Abstract
Background
Methylphenidate is among the most prescribed medications for treating attention-deficit/hyperactivity disorder (ADHD). However, nearly half of pediatric patients with ADHD do not respond to methylphenidate treatment. Pharmacogenetic testing can aid in identifying patients for whom methylphenidate is unlikely to be safe or effective, leading to improved methylphenidate outcomes and increased use of alternative treatment options for ADHD. This article aimed to summarize findings from studies of the ADRA2A gene variant, rs1800544, and its association with methylphenidate outcomes in ADHD.
Methods
We systematically reviewed and meta-analyzed available literature on the impact of rs1800544 on methylphenidate outcomes in ADHD.
Results
Fourteen studies met inclusion criteria for review, 9 of which were eligible for meta-analysis. The included studies compared methylphenidate outcomes in patients with ADHD categorized by rs1800544 genotype. G-allele carriers experienced significantly greater improvements in ADHD symptom scores (Swanson, Nolan, and Pelham Version-IV Scale or ADHD Rating Scale-IV) relative to noncarriers (odds ratio 3.08, 95% confidence interval 1.71–5.56, p = .0002) and greater response rates as measured by a ≥50% improvement in symptom scores (odds ratio 2.68, 95% confidence interval 1.23–5.82, p = .01); no significant difference in response rate as measured by Clinical Global Impressions score ≤2 was found. Stouffer’s z-score method showed significant improvement across all methylphenidate outcomes in G-allele carriers relative to noncarriers (z = 3.03, p = .002).
Conclusions
These findings suggest that carriers of rs1800544 may have improved ADHD outcomes following methylphenidate treatment. However, the extent to which these improvements are clinically impactful remain unclear. Additional studies are required to determine if rs1800544 carrier status should influence clinical recommendations for treatment of ADHD symptoms.
Keywords: ADHD, ADRA2A, Meta-analysis, Methylphenidate, Pharmacogenetics, Stimulants
Methylphenidate, a central nervous system stimulant, is among the most commonly prescribed medications in clinical practice for treatment of attention-deficit/hyperactivity disorder (ADHD) and has proven to be highly efficacious relative to placebo in both children and adults (1). Despite this, nearly half of pediatric patients with ADHD do not respond to methylphenidate treatment, and tolerability of stimulant medications remains an issue (2). The psychostimulant effects of methylphenidate are thought to be achieved through inhibition of the reuptake of norepinephrine as well as dopamine within the central nervous system. This reuptake inhibition is the result of the inhibitory binding affinity of methylphenidate at the dopamine and norepinephrine transporters.
Millions of children in the United States have an ADHD diagnosis, and more than 20% of pediatric patients are not receiving medication treatment or behavioral treatment (3). Pharmacogenetic predictors of methylphenidate outcomes in ADHD may help identify patients for whom methylphenidate is unlikely to be safe or effective, leading to an improvement in both methylphenidate outcomes and the use of alternative treatment options for ADHD symptoms. Multiple pharmacogenetic predictors of methylphenidate outcomes in ADHD have been evaluated in the literature, such as rs5569 and rs28386840 of the norepinephrine transporter gene, the variable number of tandem repeats in the 3′-untranslated region of the DAT1 gene as well as that of the DRD4 gene, rs71647871 of the CES1 gene, rs4680 of the COMT gene, rs1800544 of the ADRA2A gene, and the serotonin transporter–linked polymorphic region of the SLC6A4 gene. Of the pharmacogenetic predictors examined in the literature, rs1800544 of the ADRA2A gene is one of the more commonly studied variants in association with methylphenidate outcomes, including 14 studies presented in this review. rs1800544 is located within the upstream promoter region of ADRA2A and has uncertain functional significance. The C allele of rs1800544 is most common in European populations, with an allele frequency of approximately 73% to 75%, and least common in African populations, with an allele frequency as low as 27% (4). Given the role of norepinephrine in the mechanism of action of methylphenidate, it is plausible that genetic variation in ADRA2A, a receptor for norepinephrine, could impact methylphenidate response.
Currently, there is no pharmacogenetic guidance for ADRA2A and methylphenidate from the Clinical Pharmacogenetics Implementation Consortium, an organization that creates regularly updated pharmacogenetic clinical practice guidelines, or the U.S. Food and Drug Administration (5,6). Therefore, the purpose of the present review was to evaluate the association between rs1800544 of the ADRA2A gene and methylphenidate outcomes in the treatment of ADHD.
Methods and Materials
Literature Search
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (7) guidelines were followed to identify studies that evaluated methylphenidate outcomes in individuals with the rs1800544 variant of the ADRA2A gene. Two independent reviewers (DTH and RAL) searched MEDLINE/PubMed using the following Boolean search string: “Methylphenidate AND (ADRA2A OR rs1800544).” Studies that were published up to September 2020 were included, and PubMed search filters were applied to exclude studies that were not conducted in human subjects and/or were not written in English. Non–peer-reviewed works were excluded from review. Additionally, the references of the articles found were reviewed to ensure that all relevant articles were included.
