Abstract
Background:
Polychlorinated biphenyls (PCBs) are biopersistent chemicals classified as human carcinogens. This classification is primarily based on evidence on higher-chlorinated PCBs found in food. The carcinogenic potential of airborne lower-chlorinated PCBs remains largely unexplored.
Objectives:
We aimed to investigate cancer risk following residential exposure to airborne PCBs.
Methods:
Cancer risk was examined in the Health Effects of PCBs in Indoor Air (HESPAIR) cohort of 38,613 residents of two partly PCB-contaminated residential areas in Greater Copenhagen, identified by nationwide registries. PCB exposure was based on relocation dates and indoor air PCB measurements in subsets of apartments. Cancer diagnoses were extracted from the Danish Cancer Registry for the follow-up period of 1970–2018. We estimated adjusted hazard ratios with time-varying cumulative exposure and a 10-y lag using Cox regression.
Results:
Overall risk of cancer was not associated with , [hazard ratio (HR) for high-exposed vs. low-exposed ; 95% confidence interval (CI): 0.88, 1.09], but residents exposed to had higher risk of liver cancer (HR ; 95% CI: 1.28, 6.15) and meningiomas (HR ; 95% CI: 1.84, 6.64), with indications of exposure–response relationships. Results were suggestive of a higher risk of pancreatic cancer (HR ; 95% CI: 0.95, 2.64) at the highest aggregated PCB level. For testis cancer, a higher risk was observed among residents exposed to relative to residents exposed to (HR ; 95% CI: 1.41, 6.28), but the risk was not higher for residents exposed to . Apart from this, the risk of specific cancers was similar across exposure groups.
Discussion:
In this, to our knowledge, first population-based cohort study of residential exposure to airborne PCBs, we found no association between exposure to PCBs in indoor air in private homes and the risk for most of the specific cancers. Higher risk of liver cancer and meningiomas were observed. https://doi.org/10.1289/EHP10605
Introduction
Polychlorinated biphenyls (PCBs) constitute a group of manmade, persistent chemicals widely used in building materials during the 1950s–1970s. Food has been considered the main PCB source, but exposure to PCBs evaporating from building materials to indoor air was recently found to contribute substantially to human exposure.1–5 Highly elevated blood concentrations among people working and living in contaminated buildings have been reported.6–8
PCBs are classified as “Carcinogenic to Humans” by the International Agency for Research on Cancer (IARC), based on sufficient evidence for malignant melanoma and limited evidence for non-Hodgkin lymphoma (NHL) and breast cancer.9 This classification is mainly based on studies of higher-chlorinated PCBs (HC-PCBs) typically found in food, rather than the lower-chlorinated PCBs (LC-PCBs) dominating indoor air in contaminated buildings.9 Studies of HC-PCBs may not, however, be directly used for risk assessment of LC-PCBs, due to potential differences in mechanism of action.9–12 Carcinogenesis of HC-PCBs is mostly driven by dioxin-like PCBs through aryl hydrocarbon receptor (AhR) activation, whereas the mostly nondioxin-like LC-PCBs act through AhR-independent pathways, probably through metabolic activation and metabolites.9,11–13
We know of no previous human studies that have investigated cancer risk following exposure to LC-PCBs in indoor air specifically. Occupational studies of high PCB exposure mainly through inhalation do not indicate higher risk of malignant melanoma, NHL, and breast cancer14–17 but find indications of higher mortality from liver, gall bladder, and biliary tract cancers, although based on small numbers.18–20 Animal studies support a carcinogenic effect of LC-PCBs in the liver,11,12 suggesting that exposure to airborne PCBs (predominantly LC-PCBs) may be associated with cancer in other organs than foodborne PCBs (primarily HC-PCBs).
We aimed to determine cancer risk following airborne PCB exposure in a cohort of residents of two partly PCB-contaminated residential areas. This natural experimental design enables comparisons of high- and low-level exposed populations with balanced background exposure and sociodemographic characteristics. Cancers previously associated with PCB exposure were of a priori interest, namely malignant melanoma, NHL, breast, liver, gall bladder, and biliary tract cancer.
Methods
Setting
The study uses the Health Effects of PCBs in indoor Air (HESPAIR) cohort of residents of two residential areas in Greater Copenhagen in Denmark, Farum Midtpunkt (Farum), and Brøndby Strand Parkerne (Brøndby), erected 1969–1974. PCB-containing building materials were used during the first stages of construction but were replaced with PCB-free materials in later stages. Farum comprises 27 buildings with 1,645 apartments, of which 297 were PCB-contaminated.2 After high levels of PCB concentrations were documented in indoor air in contaminated apartments in 2011, the contaminated apartments were remediated in 2012–2015, which reduced air PCB levels to below the Danish action limit of .21,22 Brøndby comprises 12 identical 15-storey high-rise buildings with 885 apartments surrounded by lower apartment buildings and family houses. Five of the high-rises were constructed with PCB-containing sealants. In one of the five contaminated buildings, the use of PCB-containing building materials was terminated when the 10th floor was reached, and the 11th–15th floors were therefore built with PCB-free materials.1,7
In previous studies, sample measurements from both residential areas documented markedly increased PCB concentrations in the indoor air of contaminated apartments,1,2 dominated by LC-PCBs, especially PCB-18, 28, and 52. HC-PCBs were rarely detected.1,2 In subsamples of residents in both residential areas, LC-PCBs in blood were highly elevated among residents of contaminated apartments.6,7 The largest relative difference was observed for PCB-28, with median serum levels being 98 times higher among residents in contaminated vs. reference apartments in Farum [ (5th–95th percentile 0.216–5.279) and (5th–95th percentile ), respectively]6 and 70 times higher in Brøndby [ (5th–95th percentile 47.2–850) vs. (5th–95th percentile ), respectively.]7
Study Population
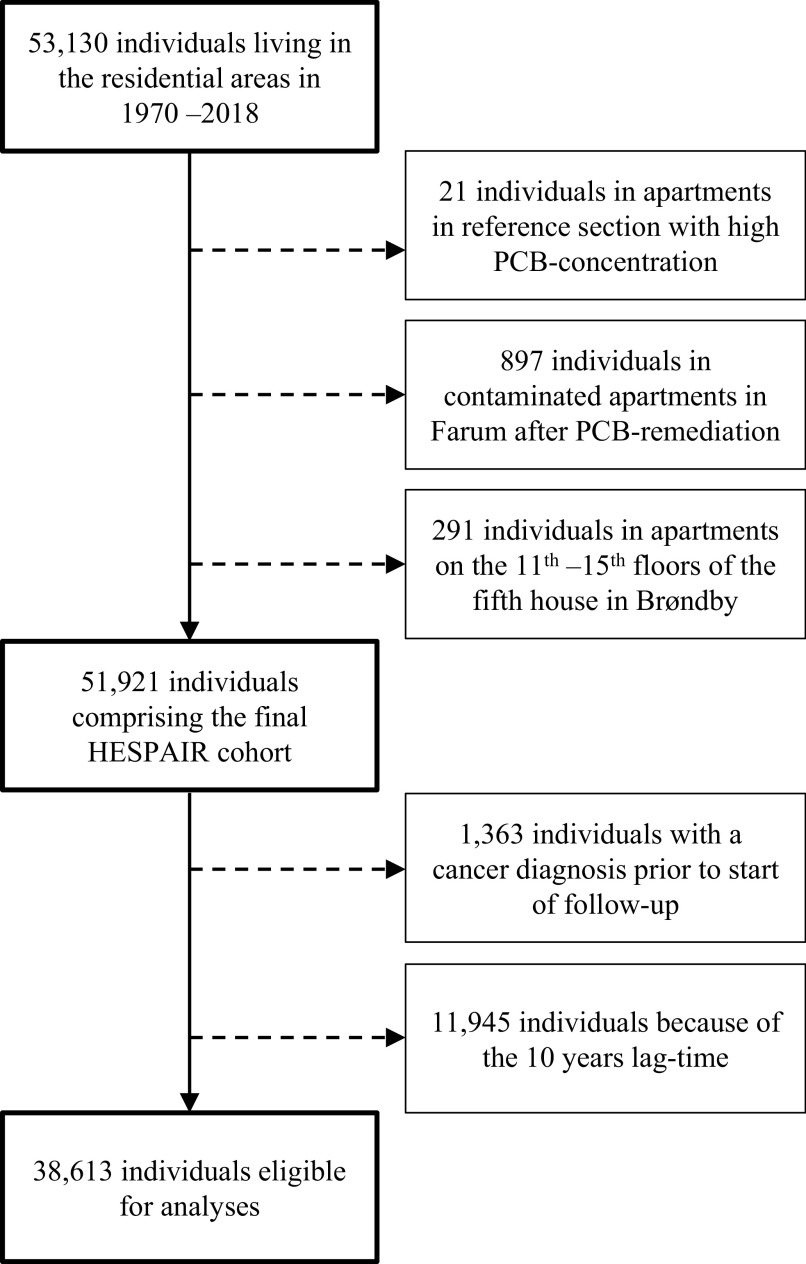
In Denmark, all residents have a unique personal identification number (CPR), introduced with the Danish Civil Registration System (CRS) in 1968.23 The CRS contains historical information on residential addresses and relocation dates on all individuals. By linkage of the Farum and Brøndby postal addresses in the CRS, we identified 53,130 former and current residents who lived in the residential areas at least once from 1970 to 2018. We excluded 21 residents of two apartments in the reference section with documented high PCB concentrations; possible explanations for the high concentrations have been described previously.2 Further, 897 Farum residents were excluded because they moved into previously contaminated apartments after PCB remediation in 2012–2015, as described in the section above. Finally, we excluded 291 residents living in apartments on the 11th–15th floors in the contaminated building in Brøndby where PCB-containing materials were not used. The final study population comprised 51,921 residents (Figure 1).
