Abstract
Background
Globally, 5 billion people lack access to safe surgical care with more deaths due to lack of quality care rather than lack of access. While many proven quality improvement (QI) interventions exist in high-income countries, implementing them in low/middle-income countries (LMICs) faces further challenges. Currently, theory-driven, systematically articulated knowledge of the factors that support successful scale-up of QI in perioperative care in these settings is lacking. We aimed to identify all perioperative safety and QI interventions applied at scale in LMICs and evaluate their implementation mechanisms using implementation theory.
Methods
Systematic scoping review of perioperative QI interventions in LMICs from 1960 to 2020. Studies were identified through Medline, EMBASE and Google Scholar. Data were extracted in two phases: (1) abstract review to identify the range of QI interventions; (2) studies describing scale-up (three or more sites), had full texts retrieved and analysed for; implementation strategies and scale-up frameworks used; and implementation outcomes reported.
Results
We screened 45 128 articles, identifying 137 studies describing perioperative QI interventions across 47 countries. Only 31 of 137 (23%) articles reported scale-up with the most common intervention being the WHO Surgical Safety Checklist. The most common implementation strategies were training and educating stakeholders, developing stakeholder relationships, and using evaluative and iterative strategies. Reporting of implementation mechanisms was generally poor; and although the components of scale-up frameworks were reported, relevant frameworks were rarely referenced.
Conclusion
Many studies report implementation of QI interventions, but few report successful scale-up from single to multiple-site implementation. Greater use of implementation science methodology may help determine what works, where and why, thereby aiding more widespread scale-up and dissemination of perioperative QI interventions.
Keywords: Surgery, Health services research, Health systems, Review
WHAT IS ALREADY KNOWN ON THIS TOPIC
At the point of care, various quality improvement (QI) interventions are delivered to reduce surgical morbidity and mortality.
These QI interventions are critical since, worldwide, poor-quality services cause more deaths than a lack of access to healthcare.
However, there is still poor knowledge about how to scale up QI interventions. Because resources are limited, surgical quality and safety are generally poor, and surgical outcomes are much worse in some of the low/middle-income countries (LMICs), the need to reduce the implementation gap in scaling is even higher than in high-income countries.
WHAT THIS STUDY ADDS
To the best of our knowledge this is one of the first studies to assess the scale-up of perioperative point-of-care patient safety interventions in LMICs using established implementation frameworks.
Interventions such as enhanced recovery after surgery and antimicrobial stewardship (including reducing surgical site infections) are currently under-represented in LMICs; our study reports a possibility of using theoretical implementation methodologies to better understand the relationship between contextual factors and success, as well as to discover scale-up facilitators and impediments.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
In LMICs, surgical safety science is shifting its focus from an evidence gap to a scalability gap, which will have an impact on effective coverage of health services.
Introduction
Since the publication of the Institute of Medicine’s Report ‘To Err is Human’,1 much progress has been made in improving surgical outcomes by reducing errors and improving the quality and safety of patient care. Many quality improvement (QI) interventions now exist that are proven to reduce surgical morbidity and mortality. Such QI interventions are important because globally, more deaths are due to lack of quality rather than lack of access to healthcare.2 In various healthcare and country contexts, the discussion about the quality of health services versus lack of access to healthcare extends beyond surgery. However, knowledge of how to successfully implement QI interventions at scale remains lacking.3 4 Over a decade ago, this failure to rapidly deploy QI technologies and practices widely to improve population health was described as a ‘form of waste that donors, researchers, clinicians, and most of all, communities in developing nations cannot afford.’5 Recently, the challenges of scale-up were highlighted by the Enhancing PeriOperative Care after High risk surgery trial. Despite strong evidence in favour of the intervention at local level, the benefits could not be replicated when scaled up at the national level in the UK.6 7 According to the WHO, the failure to expand successful pilot or demonstration projects around the world is a ‘major failure in global health’.8 There is increasing recognition that guideline publications, policy reform and training alone are insufficient to achieve successful and sustainable scale-up.9–11 For complex interventions, understanding the factors that facilitate or hinder implementation is critically important. The focus of surgical safety science is thus shifting from addressing an evidence gap to an implementation gap in scalability.12
In low/middle-income countries (LMICs), the imperative to close the implementation gap in scalability is even greater than in high-income countries (HICs) because resources are limited, surgical quality and safety are often poor, and outcomes are significantly worse.13–15 Failure to close the implementation gap means that potential ‘solutions’ for improving the quality of care risk remaining hidden in pockets around the globe, flourishing locally without reliably reaching those in need elsewhere.5 In 2019, the WHO Global Ministerial Patient Safety Summit declared that healthcare systems globally must urgently focus on the principles of implementation science if the momentum of the global patient safety movement is to be realised.16 This notion was also highlighted by the 2021 GlobalSurg-3 Study which shows that while surgical complication rates after cancer surgery are similar globally, the 30-day mortality is significantly greater in LMICs.17 The authors estimated that improving infrastructure and perioperative care would save an additional 10 lives per 100 complications. Since many proven perioperative QI interventions exist, one of the greatest challenges currently facing the global surgical community is how to implement them successfully at scale in LMICs.
Implementation science is a field of applied health research that could help address this challenge. Implementation science is a form of health policy and systems research that is used to study and support the scale-up and integration of interventions into health systems at the regional and national level (online supplemental appendix 1).18 Broadly, efforts have been made to define, classify and systematise the implementation science literature with the aim of enabling researchers and clinicians to better understand the current evidence base, identify knowledge gaps, and more clearly report implementation strategies and outcomes. Powell et al19 have developed a taxonomy (Expert Recommendations for Implementing Change: ERIC) to define implementation strategies, and to move away from confusing or unclear language when reporting results. Likewise, a framework for conceptualising and evaluating implementation outcomes has been proposed,20 which is used by the WHO as a means of defining ‘successful implementation’ (online supplemental appendix 4).
bmjgh-2022-010649supp001.pdf (493.1KB, pdf)
This systematic scoping review aims to address the current gap in understanding whether and how interventions proven clinically effective in reducing mortality and morbidity postoperatively have been scaled up in surgical settings in LMICs. We used concepts from the science of implementation, first to identify all perioperative QI interventions applied at scale in LMICs; and second, to evaluate the mechanisms and effectiveness of their scaled implementation using theoretical frameworks.