Exclusion Criteria and Study Characteristics
After the initial search was completed, each article abstract was screened to assess whether it should be included in this review. If the article could not be assessed for exclusion criteria from the abstract, the article was reviewed in its entirety. Included studies compared methylphenidate outcomes (symptom improvement, response, or adverse events) in patients with ADHD receiving methylphenidate across rs1800544 genotypes using dominant, recessive, or additive models. No restrictions were implemented based on the duration of methylphenidate treatment, the average age of study participants, or methylphenidate dosage. Case studies, literature reviews, and letters to the editor were excluded from this review.
Studies were excluded from quantitative analysis of genotype models if an additive gene model was used, if only adverse events (and not clinical outcomes) were reported, or if the scale used to evaluate clinical outcomes was not validated. For all articles included in the quantitative analyses, the quality of the study was evaluated using the National Institutes of Health Quality Assessment Tool for Before-After (Pre-Post) Studies With No Control Group (8).
Data Items and Collection
Data items collected from all studies included the following: the country in which the study was conducted, duration and dose of methylphenidate treatment, presence of comorbidities or concomitant medication, average age of subjects, scales or adverse event measures used to evaluate methylphenidate outcomes, gene model, and direction and statistical significance of effects on methylphenidate outcomes. All data items were collected as displayed in each article or its supplementary information.
For studies that evaluated ADHD symptom improvement on a continuous scale, the effect size (F statistic) of rs1800544 on methylphenidate outcome, the mean percent change in symptom score or adverse event scale for each genotype group, and the standard deviation of the percent change in symptom score or adverse event scale for each genotype group were collected. For studies that evaluated methylphenidate response rate using a dominant gene model, the number of responders and nonresponders who carried at least one copy or no copies of the G allele of rs1800544 were collected. For studies that evaluated methylphenidate response rate using a recessive gene model, the number of responders and nonresponders who carried two copies or either one or no copies of the G allele of rs1800544 were collected.
Summary Measures and Statistical Analysis
Principal summary measures include log odds ratio (OR), standard error of the log OR, z score, and p value. For binary outcomes, log OR was calculated as , standard error of the log OR was calculated as , z score was calculated as , and p values were calculated using Microsoft Office 365 ProPlus Excel Version 1902 (Microsoft Corp.) using . For continuous outcomes, F statistics, sample sizes, and mean percent changes with standard deviations were converted to Cohen’s d and its variance, from which ln(OR) and SE{ln(OR)} could be determined according to methods outlined by Lipsey and Wilson (9).
In statistical analyses for dominant gene models, variables were based on carrier status of rs1800544 as follows: A) the number of responders who carried at least one copy of the G allele; B) the number of nonresponders who carried at least one copy of the G allele; C) the number of responders who carried no copies of the G allele; D) the number of nonresponders who carried no copies of the G allele. For recessive gene models, variables were based on carrier status of rs180544 as follows: A) the number of responders who carried two copies of the G allele; B) the number of nonresponders who carried two copies of the G allele; C) the number of responders who carried one or no copies of the G allele; D) the number of nonresponders who carried one or no copies of the G allele.
Meta-analyses of log ORs were performed using RevMan 5.3 (10) generic inverse variance analyses with random effects; these meta-analyses were performed for three categories of methylphenidate outcomes, including symptom improvement as measured by changes in ADHD symptom scores on a continuous scale (Swanson, Nolan, and Pelham Version-IV Scale [SNAP-IV] or ADHD Rating Scale-IV [ADHD-RS-IV]), response as measured by a ≥50% improvement in symptom scores (SNAP-IV or ADHD-RS-IV), and Clinical Global Impressions–Improvement (CGI-I) and CGI–Severity (CGI-S) scales response as measured by a CGI score ≤2 (with or without additional qualifiers). Generic inverse variance meta-analyses were performed only for dominant or recessive model outcomes. Results from Unal et al. (11) were excluded from generic inverse variance meta-analyses owing to the definitions of treatment response used. For studies that did not report findings for an outcome category, but sufficient data were available to do so, independent analyses were performed using the available study data for either dominant or recessive model outcomes.
Meta-analyses of z scores were performed using Stouffer’s z-score method, according to methods outlined by Darlington and Hayes (12), prioritizing continuous outcomes over binary outcomes and prioritizing response over CGI response for studies with multiple outcomes. The z-score meta-analyses were performed only for dominant or recessive model outcomes.
Results
Literature Search
The PubMed database search identified 22 studies; there were no duplicates, and none of the studies were excluded based on title or abstract. One additional study (13) was identified through reviewing the reference sections of articles from the database search. After removing articles that did not meet inclusion criteria during abstract screening and full article review, 14 articles were included in the descriptive analysis (11,13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25). Of these, 9 studies were eligible for quantitative analysis (11,14, 15, 16, 17,20,22,23,25). Reasons for exclusion at each stage of review are summarized in Figure S1.
Descriptive Analysis
Table 1 summarizes the findings of individual studies as reported without any additional analyses (11,13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25). Of the 14 studies included in the descriptive analysis, 7 found no association between ADRA2A genotype and measured outcomes after methylphenidate, 4 found mixed results, and 3 found significant associations between ADRA2A genotype and measured outcomes after methylphenidate.
Table 1.
