Abstract
Background:
Exposure to tobacco or marijuana smoke, or e-cigarette aerosols, causes vascular endothelial dysfunction in humans and rats. We aimed to determine what constituent, or class of constituents, of smoke is responsible for endothelial functional impairment.
Methods:
We investigated several smoke constituents that we hypothesized to mediate this effect by exposing rats and measuring arterial flow-mediated dilation (FMD) pre- and post-exposure. We measured FMD before and after inhalation of sidestream smoke from research cigarettes containing normal and reduced nicotine level with and without menthol, as well as two of the main aldehyde gases found in both smoke and e-cigarette aerosol (acrolein and acetaldehyde), and inert carbon nanoparticles.
Results:
FMD was reduced by all four kinds of research cigarettes, with extent of reduction ranging from 20–46% depending on the cigarette type. While nicotine was not required for the impairment, higher nicotine levels in smoke were associated with a greater percent reduction of FMD (41.1 ± 4.5% percent reduction vs. 19.2 ± 9.5%; p=0.047). Lower menthol levels were also associated with a greater percent reduction of FMD (18.5 ± 9.8% vs. 40.5 ± 4.8%; p=0.048). Inhalation of acrolein or acetaldehyde gases at smoke-relevant concentrations impaired FMD by roughly 50% (p=0.001). However, inhalation of inert carbon nanoparticles at smoke-relevant concentrations with no gas phase also impaired FMD by a comparable amount (p<0.001). Bilateral cervical vagotomy blocked the impairment of FMD by tobacco smoke.
Conclusions:
There is no single constituent or class of constituents responsible for acute impairment of endothelial function by smoke; rather, we propose that acute endothelial dysfunction by disparate inhaled products is caused by vagus nerve signaling initiated by airway irritation.
Graphical Abstract
Introduction:
Exposure to cigarette smoke and aerosols from e-cigarettes causes endothelial dysfunction in humans.1–7 In our prior studies, we showed that acute exposure to sidestream smoke (i.e., smoke from the smoldering tip of a cigarette; a well-accepted surrogate for secondhand smoke) from tobacco cigarettes, little cigars, and marijuana cigarettes impairs endothelial function in rats.8–10 We also showed that acute exposure to aerosols from the heated tobacco product IQOS, and from e-cigarettes with freebase nicotine or nicotine salts, impaired endothelial function to the same extent as cigarette smoke.11–13 The specific cause of the endothelial dysfunction remains unclear, although oxidative stress has been implicated as an underlying mechanism.14,15
A fundamental unanswered question is whether endothelial dysfunction is caused by one or more specific chemicals in smoke or aerosol, or if it is a nonspecific response to inhalation of particulate matter. Thousands of chemicals have been identified in tobacco and marijuana smoke,16 some of which are also present in e-cigarette aerosols either as an original ingredient or as a chemical reaction product of the heating process.17 Identification of the specific factors involved would not only shed light on how inhalational exposures lead to endothelial dysfunction, but would also suggest potential approaches for remediation and treatment. Moreover, it would provide regulatory agencies like the FDA with important information about specific ingredients whose levels could be assessed or regulated in current and emerging products. For reasons delineated below, we hypothesized that endothelial dysfunction could be caused or exacerbated by nicotine, menthol, aldehyde gases, or ultrafine particles present in both combustion smoke and vaping aerosols.
Nicotine, a well-studied vasoconstrictor, can cause endothelial dysfunction in humans and animal models.18–21 The fact that marijuana smoke impairs endothelial function10 indicates that nicotine is not required for this effect, but we hypothesized that it could exacerbate it.
Menthol is a cigarette additive22 that is widely used in tobacco products, and menthol brands are promoted to African Americans, women, youth23,24, and other groups.25 Menthol addition to tobacco decreases irritation from smoke inhalation especially among new smokers26 and enhances nicotine addiction.27,28 Menthol exposure upregulates brain nicotine receptors and enhances nicotine withdrawal intensity in mice.29 Menthol interacts with smoke constituents and irritants such as acrolein through transient receptor potential ion channels and also decreases the metabolism rate of nicotine.30,31
Acrolein and acetaldehyde, two of the main aldehyde gases in smoke, can affect endothelial function by decreasing the bioavailability of nitric oxide through oxidative stress and eNOS uncoupling.32–34 These and other aldehydes are also generated in e-cigarettes as chemical reaction products of the glycerin and propylene glycol at the heating coil.35
While there are reasons to suspect that individual chemicals are responsible for endothelial dysfunction, fine particulate matter from air pollution has been implicated in cardiovascular toxicity.36–38 Given that various kinds of smoke and aerosols have all impaired endothelial function in studies by us and others, it is possible that the particulate nature of all of these inhalational exposures, rather than specific chemical constituents, are responsible for driving endothelial dysfunction.
In this study, we explored the influence of these suspected mediators on endothelial function by testing the effects of acute exposure to individual constituents on arterial flow-mediated dilation (FMD), which is a vascular functional index evaluating endothelium-dependent vasodilation that relies on nitric oxide bioavailability in response to increased shear stress at the vessel wall.39 Measurement of FMD is a validated non-invasive test to assess endothelial function in humans and is associated with overall cardiovascular health.39–41 Notably, FMD is impaired in humans by active and passive tobacco smoking as well as e-cigarette use.1–6 Here, we used a well-established method to measure FMD in rats that we previously developed42 and used to demonstrate impairment of FMD by smoke and e-cigarette aerosol,8–13 to assess the role of individual smoke constituents in causing endothelial dysfunction and identify which are primarily responsible for this effect.
Methods:
All data have been made publicly available at the Dryad repository and can be accessed initially at https://datadryad.org/stash/share/iN2IIaI8EbZxpaEXiAO864drJG-nX31YHwehLPLmrFg and then permanently at https://doi.org/10.7272/Q6VM49JF.
Animals:
We used male and female Sprague-Dawley rats (Simonsen Laboratory, CA and Charles River, MI) at 8–10 weeks of age with body weights of 200–250g, as has been the standard condition for our previous studies on smoke exposure.8–13 Rats were fed PicoLab® Rodent Diet 20, #5053 (LabDiet, St. Louis, MO). For FMD data, power calculation was based on standard deviations from within-group comparisons in several previous repeated-measures FMD experiments. It was determined that N=8 per group was sufficient to achieve 0.8 power to detect FMD change of 3.5 percentage points at a significance level of 0.05. Anesthesia consisted of intraperitoneal (i.p.) injection of ketamine (100 mg/kg)/xylazine (5 mg/kg). During all procedures, rats were kept on a heating pad to prevent hypothermia. Frequency and depth of respiration were closely monitored to ensure full anesthesia and supplemental i.m. anesthetic was given if necessary. Experiments were terminal. All procedures were approved by the University of California, San Francisco Institutional Animal Care and Use Committee.
