Abstract
The differential diagnosis of non-coeliac enteropathies (NCEs) is challenging and includes a wide range of aetiologies. Drug-induced NCEs are relatively common and characterized by duodenal villous atrophy, which resolves upon suspension of the offending drug. Immune-checkpoint inhibitors (ICIs), targeting molecules involved in the activation of cytotoxic T cells by targeting, for example, PD-1, PD-L1 and CTLA4, are increasingly used for many types of cancers. Adverse events occurring in the gastrointestinal tract have been described, predominantly in the form of immune-mediated colitis mimicking inflammatory bowel disease. Small bowel involvement whilst on ICI therapy is also possible, though less well described. Herein, we describe two cases of enteropathy with villous atrophy and negative coeliac serology due to ICIs: a 65-year-old man affected by stage IV pulmonary adenocarcinoma under treatment with pembrolizumab and an 18-year-old woman affected by stage IV auricular melanoma who was treated with nivolumab. We also provide a review of the current literature describing small bowel involvement during therapy with ICIs, alone or in combination, for different types of solid tumours. Implications for clinical practice include considering the possibility of small bowel involvement in oncological patients treated with ICIs and the inclusion of ICIs amongst the iatrogenic causes of NCE with villous atrophy. Enteropathies due to ICIs may also represent a pathogenetic model for the understanding of the molecular mechanisms leading to villous atrophy in NCE.
Keywords: immune-checkpoint inhibitors, malabsorption, non-coeliac enteropathy, villous atrophy
Introduction
Differential diagnosis of enteropathies with villous atrophy (VA) unrelated to coeliac disease, defined as non-coeliac enteropathies (NCEs), is challenging. A wide variety of aetiologies, such as immune-mediated, infectious, iatrogenic and lymphoproliferative disorders, should be considered.1,2
Iatrogenic causes are amongst the most common aetiologies for NCE, typically presenting with a severe malabsorption syndrome, a variable degree of duodenal VA and negative coeliac serology.3–7 Iatrogenic causes include angiotensin receptor blockers, most commonly olmesartan, chemotherapy, radiotherapy, methotrexate, azathioprine, graft-versus-host disease and immunomodulators such as mycophenolate mofetil.1–8
Immune-checkpoint inhibitors (ICIs) are a group of monoclonal antibodies targeting specific molecules involved in the downregulation of cytotoxic T cells such as CTLA4, PD-1 and PD-L1. ICIs promote cytotoxic T cell survival and antitumour action and have therefore become part of the standard of care for a wide range of cancers, revolutionizing outcomes. However, immune-related adverse events are common side effects of these therapies and can affect different organs, including the gastrointestinal tract. Gastrointestinal toxicity manifests predominantly as an immune-mediated colitis mimicking inflammatory bowel disease and is well recognized.9–12 On the contrary, adverse events involving the small bowel are less well studied and may be overlooked in clinical practice.13,14
Herein, we describe two cases of enteropathy with VA due to ICIs, which may broaden the differential diagnosis of NCEs and may represent a pathogenetic model for the understanding of the molecular mechanisms involved in these enteropathies. We also provide a narrative review summarizing the current evidence on the adverse events of ICIs involving the small bowel.
Methods
Case report description
The description of case reports was made in accordance with the CARE guidelines.
Literature search
English language articles were searched for in MEDLINE using the following strings: “immune checkpoint inhibitors villous atrophy”, “coeliac disease checkpoint inhibitor”, “ileitis checkpoint inhibitor” and “enteritis checkpoint inhibitor”. Articles describing patients with reported small bowel involvement due to treatment with ICIs for solid tumours were included and reviewed.
Ethics
Both patients described in this paper gave written informed consent to publish their data in anonymous form.