Summary Findings of Individual Studies
| Reference | Country | Duration | End Daily Dose (Central Tendency) | Comorbidities | Concomitant Medications | N | Average Age, Years | Scales Assessed | Measure | Model | rs1800544 Effectb | p Value | Risk Biasd |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polanczyk et al., 2007 (14) | Brazil | 1 and 3 months | 0.65 mg/kg | Yes | Yes | 106 | 10 | SNAP-IV | Δ SNAP-IV | Dominant | G beneficial | <.05 | Good |
| da Silva et al., 2008 (15) | Brazil | 4 weeks | 0.63 mg/kg | Yes | Yes | 59 | 12 | SNAP-IV | Δ SNAP-IV | Dominant | G beneficial | <.05 | Good |
| ≥50% SNAP-IV reduction | Dominant | – | NS | ||||||||||
| Cheon et al., 2009 (16) | Korea | 8 weeks | 41.07 mg (GG) 37.26 mg (C/G + CC) |
Yes | None specified | 114 | 9 | ADHD-RS-IV, CGI-I | ≥50% ADHD-RS-IV reduction | Recessive | GG beneficial | <.05 | Good |
| Δ ADHD-RS-IV | Recessive | GG beneficial | <.05 | ||||||||||
| CGI-I ≤2 | Recessive | GG beneficial | <.05 | ||||||||||
| Contini et al., 2011 (17) | Brazil | 4 weeks | 0.52 mg/kg | Yes | Yes | 165 | 35 | SNAP-IV, CGI-S | ≥30% SNAP-IV reduction and CGI-S ≤2 | Dominant | – | NS | Good |
| Froehlich et al., 2011 (18) | United Statesa | 4 weeks | 36 mg (≤25 kg) 54 mg (>25 kg) |
Yes | None specified | 89 | 8 | VADPRS, VADTRS | Δ VADPRS/VADTRS | Additive | – | NS | N/A |
| Cho et al., 2012 (19) | Korea | 12 weeks | 0.98 mg/kg | Yes | None specified | 101 | 9 | Diastolic blood pressure | Δ Diastolic blood pressure | Dominant | G beneficial | <.01 | N/A |
| Hong et al., 2012 (20) | Korea | 8 weeks | 29.1 mg | Yes | None specified | 103 | 9 | ADHD-RS-IV, CGI-I | ≥50% ADHD-RS-IV reduction and CGI-I ≤2 | Recessive | – | NS | Good |
| Kim et al., 2013 (21) | Korea | 12 weeks | 0.98 mg/kg | Yes | None specified | 101 | 9 | ADHD-RS-IV, CAT | Δ ADHD-RS-IV | Additive | – | NS | N/A |
| Δ CAT visual subscale | Additive | – | NS | ||||||||||
| Δ CAT auditory subscale | Additive | – | NS | ||||||||||
| Park et al., 2013 (22) | Korea | 8 weeks | 0.81 mg/kg | Yes | None specified | 115 | 9 | CGI-I, CPT | CGI-I ≤2 | Recessive | – | NS | Good |
| CPT subscales | Recessive | GG beneficial | Mixed | ||||||||||
| Kim et al., 2015 (23) | Korea | 8 weeks | Not specified | Yes | None specified | 78 | 10 | CGI-I | CGI-I ≤2 | Recessive | – | NS | Fair |
| Hegvik et al., 2016 (24) | Norway | NA | Not specified | Yes | Yes | 564 | 34 | Questionnaire | Response status | Dominant | – | NSc | N/A |
| Response status | Additive | – | NS | ||||||||||
| Unal et al., 2016 (11) | Turkey | 4–6 weeks | 0.7–1.1 mg/kg | Yes | None specified | 108 | 10 | CGI-S, GAS, CPT, TMT-A/B, CPRS, CTRS | ≥50% improvement on any CPRS/CTRS subscale and GAS ≥60 and ≥2 points improvement in CGI-S | Additive | – | NS | Fair |
| GG vs. CC | GG detrimental | <.05 | |||||||||||
| Gomez-Sanchez et al., 2015 (13) | Spain | 1 year | 40.5 mg | Not specified | None specified | 208 | 11 | CGI-S, CGAS | Δ CGAS or CGI-S | Additive | – | NS | N/A |
| Huang et al., 2018 (25) | Taiwan | 4 weeks | 0.9 mg/kg | Not specified | None specified | 59 | 11 | SNAP-IV | ≥25% SNAP-IV reduction | GG vs. CC | – | NS | Fair |
| ≥25% SNAP-IV reduction | CG vs. CC | – | NS | ||||||||||
| ≥25% SNAP-IV reduction vs. ≥50% SNAP-IV reduction | GG vs. CC | – | NS | ||||||||||
| ≥25% SNAP-IV reduction vs. ≥50% SNAP-IV reduction | CG vs. CC | CG beneficial | <.05 |
Descriptions of studies reviewed and their reported outcomes, including the associated measurement, genotypic model, direction of the effect, and significance of the outcome.
ADHD-RS-IV, ADHD Rating Scale-IV; CAT, Clinical Assessment of Attention Deficit; CGAS, Children’s Global Assessment Scale; CGI-I, Clinical Global Impressions–Improvement; CGI-S, CGI–Severity; CPRS, Conners’ Parent Rating Scale; CPT, Continuous Performance Test; CTRS, Conners’ Teacher Rating Scale; GAS, Global Assessment of Functioning Scale; N/A, not applicable; NS, not significant; SNAP-IV, Swanson, Nolan, and Pelham Version-IV Scale; TMT, Trail Making Test; VADPRS, Vanderbilt ADHD Diagnostic Parent Rating Scale; VADTRS, Vanderbilt ADHD Diagnostic Teacher Rating Scale.
