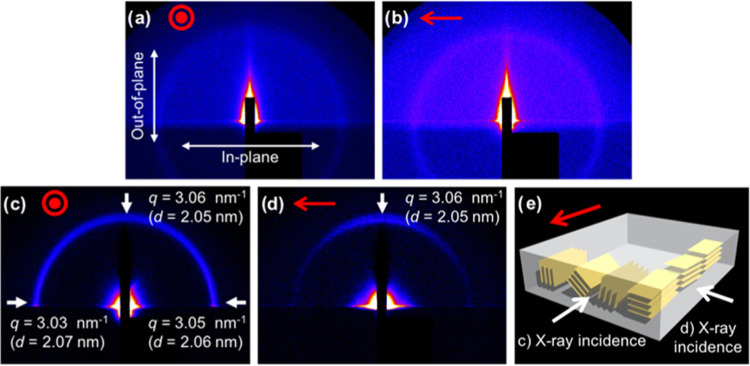
Next, grazing-incidence small-angle X-ray scattering (GI-SAXS) data were obtained for the analysis of the nanostructures of the polymerized A6CB and A0CB films. To investigate three-dimensional (3D) anisotropic structures in both in-plane and out-of-plane directions, the incident X-rays were applied either parallel or perpendicular to the scanning direction. In the case of the polymerized A6CB film, no X-ray diffraction was observed (Figure 3a,b), indicating that there was no periodic nanostructure despite the mesogen alignment in the polymerized A6CB film. By contrast, X-ray diffraction patterns were observed with the polymerized A0CB film. When the incident X-ray beam to the polymerized A0CB film was parallel to the scanning direction, a uniform X-ray diffraction pattern appeared in both in-plane and out-of-plane directions (Figure 3c). Additionally, a perpendicular incidence of the X-ray beam to the scanning direction resulted in only out-of-plane diffraction (Figure 3d). To obtain d values, representing the spacing of periodic structures, the scattering vector q was acquired from the diffractograms in Figure 3c,d, and found to be 3.03–3.06 nm–1. The d values were calculated from q values and found to be d = 2.05–2.07 nm, which agreed well with a previous report in which polymerized A0CB formed smectic layers with 2.1 nm layer spacings.49 From the GI-SAXS data for the polymerized A0CB film, we considered a 3D anisotropic nanostructure of the smectic layers in the polymerized A0CB film (Figure 3e). These layers are aligned along the scanning direction in parallel but allowed to be rotated around the axis along the scanning direction. Such layer structures do not contradict the cyanobiphenyl orientation determined from polarized UV–vis (Figure 2d) and FTIR (Figure 2f) measurements because the cyanobiphenyl moieties in the polymerized A0CB film lie normal to the smectic layers.49
Figure 3.
2D GI-SAXS diffractograms for the polymerized A6CB film using parallel (a) or perpendicular (b) X-ray incidence to the scanning direction, respectively, and for the polymerized A0CB film using parallel (c) or perpendicular (d) X-ray incidence, respectively. (e) Possible 3D nanostructures made of smectic layers (represented by yellow layers) in the polymerized A0CB film. Red circles and arrows represent the light scanning directions.