Methods
We conducted a systematic scoping review of perioperative QI interventions in LMICs. The aim of a systematic scoping review is to define and map the current evidence base for an emerging field. Such reviews can address broad questions and include varied methodologies; evidence is captured through systematic and comprehensive search, analysis and reporting.21 We followed the recommended five-step approach for such reviews, as detailed below.22 23 The review protocol was registered a priori with the Open Science Framework (https://osf.io/s6y98).
Review question
The research question was developed by using the SPIDER question format tool24: Sample, Phenomenon of Interest, Design, Evaluation and Research type. The SPIDER tool was chosen since it was designed mainly for qualitative/mixed-methods studies and therefore aligns best with the studies of interest in LMICs. The study samples belong to populations (including children and adults) from LMICs. LMICs were defined using World Bank definitions, which are based on gross national income (https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups).
The phenomenon of interest was defined as perioperative QI interventions, which are delivered at the point of care (ie, at the clinical–patient interface level). The search, therefore, excluded interfaces between health systems, such as implementing a database, designing and implementing electronic medical records, or simply engaging in a training programme. The list of perioperative QI interventions was derived from the UK Royal College of Anesthetists ‘audit recipe book’ for perioperative safety and quality improvement.25 While the ‘audit recipe book’ was predominantly designed for use in HICs, in the absence of a list designed for LMICs, it nonetheless represents a clinically relevant, established and comprehensive list on which to base the search strategy.
Search strategy
Two electronic databases—Medline and EMBASE—were searched from January 1960 to April 2020. Searches produced through Google Scholar, which is identified as a powerful search mechanism,26 were also included. The search strategy (online supplemental appendix 3) consisted of angling terms related to “surgery”, “point of care interventions”, “LMICs”, “scale-up” and a list of perioperative QI interventions which included terms such as: checklist, triage, early warning score, protocol, guideline, quality improvement, pathway, bundle, fasting, thromboprophylaxis, patient admission, airway and failure to rescue. Backward and forward citation searches of identified papers were also carried out.
Study selection
A two-stage approach was taken. Stage 1 selection was designed to identify all perioperative QI interventions implemented in LMICs, and stage 2 aimed to identify those implemented at scale. Since there is no universally agreed definition of ‘scale-up’ in the healthcare literature, we defined it using an iterative approach—as follows:
Stage 1: inclusion was based on study title, abstract and keywords. Screening was based on the phenomenon of interest, which was perioperative point-of-care QI interventions implemented in LMICs. Studies were included if they were original human studies that reported primary data. Reviews and secondary data reports were excluded. QI interventions beyond the point of care were excluded, such as implementing a database, electronic medical records or ad-hoc training programmes for healthcare workers. However, if ‘training’ was part of implementing the intervention (ie, training in how to use a checklist), then it was included. QI interventions implemented outside hospitals were excluded. Additionally, cross-sectional studies that only assessed knowledge, attitudes and behaviours of health workers for the QI interventions were also excluded. There were no restrictions imposed on types of evaluation, study designs or language.
Screening was done through the online software Rayyan. Three reviewers (MCW, SA, NS) screened a quarter of the search results in triplicate to calibrate our judgement of the inclusion criteria and establish reliable consensus for quality assurance purposes. Two authors (MCW and SA) then trained six further reviewers (IO, OC, SRM, DH, MT, JCAH) who screened the remaining articles, initially in duplicate with MCW to ensure calibration and provide quality assurance, while SA acted as arbitrator.
Stage 2: once we had identified all perioperative QI interventions reported in LMICs (stage 1), we identified the median number of hospital sites where QI interventions were applied (median=1; IQR 1–2) and pragmatically decided to define scale-up as per the upper quartile, that is, implementing an intervention in three or more sites. Stage 2 criteria were therefore not defined a priori in the registered protocol. The full texts of all articles identified by stage 2 criteria were retrieved.
Data extraction and management
At stage 1, five reviewers (IO, OC, SRM, DH, MT) extracted descriptive data including country of origin, type of QI intervention and number of hospital sites where the intervention was applied.
At stage 2, two teams of reviewers (IO and OC; DH and SRM) extracted data on implementation strategies using the ERIC framework19; implementation success using the implementation outcomes framework18 20; and scale-up approaches using Yamey’s27 and Barker et al’s11 theoretical scale-up frameworks. Brief descriptions of these frameworks and rationale for their application were as follows:
The ERIC framework19 consists of 73 discrete implementation strategies grouped into nine domains (online supplemental appendix 5): use evaluative and iterative strategies (n=10), provide interactive assistance (n=4), adapt and tailor to context (n=4), develop stakeholder inter-relationships (n=17), train and educate stakeholders (n=11), support clinicians (n=5), engage patients/service users (n=5), use financial strategies (n=9) and change infrastructure (n=8). This is currently the best-established framework for detailed analysis of strategies to support implementation, based on systematic review of the healthcare evidence base and Delphi consensus. We have recently applied this framework successfully to analyse implementation of surgical safety interventions in LMICs, including the WHO Surgical Safety Checklist28 and the use of audit and feedback to reduce surgical site infections (SSIs).29
Eight implementation outcomes are defined in the implementation science evidence base20 and adopted by the WHO18: appropriateness, adoption, acceptability, feasibility, fidelity, implementation cost, penetration/reach and sustainability. This offers a currently agreed framework for defining the results of the process of implementing an intervention. We have recently shown that these outcomes are relevant to surgical safety interventions.28 29
Yamey’s framework27 is designed for the scale-up of global health interventions. It consists of 13 discrete strategies grouped into six key components known to influence scale-up success: innovation characteristics, implementers, delivery strategy, adopting community, sociopolitical context and research context.
Barker et al’s framework11 is based on lessons learnt from large-scale QI initiatives in Africa. It consists of 14 discrete strategies, grouped into three key components known to influence scale-up success: phase of setting scale-up, environmental factors/mechanisms to foster adoption and infrastructure to support scale-up.
Data were extracted in duplicate by OC and IO (implementation strategies and outcomes), and SRM and DH (scale-up strategies) with MCW acting as an arbitrator for both groups.
Quality assessment of the included studies
Risk of bias was assessed by IO and OC using the QualSyst tool,30 which is designed to assess both quantitative and qualitative studies, including observational studies. Mixed-methods studies underwent both quantitative and qualitative assessments. Because a scoping search did not identify many randomised trials, we decided a priori that no study would be excluded based on study design or risk of bias.
