Abstract
The prevalence of chronic HBV infection differs globally and continues to be a substantial public health burden accounting for 30% of all deaths from cirrhosis and 40% of all deaths from hepatocellular carcinoma. The WHO developed guidelines in 2015 on prevention, care and treatment of chronic HBV infection targeted to country program managers in all health care settings particularly in low- and middle-income countries (LMICs). Because these guidelines were developed to target LMICs, several of the recommendations differ from those of the major Liver Societies, the American Association for the Study of Liver Diseases (AASLD), Asian Pacific Association for the Study of Liver Diseases (APASL) and the European Association for the Study of Liver Diseases (EASL). This review will highlight key differences between the AASLD and WHO guidelines and discuss the impact on management of chronic hepatitis B.
Keywords: Chronic hepatitis B virus infection, WHO guidelines, AASLD guidelines
Introduction
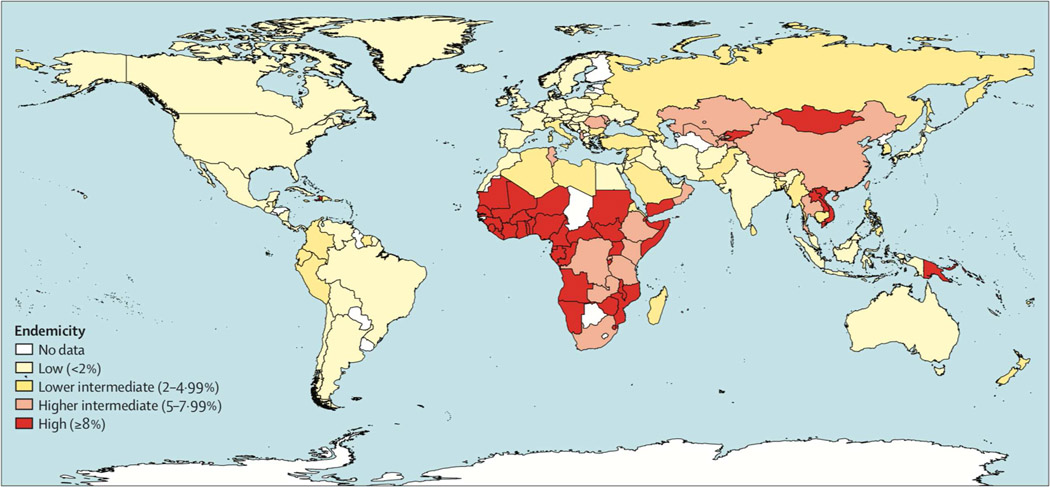
Over 2 billion people have been exposed to the hepatitis B virus (HBV) of whom an estimated 257 million people have chronic infection (1). Chronic HBV infection occurs globally but the seroprevalence of hepatitis B surface antigen (HBsAg), the marker of chronicity, varies geographically with the highest rates noted in sub-Saharan Africa and East Asia and the lowest rates are noted in North America, Western Europe and Australia, figure 1(2). Despite the availability of a safe and effective vaccine and antiviral therapy, chronic HBV infection continues to be a substantial public health burden accounting for 30% of all deaths from cirrhosis and 40% of all deaths related to hepatocellular carcinoma globally (3) As a consequence, in 2016 the World Health Organization (WHO) issued a guidance to eliminate chronic viral hepatitis as a public health problem by 2030, by reducing the incidence of chronic HBV infection by 90% and mortality by 65% (4). As part of this strategy, the WHO developed guidelines to provide evidence-based advice for the prevention, care and treatment of persons affected by chronic HBV infection (5). A driving force for development of these guidelines was to provide a global consensus on the principles of hepatitis B prevention, care and treatment, to country program managers in all health care settings particularly in low- and middle-income countries (LMICs). Because these guidelines were developed to target LMICs, several of the recommendations differ from those of the major Liver Societies, the American Association for the Study of Liver Diseases (AASLD), Asian Pacific Association for the Study of Liver Diseases (APASL) and the European Association for the Study of Liver Diseases (EASL). This review will highlight key differences between the AASLD and WHO guidelines and discuss the impact on management of chronic hepatitis B (6, 7).
Figure 1: Global Estimates of the Prevalence of HBsAg.
HBsAg Hepatitis B surface antigen. From Schweitzer A, Horn J, Mikolajczyk RT. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015 Oct 17;386:1546–55, with permission.
Prevention and Screening
Screening
Screening for chronic HBV infection is performed by testing for serum HBsAg. The presence of HBsAg for a period of at least 6 months defines chronic HBV infection. Anti-HBs should be included in screening so that unexposed persons can be identified and offered HBV vaccination. AASLD guidelines recommend screening of persons born in regions of high or intermediate HBV endemicity (HBsAg prevalence of ≥2%), US born persons not vaccinated as an infant whose parents were born in regions with HBV endemicity ≥8%), pregnant women, people needing immunosuppressive medications including chemotherapy, blood donors and patients with end stage renal disease. WHO recommends universal screening in countries with a HBsAg seroprevalence ≥ 2%. They also recommend routine testing of all pregnant women presenting to antenatal clinics and high-risk groups including sexual and household contacts of persons with chronic HBV infection, HIV infected persons, persons who infect drugs, men who have sex with men, sex workers, indigenous peoples, persons who are incarcerated and transgender persons. Blood and organ donors should be screened for HBsAg and adults presenting with signs and symptoms suspicious of viral infection. A complete list of groups at high risk for HBV infection and who should be screened is provided in Table 1.
