Abstract
Background
Hoarding disorder is a chronic psychiatric condition of increasing public health concern. Recent investigation suggests a positive association between hoarding severity and insomnia symptoms. However, these findings have yet to be replicated, and the prevalence and type of sleep impairment experienced by individuals with clinically relevant hoarding symptoms (CHSs) are not known.
Methods
This analysis of 20,473 members of the internet-based Brain Health Registry uses multivariate logistic regression modeling and structural equation modeling to evaluate the relationship between hoarding symptoms, sleep impairment, adverse health, and cognitive functioning.
Results
More than 12% of study participants endorsed CHSs or subclinical hoarding symptoms. After adjustment for demographic characteristics and psychiatric comorbidity, individuals with CHSs reported increased odds of sleep impairment in nearly all domains. The odds of poor sleep quality (adjusted odds ratio, 2.07; 95% CI, 1.83–2.34), sleep disturbances (adjusted odds ratio, 2.15; 95% CI, 1.91–2.43), and daytime dysfunction (adjusted odds ratio, 5.84; 95% CI, 5.12–6.65) were two- to fivefold higher for individuals with CHSs compared with those without. For all measures, the proportion of individuals reporting sleep impairment increased with hoarding severity. In our structural equation model, sleep impairment acted as a partial mediator on the indirect pathways from hoarding to subjective cognitive complaints and poorer quality of life.
Conclusions
Identification of sleep problems among those with hoarding symptoms is a critical component of hoarding assessment. Additional research is needed to better understand the mechanisms underlying the observed relationships, including neurobiological underpinnings, and to examine the role of sleep management in treatment for hoarding behaviors.
Keywords: Cognition, Fatigue, Hoarding disorder, Insomnia, Psychiatry, Sleep
Hoarding disorder (HD) is a chronic psychiatric condition in which individuals experience persistent difficulty discarding material possessions irrespective of their objective value (1). In the absence of external intervention, hoarding often results in an overwhelming accumulation of possessions in active living spaces, which then causes significant impairment to daily functioning. Notably, individuals with HD, particularly those who are older, frequently experience difficulty performing activities of daily living, managing personal health and hygiene, and minimizing health and safety hazards within the home (2). For example, one study of treatment-seeking individuals with HD found that more than 50% of participants experienced moderate or extreme difficulty moving around the house as a result of clutter (3), and in a study of geriatric hoarding patients, approximately one third reported difficulty sleeping in bed—facets of basic daily functioning essential to overall health and well-being (4). Indeed, psychiatric and physiological health impairment is common among individuals with HD (3,5). Nevertheless, the mechanism by which hoarding-related functional impairment relates to elevated health risk is less clear.
Difficulty managing sleep behavior and overall sleep impairment in particular have been reciprocally associated with a number of medical and psychiatric conditions. Fragmented sleep and reduced sleep efficiency are commonly reported by those with anxiety and mood disorders, and often result in negative daytime consequences, including reduced cognitive performance and decreased mood (6). Though findings are limited, there is some evidence that sleep impairment is also common among individuals with hoarding behaviors. In a recent investigation of 24 adults with HD, symptoms of insomnia, including difficulty initiating or maintaining sleep and interference of sleep with daily functioning, were positively associated with hoarding symptom severity independent of co-occurring trauma-related and mood disorders (7). Importantly, further investigation of 40 individuals with HD suggests that the association between hoarding symptom severity and sleep disturbance remains significant even when controlling for the ability to sleep in one’s bed, suggesting that hoarding symptoms (HSs) other than clutter play a large role in sleep impairment among individuals with hoarding behavior (8). To date, only one study has compared sleep impairment among individuals with hoarding with those with other psychiatric disorders and healthy control participants; findings indicate elevated insomnia symptoms and poorer sleep quality among individuals with HD compared with healthy control participants but not compared with those with obsessive-compulsive disorder (9). However, these findings have not been replicated and are limited in generalizability owing to the use of relatively small, treatment-seeking samples. Additionally, while our group has identified heightened risk of sleep apnea among those with clinically relevant HSs (CHSs) even after analytic adjustment for body mass index (BMI) (5), the prevalence and type of sleep impairment experienced by individuals with CHSs were not assessed.
There are several well-established health consequences of poor sleep quality, including emotional distress, reduced quality of life, cognitive performance deficits, mood disorders, cardiovascular and metabolic disease, and all-cause mortality (10). Though similar health outcomes have been observed among those with HD (3,5,11), few studies have considered the influence of sleep in the investigation of hoarding-related health consequences. Examination of sleep impairment as it relates to the relationship between hoarding and overall health may be of particular relevance to the improvement of treatment outcomes among those with CHSs.
The objective of this study was twofold. First, we aimed to explore the association between HSs, sleep impairment, and daytime fatigue using data collected from the Brain Health Registry (BHR), a large, online research registry of U.S. adults (12). Identification of sleep disturbances most common to those with CHSs and subclinical HSs (SCHSs) may aid clinicians in the development of targeted treatment to improve sleep quality among those with hoarding behaviors. It is hypothesized that individuals with CHSs would more frequently report difficulty initiating and maintaining sleep, poor sleep quality, and daytime fatigue than those without HSs. Additionally, as has been observed for other measures of functional impairment (3), it is hypothesized that the prevalence of sleep impairment will increase with hoarding severity. In particular, those with CHSs will most frequently report sleep impairment, followed by those with SCHSs and those without HSs. Second, we aimed to define the influence of sleep on the relationship between hoarding and various adverse health outcomes including poor mental health, cardiovascular/metabolic health, decreased cognitive functioning, and reduced quality of life. Exploration of sleep impairment as a potential intermediary on the theoretical pathway from hoarding to poor health quality may aid researchers in elucidating the etiology of health outcomes among those with CHSs and SCHSs, and may assist clinician efforts to improve overall health among those seeking treatment for hoarding-related problems. It is hypothesized that sleep impairment will mediate the relationship between hoarding and all adverse health outcomes. Further, we hypothesized that hoarding will be positively associated with all health outcomes assessed, independent of the presence or degree of sleep impairment.