Results
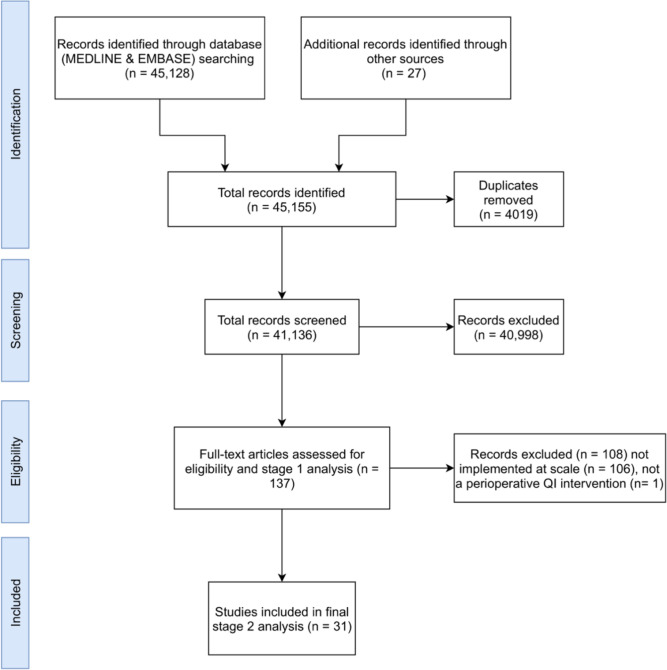
We screened 41 136 citations and included 137 papers reporting implementation of 144 point-of-care perioperative QI interventions at stage 1 of the review process (figure 1).
Figure 1.
PRISMA diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; QI, quality improvement.
Stage 1 analysis revealed that the most common QI interventions implemented in LMICs were: the WHO Surgical Safety Checklist (including safe birth checklists) (44 of 144), prevention and management of SSIs and interventions aiming to improve antimicrobial stewardship (AMS) (33 of 144), and enhanced recovery after surgery (ERAS) (27 of 144) (table 1). Most of the interventions (106 of 137, 77%) were implemented in two or fewer sites.
Table 1.
Type of QI intervention and frequency of implementation: overall and at scale (three sites or more)
| Type of QI intervention | Scale-up sites (n=31) | Implementation sites (144 intervention types from 137 studies) |
| ERAS | 1 | 27 |
| Other | 4 | 40 |
| SSI/antimicrobial stewardship | 5 | 33 |
| Surgical checklist (including maternal/birth) | 21 | 44 |
ERAS, enhanced recovery after surgery; QI, quality improvement; SSI, surgical site infection.
The median number of hospital sites undergoing QI implementation per study was 1 (range=1–120; IQR 1–2).
For stage 2 selection and final analysis, we defined scale-up as implementation at three or more sites based on the IQR of the number of study sites identified in stage 1. Therefore, studies implementing QI interventions at fewer than three sites did not meet the criteria for scale-up and were excluded. This resulted in 31 studies for final analysis (figure 1). Of these, 20 (65%) had a first author with an LMIC institutional affiliation and 11 (35%) with HIC institutional affiliation. Similarly, of last authors, 19 of 31 (61%) had LMIC institutional affiliations and 12 of 31 (39%) HIC affiliations.
Twenty-one out of 31 studies focused on safety checklists (including safe birthing checklists),31 32 five on SSI/AMS,33 34 one on ERAS35 and four36 37 38 on other interventions (table 1). The latter included implementation of guidelines to improve obstetric care, increase vaginal delivery rate by increasing access to neuraxial analgesia, management guidelines, multifaceted educational activities and a package of QI activities at intervention sites (online supplemental appendix 2 provides a summary of each study).
Analysis of implementation strategies
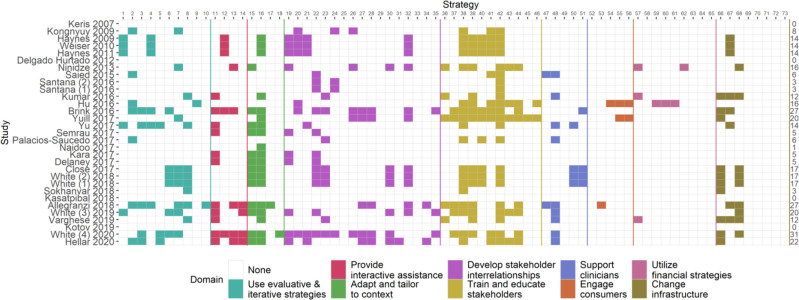
Figure 2 shows the ERIC implementation strategies used. Out of 73 different implementation strategies, 59 strategies were reported as being used across all 31 studies included in the final analysis. The median number of strategies used was 12 (range=0–31, IQR 4–17). Strategies that were most commonly reported across all included studies were from three out of nine ERIC domains: ‘train and educate stakeholders’, ‘develop stakeholder relationships’, and ‘use evaluative and iterative strategies’.
Figure 2.
ERIC implementation strategies19 (shown by the nine domains) reported in each study. ERIC, Expert Recommendations for Implementing Change.
Analysis of implementation outcomes
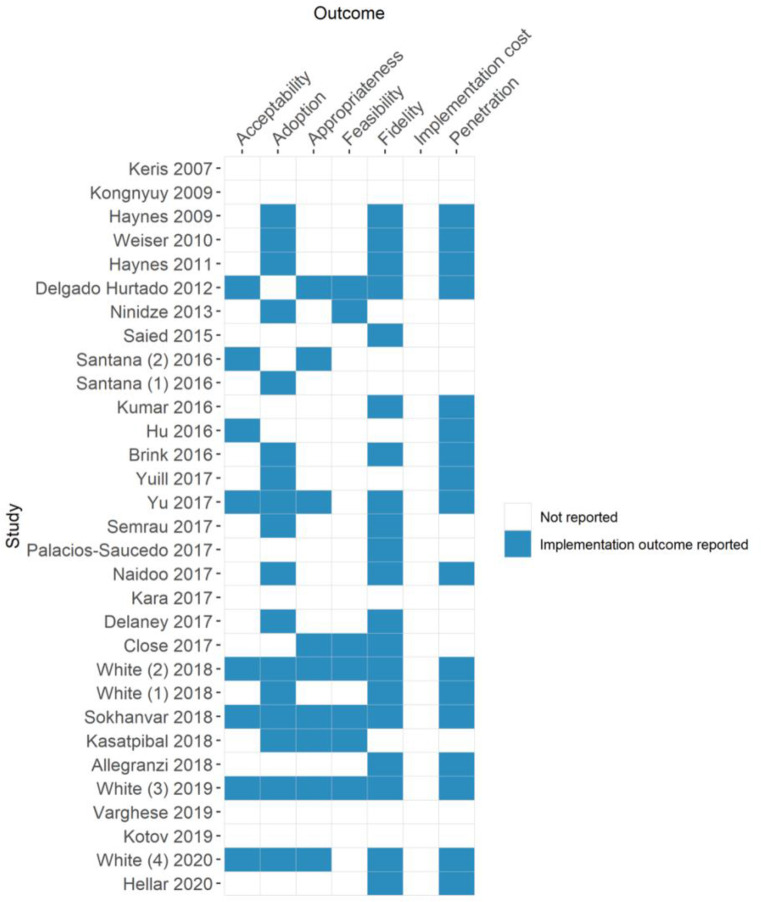
Twenty-four out of 31 studies reported one or more implementation outcomes (figure 3). The most reported implementation outcomes were fidelity (64.5%, 20 of 31), adoption (54.8%, 17 of 31) and penetration (54.8%, 17 of 31). No study reported on implementation cost or sustainability. Median length of time between QI implementation and outcome evaluation was 6 months (range=1–108 months, IQR 5–18 months). As an implementation outcome, sustainability is not defined. We pragmatically defined it as 12 months.