Table 1:
Comparison of Recommendations for Screening of Patients At Risk for Acquiring HBV (Differences between the AASLD and WHO guidelines are in italics)
| WHO | AASLD |
|---|---|
| • Household and sexual contacts of persons with HBV • Persons infected with HIV • Persons who inject drugs • Men who have sex with men • Persons who are incarcerated • Blood and organ donors • Pregnant women • General population screening in countries with high HBV endemicity |
• Persons born in regions of high or intermediate HBV endemicity (HBsAg prevalence of ≥2%)
• US born persons not vaccinated as an infant whose parents were born in regions with HBV endemicity ≥8%) • Men who have sex with men • Intravenous drug users • Individuals needing immunosuppressive therapy, including chemotherapy, immunosuppression related to transplantation, and immunosuppression for various disorders • Persons with elevated ALT or AST of unknown etiology • Organ, plasma, blood, tissue or semen donors • End stage renal disease patients needing dialysis • All pregnant women and infants born to HBsAg mothers • Persons infected with Hepatitis C and HIV • Household and sexual contacts of HBsAg positive persons • Persons requesting evaluation/treatment of sexually transmitted disease or have multiple sexual partners • Health care, public safety workers and staff of facilities for developmentally disabled persons • Persons travelling to countries with intermediate or high HBV prevalence • Inmates of correctional facilities • Unvaccinated persons with diabetes with age of 19–59 years. |
Primary Prevention
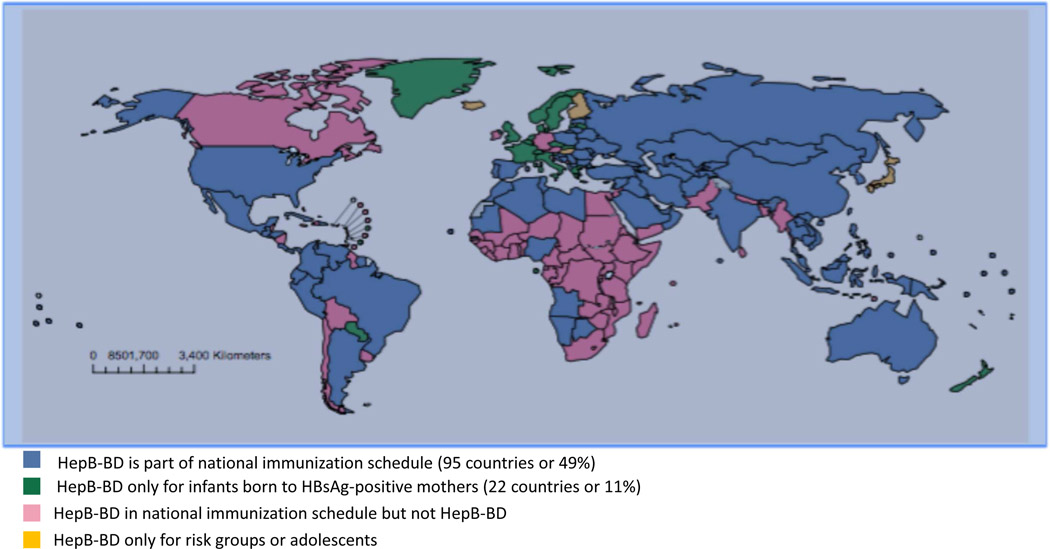
HBV is transmitted primarily through parental exposure. In endemic countries, vertical and perinatal transmission are the most important causes of chronic infection and efforts to interrupt this mode of transmission will have the greatest impact on reducing the incidence and prevalence of chronic HBV infection (8). Primary prevention of HBV infection is achieved through HBV vaccination. HBV vaccination is 90–95% effective in preventing HBV infection and transmission. Implementation of universal HBV infant vaccination programs has resulted in a dramatic decline in the incidence and prevalence of HBV and hepatocellular carcinoma (HCC) in children (9, 10). Therefore, in 2009, the WHO recommended that all countries, even those with low HBV prevalence, introduce universal hepatitis B birth dose (HepB-BD) vaccination, whereby the first dose of hepatitis B vaccine should be given as soon as possible (<24 hours) after birth, even in low birth weight infants and low-endemicity countries. Despite WHO recommendations, in 2014 only 96 (49%) out of 194 countries reported offering HepB-BD as part of their national immunization program and less than 38% of babies born worldwide received HepB-BD within 24 hours after birth, Figure 2.
Figure 2: Countries Providing Hepatitis B Birth Dose (HepB-BD) in 2014.
In 2014 only, half of all countries globally had adopted HepB-BD as part of their national immunization program and less than 38% of babies born worldwide received HepB-BD within the recommended time frame. Adapted from Schweitzer A, Horn J, Mikolajczyk RT. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015 Oct 17;386:1546–55, with permission.
The WHO released updated its recommendations for the use of HBV vaccine in 2017 (11). AASLD recommendations mirror those of the Center for Disease Control and Prevention (CDC) guidelines, released in 2012 (12). WHO recommends a three to four dose schedule with doses separated by 4 weeks. At this time, there is no data supporting the administration of booster doses after the completion of vaccination series. Vaccination given to premature and low birth infants (birth weight <2000g) does not count towards the vaccination series and these infants should receive 3 additional doses to complete the vaccination schedule.
Catch up Vaccination
Catch up vaccination of individuals who are not immune to hepatitis B hastens the development of population-based immunity, thereby decreasing the incidence of HBV. Immunity to hepatitis B can be determined by measurement of HBsAg and anti-HBs titers. WHO and AASLD guidelines both recommend vaccination of high-risk groups including household and sexual contacts of HBsAg positive patients, healthcare workers, persons with multiple sex partners and men who have sex with men. Recently, HEPLISAV-B, a two dose vaccination series given at 0 and 1 months, was approved for use in adults in the United States(13). AASLD guidelines also recommend vaccination of patients who are negative for anti-HBs in the following groups: people injecting intravenous drugs, patients with hepatitis C virus and HIV coinfection, people with elevated ALT and AST levels of unknown etiology, patients seeking evaluation or treatment of sexually transmitted diseases, patients with diabetes mellitus aged 19–59 years, prisoners, people traveling to high HBV endemic countries, and residents and staff of facilities for developmentally disabled people.
Post-Exposure Prophylaxis
AASLD guidelines recommend that infants born to HBsAg positive mothers should receive immunoprophylaxis with the combination of hepatitis B immune globulin (HBIg)(14, 15) along with HBV vaccine within 24 hours of delivery, regardless of the mother’s HBeAg status. The vaccine is given as a 3-dose series at 0, 1 and 6 months or as a 4-dose schedule administered at 0, 7, 21–30 days followed by a dose at 12 months if combined with the hepatitis A vaccine. AASLD also recommends that infants born to HBsAg positive mothers should undergo post-vaccination serologic testing at 9 to 15 months of age to determine the response to vaccine series. Non-responders to the initial vaccine series should receive a repeat 3-dose vaccination series. In contrast, WHO guidelines only recommends the combination of HBIg with HBV vaccination to infants born to mothers who are both HBsAg and HBeAg positive. HBV vaccination only is recommended in children of HBsAg positive, HBeAg negative mothers, due to concerns for low supply and high cost of HBIg. This is a reasonable recommendation in LMICs given that most vaccine failures occur when the maternal viral load is >107 IU/mL and these viral levels would be uncommon in a HBeAg negative mother.
