Abstract
Background
Though compulsive drinking is a hallmark of alcohol use disorder (AUD), little is known of the neural mechanisms driving this behavior. To further the understanding of the neural underpinnings of this compulsivity, a meta-analytic approach was used to examine gray matter (GM) volume differences related to AUD, and contrast these differences with GM volume differences in obsessive-compulsive disorder (OCD), to find common underlying regional brain differences.
Methods
We systematically meta-analyzed case-control studies investigating GM volume that used whole-brain voxel-based morphometry separately for AUD and OCD and then directly compared the results of both. Seed-based d Mapping software was used to perform the meta-analyses.
Results
The AUD meta-analysis used 19 citations, with 736 individuals with AUD and 827 control individuals. The OCD meta-analysis had 25 citations, with 995 individuals with OCD and 1177 control individuals. The AUD group showed decreased GM in areas including frontal, limbic, temporal, and cerebellar regions. The OCD group had decreased GM in frontal and insular regions but increases in the hypothalamus and brainstem. Importantly, the main outcome showed that both groups had decreased GM overlapping in the anterior cingulate cortex and insula. Brain regions were p < .05 corrected.
Conclusions
Common brain regional differences in the anterior cingulate cortex and insula that overlap between AUD and OCD suggest that interventions targeting these regions could prove to be beneficial in treating compulsive drinking related to AUD. Further research into the functional role of these brain regions in the etiology of compulsive drinking in AUD is warranted.
Keywords: ACC, AUD, Compulsivity, Meta-analysis, OCD, VBM
Alcohol misuse is a major public health concern, being the third largest cause of preventable deaths in the United States. Around 1 in 5 Americans will develop an alcohol use disorder (AUD) in their lifetime, highlighting the need for effective treatments (1, 2, 3). AUD is a maladaptive pattern of drinking that leads to clinically significant impairment or distress, often stemming from genetic, biological, developmental, and environmental risk factors. AUD has been a prominent health concern, with an estimated 14.4 million adults meeting criteria in 2018. One of the defining characteristics of AUD is compulsive alcohol use, characterized by a tendency to continue seeking and consuming alcohol despite negative or adverse consequences (4,5). Even though compulsive alcohol use is central to AUD, neural substrates underlying this behavior are not fully elucidated. One way to investigate the underlying neural mechanisms of compulsive behavior is comparing individuals with AUD with those with other disorders involving compulsive actions, such as obsessive-compulsive disorder (OCD). OCD is characterized by obsessions (intrusive thoughts, ideas, or mental images) and compulsions (urges to do ritualistic and repetitive behaviors) that can cause distress and anxiety (6).
Studies of individuals with OCD report 7.5% to 38% comorbidity with AUD, showing a strong overlap of the disorders, with most studies in the 30% range (7, 8, 9, 10). In addition, individuals with OCD have higher prevalence rates of AUD (10). A study of individuals with AUD also found a comorbidity rate with OCD of 13.5% (11). Therefore, it is likely that there is overlap of the two constructs. This is not to say that compulsive behaviors in OCD are the same as in AUD. Instead, compulsive behaviors in both disorders may share common neural substrates, which are then expressed through the specific etiologies of each disorder, giving rise to the observed behavioral differences. A model has recently been put forth comparing AUD and OCD, with compulsive behaviors in both being thought to arise with a switch from goal-directed to habitual behavior (5): that is, switching from response-outcome associations in which behavior is mediated by knowledge of the relationship between the action and its outcome to a stimulus-response association in which habitual responses are triggered directly from stimuli regardless of the consequences leading to compulsive behaviors (5). This switch is thought to largely rely on frontal and striatal brain regions.
A standardized, automated approach to investigate the brain is voxel-based morphometry (VBM), which provides measures of gray matter (GM) volume throughout the entire brain (12). Because VBM is standardized, it offers a unique opportunity to compare published studies of AUD and OCD in a meta-analytic framework. AUD has been associated with structural neurological impairments in many studies using VBM. The most consistent and frequently cited finding is loss of GM in prefrontal areas, including the dorsolateral prefrontal cortex (PFC), medial PFC, and anterior cingulate cortex (ACC) (13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28). These regions are involved with various aspects of executive function and are in line with behavioral studies showing a reduction in cognitive function in AUD (29,30). Other notable brain areas implicated include the striatum and insula, which have been associated with many functions including reward and emotion (14, 15, 16, 17, 18, 19, 20,22,23,27,28,31,32). Additional regions are the temporal lobes, including the hippocampus, which plays roles in memory, learning, and language (14,16, 17, 18,20,26,28,31,33, 34, 35). However, owing to differences in methodology and sampling, not all studies have found uniform patterns of GM loss.
Many studies have reported regional alterations in GM volume related to OCD. For instance, in many studies, decreases in GM have been shown in prefrontal regions including the ACC, dorsolateral PFC, orbitofrontal cortex, inferior frontal gyrus, and medial PFC (36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49). However, other studies have found increased GM in those same prefrontal regions except the ACC (38,42,50, 51, 52, 53). Although there are differences in directionality of GM volume, it appears that there is a role of prefrontal regions in OCD. A few studies have also reported GM differences in striatal areas including the caudate, putamen, globus pallidus, and pallidum, with all but two finding GM increases (37,39,42,44,45,49,51,52,54). In addition, there have also been GM differences seen in the thalamus related to OCD (37,39,42,49,50,52,55). These findings would be in line with common theories that OCD involves frontostriatal circuitry (56). However, there are also other commonly reported brain regions with GM differences outside the frontostriatal circuit, including the insula, temporal and parietal lobes, posterior cingulate, precentral and postcentral gyri, and last, the cerebellum, suggesting that the brain mechanisms involved with OCD are more complex (36, 37, 38, 39,41, 42, 43, 44, 45, 46, 47, 48, 49,51, 52, 53, 54,57, 58, 59).
The purpose of this study was to compare the similarities in GM differences between AUD and OCD VBM studies using meta-analyses, to further clarify the neural mechanisms of compulsive behavior in AUD. However, to our knowledge, no published investigation has directly compared brain structures in AUD and OCD populations. Specifically, we aimed to conduct separate meta-analyses for both AUD and OCD, followed by investigating the common overlap between the two. Given numerous studies of both populations have found GM alterations in prefrontal brain regions, we hypothesized that these regions would be an area of mutual overlap.
Methods and Materials
For identifying VBM studies, a systematic search method was used to locate published articles through June 2019 in PubMed, Web of Science, ScienceDirect, Scopus, and Embase. Search terms used for AUD included “alcohol dependence”; “alcoholism”; “alcohol abuse” OR “alcohol use disorder.” These keywords were then crossed with “voxel-based morphometry” or “VBM” or “morphometry” or “volumetry” or “gray matter” or “structural MRI.” Search terms for OCD included “obsessive-compulsive disorder” or “OCD,” and these keywords were then crossed with “voxel-based morphometry” or “VBM” or “morphometry” or “volumetry” or “gray matter” or “structural MRI.” The titles, abstracts, keywords, and full text of articles were searched.
Inclusion criteria were 1) a diagnosis based on the DSM or ICD-10; 2) whole-brain VBM to analyze GM differences with a single statistical threshold used across the brain, i.e., not just region-of-interest results; 3) comparison with healthy control individuals; 4) Talairach or Montreal Neurological Institute coordinates; 5) significance corrected for multiple comparisons or uncorrected with spatial extent thresholds; 6) peer-reviewed studies; and 7) results reported in English. Exclusion criteria included 1) review or meta-analysis articles, 2) studies that reanalyzed previously published data, and 3) studies with <10 patients. For articles that had patient groups overlapping, the article with the biggest sample was used. For five articles, the authors had to be directly contacted to assess if patient groups were overlapping. In four articles, subgroups of patients were compared with control individuals without combining the patient group into a single group. In these cases, each subgroup was entered separately into the meta-analysis. Last, for articles that involved magnetic resonance imaging scans pre- and posttreatment, the pretreatment scan data were used. Articles were independently reviewed by two blinded raters, were reviewed for conflicts, and followed the MOOSE (Meta-analyses Of Observational Studies in Epidemiology) guidelines for meta-analyses (60).
