Abstract
Non-dioxin-like polychlorinated biphenyls (ndl-PCBs) are a subclass of persistent bioaccumulative pollutants able to enter the food chain. Toxicokinetic models for the transfer of the six ndl-PCB congeners (PCBs 28, 52, 101, 138, 153, and 180) from contaminated feed and soil into chicken eggs and meat are presented. Three independent controlled feeding study datasets were used to estimate the model parameters and four studies for evaluating the model performance. The yolk deposition of ndl-PCBs is modeled in a novel way that mimics the physiology of yolk growth and development, resulting in improved prediction of the experimental data without introducing an ad hoc time delay between ovulation and oviposition. Using the models, the highest level of 2.4 μg/kg dry matter (DM) was calculated for the sum of ndl-PCBs in laying hen feed to ensure that the current maximum levels in meat and eggs (40 ng/g fat) will not be exceeded. It is also shown how this highest level in feed should be adapted in case soil, in addition to feed, is also a source of ndl-PCBs for free-range chickens.
Keywords: Gallus gallus domesticus, indicator PCBs, PCB metabolism, pharmacokinetics, carry-over
Introduction
Non-dioxin-like polychlorinated biphenyls (ndl-PCBs) are ubiquitous environmental pollutants that are sometimes detected in chicken eggs as a result of their transfer from contaminated feed, soil, or other materials such as bedding.1 They are associated with adverse human health effects,2 and as such, their occurrence is monitored in the food and feed chains. The European Commission set maximum levels for both food and feed.3−5 Effective risk analysis of ndl-PCBs requires toxicokinetic (TK) models to evaluate the congener-specific transfer from their major source matrices (feed and soil) into food products derived from laying hens (eggs and muscle meat). Currently, only one TK model exists that predicts the transfer of a sum of ndl-PCBs from contaminated feed into chicken eggs6 and two models that evaluate the transfer of the structurally related polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) from soil into eggs7 and from feed into eggs;8 the former cannot make congener-specific predictions and the latter two cannot be used for ndl-PCBs. We recently published a congener-specific TK model to predict the transfer of individual ndl-PCBs into the meat (muscle fat) and liver of fattening chickens after consumption of contaminated feed.9 However, that model cannot be used for laying hens due to the difference in physiology compared to fattening chickens. In this work, we close the gap and provide a set of six TK models, one for each ndl-PCB, to estimate the transfer of individual congeners from contaminated feed and soil into chicken eggs and muscle fat of laying hens.
The present TK models are based on multiple datasets for added confidence (Table 1): the first dataset comes from our recent in-house study on ndl-PCB transfer into chicken eggs during a feeding experiment using controlled amounts of a commercial compound feed accidentally contaminated with paint from the loading cells of a feed company;10 a second dataset comes from controlled free-range husbandry on soils with predefined contamination levels simulating the soils from a highly urbanized area;10,11 and the additional two datasets come from previous literature.12,13 A typical question that our models can help risk managers to answer is: “How long should a depuration period using uncontaminated feed be so that the concentrations of ndl-PCBs in laying hen body fat and eggs drop below the maximum levels?” In addition to this, the models can help risk managers to establish ndl-PCB concentrations in soil and reconsider those in feed,4 so that they will not lead to exceedance of maximum levels in eggs.3 For the sake of transparency and reusability, the model described below is included in the Supporting Information in the Food Safety Knowledge Exchange .fskx and Python .py formats.
Table 1. Details on Main Studies Used for Model Parametrization and Evaluation.
| study 110 | study 212 | study 313 | study 410,11 | study 513 | |
|---|---|---|---|---|---|
| source of contamination | paint particles | spiked soy oil | oil | feed and soil | sand |
| use of the study in this work | parametrization of “feed-yolks” model | parametrization of “feed-yolks” model | evaluation of “feed-yolks” model | parametrization of “soil-yolks” model | evaluation of “soil-yolks” model |
| examined tissues | eggs | eggs, muscle fat, ovarian fat | eggs, muscle fat, liver | eggs, muscle fat | eggs, muscle fat, liver |
| study design | 2 groups, different exposure time, similar concentrations, exposure followed by depuration period | 1 group, exposure followed by depuration period | 3 groups with different concentrations, exposure period only | 3 groups with different concentration in soil, exposure period only | 3 groups with different concentrations, exposure period only, the study compares absorption of congeners from oil and from sand |
| duration of exposure, days | 28 and 63 | 56 | 14 | 163 | 14 |
| duration of depuration, days | 100 | 56 | 0 | 0 | 0 |
| intake, ng/day | |||||
| PCB 28 | 24 | 122 | 27–66 | a | 32–75 |
| PCB 52 | 99 | 161 | 26–82 | a | 35–96 |
| PCB 101 | 154 | 357 | 67–184 | a | 112–260 |
| PCB 138 | 451 | 371 | 183–483 | a | 248–604 |
| PCB 153 | 348 | 346 | 237–641 | a | 320–778 |
| PCB 180 | 269 | 234 | 267–684 | a | 242–596 |
| total | 1347 | 1591 | 807–2140 | a | 989–2409 |
| total intake, ng | 39 032; 80 852 | 89 092 | 11 298–29 960 | a | 13 846–33 726 |
Exact value was not measured during the experimental study.
Materials and Methods (Including Safety Information)
Toxicokinetic Model
This work presents novel modeling results but does not involve any new animal experiments, so no ethical approval is required. The structure of the TK model that predicts the transfer of individual ndl-PCBs from contaminated feed and/or soil into the muscle fat and eggs was adopted from our model for fattening chickens.9 It is assumed that the ndl-PCB concentration is in equilibrium between muscle fat and other types of body fat, such as abdominal fat. This assumption is supported by other studies14 and has been used before in modeling of chickens.9,15 Due to the fact that the available datasets (Table 1) contain too few measurements of ndl-PCB concentrations in livers of laying hens,13,16 the basis of the model for fattening chickens9 is reduced to two compartments (Figure 1 and eqs 1–7): central compartment, which represents blood, and peripheral compartment, which represents total body fat excluding the brain. The values of physiological constants as well as a description of other model variables are presented in Table S1. The contaminants are absorbed into the central compartment at the fraction Fabs, which can be different for feed and soil; the rest is eliminated. The contaminants are transferred from the central compartment into the chicken ova at the rate ko. From the ova, ndl-PCBs are distributed within the growing yolks and excreted discretely with eggs. A second elimination mechanism is excretion from the central compartment and/or metabolic conversion of the parent compounds (ndl-PCBs) into their metabolites; both latter processes are lumped together with the rate constant keli. There is a mass exchange between the central and peripheral compartment according to constants k1 and k2 (Figure 1), which, as it follows from eqs 4 and 5, are equal to sMPSP × VP and sMPSP × VP × PCP, respectively.
