Abstract
Background
Childhood maltreatment affects approximately 25% of the world’s population. Importantly, the children of mothers who have been maltreated are at increased risk of behavioral problems. Thus, one important priority is to identify child neurobiological processes associated with maternal childhood maltreatment (MCM) that might contribute to such intergenerational transmission. This study assessed the impact of MCM on infant gray and white matter volumes and infant amygdala and hippocampal volumes during the first 2 years of life.
Methods
Fifty-seven mothers with 4-month-old infants were assessed for MCM, using both the brief Adverse Childhood Experiences screening questionnaire and the more detailed Maltreatment and Abuse Chronology of Exposure scale. A total of 58% had experienced childhood maltreatment. Between 4 and 24 months (age in months: mean = 12.28, SD = 5.99), under natural sleep, infants completed a magnetic resonance imaging scan using a 3T Siemens scanner. Total brain volume, gray matter volume, white matter volume, and amygdala and hippocampal volumes were extracted via automated segmentation.
Results
MCM on the Adverse Childhood Experiences and Maltreatment and Abuse Chronology of Exposure scales were associated with lower infant total brain volume and gray matter volume, with no moderation by infant age. However, infant age moderated the association between MCM and right amygdala volume, such that MCM was associated with lower volume at older ages.
Conclusions
MCM is associated with alterations in infant brain volumes, calling for further identification of the prenatal and postnatal mechanisms contributing to such intergenerational transmission. Furthermore, the brief Adverse Childhood Experiences questionnaire predicted these alterations, suggesting the potential utility of early screening for infant risk.
Keywords: Amygdala, Gray matter volume, Hippocampus, Imaging, Infant brain development, Intergenerational transmission, Maternal childhood maltreatment
The effects of childhood maltreatment (CM) can transcend generations (1), with offspring of maltreated parents at risk for adverse developmental and health outcomes (2, 3, 4). In addition, CM adversely affects brain development of the maltreated individual (5,6). However, little work has examined whether maternal CM (MCM) affects brain development of the affected mother’s offspring. To date, two studies have examined whether MCM is associated with offspring brain volume and function. Most relevant to this study, in a sample of newborns, Moog et al. (7) found that MCM was associated with lower infant intracranial volume and reduced gray matter volume (GMV) but not with differences in white matter volume (WMV), cerebrospinal fluid volume, amygdala volume, or hippocampal volume. Thus, MCM may have an effect on infant brain volume that is evident close to birth, prior to the impact of postnatal risks. In addition, Hendrix et al. (8) found that maternal childhood emotional neglect was associated with stronger infant functional connectivity between the amygdala and the dorsal anterior cingulate cortex and ventromedial prefrontal cortex, connectivity that has previously been associated with fear conditioning. This study extends the work by Moog et al. (7) by assessing how MCM is related to GMV, WMV, amygdala volume, and hippocampal volume between the ages of 4 and 24 months.
Although only one study has examined MCM and offspring brain volume (7), other studies have demonstrated that additional psychosocial risk factors are associated with child GMV and WMV. Lower socioeconomic status (SES) has been linked to lower GMV but not with lower WMV (9,10) in infants and children. Lower maternal sensitivity was associated with lower GMV in infants (11) and 8-year-old children (12), but not with combined GMV and WMV in infants (11). In addition, as reviewed elsewhere (13), higher maternal stress during pregnancy is an important risk factor related to decreased offspring GMV (14). Overall, this literature suggests that biological embedding of psychosocial adversity begins early in life and that early brain development may be linked to prenatal and postnatal adversity.
The amygdala and hippocampus may be particularly vulnerable to early stress (15). Although most studies have focused on older children, a few studies have examined associations between adversity during the first 2 years of life (institutional rearing, maltreatment, maternal depression) and amygdala and hippocampal volumes later in childhood (10,16, 17, 18). While some studies found volumetric increases to the amygdala and hippocampus (16, 17, 18), others have found volumetric decreases (19,20). The direction and magnitude of the impact of adversity on limbic volumes may depend on the type of stressor, developmental timing, and age at measurement (21,22).
Notably, Moog et al. (7) did not find associations between MCM and amygdala and hippocampal volumes in newborn infants. However, effects on these stress-sensitive brain regions may emerge later in development.
Moog et al. (7) used the Childhood Trauma Questionnaire to assess MCM in relation to infant brain volume. However, detailed assessments such as the Childhood Trauma Questionnaire can be time intensive. Given the public health priority for early identification of infants with altered brain development, in this study, maternal CM experiences were assessed using both the brief Adverse Childhood Experiences (ACE) questionnaire (23) and the more detailed Maltreatment and Abuse Chronology of Exposure (MACE) scale (24). Use of both measures allowed an evaluation of whether a brief screening measure is sensitive enough to identify associations between MCM and infant brain volume.
Study aims were as follows: 1) assess associations between MCM and indices of brain volume during the first 2 years of life, including total brain volume (TBV), GMV, and WMV (for cerebrospinal fluid volume findings, see the Supplement); 2) examine associations between MCM and infant amygdala and hippocampal volumes; 3) evaluate whether these associations were moderated by infant age at scan during the first 2 years of life; and 4) assess how the brief ACE assessment compares to the more detailed MACE in relation to aims 1, 2, and 3.
Based on previous literature (7), we hypothesized that higher MCM would be associated with lower TBV and GMV but not with WMV, starting early in the first year of life. Based on the nonsignificant finding of Moog et al. (7) regarding MCM and limbic volumes in newborns, we hypothesized that associations between MCM and amygdala and hippocampal volumes would be moderated by age, with effects emerging later in the first year. Given previous conflicting findings, specific hypotheses were not offered regarding whether MCM would be associated with increased or decreased amygdala and hippocampal volumes.
Methods and Materials
Participants
Fifty-seven mother-infant dyads were included in the magnetic resonance imaging (MRI) analyses. Dyads were drawn from a cohort of 181 families enrolled in the Harvard MIND (Mother-Infant Neurobiological Development) study. Mothers in the MIND study were recruited through prenatal classes, community flyers, and local birth records. Participants were screened and stratified such that at least 50% of mothers had experienced one or more forms of CM (physical, sexual, emotional abuse; witnessed domestic violence; physical, emotional neglect). Exclusion criteria were 1) English not a primary language spoken at home, 2) maternal age >44 years at infant birth, 3) infant <36-week gestation or <2500 g at birth, and 4) infant congenital defect or disorder. Dyads participated in behavioral assessments at infant ages 4 and 15 months.