We performed two separate meta-analyses, one for AUD and one for OCD, using the Seed-based d Mapping (SDM) package (version v6.21; https://www.sdmproject.com). The methods used have been previously described elsewhere (61, 62, 63), and a tutorial can be found at https://www.sdmproject.com/manual/. The first step involved extracting the peak coordinates and effect sizes from each published article. SDM created brain images of each study in Montreal Neurological Institute space, from which GM was recreated for each study by means of an anisotropic Gaussian kernel. This has been found to optimize the re-creation of the effect size maps and is robust because it does not depend on full width at half maximum. SDM estimated the lower and upper bounds of possible effect sizes. Second, it estimated the most likely effect sizes of each study and its standard error and created several multiple imputations based on adding noise to these estimations. Third, each imputed dataset was meta-analyzed and then Rubin's rules were used to combine these imputed meta-analyzed datasets, weighting by sample size and variance of each study and between-study heterogeneity. Statistical significance was determined using randomization tests to create a distribution of p values. Common SDM thresholds were used, namely, an uncorrected voxelwise p value of p < .005, and SDM z values of z ≥ 1 or z ≤ 1, which are proposed to optimally balance sensitivity and specificity, approximating a corrected p value <.05 in SDM, and are commonly used in dual meta-analyses comparing two populations of patients (61,64, 65, 66). Last, an extent threshold for clusters ≥10 voxels was used to omit small clusters. Jackknife sensitivity analyses were performed to assess reliability of the results by systematically omitting a single study at a time and rerunning the results. Each significant cluster was checked for heterogeneity using I2 and publication bias using SDM’s metabias test.
After performing the primary meta-analyses for both AUD and OCD, two further analyses were performed, again using the SDM package. First, and the main focus of this report, was comparing the overlap in GM volume between AUD and OCD. This was performed using the multimodal function, which allows direct comparison of two separate meta-analyses to statistically test if both meta-analyses have decreased or increased GM in the same regions of the brain (67). Second, as the purpose of this project was to identify brain regions related to compulsivity in AUD, a follow-up analysis of the OCD meta-analysis was performed. Because OCD is associated with more behavioral traits than just compulsivity, we sought to refine the results. Most studies of OCD include the Yale-Brown Obsessive Compulsive Scale (Y-BOCS), which has a subscale for compulsive behavior (68). We therefore ran a regression between the results of the OCD meta-analysis and the compulsive subscale for the studies that reported it. Therefore, if regions found in common between AUD and OCD were also found in the compulsive subscale regression, it indicates that those brain regions may be more related to compulsive behaviors instead of other behaviors associated with OCD. Once again, for both these analyses, an uncorrected voxelwise p value of p < .005, and SDM z values of z ≥ 1 or z ≤ 1 were used.
Results
For the AUD meta-analysis, there was a total of 9682 citations found. After an initial review omitting citations by title and abstract, 151 underwent a full-article review. Of these, 25 met eligibility requirements. However, five of those had participant samples overlapping with other citations and were omitted. There was one article that had a pediatric sample that was also omitted. Last, two studies compared two subgroups of participants with AUD with control individuals separately but did not combine the AUD groups together for comparison (24,26). For these studies, each subgroup analysis was entered into the final meta-analysis as a separate “study.” This resulted in 21 “studies” and a total number of 736 participants with AUD and 827 control individuals. An overview of the included journal articles is shown in Table 1, with additional information containing VBM software used, psychiatric/neurological exclusions, and length of illness in Table S3. There were 4240 citations found for the OCD meta-analysis, and after the initial review, 89 underwent a full-article review. This resulted in 42 eligible citations, but nine had overlapping samples with other citations, resulting in 33 final citations included in the meta-analysis. As with AUD, two studies used two subgroups in separate analyses, and each subgroup was entered into the meta-analysis as a separate study (37,54). In addition, eight studies were done in pediatric populations. To be more comparable to the AUD studies, we ran our main analysis with only the adult OCD studies, resulting in 27 “studies.” This resulted in 995 participants with OCD and 1177 control individuals. An overview of the articles, including the pediatric studies, is shown in Table 2, with additional information containing VBM software used, psychiatric/neurological exclusions, length of illness, and compulsive subscale scores from the Y-BOCS in Table S4. Flowsheets of the elimination processes are shown in Figures S1 and S2.
Table 1.
Overview of the Studies Included in the AUD Meta-analysis
| Study | Alcohol Diagnosis | Criteria | AUD Group (Males), N (n) | Control Group (Males), N (n) | Mean Age AUD/Control Groups, Years | p-Value Threshold | Adult Sample |
|---|---|---|---|---|---|---|---|
| Asensio et al., 2016 (13) | Abuse | DSM-IV | 24 (24) | 24 (24) | 35.62/31.91 | Cluster corr. p < .05 | Yes |
| Chanraud et al., 2007 (14) | Dependence | DSM-IV | 26 (26) | 24 (24) | 47.70/45.00 | FDR corr. p < .005 | Yes |
| Charlet et al., 2014 (31) | Dependence | DSM-IV | 33 (25) | 33 (25) | 44.80/46.10 | FWE corr. p < .05 | Yes |
| Demirakca et al., 2011 (28) | Dependence | DSM-IV/ICD-10 | 50 (27) | 66 (34) | 46.60/45.00 | FWE corr. p < .05 | Yes |
| Fein et al., 2006 (85) | Dependence | DSM-IV | 43 (24) | 58 (21) | 46.46/44.62 | FWE corr. p < .05 | Yes |
| Galandra et al., 2018 (15) | Dependence | DSM-IV | 23 (14) | 18 (10) | 45.69/44.83 | Monte Carlo corr. p < .025 | Yes |
| Grodin et al., 2013 (16) | Dependence | DSM-IV | 37 (21) | 69 (47) | 40.20/36.60 | Monte Carlo corr. p < .01 | Yes |
| Guggenmos et al., 2017 (17) | Dependence | DSM-IV/ICD-10 | 119 (101) | 97 (81) | 45.00/43.70 | FWE corr. p < .05 | Yes |
| Jang et al., 2007 (18) | Dependence | DSM-IV | 20 (20) | 20 (20) | 43.50/44.50 | FDR corr. p < .005 | Yes |
| Mechtcheriakov et al., 2007 (19) | Dependence | ICD-10 | 22 (14) | 22 (14) | 53.60/53.70 | FDR corr. p < .05 | Yes |
| Nurmedov et al., 2016 (20) | AUD | DSM-V | 24 (20) | 29 (23) | 40.79/37.45 | FWE corr. p < .05 | Yes |
| Rando et al., 2011 (21) | Dependence | DSM-IV | 45 (35) | 50 (28) | 38.20/31.14 | FWE corr. p < .025 | Yes |
| Segobin et al., 2014 (22) | Dependence | DSM-IV | 19 (17) | N/A | 44.40/46.70 | FDR corr. p < .01 | Yes |
| van Holst et al., 2012 (23) | AUD | DSM-IV | 36 (36) | 54 (54) | 43.20/35.30 | FDR corr. p < .05 | Yes |
| Wang et al., 2018 (24)a | Dependence | DSM-IV | 35 (35) | 33 (33) | 41.80/42.88 | FWE corr. p < .05 | Yes |
| Wang et al., 2018 (24)a | Dependence | DSM-IV | 21 (21) | 33 (33) | 45.95/42.88 | FWE corr. p < .05 | Yes |
| Wiers et al., 2015 (25) | Dependence | DSM-IV | 22 (22) | 21 (21) | 42.14/41.95 | FWE corr. p < .05 | Yes |
| Wu et al., 2018 (26)a | Dependence | DSM-IV | 13 (8) | 22 (13) | 44.10/44.90 | FWE corr. p < .05 | Yes |
| Wu et al., 2018 (26)a | Dependence | DSM-IV | 9 (5) | 22 (13) | 47.00/44.90 | FWE corr. p < .05 | Yes |
| Yoon et al., 2017 (86) | AUD | DSM-IV | 20 (20) | 25 (25) | 28.70/25.40 | Monte Carlo corr. p < .05 | Yes |
| Zois et al., 2017 (27) | Dependence | DSM-IV | 95 (71) | 87 (71) | 45.90/45.90 | FWE corr. p < .05 | Yes |
| Brooks et al., 2014 (33) | Dependence | DSM-IV | 58 (25) | 58 (25) | 14.90/14.70 | FWE corr. p < .05 | No |
AUD, alcohol use disorder; Corr., corrected; FDR, false discovery rate; FWE, familywise error; N/A, not available.
Only reported results for AUD subgroups, and each subgroup was entered as a separate study.
Table 2.