Case 1
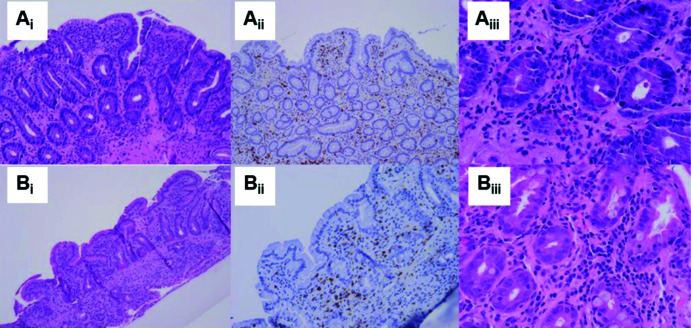
In October 2020, a 65-year-old man treated for stage IV pulmonary adenocarcinoma with pembrolizumab (KEYTRUDA) 200 mg every 3 weeks since May 2019 was admitted to hospital in Pavia, Italy, due to a severe malabsorption syndrome. This was characterized by chronic diarrhoea, weight loss of 10 kg and marked hypoalbuminaemia (1.5 g/dL), which had started 3 months previously and had gradually worsened. The patient had developed severe hypothyroidism in August 2019 as a side effect of pembrolizumab therapy and required high-dose levothyroxine replacement therapy (175 μg a day). He had also received radiotherapy (5 cycles with a total dose of 25 Gy) for bone lesions in the L3 vertebral body and left ilium in April 2019. A gastroscopy revealed markedly atrophic duodenal mucosa. Duodenal biopsies confirmed severe VA, crypt hyperplasia and normal count of intraepithelial lymphocytes (IELs) but increased eosinophilic count (Figure 1Ai–iii). The normal CD3+CD8+ phenotype of IELs was confirmed on flow cytometry. Periodic acid-Schiff (PAS) staining was negative, thus excluding Whipple’s disease. IgA tissue transglutaminase and endomysial antibodies were repeatedly negative whilst on a gluten-containing diet. HLA typing revealed DQ2.5/DQ5. Investigations for NCEs, including serum immunoglobulin profile, antienterocyte antibodies, stool parasites, HIV testing and Quantiferon, were all negative. Faecal calprotectin was markedly increased (>3000 μg/kg). Colonoscopy revealed only three small (<1 cm) polyps but no other macroscopic lesions. Colonic biopsies were unremarkable. Ileal biopsies showed normal mucosa. A capsule endoscopy was also scheduled but was cancelled after a failed patency capsule. The patient was started on budesonide 9 mg/day according to the Mayo Clinic open-capsule scheme15 with immediate and complete resolution of diarrhoea, hypoalbuminaemia and weight loss. However, duodenal biopsy repeated after 4 months showed persistence of VA despite a marked reduction of faecal calprotectin and clinical remission. Because of progression of the oncologic disease (CT performed in April 2021), pembrolizumab was stopped and the patient was started on chemotherapy (pemetrexed/cisplatin). Another gastroscopy with duodenal biopsies performed in June 2021, 1 month after pembrolizumab withdrawal, showed initial histological architectural improvement and reduction of eosinophilic count in the epithelium (Figure 1Bi–iii). Despite the complete resolution of intestinal symptoms, the patient passed away in July 2022 because of progression of the oncological disease.
Figure 1. Duodenal histopathological features of case 1 at diagnosis and during follow-up.
(Ai) Duodenal biopsy at diagnosis showing severe villous atrophy, with normal intraepithelial lymphocyte count, as highlighted by CD3 staining (Aii) and increased eosinophilic count (Aiii). (Bi–iii) Duodenal biopsy performed 1 month after the suspension of pembrolizumab showing improvement of duodenal architecture with low-grade villous atrophy and resolution of eosinophilic mucosal infiltration.
Case 2
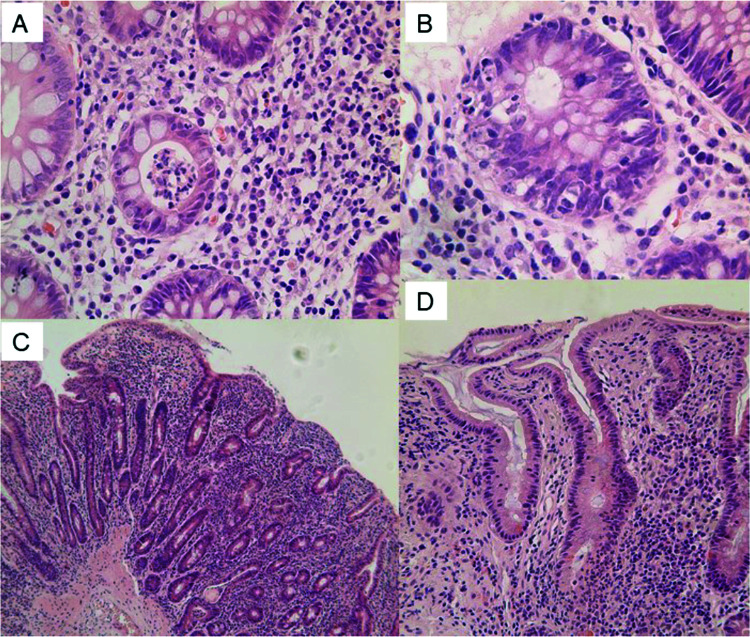