All MIND study participants were offered participation in the infant MRI assessment. Of these, 17.12% (n = 31) declined and 17.12% (n = 31) could not be scheduled or withdrew from the MRI prior to scanning. Of the consenting and available participants, 52.10% (n = 62) attempted to complete the MRI but were unsuccessful (see the Supplement). This resulted in 57 infants successfully completing the MRI. Infants with MRIs were similar to infants without MRIs on demographic characteristics and key study variables (see the Supplement). The study was approved by the institutional review board (Partners Healthcare IRB Protocol #: 2014P002522).
Infants participated in MRIs between 4 and 25 months of age (age in days: mean = 357.93, SD = 185.98; 49% male). Table 1 shows sociodemographic characteristics of the MRI sample. On study entry, the first half of participants were offered MRI scans after the 15-month assessment, while the second half of participants were offered scans after the 4-month assessment. Fifteen-month scans were offered to earlier participants owing to the long lead time needed from recruitment to the post–15-month scan. Thus, as seen in Figure 1, ages at scans clustered after the 4-month assessment and after the 15-month assessment. As shown in Table 2, age at scan was not correlated with sociodemographic variables. However, there was a moderate correlation between older ages at scan and lower ACE (r = −0.35, p < .01) and MACE (r = −0.28, p < .05) severity scores, reflecting the difficulty of obtaining successful scans from at-risk toddlers. Thus, age at scan was covaried in all analyses that included the ACE or MACE.
Table 1.
Sociodemographic Characteristics and Descriptive Statistics
| Characteristics | Mean (SD) or n (%) | Range |
|---|---|---|
| Gestational Age, Weeksa | 39.48 (1.60) | 36–42 |
| Infant Age at MRI, Days | 357.93 (185.98) | 122–750 |
| Infant Ethnicity | ||
| Hispanic | 3 (5.3%) | – |
| Non-Hispanic | 54 (94.7%) | – |
| Infant Race | ||
| Asian | 1 (1.8%) | – |
| Black | 5 (8.8%) | – |
| Multiracial | 15 (26.3%) | – |
| White | 36 (63.2%) | – |
| Maternal Education | ||
| High school | 8 (14.0%) | – |
| Associate degree | 5 (8.8%) | – |
| Bachelor’s degree | 14 (24.6%) | – |
| Master’s degree | 20 (35.1%) | – |
| Doctoral degree | 10 (17.5%) | – |
| Annual Family Income | ||
| $0–$15,000 | 4 (7.0%) | – |
| $16,000–$25,000 | 2 (3.5%) | – |
| $26,000–$50,000 | 6 (10.5%) | – |
| $51,000–$75,000 | 15 (26.3%) | – |
| $76,000–$100,000 | 10 (17.5%) | – |
| $101,000–$150,000 | 10 (17.5%) | – |
| $151,000+ | 10 (17.5%) | – |
| ACE Distribution | ||
| 0 forms of CM | 24 (42.1%) | – |
| 1–3 forms of CM | 23 (40.4%) | – |
| 4+ forms of CM | 10 (17.5%) | – |
| Emotional abuse | 26 (45.6%) | – |
| Physical abuse | 18 (31.6%) | – |
| Sexual abuse | 12 (21.1%) | – |
| Emotional neglect | 14 (24.6%) | – |
| Physical neglect | 8 (14.0%) | – |
| Witnessing domestic violence | 12 (21.1%) | – |
| ACE Severity | 1.58 (1.77) | 0–6 |
| MACE Severity | 15.72 (14.78) | 0–55 |
| EPDS | 5.81 (4.67) | 0–17 |
| TBV | 1,049,357.76 (175,853.04) | 709,725–1,519,437 |
| GMV | 541,885.60 (117,789.69) | 294,497–737,526 |
| WMV | 355,680.70 (82,719.53) | 173,861–521,082 |
| Amygdala | ||
| Right amygdala | 11,253.47 (436.85) | 621–2754 |
| Left amygdala | 1133.89 (329.91) | 603–2041.00 |
| Hippocampus | ||
| Right hippocampus | 2953.68 (630.90) | 1784–4642 |
| Left hippocampus | 3145.86 (643.06) | 1875–5438 |
Brain volume metric is mm3. Reported brain volumes have outliers removed from TBV and amygdala and hippocampal volumes.
ACE, Adverse Childhood Experiences; CM, childhood maltreatment; EPDS, Edinburgh Postnatal Depression Scale; GMV, gray matter volume; MACE, Maltreatment and Abuse Chronology of Exposure; MRI, magnetic resonance imaging; TBV, total brain volume; WMV, white matter volume.
Infants below 36 weeks’ gestation were excluded from the study.
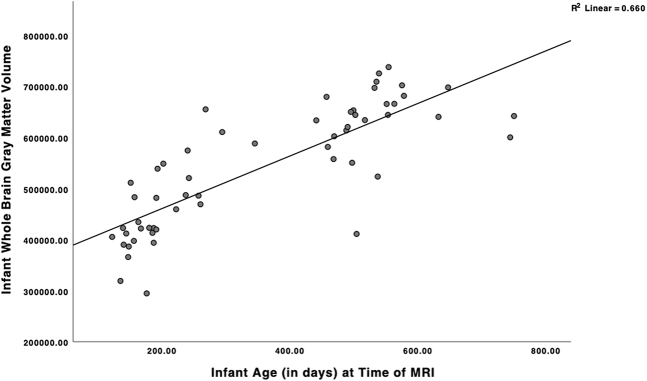
Figure 1.
Scatterplot displaying association between infant age at scan and whole-brain gray matter volume. Age in days: mean = 357.93; SD = 185.98; n = 57. MRI, magnetic resonance imaging.
Table 2.