Mother-to-Child Transmission
In countries with high endemicity, the most common mode of HBV transmission is mother-to-child transmission, usually from exposure to maternal blood and body fluids at the time of delivery (16). Transmission of HBV occurring early in life carries a much higher risk of developing chronic infection; therefore, measures to prevent mother to child transmission will have the greatest impact on reducing the burden if chronic infection. Both WHO and AASLD guidelines recommend treating HBsAg positive pregnant women if they meet the standard criteria for initiation of antiviral treatment. Breastfeeding is not contraindicated in HBV-infected mothers on antiviral therapy. There are however, some major differences between the two guidelines regarding recommendations to prevent mother-to-child HBV transmission. AASLD recommends administration of antiviral therapy (tenofovir is preferred) in the third trimester of pregnancy to women with serum HBV DNA levels >200,000 IU/ml until 4 weeks postpartum. This is based on the results of two recent randomized controlled trials comparing tenofovir with no antiviral treatment in the third trimester, which demonstrated a significant reduction in risk of mother-to-child transmission of HBV in highly viremic mothers (17, 18). WHO has not made any recommendations on the use of antiviral therapy in the third trimester to prevent mother-to-child transmission. The WHO guidelines cited the lack of evidence on the effectiveness and safety of antiviral therapy at the time of writing of the guidelines as the reason for not recommending antiviral therapy. Given the availability of updated data, the complete absence of mother-to-child transmission in women receiving antiviral therapy, the fact that therapy is administered for only short duration, and the low rates of HepB-BD vaccine in LMICs, updating the WHO guidance would have a great impact on reducing the prevalence of chronic HBV infection. Both treated and untreated HBsAg positive pregnant women should be monitored for at least 6 months postpartum for hepatitis flares and seroconversion (19, 20). AASLD suggests that HBV-infected pregnant patients with cirrhosis should be managed in a tertiary care center where high risk obstetric services are readily available. This recommendation is unlikely to be adopted by the WHO given the limited availability of these resources in LMICs. A comparison of recommendations for prevention of HBV is provided in Table 2.
Table 2:
Comparison of Recommendations for Prevention of Hepatitis B
| WHO | AASLD | |
|---|---|---|
| Primary prevention | Universal HBV vaccination to all infants | Universal HBV vaccination to all infants |
| Catch up vaccination | HBV vaccination of non-immune high-risk groups | HBV vaccination of non-immune high-risk groups |
| Prevention of mother to child transmission | HBIG + HBV vaccination to all infants born to HBsAg/HBeAg positive mothers HBV vaccination to all infants born to HBsAg positive/HBeAg negative mothers Antiviral therapy not recommended for highly viremic mothers |
HBIG + HBV vaccination within 12 hours of birth to all infants born to HBsAg positive mothers Antiviral therapy (tenofovir) initiated at 28 to 32 weeks of gestation for pregnant women with serum HBV DNA levels >200,000 IU/ml until 4 weeks postpartum |
Treatment
Who to Treat?
The outcome of chronic HBV infection is variable ranging from mild fibrosis to cirrhosis and decompensated liver disease. It is estimated that 20% to 30% of those with chronic HBV infection will be at risk for progressive liver fibrosis leading to cirrhosis and an increased risk of HCC. However, most patients will not require antiviral therapy. The primary goal of antiviral therapy is to suppress HBV DNA levels to undetectable, as this endpoint is associated with improvement in liver inflammation and fibrosis and reversal of cirrhosis, a lower risk of HCC and reduced liver-related mortality (21). However, none of the currently available therapies is curative and once treatment is started it must usually be continued lifelong. Therefore, treatment guidelines emphasize the importance of careful patient selection, initiating therapy only in those would derive the greatest benefit. Identifying which patients benefit the most from therapy is challenging. The pre-treatment assessment is based on the level of viral replication (HBeAg status and HBV DNA level), degree of inflammation (serum ALT level) and stage of disease (non-invasive test of fibrosis or liver biopsy) and additional factors such as a family history of cirrhosis and HCC, age and presence of comorbid medical conditions.
