Abstract
Background
Early-life stress is associated with alterations in telomere length, a marker of accumulated stress and aging, and a risk factor for psychiatric disorders. Nonhuman primate maternal variable foraging demand (VFD) is a validated early-life stress model, resulting in anxiety- and depressive-like symptoms in offspring. Previous studies reported increased plasma glucagon-like peptide 1 (pGLP-1) along with insulin resistance in this model. We investigated whether VFD rearing related to adult telomere length and to these neuroendocrine markers.
Methods
Adult leukocyte telomere length was measured in VFD-reared (12 males, 13 females) and non-VFD–reared (9 males, 26 females) bonnet macaques. Associations between adult telomere length and adolescent fasting pGLP-1 or insulin resistance in VFD-reared versus non-VFD–reared groups were examined using regression modeling, controlling for sex, weight, and age.
Results
VFD subjects had relatively longer telomeres than non-VFD subjects (p = .017), and females relatively longer than males (p = .0004). Telomere length was positively associated with pGLP-1 (p = .0009) and with reduced insulin sensitivity (p < .0001) in both sexes, but not as a function of rearing group.
Conclusions
Unexpectedly, VFD was associated with longer adult telomere length. Insulin resistance may lead to higher pGLP-1 levels in adolescence, which could protect telomere length in VFD offspring as adults. Associations between adult telomere length and adolescent insulin resistance and high pGLP-1 may reflect an adaptive, compensatory response after early-life stress exposure.
Keywords: Adaptive calibration, Adversity, Early-life stress, Insulin, Maltreatment, pGLP-1, Telomere
SEE COMMENTARY ON PAGE 5
Early-life stress (ELS) is related to changes in multiple biological systems conferring risk for psychiatric disorders (1). However, not every individual exposed to ELS develops psychopathology, suggesting that adaptive or compensatory processes may modify or protect against individual risk (2,3). One of these biological markers that may reflect both adaptive and risk processes is telomere length (TL), a cumulative marker of stress exposure and aging (4, 5, 6). Telomeres are DNA-protein structures located on the ends of linear chromosomes that help preserve the genome (4,7). Telomeres shorten with age and are sensitive to multiple cellular stress pathways, including those for inflammation and oxidative stress (4,8,9). Multiple studies have shown a negative relationship between ELS and TL, although some report no or a positive relationship (10, 11, 12, 13). Similarly, most studies have reported a negative relationship between TL and stress-related psychiatric disorders (7,14), although some do not replicate this finding (7,10,14,15). Such mixed results in the literature may reflect that TL is determined by multiple biological markers interacting dynamically, requiring consideration of factors shown to impact TL, including neuroendocrine function (16), inflammation (9), and oxidative stress (8).
One such biological marker is plasma glucagon-like peptide 1 (pGLP-1). pGLP-1 is a pleiotropic, neurotrophic brain-gut peptide contributing to glucose homeostasis by enhancing insulin sensitivity (17) with anti-inflammatory (18,19) and antioxidant-like (20) properties. As such, pGLP-1 may impact the relationship between ELS and TL by mitigating cellular stress pathways. Previously, we reported that pGLP-1 increased along with insulin resistance in our validated nonhuman primate model of ELS termed variable foraging demand (VFD) (21). pGLP-1 elevation persisted in VFD-exposed animals that developed insulin resistance, suggesting that increases in pGLP-1 may be a compensatory, and potentially protective, response to insulin resistance (22). Previous work has shown that insulin resistance is associated with shorter TL, while improving insulin sensitivity is associated with telomerase activation and TL increases (23). However, previous studies have not examined the relationship between TL and pGLP-1, and studies of TL in nonhuman primates using the VFD paradigm have not been reported.
Nonhuman primate models are particularly helpful in elucidating the relationship between ELS and TL because they are a homogeneous group both genetically and environmentally, minimizing baseline TL or other biological differences. Here, we examine potential associations between macaque adult leukocyte TL and VFD exposure and between adolescent pGLP-1 and adult TL. We hypothesized that TL would be negatively related to ELS. Further, we aimed to explore how pGLP-1 may relate to TL.
Methods and Materials
Colony
The subjects consisted of bonnet macaques (Macaca radiata) living in social groups of 6 to 10 females or singly housed adult males in full view of their peers. A total of 60 adult bonnet macaques (39 females, 21 males) were included in our study, of which 13 females and 12 males were reared in the VFD model described below, and 26 females and 9 males were normatively reared. All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (24). The SUNY Downstate Medical Center Institutional Animal Care and Use Committee approved the protocol.
