Recent data (10) show that enteric helminthic infections result in the secretion of type 2 cytokines (interleukin-4 [IL-4], IL-5, IL-6, IL-10) and a type 1-to-type 2 shift in cytokine profiles. The predominant production of type 2 cytokines is associated with a reduced incidence of gastritis and gastric atrophy in individuals who are coinfected with helminths and Helicobacter felis. The authors suggest that these results justify the low rate of gastric cancer observed in African individuals (the “African enigma”), a population in which helminthic infections are widespread. We are convinced that the importance of the African enigma goes well beyond the modulation of gastric cancer and that the cytokine profile observed in African individuals can justify the peculiarities that characterize human immunodeficiency virus (HIV) infection in Africa.
To summarize: an abnormal activation of the immune system has repeatedly been postulated to be involved in the pathogenesis of African HIV infection (3); and data stemming from analyses performed over an extended period of time in the Gulu District of northern Uganda, where the prevalence of HIV infection ranges between 14 and 25%, confirm that lymphocytes from African HIV-infected individuals show functional and phenotypic signs of abnormal activation. Thus, the levels of tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), and IL-10 production are increased when the level of cytokine production by antigen-stimulated peripheral blood mononuclear cells (PBMCs) of African HIV-infected individuals is compared to that of PBMCs of HIV-infected European patients; and the percentages of IL-10- and TNFα-producing CD4+ and CD8+ cells in these individuals are increased (7, 17, 18). Additionally, the numbers of CD4+ and HLA class II-expressing lymphocytes and the CD4+CD45RO+CD4+CD45RA ratio are augmented in African subjects compared to those in European subjects. Interestingly, immune activation in the African setting is not limited to HIV-infected individuals, as TNF-α, IFN-γ, and IL-10 production is greatly augmented in individuals not infected with HIV as well (7).
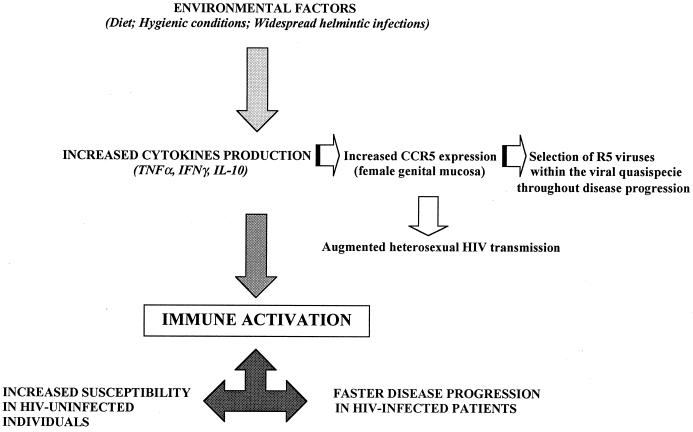
Immune activation in African subjects could result from environmental conditions, including parasitic infections, poor hygienic conditions, and dietary limitations, or could be the consequence of genetic factors (Fig. 1). Recent data have nevertheless convincingly demonstrated that environmental factors are responsible for this phenomenon. Thus, the immune responses of Ethiopians who emigrated to Israel (4) and of Ugandan individuals who had moved to Italy (7) were shown to become superimposable on those of Israeli and Italian subjects. Conversely, immune activation is detected in Italian individuals who have been living in Uganda for extended periods of time (7). Hence, the immune activation observed in the African setting is environmentally driven and is observed even in healthy individuals not infected with HIV. It is noteworthy that similar immune alterations are observed in other developing countries that harbor environmental burdens similar to those present in Africa (e.g., India and Thailand) (unpublished observations).
FIG. 1.
Schematic representation of factors influencing the immunopathogenesis of HIV infection in developing countries.
The immune activation observed in African individuals has been postulated to increase susceptibility to HIV infection in uninfected individuals exposed to the virus and the rate of disease progression in patients (8, 12, 13, 22). How could immune activation and the skewing of the cytokine profiles observed in the African setting influence the progression of HIV infection? Data derived from in vitro and ex vivo experiments show that immune activation augments the in vitro susceptibility of lymphocytes to HIV infection (20) and is associated with increases in the level of virus replication, as was demonstrated in HIV-infected patients receiving vaccines (15) or undergoing acute illness (21). Both direct and indirect cytokine-mediated effects can nevertheless account for this portion of the African enigma. To summarize: (i) TNF-α favors cachexia and has an inductive effect on HIV replication subsequent to the specific activation of the HIV long terminal repeat (9); (ii) IL-10 is known to impair antivirus specific cell-mediated immunity, and the abnormally elevated levels of this cytokine present in African individuals would result in an inefficient immune control over disease progression; and (iii) IFN-γ and IL-10 augment the expression of CCR5 (6), one of the main coreceptors for HIV on the surface of immune cells, thus favoring entry of the virus into the target cell. The impairment of cytokine production seen in African individuals would therefore augment the susceptibility of cells to HIV infection, stimulate viral replication, and alter cell-mediated immunity. Additionally, because IL-10 is produced by TH2 clones and because HIV replicates better in these clones (19), it can be argued that HIV-specific T cells in African patients mainly belong to the cellular subpopulation allowing HIV replication.