Cigarette sidestream smoke generation and exposure:
Prior to each experiment, cigarettes were humidified overnight in a chamber over 16% glycerol in water. We used Marlboro Red cigarettes to generate sidestream smoke for positive controls in experiments on aldehydes and particles. To assess the effect of nicotine and menthol on FMD, we used SPECTRUM research cigarettes from the National Institute on Drug Abuse (NIDA) that contain different nicotine yields (conventional nicotine, NRC600, 0.72 mg nicotine/cig; reduced nicotine, NRC102, 0.02 mg nicotine/cig) as well as menthol flavored cigarettes (conventional nicotine plus menthol, NRC601, 0.68 mg nicotine/cig, 7130 μg menthol/cig; reduced nicotine plus menthol, NRC103, 0.02 mg nicotine, 5980 μg menthol/cig). For sidestream smoke generation, we used a modified cigarette smoking system as described in our previous experiments.8–10 A single cigarette was placed in a holder inside a 21 L Plexiglass exposure chamber and lit for each experiment. The cigarette was smoked by the machine according to standard conditions (ISO Standard 3308:2012, one 35-mL puff of 2-s duration once/min, with filter-tip vent open) for 3 minutes and extinguished, during which time the sidestream smoke from the burning tip of the cigarette accumulated in the exposure chamber. A Sidepak AM510 aerosol monitor (TSI, Shoreview, MN), gravimetrically calibrated for measurement of tobacco smoke, was used to monitor the level of respirable suspended particles (RSP; i.e., PM2.5) in the chamber. 600 and 200 μg/m3 RSP were used for high and low RSP starting levels; the levels proceeded to decrease over the 10 minutes as we have previously reported.8,10 Rats were exposed to the sidestream smoke at the desired level through a side hole with gasket in the chamber.
Aldehyde gas generation and exposure:
We used an EcoFlex™ gas standard generator and disposable permeation tubes (KIN-TEK Analytical, Inc., La Marque, TX) to generate acrolein and acetaldehyde at 3 ppm and 10–11.5 ppm respectively (levels comparable to those found in secondhand smoke;16 see section on derivation of desired gas concentrations in the online supplement). Anesthetized rats had 10-minute continuous exposure to the generated gas through a nose cone.
Inert particle exposure:
Carbon nanoparticles with diameter <100 nm (SkySpring Nanomaterials, Inc. Houston, TX) were introduced into a plexiglass exposure chamber using a manual powder insufflator (Model 119, DeVilbiss Healthcare LLC, Toledo, OH), using that device’s intrinsic puff volume. The suspension of particles naturally and rapidly distributed in the air and then gradually settled to the bottom. A TSI Sidepak AM510 was used to monitor particle concentration just above the bottom of the exposure chamber. Rats were exposed to inert particles through a side hole at the same height as the monitor sampling tube when the concentration reached ~8192 particles/cm3, which was calculated to equal number (not the mass) of particles present in tobacco smoke at 600 μg/m3.
Endothelial function assessment:
We used our established rat model42 to measure FMD before and after each exposure to assess endothelial function. FMD is defined as the percent by which arteries vasodilate after being transiently occluded, resulting in increased blood flow and fluid shear stress that causes endothelial release of NO.39 Under anesthesia and after preparation of the groin, baseline 2 D ultrasound and Doppler images of the right femoral artery were obtained via a 35 MHz ultrasound transducer (initially Vevo660 and subsequently Vevo 3100 LT; VisualSonics, Toronto, Canada). A 5-minute transient limb ischemia was induced surgically or non-surgically (see below), and subsequent ultrasound imaging was done immediately after reperfusion and then every 30 seconds for 5 minutes. FMD was calculated as % change: (peak diameter postischemia − diameter baseline)/diameter baseline × 100. For transient limb ischemia, we used both surgical and non-surgical approaches. For non-surgical transient ischemia, we used an external occluder (In Vivo Metric, Healdsburg, CA) and occluded the femoral artery distal to the site of imaging. For surgical transient ischemia, we made a 1 cm incision on the rat’s groin and occluded the common iliac artery proximal to ultrasound probe. We inserted an arterial loop occluder consisting of a 5–0 Prolene filament under the artery and passed it through a 15 cm PE–90 tubing to enable transient occlusion of blood flow after suturing the skin. The surgical approach is what we have used for our previous studies, then the external occluder approach was used for the experiments with nicotine and menthol, but the occluders (which weaken with repeated use and needed to be replaced) were no longer available after that point so the surgical approach was used for all remaining experiments. The investigator was blinded to exposure conditions during FMD procedure, analysis of ultrasound images, and subsequent calculations.
Bilateral cervical vagotomy:
Rats were anesthetized under ketamine (100 mg/kg)/xylazine (5 mg/kg) by intraperitoneal injection and placed on a surgical pad in the supine position. After fur was removed in the neck area and skin sanitized with 70% ethanol, a midline cervical incision was made and subcutaneous tissues were separated bilaterally using blunt dissection. The two lobes of thyroid glands were pulled apart to expose the sternohyoid and sternomastoid muscles along the trachea. Blunt dissection between the sternohyoid and sternomastoid muscles was carried out to expose the carotid artery and the cervical vagus nerve on the right and left sides. The right or left vagus nerve was carefully isolated from the carotid artery and the surrounding connective tissue, then cut using microsurgical scissors, and the skin incision sutured (5–0, gut). For sham surgery, the midline cervical incision was made, subcutaneous tissues were separated using blunt dissection, and then the skin incision was sutured.
Statistics:
We used paired Student’s t-tests to compare the FMD values in each group before and after exposures. We used a two-factor ANOVA to evaluate how the extent of FMD impairment from smoke exposure was influenced by tobacco with varying nicotine and menthol levels. For comparisons involving multiple groups and times, we fit a two-factor (condition and time) repeated-measures ANOVA to all of the data at once using a mixed model estimated with restricted maximum likelihood estimation with an unstructured covariance matrix of residuals, then tested for differences over time and across condition using contrasts and pairwise comparisons, adjusting for multiple comparisons using the Šidák method. Data sets from all experiments passed Kolmogorov-Smirnov normality test and Levene’s test for homogeneity of variances. Variability is presented as SD. Calculations were done using Stata V.13.1. For all analyses, P value of <0.05 was considered statistically significant; larger P values are still provided.
Results:
Reduction of nicotine level and addition of menthol lessen the acute impairment of FMD
We exposed 8 groups of rats for 10 minutes to sidestream smoke from the following four different types of research reference cigarettes: conventional nicotine, reduced nicotine, conventional nicotine with added menthol, and reduced nicotine with added menthol. Rats of each condition were exposed to starting smoke levels of ≈600 and ≈200 μg/m3 RSP (levels decrease during the exposure period; see Methods). Because the response to both concentrations was comparable, and because variability made it difficult to achieve significance with the individual groups of n=8–10, the rats exposed to both concentrations of smoke from a given product were combined into single groups of n=16–18 for analysis. We also examined a negative control group exposed to chamber air without smoke (n=9). FMD was measured before and 10 mins after exposure in each rat.