Bivariate Correlations Among Infant Brain Volumes and Potential Covariates
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Total Brain Volume | – | |||||||||||
| 2 Gray Matter Volume | 0.90a | – | ||||||||||
| 3 White Matter Volume | 0.43a | 0.08 | – | |||||||||
| 4 RH Amygdala Volume | 0.31b | 0.23 | 0.17 | – | ||||||||
| 5 LH Amygdala Volume | 0.31b | 0.24 | 0.13 | 0.90a | – | |||||||
| 6 RH Hippocampal Volume | 0.13 | 0.15 | −0.05 | 0.84a | 0.77a | – | ||||||
| 7 LH Hippocampal Volume | 0.19 | 0.19 | −0.12 | 0.65a | 0.69a | 0.84a | – | |||||
| 8 Infant Age at MRI | 0.78a | 0.81a | 0.13 | 0.13 | 0.16 | 0.10 | 0.20 | – | ||||
| 9 Length of Pregnancy | 0.19 | 0.25 | −0.04 | 0.03 | 0.08 | 0.12 | 0.15 | −0.31b | – | |||
| 10 Infant Female Sex | −0.14 | −0.07 | −0.15 | −0.12 | −0.22 | −0.13 | −0.19 | 0.11 | 0.07 | – | ||
| 11 Higher Income | 0.05 | 0.06 | 0.17 | 0.20 | 0.10 | 0.11 | 0.03 | −0.07 | 0.13 | 0.00 | – | |
| 12 Higher Maternal Education | 0.12 | 0.21 | −0.03 | 0.02 | 0.07 | 0.16 | 0.24 | 0.12 | 0.47a | −0.16 | 0.38a | – |
| 13 Maternal EPDS Total | −0.08 | −0.06 | −0.07 | 0.09 | 0.09 | 0.22 | 0.28b | 0.04 | −0.14 | 0.13 | −0.30b | −0.06 |
n = 57, except for regions with outliers that were removed, resulting in total brain volume n = 56; RH/LH amygdala volumes n = 56; RH/LH hippocampal volumes n = 55. Infant age is measured in days; gestational age at birth is measured in weeks; infant sex (0 = male; 1 = female); maternal education is measured in years of education.
EPDS, Edinburgh Postnatal Depression Scale; LH, left hemisphere; MRI, magnetic resonance imaging; RH, right hemisphere.
p < .01.
p < .05.
Measures
Maternal Experiences of CM
Mothers completed the 10-item ACE questionnaire (23) during an initial screening call. The ACE screens for the presence of emotional, physical, and sexual abuse; emotional and physical neglect; and witnessing domestic violence (DV) prior to age 18. Although DV was originally conceptualized as a household dysfunction on the ACE scale (23), DV has since been conceptualized as falling under the threat dimension of CM (25). Thus, for consistency across MCM measures, DV was included in the ACE severity score. The ACE severity score ranged from 0 to 6, reflecting how many of the six maltreatment types were experienced. The ACE has validity in relation to maternal health and infant development (26), as well as adult medical conditions (23). The ACE also correlates highly with more detailed assessments of CM (24).
Mothers also completed the 75-item MACE scale (24). The MACE assesses the severity of seven types of parental maltreatment, including verbal abuse, nonverbal emotional abuse, physical abuse, sexual abuse, emotional neglect, physical neglect, and witnessing parental DV, as well as three less often assessed forms of nonparental maltreatment including witnessing violence to siblings, peer emotional abuse, and peer physical bullying. The overall severity score used here summed the severity of the seven types of parental maltreatment and thus had a possible range from 0 to 70. Note that these types parallel the types of CM assessed on the ACE questionnaire. MACE scores correlate highly with other measures of CM and have high test-retest reliability (24).
Covariate Assessments
Sociodemographic characteristics were assessed by maternal interview, including infant sex, infant gestational age at birth (in weeks), family income, and maternal education. Infant non-White race and infant Hispanic ethnicity were also reported. However, only 3 infants were identified as Hispanic, and most non-White participants were of mixed race (Table 1), so classification by race had unclear meaning. Therefore, race and ethnicity were not considered further.
Maternal depressive symptoms have previously been associated with infant brain volume (16,27,28), and thus, maternal depressive symptoms were considered as a covariate. Maternal depressive symptoms were assessed using the Edinburgh Postnatal Depression Scale (EPDS) (29) at infant age 4 months. The EPDS consists of 10 items that assess depressive symptoms (total score range 0–30). The EPDS is widely used for screening for postpartum depression (29).
Imaging Data Acquisition and Processing
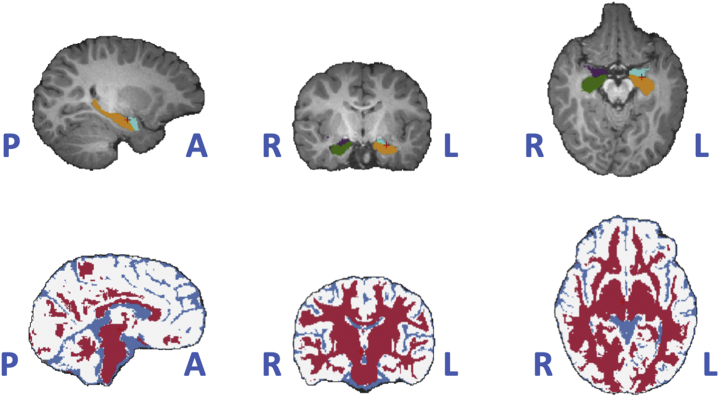
All infant MRIs were performed on a 3T Siemens Skyra scanner (Siemens Healthineers) with a 64-channel head coil. Infants were scanned during natural sleep, and no sedation was used. The T1-weighted acquisition used an advanced version of the magnetization prepared rapid acquisition gradient-echo sequence, where fast, low-resolution volumetric navigators were played for each repetition period and were used for prospective motion correction (30). The specific imaging parameters of the magnetization prepared rapid acquisition gradient-echo acquisition included voxel size = 1 × 1 × 1 mm3, repetition time = 2500 to 2540 ms, echo time = 1.65 to 2.37 ms, inversion time = 1450 to 1470 ms, field of view = 192 × 192 mm2 and between 144 and 173 slices, enough to cover the entire brain of the infant. After performing visual quality control using the Freeview software (surfer.nmr.mgh.harvard.edu), the T1-weighted volumes were manually aligned along the anterior commissure–posterior commissure plane and underwent N4 bias correction (31), field of view normalization (32) and multiatlas skull stripping (33). This was followed by automatic segmentation into cortical and subcortical regions as well as tissue type classification using a multiatlas-to-subject registration and fusion (34) that has been extensively validated (35,36) and adapted to infant brain MRIs (37,38). Quality control of the segmentations was visually performed using the FSLView software (https://fsl.fmrib.ox.ac.uk/). These segmentations enabled the extraction of TBV, GMV, WMV, cerebrospinal fluid volume (see the Supplement), and right and left hemisphere amygdala and hippocampal volumes (Figure 2).
Figure 2.
Autosegmented regions of interest and tissue types in a randomly picked subject. Top: amygdala (light blue and purple) and hippocampus (green and yellow) regions in both hemispheres in the triplanar review. Bottom: tissue types (red for gray matter, white for white matter, and blue for cerebrospinal fluid) in the same triplanar view. A, anterior; L, left; P, posterior; R, right.