Rearing Methods
As previously described (25), the VFD maternal food procurement schedule was implemented beginning at infant age 12 weeks and consisted of alternating 2-week blocks during which food was easy to find (low foraging demand) or food procurement was difficult, involving more time and effort (high foraging demand) (Figure 1). The amount of food available did not vary. Beginning with low foraging demand, the 16-week VFD experimental period consisted of four 2-week low foraging demand and four alternating 2-week high foraging demand periods. A foraging cart, in which food is either buried in wood chips (high foraging demand) or freely exposed in containers within the cart (low foraging demand), was used (26).
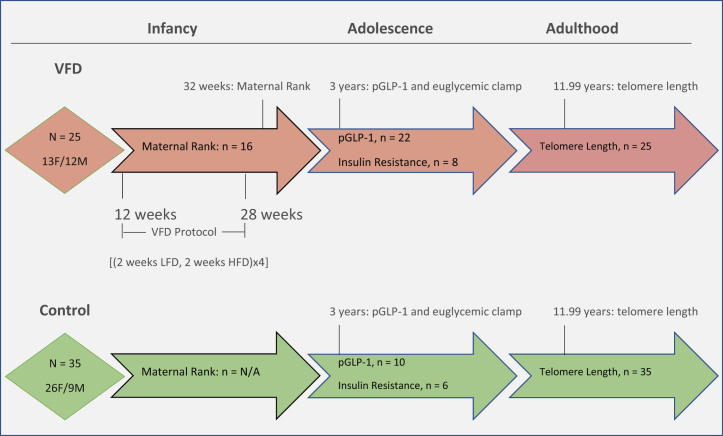
Figure 1.
Flowchart for study. A flowchart is provided depicting the three phases of the study: the infancy stage during which the early-life stress exposure occurs, the maternal VFD paradigm (red arrow) or the control condition, and the non-VFD group (green arrow) where VFD is not imposed. The number of subjects for each measurement and timing are shown in the diagram at each phase. F, female; HFD, high foraging demand; LFD, low foraging demand; M, male; N/A, not applicable; pGLP-1, plasma glucagon-like peptide 1; VFD, variable foraging demand.
Timing of Assessments
Biological and behavioral data reported in this study were assessed at different developmental time points outlined in Table 1 and Figure 1.
Table 1.
Timing of Assessments of a Bonnet Macaque Sample With Mature Adult Leukocyte Log TL Measurements
| Measure | N (Exposure) [Sex] | Offspring Age, Mean (±SE) | Stage | Reference |
|---|---|---|---|---|
| pGLP-1 | 32 (22 VFD, 10 control) [17 M, 15 F] | 3.0 (±0.5) years | Adolescent | (22) |
| Euglycemic Clamp (Insulin Resistance) | 14 (8 VFD, 6 control) [10 M, 4 F] | 3.0 (±0.5) years | Adolescent | (22) |
| TL | 60 (25 VFD, 35 control) [21 M, 39 F] | 11.99 (±0.5) years | Adulthood |
Timing of measures and sample characteristics for this longitudinal study are shown. pGLP-1 and the euglycemic clamp procedure for insulin sensitivity were assessed in adolescence, while TL was assessed in adulthood.
F, female; M, male; pGLP-1, plasma glucagon-like peptide 1; TL, telomere length; VFD, variable foraging demand.
Venipuncture and Blood Chemistry
Each macaque had two blood draws, the first as adolescents and the second as mature adults. Monkeys spontaneously entered individual carrying cages between 8:00 pm and 11:00 pm and were transferred to squeeze cages, where they were anesthetized with an intramuscular injection of ketamine (15 mg/kg). Antecubital or femoral venipuncture was used to collect blood after an overnight fast for p-GLP1 measures. Samples for pGLP-1 were immediately placed on ice and were centrifuged and stored at −80 °C within 1 hour (27). Blood for pGLP-1 was collected in nonheparinized tubes, whereas for TL, blood was collected in heparinized tubes. Blood was drawn under sterile conditions, and the field for the blood draw was swabbed carefully with alcohol to ensure that blood samples were not tainted by contaminants that could alter TL determination (28). In adolescent animals, plasma insulin and pGLP-1 were analyzed using the Human Endocrine Lincoplex kit (Linco Diagnostic Services). Venous blood was analyzed for plasma glucose by routine methods at the University Hospital of Brooklyn Clinical Chemistry Laboratory (Brooklyn, NY).