Figure 1.
Study flow chart. Dashed lines represent individuals excluded from the analysis.
PCB Measurements in Indoor Air
PCB measurements have been carried out in both contaminated apartments (apartments in the sections built first using PCB-contaminated materials) and in reference apartments (apartments in the sections using PCB-free materials). In Farum, 83 contaminated and 21 reference apartments were selected and sampled in 2011 as part of a research project investigating the indoor exposure of residents in the buildings (details in Frederiksen et al.2). In brief, air was sampled using SKC OV tubes with XAD-2 and internal filters with a flow of placed approximately 1 m above the floor for an average of 24 h (range: 16–47 h). Mean sample volume was (range: ) air. Twenty-four congeners (PCB-28, 52, 66, 74, 77, 81, 99, 101, 105, 114, 118, 123, 126, 138, 153, 156, 157, 167, 169, 170, 180, 183, 187, and 189) were analyzed from the absorbent tubes by Eurofins. In 2009, PCB contamination was documented in sealants.2 Consequently, the visible sealants were covered with aluminum tape and wooden strips to prevent direct contact and reduce emission approximately 18 months prior to sampling. This has been estimated to reduce PCB concentration in indoor air by 6% after temperature adjustment according to calculations done by the National Research Centre for the Working Environment (not published).
In Brøndby, 117 contaminated and 18 reference apartments were sampled from 2011 to 2017.24 The data were collected by a consulting company on the initiative of the owners of the buildings to investigate the indoor PCB contamination.24,25 Samples were obtained by active sampling on XAD-2 and dust filters with a flow of . Seven congeners (PCB-28, 52, 101, 118, 138, 153 and 180) were analyzed by Eurofins. Procedures changed during the period: From start to 15 December 2013, air were collected from 0400–0600 (4:00 A.M. to 6:00 A.M.) on Supelco XAD-2 tubes with external filters, and air were sampled from 0200–0600 (2:00 A.M. to 6:00 A.M.) using Supelco XAD-2 tubes with external filters, from 16 December 2013 to 1 February 2017. SKC OV tubes with XAD-2 and internal filters were used from 2 February 2017 to 26 June 2017. Table 1 shows a summary of the measurements.
Table 1.
a in indoor air (nanograms per cubic meter) in contaminated and reference apartments in Brøndby Strand Parkerne and Farum Midtpunkt.2,24
| Brøndby Strand Parkerne | Farum Midtpunkt | |||
|---|---|---|---|---|
| Contaminated apartments | Reference apartments | Contaminated apartments | Reference apartments | |
| Measurements () | 117 | 18 | 83 | 20 |
| Mean | 1,487 | 46 | 1,048 | 24 |
| Median | 1,298 | 32 | 871 | 20 |
| Minimum | 26 | 2 | 178 | 15 |
| Maximum | 4,949 | 139 | 3,901 | 44 |
.
PCB Exposure
Based on relocation dates and PCB measurements, two measures of PCB exposure were defined. First, years of living in contaminated apartments, regardless of PCB concentration, were summed for each individual as years of PCB exposure. Second, the cumulative was defined as years in an apartment multiplied by the concentration defined as . in air is defined as the sum of seven indicator congeners (PCB-28, 52, 101, 118, 138, 153 and 180) multiplied by a factor of five to compensate for all the congeners not quantified.26 This method is widely used in research and for regulatory purposes.27 was based on the mean of measurements in the specific or neighboring building, assuming a steady PCB concentration over time. Farum exposure estimates were adjusted according to an estimated 6% reduction as described above. Once exposed, residents were considered exposed for the rest of the follow-up, due to the long half-lives of PCBs.28
Cancer
Information on site-specific cancers and selected benign tumors (bladder and brain) including date of diagnosis was retrieved from the Danish Cancer Registry, which holds records of all cancers diagnosed in Denmark since 1943 with almost complete coverage.29 Prior to 1978, cancers were classified according to a Danish version of the International Classification of Diseases and Related Health Problems, 7th Revision (ICD-7) and thereafter to the International Classification of Diseases and Related Health Problems, 10th revision (ICD-10). We included all primary cancers with a minimum of five cases recorded and considered all cancers combined as well as site-specific cancers, but malignant melanoma, NHL, breast, liver, gall bladder, and biliary tract cancer were of a priori interest, because these have previously been associated with PCB exposure.9,18–20 A complete list of included cancers and ICD-10 and ICD-7 codes are provided in Table S1.
Covariates
Information on covariates was obtained from the Danish nationwide registers. Statistics Denmark provided information on ethnicity (Danish, western, nonwestern, definition according to Statistics Denmark30) marital status (married/cohabitating, unmarried, divorced/widowed), and highest attained education [low (elementary school), medium (high school), and high (university)]. Residents y of age were assigned their household’s highest attained education. Information on sex, birth date, death, disappearance, and emigration was extracted from the CRS. Information on comorbidity was obtained from the Danish National Patient Registry (NPR).31
Statistics
We estimated hazard ratios (HR) with corresponding 95% confidence intervals (95% CI) for overall (all cancer sites minus nonmelanoma skin cancers) and site-specific cancers using Cox regression with age as underlying timescale. Nonmelanoma skin cancers were included as an individual site-specific cancer but were not included in overall cancer. Analyses were performed using 10 y of latency, and consequently, start of follow-up was 10 y after date of first relocation to the residential areas. Individuals were considered at risk from age at start of follow-up and censored at age of cancer onset, death, disappearance (individuals whose residence is unknown to Danish authorities),23 emigration from Denmark, or end of follow-up (31 December 2018), whichever came first. Only the first recorded primary cancer at each of the 56 cancer sites during follow-up was included. Individuals with cancer diagnoses before start of follow-up were excluded. Each year during follow-up was assigned a year-specific exposure expressing the cumulative exposure up until that specific year. Years of PCB exposure was assessed continuously (per year) and in four categories: references (residents of reference apartments) and tertiles of cumulative years of PCB exposure ( y; 1.0–2.9 y; y). Cumulative was included in four categories: residents with levels under the Danish action level of as references and grouped in approximated tertiles (300–949; 950–2,999; ). Exposure categories were combined when there were site-specific cancer cases in individual exposure groups. To comply with national data protection regulations [General Data Protection Regulation (GDPR) (EU), 2016/679 of 25 May 2018], we calculated pseudopercentiles as the mean of the five values adjacent to the actual percentile.
Potential confounders were identified a priori using Directed Acyclic Graphs32 (Figure S1). All analyses were adjusted for calendar time in decades and sex. Analyses of breast cancer were restricted to the female population and analyses of other sex-specific cancers (cervix uteri, corpus uteri, ovary, external female genital organs and vagina, prostate, testis, other male genital organs) were restricted to the relevant sex. We further adjusted for education (as time dependent) in sensitivity analyses. Due to incomplete registration of education during the first years of follow-up, information on education was missing for a relatively high proportion of the residents, and the sensitivity analyses adjusting for education only included residents with information on education. There were no missing data on the other covariates included in the analyses.