Statistical Analyses
Statistical analyses were conducted using IBM SPSS Statistics (version 26) and Mplus (version 8; Muthén & Muthén). Linear regressions were conducted using maximum likelihood estimation with robust standard errors and full information maximum likelihood to account for missing data. Outliers were identified using boxplots in SPSS. Extreme outliers (e.g., third quartile + 3 × interquartile range) for brain volumes were removed and estimated using full information maximum likelihood to retain participants. Regression analyses were used to assess MCM associations with infant brain volumes, controlling for relevant covariates. Models were run assessing MCM and age main effects and interactions.1 Analyses were conducted using both the MACE and ACE scores to assess whether the ACE demonstrated similar strength in capturing the associations between MCM and infant brain volumes. Our rationale for not correcting for multiple comparisons is included in the Supplement.
Results
Descriptives and Covariates
There was one outlier for TBV, one for amygdala volume, and two for hippocampal volume (3 participants total). Outliers were removed and estimated using full information maximum likelihood in regression analyses. Table 1 displays descriptive statistics for all study variables, excluding outliers.
Family income, maternal education, maternal depressive symptoms, infant sex, gestational age at birth, and age at scan were assessed as potential covariates in relation to infant brain volumes of interest (Table 2). Infant age at MRI was positively correlated with GMV (Figure 1) and TBV (Figure S1). Infant sex was not a significant covariate in relation to infant brain volumes when all ages were combined. However, infant female sex was associated with smaller GMV (r = −0.46, p <.05) and hippocampal volumes (left hemisphere r = −0.48, p < .05; right hemisphere r = −0.44, p < .05) in infants older than 12 months. Given that sex is routinely covaried in brain volume studies (7), we included it as a covariate in all analyses. In addition, higher maternal depressive symptoms were significantly correlated with larger infant left hemisphere hippocampal volume (r = 0.28, p < .05). No other potential covariates were associated with infant brain volumes (Table 2). Most of the sample (68.4%) fell in the EPDS category of low probability of depression, which might account for the nonsignificant associations between the EPDS and other brain volumes. As is common in MRI analyses, we controlled for infant age at MRI and infant sex across all models. Analyses of amygdala and hippocampal volumes also controlled for total GMV. Lastly, given the significant bivariate association between depression and left hemisphere hippocampal volume, depression was included as a covariate in all left hemisphere hippocampal analyses.
MCM and Overall Infant Brain Volumes
Greater MACE severity was associated with lower TBV (β = −0.179, SE = 0.067, 95% CI −0.310 to −0.048) and GMV (β = −0.194, SE = 0.093, 95% CI −0.377 to −0.011). MACE severity was not associated with WMV (β = −0.120, SE = 0.147, 95% CI −0.410 to 0.169). The interaction of MACE severity with age was not significant for TBV (β = −0.127, SE = 0.136, 95% CI −0.394 to 0.140) or GMV (β = −0.086, SE = 0.194, 95% CI −0.466 to 0.294). However, the interaction trended toward significance for WMV (β = −0.518, SE = 0.264, p = .050, 95% CI −1.036 to −0.001).
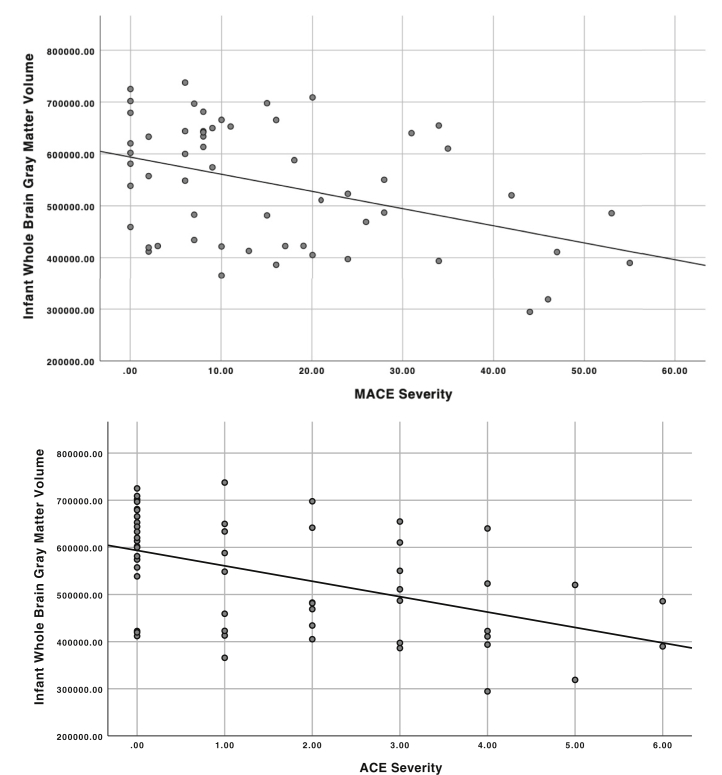
ACE severity was associated with lower TBV (β = −0.197, SE = 0.069, 95% CI −0.332 to −0.062) and GMV (β = −0.229, SE = 0.064, 95% CI −0.388 to −0.070) but not WMV (β = −0.100, SE = 0.149, 95% CI −0.391 to 0.191). There were no significant interactions between ACE severity and age at scan on TBV (β = −0.150, SE = 0.136, 95% CI −0.417 to 0.117), GMV (β = −0.112, SE = 0.168, 95% CI −0.441 to 0.216), or WMV (β = −0.432, SE = 0.269, 95% CI −0.960 to 0.096). See Figure 3 for distribution of infant GMV by ACE and MACE severity.
Figure 3.
Maltreatment and Abuse Chronology of Exposure (MACE) scale and Adverse Childhood Experiences (ACE) maternal childhood maltreatment severity in relation to infant gray matter volume. ACE severity range 0–6; MACE severity range 0–55. Gray matter volume unit mm3.
MCM and Infant Amygdala and Hippocampal Volumes
Amygdala
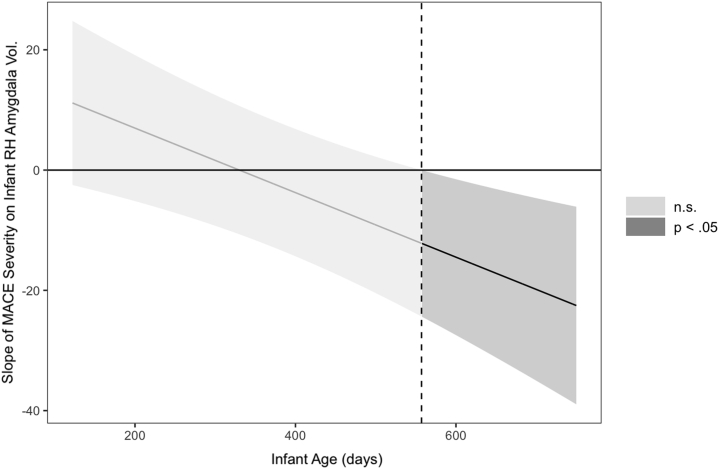
In contrast to GMV, the association between MACE severity and right amygdala volume was moderated by age (β = −0.611, SE = 0.184, 95% CI −0.973 to −0.250).2 Greater MACE severity was associated with decreased right amygdala volume as age increased. Simple slope analyses indicated that the negative association between MACE severity and right hemisphere amygdala became significant at older infant ages (β = −0.419, SE = 0.213, 95% CI −0.837 to −0.002) (Figure 4) (39).