Euglycemic Insulin Clamp
Euglycemic insulin clamps were performed to assess glucose disposal, a measure of insulin sensitivity (or, inversely, insulin resistance) expressed as mg/kg/min directly after venipuncture for pGLP-1. An intravenous 20% glucose solution was infused at a variable rate to maintain euglycemia (85 mg/dL) determined by blood samples (0.05–0.1 mL) taken every 5 minutes. Samples were centrifuged and plasma analyzed by a Beckman glucometer 2 (Beckman Instruments) (22).
Quantitative Real-Time Polymerase Chain Reaction Measurements
DNA was isolated using the QIAamp DNA isolation kit (Qiagen); reagents were added to the sample, then they were centrifuged as specified in the kit. Per the manufacturer, the kit isolates nuclear DNA with minimal to no degradation. DNA quantity and purity of all samples was determined using the Nanodrop (Thermo Scientific) absorbance measures. DNA was eluted with water and stored at −20 °C; all samples were thawed 1 or 2 times for the mentioned analyses. TL was determined using modified procedures of Cawthon et al. (29) to measure absolute TL (30). Three parallel quantitative polymerase chain reactions were performed in duplicate across two different plates to quantitate TL and the beta-hemoglobin gene as the single-copy standard, as previously described (7). Data were acquired using the ABI Prism HT79000 DNA Sequence Detection System (Applied Biosystems). Quantitative polymerase chain reactions were performed on 384-well plates in a reaction volume of 10 μL containing approximately 25 ng genomic DNA, 300 nmol/L of each primer, and 1 × Sybr Select Master Mix (Life Technologies). For TL, the reaction included 2% dimethyl sulfoxide. All plates contained wells with a 12-point serial dilution standard curve (log 0–5.25 ng) of a cloned amplicon (telomere or beta-hemoglobin amplicon) to permit absolute quantitation for telomeres or the single-copy gene in each polymerase chain reaction run. Polymerase chain reaction efficiency criteria were 99% to 104% for both measures. Coefficients of variation were calculated within each triplicate; samples with coefficients of variation >5% within or between plates were repeated (2 samples; all met quality control criteria after rerunning). The resulting average intraclass coefficient was >0.90. Modified telomere primer sequences were provided by Richard Cawthon, M.D., Ph.D. (Eccles Institute of Human Genetics, University of Utah, Salt Lake City, UT): CGG TTT GTT TGG GTT TGG GTT TGG GTT TGG GTT TGG GTT and GGC TTG CCT TAC CCT TAC CCT TAC CCT TAC CCT TAC CCT. An initial heating step of 95 °C for 30 minutes was followed by 40 cycles of 95 °C for 15 seconds and 60 °C for 1 minute. The ratio of telomere copy number to beta-hemoglobin copy number was obtained, multiplied by the length of the telomere amplicon to obtain TL per genome, and then divided by the number of chromosome ends. The sequences of the beta-hemoglobin gene forward and reverse primers were GCT TCT GAC ACA ACT GTG TTC ACT AGC and CAC CAA CTT CAT CCA CGT. An initial heating step of 95 °C for 10 minutes was followed by 40 cycles of 95 °C for 15 seconds and 60 °C for 1 minute.
Statistical Analyses
All statistical tests were two-sided with a p value of .05 considered significant. Data were tested for normality and inspected for outliers. TL was log transformed because it is derived from the exponent of Ct (30). Hypotheses were tested using general linear models with post hoc testing. In analyses, age and body mass were entered as continuous covariates given previous evidence of the importance of these in relation to TL (4,31).
Results
Rearing Group Characteristics
Table 2 provides nonhuman primate characteristics by rearing group and sex. Sex distribution did not differ between the VFD and non-VFD groups (χ21 = 3.18; p = .074), but because it approached significance, sex was included as a covariate. As expected, males showed greater body mass in comparison with females (F1,58 = 82.72, p < .0001); there were no rearing (F1,56 = 2.15, p = .14) or rearing-by-sex (F1,56 = 0.79, p = .37) effects for body mass. Females were older than males (F1,56 = 17.86, p < .0001), but the rearing groups were not distinguishable by age (F1,50 = 0.61, p = .44). When controlling for sex, rearing group, and body mass, age inversely predicted log TL (F1,56 = 4.33, p = .042).
Table 2.