An 18-year-old woman who was diagnosed with auricular melanoma in 2002 developed multifocal skeletal, small bowel and right adrenal metastases in May 2017. She was therefore started on ipilimumab 270 mg and nivolumab 90 mg for 3 cycles in June 2017. She then developed severe chronic diarrhoea. Colonoscopy revealed patchy macroscopic inflammation in the caecum, sigmoid colon and rectum. Colonic biopsies showed crypt abscesses and neutrophilic inflammation (Figure 2A,B). Ileal biopsies showed VA and chronic inflammation. Gastroscopy demonstrated hypotrophic duodenal mucosa macroscopically. Duodenal biopsies revealed severe VA with crypt hyperplasia, crypt abscesses (with presence of a few apoptotic bodies), and marked lymphoplasmacellular and neutrophilic infiltration of the lamina propria (Figure 2C,D). No viral inclusions, Giardia, other parasites or granuloma were found. HLA typing was DQ2 positive. The patient was started on systemic corticosteroids (IV methylprednisolone, IV hydrocortisone and then prednisone) with resolution of diarrhoea. Upon tapering of prednisone, symptoms recurred, prompting treatment with infliximab 5 mg/kg (2 doses, 2 weeks apart), with no further gastrointestinal symptoms. The patient then completed 2 years of nivolumab monotherapy with no further gastrointestinal events and excellent oncological response (with the exception of two bone abnormalities not interpreted as disease recurrence). Given the persistent complete clinical remission, the patient refused to undergo follow-up gastroscopy and colonoscopy. Currently, the patient is alive and well (August 2021).
Figure 2. Colonic (A, B) and duodenal (C, D) histopathological findings in patient 2.
(A) H&E ×200. Colonic biopsy showing acute colitis with crypt abscess and mild chronic inflammation. (B) H&E ×400. Colonic biopsy showing prominent crypt cell apoptosis resembling acute graft versus host disease. (C) H&E ×100. Duodenal biopsy showing total villus atrophy with acute and chronic inflammation. (D) H&E ×200. Duodenal biopsy showing flat mucosa with cryptitis and mild chronic inflammation. Note absence of intraepithelial lymphocytes in the surface epithelium.
Review
Our literature search identified 19 case reports of patients with small bowel involvement. In 10 reports (Table 1), VA was present and developed during therapy with PD/PD-L1 inhibitors for solid tumours.16–25 In the remaining 9 (Table 2), patients developed small bowel involvement other than VA.26–34 Finally, we identified 2 single-centre retrospective case series reporting cases of small bowel involvement. In the first, 2 cases of enteropathy with VA are described and, in the second, 8 cases of coeliac disease associated with ICIs and a further 9 cases of VA related to ICIs with negative tissue transglutaminase antibodies are reported.35,36 In the latter case series reporting 8 cases of coeliac disease related to ICIs, all had positive tissue transglutaminase antibodies (mean 121 IU/mL) but duodenal biopsy was performed in only 6 of these patients, of which 5 had moderate-to-severe VA.36 However, in these 2 case series, data for individual patients were not available and so we could only consider these patients as a group.
Table 1.
Case reports of patients developing enteropathy with villous atrophy whilst on immune-checkpoint inhibitors for solid tumours.