In the European Nordic countries, risk of testis cancer is highest among men age 25–45 y.33 We therefore performed sensitivity analyses stratifying residents by age at start of follow-up ( y; y). To reduce potentially higher risk of liver cancer due to hepatitis B and C or alcohol-related disorders, a sensitivity analysis adjusted for these diagnoses identified in the NPR. Because alcohol consumption and tobacco smoking are established causes of some cancers,34 we conducted sensitivity analyses combining cancers related to alcohol consumption (oral cavity, pharynx, esophagus, larynx, colo-rectum, and female breast) and tobacco smoking (oral cavity, pharynx, esophagus, stomach, colo-rectum, nasal cavity, larynx, lung, uterine cervix, ovary, urinary bladder, kidney, renal pelvis and ureter, myeloid leukemia), excluding those of interest for the present study, to indirectly evaluate potential confounding. We performed sensitivity analyses using a 5-y lag for cancers of a priori interest and cancers with observed higher risk as well as for hematological cancers because the latter may have shorter latency than other cancers. All analyses used SAS (version 9.4; SAS Institute Inc.).
Ethics Committee Approval
The establishment of the HESPAIR cohort was approved by the Knowledge Center on Data Protection Compliance under the records of processing regarding health science research projects within the Capitol Region of Denmark (BFH-2016-013, I-Suite nr.: 04461). According to Danish legislation, registry-based studies without direct contact with individuals do not require approval from the scientific ethics committee.
Results
We excluded 1,363 residents with cancer diagnoses prior to follow-up and 11,945 residents because of the applied 10-y lag time. This resulted in 38,613 residents accumulating a total of 701,542 person-years. During follow-up, 3,431 incident cancers and benign tumors were identified.
Population characteristics (including potential confounders and other sociodemographic characteristics) are presented in Table 2. Residents in contaminated apartments did not differ substantially from those in reference apartments apart from being slightly more likely to be of Danish origin. A total of 23% of the residents had lived in a contaminated apartment at least once. The median years of PCB exposure was 1.8 y ( 0.2; 10.7 y), and the median cumulative among residents exposed to was ( 343; ).
Table 2.
Characteristics of the study population in the HESPAIR cohort ().
| Total population | Residents in contaminated apartments | Residents in reference apartments | |
|---|---|---|---|
| Residents 1970–2009 [ (%)] | 38,613 | 9,015 (23.4) | 29,598 (76.7) |
| Age start [median (; )]a | 33 (10;61) | 34 (10;64) | 33 (10;60) |
| Age end [median (; )]a | 52 (21;77) | 54 (23;79) | 52 (21;77) |
| Sex distribution (% female) | 49.1 | 49.8 | 48.9 |
| Education start [ (%)] | |||
| Low (elementary school) | 11,716 (33.4) | 2,786 (34.2) | 8,930 (33.2) |
| Medium (high school) | 13,642 (38.9) | 3,144 (38.6) | 10,498 (39.0) |
| High (university) | 9,711 (27.7) | 2,215 (27.2) | 7,496 (27.8) |
| Missingb | 3,544 | 870 | 2,674 |
| Ethnicity [ (%)] | |||
| Danish | 31,610 (81.9) | 7,577 (84.1) | 24,033 (81.2) |
| Western | 1,309 (3.4) | 252 (2.8) | 1,057 (3.6) |
| Non-western | 5,688 (14.7) | 1,183 (13.1) | 4,505 (15.2) |
| Marital status start [ (%)] | |||
| Married/cohabitating | 13,502 (35.0) | 3,090 (34.3) | 10,412 (35.2) |
| Unmarried | 18,894 (48.9) | 4,351 (48.3) | 14,543 (49.1) |
| Divorced/widowed | 6,217 (16.1) | 1,574 (17.5) | 4,643 (15.7) |
| Calendar time start [ (%)] | |||
| 1970–1979 | 12,605 (32.6) | 3,232 (35.9) | 9,373 (31.7) |
| 1980–1989 | 11,455 (29.7) | 2,513 (27.9) | 8,942 (30.2) |
| 1990–1999 | 8,357 (21.6) | 1,911 (21.2) | 6,446 (21.8) |
| 2000–2009 | 6,196 (16.1) | 1,359 (15.1) | 4,837 (16.3) |
Note: HESPAIR, Health Effects of PCBs in Indoor Air.
Pseudo median and percentiles (P) calculated as the mean of the five values nearest to the actual median/percentile.
Due to incomplete registration of education during the first years of follow-up, information on education was missing for a relatively high proportion of the residents. There were no missing data on the other covariates included in the analyses.
PCB Duration
Years of PCB exposure was not associated with overall risk of cancer (HR ; 95% CI: 0.99, 1.01) (Table 3). For most of the a priori suspected cancers, including melanoma, NHL, breast, gall bladder, and biliary tract, the risk was not higher among residents in contaminated apartments (Tables 3–6). For liver cancer, meningiomas, and pancreatic cancer, residents with y of PCB exposure had a higher risk than residents of reference apartments (HR liver ; 95% CI: 1.10, 5.45; HR meningiomas ; 95% CI: 1.69, 6.02; HR pancreatic ; 95% CI: 1.07, 3.03) (Tables 3 and 5). For liver cancer and meningiomas, HRs indicated an exposure–response relationship; for pancreatic cancer, the relationship was less obvious. We observed 3-fold higher risk of testis cancer for residents with up to 1 y of PCB exposure (HR ; 95% CI: 1.70, 7.11) but not for residents with more than 1 y of PCB exposure (Table 6).
Table 3.
Hazard ratios (95% CIs) for overall and site-specific cancers by years of PCB exposure adjusted for age, sex, and time period ( Danish residents).
| Cancer site (ICD-10) | Continuous exposure | Categorical PCB exposure in years | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nonexposed | y | 1.0–2.9 y | y | |||||||
| 701,542 person-years | 545,482 person-years | 51,930 person-years | 52,020 person-years | 52,110 person-years | ||||||
| Cases () | HR (95% CI) per year | Cases () | HR (95% CI) | Cases () | HR (95% CI) | Cases () | HR (95% CI) | Cases () | HR (95% CI) | |
| All cancers (minus nonmelanoma skin cancers) | 3,431 | 1.00 (0.99, 1.01) | 2,600 | 1.00 | 251 | 1.04 (0.91, 1.18) | 235 | 0.90 (0.78, 1.02) | 345 | 1.02 (0.91, 1.14) |
| Lip (C00) | 5 | 1.03 (0.83, 1.27) | NA | NA | NA | NA | ||||
| Tongue (C01–C02) | 27 | 0.95 (0.77, 1.16) | NA | NA | NA | NA | ||||
| Salivary glands (C07–08) | 7 | 0.62 (0.16, 2.48) | NA | NA | NA | NA | ||||
| Oesophagus (C15) | 56 | 1.00 (0.92, 1.09) | 38 | 1.00 | 8 | 2.27 (1.06, 4.88) | 5 | 1.29 (0.51, 3.27) | 5 | 0.98 (0.38, 2.48) |
| Small intestine (C17) | 12 | 0.99 (0.79, 1.24) | NA | NA | NA | NA | ||||
| Colon (C18–19) | 198 | 0.99 (0.94, 1.04) | 147 | 1.00 | 17 | 1.26 (0.76, 2.09) | 13 | 0.87 (0.49, 1.53) | 21 | 0.99 (0.63, 1.57) |
| Gall bladder and biliary tract (C23–24) | 15 | 1.00 (0.85, 1.17) | NA | NA | NA | NA | ||||
| Pancreas (C25) | 106 | 1.05 (1.00, 1.10) | 73 | 1.00 | 9 | 1.32 (0.66, 2.65) | 6 | 0.80 (0.35, 1.84) | 18 | 1.80 (1.07, 3.03) |
