Abstract
Background and Aims
Facial palsy is a rare complication of the COVID‐19 infection. Herein, we conducted a systematic review of all published cases of facial palsy post‐COVID‐19 infection in an attempt to educate the general population and medical practitioners regarding the likely occurrence of facial palsy in COVID‐19 patients, its detection, effective treatment plan, and prognosis of the condition.
Methods
We searched PubMed, Google Scholar, and Directory of Open Access Journals (DOAJ) from December 1, 2019 to September 21, 2021.
Results
We included 49 studies bearing accounts of 75 cases who had facial palsy. The mean age of patients was 42.9 ± 19.59 years, with a male‐to‐female ratio of 8:7. The majority of the cases were reported from Brazil (n = 14), USA (n = 9), Turkey (n = 9), and Spain (n = 9). Noticeably, 30.14% of COVID‐19 patients were diagnosed with Guillain‐Barré syndrome. In total, 22.97% of patients complained of bilateral facial paralysis (n = 17), whereas ipsilateral paralysis was observed in 77.03% (n = 57). These were common complaints of Lagophthalmos, otalgia, facial drooping, dysarthria, and compromised forehead wrinkling. The treatment regimen mainly included the use of corticosteroids (n = 51) (69.86%), antivirals (n = 23) (31.51%), IVIG (n = 18) (24.66%), antibiotics (n = 13) (17.81%), antiretroviral (n = 9) (12.33%), and antimalarial (n = 8) (10.96%) medications. In all, 35.62% of patients (n = 26) adhered to a combination of antiviral and corticosteroid‐based therapy. Positive treatment outcomes were observed in 83.58% (n = 56) of cases. In contrast, 10 patients (14.93%) showed nonsignificant recovery, out of which 3 (4.48%) died from the disease.
Conclusion
The association of facial palsy with COVID‐19 is controversial and therefore requires further investigation and published work to confirm a causal relationship. However, physicians should not overlook the likelihood of facial palsy post‐COVID‐19 infection and treat it accordingly.
Keywords: COVID‐19, facial palsy, neurological symptoms, SARS‐CoV‐2, systematic review
1. INTRODUCTION
On December 31, 2019, a novel coronavirus was first identified in Wuhan, China, after reports of multiple cases of pneumonia among its people. 1 This was the start of an outbreak that took the shape of a pandemic over a few months, owing to its rapid transmission through respiratory droplets. As of May 10, 2022, 6.53% of the world population (n = 515,748,861) has confirmed infection with COVID‐19, while 1.21% of these have lost their lives to the complications of COVID‐19. 2
COVID‐19 patients commonly complain of fever, fatigue, nasal congestion, myalgia, anosmia, dry cough, ageusia, hemoptysis, dyspnea, and so forth. 3 Under more serious circumstances, COVID‐19 can result in severe pneumonia, acute respiratory distress syndrome, sepsis, septic shock, multiple organ failure, and so forth. 4 While these are some of the most widely reported complications of COVID‐19 infection, other less common ones have also surfaced, for example, hemophagocytic lymphohistiocytosis (HLH), vasculitis, central retinal vein occlusion, and so forth. 5 , 6 , 7 Similarly, facial palsy has emerged as an unusual yet interesting complication of COVID‐19, whose pathophysiology is yet to be known.
Numerous case reports and series documenting facial palsy as a complication of COVID‐19 have been published. In addition, some systematic reviews have discussed the association of facial palsy with COVID‐19. However, none of these reviews collectively assessed all the cases of facial palsy secondary to COVID‐19. For instance, Gupta et al. 8 included only those cases in which facial palsy was an isolated neurological finding. Therefore, in our systematic review, we aim to develop a stronger evidence base by including all the cases of facial palsy secondary to COVID‐19 that have been published to date. Moreover, we generated patient‐level data by including case reports to thoroughly evaluate the patient characteristics and clinical course of facial palsy secondary to COVID‐19. This will not only bridge the gap in literature but will also aid physicians in reaching a timely diagnosis and in devising treatment regimens that cater to the patients' individual needs.
2. METHODOLOGY
2.1. Literature review
Our work aligns with the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) checklist (Supporting Information: File S1). 9 We searched PubMed, Google Scholar, and Directory of Open Access Journals (DOAJ) from December 1, 2019 to September 21, 2021 for published accounts of cases of facial palsy as a symptom of COVID‐19. Search terms were combined using appropriate Boolean operators. Our search strategy included keywords/subject headings pertaining to COVID‐19 (e.g., SARS‐CoV‐2 OR Coronavirus Disease 2019 OR COVID‐19 OR severe acute respiratory syndrome coronavirus 2 OR coronavirus infection) and facial palsy (e.g., facial palsy OR facial weakness OR facial paresis OR bell's palsy). The Reference section of included studies was also checked for completeness' sake. Please refer to our Supporting Information: File S1 for a detailed search strategy. Furthermore, this study is registered in the International prospective register of systematic reviews (PROSPERO) and holds the unique identifying number (UIN); CRD42022324693. 10
2.2. Inclusion and exclusion criteria
Our search criteria included all case reports, case series, editorials, correspondence, and retrospective cohorts on the topic of facial palsy following COVID‐19 infection. Only work published in English and containing comprehensive detail of clinical presentation and progression of the condition in each patient was included in our systematic review. Studies bearing aggregate level data, language barriers, and incomplete detail of the condition were excluded from our study. The title, abstract, and full‐text screening were completed in duplicate and independently by two reviewers (M.K. and A.S.). Disagreements regarding the inclusion of studies for data extraction were resolved by the senior author (A.K.).
2.3. Data extraction
Duplicate work was removed after a final version of included literature was entered on excel sheets. We gathered available data on the origin of the reported case (country), date of publication, study type, relevant case within every included study, patient characteristics, age, sex, the status of Guillain‐Barré syndrome (present or absent), affected side of the face, the onset of facial palsy, the test used for detection of COVID‐19, features related to facial palsy, results of cerebrospinal fluid (CSF) analysis, COVID‐19 related symptoms, other signs/symptoms, imaging results, treatment regimen and outcome of treatment. Due to a lack of uniformity in the assessment of facial symptoms between included studies, the percentage‐wise prevalence of symptoms could not be calculated. Additionally, since follow‐up time varied between studies, treatment follow‐up results are not comparable. The terms “complete recovery,” “partial recovery,” “progressive improvement,” and “significant improvement” were regarded as positive treatment outcomes by the author of this review.
2.4. Quality assessment
The quality of included cases was assessed using Joanna Briggs institute's critical appraisal tools. 11 Selected studies were examined for inclusion criteria, sample size, description of study participants, and setting. Two reviewers independently assessed the methodological quality of each paper. Quality assessments were done with different tools based on different study designs. Each tool was modified to provide a numeric score. Tools had 8 items for case reports and 10 for case series. Included case reports (n = 39) had a mean score of 6.385 ± 1.41 with scores ranging from 2 to 8 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 10 case series had a mean score of 5.60 ± 2.01, and scores ranged from 3 to 9. 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 The detailed results of the quality assessment are provided in Supporting Information: File S1. The quality of our systematic review was assessed using AMSTAR 2 criteria. 60 The level of compliance with AMSTAR 2 came out to be “low.” We could not conduct a meta‐analysis because only case reports and case series were included in the analyses without quantitative data.
2.5. Statistical analysis
This systematic review reported descriptive information using individual‐level data of 75 cases from a total of 49 studies reported on facial palsy as a manifestation of COVID‐19. The data focused on the date and country of publication, patient's characteristics, detailed symptoms of facial palsy and COVID‐19, the status of Guillain‐Barré syndrome (present or absent), results of imaging and Cerebrospinal Fluid (CSF) analysis, treatment plan and its outcome. In addition, the continuous variable's mean, median, and SD were calculated where possible.
3. RESULTS
Our initial search provided 1408 results. After removing duplicate studies (N = 1006), 347 studies were screened individually by the two reviewers (M.K. and A.S.). Two hundred and sixteen studies were rejected after going through their titles and abstracts, while full‐text versions of the remaining articles (N = 131) were opened to ascertain their relevance to the topic. Out of these, 82 were excluded for reporting aggregate‐level data (N = 35), not being of the desired study type (N = 12), not being in English (N = 19), or for reporting insufficient data on medical manifestation (N = 16). Finally, 49 studies met our inclusion criteria and were, thus, included for systematic analysis (Figure 1).
Figure 1.