Figure 3.
International implementation outcomes20 reported in each study.
Analysis using scale-up frameworks
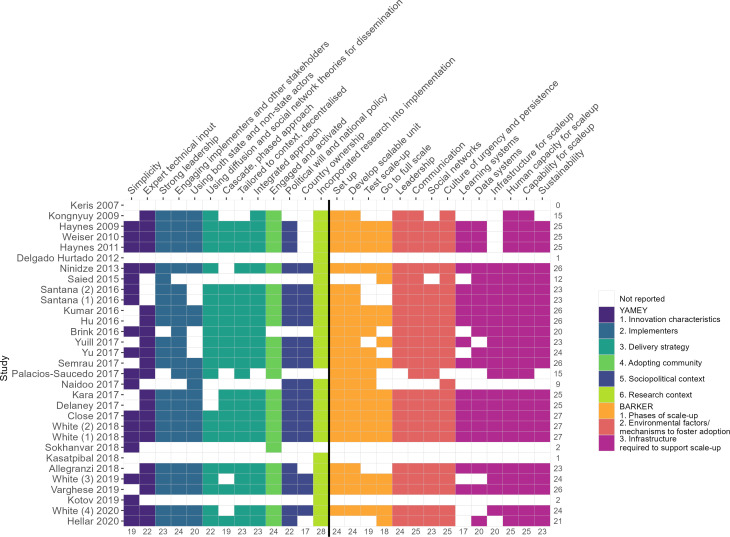
The components of both scale-up frameworks (Yamey and Barker et al)11 27 were widely reported across all included studies (Yamey: median 10, range 0–12, IQR 7–11; Barker et al: median 13, range 0–15, IQR 9–15) (figure 4). Three39–41 out of 31 studies (9.6%) reported all of the discrete strategies from the Yamey (n=13) and Barker et al (n=14) frameworks. From the two frameworks combined, 22 (71%) of the studies reported more than 20 discrete strategies.39–60 However, no study made explicit reference to either the Yamey or the Barker et al frameworks.
Figure 4.
Components of Yamey27 and Barker et al11 scale-up frameworks reported.
Discussion
To our knowledge, this is the first systematic attempt to assess the scale-up of perioperative point-of-care patient safety interventions in LMICs using established implementation frameworks. While a wide variety of QI interventions are reported as being implemented in LMICs, we found that very few (31 of 137) have been taken to scale in three or more sites. Of the interventions taken to scale, the WHO Surgical Safety Checklist is by far the most common (21 of 31) with only 5 of 31 broadly speaking related to AMS (including reduction of SSIs) and ERAS scale-up reported in a single study.
Over a decade ago in 2007, the WHO described the lack of scale-up of proven interventions to be a major failure of healthcare globally,8 and called for a focus on ways to increase the impact of proven interventions to benefit more people and foster policy development on a sustainable basis.8 Our results suggest there is much work still to be done. Innovative clinicians in surgery-designing technologies and process to improve surgical quality and safety cannot continue to operate with an implicit theory of spread—namely, that interventions proved to be successful in pilot studies or trials, will rapidly spread widely to positively impact population health simply through publication, market forces or communication networks.5 In LMICs, even when healthcare leaders and/or clinicians are aware of promising interventions, their ability to implement them is often severely constrained by limited time, resources and skill. There are several important steps between learning about a new concept, meaningful implementation in one’s own setting and subsequent widespread scale-up to other settings. Most innovative technologies and processes must be spread actively, not passively, or they may not be scaled effectively at all.61
The Yamey27 and Barker et al11 scale-up frameworks were designed to ensure active spread of innovations and they offer a guide to the key drivers for change, outlining the steps to take. We propose that they are intuitive to understand and apply for clinicians; and they may be considered as both process models and determinant frameworks for scale-up for implementation scientists. The individual components of both Yamey27 and Barker et al11 were widely reported across all studies in our review with little difference in between them making it impossible to recommend the use of one framework over the other. However, neither framework was referenced directly. This suggests that while the component parts of both scale-up frameworks may be intuitive, the frameworks themselves are less known. It could be argued that so long as scale-up occurs, it does not matter whether theoretical frameworks are cited. However, since the literature shows both high-profile failures of scale-up in the UK spanning more than a decade,6 7 62 63 as well as notable successes,64 65 and our results show scale-up is sparse in LMICs, we, along with others,16 propose that using theoretical models to guide the implementation process deserves more consideration. Theoretical models are based on observations and can highlight both barriers and facilitators to successful implementation and scale-up—but also, importantly, reasons for success or failure. We argue that mix of positive and negative results when evidenced interventions are taken to scale, in both HICs and LMICs, requires some theoretical thinking regarding, for instance, organisational and behavioural factors that may help (when present) or hinder (when absent) the success of a scale-up effort.
Theorising about what may work and reasons for it will help to develop the evidence base around the effectiveness and appropriateness of implementation and scale-up strategies in perioperative care interventions (and indeed more widely). We would further argue that, even if some scale-up models appear intuitive, they can still be useful by acting as a checklist for design, implementation and evaluation; help make the process more efficient; and reduce reliance on clinicians’ or organisations’ memory/skill set. Given that the failure of proven innovations to reach those who could benefit from them is not just a clinical failure but also a moral one as it compounds the inequity and injustice that already exists between HICs and LMICs. Theoretical models, we argue, are necessary for understanding the implementation process (including where such a process may fail) and, as a result, assisting in scaling up safety and quality measures. The standardisation that they bring allows comparison between studies and comprehensive analysis of the facilitators and barriers to scale up. If clinicians and academics are to contribute to population-wide health improvements, then it is time to pay as much attention to the mechanisms of scale-up as to the evaluation of an innovation’s effectiveness.