Overview of the Studies Included in the OCD Meta-analysis
| Study | Criteria | OCD Group (Males), N (n) | Control Group(Males), N (n) | Mean Age OCD/Control Groups, Years | p-Value Threshold | Adult Sample |
|---|---|---|---|---|---|---|
| Christian et al., 2008 (50) | DSM-IV | 14 (N/A) | 21 (15) | 38/38.9 | Uncorr. p < .001 | Yes |
| Gilbert et al., 2008 (36) | DSM-IV | 25 (13) | 20 (9) | 37.5/29.8 | FDR corr. p < .05 | Yes |
| Gonçalves et al., 2017 (57) | DSM-IV | 15 (11) | 15 (9) | 31.7/30 | Uncorr. p < .001 | Yes |
| Hashimoto et al., 2014 (37)a | DSM-IV | 24 (11) | 30 (14) | 35.7/32.5 | FDR corr. p < .05 | Yes |
| Hashimoto et al., 2014 (37)a | DSM-IV | 15 (7) | 30 (14) | 32.5/32.5 | FDR corr. p < .05 | Yes |
| Hoexter et al., 2012 (38) | DSM-IV | 38 (15) | 36 (13) | 31.5/27.8 | FWE corr. p < .05 | Yes |
| Hou et al., 2013 (39) | DSM-IV | 33 (18) | 33 (18) | 25.3/25 | FWE corr. p < .05 | Yes |
| Ikari et al., 2017 (55) | DSM-IV | 92 (42) | 146 (61) | 36.3/35 | FWE corr. p < .05 | Yes |
| Kobayashi et al., 2015 (40) | DSM-IV | 20 (10) | 30 (15) | 34.1/31.2 | FDR corr. p < .05 | Yes |
| Kopřivová et al., 2009 (41) | ICD-10 | 14 (5) | 15 (6) | 28.6/28.7 | FDR corr. p < .05 | Yes |
| Lv et al., 2017 (42) | DSM-IV | 95 (52) | 95 (49) | 30/30.4 | Monte Carlo corr. p < .05 | Yes |
| Matsumoto et al., 2010 (43) | DSM-IV | 16 (7) | 32 (14) | 32.8/32.6 | Uncorr. p < .001 | Yes |
| Moon and Jeong, 2018 (58) | DSM-IV | 18 (11) | 18 (11) | 27.6/30.7 | FWE corr. p < .05 | Yes |
| Moreira et al., 2017 (59) | DSM-IV | 40 (13) | 40 (13) | 26.3/26.5 | FWE corr. p < .05 | Yes |
| Pujol et al., 2004 (44) | DSM-IV | 72 (40) | 72 (40) | 29.8/30.1 | Corr. p < .05 | Yes |
| Real et al., 2016 (45) | DSM-IV | 124 (62) | 112 (62) | 34.9/33.8 | FWE corr. p < .05 | Yes |
| Riffkin et al., 2005 (87) | DSM-IV | 18 (8) | 18 (8) | 36.1/34.6 | Cluster corr. p < .05 | Yes |
| Spalletta et al., 2014 (88) | DSM-IV | 20 (12) | 20 (12) | 33.1/35.2 | Uncorr. p < .001 | Yes |
| Subirà et al., 2013 (54)a | DSM-IV | 30 (20) | 95 (55) | 32.2/33.9 | FWE corr. p < .05 | Yes |
| Subirà et al., 2013 (54)a | DSM-IV | 65 (29) | 95 (55) | 34.6/33.9 | FWE corr. p < .05 | Yes |
| Tan et al., 2013 (51) | DSM-IV | 28 (19) | 22 (15) | 25.4/27.9 | Uncorr. p < .001 | Yes |
| Tang et al., 2015 (46) | DSM-IV | 26 (15) | 32 (17) | 25.5/26.2 | FWE corr. p < .05 | Yes |
| Tang et al., 2016 (52) | DSM-IV | 18 (11) | 16 (10) | 27.3/26.8 | Monte Carlo corr. p < .01 | Yes |
| Togao et al., 2010 (47) | DSM-III and DSM-IV | 23 (9) | 26 (12) | 32.6/31.3 | FDR corr. p < .05 | Yes |
| Valente et al., 2005 (48) | DSM-IV | 19 (10) | 15 (7) | 32.7/32.3 | Uncorr. p < .001 | Yes |
| Wang et al., (53) 2018 | DSM-IV | 22 (11) | 22 (11) | 22.2/22.7 | Monte Carlo corr. p < .05 | Yes |
| Yoo et al., 2008 (49) | DSM-IV | 71 (47) | 71 (47) | 26.6/26.7 | Uncorr. p < .001 | Yes |
| Cabrera et al., 2019 (89) | DSM-IV | 14 (10) | 14 (10) | 11.1/11.1 | Uncorr. p < .005 | No |
| Carmona et al., 2007 (90) | DSM-IV | 18 (13) | 18 (13) | 12.9/13 | FWE corr. p < .05 | No |
| Cheng et al., 2016 (91) | DSM-IV | 30 (18) | 30 (18) | 10.8/10.5 | FWE corr. p < .05 | No |
| Gilbert et al., 2008 (92) | DSM-IV | 10 (6) | 10 (6) | 12.9/13.4 | FDR corr. p < .05 | No |
| Jayarajan et al., 2015 (93) | DSM-IV | 15 (8) | 15 (8) | 14.1/14.3 | FWE corr. p < .05 | No |
| Lázaro et al., 2009 (94) | DSM-IV | 15 (8) | 15 (8) | 13.7/14.3 | FWE corr. p < .05 | No |
| Lázaro et al., 2014 (95) | DSM-IV | 62 (36) | 46 (22) | 15.4/15.3 | FWE corr. p < .05 | No |
| Szeszko et al., 2008 (96) | DSM-IV | 37 (14) | 26 (9) | 13/13 | FWE corr. p < .05 | No |
AUD, alcohol use disorder; Corr., corrected; FDR, false discovery rate; FWE, familywise error; N/A, not available; OCD, obsessive-compulsive disorder; Uncorr., uncorrected with a cluster spatial extent.
Only reported results for AUD subgroups, and each subgroup was entered as a separate study.
The AUD meta-analysis revealed several brain regions with GM differences (Table 3 and Figure 1). The AUD group had decreased GM across all identified regional differences. It is important to note that the brain regions in Table 3 reflect the peak coordinates; however, many of the clusters were large and contained multiple brain regions. For instance, the right median cingulate cluster spanned the cingulate cortex from anterior to posterior, the medial superior frontal gyrus, and supplementary motor area. The right insula cluster spanned parts of the insula, temporal lobe, amygdala, and putamen. The left Heschl gyrus also contained the middle and superior temporal gyri, insula, and putamen. The left postcentral gyrus cluster included the precentral gyrus and inferior frontal gyrus. The right precentral gyrus also contained the inferior and middle frontal gyri. The right precuneus also spanned the cuneus and part of the posterior cingulate. Last, the cerebellum clusters contained surrounding areas of the cerebellum. The remaining clusters were retained in the reported region of the peak voxel, including the middle frontal gyrus, thalamus, parahippocampal gyrus, and hippocampus. All the significant clusters had low heterogeneity with I2 values <25%. This indicates that the identified differences are not a function of interstudy heterogeneity. In addition, none of the clusters had significant study bias, as measured by SDM’s study bias p value. Last, jackknife analysis showed very consistent results, with most the clusters remaining in 18 of the 22 studies, as shown in Table S5.
Table 3.
Regional Differences in GM Volume: AUD Versus HC Groups
| Peak MNI Coordinates |
SDM-z | Uncorrected p Value | Voxels | Brain Region | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Clusters ≥10 Voxels and SDM-z ≤ −1 and All Peak SDM-z ≤ −1 (AUD > HC) | ||||||
| No Results | ||||||
| Clusters ≥10 Voxels and SDM-z ≥ 1 and All Peak SDM-z ≥ 1 (AUD < HC) | ||||||
| 2 | −10 | 44 | 9.2 | ∼0 | 8229 | Right median cingulate gyrus |
| 38 | −4 | 6 | 6.6 | ∼0 | 4748 | Right insula |
| −38 | −22 | 6 | 6.9 | ∼0 | 3005 | Left Heschl’s gyrus |
| −48 | −12 | 40 | 7.8 | ∼0 | 1980 | Left postcentral gyrus |
| 52 | 6 | 32 | 4.6 | .000002 | 946 | Right precentral gyrus |
| −2 | −66 | 28 | 4.8 | .0000007 | 902 | Left precuneus |
| −36 | −52 | −44 | 4.5 | .000004 | 606 | Left cerebellum lobule VIII |
| 34 | −56 | −52 | 3.8 | .00007 | 586 | Right cerebellum lobule VIII |
| −28 | 40 | 30 | 3.7 | .0001 | 173 | Left middle frontal gyrus |
| −6 | −10 | 8 | 3.5 | .0002 | 159 | Left thalamus |
| 16 | −36 | −10 | 3.2 | .0006 | 118 | Right parahippocampal gyrus |
| 30 | −38 | −10 | 3.2 | .0007 | 14 | Right parahippocampal gyrus |
| −14 | −10 | −14 | 3.2 | .0008 | 11 | Left hippocampus |
AUD, alcohol use disorder; GM, gray matter; HC, healthy control; MNI, Montreal Neurological Institute; SDM, Seed-based d Mapping.