There is general consensus between the AASLD and WHO guidelines that all patients with compensated or decompensated cirrhosis require treatment independent of HBeAg status, HBV DNA and ALT levels (22). The diagnosis of cirrhosis can be made clinically by the presence of hepatosplenomegaly, reversal of AST/ALT ratio and low albumin level and low platelet count or through the use of non-invasive tests of liver fibrosis. Alternatively, more accurate tests such as liver biopsy or vibration controlled transient elastography may be used to diagnose cirrhosis, if available. The WHO recommends using an APRI cut-off of >2.0 or elevated vibration controlled elastography reading, if available, to start treatment. Tests such as vibration controlled transient elastography and liver biopsy are not widely available in LMICs and pose a challenge to accurately diagnosing cirrhosis.
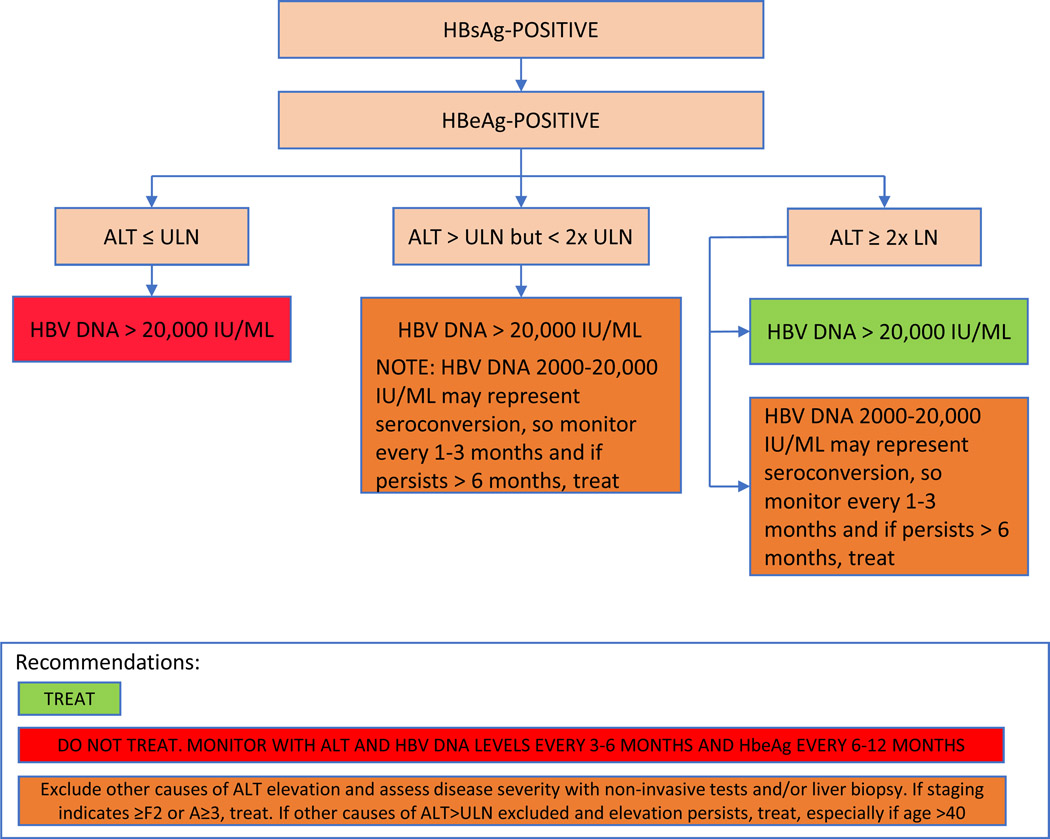
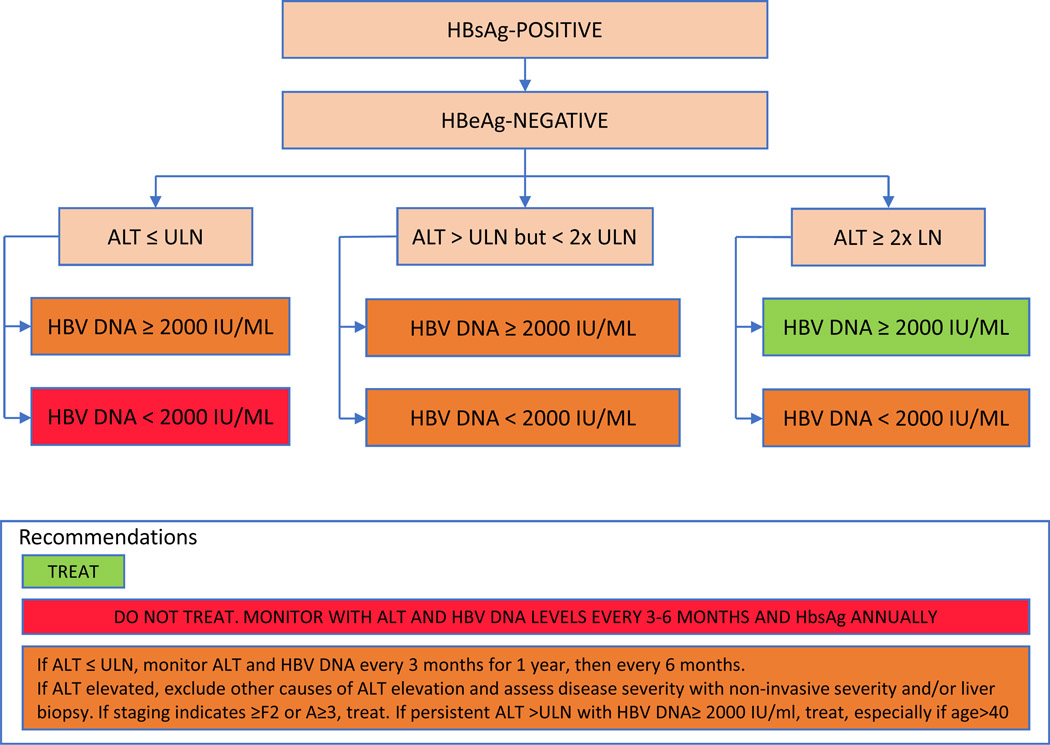
For patients without cirrhosis, the objective criteria used to determine the need for treatment differs between the two guidelines mainly because specialized testing may not be available, as highlighted in Table 3, Figures 3 and 4. For HBeAg positive patients without cirrhosis, AASLD recommends initiating treatment in patients with HBV DNA levels >20,000 IU/mL and ALT >2 X upper limit of normal (ULN) using a cutoff ALT of 35 for men and 25 for women as normal, Figure 3. For HBeAg positive patients with lower HBV DNA and ALT levels, the decision to treat should be individualized and based on presence of additional risk factors and results of a liver biopsy or non-invasive assessment of fibrosis. HBeAg positive patients with HBV DNA levels >20,000 IU/mL with normal ALT levels are not candidates for therapy. Among HBeAg negative patients, AASLD recommends treatment for patients with HBV DNA levels >2000 IU/ml and an elevated ALT>2 X ULN, Figure 3. For HBeAg negative patients with lower levels of ALT elevation (1–2 X ULN), treatment should be individualized based on results of a liver biopsy or non-invasive test of fibrosis. HBeAg negative patients with normal ALT levels and HBV DNA <2000 IU/mL are not treatment candidates.
Table 3:
Comparison of Recommendations for Treatment and Monitoring of Chronic Hepatitis B
| WHO | AASLD | |
|---|---|---|
| Who to treat | • All compensated and decompensated cirrhotics • All adults above age of 30 years with chronic HBV without clinical evidence of cirrhosis (based on APRI score ≤2 and physical examination) but have persistently abnormal ALT levels and evidence of HBV DNA >20000 IU/ml (if available) regardless of HBeAg status. |
• All compensated and decompensated cirrhotics • HBeAg positive patients with HBV DNA >20,000 IU/ml and ALT > 2xULN • HBeAg negative patients with HBV DNA >2,000 IU/ml and ALT > 2xULN |
| Criteria used for deciding treatment | • Age, • ALT levels • Fibrosis staging based on APRI score |
• HBV DNA levels • ALT level • HBeAg status • Fibrosis staging based on liver biopsy or non-invasive tests including vibration controlled transient elastography |
| Upper limit of normal for ALT | Laboratory defined upper limit of normal | • Men ALT of 35 U/L • Women ALT of 25 U/L |
| What drugs to treat with | • Tenofovir disoproxil fumarate • Entecavir |
• Tenofovir disoproxil fumarate • Entecavir • Tenofovir alafenamide • Peg-interferon alfa-2a |
| When to stop treatment | • Cirrhosis – continue treatment indefinitely • No cirrhosis-Consider discontinuing NUCs if there is HBeAg seroconversion or HBsAg loss and treatment consolidation for at least 12 months |
• Cirrhosis – continue treatment indefinitely • No cirrhosis-Consider discontinuing NUCs if there is HBeAg seroconversion or HBsAg loss and treatment consolidation for at least 12 months |
| Treatment failure | • Lamivudine, Adefovir, Telbivudine and Entecavir resistance – switch to Tenofovir | Lamivudine, Adefovir, Telbivudine and Entecavir resistance – switch to Tenofovir/Tenofovir alafenamide/add Tenofovir/ tenofovir alafenamide to ongoing therapy Tenofovir resistance-switch/add Entecavir |
| Screening for hepatocellular carcinoma | Ultrasound + AFP every 6 months | Ultrasound ± AFP every 6 months |
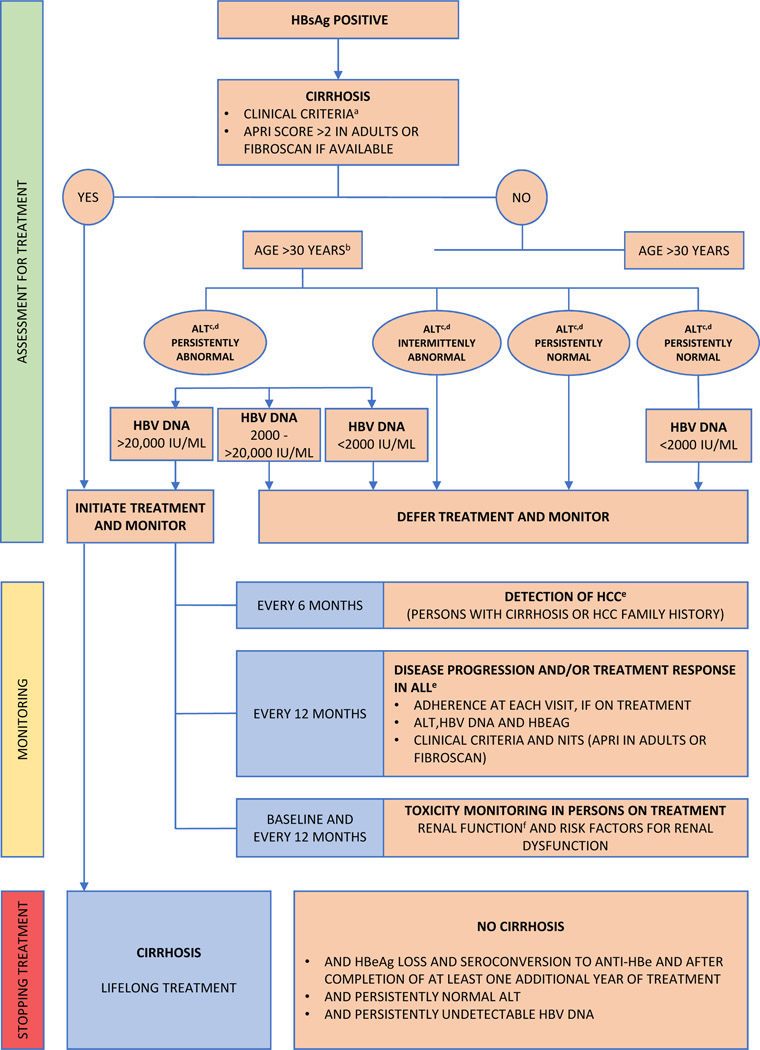
Figure 3: Algorithm of WHO Recommendations on the Management of Persons with Chronic Hepatitis B Infection.
Treatment and monitoring algorithm for patients with CHB based upon WHO guidelines. Adapted from Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection, WHO 2015.
WHO: World Health Organization; HBsAg Hepatitis B surface antigen
NITs non-invasive tests, ALT alanine aminotransferase level, APRI aspartate aminotransferase-to-platelet-ratio index
Chronic hepatitis B defined as persistence of HBsAg for six months or more.