The median number of ERIC implementation strategies reported in our review was 12 (out of a total of 73 strategies identified in the evidence base19), and all studies reported at least one implementation strategy. This is higher than that reported by White et al in a recent systematic review specifically focused on the WHO Surgical Safety Checklist implementation in LMICs29: that study showed that most checklist implementations in LMICs were single site, the median number of strategies used per study was 4 and a quarter (12 of 47) of all WHO Checklist studies reported no implementation strategies. One explanation for the greater number of strategies we found could be that when interventions are taken to scale, a larger implementation effort is necessary; hence, more attention is paid to the implementation process, which in turn leads to better reporting of the implementation strategies used.
It remains unclear which of the 73 strategies listed in ERIC are most important for successful scale-up. Evidence from the WHO Checklist implementation (ie, not scale-up per se) suggests that strategies from the following five ERIC domains: ‘train and educate stakeholders’, ‘adapt and tailor to context’, ‘provide interactive assistance’, develop stakeholder relationships’ and ‘support clinicians’ are likely to be the most important for success. Our results suggest that in the context of scale-up, strategies from the three domains: ‘train and educate stakeholders’, ‘develop stakeholder relationships’, and ‘use evaluative and iterative strategies’ may be determinants of success. The importance of stakeholders is demonstrated by two domains common to both the WHO Checklist implementation and broader QI scale-up: the domains of ‘train and educate stakeholders’ and ‘develop stakeholder relationships’ together represent almost 40% of all ERIC strategies. This adds further weight to the suggestion that stakeholder influence is a key driver of implementation and especially scale-up. Our results further show that strategies in the domain ‘use evaluative and iterative strategies’ were used more commonly than the two domains found in White et al’s review29: ‘adapt and tailor to context’ and ‘provide interactive assistance’. This may be because when an intervention is applied initially at a single site, then the adaption to context and ongoing interactive assistance is important, (ie, adaptability, acceptability and adoption); once this has been tested in a single or pilot site setting, and the intervention then progresses to scale up, other factors such as those covered under the use of evaluative and iterative strategies become more prominent for robust and lasting scale-up (ie, high fidelity, sustainable scale-up). This is an interpretation, which requires empirical evaluation. Importantly, both the Yamey and Barker et al scale-up frameworks include incorporating research into the implementation and the use of learning systems and data systems, which are important in applying iterative strategies.
The concept of scalability is relatively loosely defined and can be confused with the ability to widen the reach of an intervention, and with scant attention to continued robust performance under routine conditions (ie, fidelity and sustainability).66 In our review, fidelity was reported in two-thirds of studies and the median length of time from implementation to evaluation of scale-up was 6 months.
Limitations
This review has limitations. We did not exclude any study based on quality and therefore several potential biasing and confounding elements must be considered. Most of our data come from scale-up of the WHO Checklist, which means that the applicability of our findings to other interventions, such as ERAS and AMS, may be limited. Only two databases were searched to identify eligible studies and although we hand-searched the reference lists of included studies and review articles, it is possible that some studies were missed. There was likely under-reporting of the implementation strategies used and we did not contact individual study authors directly for further details. There may also be bias on the part of the data extraction teams in their interpretation of study methodology and mapping that to discrete implementation and scale-up strategies. Additionally, publication bias is probable and because there is no requirement to record implementation efforts a priori, the quality of the totality of implementation efforts globally may be less good than those published in per-reviewed journals and included in this study. The WHO Checklist, ERAS and interventions targeting SSI all have a strong evidence base in LMICs as well as HICs, but not all HIC interventions will be as transferable or as effective in low-resource settings so our results should be applied cautiously to interventions where evidence of clinical effectiveness in LMIC context is lacking. The World Bank Country and Lending Group classification is updated every fiscal year, and we used the classification from 2021 which means that countries that moved into the high-income classification over the last 60 years would have been excluded from the study. Lastly, our review (and any review on scale-up strategies for surgical interventions) rests on the assumption that scaling up benefits is ‘good’. This may be logical; however, scale-up may also lead to the scale-up of unintended harmful consequences; our study did not examine this.
This review also has several strengths. It describes and quantifies which perioperative QI interventions have been implemented in LMICs, specifically those taken to scale, and identifies explicitly the implementation methodologies used in scale-up. While most studies pertain to the WHO Checklist, future studies could focus on scale-up of other commonly applied interventions such as ERAS and interventions targeting AMS including the prevention of SSIs.
Despite a growing realisation in recent years of the role of implementation science in bridging the gap between research, policy and practice,16 67 68 our study suggests that use of theory-driven implementation approaches remains underused and is a missed opportunity in QI work globally. On a more positive note, since interventions such as ERAS and AMS (including reducing SSIs) are currently under-represented in LMICs, we feel we are faced with a golden opportunity for using theoretical implementation approaches to increase our understanding of the relationship between contextual factors and success and to help identify facilitators and barriers to scale up. Based on this review and recent reviews of WHO Checklist implementation29 and audit and feedback interventions to support antibiotic guidelines and reduce SSIs28 in LMICs, we propose two key recommendations. First, implementers should emphasise active stakeholder engagement when scaling perioperative QI interventions—including frontline clinicians, local academics and policymakers/managers. Second, we recommend broadening the generally clinician-driven QI implementation efforts to include a wider multidisciplinary team69 that includes implementation and improvement scientists (or at least a cadre of staff with expertise in change management, QI and behaviour or organisational change).
Conclusion
If lives can be saved by improving the quality and safety of surgical care, then clinicians and academics need to urgently address the lack of widespread scale-up of proven QI interventions, especially in LMICs. The surgical safety community has a moral and ethical duty to ensure that any new practice of merit is actively shared and implemented with those in greatest need. Improving the understanding of the process and strategies of scale-up among clinicians, health system leaders and policymakers may be one place to start.
Acknowledgments
The authors wish to thank the steering group of the Global Alliance for Surgical, Obstetric, Trauma and Anesthesia Care (G4 Alliance) International Standards and Guidelines for Quality Safe Surgery and Anesthesia Working Group (ISG-QSSA) for suggesting the topic of ‘scale-up’. The G4 Alliance is a 60-member organisation representing over 300 international federations, societies, academia and non-governmental organisations in 160 countries worldwide. In partnership with the International Society of Surgery (ISS), the alliance formed the ISG-QSSA Working Group, which is comprised of 13 members from surgical, anaesthesia, government and public health specialties with the goal of summarising the existing evidence base regarding optimal surgical, obstetric, trauma and anaesthesia systems quality improvement interventions in order to arrive at global policy recommendations for low/middle-income countries (LMICs).