FMD values were generally lower in rats exposed later in the several-week-long experiment (technical considerations limited us to ~8 rats measured per day), which accounts for some of the intergroup variability, but the percent reduction of FMD from pre-exposure to post-exposure measurement per rat remained consistent. The overall reduction in FMD measurements over the weeks was attributed to unexpected changes in the distensibility of the external occluder occurring progressively over time with reuse (see supplemental Figure S1). Exposure conditions were randomly alternated over the course of this long experiment, with measurements by a blinded investigator; thus, differences in FMD measurements between experimental conditions were not influenced by the progressive weakening of the occluders (see Supplemental Table S1 showing the order in which each rat was exposed).
FMD was decreased from pre- to post-exposure in all smoke-exposed groups (Figure 1A). The pre- vs. post-exposure FMD values (mean ± SD) for conventional nicotine were 11.2±3.3% vs. 6.0±3.7% (a 46% decrease in FMD; p=0.0001), for reduced nicotine were 11.7±4.0% vs. 7.9±4.1% (a 32% decrease in FMD; p=0.001), for conventional nicotine plus menthol were 12.8±3.4% vs. 8.8±3.9% (a 31% decrease in FMD; p=0.0001), for reduced nicotine plus menthol were 11.0±5.4% vs. 8.8±3.9% (a 20% decrease in FMD; p=0.046), and for chamber air as a negative control were 9.8±2.6% vs. 8.4±1.0% (p=0.13).
Figure 1.
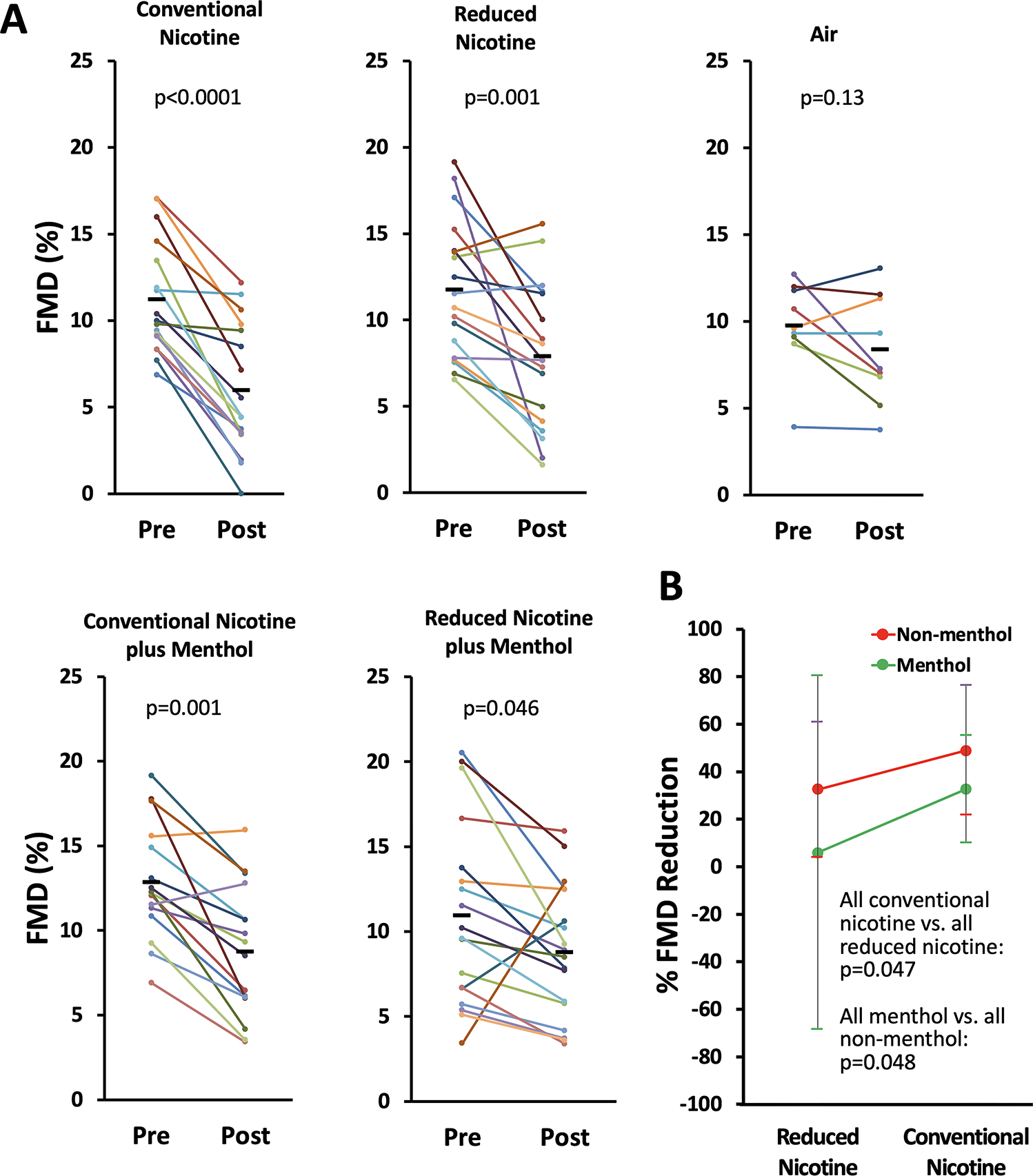
A. Impairment of FMD after 10 minute exposure to sidestream smoke from conventional and reduced-nicotine cigarettes with and without menthol addition. Each colored line shows FMD of an individual rat measured before and after exposure. See Supplemental Figure S2 for separate presentation of groups exposed to smoke levels of ≈600 and ≈200 μg/m3 RSP. B. Independent reduction in the extent of FMD impairment with decreased nicotine levels or addition of menthol. Each colored dot is the group mean of one of the four smoke exposure groups. Error bars are SD.
While each condition resulted in a significant impairment of FMD, the differences in the extent of the impairment between conditions did not reach significance. However, when we compared extent of FMD impairment in all conventional nicotine conditions to all reduced nicotine conditions, and all menthol conditions to all non-menthol conditions, an interesting pattern emerged (Figure 1B). Higher nicotine levels were associated with a greater decrease in FMD than lower nicotine levels (41.1±4.5% vs. 19.2±9.5%; p=0.047). Menthol was associated with a lower decrease in FMD than non-menthol conditions (18.5±9.8% vs. 40.5±4.8%; p=0.048). These two effects were independent (p for nicotine x menthol interaction = 0.6).