Age, Body Weight, and TL by Rearing Group and Sex
| Group | n | Age, Years, Mean ± SE | Weight, kg, Mean ± SE | Log TL, Mean ± SE (bp) |
|---|---|---|---|---|
| Non-VFD Males | 9 | 9.56 ± 0.91 | 10.37 ± 0.62 | 8.35 ± 0.14 |
| Non-VFD Females | 26 | 14.18 ± 0.65 | 5.06 ± 0.35 | 9.22 ± 0.08 |
| VFD Males | 12 | 8.89 ± 0.79 | 9.17 ± 0.54 | 8.88 ± 0.12 |
| VFD Females | 13 | 12.15 ± 0.93 | 5.36 ± 0.50 | 9.46 ± 0.11 |
bp, base pairs; SE, standard error; TL, telomere length; VFD, variable foraging demand.
TL by Rearing Groups
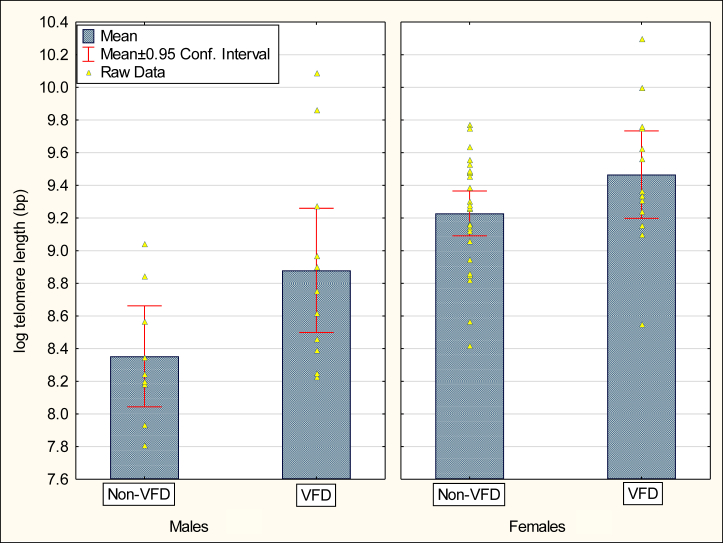
There were both group (F1,55 = 6.01, p = .017) and sex (F1,55 = 14.09, p = .0004) effects for TL. VFD subjects exhibited longer TL than non-VFD subjects, controlling for sex, age, and body mass (Figure 2). Females displayed longer TL than males. There were no rearing-by-sex effects. There were no age or weight effects in the overall model.
Figure 2.
Adult leukocyte telomere length by VFD rearing status and sex. An overall rearing group effect was observed for adult leukocyte log telomere length without evidence for a rearing group-by-sex interaction. Covariates included age and body mass. bp, base pairs; Conf., confidence; VFD, variable foraging demand.
Relationship of Adolescent pGLP-1 to Adult Leukocyte TL
Values of pGLP-1 were not normally distributed (Lilliefors p < .01); therefore, a nonlinear model was used. A positive relationship between adolescent pGLP-1 and adult TL was observed, controlling for sex, age, and body mass at the time of TL assessment (Wald Statistic [WS]1 = 11.03, p = .0009, 95% CI = −1.06 to −0.27). Effects remained significant controlling for rearing group, and there was no rearing group-by-TL interaction. There was a significant sex-by-TL interaction effect (WS1 = 33.69, p < .0001; 95% CI = 0.68–1.38), such that a positive relationship between TL and pGLP-1 was noted in males (r = 0.66; p = .003) but not in females (r = −0.27; p = .34). There were sex differences in adolescent pGLP-1 (μg/dL) means (SE) (males = 69.72 [10.87] vs. females = 98.24 [21.88]) (WS1 = 31.91; p < .0001; 95% CI = −13.70 to −6.64) adjusting for other variables in the model. There were no sex-by-group-by-TL effects.