Figure 1.
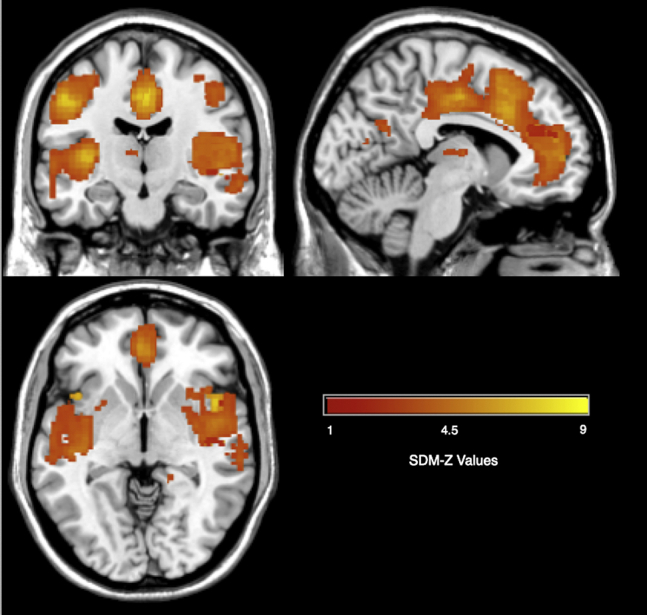
Decreased gray matter volume in alcohol use disorder vs. control groups. SDM, Seed-based d Mapping.
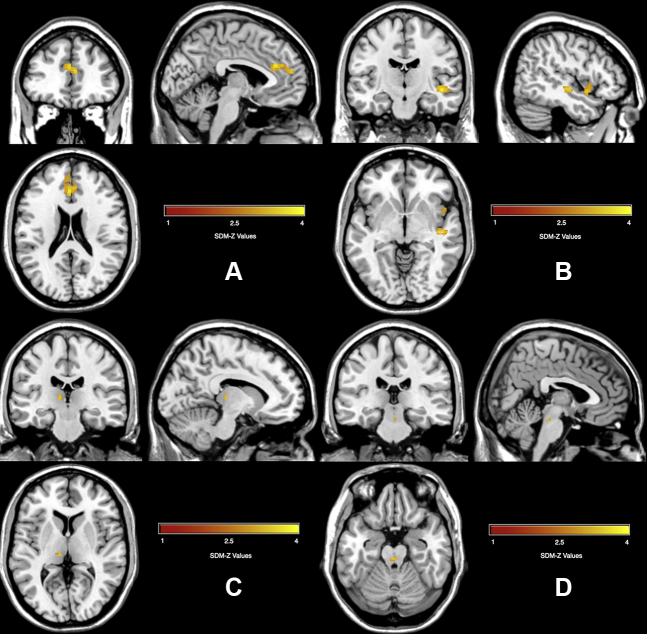
Meta-analysis of OCD revealed a relatively small number of brain regions with differences in GM volume, shown in Table 4 and Figure 2. The OCD group had increased GM volume in two small clusters, the left thalamus and bilateral corticospinal projections in the brainstem. In addition, the OCD group had decreased GM in a cluster spanning the ACC and medial superior frontal gyrus, as well as in two smaller clusters in the insula. All the significant clusters had low heterogeneity (using I2 < 25%). In addition, none of the clusters had significant study bias, as measured by SDM’s study bias p value. Last, the jackknife analysis gave fairly consistent results, with all clusters surviving in 19 to 24 of the 27 studies, shown in Table S6.
Table 4.
Regional Differences in GM Volume: OCD Versus HC Groups
| Peak MNI Coordinates | SDM-z | Uncorrected p Value | Voxels | Brain Region | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Clusters ≥10 Voxels and SDM-z ≤ −1 and All Peak SDM-z ≤ −1 (OCD > HC) | ||||||
| −10 | −24 | 6 | −3.3 | .0006 | 21 | Left thalamus |
| 0 | −28 | −20 | −3.3 | .0005 | 20 | Bilateral corticospinal projections |
| Clusters ≥10 Voxels and SDM-z ≥ 1 and All Peak SDM-z ≥ 1 (OCD < HC) | ||||||
| −2 | 34 | 22 | 3.9 | .00006 | 288 | Bilateral anterior cingulate cortex |
| 44 | −18 | −6 | 4.0 | .00003 | 115 | Right posterior insula |
| 46 | 6 | −10 | 3.6 | .0002 | 86 | Right middle insula |
GM, gray matter; HC, healthy control; MNI, Montreal Neurological Institute; OCD, obsessive-compulsive disorder; SDM, Seed-based d Mapping.
Figure 2.
Gray matter alterations found in obsessive-compulsive disorder group compared with control group. (A) Decreased gray matter in the anterior cingulate cortex; (B) decreased gray matter in the insula; (C) increased gray matter in the thalamus; (D) increased gray matter in the bilateral corticospinal projections. SDM, Seed-based d Mapping.
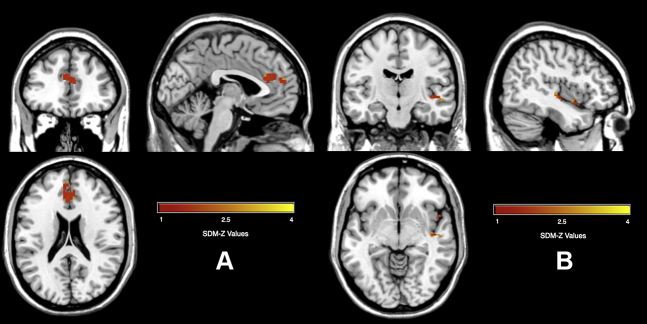
When comparing the overlap in brain volume differences between the AUD and OCD meta-analyses, there were three clusters found in common to both populations (Table 5 and Figure 3). Both groups had decreased GM in a cluster spanning the ACC and medial superior frontal gyrus. In addition, both groups had overlap in two small clusters in the posterior insula and middle insula. As mentioned above, these areas are involved in executive function and one’s own internal emotional and visceral states, and lower function in these areas could be leading to compulsive behavior in both AUD and OCD. We conducted the main analyses with only the adult OCD studies to be more comparable to the AUD studies (excluding the one AUD study that had a pediatric population). However, we also conducted an exploratory analysis including the pediatric studies and found similar overlap in the ACC and posterior insula cluster but not in the middle insula cluster. Last, given that OCD has more behavioral components than compulsions, we performed a follow-up analysis to explore if the areas of overlap between AUD and OCD, i.e., the cingulate and insula, were related to compulsive behavior. We performed a regression between the OCD meta-analysis results with the compulsive subscale of the Y-BOCS for the studies that reported it. A total of 14 studies included the subscale and are shown in Table S4 with the corresponding scores for each study. Results showed a negative correlation between GM volume differences and the compulsive subscale in the cingulate and insula among other regions, such that lower GM volume in OCD was associated with higher compulsive subscale scores. The results are shown in Figure S3 and Table S1. Last, four of the studies had medication-naïve patients. We reran the meta-analysis including a covariate for studies that were medication naïve or not, and the results were largely the same, so we used the original OCD meta-analysis to compare with AUD (Figure S4 and Table S2).
Table 5.
Regional Differences in GM Volume Common to AUD and OCD
| Center MNI Coordinates |
SDM-z | Uncorrected p Value | Voxels | Brain Region | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Volume Decreases in AUD and OCD: Clusters ≥10 Voxels and SDM-z ≥ 1 and All Peak SDM-z ≥ 1 | ||||||
| 6 | 38 | 18 | 3.8 | .00009 | 288 | Bilateral anterior cingulate cortex |
| 50 | 6 | −10 | 3.3 | .0004 | 61 | Right insula |
| 44 | −18 | −2 | 3.3 | .0004 | 51 | Right insula |
AUD, alcohol use disorder; GM, gray matter; MNI, Montreal Neurological Institute; OCD, obsessive-compulsive disorder; SDM, Seed-based d Mapping.