Figure 1.
Scheme of the TK models describing the transfer of ndl-PCBs into body fat and yolks of laying hens. Mass exchange rates are groups of constants k1 = sMPSP × VP and k2 = sMPSP × VP × PCP (eqs 4 and 5).
The model is the set of seven equations:
| 1 |
| 2 |
| 3 |
| 4 |
| 5 |
| 6 |
| 7 |
where Vx is the volume of compartment x (mL), Wx is the weight of compartment x (g), ρx is the density of compartment (g/mL), Cx is the concentration of congener in compartment x (pg/mL), Ax is the amount of congener in x (pg), amountfeed(t) is the amount of consumed feed (g), sMPSP is the specific product of membrane permeability MP and the total surface area of membranes for peripheral compartment SP (1/mL/day), PCP is the partition coefficient from central to peripheral compartments, such that PCP = CC/CP (unitless), keli is the elimination rate from the central compartment (day–1), kO is the transfer rate from the central compartment to ova (day–1), x denotes either “C” or central, “P” or peripheral, “eli” or elimination, or “O” or ova.
Chickens start laying eggs when they are ∼20 weeks old, so their body weight and body fat weight are still increasing. The weight of the laid eggs also increases gradually by up to 30% as the hens’ age. These physiological details were used to simulate the laying hens from study 4 that were still growing by the start of the experiment (Table S2). A range of physiological parameters that influence the kinetics of contaminants as well as the specific amount of feed consumed in each study were included in the TK models (Table S2 and Figures S1–S3).
Since ndl-PCBs are not detected in the egg albumen, due to their lipophilic properties, the model only simulates the transfer of contaminants into egg yolks. The equation system (eqs 1–7) formally only predicts the ndl-PCB amounts in ova AO. The predicted concentrations in egg yolks are calculated algorithmically by mimicking individual egg laying at discrete time points after the system of eqs 1–7 is solved and the time-dependent value of AO is known. The algorithm below models the physiology of yolk growth and oviposition and its influence on the time-dependent concentration of ndl-PCBs in eggs.
Algorithm to Simulate the Yolk Deposition of ndl-PCBs
The yolk deposition of ndl-PCBs is modeled in a novel way that mimics the physiology of yolk growth and development, resulting in improved prediction of the experimental data without introducing an ad hoc time delay17 between ovulation and oviposition. The ova of a mature chicken are a collection of small white yolks (immature follicles), small yellow yolks, and large yellow (preovulatory) yolks. The group of large yellow yolks consists of 8–12 yolks of distinct diameters ranging from 0.2 to 15–25 mm,18,19 which are actively growing in a hierarchical order during the 2 weeks prior to ovulation. The essential components for developing yolks are synthesized in the liver and transferred with the bloodstream in very low-density lipoprotein (VLDL) particles into the ova.19 It is assumed that these particles also contain dissolved lipophilic contaminants and serve as the only mechanism of ndl-PCB transfer into eggs.
Approximately 98% of the yolk mass is linearly deposited19 in a concentric manner20 during the final 7–11 days prior to ovulation. The deposition is interrupted once the yolk enters the oviduct, 2–3 h before ovulation.19 We assumed that deposition of yolk components lasts 11 days and the amount of large follicles in the ova remains constant. The final weight of the yolks is 15–20 g. Once the yolk becomes mature, it enters the oviduct, where fertilization and formation of the egg occur. The journey through the oviduct usually takes slightly more than 1 day. We assume that no contaminants are transferred into the yolk once it has entered the oviduct.
Single injection studies
with ampicillin, a chemical with a short
half-life in chicken, revealed that this compound can be detected
even in the yolk of the tenth egg.18 Ampicillin
does not accumulate in chicken tissues, so the amounts of the drug
deposited in the yolks are not influenced by potential drug redistribution
from chicken organs. It is assumed that the amount of ndl-PCBs that
reaches the ova during the period between two consequent ovipositions
will be distributed among yolks being formed in the same proportion
as ampicillin (see Table S3). Numerous
observations10,12,18,21 showed that the first two eggs after the
start of exposure to various contaminants contained only background
levels of corresponding contaminants, which is caused by the time
delay between subsequent ovulations and subsequent ovipositions. The
algorithm for calculation of discrete ndl-PCB deposition in the large
yellow yolks appeared in a previous version22 and has been refined here to better reflect the physiology in the
following pseudocode:
Description of Model Parameters
The model (eqs 1–7 and the yolk deposition algorithm) has six parameters, namely, fraction absorbed from feed Fabs feed, fraction absorbed from soil Fabs soil, partition coefficient from the central to peripheral compartment PCP, the specific product of membrane permeability and the total surface area of membranes for the peripheral compartment sMPSP, the elimination rate from the central compartment keli, and the transfer rate from the central compartment to ova kO. The specific product of membrane permeability and the total surface area of membranes for the peripheral compartment (sMPSP) shows how many milliliters of blood flow through membranes per day in one unit of tissue volume and is assumed to be independent of chicken age and breed. The value of sMPSP is adopted from the model for fattening chickens.9 Thus, five remaining parameters have to be fitted from the experimental datasets as discussed in the strategy below.
Strategy to Optimize and Evaluate Model Parameters from Multiple Datasets
The parameters for toxicokinetic models for contaminated feed only (step 1 below) and contaminated soil and feed (step 2) were optimized and then evaluated using similar datasets (step 3) using all five studies in Table 1 plus the study of Traag et al.16 and Huyghebaert et al.23 The last two studies had much higher feed concentrations of ndl-PCBs and are, therefore, not listed in Table 1. The same procedure was performed for each individual ndl-PCB, so each congener is described by its specific set of model parameters. The studies in Table 1 were chosen because of comparable experimental conditions and feed contaminant concentration similar to the current maximum level.4 The range of applicability was then evaluated against higher exposure datasets (step 4 below).
In the first step, the controlled studies with contaminated feed (contamination from soil excluded)10,12 (studies 1 and 2 in Table 1) were used to optimize four parameters Fabs feed, PCP, keli, and kO.