Figure 4.
Region of significance of the interaction between Maltreatment and Abuse Chronology of Exposure (MACE) scale severity and infant age in association with infant right hemisphere (RH) amygdala volume (Vol.). Region of significance plotted using the Johnson-Neyman method (39). This graph was derived using linear regression with maximum likelihood estimation with robust standard errors estimation, without full information maximum likelihood. n.s., not significant.
Results for ACE severity were similar, with age moderating the association between ACE MCM severity and right amygdala volume, such that greater ACE severity was associated with more pronounced decreased volume for older infants (β = −0.554, SE = 0.176, 95% CI −0.899 to −0.209).
There were no main effects of MACE or ACE severity on left amygdala volume, and there were no significant interactions between either MACE or ACE severity and age (MACE main effects: β = 0.023, SE = 0.179, 95% CI −0.328 to 0.373; MACE × age interaction: β = −0.291, SE = 0.201, 95% CI −0.685 to 0.102; ACE main effects: β = 0.047, SE = 0.195, 95% CI −0.334 to 0.428; ACE × age interaction: β = −0.247, SE = 0.212, 95% CI −0.662 to 0.167).
Hippocampus
Neither the main effect of MACE severity nor the interaction between MACE and infant age was significant for right hippocampal volume (MACE: β = 0.112, SE = 0.177, 95% CI −0.235 to 0.459; MACE × age interaction: β = −0.206, SE = 0.179, 95% CI −0.556 to 0.145). Results were similar for the ACE. Neither the main effect of ACE severity (β = 0.092, SE = 0.198, 95% CI −0.296 to 0.479) nor the interaction between infant age and ACE severity was significant for the right hippocampal volume (β = −0.305, SE = 0.193, 95% CI −0.683 to 0.074).
Similarly, neither MACE nor ACE severity was associated with left hippocampal volume, nor were the interactions between MACE/ACE severity and age significant (MACE main effect: β = 0.010, SE = 0.158, 95% CI −0.301 to 0.320; MACE × age interaction: β = 0.059, SE = 0.238, 95% CI −0.409 to 0.526; ACE main effect: β = −0.018, SE = 0.150, 95% CI −0.312 to 0.275; ACE × age interaction: β = 0.046, SE = 0.222, 95% CI −0.389 to 0.480).
Discussion
This study provides evidence that maternal history of CM is linked to infant brain volumes beyond the immediate postnatal period. Findings indicated that during the first 2 years of life, greater severity of MCM was associated with 1) lower infant TBV and GMV, 2) marginally lower WMV, and 3) lower infant right amygdala volume. These results emerged with similar magnitude when using either the ACE or MACE instruments.
To our knowledge, this is the first report to link MCM to lower infant TBV and GMV beyond the neonatal period. One previous study (7) found that MCM was associated with lower TBV and GMV in newborns. Similarly, our finding that MCM was associated with lower TBV seemed to be driven primarily by GMV, not WMV, effects. Notably, the relation between MCM and infant GMV was not moderated by infant age, suggesting that this association is consistent during the first 2 years of development.
Studies of typical trajectories of brain development find that overall brain volume undergoes rapid development in the latter part of gestation and early postnatal period (40). In the first year of life, GMV increases between 108% and 149%, and in the second year of life, GMV further increases by 14% to 19% (41). After controlling for infant age at MRI, the current findings suggest that infants of mothers with more severe MCM exhibit less GMV increase during the first 2 years of life.
The association between MCM severity and infant GMV during the first 2 years is consistent with both prenatal and postnatal effects on GMV. The majority of individual variation in GMV has occurred by 1 year of age (42). Epigenetic mechanisms related to endocrine and immune processes during gestation are believed to play a key role in the intergenerational consequences of MCM (1,43, 44, 45, 46, 47). Thus, given rapid neural development throughout gestation, these gestational effects may also affect postnatal brain development (1). Future work is needed to identify possible epigenetic mechanisms linking MCM and infant brain development.
In addition, given that GMV undergoes substantial growth in the first postnatal year, postnatal influences associated with MCM are also likely (41). MCM has been associated with increased maternal depression (48), suboptimal parenting (49,50), atypical maternal and infant stress responses (51, 52, 53, 54, 55), and disruptions in early mother-infant interaction (55). Finally, other work has linked reduced GMV in infancy and childhood to lower SES (9,10) and lower maternal sensitivity (11,12). The moderate to high SES of this sample may contribute to the lack of association between SES and infant brain volume. Taken together, these prenatal and postnatal factors should be explored in future work to assess whether they act as mediating or moderating mechanisms linking MCM and infant brain volumes.
These data also revealed a trend suggesting that at older infant ages, greater MCM is associated with marginally decreased WMV. Previous research has not found direct links between MCM (7) or other psychosocial risk factors (10) and WMV in infants, possibly because of the long developmental course of myelination. Future research, with larger samples, is needed to confirm a possible association between MCM and WMV at older ages.
The current data exhibited an increasing negative association between MCM severity and right amygdala volume during the first 2 years of life, which became significant at approximately 18 months of age (Figure 4). The study of newborns by Moog et al. (7) did not find a significant association between MCM severity and amygdala volume shortly after birth. Thus, associations between MCM and the amygdala may not be evident at birth but may become detectable during the period of rapid amygdala growth that occurs over the early years of life.
Given the more pronounced association between MCM and the amygdala in older infants, postnatal environmental factors associated with MCM may be particularly relevant to development of the right amygdala. Among 9- to 15-year-old children, reduced amygdala volume was related to early-life stress, including abuse, neglect, and sociodemographic risk (10). In addition, in early adolescence, fewer maternal positive behaviors were associated with smaller right amygdala volumes followed by accelerated growth in the right amygdala from early to mid adolescence (56). In contrast, other research links greater maternal availability to smaller right amygdala in 1-year-olds (57). Future work should examine both prenatal and postnatal factors that might mediate the link between MCM and right amygdala volume.