Relationship of Adolescent Insulin Resistance to Adult Leukocyte TL
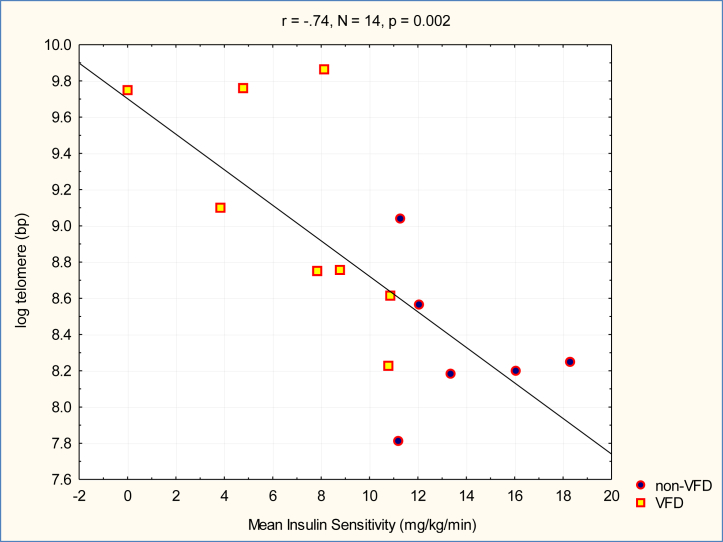
There was an inverse relationship between insulin sensitivity, as determined by the euglycemic insulin clamp procedure, and TL (WS1 = 16.64, p < .0001, n = 14; 95% CI = −0.016 to −0.005) (Figure 3), an effect that remained significant when controlling for rearing group, age, and body mass. Males exhibited greater insulin resistance than females (F1,11 = 6.26, p = .029). The association of insulin resistance and log TL remained significant after controlling for sex (WS1 = 5.40, p = .02, 95% CI = −0.58 to −0.04).
Figure 3.
Relationship of adolescent insulin sensitivity to adult leukocyte telomere length. There was an inverse relationship between adult leukocyte telomere length and mean adolescent insulin sensitivity. This effect remained significant when controlling for rearing group effects. Overall glucose disposal in adolescence was lower (= insulin resistance) in adults with relative telomere length increases. bp, base pairs; VFD, variable foraging demand.
Discussion
Here, we show that ELS-exposed macaque offspring had significantly longer adult telomeres than controls, independent of sex. Notably, after controlling for rearing group, sex, and body mass, older age in this current nonhuman primate sample was associated with shorter telomeres, consistent with the literature describing shorter telomeres as a cellular marker of aging (32,33). Adolescent pGLP-1 was associated with longer adult TL, and there was an inverse relationship between adolescent insulin sensitivity and adult TL.
We suggest the possibility that the finding of ELS-associated longer TL may suggest a compensatory response after VFD exposure. VFD subjects may be adaptively prepared with TL increases to be equipped for a future of adversity and to prevent excessive stress-induced TL shortening (34,35). Precedent for such compensatory responses following ELS in nonhuman primates has been reported (36), including the increases in pGLP-1 discussed above (22), and prefrontal plasticity following stress inoculation (37). That VFD rearing was associated with relatively longer TL is in contrast with an existing literature on ELS showing relatively shorter TL compared with control subjects (11, 12, 13,38). These findings are consistent with a recent finding of relatively longer TL in ELS-exposed preschool-aged children, however (10).
Our finding that adolescent pGLP-1 positively predicted adult TL suggests one possible mechanism for the VFD-related TL findings. pGLP-1 contributes to glucose homeostasis by enhancing insulin sensitivity (17) and has anti-inflammatory (18,19) and antioxidant-like (20) properties. Liraglutide, a GLP-1 agonist, counters production of reactive oxygen species by TNF-α, inhibits NF-κB activation, and inhibits apoptosis in a human endothelial cell model (39). Oxidative stress and inflammation are associated with relatively shorter TL (8,9). This literature, combined with our finding that higher pGLP-1 during adolescence is associated with longer adult TL, supports the view that pGLP-1 may impact TL by impacting cellular stress pathways. The finite nature of ELS exposure intrinsic to VFD rearing, a paradigm that involves an early period of circumscribed stress followed thereafter by normative rearing conditions, may have enabled recuperation and ultimately compensation.
TL was longer in females than males overall. These sex differences may reflect sex-related effects on antioxidant activity; however, further study will be required to examine this (40,41). In this study, pGLP-1 levels were also higher in females than males, as was observed in our original report in this sample (22), suggesting that elevated pGLP-1 levels could be a protective mechanism against oxidative stress, inflammation, and TL decline in females. Nevertheless, there was no rearing group-by-sex interaction for TL. Our low number of non-VFD male subjects compared with females limits statistical power for these analyses.