Methods and Materials
This analysis uses data from 20,473 BHR participants who completed internet-based assessments of hoarding and sleep behavior between February 2017 and August 2020. Since 2012, the BHR has been used to longitudinally evaluate the physical and mental health of nearly 70,000 adult participants at least 18 years of age. The BHR and its application in research related to hoarding is detailed in previous literature (12,13). In short, BHR participants are semiannually encouraged to complete online cognitive tests and self-report questionnaires assessing health history and behavior. The majority of adult volunteers enrolled in the registry are over 55 years of age and report residence from all 50 states, with overrepresentation of participants on the coasts, particularly in California. For this analysis, we examined data from the most recent time point (as of August 2020) at which participants simultaneously completed the BHR Hoarding and Clutter, Sleep, Demographics, and Medical History modules. All participants provided informed consent and the study was approved by appropriate ethical committees.
Hoarding
HSs were assessed using online adaption of the Hoarding Rating Scale–Self Report (HRS-SR), which has been validated for use in the BHR (13). The HRS-SR is a 5-item assessment of the following HSs: clutter, difficulty discarding, excessive acquisition, and hoarding-related emotional distress and impairment. Using this measure, the presence and severity of core HSs are rated on a scale ranging from 0 (none/no difficulty) to 8 (extreme/extreme difficulty). Cumulative scores range from 0 to 40, with higher scores indicating increased symptomatology. HRS-SR total scores were used to classify participants in three distinct groups: no HSs, SCHSs, and CHSs based on prior literature and validation analyses conducted in a subsample of participants included in the present study (13). Participants with scores above 14 were classified as having CHSs, those with scores between 10 and 14 were classified as having SCHSs, and those with scores <10 were classified as not having HSs. Our validation analyses indicate that these classification criteria are conservative for this sample, yet effectively differentiate those with and without clinically relevant hoarding behaviors (13).
Sleep Impairment
Impaired sleep was assessed via the Pittsburgh Sleep Quality Index (PSQI), a well-validated self-report assessment of past-month sleep patterns, sleep quality, and daytime fatigue (14). The PSQI contains 19 items that are combined to form seven summary scores: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. For all summary components, scores range from 0 to 3, with higher scores indicating greater impairment. Component scores are summed to yield one global measure of sleep impairment, with total scores ranging from 0 to 21. Validation studies indicate that a total PSQI score >5 can be used to identify poor sleepers (14). However, in this study, the average global PSQI score was 6.15 (SD = 3.56), and more than half of the participants had a PSQI global score >5 (n = 10,291, 50.3%), indicating that poor sleep was common in our sample of predominately older adults. Therefore, we dichotomously classified good and poor sleepers using a cutoff value of 13 (i.e., a PSQI score >13 indicated poor sleep), which was determined by adding two sample SDs to the sample mean [i.e., μPSQI + 2(PSQI)]. By using this conservative cutoff value, we aimed to identify individuals with substantial sleep impairment and to avoid false-positive classification of individuals with mild or moderate sleep difficulties. In addition to the global PSQI score, responses for each of the seven component scores were dichotomized as good (i.e., a summary score of 0 or 1) or poor (i.e., a summary score of 2 or 3).
Demographic, Behavioral, and Psychiatric Covariates
Demographic and behavioral covariates of interest included gender (male, female), age (categorized as <60 years of age or 60 years of age or older), race (categorized as white or other), educational attainment (categorized as less than college degree, college degree or graduate/professional degree), and lifetime use of tobacco products (yes/no). Additionally, we determined approximate BMI (categorized as underweight/normal weight, overweight, or obese) using self-reported participant height and weight. Given the categorical nature of the BHR weight measure (140–149 pounds, 150–159 pounds, 160–169 pounds, etc.), exact BMI values were not obtainable. In the case in which BMI category determination was unclear based on the reported height and weight range, participants were classified in the BMI group closest to the “normal weight” classification. For example, if the decision was between obese and overweight, the participant was classified as overweight. This classification method, which aims to be conservative, has been successfully applied in a previous investigation using data collected by the BHR (5). Last, in terms of psychiatric covariates, participants were asked to self-report lifetime history of major depressive disorder (MDD) and anxiety disorders (generalized anxiety disorder, panic disorder, or specific phobia), rated as present or absent.
Depressive Mood
Current mood was assessed using the Patient Health Questionnaire–9, a 9-item assessment of depressive symptom presence and frequency occurring over the previous two weeks (15). Individual items are scaled from 0 (not at all) to 3 (nearly every day) and are summed for a total score ranging from 0 to 27. Given our focus on sleep disturbance, Patient Health Questionnaire–9 item 3 (frequency of trouble falling or staying asleep, or sleeping too much) was removed from analyses to reduce collinearity; an adjusted Patient Health Questionnaire–9 score was calculated by subtracting the sleep item from the total score (i.e., adjusted total score ranging from 0 to 24).
Adverse Health Outcomes
Self-reported medical history, internet-based cognitive assessments, and self-report symptom questionnaires were used to examine relationship of sleep and hoarding to overall mental and physical health, cognitive functioning, and quality of life. Details pertaining to the assessment of the four health outcomes of interest (mental health, physical health, objective and subjective cognitive functioning, and quality of life) are outlined in the Supplemental Methods. In brief, participants self-reported lifetime diagnoses of psychiatric and physical health conditions and completed objective and subjective assessments of cognitive functioning [CogState Brief Battery (16,17) and the self-report version of the Everyday Cognition Scale (18,19), respectively], as well as a subjective assessment of general health and quality of life [36-item Short-Form Health Survey (20, 21, 22)].