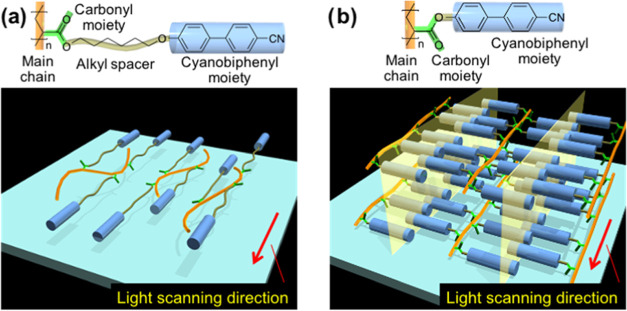
Considering all of these experimental results, we developed the molecular alignment mechanism and resultant polymer structures as follows. Photopolymerization with the 1D scanned slit light localizes the polymerized region and brings about a gradient of chemical potential between irradiated and unirradiated regions, triggering a molecular flow across the boundary of each region (i.e., flow parallel to the scanning direction). This flow then generates shear stress to align main chains along the flow direction. Here, in the case of A6CB, due to the flexibility of hexyl spacers, the mesogenic cyanobiphenyl moieties attached to the end of the side chains are aligned in the direction of the flow as well as main chains (Figure 4a). On the other hand, since A0CB has a rigid structure without alkyl spacers, the mesogens in the side chain cannot orient in the flow direction. Instead, mesogen alignment was regulated by the structural restriction from the main chain orientation. Thus, they finally lie perpendicular to the main chains like a comb (Figure 4b). The smectic layer spacing was calculated to be approximately 2.1 nm, which is less than twice the 1.33 nm length of a single A0CB side chain, indicating that the side chains form interdigitated structures, as depicted in Figure 4b.49 The layered structures might be rotated around the axis of the light scanning direction, as shown in Figure 3e. Because of the aforementioned mechanism, the scanning of slit light can also clearly align polymer main chains, governing the alignment of mesoscopic nanostructures in addition to the mesogens. This means that these molecular and mesoscopic alignments are strongly affected by the polymer main chains and flexibility of side chains.
Figure 4.
3D schematics of the possible alignment structure of the films obtained by photopolymerization with the 1D scanned slit light of (a) A6CB and (b) A0CB mixtures. Yellow planes indicate smectic layers, as depicted in Figure 3e.
Conclusions
In summary, we demonstrated that smectic layered nanostructures composed of side-chain LC molecules were mesoscopically aligned by photopolymerization with the 1D scanned slit light that generates photopolymerization-triggered molecular flow. Regardless of the presence or absence of alkyl spacers, the polymer main chains were aligned along the light scanning direction, while the cyanobiphenyl mesogens attached to the end of side chains were aligned in a different direction. We found that such polymer main chain alignment is the controlling factor governing the alignment direction of side-chain LC molecules. Note that due to the alignment of polymer main chains, the proposed photopolymerization system is able to align the higher-order structures of a LC polymer such as the smectic layered nanostructures in addition to LC molecules at a molecular scale. This photopolymerization, creating molecular flow-induced shear stress as a driving force for alignment, has excellent advantages for the inscription of precise alignment patterns over large areas in a single step. These results provide a possibility toward the development of aligning not only LC mesogens but also larger-scale anisotropic structures. Photopolymerization-triggered molecular flow is anticipated to represent an important new protocol available in optical materials science to produce highly functional soft materials and devices with sophisticated designs of hierarchical structures.
Materials and Methods
For the preparation of photopolymerizable mixtures, A6CB was mixed together with HDDMA (Wako Chemical) at the molar ratio of 97:3. A0CB and HDDMA were mixed in the molar ratio of 99.5:0.5. Additionally, Irgacure 651 photoinitiator (Tokyo Chemical Industry Co.) was doped in at a concentration of 1 mol % to the total amount of the monomers in both mixtures. These mixtures were dissolved and stirred in THF and subsequently dried to remove THF to obtain uniformly mixed materials. The Supporting Information section provides further experimental details of the optical setup and characterization methods.
Acknowledgments
This work was performed under the Cooperative Research Program of “Network Joint Research Center for Materials and Devices.” This work was performed under the Research Program of “Dynamic Alliance for Open Innovation Bridging Human, Environment and Materials” in “Network Joint Research Center for Materials and Devices.” The Tokyo Institute of Technology is thanked for the sabbatical visit funding for C.J.B. to the Shishido Labs, as Visiting Adjunct Professor.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsami.2c13299.