The net result of immune activation would be a faster rate of disease progression in HIV-infected patients. More rapid progression to AIDS as well as shortened survival times after AIDS diagnosis are indeed among the main characteristics of HIV infection in African subjects (8, 12, 13, 22). It must nevertheless be underlined that recent data seem to indicate that the pattern of AIDS progression could be similar in developing and developed countries (14); this is not the belief of people operating in the field in Africa.
The concept that immune activation in the African setting is associated with or is responsible for faster disease progression was strenghtened by recent results showing that (i) HIV replication is enhanced in the PBMCs of patients coinfected with HIV and filaria (11) and (ii) eradication of helminthic infestations, as well as treatment of Leishmania infections, results in a down-modulation of immune activation and a decrease in the HIV load (5) in antiviral-naive patients coinfected with HIV and Leishmania. Another corollary which derives from this hypothesis is that immune activation would augment susceptibility to HIV in uninfected individuals exposed to the virus. Hence, PBMCs of African individuals would be more permissive to HIV infection because of IFN-γ and TNF-α-associated immune activation. The production of massive amount of IL-10 in the same individuals would impair antivirus specific cell-mediated immunity, weakening virus-specific immune defenses. Finally, whereas the emergence of X4 strains of HIV is detected in the majority of European and U.S. HIV-infected individuals who develop AIDS (2), recent data indicate that HIV infection in Africa is mostly supported by R5 virus strains throughout the entire disease progression (1, 7). IFN-γ and IL-10, the levels of which are elevated in the African setting, stimulate the expression of CCR5 on the cell surface (6); thus, the peculiar immune activation observed in African individuals would preferentially select R5 HIV strains within the viral quasispecies via the selective induction of CCR5 expression on the surface of cells. Because CCR5-expressing cells, i.e, cells susceptible to the immunological induction of CCR5 upregulation, are concentrated in the female genital tract (16) and HIV infection in Africa is mainly transmitted by heterosexual sexual contact, the immune scenario detected in Africa could also be at least partially responsible for the facilitated transmission of HIV infection by heterosexual sexual contact.
In conclusion, the African enigma can explain the peculiar epidemiological, immunological, and clinical characteristics of African HIV infection. Because of these peculiarities, therapeutic and vaccinal strategies against HIV infection developed in the industrialized world might not be as effective in the African setting. Hence, in African patients highly active antiretroviral therapy might be significantly more effective when it is given in association with antiparasitic drugs, the dose of vaccine that induces the best immune response could be slightly different, and specific immune modulators are likely to be needed. Further extensive analyses of the qualitative peculiarities of the immune response in the developing world will nevertheless be needed to shed more light on the African enigma.
ACKNOWLEDGMENTS
This paper was supported by grants from the Istituto Superiore di Sanitá (II Programma Nazionale di Ricerca sull' AIDS 1998; grants 40B/1.23, 30B.17, and 40B.29) and from Uganda AIDS Project 667.