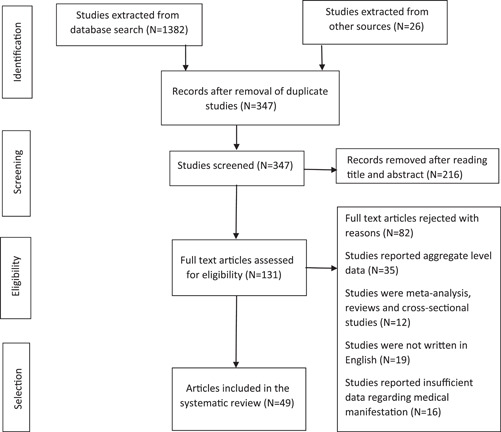
Flow chart of selected studies
3.1. Patient characteristics
A total of 75 patients gathered from 49 studies who developed facial palsy due to COVID‐19 [Table 1]. The mean age of patients was 42.91 ± 19.59 years, ranging from 15 months to 88 years. In addition, 40 out of 75 patients were males. At the same time, 35 were females, giving a slightly higher ratio of male to female sufferers (8:7). Highest number of cases were reported from Brazil (n = 14), followed by the USA (n = 9), Turkey (n = 9), and Spain (n = 7). Iran and Italy reported six cases each, whereas Singapore, Morocco, and France published accounts of three people each suffering from the condition. India, Egypt, and Japan reported two cases each. At the same time, Canada, Nepal, UK, Belgium, Qatar, Germany, Sweden, Norway, and Portugal each had one published account of a patient complaining of facial palsy due to COVID‐19. It is noteworthy that 13 out of 62 patients had hypertension (20.97%), 9 had diabetes mellitus (14.52%), and 3 of the women were pregnant (4.84%). Twenty‐seven patients had no comorbid condition (43.55%), while no information was shared for 13 patients (17.33%).
Table 1.
Patient characteristics
| Author | Country | Study type | No of cases | Patient No. | Patient characteristics | Age/Sex | GB present | Affected side of face | Facial palsy as first sign or not | Features related to facial palsy | CSF results | COVID‐19 related symptoms | Other signs/symptoms | Imaging | Treatment | Treatment outcome | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lima et al. | Brazil | Case series | 8 | 1 | None | 43/F | No | Right | Yes | Moderate (HB Grade 3) | NS | Mild symptoms | Ipsilateral abducent nerve palsy | CT Scan normal | Oral corticosteroids | PR | |
| 2 | None | 25/F | No | Right | Yes | Mild (HB Grade 2) | NS | Mild | None | Brain MRI normal | Oral corticosteroids + acyclovir | CR. | |||||
| 3 | None | 33/F | No | Right | Yes | Moderate (HB Grade 3) | NS | Mild | None | NA | Oral corticosteroids + acyclovir | PR | |||||
| 4 | None | 26/F | No | Left | No (after 2–10 days for all Nos) | Mild (HB Grade 2) | NS | Mild | None | MRI: left CN7 enhancement | Oral corticosteroids | CR | |||||
| 5 | None | 50/F | No | Left | No | Moderate (HB Grade 3) | Protein: mildly elevated; WBC: normal; SARS‐COV: negative | Mild | None | CT scan: normal | Oral corticosteroids | PR | |||||
| 6 | None | 38/F | No | Left | No | Mild (HB Grade 2) | NS | Mild | None | Brain MRI: normal | Supportive (eye lubricant) | CR | |||||
| 7 | None | 39/F | No | Right | No | Mild (HB Grade 2) | NS | Mild | None | Brain MRI: normal | Oral corticosteroids | CR | |||||
| 8 | None | 34/M | No | Left | No | Mild (HB Grade 2) | NS | Mild | None | Brain MRI: normal | IV corticosteroids | CR | |||||
| Homma et al. | Japan | Case report | 1 | 1 | Smoker | 35/F | No | Right | Yes | NA | NS | Cough, malaise, sore throat, nausea, fever, right‐sided aguesia of tongue and anosmia | None | CT scan: multiple bilateral ground‐glass opacities | Acetaminophen, Maoto, favipiravir, and inhaled Ciclesonide (corticosteroid) | CR | |
| Goh et al. | Singapore | Case report | 1 | 1 | NA | 27/M | No | Left | No (after 6 days) | Left‐sided otalgia | NS | Myalgia, cough, fever, dysguesia, left‐sided throbbing headache, and conjunctival infection | None | Chest X‐ray: unremarkable; brain MRI; left CN7 enhancement | Oral corticosteroid, valacyclovir and Lopinavir/ritonavir | No significant improvement. | |
| Figueiredo et al. | Portugal | Case report | 1 | 1 | Pregnant | 35/F | No | Left | Yes | Involuntary drooling, left‐side labial commissure deviation and ipsilateral lagophthalmos | NA | None | None | NA | Corticosteroid therapy and eye hydration therapy | No significant improvement. | |
| Caamaño et al. | Spain | Case report | 1 | 1 | None | 61/M | Yes | Bilateral | No (after 10 days) | Involuntary drooling on his right facial commissure, unresponsive blink reflex on both eyes | Protein: mildly elevated; WBC: normal; SARS‐COV: negative | Fever, cough, and pneumonia | None | Brain MRI: unremarkable; Chest X‐ray: bilateral frosted glass pneumonia | Oral corticosteroid, antimalarial and lopinavir/Ritonavir | No significant improvement. | |
| Muras et al. | Spain | Case report | 1 | 1 | None | 20/M | No | Bilateral | No (after a week) | NA | Protein: elevated; WBC: elevated; SARS‐COV: negative | Fever, significant asthenia, headache, myalgia, nausea, headache, odynophagia and vomiting | EBV coinfection | Brain MRI: confirmed bilateral facial neuritis | Levofloxacin and oral corticosteroid | CR | |
| Manganotti et al. | Italy | Case series | 3 | 1 | NA | 72/M | Yes | Right | No (after 18 days) | Mild right sided lower face weakness | Protein: elevated; WBC: normal; SARS‐COV: negative | Fever, dyspnea, hyposmia, ageusia | Flaccid tetraparesis, hypesthesia of extremities, dysuria, dysphasia, sinus arrythmia | NA | IVIG cycle, antimalarial, oseltamivir, darunavir, IV corticosteroid, and tocilizumab | NA | |
| 2 | NA | 49/F | Yes | Right | No (after 14 days) | Right‐sided hypoesthesia of the face | Protein: elevated; WBC: normal; SARS‐COV: negative | Fever, cough, dyspnea, hyposmia, and ageusia | Ophthalmoplegia with diplopia in the vertical and lateral gaze, and limb ataxia | Brain MRI: normal | IVIG cycle, antimalarial, lopinavir–ritonavir, IV corticosteroid | Progressive improvement | |||||
| 3 | NA | 76/M | Yes | Left | No (after 22 days) | Mild left‐sided lower facial deficit | Protein: elevated; WBC: normal; SARS‐COV: negative | Fever, cough, hyposmia, ageusia | Mild transient diplopia, tetraparesis and dysuria | NA | IVIG cycle, oseltamivir, darunavir, IV corticosteroid, | Progressive improvement | |||||
| Tocilizumab, | |||||||||||||||||
| meropenem, linezolid | |||||||||||||||||
| clarithromycin, | |||||||||||||||||
| doxycycline and fluconazole | |||||||||||||||||
| Khaja et al. | USA | Case report | 1 | 1 | HTN and asthma | 44/M | Yes | Bilateral | No (after 3 days) | Severe (HB Grade 5) | Protein: elevated; WBC: normal; glucose: normal | Ageusia | None | Chest X‐ray: clear; MRI brain: unremarkable | IVIG | CR | |
| Sancho‐Saldaña et al. | Spain | Case report | 1 | 1 | None | 56/F | Yes | Bilateral | No (after 20 days) | NA | Protein: elevated; WBC: normal; SARS‐COV: negative | Fever, dry cough, and dyspnea | Tetraparesis, lumbar pain, pararesthesia in both hands and oropharyngeal weakness | Chest X‐ray: lobar consolidation | Antimalarial, azithromycin and IVIG | PR | |
| Theophanous et al. | USA | Case report | 1 | 1 | Prematurely born, multiple congenital abnormalities, asthma, and gastrostomy tube feeding | 6/M | No | Right | Yes | Moderate severe (HB Grade 4) | NA | None | Tachycardiac | NA | IV acyclovir, IVIG infusion, lubricating eye drops and IV corticosteroids | Significant improvement | |
| Dahl et al. | Norway | Case report | 1 | 1 | Acute MI | 37/M | No | Right | No (after 18 days) | NA | Protein: elevated; IgG: normal; WBC: elevated | Fever, headache, dyspnea | Oliguria, hypotension, tachycardiac, tachypneic and unilateral painful neck swelling | X‐ray thorax: bibasal consolidations | IV furosemide and intermittently required low‐dose norepinephrine | CR | |
| Egilmez et al. | Turkey | Retrospective cohort | 8 | 1 | HTN, CHF | 90/M | No | Left | Yes | Moderate severe (HB Grade 4) | NA | Pneumonia | None | Thorax CT: Intense pneumonia with ground glass opacities | IV moxifloxacin and corticosteroids (dexamethasone and prednisolone) | PR | |