| Paper | Age/sex | Tumour | Oncological treatment | Symptoms | Coeliac serology | Upper GI endoscopic findings | Duodenal histology | Lower GI endoscopic findings | Lower GI histological findings | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| Alsaadi et al.16 | 74 F |
Renal cell carcinoma | Nivolumab/ipilimumab | Diarrhoea; weight loss; nausea; vomiting | Borderline TTA (12 U/mL) | Erythematous duodenopathy | Increased IELs; villous atrophy | Not performed | Not performed | Budesonide; gluten-free diet; discontinued ICIs; supportive therapy |
| Arnouk et al.17 | 79 M |
Melanoma | Pembrolizumab | Diarrhoea | Positive TTA (59U/mL) | Erosive gastropathy; normal duodenum | Increased IELs; villous atrophy | Normal | Normal | Hydrocortisone; gluten-free diet; discontinued ICIs |
| Leblanc et al.18 | 70 M |
Pleural mesothelioma | Nivolumab | Diarrhoea; nausea; vomiting | Positive TTA (128 U/mL); positive EmA | Diffuse duodenal ulcers | Increased IELs; crypt hyperplasia; villous atrophy | Normal | Normal | Proton-pump inhibitors; systemic steroids; gluten-free diet; discontinued ICIs |
| Sethi et al.19 | 63 F |
Adenocarcinoma of unknown origin (probably breast) | Carboplatin, paclitaxel and pembrolizumab | Diarrhoea; abdominal pain; weight loss | Negative TTA and EmA | Not reported | Crypt hyperplasia; villous atrophy; IELs not reported | Normal | Normal | Systemic steroids; gluten-free diet |
| Duval et al.20 | 58 M |
Renal cell carcinoma | Nivolumab | Diarrhoea; vomiting; weight loss | Negative TTA | Normal | Increased IELs; villous atrophy | Normal | Normal | Systemic steroids; antibiotics; gluten-free diet |
| Kokorian et al.21 | 65 M |
NSCLC | Nivolumab | Diarrhoea; weight loss | Negative TTA and EmA | Not reported | Increased IELs; villous atrophy | Normal | Normal | Systemic steroids; gluten-free diet; discontinued ICIs |
| Schoenfeld et al.22 | 72 F |
Pulmonary adenocarcinoma | Pembrolizumab | Diarrhoea | Positive TTA (37 U/mL) | Normal | Increased IELs; villous architecture not reporteda | Normal | Normal | Gluten-free diet |
| Hussain et al.23 | 64 F |
Pulmonary adenocarcinoma | Pembrolizumab | Diarrhoea; bloating peripheral oedema | Negative TTA and EmA | Diffuse duodenal ulcers | Normal IELs count; villous atrophy | Normal | Not reported | Systemic steroids; budesonide; discontinued ICIs |
| Messmer et al.24 | 83 M |
Melanoma | Pembrolizumab then ipilimumab | Diarrhoea; abdominal pain | Not performed | Duodenal ulcer | Acute inflammation; apoptotic bodies; villous atrophy | Normal | Inflammatory changes | Systemic steroids; antibiotics; infliximab |
| Facchinetti et al.25 | 42 F |
Lung adenocarcinoma | Nivolumab | Diarrhoea; nausea; abdominal pain | Negative TTA, EmA, AEA | Reduced duodenal plicae | Increased IELs; villous atrophy | Diffuse jejunal and ileal erosions; normal colon | Collagenous colitis | Systemic steroids; antibiotics; total parenteral nutrition |
Data on villous atrophy not reported for this patient, but histological findings reported in the paper as diagnostic of coeliac disease.
AEA, antienterocyte antibodies; EmA: IgA endomysial antibodies; F, female; GI, gastrointestinal; IELs, intraepithelial lymphocytes; M, male; NSCLC, non-small-cell lung cancer; TTA, IgA tissue transglutaminase antibodies.
Table 2.
Case reports of patients developing enteritis without villous atrophy whilst on immune-checkpoint inhibitors for solid tumours.
| Paper | Type of GI toxicity | Age/sex | Tumour | Oncological treatment | Symptoms | Upper GI endoscopic findings | Upper GI histology | Lower GI endoscopic findings | Lower GI histology | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| Mohamed et al.26 | Ileal perforation | 52 F |
Vulval melanoma | Ipilimumab/nivolumab | Abdominal pain; nausea; weight loss | Not performed | Not performed | Ileal perforation on laparotomy | Transmural ischaemic necrosis | Systemic steroids; infliximab; resection of terminal ileum |
| Smith et al.27 | Ileitis | 44 F |
Melanoma | Ipilimumab/nivolumab | Diarrhoea | Not performed | Not performed | Microerosions of terminal ileum; normal colon | Ileum; increased eosinophils; villous congestion | Systemic steroids |
| Sokal et al.28 | Diffuse enteritisa | 48 F |
Melanoma | Ipilimumab/nivolumab | Diarrhoea; fever; vomiting; weight loss | Normal | Non-specific acute duodenitis | Erythematous right colon; normal terminal ileum | Non-specific oedema on colonic and ileal biopsies | Systemic steroids |
| Sanders et al.29 | Gastritis and terminal ileitis | 43 M |
Melanoma | Ipilimumab/nivolumab | Diarrhoea; nausea; vomiting; weight loss | Gastric erythema and spontaneous oozing of blood | Gastritis; duodenal inflammation | Ileal aphthous ulcers; normal colon mucosa | Ileal lamina propria lymphoplasmacytosis with eosinophilic infiltration | Systemic steroids; infliximab |
| Yang et al.30 | Eosinophilic enteritis | 68 M |
Melanoma | Pembrolizumab, talimogene, laherparepvec, ipilimumab/nivolumab | Diarrhoea; abdominal pain; nausea; skin rash; cough | Normal | Marked eosinophilia (80–100/HPF) on duodenal biopsies | Not performed | Not performed | Systemic steroids |