Preparation procedure of a glass cell; DSC thermograms of monomers and polymers; optical setup for the photopolymerization experiments; characterization methods; polarized UV–vis absorption spectra of the polymerized films; and POM images and polarized IR absorption spectra of the films polymerized with flood illumination (PDF)
Author Contributions
M.I., K.H., and A.S. conceived the project and designed the experiment. M.I., K.H., and M.A. prepared all samples and conducted sample characterization and performed photopolymerization for fabricating molecularly aligned polymer films. M.I., K.H., and M.A. measured the optical properties of the resultant films and analyzed the data. M.I. conducted the 2D GI-SAXS measurement, and M.I. analyzed the data with the help of K.H. and M.A. M.I., K.H., C.J.B., and A.S. prepared the manuscript, incorporating contributions from all authors.
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas “Molecular Engine” (JSPS KAKENHI Grant Number JP18H05422). This work was supported by JST CREST Grant Number JPMJCR18I4, Japan. This work was supported by JSPS KAKENHI Grant Number JP22H02128.
The authors declare no competing financial interest.
Supplementary Material
References
- Lakes R. Materials with Structural Hierarchy. Nature 1993, 361, 511–515. 10.1038/361511a0. [DOI] [Google Scholar]
- White T. J.; Broer D. J. Programmable and Adaptive Mechanics with Liquid Crystal Polymer Networks and Elastomers. Nat. Mater. 2015, 14, 1087–1098. 10.1038/nmat4433. [DOI] [PubMed] [Google Scholar]
- O’Neill M.; Kelly S. M. Liquid Crystals for Charge Transport, Luminescence, and Photonics. Adv. Mater. 2003, 15, 1135–1146. 10.1002/adma.200300009. [DOI] [Google Scholar]
- Yu H. Recent Advances in Photoresponsive Liquid-Crystalline Polymers Containing Azobenzene Chromophores. J. Mater. Chem. C 2014, 2, 3047–3054. 10.1039/C3TC31991A. [DOI] [Google Scholar]
- Chigrinov V.; Kozenkov V.; Kwok H.-S.. Photoalignment of Liquid Crystalline Materials: Physics and Applications; John Wiley & Sons: England, 2008; pp 101–156. [Google Scholar]
- Kato T.; Mizoshita N.; Kishimoto K. Functional Liquid-Crystalline Assemblies: Self-Organized Soft Materials. Angew. Chem., Int. Ed. 2006, 45, 38–68. 10.1002/anie.200501384. [DOI] [PubMed] [Google Scholar]
- Kato T.; Uchida J.; Ichikawa T.; Sakamoto T. Functional Liquid Crystals towards the Next Generation of Materials. Angew. Chem., Int. Ed. 2018, 57, 4355–4371. 10.1002/anie.201711163. [DOI] [PubMed] [Google Scholar]
- Schadt M.; Seiberle H.; Schuster A. Optical Patterning of Multi-Domain Liquid-Crystal Displays with Wide Viewing Angles. Nature 1996, 381, 212–215. 10.1038/381212a0. [DOI] [Google Scholar]
- Seki T.; Nagano S.; Hara M. Versatility of Photoalignment Techniques: from Nematics to a Wide Range of Functional Materials. Polymer 2013, 54, 6053–6072. 10.1016/j.polymer.2013.08.058. [DOI] [Google Scholar]