Additional limitations of this study include our measurement of leukocyte TL at one time point without data on telomerase, a ribonucleoprotein that adds a species-dependent telomere repeat sequence to the 3′ end of telomeres (42). High telomerase activity in conjunction with shorter TL has been used as an indicator of a stressed biological system (43). Our study did not examine markers of oxidative stress or inflammation, which would further elucidate the mechanisms underlying the relationship between TL and pGLP-1. Euglycemic clamp and pGLP-1 data were available for a subset of the population only. Although one might consider our VFD laboratory model limited compared with real-world stressors, this experimental induction of an early-stress period carefully controls for other environmental exposures. VFD allows examination of early experience without many of the concomitant confounding adversities experienced by humans. While the sample size was only n = 60 for the main aim of this study, this is a large sample size compared with other developmental nonhuman primate studies (44), including one examining telomeres (33). Nonhuman primates are inbred laboratory animals and, as such, these subjects were homogeneous genetically and raised in identical environments (food, resources, exposures, and so on) other than the VFD exposure, which minimized baseline TL or other biological differences. As such, confounders inherent to human studies of TL (including baseline, genetic differences in length, culture, socioeconomic status, parental mental illness, diet, neighborhood violence/disruption) are controlled for in this model, likely reflected in the 100- to 1000-fold lower variance in these data compared with human studies (45). Further minimizing the effect of baseline telomere differences, rearing assignment was random, suggesting that any baseline telomere differences were similar between groups.
In summary, we found that ELS was associated with longer adult TL, and longer adult TL was positively associated with adolescent pGLP-1 and insulin resistance. These findings, taken together, suggest that TL may represent one indicator reflective of mechanisms such as pGLP-1 that may confer a form of biological adaptation or resilience toward allostatic overload. Such results may align with models such as adaptive calibration (34,35), which bear relevance to the current TL findings in which adaptations for survival require calibration of the organism to adequately cope with a potentially adverse future. These data highlight the importance of future work examining TL in the VFD paradigm, investigating the relationship between stress and sex hormones, pGLP-1, insulin resistance, and TL. Longitudinal assessment and prospective analysis of the developmental progression of these changes will be important to understand the biological mechanisms of risk and resilience following VFD exposure. These results highlight the importance of accounting for the timing and severity of ELS, because different ELS exposure types, severities, and developmental timings may have adaptive versus deleterious outcomes.
Acknowledgments and Disclosures
This work was supported by the National Institutes of Health (Grant No. R01-MH083990-03 [to JC]). The study sponsor had no role in the design or preparation of this manuscript.
JC has served on advisory boards for Otsuka/Lundbeck and is on the speakers’ bureau for Sunovion, Otsuka, Lundbeck, Allergan, and Neurocrine. He received grant support from National Institute of Mental Health (Grant No. R01MH59990A), NYSTEM, GlaxoSmithKline, Pfizer, and Alexza Pharmaceuticals. He is on the Pfizer advisory board and gives talks for BMS, Astra Zeneca, GSK, and Pfizer. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2021.07.006.
Supplementary Material
References
- 1.Felitti V.J., Anda R.F., Nordenberg D., Williamson D.F., Spitz A.M., Edwards V., et al. Reprint of: Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 2019;56:774–786. doi: 10.1016/j.amepre.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Han M.H., Nestler E.J. Neural substrates of depression and resilience. Neurotherapeutics. 2017;14:677–686. doi: 10.1007/s13311-017-0527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price R.B., Duman R. Neuroplasticity in cognitive and psychological mechanisms of depression: An integrative model. Mol Psychiatry. 2020;25:530–543. doi: 10.1038/s41380-019-0615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackburn E.H., Epel E.S., Lin J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350:1193–1198. doi: 10.1126/science.aab3389. [DOI] [PubMed] [Google Scholar]