During the second step, study 4 (refs (10, 11)) including contaminant transfer from both soil and feed into eggs was used to shed light on the transfer of ndl-PCBs from soil. The experiments in study 4 did not record the amount of soil consumed per laying hen. However, the amount of each ndl-PCB consumed from soil can be estimated by differences in the mass balance. The model with one free parameter amountsoil × Fabs soil was optimized, while the values Fabs feed, PCP, keli, and kO were kept fixed to the values found in the first step. To estimate the average daily amountsoil consumed by chickens, a scenario was simulated, where Fabs soil from soil constitutes 100%. Under this condition, the putative amountsoil consumed for each individual congener was estimated with the use of experimental data from study 4. After this, the values of Fabs soil of ndl-PCBs were estimated as the ratio of soil amount evaluated for this congener to the maximum value among the soil consumption estimates. These estimates constitute upper bounds for Fabs soil. In our study, we use the soil concentration and amount of ndl-PCBs as a proxy for the ndl-PCBs consumed also from the worms, insects, and herbs therein. The contribution of soil insects and herbs was not considered explicitly, also because they have been reported to be a less significant source of persistent organic pollutants than soil and chicken feed.24,25
The third step is the evaluation of parameters by testing model predictions against datasets from studies with comparable exposure of the animals. Study 3 (ref (13)) was used for evaluation of the model for contaminated feed (contamination from soil excluded) optimized in the first step. Similarly, the model including feed and soil contamination optimized in the second step was evaluated on the basis of study 5.13
In the fourth and last step, the range of applicability of the model at much higher feed concentrations is gauged. Additional model evaluations using two experiments with higher exposure levels were performed using the experimental datasets from Traag et al.16 and Huyghebaert et al.23
Fitting of Model Parameters
The fitting of model parameters was performed separately for each ndl-PCB by searching for the minimum of the function characterizing the distance between modeled and experimental values (eq 8). The distance is zero if the modeled value is within the uncertainty interval of the measured value.
| 8 |
where m and e denote modeled and experimental values, respectively; x = yolk stands for yolks or x = P for peripheral compartment; i is a particular measurement; and s is study 1 or study 2. δin = 0 if the modeled value is in the uncertainty interval of the corresponding experimental value and δin = 1 if the modeled value does not belong to the uncertainty interval of the corresponding experimental value. In studies 1 (ref (10)) and 2 (ref (12)), the expanded uncertainty of the measurement was 37 and 15%, respectively. For studies 3 (ref (13)) and 5 (ref (13)), an expanded uncertainty of the measurement of 15% was assumed. For study 4 (ref (11)), the standard deviation of the data was used in the modeling as the uncertainty interval for distance (eq 8) evaluation during the fitting procedure.
Estimation of Half-Lives, Transfer Rates, and Transfer Factors of ndl-PCBs from Feed and Soil
To illustrate the bioaccumulative potential of individual ndl-PCB congeners in chicken eggs, their transfer rates (TR) and transfer factors (TF) are calculated as a function of exposure duration. For this, model simulations were performed with defined model parameters, where the chickens with a constant body weight of 1900 g, fat fraction of 16%, and constant laying performance of 0.9 eggs per day were exposed to either contaminated feed or contaminated soil. Since different congeners require different amounts of time to reach steady state, it is necessary to specify a relevant time point for TR and TF. To visualize the time development of TR and TF, the exposure of physiologically unchanging chickens to the contaminants lasted 420 days, which corresponds to the timeslot between the start of laying and the first molt of laying chickens.26 TR (eq 9) was calculated as the ratio of the congener amount excreted with one laid egg to the amount consumed during the corresponding laying phase (time period since the last laying). TF was defined as the ratio of contaminant concentration in an egg laid at a certain time point to the concentration in the contaminated feed or soil (eq 10). The amount of contaminants excreted with laid eggs is approximated for simplicity with the amount of these contaminants transferred into the ova (i.e., excluding for this purpose the yolk deposition algorithm), so that
| 9 |
and
| 10 |
In addition to the TRs and TFs, the kinetics
of individual ndl-PCB congeners was characterized with the half-lives
during the depuration period. For this, a long-term exposure of 400
days was simulated until the ndl-PCB concentrations in eggs approached
their plateau values. The α (short) and β (long) half-lives
were calculated by approximating the concentration–time profile
of eggs during the onset and terminal periods of the depuration period
with respective monoexponential functions. To do so, the values of
concentrations were log-transformed and fitted with straight-line
equations y = kx + b. The estimation uncertainty Δk of the coefficient k was assessed using the square root of its diagonal element
in the inverted Hessian matrix, which is an output of the minimization
function. Then, the estimation uncertainty of each half-life was calculated
as 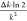 . Note that the estimation uncertainty pertains
only to the fit around the available data and does not include all
sources of uncertainty, such as that which may come from biological
differences among races or nutritional regimens.
. Note that the estimation uncertainty pertains
only to the fit around the available data and does not include all
sources of uncertainty, such as that which may come from biological
differences among races or nutritional regimens.
Estimation of the Highest Levels for the Sum of ndl-PCBs in Feed and Soil to Ensure Compliance with Maximum Levels in Eggs
Our presented TK models were used to perform simulations of 420 day (ref (26)) exposure of grown chickens to either feed or soil contaminated with one specific ndl-PCB congener. By varying the concentrations of individual congeners, the highest possible feed and soil levels that still ensure egg concentrations under the current maximum level of 40 ng/g fat for the sum were manually searched. This maximum level for ndl-PCBs is based on food occurrence data statistics and not on a human health-based guidance value.3,27 For the simulations, the chickens were assumed to consume daily either 124 g of feed or 6 g of soil contaminated with only one of the ndl-PCB congeners. The assumption of a 6 g daily soil consumption stems from the results of model simulations and is within accepted values.28 The chickens were assumed to have a constant body weight of 1900 g and 16% of body fat content, constant laying performance of 0.9 eggs per day, and a yolk weight per egg of 20 g.
The estimation uncertainty of the highest ndl-PCB concentration in feed was assessed by performing fitting of model parameters on a bootstrapped set of experimental values from studies 1 and 2, with consequent exposure simulations. The procedure was performed as follows: Step 1: The experimental values from studies 1 and 2 were combined. As chickens in these two studies were exposed to very similar levels of PCB 180 (269 and 234 ng/day, respectively), it was assumed that under these conditions, the concentrations in eggs are proportional to concentrations in feed. Therefore, to combine two datasets into one, the concentration values from study 1 were multiplied by a ratio of daily feed intake for study 1 to daily feed intake for study 2. Data related to the depuration phase were excluded from the dataset. Step 2: The model was fitted on the bootstrapped combined dataset. The new sets of model parameters were obtained. Step 3: The new sets of model parameters were used to estimate the highest levels in feed. The collected highest levels were used to calculate the estimation uncertainty. Note that the estimation uncertainty pertains only to the fit around the available data and does not include all sources of uncertainty.