| Young et al.31 | Enteritis and small bowel bleeding | 71 M |
Colon adenocarcinoma | Atezolizumab | Diarrhoea; abdominal pain; massive GI bleeding | Multiple duodenal and jejunal ulcers on enteroscopy | Non-specific abnormalities | Normal | Normal | Systemic steroids; infliximab; jejunal resection |
| Otagiri et al.32 | Enteritis | 68 M |
Pleural mesothelioma | Nivolumab | Diarrhoea; abdominal pain; melena; fever | Gastric and small bowel aphthous ulcers on OGD and CE | Not reported | Diffuse aphthous ulcers on colonoscopy | Not reported | Systemic steroids |
| Saito et al.33 | Acute duodenal haemorrhage | 66 M |
Small-cell lung cancer | Carboplatin + etoposide + atezolizumab | Haematemesis; diarrhoea | Large duodenal ulcers with pulsatile bleeding | Duodenal lymphocyte, eosinophil and plasma cell infiltrate | Normal | Not reported | Endoscopic haemostasis; supportive therapy |
| Trystram et al.34 | Diffuse ulcerative haemorrhagic enteritis | 62 M |
Melanoma | Ipilimumab/nivolumab | Fever; melena; haemorrhagic shock | Normal | Not reported | Multiple deep bleeding jejunal and ileal ulcers; ulcerated Meckel diverticulum | Non-specific ileitis | Systemic steroids; antibiotics; Meckel diverticulum resection; infliximab |
Abdominal CT scan revealed diffuse small bowel wall thickening with contrast enhancement.
CE, capsule endoscopy; F, female; GI, gastrointestinal; HPF, high-power field; M, male; OGD, oesophagogastroduodenoscopy.
Patients who developed VA whilst on ICIs
All patients who developed VA whilst on ICIs presented with diarrhoea. Weight loss was also frequently reported. It is noteworthy that three of these patients also had positive coeliac antibodies (specified in Table 1). It is also remarkable that two patients presented with diffuse ulceration of the duodenum as well as VA. Almost all patients had an increase in duodenal IELs count. Nine out of ten patients underwent colonoscopy, which did not reveal any visible lesions, though collagenous colitis was detected in one patient on colon biopsies. Systemic corticosteroids were the mainstay of therapy in these patients. Although only three patients had positive coeliac antibodies and a fourth one had borderline results, a gluten-free diet was initiated in 7 out of 10 patients. Treatment with ICIs was suspended in half of patients. Rescue therapy with infliximab was required in one patient. Only one patient amongst those who developed VA was on combination therapy with ipilimumab/nivolumab.
Patients who developed small bowel involvement other than VA whilst on ICIs
Combination therapy with ipilimumab/nivolumab was much more common in patients who developed small bowel involvement other than VA whilst on ICIs than in those presenting with VA (6/9 versus 1/10). In these patients, diarrhoea was still the most common symptom, though the clinical picture was generally more severe, in some cases presenting with life-threatening gastrointestinal bleeding or small bowel perforation. Small bowel ulcers were reported in all nine cases, with severity ranging from small aphthous ulcers to diffuse small bowel ulcers with small bowel perforation or massive gastrointestinal bleeding. Biopsy results excluding VA were reported for five of these patients, whilst this was not reported for the remaining four patients. Three of these patients required surgery and one patient required repeated endoscopic haemostasis. Eight patients were treated with systemic steroids and four required rescue therapy with infliximab (Table 2).
Discussion
Therapy with ICIs can lead to severe gastrointestinal side effects, which are mainly due to severe and potentially life-threatening forms of colitis mimicking inflammatory bowel disease.9–12 Although the term enterocolitis is usually adopted to indicate the major gastrointestinal side effects of ICIs,9–12 small bowel involvement can also occur.16–36
Herein, we have described two patients with small bowel VA due to ICIs and negative coeliac serology. Very interestingly, at diagnosis, both patients showed a normal intraepithelial lymphocyte count on duodenal biopsies and, in case 1, eosinophils in the duodenal mucosa were instead increased. In both patients, VA and clinical symptoms improved whilst on immunosuppressants and definitively resolved after suspending the therapy. As occurs with colonic involvement, we confirm that the use of steroids and infliximab can be useful to treat ICI side effects related to small bowel involvement. In particular, budesonide administered according to the Mayo Clinic open-capsule scheme appears to be an effective treatment as observed in patients with extensive small bowel involvement due to refractory coeliac disease.15 The fact that the patient in case 2 did not undergo a follow-up duodenal biopsy to ascertain histological recovery is a significant limitation. Nevertheless, given the complete well-being of the patient, it seems reasonable to assume that histological recovery also occurred.