Footnotes
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
REFERENCES
- 1.Abebe A, Demissie D, Goudsmit J, Brouwer M, Kuiken C L, Pollakis G, Schuitemaker H, Fontanet A L, Rinke de Wit T F. HIV-1 subtpe C syncytium- and non-syncytium-inducing phenotypes and coreceptor usage among Ethiopian patients with AIDS. AIDS. 1999;13:1305–1312. doi: 10.1097/00002030-199907300-00006. [DOI] [PubMed] [Google Scholar]
- 2.Asijo B. Replicative capacity of human immunodeficiency virus from patients with varying severity of infection. Lancet. 1986;ii:660–664. [PubMed] [Google Scholar]
- 3.Bentwich Z, Kalinovich A, Weisman Z. Immune activation is a dominant factor in the pathogenesis of African AIDS. Immunol Today. 1995;16:187–191. doi: 10.1016/0167-5699(95)80119-7. [DOI] [PubMed] [Google Scholar]
- 4.Bentwich Z. Pathogenesis of AIDS in Africa. Lesson from the Ethiopian immigrants in Israel. Immunologist. 1997;5:21–26. [Google Scholar]
- 5.Bentwich Z, Maartens G, Torten D, Lal A A, Lal R B. Concurrent infections and HIV pathogenesis. AIDS. 2000;14:2071–2081. doi: 10.1097/00002030-200009290-00002. [DOI] [PubMed] [Google Scholar]
- 6.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clerici M, Buttó S, Lukwiya M, Declich S, Trabattoni D, Piconi S, Ferrante P, Rizzardini G, Lopalco L. Immune activation in Africa is environmentally-driven and is associated with upregulation of CCR5. AIDS. 2000;14:2083–2092. doi: 10.1097/00002030-200009290-00003. [DOI] [PubMed] [Google Scholar]
- 8.Colebunders R, Ryder R, Francis H, Nekwei W, Bahwe Y, Lebughe I, Ndilu M, Vercauteren G, Nseka K, Perriens J. Seroconversion rate, mortality and clinical manifestations associated with the receipt of an HIV infected blood transfusion in Kinshasa, Zaire. J Infect Dis. 1991;164:450–456. doi: 10.1093/infdis/164.3.450. [DOI] [PubMed] [Google Scholar]
- 9.Folks T M, Clouse K A, Justement J, Rabson A, Duh E, Kehrl J H, Fauci A S. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci USA. 1990;87:2365–2369. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox J G, Beck P, Dangler C A, Whary M T, Wang T C, Shi H N, Nagler-Anderson C. Concurrent enteric helmintic infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat Med. 2000;6:536–542. doi: 10.1038/75015. [DOI] [PubMed] [Google Scholar]
- 11.Gopinath D R. Filarial infection augments susceptibility to HIV infection. J Infect Dis. 2000;182:1804–1808. doi: 10.1086/317623. [DOI] [PubMed] [Google Scholar]
- 12.Grant A D, Djomand G, De Cock K M. Natural history and spectrum of disease in adults with HIV/AIDS in Africa. AIDS. 1997;11:S43–S54. [PubMed] [Google Scholar]
- 13.Morgan D, Malamba S S, Maude G H, Okongo M J, Wagner H U, Mulder D W, Whitworth J A. An HIV-1 natural history cohort and survival times in rural uganda. AIDS. 1991;11:633–640. doi: 10.1097/00002030-199705000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Morgan D, Whitworth J A G. The natural history of HIV-1 infection in Africa. Nat Med. 2001;7:143–145. doi: 10.1038/84564. [DOI] [PubMed] [Google Scholar]
- 15.O'Brien W A, Grovit-Ferbas K, Namazi A, Ovcak-Derzic S, Wang H J, Park J, Yeramian C, Mao S H, Zack J A. Human immunodeficiency virus-type 1 replication can be increased in peripheral blood of seropositive patients after influenza vaccination. Blood. 1995;86:1082–1089. [PubMed] [Google Scholar]
- 16.Patterson B K, Landay A L, Andersson J, Brown C, Behbahani H, Jiyamapa D, Burki Z, Stanislawski D, Czerniewski M A, Garcia P. Repertoire of chemokine receptors in the female genital tract: implications for HIV tranmission. Am J Pathol. 1998;153:481–490. doi: 10.1016/S0002-9440(10)65591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rizzardini G, Piconi S, Ruzzante S, Fusi M L, Lukwiya M, Declich S, Villa M L, Clerici M. Immunologic activation markers in the serum of African and European HIV seropositive and seronegative individuals. AIDS. 1996;10:1535–1542. doi: 10.1097/00002030-199611000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Rizzardini G, Trabattoni D, Saresella M, Piconi S, Lukwiya M, Declich S, Ferrante P, Clerici M. Immune activation in HIV-infected African individuals. AIDS. 1998;12:2387–2396. doi: 10.1097/00002030-199818000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Romagnani S. Role of Th1/Th2 cytokines in HIV infection. Immunol Rev. 1994;140:73–92. doi: 10.1111/j.1600-065x.1994.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg Z F, Fauci A S. Induction of expression of HIV in latently or chronically infected cells. AIDS Res Hum Retrovir. 1989;15:1–8. doi: 10.1089/aid.1989.5.1. [DOI] [PubMed] [Google Scholar]
- 21.Sulkowski M S, Chaisson R E, Karp C L, Moore R D, Margolick J B, Quinn T C. The effect of acute infectious illnesses on plasma HIV-1 viral load and the expression of serologic markers of immune activation among HIV-infected adults. J Infect Dis. 1998;178:1642–1648. doi: 10.1086/314491. [DOI] [PubMed] [Google Scholar]
- 22.Van De Perre P. The epidemiology of HIV infection and AIDS in Africa. Trends Microbiol. 1995;3:217–222. doi: 10.1016/s0966-842x(00)88928-3. [DOI] [PubMed] [Google Scholar]