Statistical Analysis
Demographic and behavioral characteristics of the sample were compared between those with CHSs, SCHSs, and no HSs using Pearson’s χ2 tests and one-way analysis of variance; pairwise comparisons were conducted using χ2 and independent-sample t tests. Pearson’s χ2 tests were further used to assess variation in the prevalence of sleep impairment by hoarding group; full-sample and pairwise comparisons were conducted.
Multivariate logistic regression models adjusted for participant demographic characteristics (gender, age, race, education) and health status (BMI, lifetime history of MDD, anxiety disorders, and sleep apnea) were used to examine the relationship between HSs and sleep disturbances. In these models, the seven dichotomized PSQI component summary scores (i.e., dichotomized measures of sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction) and the dichotomized PSQI global score were used as separate outcome variables, and hoarding (i.e., CHSs, SCHSs, no HSs) was used as the primary predictor variable. In order to compare sleep impairment between those with SCHSs and CHSs, the analyses were repeated after excluding those with no HSs (effective sample size: n = 2477). Correction for multiple testing was applied to all analyses (Bonferroni adjustment for 90 statistical tests: α = 0.00056).
Structural Equation Modeling
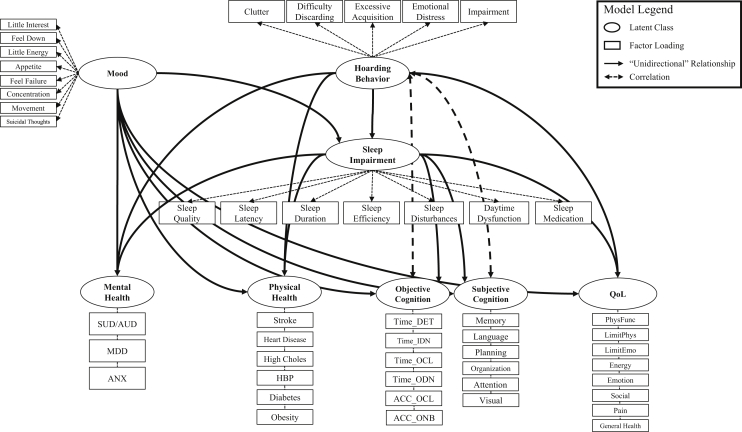
Structural equation modeling [MPlus version 8 (23)] was used to examine the mediating effect of sleep impairment on the association between hoarding and five adverse health outcomes: poor psychiatric health, cardiovascular vulnerability, decreased cognitive functioning (objective and subjective), and reduced quality of life. Our model consisted of eight latent constructs as outlined in Figure 1: HSs, current mood, sleep impairment, mental health status, cardiovascular vulnerability, objective cognitive functioning, subjective cognitive functioning, and quality of life. It was hypothesized that sleep impairment would significantly decrease (i.e., partially mediate) the association between hoarding and all five health outcomes, independent of depressive mood (Figure 1). Given the use of categorical factor loadings, weighted least squares with mean and variance adjustment was used for model estimation (24). Established cutoff criteria for the root-mean-square error of approximation (<0.08), comparative fit index (≥0.95), and Tucker-Lewis index (≥0.95) were used to assess model fit (25). Given that the model χ2 statistic is extremely sensitive to large sample size, the value was not used in the evaluation of model fit (25). However, program-generated modifications recommended to improve model fit (i.e., modification indices) that reduced the model χ2 value by 200 units or more were incorporated in the final model. Correction for multiple testing was applied (Bonferroni adjustment for 17 statistical tests: α = 0.0029).
Figure 1.
Hypothesized model for the relationship between hoarding, sleep impairment, and adverse health outcomes. Mental health factor loadings: self-reported 1) substance use disorder (SUD) or alcohol use disorder (AUD), 2) major depressive disorder (MDD), and 3) anxiety disorder (ANX). Physical health factor loadings: self-reported 1) stroke, 2) heart disease, 3) high cholesterol (High Choles), 4) high blood pressure (HBP), 5) diabetes, and 6) obesity. Objective cognition factor loadings: Detection Test reaction time (Time_DET), Identification Test reaction time (Time_IDN), One Card Learning Test reaction time (Time_OCL), One Back Test reaction time (Time_ODN), One Card Learning Test accuracy (ACC_OCL), and One Back Test accuracy (ACC_ONB). Subjective cognition factor loadings: compromised performance on Everyday Cognition subdomains, including 1) memory, 2) language, 3) planning, 4) organization, 5) divided attention, and 6) visuospatial abilities (Visual). Quality-of-life (QoL) factor loadings: 36-item Short-Form Health Survey score for 1) physical functioning (PhysFunc), 2) role limitations owing to physical health (LimitPhys), 3) role limitations owing to emotional health (LimitEmo), 4) energy, 5) emotional well-being (Emotion), 6) social functioning (Social), 7) pain, and 8) general health.
Results
Of 20,473 BHR participants included in this analysis, 1258 (6.1%) endorsed CHSs, 1219 (6.0%) endorsed SCHSs, and 17,996 (87.9%) did not report significant HSs. Statistically significant differences were observed between hoarding groups for all demographic characteristics assessed (Table 1). Of note, those with CHSs were nearly three times as likely to report a lifetime diagnosis of MDD (43.9% vs. 16.7%; p < .0001) and approximately twice as likely to report a lifetime diagnosis of an anxiety disorder (36.1% vs. 16.7%; p < .0001) or sleep apnea (34.7% vs. 16.7%; p < .0001) when compared with those without HSs. Those with CHSs were also twice as likely to report obesity as those without HSs (40.8% vs. 19.6%; p < .0001). Prevalence of all four health conditions among those with SCHSs fell intermediate to those with and without CHSs.