| 2 | None | 4/F | No | Left | No (after 7 days) | Moderate severe (HB Grade 4) | NA | Cough and fever | None | Thorax CT: normal | Oral corticosteroid | CR | |||||
| 3 | None | 17/F | No | Right | Yes | Moderate (HB Grade 3) | NA | Cough, ageusia and anosmia | None | Thorax CT: normal | Favipravir and oral corticosteroid | CR | |||||
| 4 | HTN, DM | 71/F | No | Right | Yes | Moderate severe (HB Grade 4) | NA | Fever, ageusia and anosmia | None | Thorax CT: normal | Favipravir and IV corticosteroid | CR | |||||
| 5 | None | 63/F | No | Left | Yes | Moderate severe (HB Grade 4) | NA | Fever, myalgia, ageusia and anosmia | None | Thorax CT: Mild pneumonia with ground glass appearance | Favipravir and oral corticosteroid | PR | |||||
| 6 | None | 60/F | No | Left | No (after 12 days) | Moderate severe (HB Grade 4) | NA | Fever, ageusia and anosmia | None | Thorax CT: normal | Favipravir and oral corticosteroid | PR | |||||
| 7 | HTN | 65/F | No | Left | Yes | Moderate (HB Grade 3) | NA | Ageusia and anosmia | None | Thorax CT: Mild pneumonia with ground glass opacities | IV corticosteroids | PR | |||||
| 8 | HTN, OSA | 30/M | No | Left | No (after 9 days) | Moderate (HB Grade 3) | NA | ageusia and anosmia | None | Thorax CT: Mild pneumonia with ground glass appearance; brain MRI: normal | Favipravir and oral corticosteroid (methylprednisolone and dexamethasone) | No improvement | |||||
| Engström et al. | Sweden | Case report | 1 | 1 | None | 46/F | No | Left | No (after 26 days) | Tongue deviation to left, inability to wrinkle forehead and left lagophthalmos, drooping left corner of mouth, vocal cord paresis, left‐sided paresis | NA | High fever, cough, dyspnea, dysphagia, and severe headaches | None | CT thorax: bilateral ground glass appearance. MRI brain: some edema in the parotid gland | High‐flow oxygen therapy, dalteparin, IV cefotaxime, oral and IV corticosteroids, and tear substitutes with watch bandages | Significant improvement | |
| Corrêa et al. | Brazil | Case series | 4 | 1 | None | 25/F | No | Right | No (after 2 weeks) | Right‐sided facial muscle weakness and right lagophthalmos | NA | Vertigo, mild dyspnea, and fever | Strabismus in the right eye after right CN6 palsy | Brain MRI: restricted diffusion (right CN6 nucleus) and an asymmetrical enhancement (right CN7) | Oral corticosteroids | CR | |
| 2 | None | 30/F | No | Right | No (after 10 days) | NA | NA | Mild fever and sore throat | None | Brain MRI: enhancement in right CN7 | Oral corticosteroids | CR | |||||
| 3 | OA, AF | 65/M | Yes | Bilateral | No (after 2 weeks) | NA | Protein: elevated; WBC: normal; SARS‐COV: negative | Headache, fever, and generalized myalgia | Lower limbs weakness | Brain MRI: bilateral enhancement in CN7 | IVIg | PR | |||||
| 4 | None | 33/M | No | Bilateral | No (after 2 weeks) | NA | NA | Fever | NA | Brain MRI: enhancement in CN7 | Oral corticosteroids | CR | |||||
| Chan et al. | Canada | Case report | 1 | 1 | None | 58/M | Yes | Bilateral | Yes | Dysarthria, bilateral lagophthalmos, inability to raise eyebrow, wrinkle forehead, smile, and close lips | Protein: elevated; WBC: normal; SARS‐COV: negative | None | Hypertension, tachypnea, paresthesia in his feet, and tachycardia | Chest X‐ray: bilateral infiltrates; CT: bilateral ground‐glass opacities in lung apices; Brain MRI: Bilateral CN7 enhancement | Empiric ceftriaxone, azithromycin and IVIG | PR | |
| Decio et al. | Italy | Correspondence | 1 | 1 | NA | 1.25/F | No | Right | NA | NA | NA | Mild respiratory symptoms, fever, anosmia, and ageusia | None | Brain MRI: right CN7 enhancement | Oral corticosteroids | CR | |
| Ozer et al. | Turkey | Case report | 1 | 1 | NA | 62/F | No | Left | No (after 2 days) | Total paralysis (HB Grade 6) | NA | Fatigue, chills, and myalgia | Sensorineural hearing loss | Brain MRI: CN7 and CN 8 enhancement | Oral corticosteroids, famotidine, oral favipiravir and SQ enoxaparin sodium | PR | |
| Neo et al. | Singapore | Case series | 2 | 1 | NA | 25/M | No | Left | Yes | Severe (HB Grade 5) | NA | None | None | All imaging were unremarkable | Oral corticocorticosteroids, valaciclovir and given eye care advice | CR | |
| 2 | NA | 34/M | No | Right | Yes | Moderate severe (HB Grade 4) | NA | None | None | All imaging were unremarkable | Oral corticocorticosteroids, valaciclovir and given eye care advice | PR | |||||
| Mackenzie et al. | USA | Case report | 1 | 1 | HTN, T2DM | 39/F | Yes | Bilateral | No (after 20 days) | NA | NA | Ageusia, anosmia, headache, myalgias, malaise, and cough | Left arm paresthesia, generalized flaccid areflexia, and inability to walk | NA | Enoxaparin SC, losartan, meperidine IV, antimalarial drug, oral corticosteroids and plasmapheresis | PR | |
| Bastola et al. | Nepal | Case report | 1 | 1 | DM | 48/M | No | Left | No (after 4 days) | Left‐sided facial droop with inability to wrinkle left forehead, raise left eyebrow and left laogphthalmus | NA | Mild dry cough and hyposmia | None | HRCT chest: ground‐glass opacity in the right lower lobe | Regular insulin and other antidiabetic medications, tear plus drops for dry eyes, and IV corticosteroid | Significant improvement with some residual weakness | |
| Hookham et al. | UK | Case report | 1 | 1 | Childhood asthma and HTN | 17/M | No | Right | No (after 1.5 months) | Right‐sided facial droop with right‐sided facial hypoesthesia | NA | Fever, diarrhea, vomiting, mild headache, intermittent right‐sided chest pain, myalgia and lethargy, diaphoretic and conjunctival injection (anterior uveitis) | Pediatric inflammatory multisystem syndrome, tachycardiac, tachypnea, raised blood pressure, palpitations | Brain MRI: minimal increased enhancement of a segment of right CN 7 | IV fluids, broad spectrum antibiotics, oral corticosteroids, tocilizumab, amlodipine (for HTN), aspirin and eye drops | NA | |
| Khedr et al. | Egypt | Case report | 2 | 1 | None | 49/F | Yes | Left | No | Right‐sided deviation of mouth and left lagophthalmos | NA | Fever, dysphagia, and vomiting | Flaccid areflexic quadriplegia, hoarseness of voice, and an impaired cough reflex and stock and glove hypoesthesia | CT chest: bilateral ground‐glass opacities | Plasmapheresis and IVIg | Progressive improvement | |
| 2 | None | 55/F | Yes | Bilateral | No | Bilateral inability to close eyes, with reduced blinking, inability to whistle, protrude the lips or expose the teeth. | NA | Fever, cough, and expectoration | flaccid areflexic quadriplegia, stock and glove hypoesthesia | CT chest: bilateral ground‐glass opacities | IVIg | CR | |||||
| Kumar et al. | India | Case report | 1 | 1 | pregnant and PCOS | 28/F | No | Right | No | Inability to wrinkle right forehead and close right eye, left‐sided deviation of mouth, numbness of the right side of the face and right‐sided drooling | NA | Fever, dysgeusia, and anosmia | Persistently high blood pressure (160/110), generalized weakness | NA | Oral valacyclovir and oral corticosteroid, insulin (for steroid‐induced DM) with physiotherapy and eye protective measures | CR | |
| Aasfara et al. | Morocco | Case report | 1 | 1 | Pregnant | 36/F | Yes | Bilateral | Yes | Moderate severe (HB Grade 4) | protein: elevated; WBC: normal cell count; glucose: normal | Vertigo, nausea, and vomiting | asymmetric numbness in the lower limbs and left fingers, right sensorineural hearing loss, right vestibular areflexia and nystagmus | NA | IVIg and IV corticosteroids | CR of facial palsy. | |