In Silico Derived Partition Coefficients
The values of partition coefficients can be estimated with in silico methods, for which the LSER database29 was chosen. Linear solvation energy relation (LSER) is an approach that uses the physicochemical properties of a substance to predict its partition coefficient between two phases. Those phases may be biological tissues composed of defined amounts of albumin, muscle proteins, phospholipids, storage lipids, and water. The estimated error for the predictions of partition coefficients with this method is 0.7 log units. Details on the calculation of predicted partition coefficients between blood and peripheral compartment are described by Ohlhoff et al.,9 and the resulting values are presented in Table S4.
Results and Discussion
Model Parameters for ndl-PCB Exposure from Feed
The toxicokinetic model (Figure 1) was obtained following the steps outlined under “Strategy to optimize and evaluate model parameters from multiple datasets”. In the first step, the model for only feed contamination (contamination from soil excluded) was optimized using studies 1 and 2 simultaneously, according to eq 8. The experimental data and fitted curves are shown in Figures 2 and 3, respectively. The result is a congener-specific TK model to estimate the transfer of ndl-PCBs from contaminated feed into eggs and body fat of laying hens with parameters presented in Table S5 for eqs 1–7.
Figure 2.
Model simulation of study 1,10 showing measured (dots) and modeled (line) levels in eggs. Two groups of chickens were fed contaminated feed during either 28 (S-group) or 63 (L-group) days, in both cases followed by a depuration period of 100 days. Vertical dashed lines show the switch from the accumulation phase to the depuration phase. The horizontal axis shows the time passed from the start of the experiment. Experimental data (concentration measured for 10 pooled eggs) are presented as points with an expanded measurement uncertainty of 37.7%.
Figure 3.
Model simulation of study 2.12 Chickens consumed feed prepared with contaminated oil during 56 days followed by 56 days of depuration. Horizontal axis shows the age of the chickens in days. Experimental data (points) are presented as concentrations measured for 7–24 pooled eggs or mean concentrations for abdominal fat (n = 5) with expanded measurement uncertainty of 15%, model simulations are shown as lines. Light gray: concentration of ndl-PCBs in yolks and black: concentration of ndl-PCBs in abdominal fat.
Based on the previous experimental observations13,23,30 and simulations of ndl-PCB kinetics in fattening chickens,9 the fraction absorbed was restricted to above 80% for each type of matrix (contaminated feed, oil, or paint particles). After parametrization, the resulting values for fraction absorbed and elimination rate are found to be comparable to those for fattening chickens.9 This leads to the conclusion that laying hens, besides excretion via eggs, eliminates ndl-PCBs in a similar fashion to fattening chickens. We hypothesize that this elimination (not through excretion via eggs) is predominantly due to metabolization (whereas no measurement of metabolites was performed).
For all individual congeners except PCB 28, the two experimental datasets from studies 1 and 210,12 could be combined due to successful consensus (Table S5). Interestingly, the values of absorption from contaminated oil and paint particles Fabs feed, though fitted independently, show almost identical values for all ndl-PCBs except for PCB 28. So, it shows that ndl-PCBs present similar absorption from these two matrices. For PCB 28, an unrealistically high Fabs feed of 1.7 was obtained for study 1 by Ohlhoff et al.,10 as compared to 0.9 for study 2 (Table S6). Under optimized model parameters (Table S5), the Fabs feed of 0.9 for PCB 28 was chosen. The Fabs feed for other congeners is taken from study 2 since they are very similar to those obtained from study 1 (Table S5). Complications in the modeling of PCB 28 were also observed in other studies31 and were likely associated with volatility and overlooked background contamination sources of this congener. However, this inaccuracy may not be very relevant for real-world contamination incidents since PCB 28 is found in marginal quantities relative to higher chlorinated ndl-PCBs: this tendency was observed in the studies discussed in this paper (see Table 1), as well as in other exposure scenarios.16 Care should still be taken since some ndl-PCB sources may contain PCB 28 as a major component (e.g., Aroclor 1016 and Aroclor 1242, Table S7).
In agreement with the calculations performed for the fattening chickens,9 PCB 52 and PCB 101 have the fastest elimination kinetics (Table S5, high keli). Figure 2 depicts concentrations of fast eliminated congeners with fluctuations of high amplitude. This behavior of both the data and the model is due to a high elimination constant and discontinuous feed consumption throughout the day, whereas the model simulated uniform intake of the contaminants.
Estimating ndl-PCB Absorption from Soil
In the second step of the “Strategy to optimize and evaluate model parameters from multiple datasets”, the consumption and absorption of ndl-PCBs from soil was estimated. For this, study 4 was used to optimize one free parameter, namely, amountsoil × Fabs soil, while the values of other parameters were kept as in Table S5. The experiments in study 4 (ref (11)) unfortunately did not record the amountsoil consumed per laying hen. However, the amount of each ndl-PCB absorbed from soil (amountsoil × Fabs soil) can be estimated by differences in the mass balance: For each ndl-PCB, the amounts absorbed from soil together with the contribution from feed have to be equal to the amounts stored in the fat compartment, transferred into eggs, eliminated (metabolized), and unabsorbed from feed. Furthermore, the soil absorption coefficient of each PCB relative to the highest absorbed PCB (i.e., PCB 153) can also be established. If 100% absorption of PCB 153 from soil is postulated, then it follows that the laying hens consumed 6 g of soil (Table S6) per day to explain the observed concentrations in abdominal fat and eggs of the hens. A consumption of 6 g/day is reasonable for layers provided with nutritionally balanced feed28 and kept in the beginning of this study on an area with dense grass coverage.24 The absorption for other congeners was calculated on this basis. Insects, earthworms, and herbs were not included in the modeling as a separate source of ndl-PCBs for free-range chickens because of their minor contribution to exposure,24,25 absence of congener-specific data on soil-to-worm bioaccumulation factors, and high variability of PCB bioaccumulation factors for different types of worms.32−36 The result in Table S6 can be interpreted as the absorption of each congener relative to PCB 153 and as such as an upper bound/worst case for Fabs soil. For PCBs 101, 138, and 180, the maximum absorption rates of 67, 83, and 67%, respectively, were calculated, assuming 6 gram of soil intake and based on 100% absorption for PCB 153. As for the model parameter, the values from Table S6 in grams were used and interpreted as amountsoil × Fabs soil, meaning the product of an actual amount of consumed soil in grams and the actual absorption coefficient of corresponding ndl-PCBs in study 4. For PCBs 28 and 52, the absorption coefficients from soil could not be determined because the apparent mass balance is more than 100% considering the amount of contaminants consumed with feed alone. The final mass balance that uses the upper bound of absorption coefficients from Table S6 and the assumption of 6 g daily soil consumption is shown in Figure S4. More detailed and accurate experiments are required to evaluate the absorption of PCBs from soil, especially for PCBs 28 and 52.