Table 1.
Sample Characteristics, by HD Status
| Full Sample (N = 20,473) | HD (n = 1258) | Subclinical HD (n = 1219) | No HD (n = 17,996) | p | |
|---|---|---|---|---|---|
| Gender | <.0001 | ||||
| Female | 15,306 (74.8) | 1007 (80.0) | 910 (74.6) | 13,389 (74.4) | |
| Male | 5167 (25.2) | 251 (20.0)a | 309 (25.4) | 4607 (25.6) | |
| Race | <.0001 | ||||
| Other | 2222 (10.8) | 187 (14.9) | 183 (15.0) | 1852 (10.3) | |
| White | 18,251 (89.2) | 1071 (85.1)a | 1036 (85.0)a | 16,144 (89.7) | |
| Age | <.0001 | ||||
| <60 years of age | 7317 (35.7) | 528 (42.0)a | 519 (42.6)a | 6270 (34.8) | |
| 60 years of age and older | 13,156 (64.3) | 730 (58.0) | 700 (57.4) | 11,726 (65.2) | |
| Education | <.0001 | ||||
| Less than college | 3593 (17.6) | 291 (23.1)a | 257 (21.1)a | 3045 (16.9) | |
| College | 8210 (40.1) | 543 (43.2) | 504 (41.3) | 7163 (39.8) | |
| Graduate/professional | 8670 (42.3) | 424 (33.7) | 458 (37.6) | 7788 (43.3) | |
| Tobacco Use (Yes) | 7593 (37.1) | 520 (41.3) | 496 (40.7) | 6577 (36.6) | .0001 |
| BMI | <.0001 | ||||
| Normal weightc | 9989 (48.8) | 414 (32.9)a,b | 442 (36.3)a | 9133 (50.8) | |
| Overweight | 6070 (29.6) | 331 (26.3) | 404 (33.1) | 5335 (29.6) | |
| Obese | 4414 (21.6) | 513 (40.8) | 373 (30.6) | 3528 (19.6) | |
| Lifetime MDD (Yes) | 3933 (19.2) | 552 (43.9)a,b | 373 (30.6)a | 3008 (16.7) | <.0001 |
| Lifetime Anxiety Disorder (Yes) | 3822 (18.7) | 454 (36.1)a,b | 358 (29.4)a | 3010 (16.7) | |
| Lifetime Sleep Apnea (Yes) | 3705 (18.1) | 436 (34.7)a,b | 293 (24.0)a | 2976 (16.5) | <.0001 |
Values are n (%).
BMI, body mass index; HD, hoarding disorder; MDD, major depressive disorder.
Significantly different from individuals with no HD (p < .0001).
Significantly different from individuals with subclinical HD (p < .0001).
And underweight.
For all PSQI component summary measures, the proportion of individuals reporting poor sleep increased with hoarding severity (Table 2). Compared with those without HSs, those with CHSs were twice as likely to report poor sleep quality, decreased sleep duration, impaired sleep efficiency, and increased sleep disturbances. Additionally, the rate of daytime dysfunction was five times higher among individuals with CHSs than among those without HSs (49.5% vs. 9.9%; p < .0001), and three times higher among individuals with SCHSs when compared with those without HSs (30.9% vs. 9.9%; p < .0001).
Table 2.
Prevalence of Sleep Impairment, by HD Status
| Full Sample (N = 20,473) | HD (n = 1258) | Subclinical HD (n = 1219) | No HD (n = 17,996) | p | |
|---|---|---|---|---|---|
| Poor Sleep Quality (Subjective) | 4290 (21.0) | 491 (39.0)a | 387 (31.8)a | 3412 (19.0) | <.0001 |
| Increased Sleep Latency | 4617 (22.6) | 458 (36.4)a | 364 (29.9)a | 3795 (21.1) | <.0001 |
| Decreased Sleep Duration | 2287 (11.2) | 275 (21.9)a | 218 (17.9)a | 1794 (10.0) | <.0001 |
| Impaired Sleep Efficiency | 3454 (16.9) | 369 (29.3)a | 288 (23.6)a | 2797 (15.5) | <.0001 |
| Increased Sleep Disturbances | 5943 (29.0) | 648 (51.5)a,b | 514 (42.2)a | 4781 (26.6) | <.0001 |
| Increased Use of Sleep Medication | 5202 (25.4) | 406 (32.3)a,b | 351 (28.8) | 4445 (24.7) | <.0001 |
| Increased Daytime Dysfunction | 2774 (13.6) | 619 (49.2)a,b | 368 (30.2)a | 1787 (9.9) | <.0001 |
| “Poor” Sleep (PSQI Total Score >13) | 845 (4.1) | 169 (13.4)a,b | 98 (8.0)a | 578 (3.2) | <.0001 |
Values are n (%).
HD, hoarding disorder; PSQI, Pittsburgh Sleep Quality Index.
Significantly different from individuals with no HD (p < .0001).
Significantly different from individuals with subclinical HD (p < .0001).