Subjects were 79% White.
Beneficial implies that rs1800544 variant alleles improved methylphenidate outcomes; detrimental implies that rs1800544 variant alleles were detrimental to methylphenidate outcomes.
After correction for multiple testing, as reported by Hegvik et al. (24).
Overall quality rating based on the National Institutes of Health Quality Assessment Tool for Before-After (Pre-Post) Studies With No Control Group (8).
Five studies included in the descriptive analysis were excluded from meta-analyses and are therefore discussed here in brief. Cho et al. (19) reported on adverse events rather than clinical outcomes and demonstrated a significant protective effect of rs1800544 carrier status against increases in diastolic blood pressure following methylphenidate treatment (p = .009). Hegvik et al. (24) used a nonvalidated clinician-rated response questionnaire to evaluate clinical outcomes and found no association between rs1800544 genotype and methylphenidate outcomes. Froelich et al. (18), Kim et al. (21), and Gomez-Sanchez et al. (13) examined additive models of rs1800544 and as such were not included in any meta-analyses; all 3 studies showed no association between rs1800544 genotype and methylphenidate outcomes.
Quality Assessment
The quality assessment ratings are shown in Table 1. Of the 9 studies assessed, 6 were rated as “good” (14, 15, 16, 17,20,22), and 3 were rated as “fair” (11,23,25). None of the included studies used multiple measurements for the same outcome (e.g., an interrupted time-series design), which could have potentially increased confidence that outcomes were accurately measured. A majority of the studies (11,15, 16, 17,22,23,25) presented an issue with sample size reporting; only 2 studies (14,20) specifically commented on effect size. The 3 studies that were rated as “fair” (11,23,25) did not specify whether the raters assessing outcomes were blinded; if studies were not blinded, the raters’ assessment could have been biased by participant genotypes. However, all studies clearly stated the objective, appropriately described inclusion criteria for participants, represented clinical populations of interest, enrolled all eligible (consenting) participants, clearly described patient genotyping and outcomes, had high follow-up rates, and statistically examined outcome measures before and after methylphenidate treatment.
Independent Analyses
Independent analyses were performed for 3 studies (11,16,25) using data that were available in each publication to obtain ORs. The results are summarized in Table 2. Cheon et al. (16) reported recessive model outcomes but did not analyze their data using a dominant model, which was performed independently for response and CGI response. Huang et al. (25) compared various genotype distributions across nonresponders (<25% SNAP-IV reduction), moderate responders (≥25% SNAP-IV reduction), and better responders (≥50% SNAP-IV reduction) but did not analyze their data using a dominant or recessive model, which was performed independently for response. Unal et al. (11) reported additive model outcomes and genotype comparisons but did not analyze their data using dominant or recessive models, which was performed independently using the provided definition of treatment response. Overall, the findings from our independent analyses were similar to the results reported in the original studies (Table 1).
Table 2.
Independent Analyses
| Reference | Country | Duration | N | Average Age, Years | Scales Assessed | Measure | Model | rs1800544 Effecta | OR (95% CI)b | p Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Cheon et al., 2009 (16) | Korea | 8 weeks | 114 | 9 | ADHD-RS-IV, CGI-I | ≥50% ADHD-RS-IV reduction | Dominant | – | 2.26 (0.67–7.62) | NS |
| CGI-I ≤2 | Dominant | G beneficial | 3.83 (1.08–13.60) | <.05 | ||||||
| Huang et al., 2018 (25) | Taiwan | 4 weeks | 59 | 11 | SNAP-IV | ≥50% SNAP-IV reduction | Dominant | – | 3.45 (0.36–32.90) | NS |
| ≥50% SNAP-IV reduction | Recessive | – | 1.44 (0.49–4.25) | NS | ||||||
| Unal et al., 2016 (11) | Turkey | 4–6 weeks | 108 | 10 | CGI-S, GAS, CPT, TMT-A/B, CPRS, CTRS | ≥50% improvement on any CPRS/CTRS subscale and GAS ≥60 and ≥2 points improvement in CGI-S | Dominant | – | 0.89 (0.40–2.00) | NS |
| Recessive | – | 0.27 (0.06–1.20) | NS |
Descriptions of studies from which unreported outcomes were generated, including the associated measurement, genotypic model, direction of the effect, and significance of the outcome.
ADHD-RS-IV, ADHD Rating Scale-IV; CGI-I, Clinical Global Impressions–Improvement; CGI-S, CGI–Severity; CI, confidence interval; CPRS, Conners’ Parent Rating Scale; CPT, Continuous Performance Test; CTRS, Conners’ Teacher Rating Scale; GAS, Global Assessment of Functioning Scale; NS, not significant; OR, odds ratio; SNAP-IV, Swanson, Nolan, and Pelham Version-IV Scale; TMT, Trail Making Test.
Beneficial implies that rs1800544 variant alleles improved methylphenidate outcomes; detrimental implies that rs1800544 variant alleles were detrimental to methylphenidate outcomes.
Odds of response among carriers of rs1800544 (dominant models) or among homozygous carriers of rs1800544 (recessive models).