| Nasal cavity (C30–31) | NA | NA | NA | NA | NA | |||||
| Larynx (C32) | 25 | 0.97 (0.81, 1.17) | NA | NA | NA | NA | ||||
| Lung (C33–34, C39) | 476 | 1.02 (0.99, 1.05) | 349 | 1.00 | 38 | 1.18 (0.84, 1.65) | 27 | 0.76 (0.51, 1.12) | 62 | 1.29 (0.98, 1.69) |
| Thymus (C37) | NA | NA | NA | NA | NA | |||||
| Heart (C380–383, C388) | NA | NA | NA | NA | NA | |||||
| Pleura (C384) | NA | NA | NA | NA | NA | |||||
| Bones (C40–41) | NA | NA | NA | NA | NA | |||||
| Melanoma of skin (C43) | 177 | 1.00 (0.95, 1.06) | 135 | 1.00 | 15 | 1.18 (0.69, 2.01) | 13 | 0.99 (0.56, 1.75) | 14 | 0.92 (0.53, 1.61) |
| Nonmelanoma skin cancers (C44, C460) | 1,090 | 1.00 (0.98, 1.02) | 803 | 1.00 | 80 | 1.08 (0.86, 1.36) | 90 | 1.13 (0.91, 1.40) | 117 | 1.10 (0.91, 1.34) |
| Mesothelium (C450–459) | 8 | 1.10 (0.98, 1.22) | NA | NA | NA | NA | ||||
| Peripheral nerves and autonomic nervous system (C47) | NA | NA | NA | NA | NA | |||||
| Peritoneum and retroperitoneum (C48) | NA | NA | NA | NA | NA | |||||
| Connective tissue (C49, C461, C463, C467, C468, C469, B210) | 16 | 1.01 (0.83, 1.23) | 15 | 1.00 | NA | NA | NA | |||
| Breast (C50)a | 615 | 1.00 (0.97, 1.03) | 480 | 1.00 | 42 | 0.92 (0.67, 1.26) | 37 | 0.77 (0.55, 1.07) | 56 | 0.91 (0.69, 1.20) |
| External female genital organs and vagina (C51–52)a | 15 | 0.95 (0.77, 1.17) | 1.00 | NA | NA | NA | ||||
| Other and unspecified female genital organs (C577–C579)a | NA | NA | NA | NA | NA | |||||
| Prostate (C61)b | 298 | 0.97 (0.93, 1.01) | 226 | 1.00 | 15 | 0.73 (0.44, 1.24) | 32 | 1.43 (0.99, 2.08) | 25 | 0.79 (0.52, 1.19) |
| Other male genital organs (C60, C63)b | 7 | 0.60 (0.14, 2.53) | 1.00 | NA | NA | NA | ||||
| Renal pelvis and ureter (C65–66, D301–D302, D411–D412) | 11 | 0.94 (0.69, 1.28) | 1.00 | NA | NA | NA | ||||
| Urinary bladder (C67, D090, D303, D414) | 157 | 1.01 (0.96, 1.06) | 118 | 1.00 | 10 | 0.89 (0.47, 1.70) | 11 | 0.88 (0.48, 1.64) | 18 | 1.08 (0.66, 1.78) |
| Other urinary organs (C68, D091, D304–D309, D413, D417–D419) | NA | NA | NA | NA | NA | |||||
| Eye (C69) | NA | NA | NA | NA | NA | |||||
| Brain (C71, C751–753, D330–332, D430–432, D352–354, D443–445) | 103 | 1.00 (0.92, 1.08) | 78 | 1.00 | 9 | 1.24 (0.62, 2.47) | 9 | 1.20 (0.60, 2.40) | 7 | 0.80 (0.37, 1.73) |
| Adrenal gland (C74) | NA | NA | NA | NA | NA | |||||
| Other endocrine glands (C750; C754–C759) | NA | NA | NA | NA | NA | |||||
| Hodgkin lymphoma (C81) | 7 | 1.02 (0.75, 1.37) | NA | NA | NA | NA | ||||
| Monocytic leukemia (C93) | NA | NA | NA | NA | NA | |||||
| Other and unspecified leukemia (C94–95) | NA | NA | NA | NA | NA | |||||
| Other, unspecified in lymphatic and hematopoietic tissue (C96) | NA | NA | NA | NA | NA | |||||
| Ill-defined and unspecified cancer (C76–80) | 103 | 1.00 (0.94, 1.07) | 75 | 1.00 | 9 | 1.25 (0.63, 2.51) | 9 | 1.13 (0.57, 2.26) | 10 | 0.97 (0.50, 1.88) |
Note: CI, confidence interval; HR hazard ratio; ICD-10, International Statistical Classification of Diseases and Related Health Problems, 10th Revision; NA, not applicable.
Restricted to female population.
Restricted to male population.
Table 6.
Hazard ratios (95% CIs) for site-specific cancers by years of PCB exposure with combined exposure categories due to small number of cases adjusted for age, sex, and time period ( Danish residents).
| Cancer site (ICD-10) | Continuous | Nonexposed | y | y | ||||
|---|---|---|---|---|---|---|---|---|
| Cases (n) | HR (95% CI) | Cases (n) | HR (95% CI) | Cases (n) | HR (95% CI) | Cases (n) | HR (95% CI) | |
| Cervix uteri (C53)a | 84 | 0.97 (0.88, 1.08) | 67 | 1.00 | 7 | 1.04 (0.48, 2.27) | 10 | 0.72 (0.37, 1.40) |
| Testis (C62)b | 46 | 0.94 (0.79, 1.14) | 31 | 1.00 | 10 | 3.48 (1.70, 7.11) | 5 | 0.87 (0.34, 2.24) |
| Other parts of CNS (C72, D333–339, D433–D439) | 37 | 0.86 (0.66, 1.12) | 26 | 1.00 | 6 | 2.42 (1.00, 5.88) | 5 | 0.92 (0.35, 2.39) |
Note: CI, confidence interval; CNS, central nervous system; HR, hazard ratio; ICD-10, International Statistical Classification of Diseases and Related Health Problems, 10th Revision.
aRestricted to female population.
bRestricted to male population.
Table 4.
Hazard ratios (95% CIs) for site-specific cancers by years of PCB exposure with combined exposure categories due to small number of cases adjusted for age, sex, and time period ( Danish residents).
| Cancer site (ICD-10) | Continuous | Nonexposed | Exposed | |||
|---|---|---|---|---|---|---|
| Cases (n) | HR (95% CI) | Cases (n) | HR (95% CI) | Cases (n) | HR (95% CI) | |
| Mouth (C03–06, C462) | 31 | 1.00 (0.88, 1.15) | 23 | 1.00 | 8 | 1.11 (0.49, 2.47) |
| Pharynx (C09–14) | 50 | 0.86 (0.69, 1.08) | 41 | 1.00 | 9 | 0.70 (0.34, 1.43) |
| Stomach (C16) | 50 | 0.98 (0.88, 1.08) | 40 | 1.00 | 10 | 0.76 (0.38, 1.52) |
| Anus (C21, C26) | 25 | 0.93 (0.75, 1.15) | 18 | 1.00 | 7 | 1.19 (0.50, 2.85) |
| Thyroid gland (C73) | 40 | 0.79 (0.55, 1.13) | 34 | 1.00 | 6 | 0.60 (0.25, 1.43) |
| Non-Hodgkin lymphoma (C82–85, C883–889) | 79 | 0.95 (0.86, 1.05) | 66 | 1.00 | 13 | 0.62 (0.34, 1.13) |
| Multiple myeloma (C90, C880–882) | 32 | 0.75 (0.49, 1.13) | 27 | 1.00 | 5 | 0.57 (0.22, 1.48) |
| Lymphatic leukemia (C91) | 47 | 0.93 (0.80, 1.08) | 37 | 1.00 | 10 | 0.81 (0.40, 1.64) |
| Myeloid leukemia (C92) | 15 | 0.90 (0.66, 1.23) | 9 | 1.00 | 6 | 2.03 (0.72, 5.73) |
Note: CI, confidence interval; HR, hazard ratio; ICD-10, International Statistical Classification of Diseases and Related Health Problems, 10th Revision.
Table 5.
Hazard ratios (95% CIs) for site-specific cancers by years of PCB exposure with combined exposure categories due to small number of cases adjusted for age, sex, and time period ( Danish residents).
| Cancer site (ICD-10) | Continuous | Nonexposed | 0–2.9 y | y | ||||
|---|---|---|---|---|---|---|---|---|
| Cases (n) | HR (95% CI) | Cases (n) | HR (95% CI) | Cases (n) | HR (95% CI) | Cases (n) | HR (95% CI) | |
| Rectum (C20) | 111 | 1.01 (0.95, 1.07) | 85 | 1.00 | 10 | 0.60 (0.31, 1.16) | 16 | 1.41 (0.82, 2.42) |
| Liver (C22) | 40 | 1.08 (1.02, 1.15) | 25 | 1.00 | 7 | 1.48 (0.64, 3.43) | 8 | 2.45 (1.10, 5.45) |
| Corpus uteri (C54–55, C58)a | 70 | 1.03 (0.97, 1.09) | 53 | 1.00 | 7 | 0.69 (0.31, 1.51) | 10 | 1.27 (0.64, 2.52) |
| Ovary, fallopian tube, and broad ligament (C56, C570–C574)a | 73 | 1.00 (0.93, 1.09) | 60 | 1.00 | 7 | 0.60 (0.28, 1.32) | 6 | 0.74 (0.32, 1.72) |
| Kidney (C64) | 86 | 1.01 (0.94, 1.09) | 65 | 1.00 | 14 | 1.11 (0.62, 1.97) | 7 | 0.93 (0.42, 2.03) |
| Meningiomas (C70, D32, D42) | 53 | 1.06 (1.00, 1.12) | 32 | 1.00 | 7 | 1.14 (0.50, 2.58) | 14 | 3.19 (1.69, 6.02) |
Note: CI, confidence interval; HR, hazard ratio; ICD-10, International Statistical Classification of Diseases and Related Health Problems, 10th Revision.
aRestricted to female population.
