Abstract
Noninvasive neuroimaging is a powerful tool for quantifying diverse aspects of brain structure and function in vivo, and it has been used extensively to map the neural changes associated with various brain disorders. However, most neuroimaging techniques offer only indirect measures of underlying pathological mechanisms. The recent development of anatomically comprehensive gene expression atlases has opened new opportunities for studying the transcriptional correlates of noninvasively measured neural phenotypes, offering a rich framework for evaluating pathophysiological hypotheses and putative mechanisms. Here, we provide an overview of some fundamental methods in imaging transcriptomics and outline their application to understanding brain disorders of neurodevelopment, adulthood, and neurodegeneration. Converging evidence indicates that spatial variations in gene expression are linked to normative changes in brain structure during age-related maturation and neurodegeneration that are in part associated with cell-specific gene expression markers of gene expression. Transcriptional correlates of disorder-related neuroimaging phenotypes are also linked to transcriptionally dysregulated genes identified in ex vivo analyses of patient brains. Modeling studies demonstrate that spatial patterns of gene expression are involved in regional vulnerability to neurodegeneration and the spread of disease across the brain. This growing body of work supports the utility of transcriptional atlases in testing hypotheses about the molecular mechanism driving disease-related changes in macroscopic neuroimaging phenotypes.
Keywords: Brain imaging, Connectome, Gene expression, Neurodegeneration, Neurodevelopment, Psychiatric disorders
SEE COMMENTARY ON PAGE 311
The advent of magnetic resonance imaging (MRI) ushered in a new era of biological psychiatry, arming researchers with a powerful tool for studying the neural correlates of mental illness noninvasively. Thousands of research articles published in the intervening years have shed important light on the disruptions of brain structure and function that occur in association with a diverse range of psychiatric disorders. However, owing to the limited spatiotemporal resolution of current MRI methods and the biophysical properties of the acquired signals, the resulting imaging-derived phenotypes (IDPs) often represent indirect proxies for the cellular and molecular processes that ultimately drive disease pathophysiology. As a result, in vivo neuroimaging is an excellent tool for mapping where changes occur in the brain and at which specific stage of disease, but it can offer only limited insight into the physiological mechanisms that underpin these changes.
Over the past 2 decades, imaging genetics has offered a means for studying the molecular basis of disease-related IDPs. A growing body of evidence indicates that genetics plays a substantial role in shaping how the brain is organized (1, 2, 3, 4, 5, 6, 7). Heritability studies show that a range of IDPs are highly heritable (6, 8, 9, 10, 11, 12) and genome-wide association studies (GWASs) have begun to identify robust links to DNA variation (13, 14, 15, 16), uncovering hundreds of associations between single nucleotide polymorphisms and diverse measures of brain structure, function, and connectivity (13, 14, 15,17,18). The general assumption is that DNA variants implicated in GWASs or variants in linkage disequilibrium affect protein expression and/or function, thereby influencing cellular form and physiology to give rise to phenotypic variability. However, multiple factors can influence the transcriptional activity of a gene and the subsequent abundance of its protein product (19, 20, 21), meaning that allelic variations of DNA can be somewhat distant from the molecular mechanisms influencing a particular phenotype. Studies of gene expression can more directly interrogate the transcriptional activity of genes, as measured in postmortem specimens. Because of the complexities of tissue curation, processing, and analysis, such investigations have historically been limited to small subsets of brain regions, precluding an opportunity to link disease-related changes in gene expression to brainwide IDPs. Over the past decade, advances in high-throughput tissue processing and analysis pipelines have facilitated the development of brainwide gene expression atlases (22,23), opening new opportunities to investigate how the spatial patterns of gene expression relate to anatomical variations in brain structure and function in both health and disease [for a review, see (3)]. Of particular relevance to psychiatry, this emerging field of imaging transcriptomics makes it possible to generate and test hypotheses about the transcriptional correlates of disease-related changes in IDPs, offering a bridge between molecular organization and macroscale measures of brain dysfunction.