| Paybast et al. | Iran | Case report | 1 | 1 | HTN | 38/M | Yes | Bilateral | Yes | Bilateral facial droop, drooling, and slurred speech | Glucose: normal; WBC: normal; protein: elevated | Band‐like headache, dysphagia, and mild dizziness | quadriparesthesia, decrease in all sensation modalities in four limbs affecting the distal parts up to ankle and elbow joints, tachycardia, blood pressure instability | NA | Plasmapharesis and labetalol (for HTN) | No significant improvement | |
| Bigaut et al. | France | Case report | 2 | 1 | None | 43/M | Yes | Right | No | NA | WBC: normal; protein: elevated | Cough, anosmia, ageusia, and diarrhea | Flaccid paraparesis, generalized areflexia, hypoesthesia, fore limb paresthesia, ataxia, myalgias in legs | Chest CT: bilateral ground‐glass opacities; MRI: CN 3, 5, 6, 7, and 8 neuritis | IVIg | Progressive improvement | |
| 2 | Obesity | 70/M | Yes | Left | No | NA | WBC: normal; protein: elevated | anosmia, ageusia, diarrhea, dyspnea | Flaccid tetraparesis, generalized areflexia, forelimb paresthesia | Chest CT: bilateral moderate ground‐glass opacities | IVIg and physiotherapy | No significant improvement. | |||||
| Ottaviani et al. | Italy | Case report | 1 | 1 | Mild HTN | 66/F | NA | left | Yes | NA | Protein: elevated; rest; normal | Acute fatigue, mild fever, and cough | Paraplegia, transient pruriginous dorsal rash, initial distal weakness in the upper limbs and diffuse areflexia | Lung CT: bilateral ground‐glass opacities | IVIg, lopinavir/ritonavir and antimalarial | NA | |
| Casas et al. | Spain | Case report | 1 | 1 | vWB | 32/M | No | Left | No | Moderate severe (HB Grade 4) | NA | Malaise, fever, dry cough, and headache | None | Brain MRI: asymmetric contrast uptake in a segment of Left CN7 | acetaminophen, metamizole, physiotherapy and ocular hydration | CR. | |
| Hutchins et al. | USA | Case report | 1 | 1 | HTN, prediabetes, Class I obesity | 21/M | Yes | Bilateral | No | Dysarthria, hypogeusia, and facial numbness | Protein: mildly elevated; WBC: normal; SARS‐COV: negative | Fever, cough, dyspnea, diarrhea, nausea, headache, and sinonasal congestion, dizziness, hypogeusia | Tachycardic, bilateral lower extremity weakness, bilateral upper extremity paranesthesia, Grade 4/5 weakness in the deltoids and hip flexors bilaterally, diffuse areflexia | Chest X‐ray: increased bilateral air space opacities; brain MRI: abnormal bilateral enhancement of CN 6 and 7, alongside right CN 3 | Plasmapheresis | Nonsignificant improvement | |
| Abolmaali et al. | Iran | Case series | 3 | 1 | HTN | 88/F | Yes | Left | Yes | Left lagophthalmos and neck flexion weakness | Protein: elevated; rest: normal | Fatigue | Quadriparesis, low back and thigh pain, impaired proprioception | CT: pneumonia with a ground‐glass pattern | Plasmapharesis, intubation, corticosteroids, antimalarial and lopinavir/ritonavir | No significant improvement. | |
| 2 | NA | 47/M | Yes | Bilateral | No | Weakness of neck flexors and dysarthria | Protein: elevated; rest: normal | Fatigue, dyspnea, and cough | Generalized hyporeflexia, urinary retention, quadriparesis, low back pain | CT: ground‐glass opacities | Plasmapharesis, intubation, corticosteroids, antimalarial and lopinavir/ritonavir | Death | |||||
| 3 | NA | 58/M | Yes | NA | No | NA | Protein: elevated; rest: normal | Progressive dyspnea, dry cough, and dizziness | Muscle weakness, gait disturbance and areflexia. | CT: ground‐glass opacities | Plasmapharesis, IVIg, remdesivir, antimalarial, favipiravir and lopinavir/ritonavir | Death | |||||
| Oke et al. | USA | Case report | 1 | 1 | history of nephrolithiasis | 36/M | No | Right | No | Moderate severe (HB Grade 4) | NA | Fever and body aches | NA | Brain MRI: asymmetric enhancement of the right CN7 | Oral valacyclovir, corticosteroid, eye patch and artificial tears | Significant improvement | |
| Derollez et al. | France | Case report | 1 | 1 | Overweight | 57/F | NA | Left | No | NA | NA | Fatigue, myalgia, chills, and moderate cough | NA | Chest X‐ray: infiltrates | Ocular protection | CR | |
| Hasibi et al. | Iran | Case report | 1 | 1 | Class 1 obesity | 52/M | No | Right | No | Severe (HB Grade 5) | NA | Fever, malaise, dry cough, and anorexia | NA | CT: multiple bilateral peripheral ground glass opacities | Oral and corticosteroid, favipiravir, remdesivir, arbidol and NSAID | CR | |
| Taouihar et al | Morocco | Case report | 2 | 1 | DM, CML | 39/M | No | Right | Yes | Facial asymmetry, dysarthria, and difficulty chewing | NA | Dyspnea | NA | NA | Azithromycin, zinc, vitamin C, oral corticosteroid, preventive anticoagulation | CR of facial palsy | |
| 2 | DM, HTN | 57/M | No | Right | Yes | Dysarthria, facial asymmetry, swallowing disorder, and left‐sided deviation of mouth | NA | Dyspnea | NA | NA | Azithromycin, zinc, vitamin C, oral corticosteroid, and preventive anticoagulation | Significant improvement | |||||
| Kaplan et al. | USA | Case report | 1 | 1 | DM | 48/F | No | Left | No | Asymmetric forehead folds, dry eye, inability to raise the left eyebrow and left facial droop | NA | Fever, chills, headaches, fatigue, myalgia, and weakness | NA | CT: bilateral ground‐glass opacities | Oral corticosteroids, valacyclovir, and doxycycline | Significant improvement | |
| Kerstens et al. | Belgium | Case report | 1 | 1 | NA | 27/M | No | Bilateral | Yes | Severe (HB Grade 5) | IgG: elevated; rest: normal | Ageusia | None | MRI: bilateral CN7 contrast enhancement | Valaciclovir, artificial tears and oral corticosteroids | CR | |
| Kakumoto et al. | Japan | Case report | 1 | 1 | NA | 22/M | Yes | Bilateral | No | Dysarthria | Protein: elevated; rest: normal | Fever and dysphagia | Tetraparesis, hypesthesia of extremities, dysuria, inability to defecate, dyschezia, sinus arrythmia. | Head MRI: bilateral CN7 contrast enhancement | IVIG, intubated and managed on a ventilator | CR | |
| Al‐Mashdali et al. | Qatar | Case report | 1 | 1 | Atrial septal defect | 21/M | No | Right | No | NA | NA | Fever, cough, watery diarrhea, vomiting, conjunctivitis, and abdominal pain | Acute myocarditis | CT: Bilateral ground‐glass opacities and pleural effusion | IV corticosteroids and ocular lubricant | Significant improvement | |
| Judge et al. | USA | Case report | 1 | 1 | NA | 64/M | No | Bilateral | No | Dysarthria and subjective facial paresthesia | WBC: elevated; protein: elevated; Glucose: normal | Cough, fever, and chills | None | NA | NA | Progressive improvement | |
| Tran et al. | USA | Case report | 1 | 1 | DM | 42/M | Yes | Right | Yes | Right‐sided hypesthesia, dysarthria, diplopia, ptosis, and inability to raise eyebrows or smile | protein: elevated; WBC: normal; glucose: elevated | None | Right lower extremity weakness | Chest X‐ray: bibasilar infiltrates; CT: ground‐glass opacities | IV corticosteroids, electrolyte replacement for hypokalemia, IVIg, physical, occupational, and speech therapy | CR | |
| Silveira et al. | Brazil | Case report | 1 | 1 | DM, HTN | 65/M | No | Left | Yes | Facial asymmetry, otalgia, and ophthalmoplegia | NA | Fever, dry cough, and dyspnea | Clear left eye proptosis and blindness, otorrhea and complete hearing loss on the left and partial hearing on the right | Initial CT: erosion of the anterior wall of the left external ear conduct and left mandible condyle; brain MRI: compression of left CN 2, 3, 4 and 6 | IV Meropenem, IV vancomycin, IV Ciprofloxacin, and mastoidectomy | Death | |