The presence of CHSs or SCHSs significantly increased the odds of nearly all measures of sleep impairment after controlling for participant demographics (gender, age, race, education) and health history (BMI, lifetime history of MDD, generalized anxiety disorder, and sleep apnea; Table 3). Of note, hoarding symptomatology increased the odds of daytime dysfunction more than fivefold (adjusted odds ratio [aOR], 5.84; 95% CI, 5.12–6.65) and doubled the odds of experiencing poor sleep quality (aOR, 2.07; 95% CI, 1.83–2.34), a greater number of sleep disturbances (aOR, 2.15; 95% CI, 1.91–2.43), and shorter sleep duration (aOR, 1.93; 95% CI, 1.66–2.34). Additionally, CHSs increased the odds of poor sleep more than 2.5-fold (aOR, 2.75; 95% CI, 2.26–3.35). SCHSs also increased the odds of sleep impairment, though to a lesser extent than clinically meaningful symptoms. Nevertheless, SCHSs were associated with a threefold increase in the odds of daytime dysfunction (aOR, 3.01; 95% CI, 2.62–3.46) and a near twofold increase in poor sleep (aOR, 1.86; 95% CI, 1.48–2.34). In models comparing individuals with CHSs and SCHSs, those with CHSs reported slightly greater sleep impairment in all domains except sleep medication use. For most measures of sleep impairment, the size of the effect for the adjusted comparison between individuals with CHSs and SCHSs was small (Table S1). However, endorsement of CHSs, rather than of SCHSs, nearly doubled the odds of daytime dysfunction (aOR, 1.97; 95% CI, 1.65–2.35) and increased the odds of poor sleep by more than 50% (aOR, 1.54; 95% CI, 1.17–2.02).
Table 3.
Adjusted Logistic Regression Models Predicting Sleep Impairment
| HD | Subclinical HD | |
|---|---|---|
| Poor Sleep Quality (Subjective) | 2.07 (1.83–2.34) | 1.66 (1.46–1.89) |
| Increased Sleep Latency | 1.59 (1.40–1.81) | 1.34 (1.17–1.53) |
| Decreased Sleep Duration | 1.93 (1.66–2.24) | 1.64 (1.40–1.92) |
| Impaired Sleep Efficiency | 1.72 (1.50–1.97) | 1.44 (1.24–1.66) |
| Increased Sleep Disturbances | 2.15 (1.91–2.43) | 1.71 (1.51–1.93) |
| Increased Use of Sleep Medication | 1.10 (0.97–1.26) | 1.07 (0.94–1.23) |
| Increased Daytime Dysfunction | 5.84 (5.12–6.65) | 3.01 (2.62–3.46) |
| “Poor” Sleep (PSQI Total Score >13) | 2.75 (2.26–3.35) | 1.86 (1.48–2.34) |
Values are adjusted odds ratio (95% confidence interval). Separate logistic regression models adjusted for gender, age, race, educational attainment, body mass index, self-reported lifetime psychiatric conditions (major depressive disorder, anxiety disorders), and self-reported sleep apnea.
HD, hoarding disorder; PSQI, Pittsburgh Sleep Quality Index.
Hoarding and Adverse Health Outcomes: Mediation by Sleep Impairment
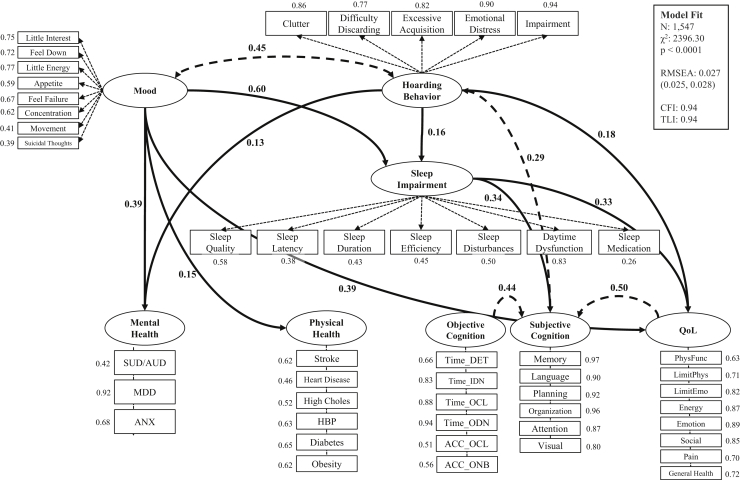
Structural equation modeling was used to examine the direct pathways from hoarding to poor mental health, cardiovascular vulnerability, decreased objective and subjective cognitive functioning, and reduced quality of life, as well as to examine the indirect influence of sleep impairment on the relationships between HSs and these adverse health outcomes. Modifications to the model as suggested by the fit statistics included the addition of a correlation between current mood and hoarding (β = 0.45, p < .0001) and the addition of correlations between subjective cognitive functioning and 1) objective cognitive functioning (β = 0.44, p < .0001) and 2) self-reported quality of life (β = 0.50, p < .0001). The final structural equation model, including significant direct and indirect standardized path coefficients, is displayed in Figure 2. Independent of current mood, HSs were directly associated with three of five of the adverse health outcomes assessed: mental health (β = 0.13, p < .0001), subjective cognitive functioning (β = 0.29, p < .0001), and quality of life (β = 0.18, p < .0001). Additionally, HSs were directly associated with sleep impairment (β = 0.16, p < .0001), which contributed to worsened cognitive functioning (subjective; β = 0.34, p < .0001) and reduced quality of life (β = 0.33, p < .0001). Notably, impaired sleep partially mediated the relationship between HSs and decreased subjective cognition (standardized indirect effect: β = 0.053, p < .0001) and the relationship between HSs and reduced quality of life (standardized indirect effect: β = 0.052, p < .0001). However, for both hypothesized pathways, the direct relationship between HSs and adverse health was stronger than the indirect relationship mediated by sleep (Table 4). Sleep impairment also partially mediated the relationship between current mood and reduced quality of life (standardized indirect effect: β = 0.200, p < .0001) and fully mediated the relationship between current mood and decreased subjective cognitive functioning (standardized indirect effect: β = 0.204, p < .0001). However, sleep was not a significant mediator of the relationships between HSs, mood, and mental health outcomes (indirect effect of sleep on HD-Mental Health: β = 0.011, p = .269; indirect effect of sleep on Current Mood-Mental Health: β = 0.043, p = .244). Acceptable model fit was determined via model fit statistics (root-mean-square error of approximation = 0.027 [95% CI, 0.25–0.028]; comparative fit index = 0.94; Tucker-Lewis index = 0.94).