Inhalation of acrolein and acetaldehyde impairs FMD
To determine if acrolein or acetaldehyde gas components of smoke and aerosols could be at least partially responsible for the impairment of FMD, we exposed 4 groups of rats for 10 minutes to 3 ppm acrolein gas, 10–11.5 ppm acetaldehyde gas, Marlboro Red cigarette smoke (600 μg/m3 RSP), or clean air. FMD was measured pre- and 10 min post-exposure in each individual rat. Impairment of FMD was observed for acrolein (pre-exposure 10.8±1.7% vs. post-exposure 5.8±2.9%; p=.001), acetaldehyde (8.8±2.0% vs. 6.0±2.5%; p=.001), and cigarette smoke (9.4±2.9% vs. 5.8±2.0%; p=.002), but not for air (7.9±2.0% vs. 9±3.2%; p=.44) (Figure 2).
Figure 2.
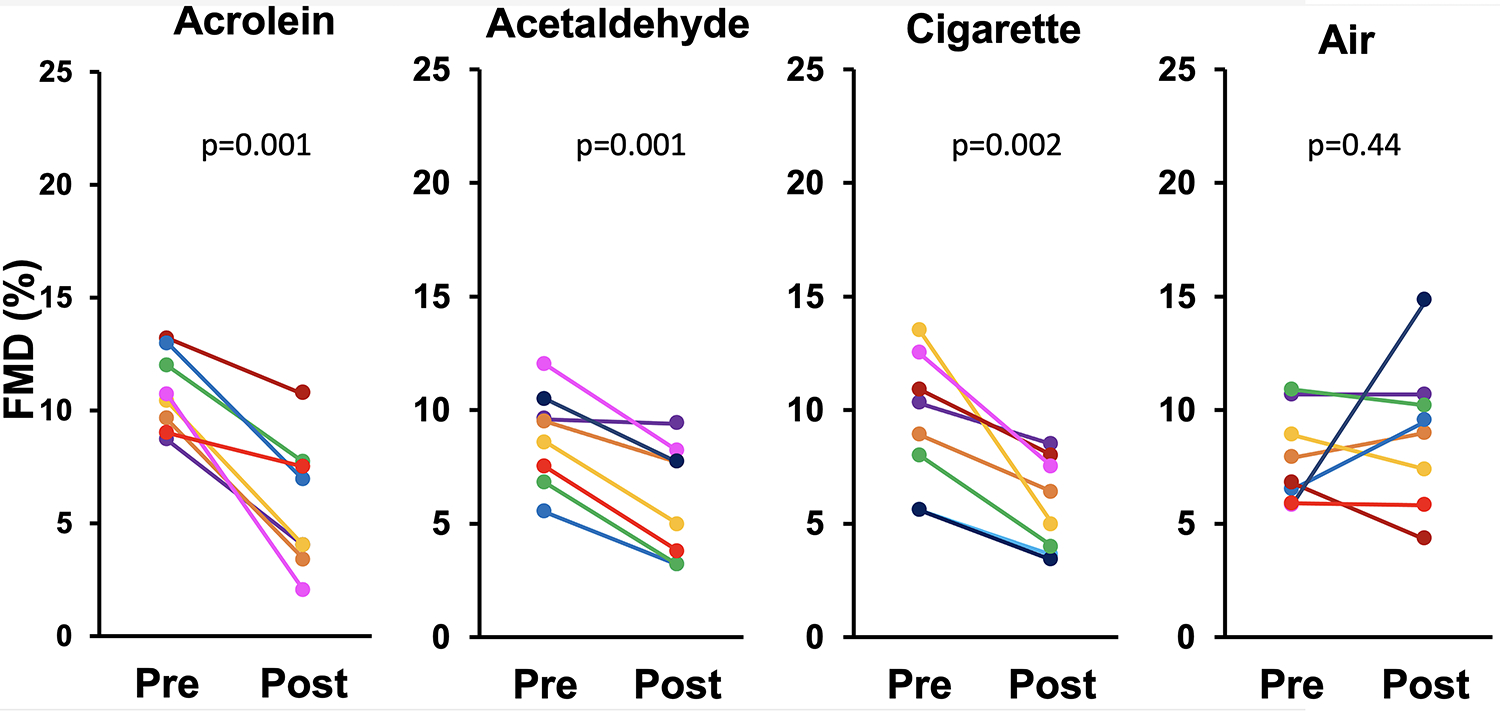
Impairment of FMD after a 10-minute exposure to acrolein, acetaldehyde, or sidestream smoke from Marlboro Red cigarettes. Each colored line represents one rat; black bars show group means.
Inhalation of inert carbon nanoparticles impairs FMD
Despite having shown that gas phase components can impair FMD, we still wanted to determine whether the particulate phase of smoke or aerosols could independently exert a similar effect. We exposed 3 groups of rats for 10 minutes to inert carbon nanoparticles at the level of ~8192 particles/cm3 (comparable to 600 μg/m3 RSP smoke), Marlboro Red cigarette sidestream smoke at 600 μg/m3 RSP, and clean air. FMD was quantified in each rat pre- and post-exposure. FMD was impaired in the particle group (11.5±5.2% vs. 3.9±2.6%; p=0.009) and the smoke group (11.9±4.8% vs. 6.1±5.2%; p=0.03), but not in the air group (10.0±3.8% vs. 8.4±2.5%; p=0.47) (Figure 3A). To determine if recovery occurred within ~30 minutes as we have previously shown for tobacco smoke,8–10 we subsequently exposed an additional two groups of rats to particles or air and measured FMD pre-exposure, 10 minutes post (end of) exposure, and 40 minutes post-exposure. In rats exposed to particles, FMD was impaired (9.1±1.8% vs 7.1±2.3%; p=0.004) and recovered after 40 minutes (7.1±2.3% vs. 9.0±1.8%; p=0.006). In rats exposed to air, there was no significant difference in FMD pre-, 10 minutes post- and 40 minutes post-exposure (Figure 3B).
Figure 3.