The experimental data from study 4 (ref (11)) for PCBs 138, 153, and 180 show apparent outliers that cannot be explained by our TK models (Figure 4, measurements at age of 266 and 308 days). These unpredictably high concentrations were measured for muscle fat and egg yolk collected after 126 and 168 days of exposure. The most plausible explanation for the observed rapid increase of the levels at the end of the experiment would be changed experimental conditions that were not properly documented and therefore not considered in the simulations, rather than physiological processes. The factors that the model simulations reckoned without could include increased soil intake in winter associated with decreased grass cover density, higher availability of earthworms caused by rains, or change of a feed batch to one with a higher level of contamination. Observed concentrations in muscle fat and eggs could be simulated under the assumption that chickens started to consume 2–3 times higher amounts of soil in the second part of the experiment, which is still in the range of the expected soil intake of 2–30 g.28
Figure 4.
Model simulation of study 4.11 Chickens were exposed to three types of contaminated soils (data related to corresponding groups depicted in blue, burgundy, and gray) and consumed contaminated feed. (A) experimental data and model simulations for eggs and (B) experimental data and model simulations for muscle fat. Orange crosses show the measurements that are considered to be outliers due to the putative presence of additional undocumented sources of contaminants.
The models are freely available in code form as part of the Supporting Information. They are also available from the Open Food Safety Model Repository and as a module for implementation within the ConTrans suite of contaminant transfer estimation.
Model Performance and Evaluation
In the third step of the “Strategy to optimize and evaluate model parameters from multiple datasets”, the resulting TK models were evaluated against similar datasets. The model accounting only for exposure via feed (step 1) was evaluated against experimental data from the three groups of hens from study 313 that received feed contaminated with oil at three different levels (Figure S5), mixed in addition with clean sand. For PCBs 138, 153, and 180, the model predictions are within one standard deviation of the measured values. However, contrary to the studies used for parameter fitting (Figures 2 and 3), the TK models show a worse performance for the lower chlorinated PCBs 28, 52, and 101, and the model predictions are 3 times lower than the lower limit of the uncertainty range of the experimental values.
The toxicokinetic model including exposure via soil was evaluated for its ability to predict the transfer of ndl-PCBs against experimental data from study 5,13 in which three groups of hens received clean feed mixed with three levels of contaminated soil. The evaluation is depicted in Figure S6, where the model prediction of the feeding scenario from study 5 is shown as a range (filled area) to highlight the uncertainty in the estimation of the product amountsoil × Fabs soil. As study 4, used for the parametrization, could not shed light on the absorption properties of PCBs 28 and 52, Figure S6 shows predictions for these two congeners with an absorption coefficient varying from 0 to 100%.
As the fourth and last step in the “Strategy to optimize and evaluate model parameters from multiple datasets”, additional model evaluations using two experiments with much higher exposure levels were performed. The purpose was to test the applicability for higher exposure levels. In the experiment of Traag et al.,16 laying hens were exposed to feed levels of around 240-fold the current maximum level.4 The models presented here can describe the data on PCBs 138, 153, and 180 within the error of 15%, as well as the fast eliminated congeners PCBs 52 and 101 with less than 50% error (Figure S7). The prediction for PCB 28 overestimates the experimental data by more than 200%. Huyghebaert et al.23 exposed the laying hens via feed with 1650-fold the current maximum level;4 the models cannot accurately describe the observed levels of any of the ndl-PCBs in this study (Figure S8).
Accurate estimation of the actual chicken body fat proportion is important to obtain reliable results when simulating a specific scenario. As shown in the simulations (see the “Influence of total fat percentage of the chickens on the modeling results” section, Supporting Information), variation of the fat content from 10 to 20% of the total body weight leads to deviations in modeled ndl-PCB levels of up to 20% in the chicken eggs and 30% in chicken fat. This effect is most pronounced during the first few weeks of exposure, which was the case in study 3. As shown in Figure S9, the fat content of the body influences the ndl-PCB kinetics. As the reported fat content values of chickens from study 3 were unusually low,37 our model results include additionally a prediction error of 20–30% for the values measured 14 days after the onset of the exposure (e.g., short duration of a feed incident). Interestingly, knowledge of the fat weight of the laying hens becomes insignificant for the model simulation results after chronic exposure. After 150 days, the differences in model predictions with different fat contents vary by less than 5% (Figure S9). This means that for simulations of scenarios with constant background contamination, the developed models can be implemented, and the results will not be drastically influenced by the variability in constitution of individual hens.
Half-Lives in Eggs and Body Fat, Transfer Factors, and Transfer Rates
The studied ndl-PCBs display different kinetic properties and thus require different time periods until their concentration in eggs approaches the steady state under the condition of constant ndl-PCB intake. Table S8 summarizes the duration of exposure necessary to approach steady state and the half-lives in eggs and body fat. Fast eliminated congeners (PCBs 52 and 101) have the shortest α-half-life of 3 days. In addition to this, they reach a maximum concentration in eggs already after 85 days.
Transfer rates and transfer factors for individual ndl-PCBs as a function of exposure duration are presented in Figures S10 and S11, respectively. PCB 138 has very similar transfer rates from feed and from soil into eggs. Other slowly eliminated congeners display transfer rates from soil that are 30% higher than for feed. Fast eliminated congeners (PCB 52 and PCB 101) have transfer rates of around 0.05 for both feed and soil.
Highest Levels in Feed and Soil that Ensure Compliance with Maximum Levels in Edible Products
Ensuring compliance with the maximum levels for eggs is more critical than for muscle fat because the concentrations of all ndl-PCBs in muscle fat (except for PCB 28) are lower than those in eggs (on fat basis), whereas the maximum level is the same for both food products. Compared to the fattening chickens,9 slowly eliminated congeners (PCBs 28, 138, 153, and 180) have slightly different kinetic profiles, with PCB 180 being the most bioaccumulative, and therefore, the most critical component in the mixture (Figure 5). Coincidentally, our model showed the most accurate prediction for PCB 180 during the model evaluation (Figures S5 and S7) with studies, where the feed burden concentrations varied by 240 times.
Figure 5.