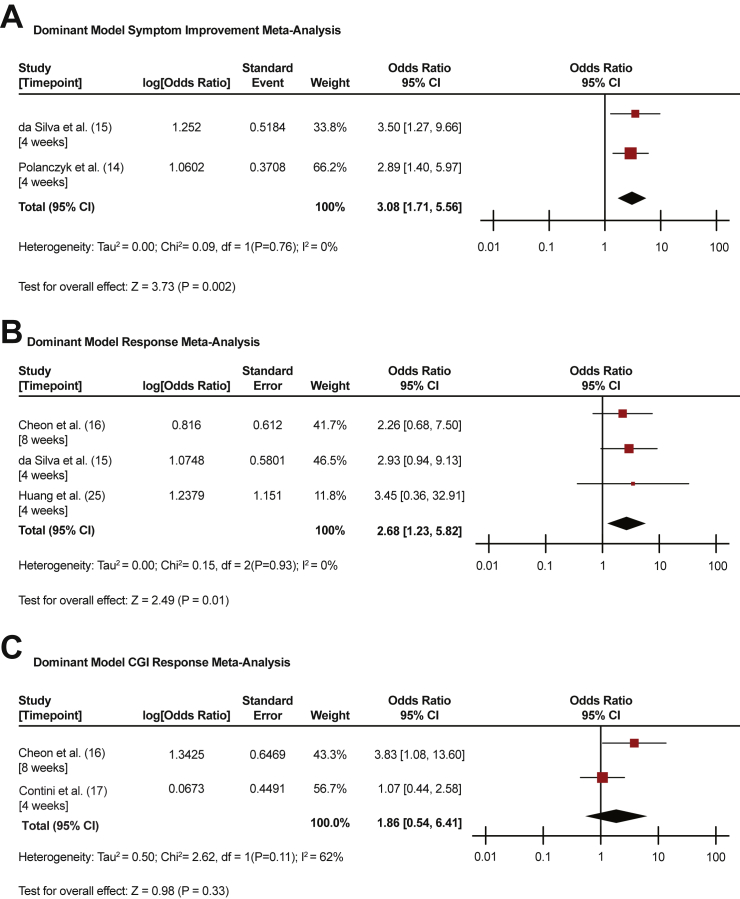
Clinical Outcomes Using a Dominant Gene Model
Figure 1 shows the results of the generic inverse variance meta-analyses for symptom improvement (Figure 1A), response (Figure 1B), and CGI response (Figure 1C), respectively, for studies which evaluated a dominant model. The observed heterogeneity was not significant for symptom improvement (I2 = 0%, p = .76), response (I2 = 0%, p = .93), or CGI response (I2 = 62%, p = .11). rs1800544 carrier status was significantly associated with symptom improvement (OR 3.08, 95% confidence interval [CI] 1.71–5.56, p = .002) and response (OR 2.68, 95% CI 1.23–5.82, p = .01), but not CGI response (OR 1.86, 95% CI 0.54–6.41, p = .33), following methylphenidate treatment.
Figure 1.
Dominant model meta-analyses. (A) Odds ratio for symptom improvement in carriers of rs1800544. (B) Odds ratio for response in carriers of rs1800544. (C) Odds ratio for Clinical Global Impressions (CGI) response in carriers of rs1800544. CI, confidence interval.
Table 3 shows the results of Stouffer’s z-score combined meta-analysis (symptom improvement, response, and CGI response) for studies that evaluated a dominant model. One dominant model outcome was taken from each study, if available, prioritizing symptom improvement over response over CGI response. There was a significant association between rs1800544 carrier status (z = 3.03, p = .002), with improved methylphenidate outcomes in carriers of the G allele.
Table 3.
Dominant Model z-Score Meta-analysis
| Reference | Country | Duration | N | Average Age, Years | Scales Assessed | Measure | OR (95% CI)a | z Score | Stouffer’s z | p Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Cheon et al., 2009 (16) | Korea | 8 weeks | 114 | 9 | ADHD-RS-IV, CGI-I | ≥50% ADHD-RS-IV reduction | 2.26 (0.67–7.62) | 1.32 | 3.03 | .002 |
| Huang et al., 2018 (25) | Taiwan | 4 weeks | 59 | 11 | SNAP-IV | ≥50% SNAP-IV reduction | 3.45 (0.36–32.90) | 1.08 | ||
| Unal et al., 2016 (11) | Turkey | 4–6 weeks | 108 | 10 | CGI-S, GAS, CPT, TMT-A/B, CPRS, CTRS | ≥50% improvement on any CPRS/CTRS subscale and GAS ≥60 and ≥2 points improvement in CGI-S | 0.89 (0.40–2.00) | −0.28 | ||
| Polanczyk et al., 2007 (14) | Brazil | 1 and 3 months | 106 | 10 | SNAP-IV | Δ SNAP-IV | 2.89 (1.40–5.97) | 2.88 | ||
| da Silva et al., 2008 (15) | Brazil | 4 weeks | 59 | 12 | SNAP-IV | Δ SNAP-IV | 3.50 (1.27–9.66) | 2.41 | ||
| Contini et al., 2011 (17) | Brazil | 4 weeks | 165 | 35 | SNAP-IV, CGI-S | ≥30% SNAP-IV reduction and CGI-S and CGI-S ≤2 | 1.07 (0.44–2.58) | 0.00 |
Stouffer’s z score for improved outcomes in G-allele carriers relative to CC subjects.