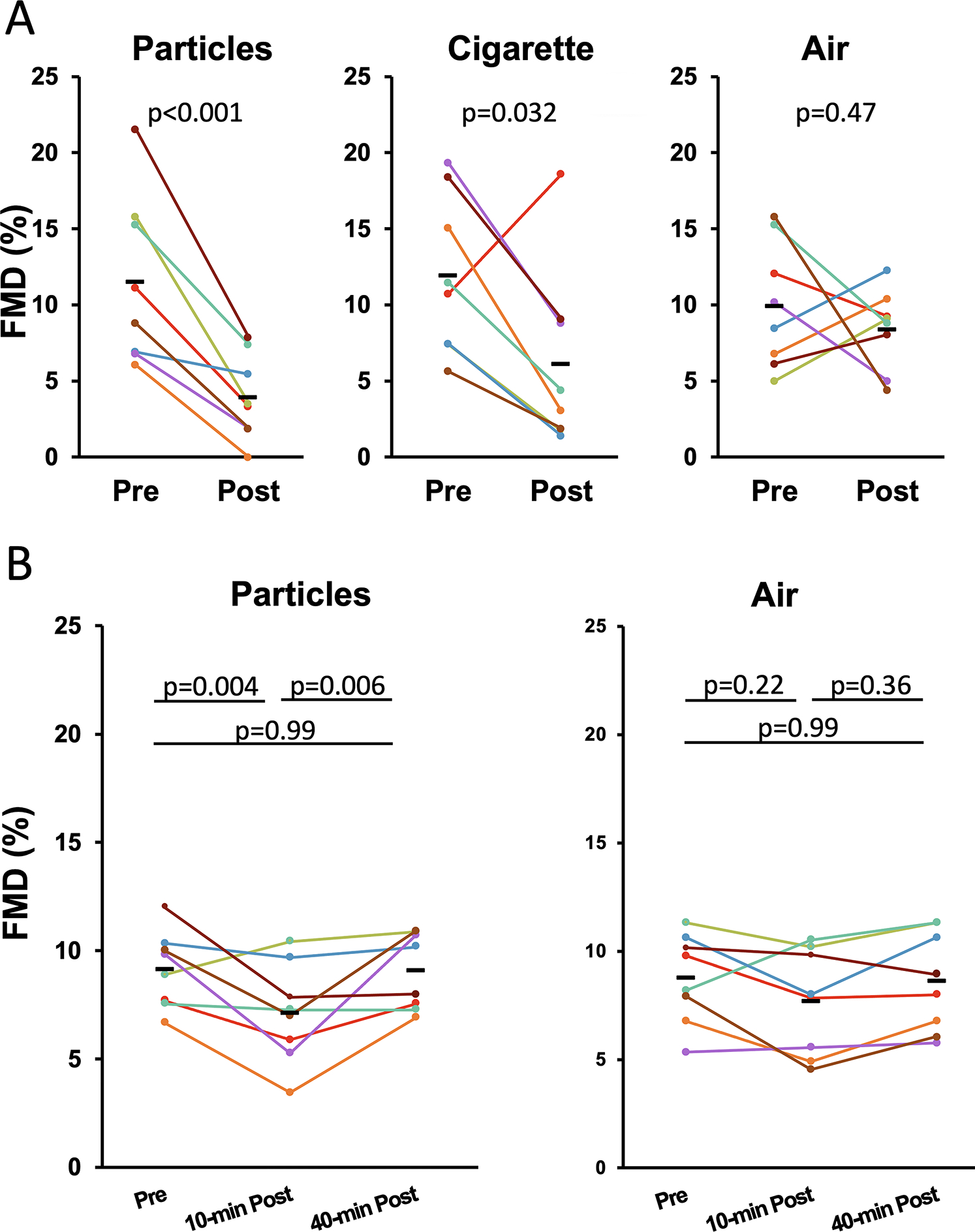
A. Impairment of FMD after a 10-minute exposure to carbon nanoparticles or sidestream cigarette smoke. The smoke group showed significant impairment even without removing the one outlier. B. Impairment of FMD after a 10-minute exposure to carbon nanoparticles was recovered 40 minutes post exposure. Each colored line represents one rat.
Impairment of FMD by cigarette sidestream smoke is dependent on an intact vagus nerve
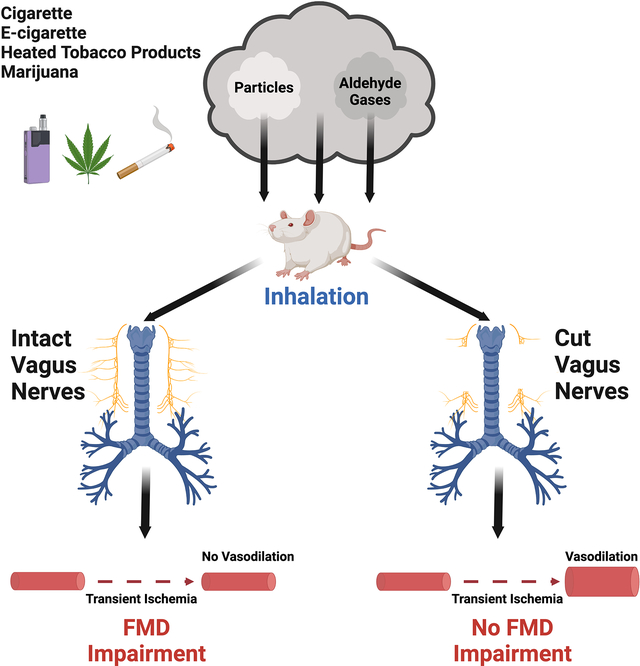
Because FMD was impaired by whole smoke, gas phase components of smoke, and plain carbon particles, with no single constituent or class of constituents uniquely responsible, we explored whether the mechanism involved a common irritation response from the airway mediated by the vagus nerve.43 We performed a bilateral cervical vagotomy, severing the right and left vagus nerves above the level of the heart and lungs, as well as a sham surgical procedure without severing vagus nerves, and determined the effect of the vagotomy on FMD before and after a 10-minute exposure to sidestream smoke. While the vagotomy itself did not affect pre-exposure baseline FMD, and FMD was impaired by smoke in the sham group as expected, the impairment of FMD by smoke was completely abrogated in the vagotomized group (Figure 4A), with no discernable gender effect (Supplemental Figure S4).
Figure 4.
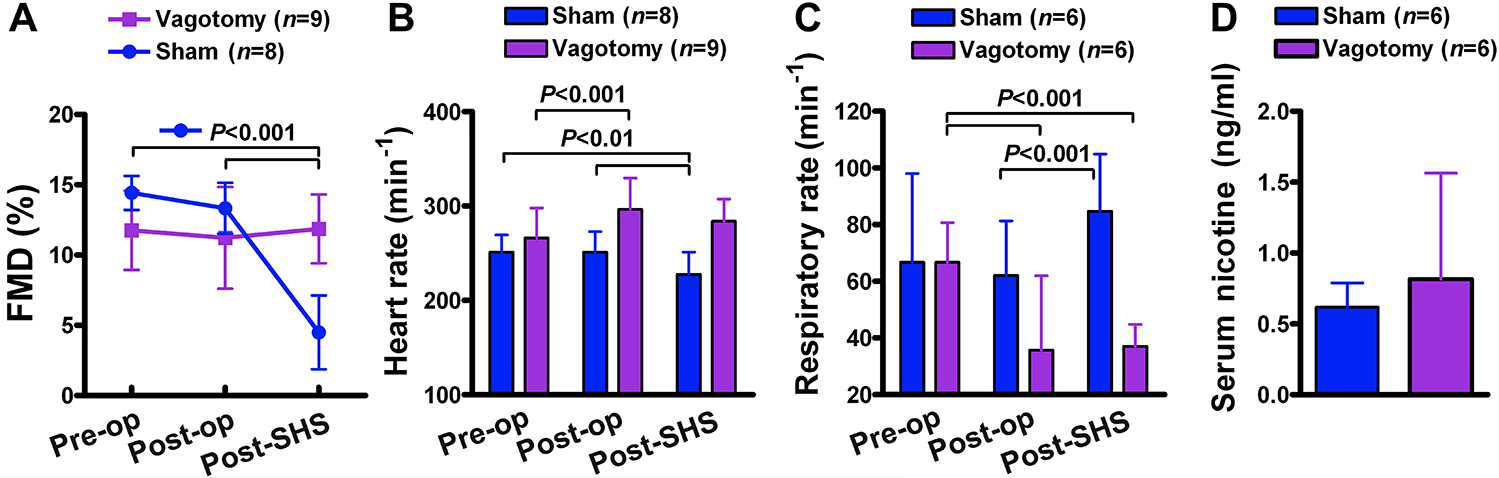
Bilateral cervical vagotomy prevented impairment of FMD by cigarette smoke. Anesthetized rats underwent bilateral cervical vagotomy or sham operation before exposure to 10 min of sidestream smoke. Average starting concentration of respirable suspended particles (RSP) were 790±18 μg/m3 for the vagotomy group and 808±16 μg/m3 for sham-operation group (see Supplemental Figure S3 for decreasing RSP levels over the exposure period. Vagotomy group consisted of 4 male and 5 female (n=9); sham-operation group consisted of 4 male and 4 female (n=8). “Pre-op” = pre-operation, “post-op” = post-operation, “post-SHS” = post-sidestream smoke. Data are mean±SD. (A) Changes in FMD (see Supplemental Figure S4 for breakdown by gender). (B,C) Changes in heart rate and respiratory rate. (D) Serum nicotine at 20 min post-end-of-exposure in subsequent control experiment. Group sizes for panel C are smaller than those in A,B because we did not measure respiratory rate for the first several rats. Group sizes in panel D are the same as those in panel C by coincidence.