PCB Concentration
Overall risk of cancer was not associated with , nor was the risk for melanoma, NHL, breast, gall bladder and biliary tract cancers (Table 7–10). Compared with residents exposed to , residents exposed had higher risk of liver cancer (HR ; 95% CI: 1.28, 6.15) and meningiomas (HR ; 95% CI: 1.84, 6.64), and the HRs suggested an exposure–response relationship (Table 7). For pancreatic cancer, results were suggestive of a higher risk (HR ; 95% CI: 0.95, 2.64) at the highest aggregated PCB level (Table 7). For testis cancer, higher risk was observed among residents exposed to (HR ; 95% CI: 1.41, 6.28) but not to (Table 10).
Table 7.
Hazard ratios (95% CIs) for overall and site-specific cancers by cumulative (a) adjusted for age, sex, and time period ( Danish residents).
| Cancer site (ICD-10) | () in categories | |||||||
|---|---|---|---|---|---|---|---|---|
| × year | × year | × year | × year | |||||
| 534,466 person-years | 54,114 person-years | 53,004 person-years | 59,959 person-years | |||||
| Cases () | HR (95% CI) | Cases () | HR (95% CI) | Cases () | HR (95% CI) | Cases () | HR (95% CI) | |
| All cancers (minus nonmelanoma skin cancers) | 2,446 | 1.00 | 331 | 1.01 (0.90, 1.14) | 277 | 1.02 (0.90, 1.16) | 377 | 0.98 (0.88, 1.09) |
| Lip (C00) | NA | NA | NA | NA | ||||
| Tongue (C01–02) | NA | NA | NA | NA | ||||
| Salivary glands (C07–08) | NA | NA | NA | NA | ||||
| Esophagus (C15) | 35 | 1.00 | 9 | 1.88 (0.90, 3.92) | 7 | 1.77 (0.79, 3.99) | 5 | 0.89 (0.35, 2.27) |
| Small intestine (C17) | NA | NA | NA | NA | ||||
| Colon (C18–19) | 142 | 1.00 | 17 | 0.83 (0.50, 1.37) | 19 | 1.17 (0.72, 1.89) | 20 | 0.80 (0.50, 1.28) |
| Rectum (C20) | 78 | 1.00 | 10 | 0.95 (0.49, 1.84) | 7 | 0.80 (0.37, 1.73) | 16 | 1.27 (0.74, 2.19) |
| Liver (C22) | 21 | 1.00 | 5 | 1.88 (0.71, 5.00) | 5 | 2.16 (0.81, 5.73) | 9 | 2.81 (1.28, 6.15) |
| Gall bladder and biliary tract (C23–24) | NA | NA | NA | NA | ||||
| Pancreas (C25) | 73 | 1.00 | 6 | 0.62 (0.27, 1.43) | 8 | 0.98 (0.47, 2.03) | 19 | 1.59 (0.95, 2.64) |
| Nasal cavity (C30–31) | NA | NA | NA | NA | ||||
| Lung (C33–34, C39) | 326 | 1.00 | 52 | 1.15 (0.86, 1.54) | 34 | 0.92 (0.65, 1.32) | 64 | 1.18 (0.90, 1.55) |
| Thymus (C37) | NA | NA | NA | NA | ||||
| Heart (C380–383, C388) | NA | NA | NA | NA | ||||
| Pleura (C384) | NA | NA | NA | NA | ||||
| Bones (C40–41) | NA | NA | NA | NA | ||||
| Melanoma of skin (C43) | 131 | 1.00 | 16 | 1.01 (0.60, 1.70) | 14 | 1.02 (0.59, 1.77) | 16 | 0.92 (0.55, 1.55) |
| Nonmelanoma skin cancers (C44, C460) | 753 | 1.00 | 112 | 1.07 (0.88, 1.31) | 89 | 1.06 (0.85, 1.33) | 136 | 1.14 (0.94, 1.36) |
| Mesothelium (C450–459) | NA | NA | NA | NA | ||||
| Peripheral nerves and autonomic nervous system (C47) | NA | NA | NA | NA | ||||
| Peritoneum and retroperitoneum (C48) | NA | NA | NA | NA | ||||
| Connective tissue (C49, C461, C463, C467, C468, C469, B210) | NA | NA | NA | NA | ||||
| Breast (C50)b | 449 | 1.00 | 61 | 1.02 (0.78, 1.34) | 43 | 0.88 (0.64, 1.20) | 62 | 0.90 (0.69, 1.17) |
| External female genital organs and vagina (C51–52)b | NA | NA | NA | NA | ||||
| Cervix uteri (C53)b | 64 | 1.00 | 6 | 0.82 (0.35, 1.90) | 8 | 1.20 (0.57, 2.50) | 6 | 0.72 (0.31, 1.67) |
| Other and unspecified female genital organs (C577–C579)b | NA | NA | NA | NA | ||||
| Prostate (C61)c | 216 | 1.00 | 20 | 0.66 (0.42, 1.05) | 31 | 1.29 (0.88, 1.88) | 31 | 0.86 (0.59, 1.25) |
| Other male genital organs (C60, C63)c | NA | NA | NA | NA | ||||
| Renal pelvis and ureter (C65–66, D301–D302, D411–D412) | NA | NA | NA | NA | ||||
| Urinary bladder (C67, D090, D303, D414) | 109 | 1.00 | 16 | 1.07 (0.63, 1.81) | 12 | 0.95 (0.52, 1.72) | 20 | 1.08 (0.67, 1.74) |
| Other urinary organs (C68, D091, D304–D309, D413, D417–D419) | NA | NA | NA | NA | ||||
| Eye (C69) | NA | NA | NA | NA | ||||
| Meningiomas (C70, D32, D42) | 26 | 1.00 | 5 | 1.38 (0.53, 3.60) | 7 | 2.42 (1.05, 5.57) | 15 | 3.49 (1.84, 6.64) |
| Brain (C71, C751–753, D330–332, D430–432, D352–354, D443–445) | 74 | 1.00 | 10 | 1.16 (0.59, 2.24) | 10 | 1.31 (0.68, 2.55) | 9 | 0.91 (0.46, 1.83) |
| Adrenal gland (C74) | NA | NA | NA | NA | ||||
| Other endocrine glands (C750; C754–C759) | NA | NA | NA | NA | ||||
| Hodgkin lymphoma (C81) | NA | NA | NA | NA | ||||
| Monocytic leukaemia (C93) | NA | NA | NA | NA | ||||
| Other and unspecified leukemia (C94–95) | NA | NA | NA | NA | ||||
| Other, unspecified in lymphatic and hematopoietic tissue (C96) | NA | NA | NA | NA | ||||
| Ill-defined and unspecified cancer (C76–80) | 66 | 1.00 | 16 | 1.90 (1.10, 3.29) | 11 | 1.47 (0.78, 2.79) | 10 | 0.91 (0.47, 1.79) |
Note: CI, confidence interval; HR, hazard ratio; ICD-10, International Statistical Classification of Diseases and Related Health Problems, 10th Revision; NA, not applicable.
.
Restricted to female population.
Restricted to male population.
Table 10.
Hazard ratios (95% CIs) for site-specific cancers by cumulative (a) with combined exposure categories due to small number of cases adjusted for age, sex, and time period ( Danish residents).
| Cancer site (ICD-10) | () in categories | |||||
|---|---|---|---|---|---|---|
| × year | × year | × year | ||||
| Cases () | HR (95% CI) | Cases () | HR (95% CI) | Cases () | HR (95% CI) | |
| Ovary, fallopian tube, and broad ligament (C56, C570–C574)b | 57 | 1.00 | 7 | 0.90 (0.41, 1.99) | 9 | 0.58 (0.29, 1.17) |
| Testis (C62)c | 30 | 1.00 | 9 | 2.97 (1.41, 6.28) | 7 | 1.15 (0.50, 2.61) |
| Kidney (C64) | 64 | 1.00 | 10 | 1.29 (0.66, 2.51) | 12 | 0.76 (0.41, 1.41) |
| Other parts of CNS (C72, D333–339, D433–D439) | 26 | 1.00 | 6 | 2.02 (0.83, 4.92) | 5 | 0.82 (0.31, 2.14) |
Note: CI, confidence interval; CNS, central nervous system; HR, hazard ratio; ICD-10, International Statistical Classification of Diseases and Related Health Problems, 10th Revision.