ADHD-RS-IV, ADHD Rating Scale-IV; CGI-I, Clinical Global Impressions–Improvement; CGI-S, CGI–Severity; CI, confidence interval; CPRS, Conners’ Parent Rating Scale; CTRS, Conners’ Teacher Rating Scale; GAS, Global Assessment of Functioning Scale; OR, odds ratio; SNAP-IV, Swanson, Nolan, and Pelham Version-IV Scale; TMT, Trail Making Test.
Odds of improved outcome among carriers of rs1800544.
Clinical Outcomes Using a Recessive Gene Model
Figure 2 shows the results of the generic inverse variance meta-analyses for response (Figure 2A) and CGI response (Figure 2B) for studies that evaluated a recessive model. The degrees of heterogeneity for response and CGI response were not significant (response: I2 = 55%, p = .13; CGI response: I2 = 53%, p = .09). rs1800544 homozygous carrier status was not significantly associated with response (OR 2.57, 95% CI 0.94–7.04, p = .07) or CGI response (OR 1.15, 95% CI, 0.63–2.13, p = .65) following methylphenidate treatment. Only one study by Cheon et al. (16) evaluated rs1800544 homozygous carrier status and symptom improvement after methylphenidate treatment, showing a significant beneficial effect (p = .003).
Figure 2.
Recessive model meta-analyses. (A) Odds ratio for response in homozygous carriers of rs1800544. (B) Odds ratio for Clinical Global Impressions (CGI) response in homozygous carriers of rs1800544. CI, confidence interval.
Table 4 shows the results of the Stouffer’s z-score combined meta-analysis (symptom improvement, response, and CGI response) for recessive model outcomes. One recessive model outcome was taken from each study, if available, prioritizing symptom improvement over response over CGI response There was no significant association between rs1800544 homozygous carrier status (z = 0.00, p = 1.00).
Table 4.
Recessive Model z-Score Meta-analysis
| Reference | Country | Duration | N | Average Age, Years | Scales Assessed | Measure | OR (95% CI)a | z Score | Stouffer’s z | p Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Huang et al., 2018 (25) | Taiwan | 4 weeks | 59 | 11 | SNAP-IV | ≥50% SNAP-IV reduction | 1.44 (0.49–4.25) | 0.66 | 0.00 | 1.00 |
| Unal et al., 2016 (11) | Turkey | 4–6 weeks | 108 | 10 | CGI-S, GAS, CPT, TMT-A/B, CPRS, CTRS | ≥50% improvement on any CPRS/CTRS Subscale and GAS ≥60 and ≥2 points improvement in CGI-S | 0.27 (0.06–1.20) | −1.72 | ||
| Cheon et al., 2009 (16) | Korea | 8 weeks | 114 | 9 | ADHD-RS-IV, CGI-I | Δ ADHD-RS-IV | 2.12 (1.08–4.17) | 2.19 | ||
| Hong et al., 2012 (20) | Korea | 8 weeks | 103 | 9 | ADHD-RS-IV, CGI-I | ≥50% ADHD-RS-IV reduction and CGI-I ≤2 | 1.08 (0.43–2.69) | 0.16 | ||
| Park et al., 2013 (22) | Korea | 8 weeks | 115 | 9 | CGI-I, CPT | CGI-I ≤2 | 1.21 (0.57–2.55) | 0.18 | ||
| Kim et al., 2015 (23) | Korea | 8 weeks | 78 | 10 | CGI-I | CGI-I ≤2 | 0.50 (0.20–1.26) | −1.48 |
Stouffer’s z score for improved outcomes in GG subjects relative to C-allele carriers.
ADHD-RS-IV, ADHD Rating Scale-IV; CGI-I, Clinical Global Impressions–Improvement; CGI-S, CGI–Severity; CI, confidence interval; CPRS, Conners’ Parent Rating Scale; CPT, Continuous Performance Test; CTRS, Conners’ Teacher Rating Scale; GAS, Global Assessment of Functioning Scale; OR, odds ratio; SNAP-IV, Swanson, Nolan, and Pelham Version-IV Scale; TMT, Trail Making Test.
Odds of improved outcome among homozygous carriers of rs1800544.
Discussion
After a systematic review of the literature, the totality of evidence from the individual studies was mixed, with many studies reporting no association between rs1800544 and methylphenidate outcomes. Owing to the variety of symptom scales and modeling approaches used among the studies, different meta-analysis methods were used to evaluate dominant and recessive gene models separately across all outcomes (Stouffer’s z score) and across specific symptom scales (generic inverse variance meta-analyses). Similar to the evidence from individual studies, the meta-analyses presented here also revealed mixed results. Dominant model meta-analyses revealed significant associations between rs1800544 carrier status and methylphenidate response for some outcome categories. Recessive models showed no association with rs1800544 homozygous carriers and methylphenidate response.
In the dominant gene model meta-analyses, symptom improvement and response were significantly associated with rs1800544 carrier status. Despite differences in methodology between the studies, the observed heterogeneity was not significant for both symptom improvement and response. For the dominant gene model CGI response meta-analysis, no significant association was found, and the observed heterogeneity was not significant. Taken together, these findings suggest a potential association between rs1800544 carrier status and methylphenidate outcomes in ADHD. However, given the small number of studies eligible for dominant gene model meta-analyses (symptom improvement, n = 2; response, n = 3; CGI response, n = 2), additional studies on this topic will be needed to determine the clinical significance of these findings.