| Liberatore et al. | Italy | Case report | 1 | 1 | HTN and history of testicular seminoma | 49/M | Yes | Left | No | NA | Glucose: normal; protein: slight elevation; WBC: normal | Fever, cough | Symmetric weakness in the upper limbs with flaccid tone, reduced tendon reflexes, and respiratory insufficiency; gastroparesis, alternating episodes of tachy‐/bradyarrhythmia, and frequent hypertensive crises) | Chest CT: multifocal ground‐glass opacities; Brain MRI: normal | Antimalarial, lopinavir/ritonavir, and ceftriaxone | NA | |
| Shinde et al. | India | Case report | 1 | 1 | HTN | 64/M | No | Right | Yes | Severe (HB Grade 5) | NA | None | Macular erythematous rash along zygomatic arch, maxillary and mandibular division of trigeminal nerve | Chest X‐ray: normal | Eye care, acyclovir, corticosteroid, and methyl cobalamin | PR | |
| Ochoa‐Fernández et al. | Spain | Case report | 1 | 1 | None | 6/F | No | Left | Yes | Moderate (HB Grade 3) | NA | None | None | NA | Eye protection and oral corticosteroids | CR | |
| Zain et al. | USA | Case report | 1 | 1 | None | 2/F | No | Right | Yes | Right lagophthalmos, ptosis, and drooping of corner of mouth, flattening of the nasiolabial fold, dryness of the eye and tearing | Glucose: normal; protein: normal; WBC: normal | None | EBV coinfection and contact dermatitis | Brain MRI: abnormal enhancement of the canalicular segment of right CN7 | IV corticosteroids | CR | |
| Ribeiro et al. | Brazil | Case report | 1 | 1 | None | 26/M | No | Right | No (on 8th day from first onset of symptoms) | Right facial weakness | NA | Cough and fever | None | Chest CT: multiple bilateral ground‐glass opacities and some superimposed intralobular septal thickening; Brain MRI: enhancement of the right CN7 | NA | NA | |
| González‐Castro et al. | Spain | Case series | 2 | 1 | Obesity | 40/F | No | Left | No (after 2nd day of ward admission) | Moderate (HB Grade 3) | NA | NA | None | MRI: poorly defined contrast uptake in the left hemifacial/malar subcutaneous region | High‐flow oxygen therapy | NA | |
| 2 | DM, smoker and Parkinson's disease patient | 65/M | No | Left | No | Moderate (HB Grade 3) | NA | NA | None | NA | High‐flow oxygen therapy | NA | |||||
| Pelea et al. | Germany | Case report | 1 | 1 | HTN, hypothereosis | 56/F | yes | Bilateral | No | Severe (HB Grade 5) | Protein: elevated; glucose: normal; WBC: elevated; SARS‐COV: negative | Dry cough, mild fever, and a general weakness | Tingling sensation in all fingertips and toes, flaccid tetraparesis, arreflexia and tachycardia | Chest CT: leaky infiltrates in the right lower lobe | IVIG and Plasmapharesis | PR | |
| Karimi‐Galougahi | Iran | Letter to the editor | 1 | 1 | None | 60/M | No | Right | No | Right‐sided facial nerve palsy, involving mouth, eye, and forehead | NA | Fever, cough, and dyspnea | None | Chest CT: ground‐glass opacitiesAbbr | Remdesivir, corticosteroid, and oxygen therapy | NA | |
Abbreviations: CR, complete recovery; CT, computed tomography; IVIg, intravenous immunoglobulin; MRI, magnetic resonance imaging; NA, not applicable; PR, partial recovery.
3.2. Symptom presentation
Noticeably, 30.14% of the patients were diagnosed with Guillain‐Barré Syndrome associated with COVID‐19. Facial palsy was observed as an initial symptom in 26/74 (35.14%) patients. In all, 22.97% of patients complained of bilateral facial paralysis, whereas ipsilateral paralysis was observed in 77.03%. Out of this 77.03% of patients, left (n = 29) and right (n = 28) sided involvement was observed in an almost equal number of patients. In all, 76.47% of bilateral facial paralysis patients also had GBS. Varying intensity of facial paralysis was seen among the 75 COVID‐19‐inflicted patients. While some experienced only mild facial deficit, weakness, or hypesthesia of the face, others complained of complete facial paralysis (n = 1). Most people complained of moderate‐severe facial dysfunction (n = 10) based on the House Brackmann scale. Among sufferers, Lagophthalmos, otalgia, facial drooping, dysarthria, and compromised forehead wrinkling were common complaints. Most patients witnessed mild to moderate COVID‐19‐specific symptoms. These included complaints of fever, fatigue, cough, ageusia, and headache.
3.3. Diagnostic results
Polymerase chain reaction (PCR), reverse transcriptase (RT)‐PCR, and serology were the most used tests to confirm COVID‐19 infection among 75 individuals. CSF analysis was performed in only half (48%) of the patients, 55.56% of whom had elevated CSF protein levels. CSF SARS‐CoV‐2 result was negative in all (100%), while approximately 1 in every 7 patients (13.5%) had elevated WBC levels on CSF report. Moreover, some patients underwent radiologic imaging to reach a diagnosis. Common findings on chest X‐ray, computer tomography (CT) thorax, and magnetic resonance imaging (MRI) brain included the presence of infiltrates/consolidations (50%), ground glass opacities (71.88%), and enhancement of CN 7 of the affected side (51.52%), respectively.
3.4. Treatment regimen and disease prognosis
Data on treatment plans were shared for 73 (97.33%) out of 75 patients. Although every individual had a treatment plan tailored according to his age, comorbidities, severity of the condition, availability of resources, and so forth, a handful of overlapping medications were prescribed too. Treatment regimen mainly included the use of corticosteroids (69.86%), antivirals (31.51%), IVIG (24.66%), antibiotics (17.81%), antiretroviral (12.33%), and antimalarial (10.96%) medications. Lopinavir/Ritonavir was the most readily prescribed antiretrovirals, whereas hydrochloroquine (100%) was the only antimalarial advised to patients. Three antivirals, namely, acyclovir, valacyclovir, and favipiravir, were predominantly administered to these patients. In total, 35.62% of patients (n = 26) adhered to a combination of antiviral‐corticosteroid‐based therapy. Furthermore, in some cases, eye care (19.18%) medications, for example, lubricants, artificial tears and watch bandages, and so forth, were also encouraged eye care. Physiotherapy (5.48%) and plasmapheresis (10.96%), though less common, were also a part of the treatment plan of some patients. The outcome of treatment was provided for 67 (89.33%) patients. Positive treatment outcomes were observed in 83.58% of cases. In contrast, 10 patients (14.93%) showed nonsignificant recovery, out of which 3 (4.48%) died from the disease.
4. DISCUSSION
Systematic reviews have been conducted in the past that discussed the association of facial palsy with COVID‐19. The reviews indirectly discussed facial palsy concerning COVID‐19 by establishing the correlation of COVID‐19 with GBS, while some only explored cases in which facial palsy was an isolated neurological finding. 8 , 61 , 62 However, none of them collectively assessed all the cases of facial palsy seconCarrillodary to COVID‐19. In our study, we could summate the vast amount of literature available by including all the cases of peripheral facial palsy secondary to COVID‐19 regardless of associated conditions. Thus, we were able to collate a stronger evidence base to support our findings concerning facial palsy and COVID‐19.