It remains unclear why MCM findings were lateralized to the right amygdala. However, the right-sided effect found here converges with previous research in adults showing that CM and posttraumatic stress disorder are more consistently associated with right-sided differences in adult amygdala volume (21,58, 59, 60, 61, 62). Continued work is needed to identify environmental or epigenetic factors that differentially affect the right versus left amygdala.
Finally, the association of MCM and lower GMV using the more complex MACE assessment was similar when using the six yes/no maltreatment questions from the brief ACE questionnaire. These ACE results have important public health implications because the ACE scale is more easily administered in clinical settings. Replications of these findings would support the screening of pregnant women and new mothers for CM histories as part of routine care to provide early support for maltreated mothers and their infants.
Limitations
Study limitations are also important to note. First, this study included a relatively small sample of infants assessed between 4 and 24 month of age. Because the study was cross-sectional, no conclusions can be drawn regarding changes in brain volume over time. Future work should examine longitudinal trajectories of infant brain growth related to MCM. In addition, owing to the small sample, we were not able to explore differential correlates of maternal childhood abuse versus neglect. Future work with larger samples should assess effects related to the type and timing of MCM on infant brain volumes. This sample also demonstrated relatively high income and education, which might explain why SES was not associated with infant brain volumes. Measures of MCM also rely on self-report and may be subject to biased recall, although similar measures have shown good validity in relation to child protective service reports (63, 64, 65) and health outcomes (23,26). Finally, there was a modest correlation between age at scan and lower severity of MCM in this study. Age at scan was controlled in all analyses, however, and the tendency for older infants to be at lower risk would be conservative to the finding that MCM was related to lower amygdala volume at older ages. Nevertheless, further work in larger, more diverse samples is needed to corroborate and generalize study findings.
Conclusions
MCM is associated with decreased infant TBV and GMV during the first 2 years of life and with decreased volume in the right amygdala among infants 18 months and older. Findings using the comprehensive MACE instrument (24) were similar when using the brief ACE questionnaire (23). Thus, using the ACE questionnaire in pregnancy may allow for early identification and intervention for maltreated mothers and their infants at risk for altered neurobiological development. Further research is needed to identify the contributing prenatal and postnatal biological and behavioral mechanisms that might account for the influence of MCM on infant TBV, GMV, and right amygdala volume so that appropriate interventions may be developed.
Acknowledgments and Disclosures
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grant No. R01HD079484 [to KL-R, PEG, MBE, and MHT]).
We would like to thank the families whose interest and participation made this work possible and also express our appreciation for the hard work and dedication of the study staff.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Given that Mplus will not run models when the variances of variables are too large, all brain volumes were divided by 1000, and age (in days) was divided by 100 to reduce variance of the age by MCM interactions.
In models excluding the significant interaction terms, the main effects of MACE severity (β = 0.074, SE = 0.191, CI −0.601 to 0.297) and ACE severity (β = 0.122, SE = 0.198, CI −0.068 to 0.128) were not significant.
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2021.09.005.
Supplementary Material
References
- 1.Buss C., Entringer S., Moog N.K., Toepfer P., Fair D.A., Simhan H.N., et al. Intergenerational transmission of maternal childhood maltreatment exposure: Implications for fetal brain development. J Am Acad Child Adolesc Psychiatry. 2017;56:373–382. doi: 10.1016/j.jaac.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noll J.G., Trickett P.K., Harris W.W., Putnam F.W. The cumulative burden borne by offspring whose mothers were sexually abused as children: Descriptive results from a multigenerational study. J Interpers Violence. 2009;24:424–449. doi: 10.1177/0886260508317194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts A.L., Galea S., Austin S.B., Corliss H.L., Williams M.A., Koenen K.C. Women’s experience of abuse in childhood and their children’s smoking and overweight. Am J Prev Med. 2014;46:249–258. doi: 10.1016/j.amepre.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plant D.T., Pawlby S., Pariante C.M., Jones F.W. When one childhood meets another—Maternal childhood trauma and offspring child psychopathology: A systematic review. Clin Child Psychol Psychiatry. 2018;23:483–500. doi: 10.1177/1359104517742186. [DOI] [PubMed] [Google Scholar]
- 5.Gallo E.A.G., Munhoz T.N., Loret de Mola C., Murray J. Gender differences in the effects of childhood maltreatment on adult depression and anxiety: A systematic review and meta-analysis. Child Abuse Negl. 2018;79:107–114. doi: 10.1016/j.chiabu.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Hughes K., Bellis M.A., Hardcastle K.A., Sethi D., Butchart A., Mikton C., et al. The effect of multiple adverse childhood experiences on health: A systematic review and meta-analysis. Lancet Public Health. 2017;2:e356–e366. doi: 10.1016/S2468-2667(17)30118-4. [DOI] [PubMed] [Google Scholar]
- 7.Moog N.K., Entringer S., Rasmussen J.M., Styner M., Gilmore J.H., Kathmann N., et al. Intergenerational effect of maternal exposure to childhood maltreatment on newborn brain anatomy. Biol Psychiatry. 2018;83:120–127. doi: 10.1016/j.biopsych.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hendrix C.L., Dilks D.D., McKenna B.G., Dunlop A.L., Corwin E.J., Brennan P.A. Maternal childhood adversity associates with frontoamygdala connectivity in neonates. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6:470–478. doi: 10.1016/j.bpsc.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betancourt L.M., Avants B., Farah M.J., Brodsky N.L., Wu J., Ashtari M., Hurt H. Effect of socioeconomic status (SES) disparity on neural development in female African-American infants at age 1 month. Dev Sci. 2016;19:947–956. doi: 10.1111/desc.12344. [DOI] [PubMed] [Google Scholar]