Figure 3.
Common overlap in gray matter alterations between alcohol use disorder and obsessive-compulsive disorder. (A) Decreased gray matter in the anterior cingulate cortex; (B) decreased gray matter in two clusters in the right insula. SDM, Seed-based d Mapping.
Discussion
The aim of this study was to investigate regional differences in GM volume related to compulsive behavior in AUD. This was accomplished by running separate meta-analyses examining GM differences in both AUD and OCD and comparing the results. The main findings of interest were that both AUD and OCD had decreased GM in the ACC and insula, suggesting that these areas might be related to compulsive behavior in AUD. In addition, the ACC and insula were found to be related to compulsive behaviors in OCD as measured by the Y-BOCS. Results of the AUD meta-analysis showed decreased GM in many areas including the traditional limbic system, frontal lobe regions, and the cerebellum among other regions. The OCD meta-analysis showed decreased GM in the ACC and insula and increased GM in the thalamus and brainstem.
The shared findings of decreased GM in the ACC and insula helps shed light on compulsive behaviors in AUD. In addition to the shared findings, a supplementary analysis also showed that these regions were related to compulsive behavior in OCD as measured by the Y-BOCS. The ACC is associated with many functions, generally falling under the umbrellas of cognitive control and conflict resolution (69). This includes not only cognitive processes, but also affective processes. For instance, it has been associated with reward-based decision making (69). In relation to compulsive drinking in AUD, alterations in the ACC could lower their ability to evaluate and make decisions concerning the conflict of drinking or not when faced with the rewarding properties of alcohol. The insula is known for roles in emotion and pleasurable bodily states caused by drugs and alcohol (70). Therefore, alterations in the insula in AUD could lead to enhanced cravings for alcohol. In relation to compulsive drinking seen in AUD, these findings point to a double-edged sword. The alterations seen in the insula could lead to greater cravings, but with less control of the ACC to downregulate those cravings when faced with the conflict of drinking or not.
Results from the individual AUD meta-analysis supports previous meta-analyses and current theories of the brain regions associated with AUD (71,72). The results show that AUD is associated with GM decreases in numerous parts of the brain, many contained in the traditional definition of the limbic system, as well as the frontal brain regions such as the ACC. The first meta-analysis included nine articles, all of which were included in the current analysis except for one, as it was written in Chinese (71,73). The second meta-analysis included 12 articles, three of which were not in the current analysis (72). One was omitted for being a pediatric population, the second did not meet our criteria of being a whole-brain analysis, and the third did not use the DSM or ICD-10 for diagnosis (33,74,75). Both prior meta-analyses and the current one found widespread GM reductions including frontal, temporal, partial, insular, and subcortical regions. In addition, the large-scale brain imaging consortium, the ENIGMA Addiction Working Group, also found similar widespread decreases of cortical thickness in AUD, further supporting our findings (76). As far as theories of AUD, a predominant model was put forth by Koob and Volkow (77) and includes an interactive network of brain regions involved with different states of addiction. During an initial stage, called binge intoxication, midbrain structures largely composed of the striatum are involved. Our results did show decreased GM in these regions, especially in the putamen. The second stage of addiction is called withdrawal negative affect and largely involves the amygdala, in which we observed GM alterations. The last stage of their model is called preoccupation anticipation and is hypothesized to include many brain regions including the frontal cortex, insula, and hippocampus. We observed alterations in all of these regions, further supporting their role in AUD. Given that the current study was investigating GM volume differences through meta-analysis, it was unable to address the stages put forth in their theory. However, the brain regions in which we observed differences highly overlap with their theory, further supporting that these regions are involved in AUD.
Investigation of the OCD meta-analysis somewhat fit current theories about OCD and a previous meta-analysis (56,78). OCD is thought to largely rely on fronto-striatal-thalamus circuitry. We did observe decreased GM in the ACC and medial PFC and increased GM in the thalamus. Given that the thalamus is a major sensory relay center, having greater GM could increase its sensitivity to sensory information. In addition, decreased GM in the insula could reflect less of an ability to control internal emotional states. Having decreased GM in the ACC could lead to decreased executive function and ability to resolve cognitive conflict. However, there were no differences seen in the striatum, while that previous meta-analysis did find striatal differences (78). That meta-analysis included 18 articles, all but three of which were in the current meta-analysis. Two of the three excluded articles had overlapping samples with other articles, and the third did not use a whole-brain VBM approach (79, 80, 81). In addition, results published from the large database of the ENIGMA-OCD Working Group consortium found decreased cortical thinness in the ACC, as well as altered graph theory metrics in the cingulate, caudate, and thalamus (82,83). Looking at the individual articles in the current meta-analysis, many reported differences in the striatum, with some finding increases and others finding decreases (37,39,42,44,45,49,51,52,54). With studies reporting opposing directionality, it could cause effects to wash out in the current meta-analysis. Additionally, the absence of striatal differences could result from the recommended statistical threshold for the SDM program. In an exploratory analysis using a more liberal threshold of p < .03, we do observe increases in the striatum. Therefore, differences in the striatum could exist in OCD, but we cannot conclude so at the recommended statistical thresholds.
The major limitation with the current study is the question of the GM differences observed either being caused by the disorders or being related to risk for the disorder, especially for AUD. Because all the subjects in the studies included were already diagnosed, we have no way of parsing this question with the current study. However, it does point to brain regions associated with the disorders, and current longitudinal studies, such as the Adolescent Brain Cognitive Development Study (https://abcdstudy/.org), can help explain the exact role that the brain regions observed play in the development and maintenance of the disorders. Last, even though both the AUD and OCD meta-analyses passed the publication bias tests, because these are statistical methods, we cannot verify that there is no bias, just that a minimal amount exists.
The clinical significance of this report suggests that treatments addressing compulsive drinking in AUD might benefit from targeting the ACC and/or insula. As this is a meta-analytic approach, cause and effect cannot be shown. Therefore, further targeted studies are warranted to investigate if these brain regions are truly involved in compulsive behaviors in AUD, especially in a clinically useful way. If so, using behavioral therapies or therapeutics known to enhance the ACC could help curb compulsive drinking. For instance, recent techniques that can specifically alter ACC function could be of use, such as deep transcranial magnetic stimulation, which has already been approved by the Food and Drug Administration for use with OCD. It has recently been shown to improve ACC function not only neuronally, but also behaviorally, and could therefore potentially be researched to help with compulsive drinking in AUD (84).
Acknowledgments and Disclosures
This work was supported by the National Institute on Alcohol Abuse and Alcoholism Division of Intramural Clinical and Biological Research (Grant No. Z1A AA000466 [to VAR]).
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2021.11.010.