Vagotomy caused significant increases in heart rate and significant decreases in respiratory rate, as expected. Respiratory rate and heart rate in the vagotomy group did not significantly change post-exposure. In contrast, sham surgery animals showed an increase in respiratory rate decrease in heart rate post-exposure (Figure 4B,C).
The slower respiration in the vagotomized group raised the possibility that the lack of FMD impairment might not reflect vagal influence, but instead might simply be a result of having inhaled too little smoke to impair FMD. Therefore, a subsequent control experiment was performed in which vagotomized rats and sham rats were subjected to the same smoke exposure conditions as before, and blood was collected 20 minutes after end of exposure and assayed for serum nicotine levels to assess the influence of vagotomy on overall smoke intake. (A substantial amount of nicotine remains in the circulation of rats 20 minutes after end of exposure and different nicotine exposure levels are reflected by similarly different serum nicotine levels.11) Serum levels of nicotine were not significantly different between the groups (Figure 4D), indicating that the prevention of FMD impairment was a direct result of the vagotomy.
Discussion:
The goal of this project was to determine why a growing number of inhaled tobacco and marijuana products, including combustible products, dry heat vaporizer and heated tobacco products, and e-cigarettes, all acutely impair endothelial function despite fundamental differences in the products. Given that aldehyde gases and inert carbon particles both exert similar impairment effects despite representing completely different chemical and physical components of smoke, we reached the unexpected conclusion that there is not such a convenient substance or property (e.g., particulate nature) responsible. This conclusion implies that either there are multiple mechanisms of FMD impairment mediated by distinct components in these inhalational products, or that all of the products exert their acute effects through a common pathway, such as the inhalation of irritants.
Our results thus suggest that it is unlikely that design changes to different tobacco products (such as cigarettes vs. heated tobacco products11 or e-cigarettes12,13) avoid or substantially reduce the endothelial dysfunction that accompanies using inhaled tobacco (and marijuana) products. The lack of a specific toxin that accounts for FMD impairment means that regulatory agencies cannot rely on imposing product design rules based on prohibiting specific ingredients to avoid adverse effects of inhaled products on endothelial function.
Our study showed that reduction of nicotine level and addition of menthol each independently lessen the acute impairment of FMD. Prior studies in humans showed that exposure to nicotine causes airway irritation and cough with more intense response when exposed to high nicotine smoke as opposed to low nicotine.44 The nicotine-induced cough is mediated through vagal bronchopulmonary C-fibers and rapidly adapting receptors as well as activation of neuronal nicotinic acetylcholine receptors on pulmonary sensory nerves.44–46 This study, as well as our prior studies involving marijuana smoke and cannabis leaf vaporizer aerosols10,47 reveal that nicotine is not required for FMD impairment. However, with lower nicotine level and menthol addition causing less respiratory irritation, our finding suggests a separate mechanism for FMD impairment through respiratory irritation and pulmonary sensory nerves.
The finding that smoke from “reduced nicotine” cigarettes impaired FMD to a lesser extent than that from “conventional nicotine” cigarettes raises the question of whether government-mandated reduction in nicotine to non-addictive levels might also benefit vascular health as a direct result of the lower nicotine level. Notably, the “reduced nicotine” research cigarettes used here contain such a low amount of nicotine that they are practically nicotine-free (yield of 0.02 mg nicotine/cig compared to 0.72 mg nicotine/cig for the conventional nicotine product); presumably far lower than any commercial reduced nicotine product. Therefore, our findings do not suggest any direct vascular benefit from using commercial reduced nicotine products.
It should be emphasized that our finding that addition of menthol reduces the severity of FMD impairment should not be over-interpreted to suggest that menthol is a beneficial additive in smoking and vaping products. Menthol inhibition of respiratory irritation could hypothetically lead to greater exposure to harmful constituents in tobacco products. Moreover, menthol plays a major role in facilitating nicotine addiction, including in youth and specific populations, notably in the African-American and LGBTQ communities, resulting in the FDA recently proposing rules to end menthol in cigarettes and cigars.48 Any incremental benefit to reducing (but not preventing) the acute impairment of FMD by smoke with menthol should be viewed in the context of its addiction-enhancing qualities.
Given the implication that many inhalational products impair FMD via a common pathway not mediated by specific constituents, an important finding of our study is that smoke exposure in vagotomized rats does not impair FMD. As shown previously, there are both NO-mediated and non-NO-mediated mechanisms for FMD.41 The requirement for an intact vagus nerve in the underlying mechanism of FMD impairment suggests that airway irritation mediates downstream inhibitory effects on endothelial function, potentially via neurogenic inflammation43 in a manner that leads to oxidative stress. Such an interpretation is consistent with the findings of Jin et al.,49 who reported airway irritation and endothelial dysfunction in mice exposed to aldehydes found in e-cigarette aerosol. Of note, they did not observe these effects to be caused specifically by acetaldehyde whereas we did. This discrepancy may be due to higher acetaldehyde levels in our study (>10 ppm vs. 5 ppm). Still, our recent report13 showing almost identical extents of FMD impairment from smoke, IQOS, and multiple types of e-cigarettes with and without nicotine indicate a threshold effect that would be unexpected if the culprit was a specific smoke/aerosol constituent, but is not surprising if the effect is caused by an irritation response. The nature of the vagal link from smoking or vaping to acute endothelial dysfunction, and its relationship to the effects of chronic smoking/vaping, are the subject of future studies.