Figure 2.
Final structural equation model for the relationship between hoarding, sleep disturbance, and adverse health outcomes. The model depicts standardized estimates for latent class factor loadings (all p < .0001) and significant (p < .0029 after Bonferroni adjustment) pathways between latent class variables. Mental health factor loadings: self-reported 1) substance use disorder (SUD) or alcohol use disorder (AUD), 2) major depressive disorder (MDD), and 3) anxiety disorder (ANX). Physical health factor loadings: self-reported 1) stroke, 2) heart disease, 3) high cholesterol (High Choles), 4) high blood pressure (HBP), 5) diabetes, and 6) obesity. Objective cognition factor loadings: Detection Test reaction time (Time_DET), Identification Test reaction time (Time_IDN), One Card Learning Test reaction time (Time_OCL), One Back Test reaction time (Time_ODN), One Card Learning Test accuracy (ACC_OCL), and One Back Test accuracy (ACC_ONB). Subjective cognition factor loadings: compromised performance on Everyday Cognition subdomains, including 1) memory, 2) language, 3) planning, 4) organization, 5) divided attention, and 6) visuospatial abilities (Visual). Quality-of-life (QoL) factor loadings: 36-item Short-Form Health Survey score for 1) physical functioning (PhysFunc), 2) role limitations owing to physical health (LimitPhys), 3) role limitations owing to emotional health (LimitEmo), 4) energy, 5) emotional well-being (Emotion), 6) social functioning (Social), 7) pain, and 8) general health. CFI, comparative fit index; RMSEA, root-mean-square error of approximation; TLI, Tucker-Lewis index.
Table 4.
Standardized Effects for the Relationships Between HD, Sleep Impairment, and Adverse Health and for the Relationships Between Mood, Sleep Impairment, and Adverse Health
| HD, Sleep Impairment, and Adverse Health |
Mood, Sleep Impairment, and Adverse Health |
|||
|---|---|---|---|---|
| Standardized Estimate | p | Standardized Estimate | p | |
| Mental Health | ||||
| Total | 0.139 | .002 | 0.435 | <.0001 |
| Direct | 0.128 | .007 | 0.392 | <.0001 |
| Indirect (i.e., sleep as a mediator) | 0.011 | .269 | 0.043 | .244 |
| Physical Health | ||||
| Total | 0.079 | .108 | 0.167 | <.0001 |
| Direct | 0.073 | .150 | 0.146 | .012 |
| Indirect (i.e., sleep as a mediator) | 0.006 | .583 | 0.021 | .581 |
| Quality of Life | ||||
| Total | 0.230 | <.0001 | 0.594 | <.0001 |
| Direct | 0.178 | <.0001 | 0.394 | <.0001 |
| Indirect (i.e., sleep as a mediator) | 0.052 | <.0001 | 0.200 | <.0001 |
| Objective Cognitive Functioning | ||||
| Total | 0.022 | .728 | 0.110 | .039 |
| Direct | 0.033 | .595 | 0.154 | .040 |
| Indirect (i.e., sleep as a mediator) | −0.012 | .370 | −0.044 | .366 |
| Subjective Cognitive Functioning | ||||
| Total | 0.340 | <.0001 | 0.346 | <.0001 |
| Direct | 0.287 | <.0001 | 0.142 | .013 |
| Indirect (i.e., sleep as a mediator) | 0.053 | <.0001 | 0.204 | <.0001 |
HD, hoarding disorder.
Discussion
Consistent with a previous investigation (9), our findings suggest that individuals with HSs report sleep impairment at significantly higher rates than those without hoarding. Although all measures of sleep impairment were more frequently reported by participants with CHSs, the odds of poor sleep quality, sleep disturbances, and daytime dysfunction in particular were two- to fivefold higher for individuals with CHSs compared with those without, even after adjustment for demographic characteristics and health status. Importantly, those with SCHSs also reported impaired sleep, increasing the odds of reduced sleep quality and daytime dysfunction between 1.5- and 3-fold. Though it is frequently reported that excessive clutter may impede on the ability to sleep in bed, we found no evidence that clutter is the primary feature underlying the relationship between HSs and poor sleep outcomes, as evidenced by the high correlations between the hoarding latent class variable and each of the five observed HSs in our structural equation model (i.e., difficulty discarding, excessive acquisition, impairment, emotional distress, and clutter). This finding is consistent with previous investigation of insomnia among patients with HD, which found no evidence that insomnia was specifically associated with the clutter domain of hoarding despite the significant, positive association between hoarding severity and sleep disturbances (7,8).
In our structural equation model, we observed direct associations between HSs and three of five adverse health outcomes assessed: 1) poor mental health, 2) reduced quality of life, and 3) decreased subjective cognitive functioning. Additionally, the model identified a direct relationship between HSs and sleep, which was independently associated with decreased subjective cognitive functioning and reduced quality of life. Sleep impairment thus acted as a partial mediator for the associations between hoarding and subjective cognitive functioning, and between hoarding and reduced quality of life. Similarly, sleep impairment mediated the relationships between depressed mood, decreased subjective cognitive functioning, and reduced quality of life. The effects of sleep impairment on the relationship between psychiatric symptoms (i.e., HSs and depressed mood) and quality of life were clear: sleep impairment mediated between 25% and 35% of the observed relationships between 1) HSs and quality of life and 2) current mood and quality of life, respectively. However, the mediating effects of sleep on the relationships between hoarding, mood, and subjective cognition provided a more nuanced picture of adverse health among individuals with hoarding behaviors. Though sleep impairment fully explained the relationship between current mood and subjective cognition, it only partially explained the relationship between HSs and decreased cognitive functioning. Thus, unlike cognitive impairment among those with depressed mood, self-reported cognitive impairment among individuals with HSs is not entirely a product of impairment in other domains. Rather, HSs are associated with increased subjective cognitive dysfunction independent of other psychiatric and physical health symptoms (i.e., depressed mood and poor sleep), though the underlying mechanisms of this relationship are not clear. As researchers work to identify other modifiable behaviors that affect subjective cognitive impairment (and quality of life) among those with CHSs and SCHSs, addressing impairments to sleep habits and quality may improve health outcomes among those with HSs, especially in cognitive domains.