The findings presented here are consistent with a previous meta-analysis that reported an improved methylphenidate response in association with the G allele (26). Myer et al. (26) meta-analyzed 4 studies (14,15,18,21), all of which are also reported here. However, owing to differences in clinical tools used to measure outcomes and the type of gene model used, only 2 of the studies meta-analyzed by Myer et al. (26) were eligible for quantitative analysis in the present study; the remaining 2 studies were presented only in the descriptive analysis (Table 1). Because of the inclusion criteria applied in the present study, the studies combined for individual meta-analyses were more methodologically similar than the studies combined in the meta-analysis performed by Myer et al. (26). As such, there was a large difference between the degree of heterogeneity reported here for both symptom improvement and response (dominant model meta-analyses; 0%) and that reported by Myer et al. (80%) (26). Despite the differences in approach and heterogeneity, the conclusions between these 2 studies are consistent. This further supports that the association between methylphenidate outcomes and rs1800544 is an important area of study, and rs1800544 may be a potential gene of interest in ADHD pharmacogenomics. Additionally, more methodologically consistent studies are needed in this area to allow for future meta-analyses and to provide a framework for any relevant clinical recommendations.
This study had some limitations. Age restrictions were not considered, such that results from pediatric and adult ADHD populations were combined, and ADHD subtypes were not evaluated. Study methodology varied considerably among the individual studies, including variations in treatment duration, symptom scales, and definitions of treatment response. Despite these limitations, the meta-analyses presented here provide relevant information, as individual studies can sometimes be underpowered. For example, individual studies included in the dominant gene model for response did not report statistically significant associations between methylphenidate outcomes and rs1800544 carrier status; however, when these studies were meta-analyzed, a significant association was found.
Additional studies are needed to provide more definitive evidence that rs1800544 is associated with methylphenidate outcomes. The Clinical Pharmacogenetics Implementation Consortium prioritizes guideline implementation for gene–drug interactions based on a variety of criteria. Studies that focus on prescribing actionability, adverse effects in the absence of pharmacogenetic-informed prescribing, and frequency of high-risk gene variants will be needed for consideration of actionable recommendations by the Clinical Pharmacogenetics Implementation Consortium (27). Additionally, given the complex mechanism of action of methylphenidate, further research may likely reveal that variation in other genes plays an important role in identifying either methylphenidate nonresponders or patients who may be at increased risk of adverse events following methylphenidate treatment.
While methylphenidate is a highly effective option for the treatment of ADHD, nearly half of pediatric patients are nonresponders (2). Pharmacogenetic predictors of methylphenidate outcomes offer an opportunity to improve the use of methylphenidate in treatment of ADHD. The meta-analysis findings highlighted here suggest that carriers of rs1800544 may have improved ADHD outcomes following methylphenidate treatment. However, it is unclear to what extent the improvements observed in this review are clinically meaningful. Individual testing laboratories and health care providers will need to evaluate this information and how it fits into clinical practice. Additionally, further research in this area will help determine whether noncarriers of rs1800544 may benefit more from an alternative option to methylphenidate for the treatment of ADHD symptoms.
Acknowledgments and Disclosures
This work was supported by Myriad Genetics.
We thank Brenda Rubalcaba for assistance with manuscript preparation.
All authors are employed by Myriad Genetics.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2021.07.009.
Supplementary Material
References
- 1.Cortese S., Adamo N., Del Giovane C., Mohr-Jensen C., Hayes A.J., Carucci S., et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: A systematic review and network meta-analysis. Lancet Psychiatry. 2018;5:727–738. doi: 10.1016/S2215-0366(18)30269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newcorn J.H., Kratochvil C.J., Allen A.J., Casat C.D., Ruff D.D., Moore R.J., et al. Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: Acute comparison and differential response. Am J Psychiatry. 2008;165:721–730. doi: 10.1176/appi.ajp.2007.05091676. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Data and statistics about ADHD. https://www.cdc.gov/ncbddd/adhd/data.html Available at:
- 4.National Library of Medicine, National Center for Biotechnology Information: Reference SNP (rs) Report. rs1800544. https://www.ncbi.nlm.nih.gov/snp/rs1800544?vertical_tab=true Available at:
- 5.CPIC: Guidelines. https://cpicpgx.org/guidelines/ Available at:
- 6.U.S. Food and Drug Administration: Table of Pharmacogenetic Associations. https://www.fda.gov/medical-devices/precision-medicine/table-pharmacogenetic-associations Available at:
- 7.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 8.National Institutes of Health: Study Quality Assessment Tools: Quality Assessment Tool for Before-After (Pre-Post) Studies With No Control Group. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools Available at:
- 9.Lipsey M.W., Wilson D.B. SAGE Publications; Thousand Oaks, CA: 2001. Practical Meta-Analysis. [Google Scholar]
- 10.The Cochrane Collaboration . The Nordic Cochrane Centre; Copenhagen: 2014. Review Manager (RevMan) [Google Scholar]
- 11.Unal D., Unal M.F., Alikasifoglu M., Cetinkaya A. Genetic variations in attention deficit hyperactivity disorder subtypes and treatment resistant cases. Psychiatry Investig. 2016;13:427–433. doi: 10.4306/pi.2016.13.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darlington R.B., Hayes A.F. Combining independent p values: Extensions of the Stouffer and binomial methods. Psychol Methods. 2000;5:496–515. doi: 10.1037/1082-989x.5.4.496. [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Sanchez C.I., Riveiro-Alvarez R., Soto-Insuga V., Rodrigo M., Tirado-Requero P., Mahillo-Fernandez I., et al. Attention deficit hyperactivity disorder: Genetic association study in a cohort of Spanish children. Behav Brain Funct. 2015;12:2. doi: 10.1186/s12993-015-0084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polanczyk G., Zeni C., Genro J.P., Guimaraes A.P., Roman T., Hutz M.H., et al. Association of the adrenergic alpha2A receptor gene with methylphenidate improvement of inattentive symptoms in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007;64:218–224. doi: 10.1001/archpsyc.64.2.218. [DOI] [PubMed] [Google Scholar]
- 15.da Silva T., Pianca T., Roman T., Hutz M., Faraone S., Schmitz M., et al. Adrenergic α2A receptor gene and response to methylphenidate in attention-deficit/hyperactivity disorder-predominantly inattentive type. J Neural Transm (Vienna) 2008;115:341–345. doi: 10.1007/s00702-007-0835-0. [DOI] [PubMed] [Google Scholar]
- 16.Cheon K.A., Cho D.Y., Koo M.S., Song D.H., Namkoong K. Association between homozygosity of a G allele of the alpha-2a-adrenergic receptor gene and methylphenidate response in Korean children and adolescents with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2009;65:564–570. doi: 10.1016/j.biopsych.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Contini V., Victor M.M., Cerqueira C.C., Polina E.R., Grevet E.H., Salgado C.A., et al. Adrenergic α2A receptor gene is not associated with methylphenidate response in adults with ADHD. Eur Arch Psychiatry Clin Neurosci. 2011;261:205–211. doi: 10.1007/s00406-010-0172-4. [DOI] [PubMed] [Google Scholar]
- 18.Froehlich T.E., Epstein J.N., Nick T.G., Melguizo Castro M.S., Stein M.A., Brinkman W.B., et al. Pharmacogenetic predictors of methylphenidate dose-response in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2011;50:1129–1139.e2. doi: 10.1016/j.jaac.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho S.C., Kim B.N., Cummins T.D., Kim J.W., Bellgrove M.A. Norepinephrine transporter −3081 (A/T) and alpha-2A-adrenergic receptor MspI polymorphisms are associated with cardiovascular side effects of OROS-methylphenidate treatment. J Psychopharmacol. 2012;26:380–389. doi: 10.1177/0269881111405356. [DOI] [PubMed] [Google Scholar]
- 20.Hong S.B., Kim J.W., Cho S.C., Shin M.S., Kim B.N., Yoo H.J. Dopaminergic and noradrenergic gene polymorphisms and response to methylphenidate in Korean children with attention-deficit/hyperactivity disorder: Is there an interaction? J Child Adolesc Psychopharmacol. 2012;22:343–352. doi: 10.1089/cap.2011.0076. [DOI] [PubMed] [Google Scholar]
- 21.Kim B.N., Kim J.W., Cummins T.D., Bellgrove M.A., Hawi Z., Hong S.B., et al. Norepinephrine genes predict response time variability and methylphenidate-induced changes in neuropsychological function in attention deficit hyperactivity disorder. J Clin Psychopharmacol. 2013;33:356–362. doi: 10.1097/JCP.0b013e31828f9fc3. [DOI] [PubMed] [Google Scholar]
- 22.Park S., Kim J.W., Kim B.N., Hong S.B., Shin M.S., Yoo H.J., et al. No significant association between the alpha-2A-adrenergic receptor gene and treatment response in combined or inattentive subtypes of attention-deficit hyperactivity disorder. Pharmacopsychiatry. 2013;46:169–174. doi: 10.1055/s-0033-1343485. [DOI] [PubMed] [Google Scholar]
- 23.Kim J.W., Sharma V., Ryan N.D. Predicting methylphenidate response in ADHD using machine learning approaches. Int J Neuropsychopharmacol. 2015;18:pyv052. doi: 10.1093/ijnp/pyv052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hegvik T.A., Jacobsen K.K., Fredriksen M., Zayats T., Haavik J. A candidate gene investigation of methylphenidate response in adult attention-deficit/hyperactivity disorder patients: Results from a naturalistic study. J Neural Transm. 2016;123:859–865. doi: 10.1007/s00702-016-1540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang H.C., Wu L.S., Yu S.C., Wu B.J., Lua A.C., Lee S.M., et al. The alpha-2A adrenergic receptor gene-1291C/G single nucleotide polymorphism is associated with the efficacy of methylphenidate in treating Taiwanese children and adolescents with attention-deficit hyperactivity disorder. Psychiatry Investig. 2018;15:306–312. doi: 10.30773/pi.2017.07.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myer N., Boland J., Faraone S. Pharmacogenetics predictors of methylphenidate efficacy in childhood ADHD. Mol Psychiatry. 2018;23:1929–1936. doi: 10.1038/mp.2017.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.CPIC: Prioritization: Assignment of CPIC Levels for Genes/Drugs. https://cpicpgx.org/prioritization/ Available at:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.