Supplementary Material
References
- 1.Grant B.F., Goldstein R.B., Saha T.D., Chou S.P., Jung J., Zhang H., et al. Epidemiology of DSM-5 alcohol use disorder results from the national epidemiologic survey on alcohol and related conditions III. JAMA Psychiatry. 2015;72:757–766. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention . Centers for Disease Control and Prevention; Atlanta: 2013. Alcohol and Public Health: Alcohol-Related Disease Impact (ARDI): Average for United States 2006-2010: Alcohol-Attributable Deaths Due to Excessive Alcohol Use. [Google Scholar]
- 3.Hasin D.S., Stinson F.S., Ogburn E., Grant B.F. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- 4.Wise R.A., Koob G.F. The development and maintenance of drug addiction. Neuropsychopharmacology. 2014;39:254–262. doi: 10.1038/npp.2013.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burchi E., Makris N., Lee M.R., Pallanti S., Hollander E. Compulsivity in alcohol use disorder and obsessive compulsive disorder: Implications for neuromodulation. Front Behav Neurosci. 2019;13:70. doi: 10.3389/fnbeh.2019.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abramowitz J.S., Taylor S., McKay D. Obsessive-compulsive disorder. Lancet. 2009;374:491–499. doi: 10.1016/S0140-6736(09)60240-3. [DOI] [PubMed] [Google Scholar]
- 7.Mancebo M.C., Grant J.E., Pinto A., Eisen J.L., Rasmussen S.A. Substance use disorders in an obsessive compulsive disorder clinical sample. J Anxiety Disord. 2009;23:429–435. doi: 10.1016/j.janxdis.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gentil A.F., de Mathis M.A., Torresan R.C., Diniz J.B., Alvarenga P., do Rosário M.C., et al. Alcohol use disorders in patients with obsessive-compulsive disorder: The importance of appropriate dual-diagnosis. Drug Alcohol Depend. 2009;100:173–177. doi: 10.1016/j.drugalcdep.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Ruscio A.M., Stein D.J., Chiu W.T., Kessler R.C. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15:53–63. doi: 10.1038/mp.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osland S., Arnold P.D., Pringsheim T. The prevalence of diagnosed obsessive compulsive disorder and associated comorbidities: A population-based Canadian study. Psychiatry Res. 2018;268:137–142. doi: 10.1016/j.psychres.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 11.Gungor B.B., Askin R., Taymur I., Sari S. Obsessive compulsive disorder and impulse control disorder comorbidity and evaluation of impulsivity and compulsivity in alcohol dependent patients. Dusunen Adam. 2014;27:233–241. [Google Scholar]
- 12.Ashburner J., Friston K.J. Voxel-based morphometry--The methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 13.Asensio S., Morales J.L., Senabre I., Romero M.J., Beltran M.A., Flores-Bellver M., et al. Magnetic resonance imaging structural alterations in brain of alcohol abusers and its association with impulsivity. Addict Biol. 2016;21:962–971. doi: 10.1111/adb.12257. [DOI] [PubMed] [Google Scholar]
- 14.Chanraud S., Martelli C., Delain F., Kostogianni N., Douaud G., Aubin H.-J., et al. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32:429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- 15.Galandra C., Basso G., Manera M., Crespi C., Giorgi I., Vittadini G., et al. Salience network structural integrity predicts executive impairment in alcohol use disorders. Sci Rep. 2018;8:14481. doi: 10.1038/s41598-018-32828-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grodin E.N., Lin H., Durkee C.A., Hommer D.W., Momenan R. Deficits in cortical, diencephalic and midbrain gray matter in alcoholism measured by VBM: Effects of co-morbid substance abuse. NeuroImage Clin. 2013;2:469–476. doi: 10.1016/j.nicl.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guggenmos M., Schmack K., Sekutowicz M., Garbusow M., Sebold M., Sommer C., et al. Quantitative neurobiological evidence for accelerated brain aging in alcohol dependence. Transl Psychiatry. 2017;7:1279. doi: 10.1038/s41398-017-0037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jang D.-P., Namkoong K., Kim J.-J., Park S., Kim I.-Y., Kim S.I., et al. The relationship between brain morphometry and neuropsychological performance in alcohol dependence. Neurosci Lett. 2007;428:21–26. doi: 10.1016/j.neulet.2007.09.047. [DOI] [PubMed] [Google Scholar]
- 19.Mechtcheriakov S., Brenneis C., Egger K., Koppelstaetter F., Schocke M., Marksteiner J. A widespread distinct pattern of cerebral atrophy in patients with alcohol addiction revealed by voxel-based morphometry. J Neurol Neurosurg Psychiatry. 2007;78:610–614. doi: 10.1136/jnnp.2006.095869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nurmedov S., Noyan O., Metin B., Ekmen S., Avcil C., Kose S. Extensive gray matter volume reduction and correlations with neuropsychological performance in alcohol use disorder patients. Klinik Psikofarmakol Bulteni. 2016;26:355–363. [Google Scholar]
- 21.Rando K., Hong K., Bhagwagar Z., Li C.R., Bergquist K., Guarnaccia J., Sinha R. Association of frontal and posterior cortical gray matter volume with time to alcohol relapse: A prospective study. Am J Psychiatry. 2011;168:183–192. doi: 10.1176/appi.ajp.2010.10020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segobin S.H., Chételat G., Le Berre A.-P., Lannuzel C., Boudehent C., Vabret F., et al. Relationship between brain volumetric changes and interim drinking at six months in alcohol-dependent patients. Alcohol Clin Exp Res. 2014;38:739–748. doi: 10.1111/acer.12300. [DOI] [PubMed] [Google Scholar]
- 23.van Holst R.J., de Ruiter M.B., van den Brink W., Veltman D.J., Goudriaan A.E. A voxel-based morphometry study comparing problem gamblers, alcohol abusers, and healthy controls. Drug Alcohol Depend. 2012;124:142–148. doi: 10.1016/j.drugalcdep.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 24.Wang J., Fan Y., Dong Y., Ma M., Dong Y., Niu Y., et al. Combining gray matter volume in the cuneus and the cuneus-prefrontal connectivity may predict early relapse in abstinent alcohol-dependent patients. PLoS One. 2018;13 doi: 10.1371/journal.pone.0196860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiers C.E., Gawron C.K., Gröpper S., Spengler S., Stuke H., Lindenmeyer J., et al. Decreased gray matter volume in inferior frontal gyrus is related to stop-signal task performance in alcohol-dependent patients. Psychiatry Res. 2015;233:125–130. doi: 10.1016/j.pscychresns.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Wu G.-R., Baeken C., Van Schuerbeek P., De Mey J., Bi M., Herremans S.C. Accelerated repetitive transcranial magnetic stimulation does not influence grey matter volumes in regions related to alcohol relapse: An open-label exploratory study. Drug Alcohol Depend. 2018;191:210–214. doi: 10.1016/j.drugalcdep.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Zois E., Vollstädt-Klein S., Hoffmann S., Reinhard I., Charlet K., Beck A., et al. Orbitofrontal structural markers of negative affect in alcohol dependence and their associations with heavy relapse-risk at 6 months post-treatment. Eur Psychiatry. 2017;46:16–22. doi: 10.1016/j.eurpsy.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 28.Demirakca T., Ende G., Kämmerer N., Welzel-Marquez H., Hermann D., Heinz A., Mann K. Effects of alcoholism and continued abstinence on brain volumes in both genders. Alcohol Clin Exp Res. 2011;35:1678–1685. doi: 10.1111/j.1530-0277.2011.01514.x. [DOI] [PubMed] [Google Scholar]
- 29.Niendam T.A., Laird A.R., Ray K.L., Dean Y.M., Glahn D.C., Carter C.S. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12:241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Berre A.-P., Fama R., Sullivan E.V. Executive functions, memory, and social cognitive deficits and recovery in chronic alcoholism: A critical review to inform future research. Alcohol Clin Exp Res. 2017;41:1432–1443. doi: 10.1111/acer.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charlet K., Schlagenhauf F., Richter A., Naundorf K., Dornhof L., Weinfurtner C.E.J., et al. Neural activation during processing of aversive faces predicts treatment outcome in alcoholism. Addict Biol. 2014;19:439–451. doi: 10.1111/adb.12045. [DOI] [PubMed] [Google Scholar]
- 32.Menon V., Uddin L.Q. Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brooks S.J., Dalvie S., Cuzen N.L., Cardenas V., Fein G., Stein D.J. Childhood adversity is linked to differential brain volumes in adolescents with alcohol use disorder: A voxel-based morphometry study. Metab Brain Dis. 2014;29:311–321. doi: 10.1007/s11011-014-9489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeWitt I., Rauschecker J.P. Phoneme and word recognition in the auditory ventral stream. Proc Natl Acad Sci U S A. 2012;109:505–514. doi: 10.1073/pnas.1113427109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eichenbaum H., Sauvage M., Fortin N., Komorowski R., Lipton P. Towards a functional organization of episodic memory in the medial temporal lobe. Neurosci Biobehav Rev. 2012;36:1597–1608. doi: 10.1016/j.neubiorev.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilbert A.R., Mataix-Cols D., Almeida J.R.C., Lawrence N., Nutche J., Diwadkar V., et al. Brain structure and symptom dimension relationships in obsessive-compulsive disorder: A voxel-based morphometry study. J Affect Disord. 2008;109:117–126. doi: 10.1016/j.jad.2007.12.223. [DOI] [PubMed] [Google Scholar]