aClinical features of decompensated cirrhosis: ascites, variceal hemorrhage and hepatic encephalopathy), coagulopathy or jaundice. Other clinical features of cirrhosis include hepatomegaly, splenomegaly, pruritus, fatigue, arthralgias, palmar erythema, peripheral edema.
bThe age cutoff of >30 years is not absolute and some persons with CHB less than 30 years may also meet criteria for antiviral treatment.
cALT levels fluctuate in persons with chronic hepatitis B and require longitudinal monitoring to determine the trend. Upper limits for normal ALT have been defined as below 30 U/L for men and 19 U/L for women, though local laboratory normal ranges should be applied. Persistently normal/abnormal may be defined as three ALT determinations below or above the upper limit of normal, made at unspecified intervals during a 6–12-month period or predefined intervals during 12-month period.
dWhere HBV DNA testing is not available, treatment may be considered based on persistently abnormal ALT levels, but other common causes of persistently raised ALT levels such as impaired glucose tolerance, dyslipidemia and fatty liver should be excluded.
eAll persons with CHB should be monitored regularly for disease activity/progression and detection of HCC, and after stopping treatment for evidence of reactivation. More frequent monitoring maybe required in those with more advanced liver disease, during the first year of treatment or where adherence is a concern, and in those with abnormal ALT and HBV DNA levels >2000 IU/mL, not yet on treatment.
fBefore initiation, assessment should be done of renal function (serum creatinine level, estimated glomerular filtration rate, urine dipsticks for proteinuria and glycosuria, and risk factors for renal dysfunction (decompensated cirrhosis, CrCl <50 mL/min, poorly controlled hypertension, proteinuria, uncontrolled diabetes, active glomerulonephritis, concomitant nephrotoxic drugs, solid organ transplantation, older age, BMI <18.5 kg/m2 (or body weight <50 kg), concomitant use of nephrotoxic drugs or a boosted protease inhibitor (PI) for HIV). Monitoring should be more frequent in those at higher risk of renal dysfunction.
Figure 4: (A) AASLD Guidelines on Management of Persons with HBeAg positive chronic hepatitis B (B) AASLD Guidelines on Management of Persons with HBeAg negative chronic hepatitis B.
Treatment algorithm for patients with HBeAg positive and HBeAg negative chronic hepatitis B based upon AASLD guidelines. Adapted from NA Terrault, AS Lok, BJ McMahon, et al. Update on Prevention, Diagnosis, and Treatment and of Chronic Hepatitis B: AASLD 2018 Hepatitis B Guidance. Hepatology. 2018 Apr; 67(4): 1560–1599, with permission.
In LMICs, access to nucleic acid and serological testing may be limited. To circumvent this issue the WHO recommends using age, ALT levels and non-invasive testing in the decision algorithm to start treatment, Figure 4. For patients without cirrhosis (APRI <2.0) where specialized testing is unavailable, WHO recommends treating all adults above the age of 30 years who have persistently abnormal ALT levels. Persistently abnormal ALT levels are defined as three ALT determinations above the upper limit of normal made at unspecified intervals during a 6 to 12-month period or pre-defined intervals during a 12-month period. Notably the WHO uses laboratory-defined upper limits whereas the AASLD uses gender-specific ALT cut-offs. If HBV DNA testing is available, the cut-offs to begin treatment are the same as those for AASLD. The WHO acknowledges that the evidence supporting the recommendations are imprecise and relying solely on age and ALT may mean that some patients with chronic hepatitis B with ALT elevations due to other causes may be inadvertently exposed to HBV treatment without direct benefit and the requirement for 3 ALT levels over 6 to 12 months may mean there is a delay in initiating treatment. However, the rationale of the WHO recommendations was to strike a balance between the potential benefits of treatment against the need for long-term therapy and the associated toxicities. Recently a simple score consisting of HBeAg and four categories of ALT was shown to correctly identify 82% to 85% of patients meeting the international treatment criteria based on the conventional reference tests (serum HBV DNA and liver biopsy or vibration controlled elastography), and 77% to 78% of patients who do not meet treatment criteria (23). It is anticipated that advances in rapid detection technology (24) will improve care by providing greater access to testing and will allow on-treatment monitoring to better align management of patients in LMICs with those in developed countries.