[ (PCB-28, 52, 101, 118, 138, 153, 180)].
Restricted to female population.
Restricted to male population.
Table 8.
Hazard ratios (95% CIs) for site-specific cancers by cumulative (a) with combined exposure categories due to small number of cases adjusted for age, sex, and time period ( Danish residents).
| Cancer site (ICD-10) | () in categories | |||
|---|---|---|---|---|
| × year | × year | |||
| Cases () | HR (95% CI) | Cases () | HR (95% CI) | |
| Mouth (C03–06, C462) | 22 | 1.00 | 9 | 1.10 (0.50, 2.40) |
| Pharynx (C09–14) | 40 | 1.00 | 10 | 0.67 (0.34, 1.35) |
| Anus (C21, C26) | 17 | 1.00 | 8 | 1.17 (0.50, 2.73) |
| Larynx (C32) | 20 | 1.00 | 5 | 0.68 (0.26, 1.82) |
| Thyroid gland (C73) | 31 | 1.00 | 9 | 0.89 (0.42, 1.87) |
| Multiple myeloma (C90, C880–882) | 25 | 1.00 | 7 | 0.70 (0.30, 1.63) |
| Myeloid leukemia (C92) | 8 | 1.00 | 7 | 2.14 (0.76, 6.01) |
[ (PCB-28, 52, 101, 118, 138, 153, 180)].
Table 9.
Hazard ratios (95% CIs) for site-specific cancers by cumulative (a) with combined exposure categories due to small number of cases adjusted for age, sex, and time period ( Danish residents).
| Cancer site (ICD-10) | () in categories | |||||
|---|---|---|---|---|---|---|
| × year | × year | × year | ||||
| Cases () | HR (95% CI) | Cases () | HR (95%CI) | Cases () | HR (95% CI) | |
| Stomach (C16) | 39 | 1.00 | 5 | 0.50 (0.20, 1.27) | 6 | 0.90 (0.38, 2.14) |
| Corpus uteri (C54–55, C58)b | 48 | 1.00 | 11 | 0.87 (0.45, 1.67) | 11 | 1.26 (0.65, 2.45) |
| Non-Hodgkin lymphoma (C82–85, C883–889) | 61 | 1.00 | 9 | 0.58 (0.29, 1.17) | 9 | 0.95 (0.47, 1.92) |
| Lymphatic leukemia (C91) | 35 | 1.00 | 7 | 0.82 (0.36, 1.85) | 5 | 0.87 (0.34, 2.24) |
Note: CI, confidence interval; HR, hazard ratio; ICD-10, International Statistical Classification of Diseases and Related Health Problems, 10th Revision.
[ (PCB-28, 52, 101, 118, 138, 153, 180)].
Restricted to female population.
Sensitivity Analyses
Adjustment for education and hepatitis and alcohol-related disorders did not substantially alter the results (Tables S2–S7). When stratifying analyses of testis cancer by age, men y of age at start of follow-up had the highest risk (Tables S8–S9). For cancers associated with alcohol consumption or smoking (excluding liver and pancreas cancers), the risk was not higher among PCB-exposed residents (HR for high-exposed vs. residents of reference apartments: Alcohol 0.89; 95% CI: 0.73, 1.08; smoking 0.97; 95% CI: 0.82, 1.14) (Table S10). Using a 5-y lag time for selected cancers did not alter the results considerably (Tables S11–S12).
Discussion
In this, to the best of our knowledge, first-ever human study of cancer risk following residential exposure to airborne PCBs, we found no association with overall cancer morbidity, but risks of liver cancer and meningiomas (primarily benign) were higher among residents in PCB-contaminated apartments, with indication of an exposure–response relationship. Higher risks of pancreatic and testis cancers were suggestive, but effect estimates were inconsistent. Previously observed associations between HC-PCBs and malignant melanoma, NHL, and breast cancer were not confirmed.
No previous studies have, as far as we know, investigated the carcinogenic risk of living in PCB-contaminated indoor air. Occupational cohorts indicate higher risk of liver cancer mortality among highly exposed workers, mainly through inhalation, when compared with local and national figures.18–20 In contrast, a case–control study nested in two prospective general population cohorts found serum levels of HC-PCBs, but not LC-PCBs, associated with liver cancer.35 As opposed to our study, that study did not include populations highly exposed to airborne PCBs. Further, because LC-PCBs are more easily metabolized and may act through their metabolites, serum measurements of parent congeners may underestimate the actual risk of LC-PCBs.13 Associations with liver cancer are supported by rodent studies, where commercial PCB mixtures have been reported to be liver carcinogenic,9 and LC-PCBs and their metabolites may increase liver mutations.11,12 Because the higher risk of liver cancer persisted after adjustment for hepatitis and alcohol-related disorders, these diseases unlikely account for the observed higher risk. Further, the risk of liver cirrhosis did not differ between exposure groups. Our study did not confirm the previously reported higher risk of gall bladder and biliary tract cancer observed in PCB-exposed workers,18,20 but case numbers were small.
To our knowledge, we are the first to report a markedly higher risk of (primarily benign) meningiomas following PCB exposure. The etiology of meningiomas is unclear, and considering the multiple comparisons performed, it could be a chance finding, although there were rather robust indications of an exposure–response relationship.
Risk of pancreatic cancer was higher among highly PCB-exposed residents, but with an inconsistent exposure–response relationship. A meta-analysis of occupational exposure to chlorinated solvents suggested a weak association between PCB exposure and pancreatic cancer mortality,36 heavily driven by one study of Canadian transformer manufacturing workers. No excess risk was found among electrical capacitor manufacturing workers in four other occupational studies.36 Considering the inconsistent exposure–response relationship, this might also be a random finding.
Our observation of higher testis cancer risk is in agreement with a recent case–control study, where higher serum levels of potentially estrogenic PCBs (including PCB-28 and 52) were associated with testis cancer.37 Previous results have been conflicting: an Italian case–control study found the sum of nine PCB congeners, including PCB-28, 31, and 52, to be significantly higher in 125 cases in comparison with 103 controls38; two case–control studies, one Norwegian39 and one Swedish,40 found no association between some LC-PCBs measured in blood and testis cancer. The few available studies indicate LC-PCBs exert estrogenic effects, which may be even stronger for their metabolites, that might have direct toxic, reproductive, and carcinogenic consequences.10,13,41 As expected, we observed the highest risk among men y of age at start of follow-up, suggesting prenatal and childhood exposure may be important in the etiology of testis cancer, as suggested by others.40 Our results did however not show a exposure–response relationship nor a higher risk among long-term exposed residents.
A possible explanation for the discrepancy between the IARC’s conclusion and our results could be that the IARC evaluation was based on mixtures primarily including HC-PCBs that likely have different mechanisms than LC-PCBs.13 This explanation is supported by three more recent meta-analyses that reported no strong support for an association between occupational PCB exposure, mainly by inhalation, and malignant melanoma and NHL.14,15,17 Together with the current study, this emphasizes that evidence on HC-PCBs may be inappropriate for evaluation of LC-PCBs because they may have other carcinogenic effects.
Methodological Considerations
This HESPAIR study builds on a natural experimental design, where residents have unknowingly relocated themselves to contaminated apartments. We therefore expect factors like socioeconomic status, lifestyle, and background PCB exposure to be similar between exposure groups, which is supported by previous studies on subsamples of the population.6,7 Longstanding and virtually complete national registries supplied valid information on cancer diagnoses and long-term follow-up.29 During the long follow-up, some factors changed: Diagnostic tools have improved, smoking decreased, and the residential areas have become less coveted. The chance of living in a contaminated apartment was higher during the first years because those apartments were built first. Consequently, there might be fewer valid diagnoses and higher proportions of smokers and socioeconomically advantaged among the exposed. We therefore adjusted for calendar time and education but could not adjust for other potential confounders. Although we do not consider body mass index (BMI) or health behaviors, such as smoking, to be associated with living in a PCB-contaminated apartment, they could be related to the susceptibility and body burden of PCBs,42,43 and because they are strong risk factors for some cancers, they could potentially confound or interact with the association. However, due to the natural experiment design, we assume potential confounding factors to be equally distributed between exposure groups and expect potential confounding to be minimal. This is supported by observation of equal distribution of BMI in subsets of residents.6,7 Adjustment for education would also indirectly reduce confounding from BMI and health behaviors because these are closely related. In sensitivity analyses of liver cancer, we adjusted for alcohol-related disorders, without alteration of the results of the main analyses. Further, in the sensitivity analyses the risk was not higher for other alcohol- and smoking-related cancers combined. This finding suggests that alcohol consumption and tobacco smoking are unlikely to explain the observed higher risk of liver cancer in this cohort.