Footnotes
Handling editor: Seye Abimbola
Twitter: @mcwdoc, @JaymieClaire
Contributors: MCW and SA—study concept and design, data analysis, visualisation and interpretation, drafting and revising the article critically for important intellectual content. MCW and SA are both responsible for overall content as a guarantor. KP—data acquisition and interpretation, data analysis and revising the article critically for important intellectual content. SRM, DH, IO, OC, MT and JCAH—data acquisition and interpretation, and revising the article critically for important intellectual content. NS—study concept and design, data acquisition and interpretation, drafting and revising the article critically for important intellectual content. All authors give final approval of the submitted version of the article to be reviewed and published.
Funding: NS is supported by the National Institute for Health Research (NIHR) Applied Research Collaboration (ARC) South London at King’s College Hospital NHS Foundation Trust. NS is a member of King’s Improvement Science, which offers co-funding to the NIHR ARC South London and comprises a specialist team of improvement scientists and senior researchers based at King’s College London. Its work is funded by King’s Health Partners (Guy’s and St Thomas’ NHS Foundation Trust, King’s College Hospital NHS Foundation Trust, King’s College London and South London and Maudsley NHS Foundation Trust), Guy’s and St Thomas’ Charity and the Maudsley Charity. NS is funded by the NIHR Global Health Research Unit on Health System Strengthening in Sub-Saharan Africa, King’s College London (GHRU 16/136/54) using UK aid from the UK Government to support global health research. NS and SA are further supported by the ASPIRES research programme in LMICs (Antibiotic use across Surgical Pathways–Investigating, Redesigning and Evaluating Systems), funded by the Economic and Social Research Council.
Disclaimer: The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Competing interests: None declared.
Patient and public involvement: The early stages of the review (that is, protocol), to develop the review question and scope, were informed by MW's field experience in Sub-Saharan Africa, which included consultations with patients.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
We have adhered to the principles of open and transparent reporting. This scoping review did not require permission from any specific ethical body.
References
- 1.Institute of Medicine . (US) Committee on Quality of Health Care in America. In: Kohn LT, Corrigan JM, Donaldson MS, eds. To err is human: building a safer health system. Washington (DC): National Academies Press (US), 2000. [PubMed] [Google Scholar]
- 2.Kruk ME, Gage AD, Arsenault C, et al. High-quality health systems in the sustainable development goals era: time for a revolution. Lancet Glob Health 2018;6:e1196–252. 10.1016/S2214-109X(18)30386-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howell A-M, Panesar SS, Burns EM, et al. Reducing the burden of surgical harm: a systematic review of the interventions used to reduce adverse events in surgery. Ann Surg 2014;259:630–41. 10.1097/SLA.0000000000000371 [DOI] [PubMed] [Google Scholar]
- 4.Montroy J, Breau RH, Cnossen S, et al. Change in adverse events after enrollment in the National surgical quality improvement program: a systematic review and meta-analysis. PLoS One 2016;11:e0146254. 10.1371/journal.pone.0146254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCannon CJ, Berwick DM, Massoud MR. The science of large-scale change in global health. JAMA 2007;298:1937–9. 10.1001/jama.298.16.1937 [DOI] [PubMed] [Google Scholar]
- 6.Peden CJ, Stephens T, Martin G, et al. Effectiveness of a national quality improvement programme to improve survival after emergency abdominal surgery (epoch): a stepped-wedge cluster-randomised trial. Lancet 2019;393:2213–21. 10.1016/S0140-6736(18)32521-2 [DOI] [PubMed] [Google Scholar]
- 7.Stephens TJ, Peden CJ, Pearse RM, et al. Improving care at scale: process evaluation of a multi-component quality improvement intervention to reduce mortality after emergency abdominal surgery (epoch trial). Implement Sci 2018;13:142. 10.1186/s13012-018-0823-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simmons R, Fajans P, Ghiron L. Scaling up helath service delivery: from pilot innovations to policies and programmes, 2007. Available: https://apps.who.int/iris/handle/10665/43794
- 9.World Health Organisation . Beginning with the end in mind: planning pilot projects and other programmatic research for successful scaling up; 2011. https://www.who.int/reproductivehealth/publications/strategic_approach/9789241502320/en/
- 10.World Health Organisation . Nine steps for developing a scaling-up strategy; 2010. https://www.who.int/reproductivehealth/publications/strategic_approach/9789241500319/en/
- 11.Barker PM, Reid A, Schall MW. A framework for scaling up health interventions: lessons from large-scale improvement initiatives in Africa. Implement Sci 2016;11:12. 10.1186/s13012-016-0374-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delgado Hurtado JJ, Jiménez X, Peñalonzo MA, et al. Acceptance of the who surgical safety checklist among surgical personnel in hospitals in Guatemala city. BMC Health Serv Res 2012;12:169. 10.1186/1472-6963-12-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biccard BM, Madiba TE, Kluyts H-L, et al. Perioperative patient outcomes in the African surgical outcomes study: a 7-day prospective observational cohort study. Lancet 2018;391:1589–98. 10.1016/S0140-6736(18)30001-1 [DOI] [PubMed] [Google Scholar]
- 14.GlobalSurg Collaborative . Surgical site infection after gastrointestinal surgery in high-income, middle-income, and low-income countries: a prospective, international, multicentre cohort study. Lancet Infect Dis 2018;18:516–25. 10.1016/S1473-3099(18)30101-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.GlobalSurg Collaborative . Mortality of emergency abdominal surgery in high-, middle- and low-income countries. Br J Surg 2016;103:971–88. 10.1002/bjs.10151 [DOI] [PubMed] [Google Scholar]
- 16.Global Ministerial Summit on Patient Safety . Jeddah Declaration on patient safety, 2019. Available: https://www.who.int/news-room/events/detail/2019/03/02/default-calendar/global-ministerial-summit-on-patient-safety