What Drugs to Treat With?
Currently, there are 8 drugs approved for the treatment of chronic hepatitis B which can be categorized into two main classes: interferon preparations (standard interferon alfa-2b, peginterferon alfa 2a [peg-alfa 2a]) and nucleos(t)ide analogues (lamivudine, adefovir, entecavir, telbivudine, tenofovir disoproxil fumarate and tenofovir alafenamide). Both guidelines recommend initiating treatment with a potent agent that has a high barrier to resistance and a low rate and antiviral resistance. Thus, entecavir and tenofovir are recommended as first line therapy for treatment of chronic HBV infection. Entecavir is licensed for use in children older than 2 years and tenofovir in children aged 12 years and older. AASLD also includes tenofovir alafenamide, which is an orally available prodrug of tenofovir with similar antiviral efficacy but lower renal and bone toxicity (25, 26). Peginterferon alfa-2a which is also considered a first line option in the AASLD guidelines in patients with suitable pre-treatment characteristics is not recommended in the WHO guidelines due to the need for additional monitoring and toxicity.
When to Stop Treatment?
Peg-alfa 2a is administered for a fixed duration of 48 weeks when used for treatment of chronic HBV infection. Early stopping rules at week 12 and 24 exist for HBeAg positive and negative patients based on tolerability, HBV genotype and decline in HBsAg and HBV DNA levels. Stopping nucleoside analogue therapy requires careful consideration of the benefits and risks of doing so. Benefits include reduced financial burden and the need to take a medication daily that carries a risk of drug toxicity and antiviral resistance. Risks include virologic relapse, hepatic decompensation, HCC and death. Both guidelines recommend careful selection of patients after consideration of the risk/benefit ratio the patients’ ability to comply with the additional monitoring in the initial 6 months of stopping treatment and with the need for lifelong monitoring.
AASLD and WHO guidelines recommend that all patients with compensated and decompensated cirrhosis treated with a nucleos(t)ide analogue require lifelong therapy and should not discontinue treatment. This is to avoid the risk of HBV reactivation leading to hepatic decompensation and death (27). Both guidelines recommend discontinuation of treatment in patients who achieve HBsAg loss or HBeAg positive patients undergo HBeAg seroconversion and who receive a minimum of 12 months of treatment consolidation and have normal ALT levels and persistently undetectable HBV DNA levels.
It is imperative that if treatment is stopped, patients are carefully monitored every 3 months or more frequently as necessary for at least a year to detect episodes of recurrent viremia, ALT flares, clinical decompensation and HBsAg and HBeAg seroreversion. Both AASLD and WHO recommend lifelong treatment of patients with HBeAg-negative chronic hepatitis B unless they achieve HBsAg loss (28). Recently, there has been a recommendation by EASL and APASL to consider discontinuing therapy in selected patients with HBeAg negative chronic hepatitis B without cirrhosis who have maintained undetectable HBV DNA and normal ALT levels for a period of two to three years. Small series have reported rates of HBsAg loss around 20%, but ~ 50% of patients require re-initiation of therapy and 10% to 20% may experience withdrawal flares with/without hepatic decompensation. Given the need for close surveillance and frequent testing after stopping treatment this approach is not recommended for LMICs.
Management of Treatment Failure
Primary treatment failure is defined as the failure to reduce HBV DNA levels by ≥ 1 × log10 IU/ml within 3 months from the start of therapy. Secondary treatment failure is defined as increase of HBV DNA by ≥ 1 × log10 IU/ml from baseline in persons with initial treatment response (≥ 1 × log10 IU/ml reduction in HBV DNA). An increase in HBV DNA levels on treatment may signify non-compliance or antiviral resistance. In these clinical circumstances it is important to review medication compliance with the patient and if adherence is confirmed, antiviral resistance testing should be considered. In LMICs, access to HBV DNA testing remains a limiting factor in diagnosing antiviral resistance. In settings without access to HBV DNA testing or antiviral resistance testing, assessment of resistance is largely based on clinical suspicion and should be considered in the following circumstances: (1) patients receiving antiviral drugs with low barrier to resistance in combination with poor adherence, (2) increase in levels of serum aminotransferases and (3) evidence of progression in liver disease based on physical examination or APRI score. Clinical relapse manifested by elevation in ALT levels tends to lag virological relapse and therefore is not a sensitive marker of resistance.
Primary treatment failure is rarely seen in patients who are adherent to treatment with entecavir and tenofovir and adherence to treatment should be reinforced (29). If antiviral resistance is suspected or confirmed to lamivudine, adefovir, telbivudine or entecavir, the preferred management strategy of both guidelines is switching to tenofovir; tenofovir alafenamide is an alternative option in the AASLD guidelines (30, 31). AASLD also recommends adding tenofovir to the ongoing nucleos(t)ide analogue however, the WHO does not recommend this option as of all the available nucleos(t)ide analogues tenofovir is associated with the highest probability at one year of achieving low or undetectable HBV DNA levels. For patients with resistance to tenofovir, switching to or adding entecavir to ongoing tenofovir are recommended options. AASLD recommends that patients with low level viremia on entecavir of tenofovir should continue monotherapy regardless of ALT levels. Both guidelines emphasize the importance of counselling patients regularly in clinic visits about treatment adherence as non-compliance is a major contributor to antiviral resistance.
Monitoring
Monitoring of Patients Receiving Treatment
Patients on antiviral treatment require monitoring to assess compliance and efficacy of treatment, liver disease progression, development of antiviral resistance and complications of treatment. Treatment efficacy is best assessed by monitoring HBV DNA levels. There are no clear-cut guidelines regarding the frequency of testing HBV DNA levels. However, given the excellent efficacy and low rate of antiviral resistance in adherent patients receiving entecavir and tenofovir, monitoring can be relatively infrequent. AASLD recommends testing HBV DNA levels every 3 months for the first 6 months of therapy to establish efficacy and then increase the testing interval to every 3 to 6 months. AASLD also recommends obtaining ALT, HBeAg, anti-HBe, and HBsAg every 6 to 12 months. In patients at risk for renal toxicity, creatinine clearance, serum phosphate, urine glucose and protein should be assessed annually. A bone density study should be considered at baseline and periodically during treatment in patients with a history of fracture or at risk for osteopenia. Lactic acid levels should be tested if there is clinical suspicion of mitochondrial injury.