Cranial nerve involvement in GBS most commonly results in bilateral facial palsy, rarely unilateral involvement, and facial palsy mostly occurs in the early stage of the disease. 63 , 64 The findings of our study corroborate this observation. Of the 17 patients who demonstrated bilateral facial nerve palsy, 13 (76.5%) had accompanying GBS. Thus, cases of bilateral facial palsy are mostly attributed to GBS.
4.1. Pathophysiology
Pathomechanisms of nervous tissue involvement have been discussed in great detail in the literature. 65 , 66 , 67 , 68 , 69 , 70 , 71 Some of these mechanisms have been highlighted in Figure 2. In our study, unilateral facial palsy patients demonstrated right and left side involvement in almost equal proportions. This shows that SARS‐CoV‐2 has an equivalent predilection for right and left facial nerves.
Figure 2.
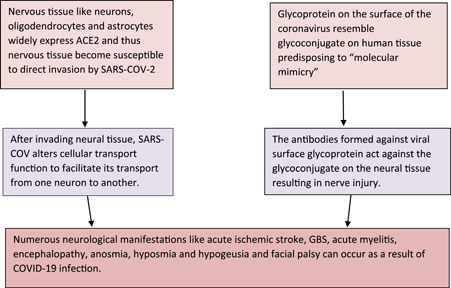
Pathophysiology behind the onset of facial palsy in COVID‐19 patients
In clinical practice, various other viruses have been observed to be associated with facial palsy as well, which include echovirus, enterovirus, herpes simplex virus, Epstein‐Barr virus, cytomegalovirus, human herpesvirus 6, human immunodeficiency virus, mumps, rubella, poliomyelitis, and varicella zoster virus. 72 , 73 , 74 Thus, this further corroborates that a virus like SARS‐CoV‐2 could be behind the etiopathogenesis of facial palsy.
Classically, facial palsy is known to show a predominance in females, which is evident in the prevalence studies conducted. 66 , 75 Moreover, a systematic review involving studies in which Bell's palsy was the only major neurological manifestation in COVID‐19 patients also showed a female preponderance. 8 However, our study demonstrated a slightly high male preponderance. This is because our study included a significantly high number (30.14%) of patients with accompanying GBS, and previous reviews evaluating the relationship between COVID‐19 and GBS have demonstrated a high male preponderance. 61 , 62 , 76 In addition, approximately 21% of the patients had hypertension, and 14.5% demonstrated diabetes mellitus. This finding is corroborated by a study conducted in Korea which demonstrated that facial palsy was associated with age, gender, smoking status, alcohol drinking, history of hypertension, stroke, CVD, diabetes mellitus, total cholesterol level in the blood, and hearing loss through a univariable analysis. 75
Furthermore, Paolino and colleagues 77 , 78 reported a greater frequency of arterial hypertension and lipid disorders in patients with Bell's palsy than in controls. Moreover, a study showed that the risk of Bell's palsy was increased in diabetes. 79 Also, patients with underlying comorbidities such as DM, obesity, hypertension, respiratory distress, or advanced age are at higher risk of developing COVID‐19. 80 Thus, all these factors contribute to the findings of our study.
The treatment regimen mainly involved corticosteroids (69.86%), and 35.62% of patients adhered to a combination of antiviral‐corticosteroid‐based therapy. This finding corroborates a meta‐analysis demonstrating significant benefits of treating Bell's palsy with corticosteroids. 81 Moreover, a network meta‐analysis showed that combined therapy remains the best regimen for a good recovery outcome, supporting its use by a significant 35.62% of patients. 82 However, only two patients used antivirals without corticosteroids to treat facial palsy. A systematic review supports this finding by demonstrating that corticosteroids alone were superior to antivirals alone in treating facial palsy. There was no clear benefit from antivirals alone over placebo. 83 Our findings show that the successful regimens in treating facial palsy due to other etiologies are also effective in treating facial palsy secondary to COVID‐19.
Positive treatment outcomes were observed in 83.58% of patients. This corroborates the effectiveness of the treatment regimens used in the case reports to treat facial palsy secondary to COVID‐19. A favorable response to treatment has also been shown in other complications that arise secondary to COVID‐19, such as central retinal vein occlusion. 7 However, some complications, such as hypoxic encephalopathy, have also shown a poor prognosis. 84 Thus, this highlights that many distinct complications can arise due to COVID‐19 with differing pathogenesis and severity.
There were some limitations in our study. Due to lack of provision of pertinent analytical data, no meta‐analysis could be conducted on the topic to confirm the relationship between COVID‐19 and facial palsy. Our review only comprised of case reports/series in which a limited number of patients were assessed. Therefore, large‐scale studies with more patients and longer follow‐ups are warranted to reliably draw the correlation between COVID‐19 and facial palsy. Moreover, studies in languages other than English were excluded from the analyses. Lastly, adequate representation of most countries was not seen in our review, which implies that many cases went unreported there, so they could not be included in our analyses. Despite the limitations, we tried to include all relevant cases to date and demonstrated an in‐depth comparison of clinical, radiological, and diagnostic features of COVID‐19 and concomitant facial palsy in our patient‐level analyses.
5. CONCLUSION
To the best of our knowledge, this is the most updated review of facial palsy cases following COVID‐19 infection. Although our patient‐level systematic review successfully collated published accounts of facial palsy cases post COVID‐19 infection while theorizing the pathophysiology behind COVID‐19 and subsequent onset of facial palsy, the likelihood of the association being purely coincidental cannot be overlooked. Therefore, large‐scale studies are still warranted to thoroughly understand the association between COVID‐19 and concomitant facial palsy. Systematic reviews involving studies with large sample sizes, such as retrospective cohorts, should be conducted, generating a large patient pool for analyses. This would allow us to develop a clearer understanding of patient characteristics and devise more effective treatment regimens that cater to the needs of individual patients.
AUTHOR CONTRIBUTIONS
Aiman Khurshid: Conceptualization, methodology, writing – original draft preparation, writing – review & editing. Maman Khurshid, Aruba Sohail, Imran Mansoor Raza, Muhammad Khubab Ahsan, Mir Umer Farooq Alam Shah, Anab Rehan Taseer, Abdulqadir J. Nashwan, Irfan Ullah: Data curation, writing – review & editing. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
Abdulqadir J. Nashwan is an Editorial Board member of Health Science Reports and co‐author of this article. He is excluded from editorial decision‐making related to the acceptance of this article for publication in the journal. The remaining authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead author Abdulqadir J. Nashwan affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
Open Access funding was provided by the Qatar National Library.
Khurshid A, Khurshid M, Sohail A, et al. Facial palsy as a manifestation of COVID‐19: A systematic review of cases. Health Sci Rep. 2022;5:e887. 10.1002/hsr2.887
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article and its Supporting Information.