- 17.GlobalSurg Collaborative and National Institute for Health Research Global Health Research Unit on Global Surgery . Global variation in postoperative mortality and complications after cancer surgery: a multicentre, prospective cohort study in 82 countries. Lancet 2021;397:387–97. 10.1016/S0140-6736(21)00001-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters DH, Tran NT, Adam T. Implementation research in health: a practical guide. Geneva: Alliance for Health Policy and Systems Research, World Health Organization; 2013. https://apps.who.int/iris/bitstream/handle/10665/91758/9789241506212_eng.pdf?sequence=1 [Google Scholar]
- 19.Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the expert recommendations for implementing change (ERIC) project. Implement Sci 2015;10:21. 10.1186/s13012-015-0209-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health 2011;38:65–76. 10.1007/s10488-010-0319-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munn Z, Peters MDJ, Stern C, et al. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol 2018;18:1. 10.1186/s12874-018-0611-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implementation Science 2010;5:1. 10.1186/1748-5908-5-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005;8:19–32. 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 24.Cooke A, Smith D, Booth A. Beyond PICO. Qual Health Res 2012;22:1435–43. 10.1177/1049732312452938 [DOI] [PubMed] [Google Scholar]
- 25.Royal College of Anaesthetists . Raising the Standards: rcoA quality improvement compendium. Available: https://rcoa.ac.uk/safety-standards-quality/quality-improvement/raising-standards-rcoa-quality-improvement-compendium
- 26.Haddaway NR, Collins AM, Coughlin D, et al. The role of google scholar in evidence reviews and its applicability to grey literature searching. PLoS One 2015;10:e0138237. 10.1371/journal.pone.0138237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamey G. Scaling up global health interventions: a proposed framework for success. PLoS Med 2011;8:e1001049. 10.1371/journal.pmed.1001049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahuja S, Peiffer-Smadja N, Peven K, et al. Use of feedback data to reduce surgical site infections and optimize antibiotic use in surgery: a systematic scoping review. Ann Surg 2022;275:e345–52. 10.1097/SLA.0000000000004909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White MC, Peven K, Clancy O, et al. Implementation strategies and the uptake of the World Health organization surgical safety checklist in low and middle income countries: a systematic review and meta-analysis. Ann Surg 2021;273:e196–205. 10.1097/SLA.0000000000003944 [DOI] [PubMed] [Google Scholar]
- 30.Kmet LM, Cook LS, Lee RC, et al. Standard quality assessment criteria for evaluating primary research papers from a variety of fields (QualSyst), 2004. https://era.library.ualberta.ca/items/48b9b989-c221-4df6-9e35-af782082280e [Google Scholar]
- 31.Kasatpibal N, Sirakamon S, Punjasawadwong Y, et al. An exploration of surgical team perceptions toward implementation of surgical safety checklists in a non-native English-speaking country. Am J Infect Control 2018;46:899–905. 10.1016/j.ajic.2017.12.003 [DOI] [PubMed] [Google Scholar]
- 32.Kongnyuy EJ, Mlava G, van den Broek N. A criterion based audit of the management of obstructed labour in Malawi. Arch Gynecol Obstet 2009;279:649–54. 10.1007/s00404-008-0786-1 [DOI] [PubMed] [Google Scholar]
- 33.Saied T, Hafez SF, Kandeel A, et al. Antimicrobial stewardship to optimize the use of antimicrobials for surgical prophylaxis in Egypt: a multicenter pilot intervention study. Am J Infect Control 2015;43:e67–71. 10.1016/j.ajic.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 34.Palacios-Saucedo GdelC, de la Garza-Camargo M, Briones-Lara E, et al. Evaluación del uso de antibióticos e impacto de una intervención dirigida a modificar la conducta prescriptiva en profilaxis quirúrgica en 6 hospitales del área metropolitana de monterrey. Cirugía y Cirujanos 2017;85:459–70. 10.1016/j.circir.2016.10.033 [DOI] [PubMed] [Google Scholar]
- 35.Kotov SVK, Khachatryan ALK, Kotova DPK, et al. Analysis of the results of ERas protocol in real-life clinical practice after radical cystectomy (the first prospective multicenter study in Russia). Urologiia 2020;6_2020:60–6. 10.18565/urology.2019.6.60-66 [DOI] [PubMed] [Google Scholar]
- 36.Naidoo M, Moodley J, Gathiram P, et al. The impact of a modified World Health organization surgical safety checklist on maternal outcomes in a South African setting: a stratified cluster-randomised controlled trial. S Afr Med J 2017;107:248–57. 10.7196/SAMJ.2017.v107i3.11320 [DOI] [PubMed] [Google Scholar]
- 37.Keris V, Lavendelis E, Macane I. Association between implementation of clinical practice guidelines and outcome for traumatic brain injury. World J Surg 2007;31:1354–7. 10.1007/s00268-007-9002-x [DOI] [PubMed] [Google Scholar]
- 38.Sokhanvar M, Kakemam E, Goodarzi N. Implementation of the surgical safety checklist in hospitals of Iran; operating room personnel's attitude, awareness and acceptance. Int J Health Care Qual Assur 2018;31:609–18. 10.1108/IJHCQA-03-2017-0051 [DOI] [PubMed] [Google Scholar]
- 39.Close KL, Baxter LS, Ravelojaona VA, et al. Overcoming challenges in implementing the who surgical safety checklist: lessons learnt from using a checklist training course to facilitate rapid scale up in Madagascar. BMJ Glob Health 2017;2:e000430. 10.1136/bmjgh-2017-000430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White MC, Baxter LS, Close KL, et al. Evaluation of a countrywide implementation of the world health organisation surgical safety checklist in Madagascar. PLoS One 2018;13:e0191849. 10.1371/journal.pone.0191849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White MC, Randall K, Ravelojaona VA, et al. Sustainability of using the who surgical safety checklist: a mixed-methods longitudinal evaluation following a nationwide blended educational implementation strategy in Madagascar. BMJ Glob Health 2018;3:e001104. 10.1136/bmjgh-2018-001104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allegranzi B, Bischoff P, de Jonge S, et al. New who recommendations on preoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis 2016;16:e276–87. 10.1016/S1473-3099(16)30398-X [DOI] [PubMed] [Google Scholar]
- 43.Brink AJ, Messina AP, Feldman C, et al. From guidelines to practice: a pharmacist-driven prospective audit and feedback improvement model for peri-operative antibiotic prophylaxis in 34 South African hospitals. J Antimicrob Chemother 2017;72:1227–34. 10.1093/jac/dkw523 [DOI] [PubMed] [Google Scholar]