Simulation of the ndl-PCB levels in eggs and body fat (foods of animal origin) for the proposed highest levels in feed (2.4 μg/kg) and soil (45 μg/g soil) that ensure compliance with the maximum levels in foods (40 ng ndl-PCB/g fat). The estimation uncertainty for the highest levels was calculated to be 13%. The simulations present the chicken life phase from the start of laying eggs to the first molt. (A, B) Feed is contaminated with only one of the six congeners. In this case, concentrations of PCB 180 will result in egg levels equal to the maximum level in foods (40 ng/g fat). Chickens consumed daily 124 g of feed, being the only source of ndl-PCBs. Each line is a result of an individual simulation, where the feed was contaminated with a single ndl-PCB congener at a concentration of 2.4 μg/kg feed 88% DM. (C, D) Chickens are assumed to consume daily 6 g of soil, being the only source of ndl-PCBs. Each line is a result of an individual simulation, where the soil was contaminated with a single ndl-PCB congener at a concentration of 45 μg/g soil.
Therefore, a simulation was performed, where the feed was contaminated exclusively with PCB 180 and its concentration adjusted to the value that results in an egg concentration of 40 ng/g fat. The simulation results in a feed concentration of 2.4 μg/kg feed 88% DM for PCB 180 suggested as the highest level protective for any mixture of six indicator ndl-PCBs. Figure 5 shows the results of six model simulations, where the feed was contaminated with a single ndl-PCB at a concentration of 2.4 μg/kg feed DM.
As the result of the data analysis on transfer of ndl-PCBs from soil into chicken eggs, four models are presented that simulate the kinetics of PCBs 101, 138, 153, and 180 and can be used to evaluate the critical levels for soil. A daily intake of 6 g of soil was used to simulate chronic exposure. Four independent simulations were performed, where the soil was the only source and was contaminated with only one ndl-PCB congener (Figure 5). The modeled exposure lasted 420 days.26 The chickens had a constant body weight of 1900 g and laying performance of 0.9 eggs per day. In contrast to the feed, maximum estimated absorption constants for ndl-PCBs from soil vary from 0.67 to 1 with PCB 153 being most readily available. Therefore, it is suggested to limit the highest level of ndl-PCBs in soil to the concentration for PCB 153 of 45 μg/g soil that still ensures egg concentrations below 40 ng/g fat. Figure 5 shows how individual ndl-PCBs accumulate in the eggs after the chickens were exposed to soil with a concentration of 45 μg/g for each congener. Simulations for PCBs 28 and 52 could not be performed for study 4 because sources other than soil (feed and environment) were more important for exposure to these congeners, thus making the parametrization of absorption from soil unfeasible.
Under actual field conditions, free-range chickens may be exposed via both feed and soil with background contamination with ndl-PCBs. However, the highest levels for feed and soil were calculated for the situation, where chickens are exposed via only one contamination source. Therefore, using the highest levels for feed and for soil suggested here can still result in eggs with concentrations of ndl-PCBs exceeding the ML of 40 ng/g fat when both sources play a significant role. To consider the influence of both contamination sources on the final concentrations in eggs, an equation is proposed that takes into account the highest levels in feed and soil (eq 11)
| 11 |
Coefficients are the transfer factors shown in Figure S11. Input from the soil and feed matrices are characterized with the maximum transfer factors that were reached after 480 days of constant exposure. In fact, for young chickens, the transfer factors under the real conditions might be up to 40% lower than the values used in the equation. However, chickens kept at private households can reach an age of several years, and for them, modeling of molting phases and variation on laying performance are required to accurately predict transfer of ndl-PCBs from contaminated matrices into eggs. The controlling procedure can be simplified using eq 11 or Figure S12 to show the pairs of feed and soil concentrations that ensure levels below the current maximum level for eggs of 40 ng/g fat.
Our calculations of maximum levels considered high laying performance (0.9 eggs per day) and constant values of background contamination during the whole laying period. However, both parameters vary with time, which should be taken into account when calculations for a specific case are performed. Whenever the data are available, it is recommended to run a customized simulation rather than to use the default setting of the model script.
Though there are currently no EU regulations on ndl-PCB levels in soils, some countries have introduced national action levels for different soil types. For example, the Netherlands and the UK have a background (optimum) value for residential soils of 0.02 μg/g for the sum of PCBs.38 Higher levels are permitted in Canada: 0.5 and 1.3 μg/g for agricultural and residential soils, respectively. Studies on background PCB levels in soil in Denmark report a range from 0.1 to 10 ng/g dry matter for agricultural and rural areas.38 Consequently, our estimation of soil highest levels in the range of 0–45 μg/g is relevant only for considerably contaminated soils.
This theoretical study presents a congener-specific toxicokinetic model for the transfer of ndl-PCBs from contaminated feed and soil into muscle fat and eggs of laying hens. The appeal of the model results from the fact that it is based on three independent datasets and evaluated on four studies. The model includes a novel way of mimicking the gradual and concentric deposition of contaminants into the growing yolks over several days. Based on the performed simulations, the highest levels of ndl-PCBs in feed and soil are suggested to ensure compliance with MLs in eggs and body fat. The models do not consider biotransformation of parent ndl-PCB compounds into potentially toxic and potentially bioaccumulative PCB metabolites because of the lack of data. Further studies are necessary to close this gap.
Acknowledgments
The authors would like to thank Leonie Lautz for critically reading the manuscript.
Glossary
Abbreviations
- DM
dry matter
- LSER
linear solvation energy relation
- ML
maximum level
- ndl-PCB
non-dioxin-like polychlorinated biphenyls
- PCDD/Fs
dibenzo-p-dioxins and dibenzofurans
- TF
transfer factor
- TK
toxicokinetic model
- TR
transfer rate
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jafc.2c04396.
Python.py script of the default model simulation; Food Safety Knowledge Exchange format.fskx file of the model that can be run in the KNIME environment for various exposure scenario simulations including visualization (ZIP)
Details on model script parameters; modeling of chicken physiology; modeling of transfer of contaminants into growing yolks; partition coefficients, model parameters; evaluation of ndl-PCB absorption from soil and distribution of ndl-PCB congeners in ndl-PCB containing commercial sources; mass balance for study 4; model evaluation with the data from study 3 and study 5; additional model evaluation using two experiments with much higher exposure levels; influence of total fat percentage of the chickens on the modeling results; half-lives, transfer rates, and transfer factors of ndl-PCBs; model assumptions, limitations, and comparison with the model of Van Eijkeren et al.; practical levels of ndl-PCBs for both feed and soil to ensure the current maximum levels for eggs; and comparison of the models for laying hens with the models for fattening chickens (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Hoogenboom R. L.; Malisch R.; van Leeuwen S. P. J.; Vanderperren H.; Hove H.; Fernandes A.; Schachtele A.; Rose M. Congener Patterns of Polychlorinated Dibenzo-p-Dioxins, Dibenzofurans and Biphenyls as a Useful Aid to Source Identification during a Contamination Incident in the Food Chain. Sci. Total Environ. 2020, 746, 141098 10.1016/j.scitotenv.2020.141098. [DOI] [PubMed] [Google Scholar]
- WHO . Safety Evaluation of Certain Food Additives and Contaminants Supplement 1: Non-Dioxin-like Polychlorinated Biphenyls, 2016.