In this review, we consider how imaging transcriptomics can shed light on putative pathophysiological mechanisms in psychiatric disorders, primarily focusing on studies using brainwide gene expression data derived from the Allen Human Brain Atlas (AHBA), which offers the most anatomically comprehensive expression atlas. We first provide an overview of some fundamental methods in the field and then examine applications of these methods to understand neurodevelopment, neuropsychiatric illness, and neurodegeneration. These studies indicate that regional gene expression patterns are related to normative changes in brain structure during age-related maturation and neurodegeneration. Genes related to synaptic signaling, metabolism, and other neurobiologically relevant categories are consistently associated with clinical alterations in neuropsychiatric disorders. Disorder-associated expression signatures tend to involve transcriptionally dysregulated genes in postmortem brain tissues of patients. Moreover, spatial patterns of gene expression play a role in both regional vulnerability to neurodegeneration and the spread of neurodegeneration across the brain. Finally, we discuss some methodological challenges and consider future directions for the field.
Fundamental Methods of Imaging Transcriptomics
Brainwide imaging transcriptomic studies rely on transcriptional atlases that assay gene expression across multiple locations in the brain. The methods available for quantifying gene expression depend on a range of factors, including the organism of interest, tissue availability, and the required spatial resolution. In smaller species such as mice, for which specimens are more readily available, gene expression can be measured using in situ hybridization (23) in which the transcriptional activity of each gene is measured in separate tissue samples at a cellular resolution. Tissue availability in humans is very limited and bulk tissue microarray (24) remains the most accessible method for high-throughput spatial transcriptomics (22). With microarray, the expression levels of thousands of genes are quantified simultaneously using a single tissue sample. The AHBA provides the most anatomically comprehensive gene expression data for the human brain, quantifying expression levels of more than 20,000 genes in 3702 different regions using microarray (22). Although atlases for developmental (25) and human-specific spatiotemporal gene expression patterns (26) are also available, the spatial coverage of these atlases is much sparser compared with that of AHBA, extending across only 16 brain regions [for an overview of different gene expression atlases, see (27)]. The AHBA therefore represents the most readily accessible resource for linking measures of gene expression to a given brainwide IDP.
Several considerations about the AHBA must be borne in mind. First, the atlas comprises data from only 6 individuals, with the majority of samples derived from the left hemisphere, precluding detailed investigations of individual variability in regional gene expression and the lateralization of expression patterns across hemispheres. While it appears that expression differences are greater across regions than between people (28), the full extent and functional significance of interindividual variation in gene expression remains unclear. Second, all measures are derived from adult brains, with donors ranging in age from 24 to 57 years; variations in expression may therefore be related to the myriad endogenous and exogenous factors that can influence transcriptional activity throughout life (29). While many genes show neotenous patterns of expression (30), many others show marked changes in transcriptional activity across the life span (31). These changes can be tracked for some brain regions through developmental gene expression atlases (25,32), but our understanding of regional differences in the transcriptional dynamics of different genes remains incomplete. Third, as gene expression in the AHBA is assayed using bulk tissue microarray, regional differences in gene expression could reflect variations in the cellular, functional, or anatomical composition of that region. Single-cell transcriptomics offers a way of disentangling these contributions, but the anatomical coverage of these atlases remains limited in comparison (33, 34, 35). Finally, the relationship between the transcriptional and translational activity of a gene is complex and influenced by multiple factors (36,37). It is ultimately the latter that determines protein form and abundance and thus cellular function. Transcriptional atlases thus offer an indirect marker of more proximal molecular drivers of phenotypic variation.
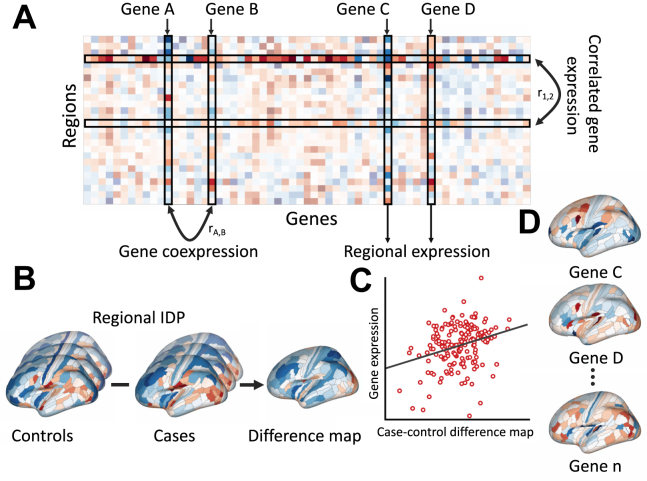
Three main categories of analyses are performed in imaging transcriptomics (Figure 1A). One focuses on regional gene expression patterns and aims to identify correlations between spatial variations in gene expression and anatomically defined IDPs across spatial locations (38,39). A second category of analyses considers correlated gene expression (CGE), which quantifies the transcriptional similarity between pairs of regions across a set of genes. CGE can then be related to IDPs defined at the level of pairs of brain regions, such as measures of structural or functional connectivity (8,40, 41, 42). A third analysis category examines gene coexpression, which quantifies the similarity of spatial expression profiles between pairs of genes, resulting in a gene × gene matrix. Statistical summaries of these gene × gene matrices, such as eigengene measures of coherent subsets of coexpressed genes, have been computed and related to IDPs (43,44). Note that in the literature, “gene coexpression” has also been used to refer to analyses of CGE (41,45).