- 5.Epel E.S., Blackburn E.H., Lin J., Dhabhar F.S., Adler N.E., Morrow J.D., Cawthon R.M. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridout S.J., Ridout K.K., Kao H.T., Carpenter L.L., Philip N.S., Tyrka A.R., Price L.H. Telomeres, early-life stress and mental illness. Adv Psychosom Med. 2015;34:92–108. doi: 10.1159/000369088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ridout K.K., Ridout S.J., Price L.H., Sen S., Tyrka A.R. Depression and telomere length: A meta-analysis. J Affect Disord. 2016;191:237–247. doi: 10.1016/j.jad.2015.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnes R.P., Fouquerel E., Opresko P.L. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech Ageing Dev. 2019;177:37–45. doi: 10.1016/j.mad.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong J.Y.Y., De Vivo I., Lin X., Fang S.C., Christiani D.C. The relationship between inflammatory biomarkers and telomere length in an occupational prospective cohort study. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ridout K.K., Parade S.H., Kao H.T., Magnan S., Seifer R., Porton B., et al. Childhood maltreatment, behavioral adjustment, and molecular markers of cellular aging in preschool-aged children: A cohort study. Psychoneuroendocrinology. 2019;107:261–269. doi: 10.1016/j.psyneuen.2019.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanssen L.M., Schutte N.S., Malouff J.M., Epel E.S. The relationship between childhood psychosocial stressor level and telomere length: A meta-analysis. Health Psychol Res. 2017;5:6378. doi: 10.4081/hpr.2017.6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z., He Y., Wang D., Tang J., Chen X. Association between childhood trauma and accelerated telomere erosion in adulthood: A meta-analytic study. J Psychiatr Res. 2017;93:64–71. doi: 10.1016/j.jpsychires.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Ridout K.K., Levandowski M., Ridout S.J., Gantz L., Goonan K., Palermo D., et al. Early life adversity and telomere length: A meta-analysis. Mol Psychiatry. 2018;23:858–871. doi: 10.1038/mp.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darrow S.M., Verhoeven J.E., Révész D., Lindqvist D., Penninx B.W.J.H., Delucchi K.L., et al. The association between psychiatric disorders and telomere length: A meta-analysis involving 14,827 persons. Psychosom Med. 2016;78:776–787. doi: 10.1097/PSY.0000000000000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boks M.P., van Mierlo H.C., Rutten B.P.F., Radstake T.R.D.J., De Witte L., Geuze E., et al. Longitudinal changes of telomere length and epigenetic age related to traumatic stress and post-traumatic stress disorder. Psychoneuroendocrinology. 2015;51:506–512. doi: 10.1016/j.psyneuen.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Gotlib I.H., LeMoult J., Colich N.L., Foland-Ross L.C., Hallmayer J., Joormann J., et al. Telomere length and cortisol reactivity in children of depressed mothers. Mol Psychiatry. 2015;20:615–620. doi: 10.1038/mp.2014.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holst J.J. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 18.Kim S., Jeong J., Jung H.S., Kim B., Kim Y.E., Lim D.S., et al. Anti-inflammatory effect of glucagon like peptide-1 receptor agonist, exendin-4, through modulation of IB1/JIP1 expression and JNK signaling in stroke. Exp Neurobiol. 2017;26:227–239. doi: 10.5607/en.2017.26.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krasner N.M., Ido Y., Ruderman N.B., Cacicedo J.M. Glucagon-like peptide-1 (GLP-1) analog liraglutide inhibits endothelial cell inflammation through a calcium and AMPK dependent mechanism. PLoS One. 2014;9 doi: 10.1371/journal.pone.0097554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen K.E., Rakipovski G., Raun K., Lykkesfeldt J. Does glucagon-like peptide-1 ameliorate oxidative stress in diabetes? evidence based on experimental and clinical studies. Curr Diabetes Rev. 2016;12:331–358. doi: 10.2174/1573399812666150918150608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coplan J.D., Syed S., Perera T.D., Fulton S.L., Banerji M.A., Dwork A.J., Kral J.G. Glucagon-like peptide-1 as predictor of body mass index and dentate gyrus neurogenesis: Neuroplasticity and the metabolic milieu. Neural Plast. 2014;2014:917981. doi: 10.1155/2014/917981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufman D., Banerji M.A., Shorman I., Smith E.L.P., Coplan J.D., Rosenblum L.A., Kral J.G. Early-life stress and the development of obesity and insulin resistance in juvenile bonnet macaques. Diabetes. 2007;56:1382–1386. doi: 10.2337/db06-1409. [DOI] [PubMed] [Google Scholar]
- 23.Kirchner H., Shaheen F., Kalscheuer H., Schmid S.M., Oster H., Lehnert H. The telomeric complex and metabolic disease. Genes. 2017;8:176. doi: 10.3390/genes8070176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Research Council . National Academies Press; Washington, DC: 2010. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- 25.Rosenblum L.A., Paully G.S. The effects of varying environmental demands on maternal and infant behavior. Child Dev. 1984;55:305–314. [PubMed] [Google Scholar]