- Wani O. M.; Zeng H.; Priimagi A. A Light-Driven Artificial Flytrap. Nat. Commun. 2017, 8, 15546 10.1038/ncomms15546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palagi S.; Mark A. G.; Reigh S. Y.; Melde K.; Qiu T.; Zeng H.; Parmeggiani C.; Martella D.; Sanchez-Castillo A.; Kapernaum N.; Giesselmann F.; Wiersma D.; Lauga E.; Fischer P. Structured Light Enables Biomimetic Swimming and Versatile Locomotion of Photoresponsive Soft Microrobots. Nat. Mater. 2016, 15, 647–653. 10.1038/nmat4569. [DOI] [PubMed] [Google Scholar]
- Woltman S. J.; Jay G. D.; Crawford G. P. Liquid-Crystal Materials Find a New Order in Biomedical Applications. Nat. Mater. 2007, 6, 929–938. 10.1038/nmat2010. [DOI] [PubMed] [Google Scholar]
- Ichimura K.; Suzuki Y.; Seki T.; Hosoki A.; Aoki K. Reversible Change in Alignment Mode of Nematic Liquid Crystals Regulated Photochemically by Command Surfaces Modified with an Azobenzene Monolayer. Langmuir 1988, 4, 1214–1216. 10.1021/la00083a030. [DOI] [Google Scholar]
- Natansohn A.; Rochon P. Photoinduced Motions in Azo-Containing Polymers. Chem. Rev. 2002, 102, 4139–4175. 10.1021/cr970155y. [DOI] [PubMed] [Google Scholar]
- Seki T. New Strategies and Implications for the Photoalignment of Liquid Crystalline Polymers. Polym. J. 2014, 46, 751–768. 10.1038/pj.2014.68. [DOI] [Google Scholar]
- Seki T. A Wide Array of Photoinduced Motions in Molecular and Macromolecular Assemblies at Interfaces. Bull. Chem. Soc. Jpn. 2018, 91, 1026–1057. 10.1246/bcsj.20180076. [DOI] [Google Scholar]
- Kawatsuki N. Photoalignment and Photoinduced Molecular Reorientation of Photosensitive Materials. Chem. Lett. 2011, 40, 548–554. 10.1246/cl.2011.548. [DOI] [Google Scholar]
- Schadt M.; Schmitt K.; Kozinkov V.; Chigrinov V. Surface-Induced Parallel Alignment of Liquid Crystals by Linearly Polymerized Photopolymers. Jpn. J. Appl. Phys. 1992, 31, 2155–2164. 10.1143/JJAP.31.2155. [DOI] [Google Scholar]
- Priimagi A.; Barrett C. J.; Shishido A. Recent Twists in Photoactuation and Photoalignment Control. J. Mater. Chem. C 2014, 2, 7155–7162. 10.1039/C4TC01236D. [DOI] [Google Scholar]
- Shishido A. Rewritable Holograms Based on Azobenzene-Containing Liquid-Crystalline Polymers. Polym. J. 2010, 42, 525–533. 10.1038/pj.2010.45. [DOI] [Google Scholar]
- Fridrikh S. V.; Terentjev E. M. Polydomain-Monodomain Transition in Nematic Elastomers. Phys. Rev. E 1999, 60, 1847–1857. 10.1103/PhysRevE.60.1847. [DOI] [PubMed] [Google Scholar]
- Pujolle-Robic C.; Noirez L. Observation of Shear-Induced Nematic–Isotropic Transition in Side-Chain Liquid Crystal Polymers. Nature 2001, 409, 167–171. 10.1038/35051537. [DOI] [PubMed] [Google Scholar]
- Agrawal A.; Chipara A. C.; Shamoo Y.; Patra P. K.; Carey B. J.; Ajayan P. M.; Verduzco R.; Agrawal A.; Chipara A. C.; Shamoo Y.; Patra P. K.; Carey B. J.; Ajayan P. M.; Chapman W. G.; Verduzco R. Dynamic Self-Stiffening in Liquid Crystal Elastomers. Nat. Commun. 2013, 4, 1739 10.1038/ncomms2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongladarom K.; Ugaz V. M.; Cinader D. K.; Burghardt W. R.; Quintana J. P.; Hsiao B. S.; Butler P. D.; Dadmun M. D.; Hamilton W. A. Birefringence, X-ray Scattering, and Neutron Scattering Measurements of Molecular Orientation in Sheared Liquid Crystal Polymer Solutions. Macromolecules 1996, 29, 5346–5355. 10.1021/ma960171h. [DOI] [Google Scholar]