WHO guidelines advise at a minimum, annual monitoring of ALT, HBsAg, HBeAg, HBV DNA levels (if available), APRI score to assess for the presence of cirrhosis in those without cirrhosis at baseline, along with baseline and annual testing of renal function with urine dipstick and creatinine measurement. WHO additionally recommends frequent monitoring of these parameters for at least 3 months for the first year in patients with cirrhosis and HIV coinfection. Tests to assess bone density are not widely available in LMICs.
Monitoring of Untreated Patients
Chronic HBV is a dynamic disease with a fluctuating course of disease (32). The primary goal of monitoring is to identify clinical changes that might indicate disease progression necessitating treatment. In general, the frequency of monitoring should be appropriate for activity and stage of the liver disease. It is therefore important to remind untreated patients of the need for lifelong monitoring. Patients who do not presently require treatment may need treatment in the future due to the change in the clinical status, which can be recognized by rise in ALT and HBV DNA levels. AASLD guidelines recommend that patients who are inactive carriers and those with immune tolerant disease, the two groups in whom treatment is not recommended, should be tested periodically for disease transition and HBeAg (if applicable) and HBsAg loss. Inactive carriers should be monitored with HBV DNA and ALT every 3 months for first year and every 6 to 12 months thereafter and HBsAg tested annually. Patients with immune tolerant disease should be monitored with HBV DNA and ALT levels and HBeAg at least every 6 months. WHO guidelines recommend annual monitoring of HBeAg, HBsAg, serum ALT, HBV DNA levels and APRI scores for all patients without cirrhosis at baseline who do not qualify for treatment.
Screening for HCC
HBV accounts for the largest proportion of HCCs globally. In LMICs, with high HBV seroprevalence, patients are sometimes diagnosed with HBV only after they present with advanced HCC. In patients with HBV, HCC can occur even in the absence of cirrhosis (33). Therefore, surveillance is required even in patients without cirrhosis to detect HCC at an early stage to increase the chances of survival. AASLD and WHO guidelines recommend a screening ultrasound every 6 months for patients with cirrhosis and family history of HCC. WHO recommends obtaining an alpha-fetoprotein in addition to ultrasound (34, 35) whereas alpha-fetoprotein testing is optional in AASLD guidelines. Where ultrasound is not available, screening with alpha-fetoprotein every 6 months is recommended. There are minor differences in the age to initiate screening between the two guidelines. WHO recommends screening for HCC in all patients over 40 years of age with HBV DNA levels > 2000 IU/ml. AASLD recommends HCC screening only for Asian or African American men over 40 years of age and to begin screening Asian women at 50 years of age (36). The screening interval recommended by WHO is appropriate for the prevalence of HBV-related HCC in LMICs. The inclusion of alpha-fetoprotein in the WHO recommendations may result in unnecessary and costly interventions and the need for additional screening visits due to false positive results. In LMICs for surveillance to be effective in improving survival there must be access to treatment of early stage HCC. These include alcohol injection, ablation, chemoembolization and resection. However, there is limited access to these interventions in LMICs and this is a challenge that must be addressed.
Summary
The 2016 WHO guidelines on testing for HBV are the first global evidence-based guidelines that complement the 2015 guidelines on prevention, care and treatment of chronic HBV infection. Their main focus is to target LMICs to improve their regional and national testing strategies. Unlike the other clinician-focused international guidelines from AASLD, APASL and EASL(37, 38), the target audience for the WHO guidelines are national program managers and health policy makers who are responsible for the development of national hepatitis testing and treatment policies.(39)
The WHO guidelines are created around the limitations of resources, accessibility and affordability to measure of HBV DNA levels. This expensive assay requires highly advanced equipment and facilities. However, HBV DNA is a major predictor for development of HBV-related diseases including HCC and a key marker to identify response to treatment and decide eligibility for antiviral therapy. In the absence of HBV DNA assays, WHO relies on serial ALT measurements, which is problematic as it requires several blood tests and medical visits before a decision to treat can be made. With the increasing availability of HBV DNA testing, and novel rapid detection technology patients around the world will be able to be screened, treated and monitored more effectively. However, under the present circumstances, prevention of chronic hepatitis B through vaccination and post-exposure prophylaxis remain the cornerstone for reducing the incidence of the disease and its related mortality.