- 26.Andrews M.W., Rosenblum L.A. Attachment in monkey infants raised in variable- and low-demand environments. Child Dev. 1991;62:686–693. [PubMed] [Google Scholar]
- 27.Coplan J.D., Gopinath S., Abdallah C.G., Berry B.R. A neurobiological hypothesis of treatment-resistant depression - Mechanisms for selective serotonin reuptake inhibitor non-efficacy. Front Behav Neurosci. 2014;8:189. doi: 10.3389/fnbeh.2014.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denham J., Marques F.Z., Charchar F.J. Leukocyte telomere length variation due to DNA extraction method. BMC Res Notes. 2014;7:877. doi: 10.1186/1756-0500-7-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cawthon R.M., Smith K.R., O’Brien E., Sivatchenko A., Kerber R.A. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 30.O’Callaghan N.J., Fenech M. A quantitative PCR method for measuring absolute telomere length. Biol Proced Online. 2011;13:3. doi: 10.1186/1480-9222-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seluanov A., Chen Z., Hine C., Sasahara T.H.C., Ribeiro A.A.C.M., Catania K.C., et al. Telomerase activity coevolves with body mass not lifespan. Aging Cell. 2007;6:45–52. doi: 10.1111/j.1474-9726.2006.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang E., Harley C.B. Telomere length and replicative aging in human vascular tissues. Proc Natl Acad Sci U S A. 1995;92:11190–11194. doi: 10.1073/pnas.92.24.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drury S.S., Howell B.R., Jones C., Esteves K., Morin E., Schlesinger R., et al. Shaping long-term primate development: Telomere length trajectory as an indicator of early maternal maltreatment and predictor of future physiologic regulation. Dev Psychopathol. 2017;29:1539–1551. doi: 10.1017/S0954579417001225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hostinar C.E., Gunnar M.R. The developmental effects of early life stress: An overview of current theoretical frameworks. Curr Dir Psychol Sci. 2013;22:400–406. doi: 10.1177/0963721413488889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Del Giudice M., Ellis B.J., Shirtcliff E.A. The adaptive calibration model of stress responsivity. Neurosci Biobehav Rev. 2011;35:1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyons D.M., Parker K.J. Stress inoculation-induced indications of resilience in monkeys. J Trauma Stress. 2007;20:423–433. doi: 10.1002/jts.20265. [DOI] [PubMed] [Google Scholar]
- 37.Lyons D.M., Parker K.J., Katz M., Schatzberg A.F. Developmental cascades linking stress inoculation, arousal regulation, and resilience. Front Behav Neurosci. 2009;3:32. doi: 10.3389/neuro.08.032.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ridout K.K., Khan M., Ridout S.J. Adverse childhood experiences run deep: Toxic early life stress, telomeres, and mitochondrial DNA copy number, the biological markers of cumulative stress. Bioessays. 2018;40 doi: 10.1002/bies.201800077. [DOI] [PubMed] [Google Scholar]
- 39.Shiraki A., Oyama J-i, Komoda H., Asaka M., Komatsu A., Sakuma M., et al. The glucagon-like peptide 1 analog liraglutide reduces TNF-α-induced oxidative stress and inflammation in endothelial cells. Atherosclerosis. 2012;221:375–382. doi: 10.1016/j.atherosclerosis.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 40.Kander M.C., Cui Y., Liu Z. Gender difference in oxidative stress: A new look at the mechanisms for cardiovascular diseases. J Cell Mol Med. 2017;21:1024–1032. doi: 10.1111/jcmm.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller A.A., Drummond G.R., Mast A.E., Schmidt H.H., Sobey C.G. Effect of gender on NADPH-oxidase activity, expression, and function in the cerebral circulation: Role of estrogen. Stroke. 2007;38:2142–2149. doi: 10.1161/STROKEAHA.106.477406. [DOI] [PubMed] [Google Scholar]
- 42.Greider C.W., Blackburn E.H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989;337:331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- 43.Epel E.S., Lin J., Wilhelm F.H., Wolkowitz O.M., Cawthon R., Adler N.E., et al. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31:277–287. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 44.Huber H.F., Jenkins S.L., Li C., Nathanielsz P.W. Strength of nonhuman primate studies of developmental programming: Review of sample sizes, challenges, and steps for future work. J Dev Orig Health Dis. 2020;11:297–306. doi: 10.1017/S2040174419000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tyrka A.R., Parade S.H., Price L.H., Kao H.T., Porton B., Philip N.S., et al. Alterations of mitochondrial DNA copy number and telomere length with early adversity and psychopathology. Biol Psychiatry. 2016;79:78–86. doi: 10.1016/j.biopsych.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.