- 44.Marx Delaney M, Maji P, Kalita T, et al. Improving adherence to essential birth practices using the who safe childbirth checklist with peer coaching: experience from 60 public health facilities in Uttar Pradesh, India. Glob Health Sci Pract 2017;5:217–31. 10.9745/GHSP-D-16-00410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haynes AB, Weiser TG, Berry WR, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med 2009;360:491–9. 10.1056/NEJMsa0810119 [DOI] [PubMed] [Google Scholar]
- 46.Hellar A, Tibyehabwa L, Ernest E, et al. A team-based approach to introduce and sustain the use of the who surgical safety checklist in Tanzania. World J Surg 2020;44:689–95. 10.1007/s00268-019-05292-5 [DOI] [PubMed] [Google Scholar]
- 47.Hu L-Q, Flood P, Li Y, et al. No pain labor & delivery: a global health initiative's impact on clinical outcomes in China. Anesth Analg 2016;122:1931–8. 10.1213/ANE.0000000000001328 [DOI] [PubMed] [Google Scholar]
- 48.Kara N, Firestone R, Kalita T, et al. Update of: Kara et al., the Betterbirth program: pursuing effective adoption and sustained use of the who safe childbirth checklist through Coaching-Based implementation in Uttar Pradesh, India. Glob Health Sci Pract 2018;6:225–6. 10.9745/GHSP-D-18-00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar S, Yadav V, Balasubramaniam S, et al. Effectiveness of the who SCC on improving adherence to essential practices during childbirth, in resource constrained settings. BMC Pregnancy Childbirth 2016;16:345. 10.1186/s12884-016-1139-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ninidze N, Bodin S, Ivester T, et al. Advancing obstetric anesthesia practices in georgia through clinical education and quality improvement methodologies. Int J Gynaecol Obstet 2013;120:296–300. 10.1016/j.ijgo.2012.09.027 [DOI] [PubMed] [Google Scholar]
- 51.Santana HT, Rodrigues MCS, do Socorro Nantua Evangelista M. Surgical teams' attitudes and opinions towards the safety of surgical procedures in public hospitals in the Brazilian federal district. BMC Res Notes 2016;9:276. 10.1186/s13104-016-2078-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santana HT, de Freitas MR, Ferraz EM, et al. Who safety surgical checklist implementation evaluation in public hospitals in the Brazilian federal district. J Infect Public Health 2016;9:586–99. 10.1016/j.jiph.2015.12.019 [DOI] [PubMed] [Google Scholar]
- 53.Semrau KEA, Hirschhorn LR, Marx Delaney M, et al. Outcomes of a coaching-based who safe childbirth checklist program in India. N Engl J Med 2017;377:2313–24. 10.1056/NEJMoa1701075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Varghese B, Copas A, Kumari S, et al. Does the safe childbirth checklist (SCC) program save newborn lives? Evidence from a realistic quasi-experimental study, Rajasthan, India. Matern Health Neonatol Perinatol 2019;5:3. 10.1186/s40748-019-0098-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.White MC, Randall K, Capo-Chichi NFE, et al. Implementation and evaluation of nationwide scale-up of the surgical safety checklist. Br J Surg 2019;106:e91–102. 10.1002/bjs.11034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.White MC, Daya L, Karel FKB, et al. Using the knowledge to action framework to describe a nationwide implementation of the who surgical safety checklist in cameroon. Anesth Analg 2020;130:1425–34. 10.1213/ANE.0000000000004586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu X, Huang Y, Guo Q, et al. Clinical motivation and the surgical safety checklist. Br J Surg 2017;104:472–9. 10.1002/bjs.10446 [DOI] [PubMed] [Google Scholar]
- 58.Yuill G, Amroyan A, Millar S, et al. Establishing obstetric anesthesiology practice guidelines in the republic of armenia: a global health collaboration. Anesthesiology 2017;127:220–6. 10.1097/ALN.0000000000001707 [DOI] [PubMed] [Google Scholar]
- 59.Haynes AB, Weiser TG, Berry WR, et al. Changes in safety attitude and relationship to decreased postoperative morbidity and mortality following implementation of a checklist-based surgical safety intervention. BMJ Qual Saf 2011;20:102–7. 10.1136/bmjqs.2009.040022 [DOI] [PubMed] [Google Scholar]
- 60.Weiser TG, Haynes AB, Dziekan G, et al. Effect of a 19-item surgical safety checklist during urgent operations in a global patient population. Ann Surg 2010;251:976–80. 10.1097/SLA.0b013e3181d970e3 [DOI] [PubMed] [Google Scholar]
- 61.Berwick DM. Disseminating innovations in health care. JAMA 2003;289:1969–75. 10.1001/jama.289.15.1969 [DOI] [PubMed] [Google Scholar]
- 62.Bion J, Richardson A, Hibbert P, et al. 'Matching michigan': a 2-year stepped interventional programme to minimise central venous catheter-blood stream infections in intensive care units in England. BMJ Qual Saf 2013;22:110–23. 10.1136/bmjqs-2012-001325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dixon-Woods M, Leslie M, Tarrant C, et al. Explaining matching michigan: an ethnographic study of a patient safety program. Implement Sci 2013;8:70. 10.1186/1748-5908-8-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.The AHSN Network . Emergency laparotomy collaborative. improving standards of care for patients undergoing emergency laparotomy surgery, 2020. Available: https://www.ahsnnetwork.com/about-academic-health-science-networks/national-programmes-priorities/emergency-laparotomy-collaborative
- 65.Royal College of Pshycicians . Final evaluation report scaling up improvement round 2- scaling up for safety: standardising the lessons learnt from HipQIP, 2019. Available: file:///Users/shaliniahuja/Downloads/HQIP%20Final%20Evaluation%20Report%202_0.pdf
- 66.Zamboni K, Schellenberg J, Hanson C, et al. Assessing scalability of an intervention: why, how and who? Health Policy Plan 2019;34:544–52. 10.1093/heapol/czz068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Theobald S, Brandes N, Gyapong M, et al. Implementation research: new imperatives and opportunities in global health. Lancet 2018;392:2214–28. 10.1016/S0140-6736(18)32205-0 [DOI] [PubMed] [Google Scholar]
- 68.Hull L, Athanasiou T, Russ S. Implementation science: a neglected opportunity to accelerate improvements in the safety and quality of surgical care. Ann Surg 2017;265:1104–12. 10.1097/SLA.0000000000002013 [DOI] [PubMed] [Google Scholar]
- 69.Davis R, D'Lima D. Building capacity in dissemination and implementation science: a systematic review of the academic literature on teaching and training initiatives. Implement Sci 2020;15:97. 10.1186/s13012-020-01051-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2022-010649supp001.pdf (493.1KB, pdf)
Data Availability Statement
Data are available upon reasonable request.