Figure 1.
Approaches for relating gene expression to neuroimaging data. (A) Transcriptional atlas data can be collated into a region × gene matrix, from which different estimates of gene expression can be quantified. In this example, the regional expression profile of any given gene, indicating the spatial patterning of the gene’s transcriptional activity, corresponds to a single column of the matrix. A given region’s expression profile across genes corresponds to a row of the matrix. Correlated gene expression is therefore calculated by correlating pairs of rows (resulting in region × region similarity matrix), while gene coexpression is calculated by correlating pairs of columns (resulting in gene × gene similarity matrix). (B) When applied to brain disorders, spatial maps of a given IDP measure in cases and controls are compared to yield some kind of difference map. This difference map is then correlated (C) with the spatial maps of each gene (D) in a hypothesis- or data-driven method. Genes associated with high spatial correlations to the difference map represent transcriptional correlates of the IDP. IDP, imaging-derived phenotype.
An important consideration in any of these analyses is that both neuroimaging and transcriptional measures typically exhibit some degree of spatial autocorrelation, meaning that values in different brain regions are not independent of each other. This spatial autocorrelation commonly takes the form of a dependence between regional expression values that decays with distance, such that regions physically closer to each other have values that are more highly correlated than pairs of distant regions (46,47). This dependence must be accounted for to ensure valid inference, and multiple approaches have been used (8,41,46,48, 49, 50, 51, 52). A comprehensive comparison of the efficacy of some of these approaches is provided by Markello and Misic (53).
Imaging transcriptomics has been most commonly deployed in clinical contexts to investigate associations at the level of brain regions. More specifically, these analyses involve comparing a spatial map that quantifies case-control differences in a given IDP to the spatial expression pattern of each gene in either a hypothesis-driven or data-driven way (Figure 1B). Hypothesis-driven analyses typically focus on specific sets of genes identified a priori, whereas data-driven approaches aim to identify patterns across thousands of genes, which involves scoring each gene based on its association with a given phenotype, using either mass univariate, gene-specific analyses or multivariate approaches that relate IDPs to linear combinations of genes (39,41,42,54,55). Based on experimental and theoretical evidence, genes have been grouped according to their biological and molecular functions, making it possible to test for the enrichment or over-representation of certain classes of genes using established annotation systems such as the Gene Ontology (GO) (56) or the Kyoto Encyclopedia of Genes and Genomes (57) through gene category enrichment analysis (GCEA). A range of statistical methods have been developed to accommodate different implementations of these analyses. For instance, when continuous gene scores are used, GCEA tests whether a summary score for each category is higher than expected, given some null distribution (58). Over-representation analysis, in contrast, is used for thresholded lists of genes, when the purpose of the analysis is to determine whether there are any gene sets that are statistically over-represented in that list compared with chance. Appropriate null models for such analyses are an active area of development (49,52,59). In a recent study, Fulcher et al. (59) showed that standard null models can dramatically inflate false positive rates across GO categories. We discuss some of these issues in more detail below, but the caveats are important to keep in mind when interpreting the findings presented in the literature. A further complication is that the gene expression data must be processed before they can be related to imaging measures. This processing involves multiple steps, each requiring choices between different processing options (60) that can have a substantial effect on the final results (50,61). The recently developed abagen toolbox can assist in running consistent workflows (61).