Our review of the literature has identified several points worth noting. Firstly, we identified two main clinical phenotypes of small bowel involvement related to ICIs. The first is characterized by an enteropathy with VA, either related or unrelated to coeliac disease, which generally occurred in patients on ICI monotherapy. The second group of patients was instead characterized by generally severe ulcerative enteritis, sometimes with massive gastrointestinal bleeding or small bowel perforation, but with no mention of VA. This latter group was most frequently on combination therapy with ipilimumab/nivolumab and often required either rescue therapy with infliximab or surgery. No patients were reported to have died directly as a result of gastrointestinal toxicity of ICIs though, in one patient who died 2 months from onset, it may have contributed to a poor outcome. Finally, it appears that concomitant small bowel and colonic involvement is rare, though it should be kept in mind as a possibility, as suggested by the observations of case 2.
Patients with VA were frequently started on a gluten-free diet. However, in many of these patients, coeliac serology was negative and, in others, only low levels of tissue transglutaminase antibodies were detected with no confirmatory endomysial antibody testing performed. Regrettably, in most of these patients, HLA typing, which is very useful to exclude coeliac disease,1–3 was not available. Furthermore, even in the cases where the diagnosis of coeliac disease was certain beyond a doubt, it is unclear whether the disease was induced by ICIs, as it has been previously reported for other immune-related disorders,37 or if an underlying subclinical enteropathy was been exacerbated by ICIs, leading to overt clinical manifestations. This is an area warranting further investigation.
We believe that these results can have important implications for patients on ICIs. Clinicians should maintain a high index of suspicion for ICI toxicity should these patients develop chronic diarrhoea. The possibility of colonic or small bowel toxicity must be considered after the exclusion of major infectious causes for diarrhoea (Clostridium difficile, Salmonella, and other bacterial, parasitic or viral infections) and cancer progression. Moreover, we have shown here that small bowel involvement frequently occurs in the absence of a concomitant colonic involvement. Therefore, the possibility of a toxicity involving the small bowel must be considered, regardless of whether colonic involvement is present or not. Although the prevalence of immune-related toxicity on the small bowel in patients under treatment with ICIs is difficult to ascertain, timely recognition and treatment of a severe and potentially life-threatening malabsorption syndrome is nonetheless crucial for these patients with cancer. Therefore, we suggest that ICIs should be definitely included amongst the iatrogenic causes of NCEs.
Although NCEs are usually burdened by poor prognosis, long-term outcomes of enteropathies due to identifiable and potentially reversible causes, including iatrogenic causes, are usually good in our clinical experience.3,4 Our review of the literature also supports this hypothesis as patients generally recovered from gastrointestinal toxicity due to ICIs with appropriate treatment.
Finally, we believe that these reports may help shed some light on the underlying molecular mechanisms leading to enteropathies characterized by VA and negative coeliac serology. Future perspectives also include a comprehensive study of the epidemiology of small bowel toxicity due to ICIs and the identification of patients at higher risk of developing these side effects. Therefore, correct identification and targeted management of these enteropathies may help improve the general conditions of these patients with cancer.
Acknowledgements
None.