REFERENCES
- 1. World Health Organization . Archived: WHO Timeline – COVID‐19. 2020. https://www.who.int/news/item/27-04-2020-who-timeline---covid-19
- 2.WHO Coronavirus (COVID‐19) Dashboard. WHO coronavirus (COVID‐19) Dashboard with Vaccination Data. World Health Organization; 2022. https://covid19.who.int/
- 3. Tsang HF, Chan LWC, Cho WCS, et al. An update on COVID‐19 pandemic: the epidemiology, pathogenesis, prevention and treatment strategies. Expert Rev Anti Infect Ther. 2021;19(7):877‐888. https://www.tandfonline.com/action/journalInformation?journalCode=ierz20 [DOI] [PubMed] [Google Scholar]
- 4.Respiratory management of COVID 19 – physiopedia. Physiopedia. Accessed March 1, 2022. https://www.physio-pedia.com/Respiratory_Management_of_COVID_19
- 5. Soy M, Atagündüz P, Atagündüz I, Sucak GT. Hemophagocytic lymphohistiocytosis: a review inspired by the COVID‐19 pandemic. Rheumatol Int. 2020;41(1):7‐18. https://pubmed.ncbi.nlm.nih.gov/32588191/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wong K, Shah MUFA, Khurshid M, Ullah I, Tahir MJ, Yousaf Z. COVID‐19 associated vasculitis: a systematic review of case reports and case series. Ann Med Surg. 2022;74:103249. https://pubmed.ncbi.nlm.nih.gov/35039779/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ullah I, Sohail A, Shah MUFA, et al. Central retinal vein occlusion in patients with COVID‐19 infection: a systematic review. Ann Med Surg. 2021;71:102898. https://pubmed.ncbi.nlm.nih.gov/34659743/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gupta S, Jawanda MK, Taneja N, Taneja T. A systematic review of Bell's Palsy as the only major neurological manifestation in COVID‐19 patients. J Clin Neurosci. 2021;90:284‐292. [DOI] [PubMed] [Google Scholar]
- 9. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 10. Systematic Reviews Registry . PROSPERO. 2022. https://www.crd.york.ac.uk/PROSPERO/#recordDetails
- 11. Critical Appraisal Tools . Joanna Briggs Institute. https://jbi.global/critical-appraisal-tools
- 12. Homma Y, Watanabe M, Inoue K, Moritaka T. Coronavirus Disease‐19 pneumonia with facial nerve palsy and olfactory disturbance. Intern Med. 2020;59(14):1773‐1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goh Y, Beh DLL, Makmur A, Somani J, Chan ACY. Pearls & oysters: facial nerve palsy in COVID‐19 infection. Neurology. 2020;95(8):364‐367. [DOI] [PubMed] [Google Scholar]
- 14. Figueiredo R, Falcão V, Pinto MJ, Ramalho C. Case report: peripheral facial paralysis as presenting symptom of COVID‐19 in a pregnant woman. BMJ Case Rep. 2020;13(8):237146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Juliao Caamaño DS, Alonso Beato R. Facial diplegia, a possible atypical variant of Guillain‐Barré syndrome as a rare neurological complication of SARS‐CoV‐2. J Clin Neurosci. 2020;77:230‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cabrera Muras A, Carmona‐Abellán MM, Collía Fernández A, Uterga Valiente JM, Antón Méndez L, García‐Moncó JC. Bilateral facial nerve palsy associated with COVID‐19 and Epstein‐Barr virus co‐infection. Eur J Neurol. 2021;28:358‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khaja M, Gomez GPR, Santana Y, et al. A 44‐year‐old hispanic man with loss of taste and bilateral facial weakness diagnosed with Guillain‐Barré syndrome and Bell's Palsy associated with SARS‐CoV‐2 infection treated with intravenous immunoglobulin. Am J Case Rep. 2020;21:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sancho‐Saldaña A, Lambea‐Gil Á, Liesa JLC, et al. Guillain–Barré syndrome associated with leptomeningeal enhancement following SARS‐CoV‐2 infection. Clin Med (Northfield Il). 2020;20(4):e93‐e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Theophanous C, Santoro JD, Itani R. Bell's palsy in a pediatric patient with hyper IgM syndrome and severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Brain Dev. 2021;43(2):359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dahl EH, Mosevoll KA, Cramariuc D, Vedeler CA, Blomberg B. COVID‐19 myocarditis and postinfection Bell's palsy. BMJ Case Rep. 2021;14(1):e2400955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Engström K, Saber A. Kranialnervspareser uppstod hos Iva‐vårdad covid‐19‐patient. Lakartidningen. 2021;118:21079. https://lakartidningen.se/klinik-och-vetenskap-1/artiklar-1/fallbeskrivning/2021/07/kranialnervspareser-uppstod-hos-iva-vardad-covid-19-patient/ [PubMed] [Google Scholar]
- 22. Chan JL, Ebadi H, Sarna JR. Guillain‐Barré syndrome with facial diplegia related to SARS‐CoV‐2 infection. Can J Neurol Sci. 2020;47(6):852‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Decio A, Mazza A, Quadri V, et al. Neurological manifestations of COVID‐19 in children: a case of facial nerve palsy. Pediatr Neurol. 2021;116:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ozer F, Alkan O. Simultaneous sudden hearing loss and peripheral facial paralysis in a patient with Covid‐19. Ear Nose Throat J. 2021:014556132110280. [DOI] [PubMed]
- 25. Mackenzie N, Lopez‐Coronel E, Dau A, et al. Concomitant Guillain‐Barré syndrome with COVID‐19: a case report. BMC Neurol. 2021;21:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bastola A, Sah R, Nepal G, et al. Bell's palsy as a possible neurological complication of COVID‐19: a case report. Clin Case Rep. 2021;9(2):747‐750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hookham L, Teoh P, Stern W, Goodman AL. Can PIMS‐TS lead to a facial nerve palsy? BMJ Case Rep. 2021;14(6):e242887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kumar V, Narayanan P, Shetty S, Mohammed AP. Lower motor neuron facial palsy in a postnatal mother with COVID‐19. BMJ Case Rep. 2021;14(3):e240267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aasfara J, Hajjij A, Bensouda H, Ouhabi H, Benariba F. A unique association of bifacial weakness, paresthesia and vestibulocochlear neuritis as post‐COVID‐19 manifestation in pregnant women: a case report. Pan Afr Med J. 2021;38:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Paybast S, Gorji R, Mavandadi S. Guillain‐Barré syndrome as a neurological complication of novel COVID‐19 infection: a case report and review of the literature. Neurologist. 2020;25(4):101‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ottaviani D, Boso F, Tranquillini E, et al. Early Guillain‐Barré syndrome in coronavirus disease 2019 (COVID‐19): a case report from an Italian COVID‐hospital. Neurol Sci. 2020;41:1351‐1354. 10.1007/s10072-020-04449-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Casas E, Barbosa A, Rubio‐García E, et al. [Isolated peripheral facial paralysis in a patient with COVID‐19]. Rev Neurol. 2020;71(1):40‐41. [DOI] [PubMed] [Google Scholar]
- 33. Ribeiro BN, de F, Marchiori E. Facial palsy as a neurological complication of SARS‐CoV‐2. Arq Neuropsiquiatr. 2020;78(10):667. [DOI] [PubMed] [Google Scholar]
- 34. Hutchins KL, Jansen JH, Comer AD, et al. COVID‐19–Associated bifacial weakness with paresthesia subtype of Guillain‐Barré syndrome. AJNR Am J Neuroradiol. 2020;41(9):1707‐1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Karimi‐Galougahi M, Yousefi‐Koma A, Raygani N, Bakhshayeshkaram M, Haseli S. 18FDG‐PET/CT assessment of COVID‐19‐induced Bell's Palsy. Acad Radiol. 2021;28(1):144‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oke IO, Oladunjoye OO, Oladunjoye AO, Paudel A, Zimmerman R. Bell's palsy as a late neurologic manifestation of COVID‐19 infection. Cureus. 2021;13(3):e13881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Derollez C, Alberto T, Leroi I, Mackowiak MA, Chen Y. Facial nerve palsy: an atypical clinical manifestation of COVID‐19 infection in a family cluster. Eur J Neurol. 2020;27(12):2670‐2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hasibi M, Ahadi MS, Abdollahi H, Jafari M. Protracted COVID‐19 during treatment of facial palsy. Case Rep Neurol Med. 2021;2021:5569841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zain S, Petropoulou K, Mirchia K, Hussien A, Mirchia K. COVID‐19 as a rare cause of facial nerve neuritis in a pediatric patient. Radiol Case Rep. 2021;16(6):1400‐1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Taouihar S, Bouabdallaoui A, Aabdi M, et al. Peripheral facial paralysis as the only symptom revealing sars CoV 2 infection: case report. Ann Med Surg. 2021;68:102550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kerstens J, Deschuytere L, Schotsmans K, Maréchal E. Bilateral peripheral facial palsy following asymptomatic COVID‐19 infection: a case report. Acta Neurol Belg. 2021;121(3):815‐816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. GO‐F E, Víllora‐Morcillo N, Taboas‐Pereira MA. [Peripheral facial palsy in a paediatric patient with no risk factors within the context of infection by SARS‐CoV‐2]. Rev Neurol. 2021;72(5):177‐178. [DOI] [PubMed] [Google Scholar]