In conclusion, there is no single constituent or class of constituents responsible for acute impairment of endothelial function; instead, we propose that acute endothelial dysfunction by disparate products is caused by a vagus nerve dependent mechanism resulting from airway irritation. Thus, rather than assessing endothelial toxicity of new tobacco products on the basis of a specific compound or compounds, regulatory agencies may need to evaluate the physiological effects directly and recognize that all inhaled products are likely to have similar effects on vascular function.
Supplementary Material
Highlights:
Nicotine was not required for impairment of vascular endothelial function (FMD) by cigarette smoke, but higher nicotine levels increased the extent of impairment, whereas menthol in cigarette smoke decreased the extent of impairment.
Inhalation of acrolein or acetaldehyde gases at concentrations found in smoke impaired FMD.
Inhalation of inert carbon particles at smoke-relevant concentrations also impaired FMD.
Impairment of FMD by smoke was completely abrogated by severing the vagus nerve.
There is no single constituent or class of constituents responsible for acute impairment of endothelial function by smoke; rather, we propose that acute endothelial dysfunction by disparate inhaled products is caused by vagus nerve signaling initiated by airway irritation.
Acknowledgments:
We thank Trisha Mao and Tina Won for running serum nicotine assays. We also appreciate Alexis Parker-Vega for helping in assembling the Ecoflex Kintek gas generator system. The graphic abstract for this paper was created with BioRender.com.
Sources of Funding:
This work was supported by grants R01HL120062 and U54HL147127 from the National Heart, Lung, and Blood Institute at the National Institutes of Health (NIH) and the US Food and Drug Administration (FDA) Center for Tobacco Products, grant P50CA180890 from the National Cancer Institute at the NIH and FDA CTP, and generous support from the Elfenworks Foundation (in memory of Deb O’Keefe) and the Roy E Thomas Medical Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA.
Non-standard Abbreviations and Acronyms:
- SHS
Secondhand smoke
- FMD
Arterial flow-mediated dilation
Footnotes
Disclosures: None.
Supplemental Material:
- Detailed Supplemental Methods for Acrolein and acetaldehyde exposure conditions
- Table S1
- Figures S1–S4
References:
- 1.Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, Deanfield JE. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88:2149–2155. [DOI] [PubMed] [Google Scholar]
- 2.Celermajer DS, Adams MR, Clarkson P, Robinson J, McCredie R, Donald A, Deanfield JE. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med. 1996;334:150–154. [DOI] [PubMed] [Google Scholar]
- 3.Kato T, Inoue T, Morooka T, Yoshimoto N, Node K. Short-term passive smoking causes endothelial dysfunction via oxidative stress in nonsmokers. Can J Physiol Pharmacol. 2006;84:523–529. [DOI] [PubMed] [Google Scholar]
- 4.Carnevale R, Sciarretta S, Violi F, Nocella C, Loffredo L, Perri L, Peruzzi M, Marullo AG, De Falco E, Chimenti I, et al. Acute Impact of Tobacco vs Electronic Cigarette Smoking on Oxidative Stress and Vascular Function. Chest. 2016;150:606–612. [DOI] [PubMed] [Google Scholar]
- 5.Caporale A, Langham MC, Guo W, Johncola A, Chatterjee S, Wehrli FW. Acute Effects of Electronic Cigarette Aerosol Inhalation on Vascular Function Detected at Quantitative MRI. Radiology. 2019;293:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heiss C, Amabile N, Lee AC, Real WM, Schick SF, Lao D, Wong ML, Jahn S, Angeli FS, Minasi P, et al. Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: sustained vascular injury and blunted nitric oxide production. J Am Coll Cardiol. 2008;51:1760–1771. [DOI] [PubMed] [Google Scholar]
- 7.Frey PF, Ganz P, Hsue PY, Benowitz NL, Glantz SA, Balmes JR, Schick SF. The exposure-dependent effects of aged secondhand smoke on endothelial function. J Am Coll Cardiol. 2012;59:1908–1913. [DOI] [PubMed] [Google Scholar]
- 8.Pinnamaneni K, Sievers RE, Sharma R, Selchau AM, Gutierrez G, Nordsieck EJ, Su R, An S, Chen Q, Wang X, et al. Brief exposure to secondhand smoke reversibly impairs endothelial vasodilatory function. Nicotine Tob Res. 2014;16:584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Wang X, Narayan S, Glantz SA, Schick SF, Springer ML. Impairment of Endothelial Function by Little Cigar Secondhand Smoke. Tob Regul Sci. 2016;2:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Derakhshandeh R, Liu J, Narayan S, Nabavizadeh P, Le S, Danforth OM, Pinnamaneni K, Rodriguez HJ, Luu E, et al. One Minute of Marijuana Secondhand Smoke Exposure Substantially Impairs Vascular Endothelial Function. J Am Heart Assoc. 2016;5: e003858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nabavizadeh P, Liu J, Havel CM, Ibrahim S, Derakhshandeh R, Jacob Iii P, Springer ML. Vascular endothelial function is impaired by aerosol from a single IQOS HeatStick to the same extent as by cigarette smoke. Tob Control. 2018;27:s13–s19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao P, Liu J, Springer ML. JUUL and Combusted Cigarettes Comparably Impair Endothelial Function. Tob Regul Sci. 2020;6:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao P, Han DD, Tan K, Mohammadi L, Derakhshandeh R, Navabzadeh M, Goyal N, Springer ML. Comparable Impairment of Vascular Endothelial Function by a Wide Range of Electronic Nicotine Delivery Devices. Nicotine Tob Res. 2022;24:1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barua RS, Ambrose JA, Eales-Reynolds LJ, DeVoe MC, Zervas JG, Saha DC. Dysfunctional endothelial nitric oxide biosynthesis in healthy smokers with impaired endothelium-dependent vasodilatation. Circulation. 2001;104:1905–1910. [DOI] [PubMed] [Google Scholar]
- 15.Barua RS, Ambrose JA, Srivastava S, DeVoe MC, Eales-Reynolds LJ. Reactive oxygen species are involved in smoking-induced dysfunction of nitric oxide biosynthesis and upregulation of endothelial nitric oxide synthase: an in vitro demonstration in human coronary artery endothelial cells. Circulation. 2003;107:2342–2347. [DOI] [PubMed] [Google Scholar]
- 16.Moir D, Rickert WS, Levasseur G, Larose Y, Maertens R, White P, Desjardins S. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem Res Toxicol. 2008;21:494–502. [DOI] [PubMed] [Google Scholar]
- 17.Herrington JS, Myers C. Electronic cigarette solutions and resultant aerosol profiles. J Chromatogr A. 2015;1418:192–199. [DOI] [PubMed] [Google Scholar]
- 18.Neunteufl T, Heher S, Kostner K, Mitulovic G, Lehr S, Khoschsorur G, Schmid RW, Maurer G, Stefenelli T. Contribution of nicotine to acute endothelial dysfunction in long-term smokers. J Am Coll Cardiol. 2002;39:251–256. [DOI] [PubMed] [Google Scholar]
- 19.Chalon S, Moreno H, Jr., Benowitz NL, Hoffman BB, Blaschke TF. Nicotine impairs endothelium-dependent dilatation in human veins in vivo. Clin Pharmacol Ther. 2000;67:391–397. [DOI] [PubMed] [Google Scholar]
- 20.Mayhan WG, Sharpe GM. Chronic exposure to nicotine alters endothelium-dependent arteriolar dilatation: effect of superoxide dismutase. J Appl Physiol (1985). 1999;86:1126–1134. [DOI] [PubMed] [Google Scholar]
- 21.Mayhan WG, Sharpe GM. Superoxide dismutase restores endothelium-dependent arteriolar dilatation during acute infusion of nicotine. J Appl Physiol (1985). 1998;85:1292–1298. [DOI] [PubMed] [Google Scholar]
- 22.Krusemann EJ, Visser WF, Cremers JW, Pennings J, Talhout R. Identification of flavour additives in tobacco products to develop a flavour library. Tob Control. 2018;27:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villanti AC, Mowery PD, Delnevo CD, Niaura RS, Abrams DB, Giovino GA. Changes in the prevalence and correlates of menthol cigarette use in the USA, 2004–2014. Tob Control. 2016;25:ii14–ii20. [DOI] [PubMed] [Google Scholar]
- 24.D’Silva J, Cohn AM, Johnson AL, Villanti AC. Differences in Subjective Experiences to First Use of Menthol and Nonmenthol Cigarettes in a National Sample of Young Adult Cigarette Smokers. Nicotine Tob Res. 2018;20:1062–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CDC. Menthol and Cigarettes. https://www.cdc.gov/tobacco/basic_information/tobacco_industry/menthol-cigarettes/index.html. 2020.