Given the multiple outcomes examined, associations could occur by chance. Findings are, therefore, interpreted with caution and weighed against a priori hypotheses, and exposure–response relationships are emphasized. Findings for cancer sites with limited existing evidence should be considered basis for generation of hypotheses. Although this is the largest population study of airborne LC-PCBs to date, it was underpowered for many rare cancers. When numbers of cases were too small, exposure categories were collapsed. This diminished exposure contrast and could have caused nondifferential misclassification, attenuating effects.
Years of PCB exposure were based on years of living in contaminated apartments, with residents of reference apartments serving as references. This was based on the considerable contrast in PCB concentration between contaminated and reference apartments which, together with reported 30–52 times higher LC-PCBs serum levels in subsets of residents,1,2,6,7 indicate minimal misclassification. However, in Brøndby the maximum in reference apartments exceeded the minimum in contaminated apartments (Table 1), which could imply some nondifferential misclassification with potential attenuations toward the null. was based on air measurements extrapolated from relatively few measurements over several years. Because temporal development of PCB in indoor air is not fully described, and factors like maintenance activities, cleaning, temperature, season, and ventilation may influence concentrations,2,25,44 this extrapolation could potentially imply further nondifferential misclassification. It would have been ideal to have biological PCB measurements of all residents, because residents may have interacted with each other or spent considerable time outside their residential areas, which could have affected their PCB body burden. However, by design, we expect this to be equally distributed across exposure groups.
Our study examines the overall effect of airborne PCBs but not of specific LC-PCBs, because their concentrations are highly correlated.1,2 Although differences between contaminated and reference apartments in air and serum samples in a subset of residents were mostly attributed to LC-PCBs, the serum showed significantly elevated levels of some HC-PCBs among exposed residents,1,2,6,7 meaning that HC-PCBs or an interaction between HC- and LC-PCBs may contribute to observed associations.
Because the use of PCB-containing materials ceased throughout the construction, the buildings constructed during later stages were built using PCB-free materials. We are, however, not aware of which materials replaced the PCB-containing materials, but we have no reasons to expect higher levels of other chemicals in the buildings built without PCB.
In conclusion, residents exposed to airborne PCBs in their private homes had higher risk of liver cancer and meningiomas, although the risk for the majority of cancers was not higher. Our findings suggest a potential carcinogenic effect of LC-PCBs that is different from that of HC-PCBs. Even larger cohort studies, with biologically measured LC-PCBs and their metabolites, are warranted to enable firm conclusions about the potential human carcinogenicity of airborne LC-PCBs.
Supplementary Material
Acknowledgments
This study was funded by The National Building Foundation, Realdania (Ref. No. PRJ-2017-00176), and The Landowners’ Investment Foundation (Ref. No. 18-58). K.S.H.’s contribution to the present study was supported by the Focused Research Effort on Chemicals in the Working Environment (FFIKA) from the Danish Government.
Funding was acquired by J.P.B., H.W.M., and S.S.T. Material preparation and data collection were performed by S.S.T., L.D., and J.P.B. T.H., M.F., L.G., H.W.M., and H.V.A. provided PCB exposure data and contributed with knowledge of the contaminated buildings. All authors contributed to the study conception and design. S.S.T. and L.D. had full access to and verified the underlying study data. Statistical analyses and first draft of the manuscript were performed by L.D. All authors critically revised several versions of the manuscripts and approved the final manuscript.
Data may be obtained from a third party and are not publicly available. The authors do not have the permission to share the data used in this study due to data protection regulations. Deidentified individual data are available from Statistics Denmark and The Danish Health and Medicines Authority on reasonable request.
References
- 1.Andersen HV, Gunnarsen L, Knudsen LE, Frederiksen M. 2020. PCB in air, dust and surface wipes in 73 Danish homes. Int J Hyg Environ Health 229:113429, PMID: , 10.1016/j.ijheh.2019.113429. [DOI] [PubMed] [Google Scholar]
- 2.Frederiksen M, Meyer HW, Ebbehøj NE, Gunnarsen L. 2012. Polychlorinated biphenyls (PCBs) in indoor air originating from sealants in contaminated and uncontaminated apartments within the same housing estate. Chemosphere 89(4):473–479, PMID: , 10.1016/j.chemosphere.2012.05.103. [DOI] [PubMed] [Google Scholar]
- 3.Harrad S, Hazrati S, Ibarra C. 2006. Concentrations of polychlorinated biphenyls in indoor air and polybrominated diphenyl ethers in indoor air and dust in birmingham, United Kingdom: implications for human exposure. Environ Sci Technol 40(15):4633–4638, PMID: , 10.1021/es0609147. [DOI] [PubMed] [Google Scholar]
- 4.Lehmann GM, Christensen K, Maddaloni M, Phillips LJ. 2015. Evaluating health risks from inhaled polychlorinated biphenyls: research needs for addressing uncertainty. Environ Health Perspect 123(2):109–113, PMID: , 10.1289/ehp.1408564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marek RF, Thorne PS, Herkert NJ, Awad AM, Hornbuckle KC. 2017. Airborne PCBs and OH-PCBs Inside and Outside urban and rural U.S. Schools. Environ Sci Technol 51(14):7853–7860, PMID: , 10.1021/acs.est.7b01910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer HW, Frederiksen M, Göen T, Ebbehøj NE, Gunnarsen L, Brauer C, et al. 2013. Plasma polychlorinated biphenyls in residents of 91 PCB-contaminated and 108 non-contaminated dwellings–an exposure study. Int J Hyg Environ Health 216(6):755–762, PMID: , 10.1016/j.ijheh.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Frederiksen M, Andersen HV, Haug LS, Thomsen C, Broadwell SL, Egsmose EL, et al. 2020. PCB in serum and hand wipes from exposed residents living in contaminated high-rise apartment buildings and a reference group. Int J Hyg Environ Health 224:113430, PMID: , 10.1016/j.ijheh.2019.113430. [DOI] [PubMed] [Google Scholar]
- 8.Herrick RF, Meeker JD, Altshul L. 2011. Serum PCB levels and congener profiles among teachers in PCB-containing schools: a pilot study. Environ Heal 10(1):1–10, 10.1186/1476-069X-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.IARC (International Agency for Research on Cancer). 2016. Polychlorinated Biphenyls and Polybrominated Biphenyls – IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 107. Lyon, France: IARC. [PMC free article] [PubMed] [Google Scholar]
- 10.Plísková M, Vondrácek J, Canton RF, Nera J, Kocan A, Petrík J, et al. 2005. Impact of polychlorinated biphenyls contamination on estrogenic activity in human male serum. Environ Health Perspect 113(10):1277–1284, PMID: , 10.1289/ehp.7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ludewig G, Lehmann L, Esch H, Robertson LW. 2008. Metabolic activation of PCBs to carcinogens in vivo–a review. Environ Toxicol Pharmacol 25(2):241–246, PMID: , 10.1016/j.etap.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robertson LW, Ludewig G. 2011. Polychlorinated biphenyl (PCB) carcinogenicity with special emphasis on airborne PCBs. Gefahrst Reinhalt Luft 71(1–2):25–32, PMID: . [PMC free article] [PubMed] [Google Scholar]
- 13.Grimm FA, Hu D, Kania-Korwel I, Lehmler H-J, Ludewig G, Hornbuckle KC, et al. 2015. Metabolism and metabolites of polychlorinated biphenyls. Crit Rev Toxicol 45(3):245–272, PMID: , 10.3109/10408444.2014.999365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boffetta P, Catalani S, Tomasi C, Pira E, Apostoli P. 2018. Occupational exposure to polychlorinated biphenyls and risk of cutaneous melanoma: a meta-analysis. Eur J Cancer Prev 27(1):62–69, PMID: , 10.1097/CEJ.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 15.Catalani S, Donato F, Tomasi C, Pira E, Apostoli P, Boffetta P. 2019. Occupational and environmental exposure to polychlorinated biphenyls and risk of non-Hodgkin lymphoma: a systematic review and meta-analysis of epidemiology studies. Eur J Cancer Prev 28(5):441–450, PMID: , 10.1097/CEJ.0000000000000463. [DOI] [PubMed] [Google Scholar]
- 16.Silver SR, Whelan EA, Deddens JA, Steenland NK, Hopf NB, Waters MA, et al. 2009. Occupational exposure to polychlorinated biphenyls and risk of breast cancer. Environ Health Perspect 117(2):276–282, PMID: , 10.1289/ehp.11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zani C, Ceretti E, Covolo L, Donato F. 2017. Do polychlorinated biphenyls cause cancer? A systematic review and meta-analysis of epidemiological studies on risk of cutaneous melanoma and non-Hodgkin lymphoma. Chemosphere 183:97–106, PMID: , 10.1016/j.chemosphere.2017.05.053. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen EB, Jacobsen P, Jensen AA, et al. 2013. Risk of Disease Following Occupational Exposure to Polychlorinated Biphenyls. [In Danish] Copenhagen, Denmark: Bispebjerg Hospital.