- 10.Hanson J.L., Hair N., Shen D.G., Shi F., Gilmore J.H., Wolfe B.L., Pollak S.D. Family poverty affects the rate of human infant brain growth. PLoS One. 2013;8 doi: 10.1371/journal.pone.0080954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sethna V., Pote I., Wang S., Gudbrandsen M., Blasi A., McCusker C., et al. Mother–infant interactions and regional brain volumes in infancy: An MRI study. Brain Struct Funct. 2017;222:2379–2388. doi: 10.1007/s00429-016-1347-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kok R., Thijssen S., Bakermans-Kranenburg M.J., Jaddoe V.W., Verhulst F.C., White T., et al. Normal variation in early parental sensitivity predicts child structural brain development. J Am Acad Child Adolesc Psychiatry. 2015;54:824–831.e1. doi: 10.1016/j.jaac.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Charil A., Laplante D.P., Vaillancourt C., King S. Prenatal stress and brain development. Brain Res Rev. 2010;65:56–79. doi: 10.1016/j.brainresrev.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Buss C., Davis E.P., Muftuler L.T., Head K., Sandman C.A. High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6–9-year-old children. Psychoneuroendocrinology. 2010;35:141–153. doi: 10.1016/j.psyneuen.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dedovic K., Duchesne A., Andrews J., Engert V., Pruessner J.C. The brain and the stress axis: The neural correlates of cortisol regulation in response to stress. Neuroimage. 2009;47:864–871. doi: 10.1016/j.neuroimage.2009.05.074. [DOI] [PubMed] [Google Scholar]
- 16.Lupien S.J., Parent S., Evans A.C., Tremblay R.E., Zelazo P.D., Corbo V., et al. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc Natl Acad Sci U S A. 2011;108:14324–14329. doi: 10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta M.A., Golembo N.I., Nosarti C., Colvert E., Mota A., Williams S.C., et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: The English and Romanian Adoptees study pilot. J Child Psychol Psychiatry. 2009;50:943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- 18.Tottenham N., Hare T.A., Quinn B.T., McCarry T.W., Nurse M., Gilhooly T., et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. 2010;13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanson J.L., Nacewicz B.M., Sutterer M.J., Cayo A.A., Schaefer S.M., Rudolph K.D., et al. Behavioral problems after early life stress: Contributions of the hippocampus and amygdala. Biol Psychiatry. 2015;77:314–323. doi: 10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodel A.S., Hunt R.H., Cowell R.A., Van Den Heuvel S.E., Gunnar M.R., Thomas K.M. Duration of early adversity and structural brain development in post-institutionalized adolescents. Neuroimage. 2015;105:112–119. doi: 10.1016/j.neuroimage.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teicher M.H., Samson J.A. Annual research review: Enduring neurobiological effects of childhood abuse and neglect. J Child Psychol Psychiatry. 2016;57:241–266. doi: 10.1111/jcpp.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teicher M.H., Samson J.A., Anderson C.M., Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci. 2016;17:652–666. doi: 10.1038/nrn.2016.111. [DOI] [PubMed] [Google Scholar]
- 23.Felitti V.J., Anda R.F., Nordenberg D., Williamson D.F., Spitz A.M., Edwards V., et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) study. Am J Prev Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 24.Teicher M.H., Parigger A. The ‘Maltreatment and Abuse Chronology of Exposure’ (MACE) Scale for the retrospective assessment of abuse and neglect during development. PLoS One. 2015;10 doi: 10.1371/journal.pone.0117423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLaughlin K.A., Sheridan M.A., Lambert H.K. Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neurosci Biobehav Rev. 2014;47:578–591. doi: 10.1016/j.neubiorev.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Racine N., Plamondon A., Madigan S., McDonald S., Tough S. Maternal adverse childhood experiences and infant development. Pediatrics. 2018;141 doi: 10.1542/peds.2017-2495. [DOI] [PubMed] [Google Scholar]
- 27.Sethna V., Siew J., Gudbrandsen M., Pote I., Wang S., Daly E., et al. Maternal depression during pregnancy alters infant subcortical and midbrain volumes. J Affect Disord. 2021;291:163–170. doi: 10.1016/j.jad.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Wen D.J., Poh J.S., Ni S.N., Chong Y.S., Chen H., Kwek K., et al. Influences of prenatal and postnatal maternal depression on amygdala volume and microstructure in young children. Transl Psychiatry. 2017;7 doi: 10.1038/tp.2017.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox J.L., Holden J.M., Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 30.Tisdall M.D., Hess A.T., Reuter M., Meintjes E.M., Fischl B., van der Kouwe A.J. Volumetric navigators for prospective motion correction and selective reacquisition in neuroanatomical MRI. Magn Reson Med. 2012;68:389–399. doi: 10.1002/mrm.23228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tustison N.J., Avants B.B., Cook P.A., Zheng Y., Egan A., Yushkevich P.A., Gee J.C. N4ITK: Improved N3 bias correction. IEEE Trans Med Imaging. 2010;29:1310–1320. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ou Y., Zöllei L., Da X., Retzepi K., Murphy S.N., Gerstner E.R., et al. Field of view normalization in multi-site brain MRI. Neuroinformatics. 2018;16:431–444. doi: 10.1007/s12021-018-9359-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doshi J., Erus G., Ou Y., Gaonkar B., Davatzikos C. Multi-atlas skull-stripping. Acad Radiol. 2013;20:1566–1576. doi: 10.1016/j.acra.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doshi J., Erus G., Ou Y., Resnick S.M., Gur R.C., Gur R.E., et al. MUSE: MUlti-atlas region Segmentation utilizing Ensembles of registration algorithms and parameters, and locally optimal atlas selection. Neuroimage. 2016;127:186–195. doi: 10.1016/j.neuroimage.2015.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ou Y., Akbari H., Bilello M., Da X., Davatzikos C. Comparative evaluation of registration algorithms in different brain databases with varying difficulty: Results and insights. IEEE Trans Med Imaging. 2014;33:2039–2065. doi: 10.1109/TMI.2014.2330355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ou Y., Sotiras A., Paragios N., Davatzikos C. DRAMMS: Deformable registration via attribute matching and mutual-saliency weighting. Med Image Anal. 2011;15:622–639. doi: 10.1016/j.media.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morton S.U., Vyas R., Gagoski B., Vu C., Litt J., Larsen R.J., et al. Maternal dietary intake of omega-3 fatty acids correlates positively with regional brain volumes in 1-month-old term infants. Cereb Cortex. 2020;30:2057–2069. doi: 10.1093/cercor/bhz222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ou Y., Zöllei L., Retzepi K., Castro V., Bates S.V., Pieper S., et al. Using clinically acquired MRI to construct age-specific ADC atlases: Quantifying spatiotemporal ADC changes from birth to 6-year old. Hum Brain Mapp. 2017;38:3052–3068. doi: 10.1002/hbm.23573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson P.O., Neyman J. Tests of certain linear hypotheses and their application to some educational problems. Stat Res Mem. 1936;1:57–93. [Google Scholar]