- Rey A. D.; Denn M. M. Dynamical Phenomena in Liquid-Crystalline Materials. Annu. Rev. Fluid Mech. 2002, 34, 233–266. 10.1146/annurev.fluid.34.082401.191847. [DOI] [Google Scholar]
- Larson R. G.; Mead D. W. The Ericksen Number and Deborah Number Cascades in Sheared Polymeric Nematics. Liq. Cryst. 1993, 15, 151–169. 10.1080/02678299308031947. [DOI] [Google Scholar]
- Hamley I. W.; Krysmann M. J.; Kelarakis A.; Castelletto V.; Noirez L.; Hule R. A.; Pochan D. J. Nematic and Columnar Ordering of a PEG–Peptide Conjugate in Aqueous Solution. Chem. - Eur. J. 2008, 14, 11369–11375. 10.1002/chem.200800817. [DOI] [PubMed] [Google Scholar]
- Kim B. G.; Jeong E. J.; Chung J. W.; Seo S.; Koo B.; Kim J. A Molecular Design Principle of Lyotropic Liquid-Crystalline Conjugated Polymers with Directed Alignment Capability for Plastic Electronics. Nat. Mater. 2013, 12, 659–664. 10.1038/nmat3595. [DOI] [PubMed] [Google Scholar]
- Ohm C.; Serra C.; Zentel R. A Continuous Flow Synthesis of Micrometer-Sized Actuators from Liquid Crystalline Elastomers. Adv. Mater. 2009, 21, 4859–4862. 10.1002/adma.200901522. [DOI] [PubMed] [Google Scholar]
- Ohm C.; Brehmer M.; Zentel R. Liquid Crystalline Elastomers as Actuators and Sensors. Adv. Mater 2010, 22, 3366–3387. 10.1002/adma.200904059. [DOI] [PubMed] [Google Scholar]
- Ohm C.; Kapernaum N.; Nonnenmacher D.; Giesselmann F.; Serra C.; Zentel R. Microfluidic Synthesis of Highly Shape-Anisotropic Particles from Liquid Crystalline Elastomers with Defined Director Field Configurations. J. Am. Chem. Soc. 2011, 133, 5305–5311. 10.1021/ja1095254. [DOI] [PubMed] [Google Scholar]
- Hisano K.; Kurata Y.; Aizawa M.; Ishizu M.; Sasaki T.; Shishido A. Alignment Layer-Free Molecular Ordering Induced by Masked Photopolymerization with Non-Polarized Light. Appl. Phys. Express 2016, 9, 072601 10.7567/APEX.9.072601. [DOI] [Google Scholar]
- Hisano K.; Aizawa M.; Ishizu M.; Kurata Y.; Nakano W.; Akamatsu N.; Barrett C. J.; Shishido A. Scanning Wave Photopolymerization Enables Dye-Free Alignment Patterning of Liquid Crystals. Sci. Adv. 2017, 3, e1701610 10.1126/sciadv.1701610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa M.; Hisano K.; Ishizu M.; Akamatsu N.; Barrett C. J.; Shishido A. Unpolarized Light-Induced Alignment of Azobenzene by Scanning Wave Photopolymerization. Polym. J. 2018, 50, 753–759. 10.1038/s41428-018-0058-2. [DOI] [Google Scholar]
- Ishizu M.; Aizawa M.; Akamatsu N.; Hisano K.; Fujikawa S.; Barrett C. J.; Shishido A. Effect of Surface Treatment on Molecular Alignment Behavior by Scanning Wave Photopolymerization. Appl. Phys. Express 2019, 12, 041004 10.7567/1882-0786/ab040d. [DOI] [Google Scholar]
- Aizawa M.; Ota M.; Hisano K.; Akamatsu N.; Sasaki T.; Barrett C. J.; Shishido A. Direct fabrication of a Q-Plate Array by Scanning Wave Photopolymerization. J. Opt. Soc. Am. B 2019, 36, D47–D51. 10.1364/JOSAB.36.000D47. [DOI] [Google Scholar]