- 19.Mallin K, McCann K, D’Aloisio A, Freels S, Piorkowski J, Dimos J, et al. 2004. Cohort mortality study of capacitor manufacturing workers, 1944–2000. J Occup Environ Med 46(6):565–576, PMID: , 10.1097/01.jom.0000128156.24767.12. [DOI] [PubMed] [Google Scholar]
- 20.Prince MM, Ruder AM, Hein MJ, Waters MA, Whelan EA, Nilsen N, et al. 2006. Mortality and exposure response among 14,458 electrical capacitor manufacturing workers exposed to polychlorinated biphenyls (PCBs). Environ Health Perspect 114(10):1508–1514, PMID: , 10.1289/ehp.9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pangel LM, Lundsgaard C. 2013. PCB i Luft Hos Genhusede Beboere – Afgasning Fra Indbo - Farum Midtpunkt [PCB in Air at Rehoused Residents] [In Danish]. Hørsholm, Denmark: Scandinavian Biomedical Institute (SBMI).
- 22.Enemærke, Petersen. 2014. PCB-Sanering i Farum Sat Effektivt i System Farum Midtpunkt Bliver PCB-Problemet Kvit [PCB Remediation in Farum Midtpunkt] [In Danish]. Farum, Denmark: Enemærke & Petersen a/s.
- 23.Pedersen CB. 2011. The Danish Civil Registration System. Scand J Public Health 39(suppl 7):22–25, PMID: , 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 24.Golder Associates. 2015. Resultatoversigter over PCB-Målinger i Indeluften. Revision 7 [Consultancy Report: Summary of PCB Measurements for Indoor Air (In Danish)]. Kgs. Lyngby, Denmark: Golder Associates A/S.
- 25.Andersen HV, Kolarik B, Nielsen NS, Hougaard T, Gunnarsen L, Knudsen LE, et al. 2021. Indoor air concentrations of PCB in a contaminated building estate and factors of importance for the variance. Build Environ 204:108135, 10.1016/j.buildenv.2021.108135. [DOI] [Google Scholar]
- 26.Verein Deutscher Ingenieure. 2009. Ambient Air Measurement – Indoor Air Measurement – Measurement of Polychlorinated Biphenyls (PCBs) – GC/MS Method for PCB 28, 52, 101,138, 153, 180, Part 1 (VDI 2464). Düsseldorf, Germany: VDI – The Association of German Engineers. [Google Scholar]
- 27.Jensen AA. 2013. Health Risks of PCB in the Indoor Climate in Denmark - Background for Setting Recommended Action Levels. Copenhagen, Denmark: Danish Health and Medicines Authority.
- 28.Ritter R, Scheringer M, MacLeod M, Moeckel C, Jones KC, Hungerbühler K. 2011. Intrinsic human elimination half-lives of polychlorinated biphenyls derived from the temporal evolution of cross-sectional biomonitoring data from the United Kingdom. Environ Health Perspect 119(2):225–231, PMID: , 10.1289/ehp.1002211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gjerstorff ML. 2011. The Danish Cancer Registry. Scand J Public Health 39(suppl 7):42–45, PMID: , 10.1177/1403494810393562. [DOI] [PubMed] [Google Scholar]
- 30.Statistics Denmark. 2019. Indvandrere i Danmark 2019 [Immigrants in Denmark] [In Danish]. Copenhagen, Denmark: Statistics Denmark.
- 31.Lynge E, Sandegaard JL, Rebolj M. 2011. The Danish National Patient Register. Scand J Public Health 39(suppl 7):30–33, PMID: , 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 32.Greenland S, Pearl J, Robins JM. 1999. Causal diagrams for epidemiologic research. Epidemiology 10(1):37–48, PMID: , 10.1097/00001648-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 33.NORDCAN. 2019. Cancer Stat Fact Sheets - Nordic Countries - Testis. https://www-dep.iarc.fr/nordcan/English/StatsFact.asp?cancer=271&country=0 [accessed 15 February 2021].
- 34.Secretan B, Straif K, Baan R, Grosse Y, El Ghissassi F, Bouvard V, et al. 2009. A review of human carcinogens—part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol 10(11):1033–1034, PMID: , 10.1016/S1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 35.Niehoff NM, Zabor EC, Satagopan J, Widell A, O’Brien TR, Zhang M, et al. 2020. Prediagnostic serum polychlorinated biphenyl concentrations and primary liver cancer: a case-control study nested within two prospective cohorts. Environ Res 187(May):109690, PMID: , 10.1016/j.envres.2020.109690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ojajärvi A, Partanen T, Ahlbom A, Boffetta P, Hakulinen T, Jourenkova N, et al. 2001. Risk of pancreatic cancer in workers exposed to chlorinated hydrocarbon solvents and related compounds: a meta-analysis. Am J Epidemiol 153(9):841–850, PMID: , 10.1093/aje/153.9.841. [DOI] [PubMed] [Google Scholar]
- 37.Cheng Z, Zhang X, Bassig B, Hauser R, Holford TR, Zheng E, et al. 2021. Serum polychlorinated biphenyl (PCB) levels and risk of testicular germ cell tumors: a population-based case-control study in Connecticut and Massachusetts. Environ Pollut 273:116458, PMID: , 10.1016/j.envpol.2021.116458. [DOI] [PubMed] [Google Scholar]
- 38.Paoli D, Giannandrea F, Gallo M, Turci R, Cattaruzza MS, Lombardo F, et al. 2015. Exposure to polychlorinated biphenyls and hexachlorobenzene, semen quality and testicular cancer risk. J Endocrinol Invest 38(7):745–752, PMID: , 10.1007/s40618-015-0251-5. [DOI] [PubMed] [Google Scholar]
- 39.Purdue MP, Engel LS, Langseth H, Needham LL, Andersen A, Barr DB, et al. 2009. Prediagnostic serum concentrations of organochlorine compounds and risk of testicular germ cell tumors. Environ Health Perspect 117(10):1514–1519, PMID: , 10.1289/ehp.0800359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hardell L, Van Bavel B, Lindström G, Carlberg M, Eriksson M, Dreifaldt AC, et al. 2004. Concentrations of polychlorinated biphenyls in blood and the risk for testicular cancer. Int J Androl 27(5):282–290, PMID: , 10.1111/j.1365-2605.2004.00489.x. [DOI] [PubMed] [Google Scholar]
- 41.Pěnčíková K, Svržková L, Strapáčová S, Neča J, Bartoňková I, Dvořák Z, et al. 2018. In vitro profiling of toxic effects of prominent environmental lower-chlorinated PCB congeners linked with endocrine disruption and tumor promotion. Environ Pollut 237:473–486, PMID: , 10.1016/j.envpol.2018.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lan T, Liu B, Bao W, Thorne PS. 2021. BMI modifies the association between dietary intake and serum levels of PCBs. Environ Int 156:106626, PMID: , 10.1016/j.envint.2021.106626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee DH, Lind L, Jacobs DR, Salihovic S, Van Bavel B, Monica Lind P. 2014. Does mortality risk of cigarette smoking depend on serum concentrations of persistent organic pollutants? Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. PLoS One 9(5):e95937–8, 10.1371/journal.pone.0095937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolarik B, Frederiksen M, Meyer HW, Ebbehøj NE, Gunnarsen LB. 2016. Investigation of the importance of tertiary contamination, temperature and human behaviour on PCB concentrations in indoor air. Indoor Built Environ 25(1):229–241, 10.1177/1420326X14543505. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.