- 43. Kakumoto T, Kobayashi S, Yuuki H, et al. Cranial nerve involvement and dysautonomia in post‐COVID‐19 Guillain‐Barré syndrome. Intern Med. 2021;60(21):3477‐3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pelea T, Reuter U, Schmidt C, Laubinger R, Siegmund R, Walther BW. SARS‐CoV‐2 associated Guillain–Barré syndrome. J Neurol. 2021;268(4):1191‐1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shinde KJ, Karanth T, Yeolekar AM. Otoneurological presentations of COVID‐19. BMJ Case Rep. 2021;14(9):e241893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Al‐Mashdali AF, Al‐Samawi MS. A case of post COVID‐19 multisystem inflammatory syndrome and Bell's palsy in a young adult. Clin Case Rep. 2021;9(9):e04801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Judge C, Moheb N, Apolo RC, Dupont JL, Gessner ML, Yacoub HA. Facial diplegia as a rare late neurologic manifestation of SARS‐CoV‐2 infection. J Neurol Res. 2020;10(6):236‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tran C, Drury B, Yuen H‐W, Rosenthal J, Neeki MM. Miller‐Fisher syndrome presenting as facial diplegia with COVID‐19 co‐Infection. Cureus. 2021;13(8):e17060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Silveira RQ, Carvalho VT, Cavalcanti HN, et al. Multiple cranial nerve palsies in malignant external otitis: a rare presentation of a rare condition. IDCases. 2020;22:e00945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liberatore G, De Santis T, Doneddu PE, Gentile F, Albanese A, Nobile‐Orazio E. Clinical reasoning: a case of COVID‐19‐associated pharyngeal‐cervical‐brachial variant of Guillain‐Barré syndrome. Neurology. 2020;95(21):978‐983. [DOI] [PubMed] [Google Scholar]
- 51. Lima MA, Silva MTT, Soares CN, et al. Peripheral facial nerve palsy associated with COVID‐19. J Neurovirol. 2020;26(6):941‐944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Manganotti P, Bellavita G, D'acunto L, et al. Clinical neurophysiology and cerebrospinal liquor analysis to detect Guillain‐Barré syndrome and polyneuritis cranialis in COVID‐19 patients: a case series. J Med Virol. 2021;93:766‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Egilmez OK, Gündoğan ME, Yılmaz MS, Güven M. Can COVID‐19 cause peripheral facial nerve palsy? SN Compr Clin Med. 2021;3(8):1707‐1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Corrêa DG, Hygino da Cruz LC Jr., Lopes FCR, et al. Magnetic resonance imaging features of COVID‐19‐related cranial nerve lesions. J Neurovirol. 2021;27(1):171‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Neo WL, Ng JCF, Iyer NG. The great pretender—Bell's palsy secondary to SARS‐CoV‐2? Clin Case Rep. 2021;9(3):1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Khedr EM, Shoyb A, Mohamed KO, Karim AA, Saber M. Case report: Guillain–Barré syndrome associated with COVID‐19. Front Neurol. 2021;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. González‐Castro A, Rodríguez‐Rodríguez E, Arnaiz F, Ferrer‐Pargada D. [Peripheral facial paralysis in patients with SARS‐CoV‐2 in prono position]. Rev Neurol. 2021;72(8):296‐297. [DOI] [PubMed] [Google Scholar]
- 58. Abolmaali M, Heidari M, Zeinali M, et al. Guillain–Barré syndrome as a parainfectious manifestation of SARS‐CoV‐2 infection: a case series. J Clin Neurosci. 2021;83:119‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kaplan AC. Noteworthy neurological manifestations associated with COVID‐19 infection. Cureus. 2021;13(4):e14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shea BJ, Reeves BC, Wells G, et al Amstar 2: a critical appraisal tool for systematic reviews that include randomised or non‐randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Carrillo‐Larco RM, Altez‐Fernandez C, Ravaglia S, Vizcarra JA. COVID‐19 and Guillain‐Barré syndrome: a systematic review of case reports. Wellcome Open Res. 2020;5:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Uncini A, Vallat J‐M, Jacobs BC. Guillain‐Barré syndrome in SARS‐CoV‐2 infection: an instant systematic review of the first six months of pandemic. J Neurol Neurosurg Psychiatry. 2020;91(10):1105‐1110. https://jnnp.bmj.com/content/91/10/1105 [DOI] [PubMed] [Google Scholar]
- 63. Bhargava A, Banakar BF, Pujar GS, Khichar S. A study of Guillain‐Barré syndrome with reference to cranial neuropathy and its prognostic implication. J Neurosci Rural Pract. 2014;5(Suppl 1):S43‐S47. 10.4103/0976-3147.145200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ropper AH. The Guillain‐Barré syndrome. N Engl J Med. 1992;326:1130‐1136. [DOI] [PubMed] [Google Scholar]
- 65. Paliwal VK, Garg RK, Gupta A, Tejan N. Neuromuscular presentations in patients with COVID‐19. Neurol Sci. 2020;41(11):3039–3056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mustafa AHK, Sulaiman AM. The epidemiology and management of Bell's Palsy in the Sudan. Open Dent J. 2018;12:827‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280.e8. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Conde Cardona G, Quintana Pájaro LD, Quintero Marzola ID, Ramos Villegas Y, Moscote Salazar LR. Neurotropism of SARS‐CoV 2: mechanisms and manifestations. J Neurol Sci. 2020;412:116824. 10.1016/j.jns.2020.116824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bohmwald K, Gálvez NMS, Ríos M, Kalergis AM. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 2018;12:386. 10.3389/fncel.2018.00386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dubé M, Le Coupanec A, Wong AHM, Rini JM, Desforges M, Talbot PJ. Axonal transport enables neuron‐to‐neuron propagation of human coronavirus OC43. J Virol. 2018;92(17):e00404‐e00418. 10.1128/JVI.00404-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sedaghat Z, Karimi N. Guillain‐Barré syndrome associated with COVID‐19 infection: a case report. J Clin Neurosci. 2020;76:233‐235. 10.1016/j.jocn.2020.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Song K, Chang S, Lee J, Shin SA, Lee HY. Clinical characteristics of dizziness associated with acute peripheral facial palsy. J Audiol Otol. 2018;22(3):148‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rath B, Gidudu JF, Anyoti H, et al. All that palsies is not Bell's ‐the need to define Bell's palsy as an adverse event following immunization. Vaccine. 2007;26(1):1‐14. [DOI] [PubMed] [Google Scholar]
- 74. Zimmermann J, Jesse S, Kassubek J, Pinkhardt E, Ludolph AC. Differential diagnosis of peripheral facial nerve palsy: a retrospective clinical, MRI and CSF‐based study. J Neurol. 2019;266(10):2488‐2494. [DOI] [PubMed] [Google Scholar]
- 75. Chang Y‐S, Choi JE, Kim SW, Baek S‐Y, Cho Y‐S. Prevalence and associated factors of facial palsy and lifestyle characteristics: data from the Korean National Health and Nutrition Examination Survey 2010–2012. BMJ Open. 2016;6(11):e012628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sheikh AB, Chourasia PK, Javed N, et al. Association of Guillain‐Barré syndrome with COVID‐19 infection: an updated systematic review. J Neuroimmunol. 2021;355:577577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Katusic SK, Beard CM, Wiederholt WC, Bergstralh EJ, Kurland LT. Incidence, clinical features, and prognosis in Bell's palsy, Rochester, Minnesota, 1968‐1982. Ann Neurol. 1986;20(5):622‐627. 10.1002/ana.410200511 [DOI] [PubMed] [Google Scholar]
- 78. Paolino E, Granieri E, Tola MR, Panarelli MA, Carreras M. Predisposing factors in Bell's palsy: a case‐control study. J Neurol. 1985;232(6):363‐365. 10.1007/BF00313837 [DOI] [PubMed] [Google Scholar]
- 79. Yazdi AR, Vashegani A, Sadeghi M, Sadr‐Hoseini A, Sazgar A. Bell's palsy and diabetes mellitus in Iranian population. Acta Med Iran. 2008;46(4):333‐336. [Google Scholar]
- 80. Wolff D, Nee S, Hickey NS, Marschollek M. Risk factors for Covid‐19 severity and fatality: a structured literature review. Infection. 2020;49:1‐14. 10.1007/s15010-020-01509-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Madhok VB, Gagyor I, Daly F, et al. Corticosteroids for Bell's palsy (idiopathic facial paralysis). Cochrane Database Syst Rev. 2016;7(7):CD001942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jalali MM, Soleimani R, Soltanipour S, Jalali SM. Pharmacological treatments of bell's palsy in adults: A systematic review and network meta‐analysis. Laryngoscope. 2021;131(7):1615‐1625. [DOI] [PubMed] [Google Scholar]
- 83. Gagyor I, Madhok VB, Daly F, Sullivan F. Antiviral treatment for Bell's palsy (idiopathic facial paralysis). Cochrane Database Syst Rev. 2019;9(9):001869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nepal G, Rehrig JH, Shrestha GS, et al. Neurological manifestations of COVID‐19: a systematic review. Crit Care. 2020;24:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supporting Information.