- EU. COMMISSION REGULATION (EU) No 1259/2011 of 2 December 2011 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels for Dioxins, Dioxin-like PCBs and Non Dioxin-like PCBs in Foodstuffs Off. J. Eur. Union, 2011.
- EU. COMMISSION REGULATION (EU) No 277/2012 of 28 March 2012 Amending Annexes I and II to Directive 2002/32/EC of the European Parliament and of the Council as Regards Maximum Levels and Action Thresholds for Dioxins and Polychlorinated Biphenyls Off. J. Eur. Union, 2012.
- EU. COMMISSION REGULATION (EU) No 1067/2013 of 30 October 2013 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of the Contaminants Dioxins, Dioxin-like PCBs and Non-Dioxin-like PCBs in Liver of Terrestrial Animals Off. J. Eur. Union, 2013.
- Van Eijkeren J. C. H.; Zeilmaker M. J.; Kan C. A.; Traag W. A.; Hoogenboom L. A. A Toxicokinetic Model for the Carry-over of Dioxins and PCBs from Feed and Soil to Eggs. Food Addit. Contam. 2006, 23, 509–517. 10.1080/02652030500512045. [DOI] [PubMed] [Google Scholar]
- Schuler F.; Schmid P.; Schlatter C. The Transfer of Polychlorinated Dibenzo-p-Dioxins and Dibenzofurans from Soil into Eggs of Foraging Chicken. Chemosphere 1997, 34, 711–718. 10.1016/S0045-6535(97)00463-3. [DOI] [PubMed] [Google Scholar]
- Wang C.; Dong S.; Wang P.; Hao Y.; Wang R.; Zhang S.; Wang Y.; Fan M.; Zhang Q.; Jiang G. Reevaluation on Accumulation and Depletion of Dioxin-like Compounds in Eggs of Laying Hens: Quantification on Dietary Risk from Feed to Egg. Sci. Total Environ. 2021, 801, 149690 10.1016/j.scitotenv.2021.149690. [DOI] [PubMed] [Google Scholar]
- Ohlhoff B.; Savvateeva D.; Bernsmann T.; Spolders M.; Jahnke A.; Lüth A.; Lahrssen-Wiederholt M.; Numata J.; Pieper R. Unraveling Congener-Specific Transfer of Non-Dioxin-Like Polychlorinated Biphenyls (Ndl-PCBs) from Feed into Chicken Meat. Environ. Sci. Technol. 2021, 55, 11080–11090. 10.1021/acs.est.1c02650. [DOI] [PubMed] [Google Scholar]
- Ohlhoff B.; Savvateeva D.; Leisner J.; Hartmann F.; Südekum K.-H.; Bernsmann T.; Spolders M.; Jahnke A.; Lüth A.; Lahrssen-Wiederholt M.; Numata J.; Pieper R. Transfer of Non-Dioxin-like Polychlorinated Biphenyls (Ndl-PCBs) from Feed and Soil into Hen Eggs. J. Agric. Food Chem. 2022, 70, 8955–8962. 10.1021/acs.jafc.2c02243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südekum K.-H.; Hembrock-Heger A.; Leisner J.; Hartmann F.. Untersuchungen Zum Dioxin-Und PCB-Transfer Im Pfad Boden-Huhn-Ei Bei Hühnern Aus Freilandhaltung, 2020.
- Hoogenboom L. A. P.; Kan C. A.; Zeilmaker M. J.; Van Eijkeren J.; Traag W. A. Carry-over of Dioxins and PCBs from Feed and Soil to Eggs at Low Contamination Levels - Influence of Mycotoxin Binders on the Carry-over from Feed to Eggs. Food Addit. Contam. 2006, 23, 518–527. 10.1080/02652030500512037. [DOI] [PubMed] [Google Scholar]
- Fournier A.; Feidt C.; Travel A.; Bizec B.; Venisseau A.; Marchand P.; Jondreville C. Relative Bioavailability to Laying Hens of Indicator Polychlorobiphenyls Present in Soil. Chemosphere 2012, 88, 300–306. 10.1016/j.chemosphere.2012.02.041. [DOI] [PubMed] [Google Scholar]
- Pirard C.; De Pauw E. Uptake of Polychlorodibenzo-p-Dioxins, Polychlorodibenzofurans and Coplanar Polychlorobiphenyls in Chickens. Environ. Int. 2005, 31, 585–591. 10.1016/j.envint.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Fournier A.; Martin O.; Travel A.; Puillet L.; Feidt C.; Jondreville C. Modeling PCB Transfer into Hen Eggs: Influence of Physiological Characteristics of the Animal. Environ. Toxicol. Chem. 2015, 34, 173–183. 10.1002/etc.2781. [DOI] [PubMed] [Google Scholar]
- Traag W. A.; Kan C. A.; van der Weg G.; Onstenk C.; Hoogenboom L. A. P. Residues of Dioxins (PCDD/Fs) and PCBs in Eggs, Fat and Livers of Laying Hens Following Consumption of Contaminated Feed. Chemosphere 2006, 65, 1518–1525. 10.1016/j.chemosphere.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Gerletti P.; Von Kleist M.; Mielke H.; Kuhl T.; Pieper R.; Lahrssen-Wiederholt M.; Numata J. Transfer Kinetics of Fipronil into Chicken (Gallus Gallus Domesticus) Eggs. Comput. Toxicol. 2020, 15, 100131 10.1016/j.comtox.2020.100131. [DOI] [Google Scholar]
- Donoghue D. J. Mechanisms Regulating Drug and Pesticide Residue Uptake by Egg Yolks: Development of Predictive Models. Worlds Poult. Sci. J. 2001, 57, 373–380. 10.1079/wps20010026. [DOI] [Google Scholar]
- Nys Y.; Bain M.; Van Immerseel F.. Improving the Safety and Quality of Eggs and Egg Products. In Egg Chemistry, Production and Consumption, Elsevier, 2011. [Google Scholar]
- Grau C. R. Ring Structure of Avian Egg Yolk. Poult. Sci. 1976, 55, 1418–1422. 10.3382/ps.0551418. [DOI] [Google Scholar]