Unfortunately, investigation of sleep disturbance among patients with mood and anxiety disorders indicates that disregard of sleep symptoms as a clinically relevant problem is common among both psychiatric patients and physicians (26). Often, health service utilization among those with concurrent psychiatric conditions and insomnia symptoms does not differ from those without sleep impairment, indicating that the frequency of treatment visits may not accurately reflect additional distress related to these dual diagnoses. Despite DSM-5 recommendations that urge clinicians to emphasize the assessment and treatment of insomnia as its own condition, rather than as a symptom of poor mental health, there is often less attention given to the treatment of sleep disturbances as compared with psychiatric symptoms (1,26). Though it is not clear whether attention to sleep impairment will improve outcomes among those with HSs, there is some evidence that sleep quality enhances psychiatric well-being and treatment outcomes among those with obsessive-compulsive, mood, and anxiety disorders (26,27). While researchers work to clarify the role of sleep outcomes in the treatment of hoarding behavior, we recommend careful assessment and management of sleep behavior among those with HSs.
Limitations
Our analysis of the relationship between hoarding and sleep impairment is the first investigation to consider the association between HSs, specific sleep disturbances, and daytime fatigue. Our exploration of clinical and subclinical hoarding as it relates to sleep impairment and adverse health outcomes is bolstered by use of a large population-based registry that is representative of individuals experiencing a wide range of HSs. Nevertheless, our sample was predominated by white females of older age and high educational attainment, and the online registry model inherently has a selection bias for those with adequate computer and internet access; thus, our findings may not be generalizable to the wider population.
Information related to sleep impairment, HSs, and adverse health outcomes was measured using internet-based, self-report assessments, and there is potential for recall and misclassification bias. Though previous work in using data collected from the BHR indicates excellent validity and reliability of the internet-based HRS-SR (10), future research is needed to confirm the observed associations.
The cross-sectional nature of this investigation precludes the ability to determine the temporal nature of the association between hoarding and sleep impairment. Like other psychiatric conditions, it is likely that the relationship between hoarding and sleep impairment is complex and bidirectional in nature. Thus, regression and mediation analyses assuming a temporal link between hoarding behavior and sleep impairment should be interpreted with caution. Longitudinal research is needed to elucidate causality.
Conclusions
Consistent with the limited number of studies investigating hoarding and sleep impairment, we found a strong link between HSs, sleep disturbances and reduced sleep quality, and daytime fatigue. Importantly, the relationships between hoarding, decreased cognitive functioning, and reduced quality of life were mediated by heightened sleep impairment. Therefore, our results suggest that careful assessment and management of sleep behavior is a critical component of hoarding treatment. Additional research is needed to better understand the mechanisms underlying the observed relationship and examine the role of sleep management in the treatment of hoarding behavior.
Acknowledgments and Disclosures
This study was funded by National Institute of Mental Health Grant No. MH 117114 (to CAM and RSM).
We are grateful for the support of past and present Brain Health Registry team members.
CAM has received royalties from W.W. Norton and Company and travel funding from the Tourette Association of America; has received research funding from the National Institutes of Health, the International Obsessive Compulsive Foundation, the Tourette Association of America, and the Patient-Centered Research Outcomes Institute; and is an unpaid member of several advisory boards, including the Tourette Association of America, the International OCD Foundation, and the Family Foundation for OCD Research. RSM has received research funding from the National Institutes of Health and Janssen Research and Development. RLN has received research funding from the National Institutes of Health and Genentech, and travel funding from Mount Sinai Medical Center. MWW has served on advisory boards for Eli Lilly, Cerecin/Accera, Roche, Alzheon, Merck Sharp & Dohme, Nestle/Nestec, Patient-Centered Research Outcomes Institute/Patient-Powered Research Networks, Dolby Family Ventures, National Institute on Aging, Brain Health Registry, and Alzheimer’s Disease Neuroimaging Initiative; serves on the Editorial Boards for Alzheimer’s & Dementia, Topics in Magnetic Resonance Imaging, and Topics in Magnetic Resonance Imaging; has provided consulting and/or acted as a speaker/lecturer to Cerecin/Accera, BioClinica, Nestle/Nestec, Roche, Genentech, the National Institutes of Health, the Buck Institute for Research on Aging, FUJIFILM-Toyama Chemical (Japan), Garfield Weston, Baird Equity Capital, the University of Southern California, Cytox, the Japanese Organization for Medical Device Development, and T3D Therapeutics; holds stock options with Alzheon, Alzeca, and Anven; receives support for his work from the National Institutes of Health, the Department of Defense, the Patient-Centered Outcomes Research Institute, the California Department of Public Health, the University of Michigan, Siemens, Biogen, the Hillblom Foundation, the Alzheimer’s Association, the State of California, Johnson and Johnson, Kevin and Connie Shanahan, GE, VUmc, Australian Catholic University, the Stroke Foundation, and the Veterans Administration. All other authors have reported no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2021.10.009.