Footnotes
Contributions: AS, AP, SM, MR, MM, LV, NS, FS, FB and CD delivered clinical care to the patients. AS, AP, SM and FS retrieved the articles for the review. AS drafted the manuscript together with AP and SM. DSS, FB and CD revised the manuscript for important intellectual content. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest relevant to this manuscript. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2022/09/dic.2022-6-3-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2022 Schiepatti A, Premoli A, Maimaris S, Rizzo M, Marples M, Villani L, Scott N, Sottotetti F, Sanders DS, Biagi F, Donnellan C. https://doi.org/10.7573/dic.2022-6-3. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: Invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: 6 Green Lane Business Park, 238 Green Lane, New Eltham, London, SE9 3TL, UK.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Schiepatti A, Sanders DS, Zuffada M, et al. Overview in the clinical management of patients with seronegative villous atrophy. Eur J Gastroenterol Hepatol. 2019;3:1409–1417. doi: 10.1097/MEG.0000000000001340. [DOI] [PubMed] [Google Scholar]
- 2.Leonard MM, Lebwohl B, Rubio-Tapia A, et al. AGA clinical practice update on the evaluation and management of seronegative enteropathies: expert review. Gastroenterology. 2021;160:437–444. doi: 10.1053/j.gastro.2020.08.061. [DOI] [PubMed] [Google Scholar]
- 3.Schiepatti A, Biagi F, Fraternale G, et al. Short article: mortality and differential diagnoses of villous atrophy without coeliac antibodies. Eur J Gastroenterol Hepatol. 2017;29:572–576. doi: 10.1097/MEG.0000000000000836. [DOI] [PubMed] [Google Scholar]
- 4.Aziz I, Peerally MF, Barnes JH, et al. The clinical and phenotypical assessment of seronegative villous atrophy; a prospective UK centre experience evaluating 200 adult cases over a 15-year period (2000–2015) Gut. 2017;66:1563–1572. doi: 10.1136/gutjnl-2016-312271. [DOI] [PubMed] [Google Scholar]
- 5.DeGaetani M, Tennyson CA, Lebwohl B, et al. Villous atrophy and negative coeliac serology: a diagnostic and therapeutic dilemma. Am J Gastroenterol. 2013;108:647–653. doi: 10.1038/ajg.2013.45. [DOI] [PubMed] [Google Scholar]
- 6.Fernández-Bañares F, Crespo L, Núñez C, et al. Gamma delta+ intraepithelial lymphocytes and coeliac lymphogram in a diagnostic approach to coeliac disease in patients with seronegative villous atrophy. Aliment Pharmacol Ther. 2020;51:699–705. doi: 10.1111/apt.15663. [DOI] [PubMed] [Google Scholar]
- 7.Volta U, Caio G, Boschetti E, et al. Seronegative coeliac disease: shedding light on an obscure clinical entity. Dig Liver Dis. 2016;48:1018–1022. doi: 10.1016/j.dld.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 8.Rubio-Tapia A, Herman ML, Ludvigsson JF, et al. Severe spruelike enteropathy associated with olmesartan. Mayo Clin Proc. 2012;87:732–738. doi: 10.1016/j.mayocp.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soularue E, Lepage P, Colombel JF, et al. Enterocolitis due to immune checkpoint inhibitors: a systematic review. Gut. 2018;67:2056–2067. doi: 10.1136/gutjnl-2018-316948. [DOI] [PubMed] [Google Scholar]
- 10.Powell N, Ibraheim H, Raine T, et al. British Society of Gastroenterology endorsed guidance for the management of immune checkpoint inhibitor-induced enterocolitis. Lancet Gastroenterol Hepatol. 2020;5:679–697. doi: 10.1016/S2468-1253(20)30014-5. [DOI] [PubMed] [Google Scholar]
- 11.Bellaguarda E, Hanauer S. Checkpoint inhibitor-induced colitis. Am J Gastroenterol. 2020;115:202–210. doi: 10.14309/ajg.0000000000000497. [DOI] [PubMed] [Google Scholar]
- 12.Collins M, Michot JM, Danlos FX, et al. Inflammatory gastrointestinal diseases associated with PD-1 blockade antibodies. Ann Oncol. 2017;28:2860–2865. doi: 10.1093/annonc/mdx403. [DOI] [PubMed] [Google Scholar]
- 13.Alsaadi D, Shah NJ, Charabaty A, et al. A case of checkpoint inhibitor-induced coeliac disease. J Immunother Cancer. 2019;7:203. doi: 10.1186/s40425-019-0694-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnouk J, Mathew D, Nulton E, et al. A coeliac disease phenotype after checkpoint inhibitor exposure: an example of immune dysregulation after immunotherapy. ACG Case Rep J. 2019;6:e00158. doi: 10.14309/crj.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukewar SS, Sharma A, Rubio-Tapia A, et al. Open-capsule budesonide for refractory coeliac disease. Am J Gastroenterol. 2017;112:959–967. doi: 10.1038/ajg.2017.71. [DOI] [PubMed] [Google Scholar]