Funding:
This work was supported by the Intramural Research Program of NIDDK, NIH
Abbreviations:
- HBV
Hepatitis B virus
- HBsAg
Hepatitis B surface Ag
- WHO
World health organization
- LMICs
Low to middle income countries
- AASLD
American Association for the Study of Liver Diseases
- APASL
Asian Pacific Association for the Study of Liver Diseases
- EASL
European Association for the Study of Liver Diseases
- HepB-BD
Hepatitis B birth dose
- CDC
Center for Disease Control
- HCC
Hepatocellular carcinoma
- HBIG
Hepatitis B immune globulin
Footnotes
Financial Disclosure: The authors are employees of the U.S. Government and have no financial conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJJTL. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. 2015;386(10003):1546–55. [DOI] [PubMed] [Google Scholar]
- 2.Ott J, Stevens G, Groeger J, Wiersma SJV. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. 2012;30(12):2212–9. [DOI] [PubMed] [Google Scholar]
- 3.James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. 2018;392(10159):1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Organization WH. Global health sector strategy on viral hepatitis 2016–2021. Towards ending viral hepatitis. World Health Organization; 2016. [Google Scholar]
- 5.Organization WH. Guidelines for the Prevention Care and Treatment of Persons with Chronic Hepatitis B Infection: Mar-15: World Health Organization; 2015. [PubMed] [Google Scholar]
- 6.Terrault NA, Lok AS, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. 2018;67(4):1560–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MHJH. A ASLD guidelines for treatment of chronic hepatitis B. 2016;63(1):261–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson NP, Jamieson DJ, Murphy TVJJotPIDS. Prevention of perinatal hepatitis B virus transmission. 2014;3(suppl_1):S7–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen D-SJJoh. Hepatitis B vaccination: the key towards elimination and eradication of hepatitis B. 2009;50(4):805–16. [DOI] [PubMed] [Google Scholar]
- 10.Chang M-H, Chen C-J, Lai M-S, Hsu H-M, Wu T-C, Kong M-S, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. 1997;336(26):1855–9. [DOI] [PubMed] [Google Scholar]
- 11.Hepatitis B vaccines: WHO position paper - July 2017. Releve epidemiologique hebdomadaire. 2017;92(27):369–92. [PubMed] [Google Scholar]
- 12.Holmberg SD, Suryaprasad A, Ward JWJM, Recommendations MWR, Reports. Updated CDC recommendations for the management of hepatitis B virus–infected health-care providers and students. 2012;61(3):1. [PubMed] [Google Scholar]
- 13.Cooper C, Mackie DJErov. Hepatitis B surface antigen-1018 ISS adjuvant-containing vaccine: a review of HEPLISAV™ safety and efficacy. 2011;10(4):417–27. [DOI] [PubMed] [Google Scholar]
- 14.Sánchez-Fueyo A, Rimola A, Grande L, Costa J, Mas A, Navasa M, et al. Hepatitis B immunoglobulin discontinuation followed by hepatitis B virus vaccination: a new strategy in the prophylaxis of hepatitis B virus recurrence after liver transplantation. 2000;31(2):496–501. [DOI] [PubMed] [Google Scholar]
- 15.Beasley RP, George C- YL, Roan C-H, Hwang L-Y, Lan C-C, Huang F-Y, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. 1983;322(8359):1099–102. [DOI] [PubMed] [Google Scholar]
- 16.Ranger-Rogez S, Denis FoJEroa-it. Hepatitis B mother-to-child transmission. 2004;2(1):133–45. [DOI] [PubMed] [Google Scholar]
- 17.Pan CQ, Duan Z, Dai E, Zhang S, Han G, Wang Y, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. 2016;374(24):2324–34. [DOI] [PubMed] [Google Scholar]
- 18.Chen HL, Lee CN, Chang CH, Ni YH, Shyu MK, Chen SM, et al. Efficacy of maternal tenofovir disoproxil fumarate in interrupting mother-to-infant transmission of hepatitis B virus. 2015;62(2):375–86. [DOI] [PubMed] [Google Scholar]
- 19.Chang CY, Aziz N, Poongkunran M, Javaid A, Trinh HN, Lau DT, et al. Serum aminotransferase flares in pregnant and postpartum women with current or prior treatment for chronic hepatitis B. 2018;52(3):255–61. [DOI] [PubMed] [Google Scholar]
- 20.Chang CY, Aziz N, Poongkunran M, Javaid A, Trinh HN, Lau D, et al. Serum alanine aminotransferase and hepatitis B DNA flares in pregnant and postpartum women with chronic hepatitis B. Nature Publishing Group; 2016. [DOI] [PubMed] [Google Scholar]
- 21.Ganem D, Prince AMJNEJoM. Hepatitis B virus infection—natural history and clinical consequences. 2004;350(11):1118–29. [DOI] [PubMed] [Google Scholar]
- 22.Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. 2013;381(9865):468–75. [DOI] [PubMed] [Google Scholar]
- 23.Shimakawa Y, Njie R, Ndow G, Vray M, Mbaye PS, Bonnard P, et al. Development of a simple score based on HBeAg and ALT for selecting patients for HBV treatment in Africa. 2018;69(4):776–84. [DOI] [PubMed] [Google Scholar]
- 24.Peeling RW, Boeras DI, Marinucci F, Easterbrook PJBid. The future of viral hepatitis testing: innovations in testing technologies and approaches. 2017;17(1):699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buti M, Gane E, Seto WK, Chan HL, Chuang W-L, Stepanova T, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. 2016;1(3):196–206. [DOI] [PubMed] [Google Scholar]
- 26.Chan HL, Fung S, Seto WK, Chuang W-L, Chen C-Y, Kim HJ, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. 2016;1(3):185–95. [DOI] [PubMed] [Google Scholar]
- 27.Lim S, Wai C, Rajnakova A, Kajiji T, Guan RJG. Fatal hepatitis B reactivation following discontinuation of nucleoside analogues for chronic hepatitis B. 2002;51(4):597–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang ML, Liaw YF, Hadziyannis SJAp, therapeutics. Systematic review: cessation of long-term nucleos (t) ide analogue therapy in patients with hepatitis B e antigen-negative chronic hepatitis B. 2015;42(3):243–57. [DOI] [PubMed] [Google Scholar]
- 29.Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ, Fang J, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. 2009;49(5):1503–14. [DOI] [PubMed] [Google Scholar]
- 30.van Bömmel F, de Man RA, Wedemeyer H, Deterding K, Petersen J, Buggisch P, et al. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. 2010;51(1):73–80. [DOI] [PubMed] [Google Scholar]
- 31.Fung S, Kwan P, Fabri M, Horban A, Pelemis M, Hann H-W, et al. Tenofovir disoproxil fumarate (TDF) vs. emtricitabine (FTC)/TDF in lamivudine resistant hepatitis B: A 5-year randomised study. 2017;66(1):11–8. [DOI] [PubMed] [Google Scholar]
- 32.Shi Y-H, Shi C-HJWjogW. Molecular characteristics and stages of chronic hepatitis B virus infection. 2009;15(25):3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chayanupatkul M, Omino R, Mittal S, Kramer JR, Richardson P, Thrift AP, et al. Hepatocellular carcinoma in the absence of cirrhosis in patients with chronic hepatitis B virus infection. 2017;66(2):355–62. [DOI] [PubMed] [Google Scholar]
- 34.Aghoram R, Cai P, Dickinson JAJCDoSR. Alpha-foetoprotein and/or liver ultrasonography for screening of hepatocellular carcinoma in patients with chronic hepatitis B. 2012(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hann H-W, Fu X, Myers RE, Hann RS, Wan S, Kim SH, et al. Predictive value of alpha-fetoprotein in the long-term risk of developing hepatocellular carcinoma in patients with hepatitis B virus infection–results from a clinic-based longitudinal cohort. 2012;48(15):2319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. 2018;67(1):358–80. [DOI] [PubMed] [Google Scholar]
- 37.hepatology EAFTSOTLJJo. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. 2017;67(2):370–98. [DOI] [PubMed] [Google Scholar]
- 38.Sarin S, Kumar M, Lau G, Abbas Z, Chan H, Chen C, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. 2016;10(1):1–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chou R, Easterbrook P, Hellard M. Methodological challenges in appraising evidence on diagnostic testing for WHO guidelines on hepatitis B and hepatitis C virus infection. BMC infectious diseases. 2017;17(Suppl 1):694. [DOI] [PMC free article] [PubMed] [Google Scholar]