Supplementary Material
References
- 1.American Psychiatric Association . 5th ed. American Psychiatric Association; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 2.Ayers C.R., Ly P., Howard I., Mayes T., Porter B., Iqbal Y. Hoarding severity predicts functional disability in late-life hoarding disorder patients. Int J Geriatr Psychiatry. 2014;29:741–746. doi: 10.1002/gps.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archer C.A., Moran K., Garza K., Zakrzewski J.J., Martin A., Chou C.-Y., et al. Relationship between symptom severity, psychiatric comorbidity, social/occupational impairment, and suicidality in hoarding disorder. J Obsessive Compuls Relat Disord. 2019;21:158–164. [Google Scholar]
- 4.Ayers C.R., Schiehser D., Liu L., Loebach Wetherell J. Functional impairment in geriatric hoarding participants. J Obsessive Compuls Relat Disord. 2012;1:263–266. [Google Scholar]
- 5.Nutley S.K., Camacho M.R., Eichenbaum J., Nosheny R.L., Weiner M., Delucchi K.L., et al. Hoarding disorder is associated with self-reported cardiovascular / metabolic dysfunction, chronic pain, and sleep apnea. J Psychiatr Res. 2021;134:15–21. doi: 10.1016/j.jpsychires.2020.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kyung Lee E., Douglass A.B. Sleep in psychiatric disorders: Where are we now? Can J Psychiatry. 2010;55:403–412. doi: 10.1177/070674371005500703. [DOI] [PubMed] [Google Scholar]
- 7.Raines A.M., Portero A.K., Unruh A.S., Short N.A., Schmidt N.B. An initial investigation of the relationship between insomnia and hoarding. J Clin Psychol. 2015;71:707–714. doi: 10.1002/jclp.22161. [DOI] [PubMed] [Google Scholar]
- 8.Dozier M.E., Speed K.J., Davidson E.J., Bolstad C.J., Nadorff M.R., Ayers C.R. The association between sleep and late life hoarding. Int J Aging Hum Dev. 2021;93:931–942. doi: 10.1177/0091415020974618. [DOI] [PubMed] [Google Scholar]
- 9.Mahnke A.R., Linkovski O., Timpano K., van Roessel P., Sanchez C., Varias A.D., et al. Examining subjective sleep quality in adults with hoarding disorder. J Psychiatr Res. 2021;137:597–602. doi: 10.1016/j.jpsychires.2020.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medic G., Wille M., Hemels M.E. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;9:151–161. doi: 10.2147/NSS.S134864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tolin D.F., Das A., Hallion L.S., Levy H.C., Wootton B.M., Stevens M.C. Quality of life in patients with hoarding disorder. J Obsessive Compuls Relat Disord. 2019;21:55–59. doi: 10.1016/j.jocrd.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiner M.W., Nosheny R., Camacho M., Truran-Sacrey D., Mackin R.S., Flenniken D., et al. The Brain Health Registry: An internet-based platform for recruitment, assessment, and longitudinal monitoring of participants for neuroscience studies. Alzheimers Dement. 2018;14:1063–1076. doi: 10.1016/j.jalz.2018.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nutley S.K., Bertolace L., Vieira L.S., Nguyen B., Ordway A., Simpson H., et al. Internet-based hoarding assessment: The reliability and predictive validity of the internet-based Hoarding Rating Scale, Self-Report. Psychiatry Res. 2020;294:113505. doi: 10.1016/j.psychres.2020.113505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buysse D.J., Reynolds C.F., Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 15.Kroenke K., Spitzer R.L., Williams J.B.W. The PHQ-9. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maruff P., Thomas E., Cysique L., Brew B., Collie A., Snyder P., Pietrzak R.H. Validity of the CogState Brief Battery: Relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol. 2009;24:165–178. doi: 10.1093/arclin/acp010. [DOI] [PubMed] [Google Scholar]
- 17.Mackin R.S., Insel P.S., Truran D., Finley S., Flenniken D., Nosheny R., et al. Unsupervised online neuropsychological test performance for individuals with mild cognitive impairment and dementia: Results from the Brain Health Registry. Alzheimers Dement (Amst) 2018;10:573–582. doi: 10.1016/j.dadm.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farias S.T. The Measurement of Everyday Cognition (ECog): Scale development and psychometric properties. Neuropsychology. 2008;22:531–544. doi: 10.1037/0894-4105.22.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nosheny R.L., Camacho M.R., Jin C., Neuhaus J., Truran D., Flenniken D., et al. Validation of online functional measures in cognitively impaired older adults. Alzheimers Dement. 2020;16:1426–1437. doi: 10.1002/alz.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ware J.E., Jr. SF-36 health survey update. Spine (Phila Pa 1976) 2000;25:3130–3139. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 21.Ware J.E., Jr., Sherbourne C. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 22.Ware J.E., Jr., Kosinski M.A., Keller S.D. Health Institute, New England Medical Center; Boston: 1993. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. [Google Scholar]
- 23.Mplus (1998–2017): Mplus HTML User’s Guide. https://www.statmodel.com/html_ug.shtml Available at:
- 24.Muthén B.O., du Toit S.H.C., Spisic D. Robust inference using weighted least squares and quadratic estimating equations in latent variable modeling with categorical and continuous outcomes. 1997. https://www.statmodel.com/download/Article_075.pdf Available at:
- 25.Hooper D., Coughlan J., Mullen M. Structural equation modelling: Guidelines for determining model fit. Electron J Bus Res Methods. 2008;6:53–60. [Google Scholar]
- 26.Seow L.S.E., Verma S.K., Mok Y.M., Kumar S., Chang S., Satghare P., et al. Evaluating DSM-5 insomnia disorder and the treatment of sleep problems in a psychiatric population. J Clin Sleep Med. 2018;14:237–244. doi: 10.5664/jcsm.6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox R.C., Parmar A.M., Olatunji B.O. Sleep in obsessive-compulsive and related disorders: A selective review and synthesis. Curr Opin Psychol. 2020;34:23–26. doi: 10.1016/j.copsyc.2019.08.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.