- 37.Hashimoto N., Nakaaki S., Kawaguchi A., Sato J., Kasai H., Nakamae T., et al. Brain structural abnormalities in behavior therapy-resistant obsessive-compulsive disorder revealed by voxel-based morphometry. Neuropsychiatr Dis Treat. 2014;10:1987–1996. doi: 10.2147/NDT.S69652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoexter M.Q., De Souza Duran F.L., D’Alcante C.C., Dougherty D.D., Shavitt R.G., Lopes A.C., et al. Gray matter volumes in obsessive-compulsive disorder before and after fluoxetine or cognitive-behavior therapy: A randomized clinical trial. Neuropsychopharmacology. 2012;37:734–745. doi: 10.1038/npp.2011.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hou J., Song L., Zhang W., Wu W., Wang J., Zhou D., et al. Morphologic and functional connectivity alterations of corticostriatal and default mode network in treatment-naïve patients with obsessive-compulsive disorder. PLoS One. 2013;8 doi: 10.1371/journal.pone.0083931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobayashi T., Hirano Y., Nemoto K., Sutoh C., Ishikawa K., Miyata H., et al. Correlation between morphologic changes and autism spectrum tendency in obsessive-compulsive disorder. Magn Reson Med Sci. 2015;14:329–335. doi: 10.2463/mrms.2014-0146. [DOI] [PubMed] [Google Scholar]
- 41.Kopřivová J., Horáček J., Tintěra J., Praško J., Raszka M., Ibrahim I., Höschl C. Medial frontal and dorsal cortical morphometric abnormalities are related to obsessive-compulsive disorder. Neurosci Lett. 2009;464:62–66. doi: 10.1016/j.neulet.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 42.Lv Q., Wang Z., Zhang C., Fan Q., Zhao Q., Zeljic K., et al. Divergent structural responses to pharmacological interventions in orbitofronto-striato-thalamic and premotor circuits in obsessive-compulsive disorder. EBioMedicine. 2017;22:242–248. doi: 10.1016/j.ebiom.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsumoto R., Ito H., Takahashi H., Ando T., Fujimura Y., Nakayama K., et al. Reduced gray matter volume of dorsal cingulate cortex in patients with obsessive-compulsive disorder: A voxel-based morphometric study. Psychiatry Clin Neurosci. 2010;64:541–547. doi: 10.1111/j.1440-1819.2010.02125.x. [DOI] [PubMed] [Google Scholar]
- 44.Pujol J., Soriano-Mas C., Alonso P., Cardoner N., Menchón J.M., Deus J., Vallejo J. Mapping structural brain alterations in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:720–730. doi: 10.1001/archpsyc.61.7.720. [DOI] [PubMed] [Google Scholar]
- 45.Real E., Subirà M., Alonso P., Segalàs C., Labad J., Orfila C., et al. Brain structural correlates of obsessive–compulsive disorder with and without preceding stressful life events. World J Biol Psychiatry. 2016;17:366–377. doi: 10.3109/15622975.2016.1142606. [DOI] [PubMed] [Google Scholar]
- 46.Tang W., Huang X., Li B., Jiang X., Li F., Xu J., et al. Structural brain abnormalities correlate with clinical features in patients with drug-naïve OCD: A DARTEL-enhanced voxel-based morphometry study. Behav Brain Res. 2015;294:72–80. doi: 10.1016/j.bbr.2015.07.061. [DOI] [PubMed] [Google Scholar]
- 47.Togao O., Yoshiura T., Nakao T., Nabeyama M., Sanematsu H., Nakagawa A., et al. Regional gray and white matter volume abnormalities in obsessive-compulsive disorder: A voxel-based morphometry study. Psychiatry Res. 2010;184:29–37. doi: 10.1016/j.pscychresns.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 48.Valente A.A., Miguel E.C., Castro C.C., Amaro E., Duran F.L.S., Buchpiguel C.A., et al. Regional gray matter abnormalities in obsessive-compulsive disorder: A voxel-based morphometry study. Biol Psychiatry. 2005;58:479–487. doi: 10.1016/j.biopsych.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 49.Yoo S.Y., Roh M.-S., Choi J.-S., Kang D.-H., Tae H.H., Lee J.-M., et al. Voxel-based morphometry study of gray matter abnormalities in obsessive-compulsive disorder. J Korean Med Sci. 2008;23:24–30. doi: 10.3346/jkms.2008.23.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Christian C.J., Lencz T., Robinson D.G., Burdick K.E., Ashtari M., Malhotra A.K., et al. Gray matter structural alterations in obsessive-compulsive disorder: Relationship to neuropsychological functions. Psychiatry Res. 2008;164:123–131. doi: 10.1016/j.pscychresns.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tan L., Fan Q., You C., Wang J., Dong Z., Wang X., et al. Structural changes in the gray matter of unmedicated patients with obsessive-compulsive disorder: A voxel-based morphometric study. Neurosci Bull. 2013;29:642–648. doi: 10.1007/s12264-013-1370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang W., Zhu Q., Gong X., Zhu C., Wang Y., Chen S. Cortico-striato-thalamo-cortical circuit abnormalities in obsessive-compulsive disorder: A voxel-based morphometric and fMRI study of the whole brain. Behav Brain Res. 2016;313:17–22. doi: 10.1016/j.bbr.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y., Zou L., Xie W., Yang Z., Zhu X., Cheung E.F.C., et al. Altered grey matter volume and cortical thickness in patients with schizo-obsessive comorbidity. Psychiatry Res. 2018;276:65–72. doi: 10.1016/j.pscychresns.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 54.Subirà M., Alonso P., Segalàs C., Real E., López-Solà C., Pujol J., et al. Brain structural alterations in obsessive-compulsive disorder patients with autogenous and reactive obsessions. PLoS One. 2013;8 doi: 10.1371/journal.pone.0075273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ikari K., Nakao T., Nemoto K., Okada K., Murayama K., Honda S., et al. Morphologic and clinical differences between early- and late-onset obsessive-compulsive disorder: Voxel-based Morphometric study. J Obsessive Compuls Relat Disord. 2017;13:35–41. [Google Scholar]
- 56.van den Heuvel O.A., van Wingen G., Soriano-Mas C., Alonso P., Chamberlain S.R., Nakamae T., et al. Brain circuitry of compulsivity. Eur Neuropsychopharmacol. 2016;26:810–827. doi: 10.1016/j.euroneuro.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 57.Gonçalves OF, Sousa S., Carvalho S., Leite J., Ganho A., Fernandes-Gonçalves A., et al. Alterations of gray and white matter morphology in obsessive compulsive disorder. Psicothema. 2017;29:35–42. doi: 10.7334/psicothema2016.86. [DOI] [PubMed] [Google Scholar]
- 58.Moon C.-M., Jeong G.-W. Associations of neurofunctional, morphometric and metabolic abnormalities with clinical symptom severity and recognition deficit in obsessive–compulsive disorder. J Affect Disord. 2018;227:603–612. doi: 10.1016/j.jad.2017.11.059. [DOI] [PubMed] [Google Scholar]
- 59.Moreira P.S., Marques P., Soriano-Mas C., Magalhães R., Sousa N., Soares J.M., Morgado P. The neural correlates of obsessive-compulsive disorder: A multimodal perspective. Transl Psychiatry. 2017;7:e1224. doi: 10.1038/tp.2017.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 61.Radua J., Mataix-Cols D., Phillips M.L., El-Hage W., Kronhaus D.M., Cardoner N., Surguladze S. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry. 2012;27:605–611. doi: 10.1016/j.eurpsy.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 62.Radua J., Rubia K., Canales-Rodríguez E.J., Pomarol-Clotet E., Fusar-Poli P., Mataix-Cols D. Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Front Psychiatry. 2014;5:13. doi: 10.3389/fpsyt.2014.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Albajes-Eizagirre A., Solanes A., Vieta E., Radua J. Voxel-based meta-analysis via permutation of subject images (PSI): Theory and implementation for SDM. Neuroimage. 2019;186:174–184. doi: 10.1016/j.neuroimage.2018.10.077. [DOI] [PubMed] [Google Scholar]
- 64.Norman L.J., Carlisi C., Lukito S., Hart H., Mataix-Cols D., Radua J., Rubia K. Structural and functional brain abnormalities in attention-deficit/hyperactivity disorder and obsessive-compulsive disorder: A comparative meta-analysis. JAMA Psychiatry. 2016;73:815–825. doi: 10.1001/jamapsychiatry.2016.0700. [DOI] [PubMed] [Google Scholar]