- 16.Alsaadi D, Shah NJ, Charabaty A, et al. A case of checkpoint inhibitor-induced coeliac disease. J Immunother Cancer. 2019;7:203. doi: 10.1186/s40425-019-0694-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnouk J, Mathew D, Nulton E, et al. A coeliac disease phenotype after checkpoint inhibitor exposure: an example of immune dysregulation after immunotherapy. ACG Case Rep J. 2019;6:e00158. doi: 10.14309/crj.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leblanc J, Hoibian S, Boucraut A, et al. Coeliac disease after administration of immune checkpoint inhibitors: a case report. Front Immunol. 2021;12:799666. doi: 10.3389/fimmu.2021.799666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sethi A, Helfand A, Balikani L, et al. Association of coeliac disease with pembrolizumab. Cureus. 2021;13:e15565. doi: 10.7759/cureus.15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duval L, Habes S, Chatellier T, et al. Nivolumab-induced coeliac-like enteropathy in patient with metastatic renal cell carcinoma: case report and review of the literature. Clin Case Rep. 2019;7:1689–1693. doi: 10.1002/ccr3.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kokorian R, Grainville T, Robert L, et al. Coeliac-Like disease is a rare immune-related complication induced by nivolumab in NSCLC. J Thorac Oncol. 2020;15:e147–e148. doi: 10.1016/j.jtho.2019.12.119. [DOI] [PubMed] [Google Scholar]
- 22.Schoenfeld SR, Aronow ME, Leaf RK, et al. Diagnosis and management of rare immune-related adverse events. Oncologist. 2020;25:6–14. doi: 10.1634/theoncologist.2019-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hussain N, Robert M, Al Bawardy, et al. Open capsule budesonide for the treatment of isolated immune checkpoint inhibitor-induced enteritis. Am J Gastroenterol. 2021;116:S1261. doi: 10.14309/01.ajg.0000785744.02669.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Messmer M, Upreti S, Tarabishy Y, et al. Ipilimumab-Induced enteritis without colitis: a new challenge. Case Rep Oncol. 2016;9:705–713. doi: 10.1159/000452403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Facchinetti F, Gnetti L, Caruana P, et al. Widespread nivolumab-induced enteropathy in a long responder non-small-cell lung cancer patient. Clin Lung Cancer. 2018;19:e591–e596. doi: 10.1016/j.cllc.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Mohamed AA, Richards CJ, Boyle K, et al. Severe inflammatory ileitis resulting in ileal perforation in association with combination immune checkpoint blockade for metastatic malignant melanoma. BMJ Case Rep. 2018 doi: 10.1136/bcr-2018-224913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith SCL, Zardo D, Cannatelli R, et al. Endoscopic findings of checkpoint inhibitor-induced ileitis with use of the latest advanced endoscopic optical diagnosis: near-focus narrow-band imaging. VideoGIE. 2019;4:133–135. doi: 10.1016/j.vgie.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sokal A, de Chou CS, Delyon J, et al. Enteritis without colitis in patients treated with immune checkpoint inhibitors: a tricky diagnosis. Melanoma Res. 2018;28:483–484. doi: 10.1097/CMR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 29.Sanders D, Webber D, Chatur N. Enteritis with immune checkpoint inhibitor use. CMAJ. 2019;191:E1106. doi: 10.1503/cmaj.190244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, Lagana SM, Saenger YM, et al. Dual checkpoint inhibitor-associated eosinophilic enteritis. J Immunother Cancer. 2019;7:310. doi: 10.1186/s40425-019-0743-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young K, Lin E, Chen E, et al. Small bowel hemorrhage from check point inhibitor enteritis: a case report. BMC Gastroenterol. 2021;21:345. doi: 10.1186/s12876-021-01915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otagiri S, Katsurada T, Yamanashi K, et al. Immune checkpoint inhibitor-induced enteritis assessed using capsule endoscopy. JGH Open. 2020;4:1231–1232. doi: 10.1002/jgh3.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saito K, Nagumo H, Iwasaki M, et al. A case of severe acute hemorrhagic duodenitis after administration of immune checkpoint inhibitor. DEN Open. 2022;2:e19. doi: 10.1002/deo2.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trystram N, Laly P, Bertheau P, et al. Haemorrhagic shock secondary to a diffuse ulcerative enteritis after ipilimumab and nivolumab treatment for metastatic melanoma: a case report. Ann Palliat Med. 2022;11:837–842. doi: 10.21037/apm-21-58. [DOI] [PubMed] [Google Scholar]
- 35.Hayashi Y, Hosoe N, Takabayashi K, et al. Clinical, endoscopic, and pathological characteristics of immune checkpoint inhibitor-induced gastroenterocolitis. Dig Dis Sci. 2021;66:2129–2134. doi: 10.1007/s10620-020-06441-w. [DOI] [PubMed] [Google Scholar]
- 36.Badran YR, Shih A, Leet D, et al. Immune checkpoint inhibitor-associated coeliac disease. J Immunother Cancer. 2020;8:e000958. doi: 10.1136/jitc-2020-000958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marin-Acevedo JA, Chirila RM, Dronca RS. Immune checkpoint inhibitor toxicities. Mayo Clin Proc. 2019;94:1321–1329. doi: 10.1016/j.mayocp.2019.03.012. [DOI] [PubMed] [Google Scholar]