- Fournier A.; Feidt C.; Dziurla M. A.; Grandclaudon C.; Jondreville C. Transfer Kinetics to Egg Yolk and Modeling Residue Recovered in Yolk of Readily Metabolized Molecules: Polycyclic Aromatic Hydrocarbons Orally Administered to Laying Hens. Chemosphere 2010, 78, 1004–1010. 10.1016/j.chemosphere.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Kowalczyk J.; Göckener B.; Eichhorn M.; Kotthoff M.; Bücking M.; Schafft H.; Lahrssen-Wiederholt M.; Numata J. Transfer of Per- And Polyfluoroalkyl Substances (PFAS) from Feed into the Eggs of Laying Hens. Part 2: Toxicokinetic Results Including the Role of Precursors. J. Agric. Food Chem. 2020, 68, 12539–12548. 10.1021/acs.jafc.0c04485. [DOI] [PubMed] [Google Scholar]
- Huyghebaert G.; Daeseleire E.; Grijspeerdt K.; Van Renterghem R. The Deposition Profile of Oxy-Carotenoids, Fat and PCBs in Egg Yolks. Arch. Geflugelkd. 2002, 66, 216–223. [Google Scholar]
- Waegeneers N.; De Steur H.; De Temmerman L.; Van Steenwinkel S.; Gellynck X.; Viaene J. Transfer of Soil Contaminants to Home-Produced Eggs and Preventive Measures to Reduce Contamination. Sci. Total Environ. 2009, 407, 4438–4446. 10.1016/j.scitotenv.2008.12.041. [DOI] [PubMed] [Google Scholar]
- Kijlstra A.; Traag W. A.; Hoogenboom L. A. P. Effect of Flock Size on Dioxin Levels in Eggs from Chickens Kept Outside. Poult. Sci. 2007, 86, 2042–2048. 10.1093/ps/86.9.2042. [DOI] [PubMed] [Google Scholar]
- Bell D. D. Historical and Current Molting Practices in the U.S. Table Egg Industry. Poult. Sci. 2003, 82, 965–970. 10.1093/ps/82.6.965. [DOI] [PubMed] [Google Scholar]
- Results of the Monitoring of Non Dioxin-like PCBs in Food and Feed. EFSA J. 2010, 8, 1701 10.2903/j.efsa.2010.1701. [DOI] [Google Scholar]
- Jurjanz S.; Germain K.; Juin H.; Jondreville C. Plant and Soil Intake by Organic Broilers Reared in Tree- or Grass-Covered Plots as Determined by Means of n-Alkanes and of Acid-Insoluble Ash. Animal 2015, 9, 888–898. 10.1017/S1751731114002870. [DOI] [PubMed] [Google Scholar]
- Endo S.; Goss K. U. Applications of Polyparameter Linear Free Energy Relationships in Environmental Chemistry. Environ. Sci. Technol. 2014, 48, 12477–12491. 10.1021/es503369t. [DOI] [PubMed] [Google Scholar]
- De Vos S.; Verschueren D.; De Schrijver R. Digestibility, Retention and Incorporation of Low-Level Dietary PCB Contents in Laying Hens. Chemosphere 2005, 58, 1553–1562. 10.1016/j.chemosphere.2004.11.081. [DOI] [PubMed] [Google Scholar]
- Moenning J.; Lamp J.; Knappstein K.; Numata J.; Molkentin J.; Susenbeth A.; Dänicke S.; Fürst P.; Schenkel H.; Pieper R.; Krause T.. Transfer of Polychlorinated Dibenzo-p-Dioxins and Dibenzofurans (PCDD/Fs) and Polychlorinated Biphenyls (PCBs) into Milk of High Yielding Cows during Positive and Negative Energy Balance, 2022.
- Denyes M. J.; Langlois V. S.; Rutter A.; Zeeb B. A. The Use of Biochar to Reduce Soil PCB Bioavailability to Cucurbita Pepo and Eisenia Fetida. Sci. Total Environ. 2012, 437, 76–82. 10.1016/j.scitotenv.2012.07.081. [DOI] [PubMed] [Google Scholar]
- Denyes M. J.; Rutter A.; Zeeb B. A. In Situ Application of Activated Carbon and Biochar to PCB-Contaminated Soil and the Effects of Mixing Regime. Environ. Pollut. 2013, 182, 573–578. 10.1016/j.envpol.2013.07.016. [DOI] [PubMed] [Google Scholar]
- Belfroid A.; van den Berg M.; Seinen W.; Hermens J.; van Gestel K. Uptake, Bioavailability and Elimination of Hydrophobic Compounds in Earthworms (Eisenia Andrei) in Field-contaminated Soil. Environ. Toxicol. Chem. 1995, 14, 605–612. 10.1002/etc.5620140408. [DOI] [Google Scholar]
- White J. C.; Parrish Z. D.; Isleyen M.; Gent M. P. N.; Iannucci-Berger W.; Eitzer B. D.; Kelsey J. W.; Mattina M. I. Influence of Citric Acid Amendments on the Availability of Weathered PCBs to Plant and Earthworm Species. Int. J. Phytorem. 2006, 8, 63–79. 10.1080/15226510500507102. [DOI] [PubMed] [Google Scholar]
- Paul P.; Ghosh U. Influence of Activated Carbon Amendment on the Accumulation and Elimination of PCBs in the Earthworm Eisenia Fetida. Environ. Pollut. 2011, 159, 3763–3768. 10.1016/j.envpol.2011.07.025. [DOI] [PubMed] [Google Scholar]
- Szentirmai E.; Milisits G.; Donkó T.; Budai Z.; Ujvári J.; Fülöp T.; Repa I.; Süto Z. Comparison of Changes in Production and Egg Composition in Relation to in Vivo Estimates of Body Weight and Composition of Brown and White Egg Layers during the First Egg-Laying Period. Br. Poult. Sci. 2013, 54, 587–593. 10.1080/00071668.2013.811717. [DOI] [PubMed] [Google Scholar]
- Larsen J. C.; Nielsen E.; Boberg J.; Petersen M. A.. Evaluation of Health Hazards by Exposure to Polychlorinated Biphenyls (PCB) and Proposal of a Health-Based Quality Criterion for Soil; The Danish Environmental Protection Agency, 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