- Hisano K.; Ota M.; Aizawa M.; Akamatsu N.; Barrett C. J.; Shishido A. Single-Step Creation of Polarization Gratings by Scanning Wave Photopolymerization with Unpolarized Light. J. Opt. Soc. Am. B 2019, 36, D112–D118. 10.1364/JOSAB.36.00D112. [DOI] [Google Scholar]
- Ueda K.; Aizawa M.; Shishido A.; Vacha M. Real-Time Molecular-Level Visualization of Mass Flow during Patterned Photopolymerization of Liquid-Crystalline Monomers. NPG Asia Mater. 2021, 13, 25 10.1038/s41427-021-00292-1. [DOI] [Google Scholar]
- Krongauz V. V.; Schmelzer E. R.; Yohannan R. M. Kinetics of Anisotropic Photopolymerization in Polymer Matrix. Polymer 1991, 32, 1654–1662. 10.1016/0032-3861(91)90402-5. [DOI] [Google Scholar]
- Broer D. J.; Lub J.; Mol G. N. Wide-Band Reflective Polarizers from Cholesteric Polymer Networks with a Pitch Gradient. Nature 1995, 378, 467–469. 10.1038/378467a0. [DOI] [Google Scholar]
- Leewis C. M.; de Jong A. M.; van IJzendoorn L. J.; Broer D. J. Reaction–Diffusion Model for the Preparation of Polymer Gratings by Patterned Ultraviolet Illumination. J. Appl. Phys. 2004, 95, 4125–4139. 10.1063/1.1688458. [DOI] [Google Scholar]
- Wu Y.; Demachi Y.; Tsutsumi O.; Kanazawa A.; Shiono T.; Ikeda T. Photoinduced Alignment of Polymer Liquid Crystals Containing Azobenzene Moieties in the Side Chain. 2. Effect of Spacer Length of the Azobenzene Unit on Alignment Behavior. Macromolecules 1998, 31, 1104–1108. 10.1021/ma971035v. [DOI] [Google Scholar]
- Kozuki J.; Kondo M.; Sasaki T.; Ono H.; Kawatsuki N. Influence of Alkylene Spacer Length on Photoinduced Orientation of Liquid Crystalline Polymer with N-Benzylideneaniline Side Groups. Mol. Cryst. Liq. Cryst. 2017, 644, 61–68. 10.1080/15421406.2016.1277330. [DOI] [Google Scholar]
- Anthamatten M.; Zheng W. Y.; Hammond P. T. A Morphological Study of Well-Defined Smectic Side-Chain LC Block Copolymers. Macromolecules 1999, 32, 4838–4848. 10.1021/ma9903153. [DOI] [Google Scholar]
- Finkelmann H.; Kiechle U.; Rehage G. Behavior of Liquid Crystalline Side Chain Polymers in an Electric Field. Mol. Cryst. Liq. Cryst. 1983, 94, 343–358. 10.1080/15421408308084267. [DOI] [Google Scholar]
- Demus D.; Goodby J. W.; Gray G. W.; Spiess H.-W.; Vill V.. Handbook of Liquid Crystals; Wiley-VCH: Weinheim, 1998; Vol. 1, p 221. [Google Scholar]
- Zhao Y.; Lei H. A Polarized Infra-Red Spectroscopic Study of Mechanically Induced Orientation in Side-Chain Liquid Crystalline. Polymer 1994, 35, 1419–1424. 10.1016/0032-3861(94)90341-7. [DOI] [Google Scholar]
- Zhao Y.; Roche P.; Yuan G. Mechanically-Induced Alignment of Mesophases. Macromolecules 1996, 29, 4619–4625. 10.1021/ma951672q. [DOI] [Google Scholar]
- Âlimoglu A. K.; Ledwith A.; Gemmell P. A.; Gray G. W.; Lacy D. Polymers with Rigid Anisotropic Side Groups: 1. Side Chain Induced Crystallinity in Substituted Biphenyl Acrylates and Methacrylates. Polymer 1984, 25, 1342–1346. 10.1016/0032-3861(84)90388-4. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.