- 40.Gilmore J.H., Knickmeyer R.C., Gao W. Imaging structural and functional brain development in early childhood. Nat Rev Neurosci. 2018;19:123–137. doi: 10.1038/nrn.2018.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knickmeyer R.C., Gouttard S., Kang C., Evans D., Wilber K., Smith J.K., et al. A structural MRI study of human brain development from birth to 2 years. J Neurosci. 2008;28:12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilmore J.H., Langworthy B., Girault J.B., Fine J., Jha S.C., Kim S.H., et al. Individual variation of human cortical structure is established in the first year of life. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:971–980. doi: 10.1016/j.bpsc.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scorza P., Duarte C.S., Hipwell A.E., Posner J., Ortin A., Canino G., et al. Research Review: Intergenerational transmission of disadvantage: Epigenetics and parents’ childhoods as the first exposure. J Child Psychol Psychiatry. 2019;60:119–132. doi: 10.1111/jcpp.12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yehuda R., Lehrner A. Intergenerational transmission of trauma effects: Putative role of epigenetic mechanisms. World Psychiatry. 2018;17:243–257. doi: 10.1002/wps.20568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moog N.K., Buss C., Entringer S., Shahbaba B., Gillen D.L., Hobel C.J., Wadhwa P.D. Maternal exposure to childhood trauma is associated during pregnancy with placental-fetal stress physiology. Biol Psychiatry. 2016;79:831–839. doi: 10.1016/j.biopsych.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ellman L.M., Schetter C.D., Hobel C.J., Chicz-DeMet A., Glynn L.M., Sandman C.A. Timing of fetal exposure to stress hormones: Effects on newborn physical and neuromuscular maturation. Dev Psychobiol. 2008;50:232–241. doi: 10.1002/dev.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sandman C.A., Wadhwa P.D., Chicz-DeMet A., Porto M., Garite T.J. Maternal corticotropin-releasing hormone and habituation in the human fetus. Dev Psychobiol. 1999;34:163–173. doi: 10.1002/(sici)1098-2302(199904)34:3<163::aid-dev1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 48.Wosu A.C., Gelaye B., Williams M.A. History of childhood sexual abuse and risk of prenatal and postpartum depression or depressive symptoms: An epidemiologic review. Arch Womens Ment Health. 2015;18:659–671. doi: 10.1007/s00737-015-0533-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alink L.R.A., Cyr C., Madigan S. The effect of maltreatment experiences on maltreating and dysfunctional parenting: A search for mechanisms. Dev Psychopathol. 2019;31:1–7. [Google Scholar]
- 50.Savage L.É., Tarabulsy G.M., Pearson J., Collin-Vézina D., Gagné L.M. Maternal history of childhood maltreatment and later parenting behavior: A meta-analysis. Dev Psychopathol. 2019;31:9–21. doi: 10.1017/S0954579418001542. [DOI] [PubMed] [Google Scholar]
- 51.Fuchs A., Möhler E., Resch F., Kaess M. Sex-specific differences in adrenocortical attunement in mothers with a history of childhood abuse and their 5-month-old boys and girls. J Neural Transm (Vienna) 2016;123:1085–1094. doi: 10.1007/s00702-016-1525-6. [DOI] [PubMed] [Google Scholar]
- 52.Fuchs A., Moehler E., Resch F., Kaess M. The effect of a maternal history of childhood abuse on adrenocortical attunement in mothers and their toddlers. Dev Psychobiol. 2017;59:639–652. doi: 10.1002/dev.21531. [DOI] [PubMed] [Google Scholar]
- 53.Juul S.H., Hendrix C., Robinson B., Stowe Z.N., Newport D.J., Brennan P.A., Johnson K.C. Maternal early-life trauma and affective parenting style: The mediating role of HPA-axis function. Arch Womens Ment Health. 2016;19:17–23. doi: 10.1007/s00737-015-0528-x. [DOI] [PubMed] [Google Scholar]
- 54.Khoury J.E., Beeney J., Shiff I., Bosquet Enlow M., Lyons-Ruth K. Maternal experiences of childhood maltreatment moderate patterns of mother-infant cortisol regulation under stress. Dev Psychobiol. 2021;63:1309–1321. doi: 10.1002/dev.22109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khoury J.E., Dimitrov L., Enlow M.B., Haltigan J.D., Bronfman E., Lyons-Ruth K. Patterns of maternal childhood maltreatment and disrupted interaction between mothers and their 4-month-old infants. Child Maltreat. 2022;27:366–377. doi: 10.1177/10775595211007567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whittle S., Simmons J.G., Dennison M., Vijayakumar N., Schwartz O., Yap M.B., et al. Positive parenting predicts the development of adolescent brain structure: A longitudinal study. Dev Cogn Neurosci. 2014;8:7–17. doi: 10.1016/j.dcn.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bernier A., Dégeilh F., Leblanc É., Daneault V., Bailey H.N., Beauchamp M.H. Mother–infant interaction and child brain morphology: A multidimensional approach to maternal sensitivity. Infancy. 2019;24:120–138. doi: 10.1111/infa.12270. [DOI] [PubMed] [Google Scholar]
- 58.Pechtel P., Lyons-Ruth K., Anderson C.M., Teicher M.H. Sensitive periods of amygdala development: The role of maltreatment in preadolescence. Neuroimage. 2014;97:236–244. doi: 10.1016/j.neuroimage.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Veer I.M., Oei N.Y., van Buchem M.A., Spinhoven P., Elzinga B.M., Rombouts S.A. Evidence for smaller right amygdala volumes in posttraumatic stress disorder following childhood trauma. Psychiatry Res. 2015;233:436–442. doi: 10.1016/j.pscychresns.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 60.Weems C.F., Klabunde M., Russell J.D., Reiss A.L., Carrión V.G. Post-traumatic stress and age variation in amygdala volumes among youth exposed to trauma. Soc Cogn Affect Neurosci. 2015;10:1661–1667. doi: 10.1093/scan/nsv053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Driessen M., Herrmann J., Stahl K., Zwaan M., Meier S., Hill A., et al. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Arch Gen Psychiatry. 2000;57:1115–1122. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- 62.Schmahl C.G., Vermetten E., Elzinga B.M., Douglas Bremner J. Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Res. 2003;122:193–198. doi: 10.1016/s0925-4927(03)00023-4. [DOI] [PubMed] [Google Scholar]
- 63.Hardt J., Rutter M. Validity of adult retrospective reports of adverse childhood experiences: Review of the evidence. J Child Psychol Psychiatry. 2004;45:260–273. doi: 10.1111/j.1469-7610.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- 64.Hambrick E.P., Tunno A.M., Gabrielli J., Jackson Y., Belz C. Using multiple informants to assess child maltreatment: Concordance between case file and youth self-report. J Aggress Maltreat Trauma. 2014;23:751–771. doi: 10.1080/10926771.2014.933463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kobulsky J.M., Kepple N.J., Jedwab M. Abuse characteristics and the concordance of child protective service determinations and adolescent self-reports of abuse. Child Maltreat. 2018;23:269–280. doi: 10.1177/1077559518771743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.