- 65.Carlisi C.O., Norman L.J., Lukito S.S., Radua J., Mataix-Cols D., Rubia K. Comparative multimodal meta-analysis of structural and functional brain abnormalities in autism spectrum disorder and obsessive-compulsive disorder. Biol Psychiatry. 2017;82:83–102. doi: 10.1016/j.biopsych.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 66.Klaming R., Harlé K.M., Infante M.A., Bomyea J., Kim C., Spadoni A.D. Shared gray matter reductions across alcohol use disorder and posttraumatic stress disorder in the anterior cingulate cortex: A dual meta-analysis. Neurobiol Stress. 2019;10:100132. doi: 10.1016/j.ynstr.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Radua J., Romeo M., Mataix-Cols D., Fusar-Poli P. A general approach for combining voxel-based meta-analyses conducted in different neuroimaging modalities. Curr Med Chem. 2013;20:462–466. [PubMed] [Google Scholar]
- 68.Goodman W.K., Price L.H., Rasmussen S.A., Mazure C., Fleischmann R.L., Hill C.L., et al. The Yale-Brown obsessive compulsive scale: I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 69.Shenhav A., Cohen J.D., Botvinick M.M. Dorsal anterior cingulate cortex and the value of control. Nat Neurosci. 2016;19:1286–1291. doi: 10.1038/nn.4384. [DOI] [PubMed] [Google Scholar]
- 70.Naqvi N.H., Gaznick N., Tranel D., Bechara A. The insula: A critical neural substrate for craving and drug seeking under conflict and risk. Ann N Y Acad Sci. 2014;1316:53–70. doi: 10.1111/nyas.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xiao P., Dai Z., Zhong J., Zhu Y., Shi H., Pan P. Regional gray matter deficits in alcohol dependence: A meta-analysis of voxel-based morphometry studies. Drug Alcohol Depend. 2015;153:22–28. doi: 10.1016/j.drugalcdep.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 72.Yang X., Tian F., Zhang H., Zeng J., Chen T., Wang S., et al. Cortical and subcortical gray matter shrinkage in alcohol-use disorders: A voxel-based meta-analysis. Neurosci Biobehav Rev. 2016;66:92–103. doi: 10.1016/j.neubiorev.2016.03.034. [DOI] [PubMed] [Google Scholar]
- 73.Li J., Chen Z., Ma L. Morphometric changes of whole brain in patients with alcohol addiction: A voxel-based morphometry study. Chinese J Radiol. 2011;45:827–830. [Google Scholar]
- 74.Dalvie S., Stein D.J., Koenen K., Cardenas V., Cuzen N.L., Ramesar R., et al. The BDNF p. Val66Met polymorphism, childhood trauma, and brain volumes in adolescents with alcohol abuse. BMC Psychiatry. 2014;14:328. doi: 10.1186/s12888-014-0328-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Howell N.A., Worbe Y., Lange I., Tait R., Irvine M., Banca P., et al. Increased ventral striatal volume in college-aged binge drinkers. PLoS One. 2013;8 doi: 10.1371/journal.pone.0074164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mackey S., Allgaier N., Chaarani B., Spechler P., Orr C., Bunn J., et al. Mega-analysis of gray matter volume in substance dependence: General and substance-specific regional effects. Am J Psychiatry. 2019;176:119–128. doi: 10.1176/appi.ajp.2018.17040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koob G.F., Volkow N.D. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu X., Du M., Chen L., Li L., Zhou M., Zhang L., et al. Meta-analytic investigations of common and distinct grey matter alterations in youths and adults with obsessive-compulsive disorder. Neurosci Biobehav Rev. 2017;78:91–103. doi: 10.1016/j.neubiorev.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 79.Kim J.-J., Lee M.C., Kim J., Kim I.Y., Kim S.I., Han M.H., et al. Grey matter abnormalities in obsessive–compulsive disorder. Br J Psychiatry. 2001;179:330–334. doi: 10.1192/bjp.179.4.330. [DOI] [PubMed] [Google Scholar]
- 80.Tang W., Li B., Huang X., Jiang X., Li F., Wang L., et al. Morphometric brain characterization of refractory obsessive-compulsive disorder: Diffeomorphic anatomic registration using exponentiated Lie algebra. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:126–131. doi: 10.1016/j.pnpbp.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 81.Van Den Heuvel O.A., Remijnse P.L., Mataix-Cols D., Vrenken H., Groenewegen H.J., Uylings H.B.M., et al. The major symptom dimensions of obsessive-compulsive disorder are mediated by partially distinct neural systems. Brain. 2009;132:853–868. doi: 10.1093/brain/awn267. [DOI] [PubMed] [Google Scholar]
- 82.Boedhoe P.S.W., Schmaal L., Abe Y., Alonso P., Ameis S.H., Anticevic A., et al. Cortical abnormalities associated with pediatric and adult obsessive-compulsive disorder: Findings from the enigma obsessive-compulsive disorder working group. Am J Psychiatry. 2018;175:453–462. doi: 10.1176/appi.ajp.2017.17050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yun J.Y., Boedhoe P.S.W., Vriend C., Jahanshad N., Abe Y., Ameis S.H., et al. Brain structural covariance networks in obsessive-compulsive disorder: A graph analysis from the ENIGMA consortium. Brain. 2020;143:684–700. doi: 10.1093/brain/awaa001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.To W.T., Eroh J., Hart J., Vanneste S. Exploring the effects of anodal and cathodal high definition transcranial direct current stimulation targeting the dorsal anterior cingulate cortex. Sci Rep. 2018;8:4454. doi: 10.1038/s41598-018-22730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fein G., Landman B., Tran H., McGillivray S., Finn P., Barakos J., Moon K. Brain atrophy in long-term abstinent alcoholics who demonstrate impairment on a simulated gambling task. Neuroimage. 2006;32:1465–1471. doi: 10.1016/j.neuroimage.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yoon E.J., Choi J.-S., Kim H., Sohn B.K., Jung H.Y., Lee J.-Y., et al. Altered hippocampal volume and functional connectivity in males with Internet gaming disorder comparing to those with alcohol use disorder. Sci Rep. 2017;7:5744. doi: 10.1038/s41598-017-06057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Riffkin J., Yücel M., Maruff P., Wood S.J., Soulsby B., Olver J., et al. A manual and automated MRI study of anterior cingulate and orbito-frontal cortices, and caudate nucleus in obsessive-compulsive disorder: Comparison with healthy controls and patients with schizophrenia. Psychiatry Res. 2005;138:99–113. doi: 10.1016/j.pscychresns.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 88.Spalletta G., Piras F., Fagioli S., Caltagirone C., Piras F. Brain microstructural changes and cognitive correlates in patients with pure obsessive compulsive disorder. Brain Behav. 2014;4:261–277. doi: 10.1002/brb3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cabrera B., Romero-Rebollar C., Jiménez-Ángeles L., Genis-Mendoza A.D., Flores J., Lanzagorta N., et al. Neuroanatomical features and its usefulness in classification of patients with PANDAS. CNS Spectr. 2019;24:533–543. doi: 10.1017/S1092852918001268. [DOI] [PubMed] [Google Scholar]
- 90.Carmona S., Bassas N., Rovira M., Gispert J.-D., Soliva J.-C., Prado M., et al. Pediatric OCD structural brain deficits in conflict monitoring circuits: A voxel-based morphometry study. Neurosci Lett. 2007;421:218–223. doi: 10.1016/j.neulet.2007.05.047. [DOI] [PubMed] [Google Scholar]
- 91.Cheng B., Cai W., Wang X., Lei D., Guo Y., Yang X., et al. Brain gray matter abnormalities in first-episode, treatment-naive children with obsessive-compulsive disorder. Front Behav Neurosci. 2016;10:141. doi: 10.3389/fnbeh.2016.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gilbert A.R., Keshavan M.S., Diwadkar V., Nutche J., MacMaster F., Easter P.C., et al. Gray matter differences between pediatric obsessive-compulsive disorder patients and high-risk siblings: A preliminary voxel-based morphometry study. Neurosci Lett. 2008;435:45–50. doi: 10.1016/j.neulet.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jayarajan R.N., Agarwal S.M., Viswanath B., Kalmady S.V., Venkatasubramanian G., Srinath S., et al. A voxel based morphometry study of brain gray matter volumes in juvenile obsessive compulsive disorder. J Can Acad Child Adolesc Psychiatry. 2015;24:84–91. [PMC free article] [PubMed] [Google Scholar]
- 94.Lázaro L., Bargalló N., Castro-Fornieles J., Falcón C., Andrés S., Calvo R., Junqué C. Brain changes in children and adolescents with obsessive-compulsive disorder before and after treatment: A voxel-based morphometric MRI study. Psychiatry Res. 2009;172:140–146. doi: 10.1016/j.pscychresns.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 95.Lázaro L., Ortiz A.G., Calvo A., Ortiz A.E., Moreno E., Morer A., et al. White matter structural alterations in pediatric obsessive-compulsive disorder: Relation to symptom dimensions. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:249–258. doi: 10.1016/j.pnpbp.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 96.Szeszko P.R., Christian C., MacMaster F., Lencz T., Mirza Y., Taormina S.P., et al. Gray matter structural alterations in psychotropic drug-naive pediatric obsessive-compulsive disorder: An optimized voxel-based morphometry study. Am J Psychiatry. 2008;165:1299–1307. doi: 10.1176/appi.ajp.2008.08010033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.