- 26.Klausner K Menthol cigarettes and smoking initiation: a tobacco industry perspective. Tob Control. 2011;20 Suppl 2:ii12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362:2295–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wackowski OA, Evans KR, Harrell MB, Loukas A, Lewis MJ, Delnevo CD, Perry CL. In Their Own Words: Young Adults’ Menthol Cigarette Initiation, Perceptions, Experiences and Regulation Perspectives. Nicotine Tob Res. 2018;20:1076–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alsharari SD, King JR, Nordman JC, Muldoon PP, Jackson A, Zhu AZ, Tyndale RF, Kabbani N, Damaj MI. Effects of Menthol on Nicotine Pharmacokinetic, Pharmacology and Dependence in Mice. PloS one. 2015;10:e0137070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benowitz NL, Herrera B, Jacob P 3rd. Mentholated cigarette smoking inhibits nicotine metabolism. J Pharmacol Exp Ther. 2004;310:1208–1215. [DOI] [PubMed] [Google Scholar]
- 31.Willis DN, Liu B, Ha MA, Jordt SE, Morris JB. Menthol attenuates respiratory irritation responses to multiple cigarette smoke irritants. FASEB J. 2011;25:4434–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conklin DJ, Haberzettl P, Prough RA, Bhatnagar A. Glutathione-S-transferase P protects against endothelial dysfunction induced by exposure to tobacco smoke. Am J Physiol Heart Circ Physiol. 2009;296:H1586–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaimes EA, DeMaster EG, Tian RX, Raij L. Stable compounds of cigarette smoke induce endothelial superoxide anion production via NADPH oxidase activation. Arterioscler Thromb Vasc Biol. 2004;24:1031–1036. [DOI] [PubMed] [Google Scholar]
- 34.Lynch J, Jin L, Richardson A, Conklin DJ. Tobacco Smoke and Endothelial Dysfunction: Role of Aldehydes? Curr Hypertens Rep. 2020;22:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogunwale MA, Li M, Ramakrishnam Raju MV, Chen Y, Nantz MH, Conklin DJ, Fu XA. Aldehyde Detection in Electronic Cigarette Aerosols. ACS Omega. 2017;2:1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. [DOI] [PubMed] [Google Scholar]
- 37.Pope CA 3rd, Burnett RT, Krewski D, Jerrett M, Shi Y, Calle EE, Thun MJ. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: shape of the exposure-response relationship. Circulation. 2009;120:941–948. [DOI] [PubMed] [Google Scholar]
- 38.Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ, et al. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ Res. 2008;102:589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. [DOI] [PubMed] [Google Scholar]
- 40.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Luscher TF, Shechter M, Taddei S, et al. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126:753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol. 2005;568:357–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heiss C, Sievers RE, Amabile N, Momma TY, Chen Q, Natarajan S, Yeghiazarians Y, Springer ML. In vivo measurement of flow-mediated vasodilation in living rats using high-resolution ultrasound. Am J Physiol Heart Circ Physiol. 2008;294:H1086–1093. [DOI] [PubMed] [Google Scholar]
- 43.McDonald DM, Bowden JJ, Baluk P, Bunnett NW. Neurogenic inflammation. A model for studying efferent actions of sensory nerves. Adv Exp Med Biol. 1996;410:453–462. [PubMed] [Google Scholar]
- 44.Lee LY, Burki NK, Gerhardstein DC, Gu Q, Kou YR, Xu J. Airway irritation and cough evoked by inhaled cigarette smoke: role of neuronal nicotinic acetylcholine receptors. Pulm Pharmacol Ther. 2007;20:355–364. [DOI] [PubMed] [Google Scholar]
- 45.Lee LY, Morton RF, Frazier DT. Influence of nicotine in cigarette smoke on acute ventilatory responses in awake dogs. J Appl Physiol (1985). 1985;59:229–236. [DOI] [PubMed] [Google Scholar]
- 46.Xu J, Yang W, Zhang G, Gu Q, Lee LY. Calcium transient evoked by nicotine in isolated rat vagal pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol. 2007;292:L54–61. [DOI] [PubMed] [Google Scholar]
- 47.Liu J, Nabavizadeh P, Rao P, Derakhshandeh R, Springer ML. Abstract 14428: Impairment of Endothelial Function by Aerosol From Marijuana Leaf Vaporizers. Circulation. 2019;140:A14428–A14428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.FDA. FDA Proposes Rules Prohibiting Menthol Cigarettes and Flavored Cigars to Prevent Youth Initiation, Significantly Reduce Tobacco-Related Disease and Death. https://www.fda.gov/news-events/press-announcements/fda-proposes-rules-prohibiting-menthol-cigarettes-and-flavored-cigars-prevent-youth-initiation. 2022.
- 49.Jin L, Lynch J, Richardson A, Lorkiewicz P, Srivastava S, Theis W, Shirk G, Hand A, Bhatnagar A, Srivastava S, et al. Electronic cigarette solvents, pulmonary irritation, and endothelial dysfunction: role of acetaldehyde and formaldehyde. Am J Physiol Heart Circ Physiol. 2021;320:H1510–h1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