Neurodevelopment and Its Disorders
Age-related changes in cortical structure and function are associated with normal brain development (62, 63, 64), with cortical thinning being regarded as a morphological hallmark of cortical maturation (65,66). Brainwide changes in cortical thickness, mostly involving cortical thinning, are also associated with a range of psychiatric disorders (67, 68, 69, 70). Cortical thickness differences observed in many neurodevelopmental disorders, including schizophrenia, are thus often thought to arise from altered trajectories of brain maturation (71,72). These cortical thickness changes may be driven by changes to neuronal and/or synaptic size and/or density or the myelination of fibers penetrating the cortical mantle (73).
To shed light on the molecular correlates of brain changes that occur in both normal and aberrant neurodevelopment, imaging transcriptomic studies have used the expression profiles of specific cell-type marker genes to identify cellular corelates of these IDPs. The expression levels of cell-specific marker genes have been shown to act as indirect proxies for the relative abundance and/or function of each cell type (74,75). A correlation between the cell-type marker and the IDP is regarded as implicating that cell type in relation to the phenotype, but such correlational relationships should be interpreted with some caution. Indeed, while the relationship between gene expression and cell abundance for some cell types is very strong, not all cell-type markers demonstrate the same degree of correspondence (76).
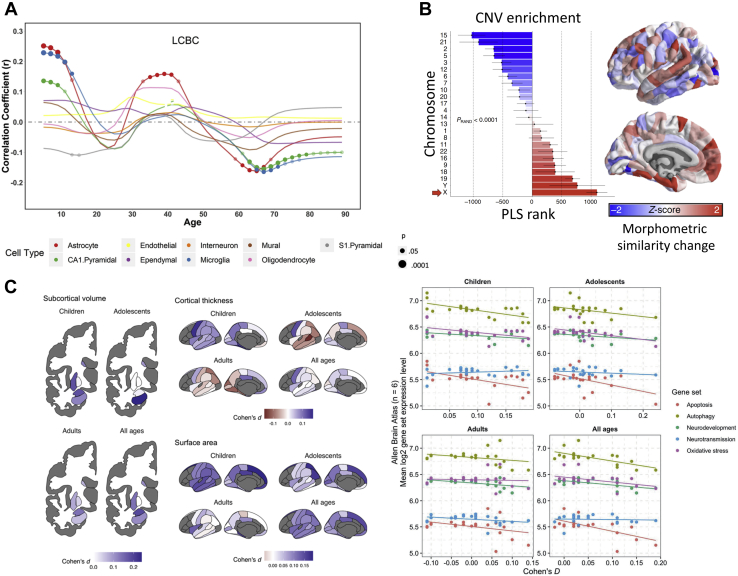
One analysis of cell-type markers found that regional variations of the magnetization transfer ratio (MTR), which is commonly used to index myelin content (77), are associated with CA1 pyramidal and ependymal cells, but not myelination, in mid-adolescence (mean age = 14 years) (78). Considering that the CA1 pyramidal gene set is enriched in genes that are also related to dendritic organization, the authors suggest that variations in the MTR across the brain could be related to regional differences in dendritic arborization. Subsequent longitudinal changes in the MTR from mid-to-late adolescence, however, were related to the expression of oligodendrocyte cell markers and genes involved in myelination (78). The involvement of oligodendrocyte cell markers in age-related cortical myelination was further confirmed in a detailed longitudinal investigation (79). In particular, oligodendrocyte gene markers were associated with age-related decreases in the ratio of myelination across the cortical depth, suggesting accumulation of myelin in mid-to-deep layers of the cortex. Similar associations implicating oligodendrocyte and neurotransmission-related genes were found in relation to changes in both the MTR and cortical thickness between 14 and 24 years through the partial least squares (PLS) regression (55). The fact that a single PLS component demonstrated associations with both measures implies that cortical myelination and thinning may act together as mechanisms of cortical consolidation that are at least in part driven by genes with correlated expression patterns. Other studies, conducted both cross-sectionally and longitudinally, indicate that transcriptional markers of astrocytes, microglia, and CA1 pyramidal cells are linked to a thicker cortex in young individuals and enhanced cortical thinning later in life, as shown in Figure 2A (80,81). This suggests that changes in cortical thickness across the life span are closely tied to the expression levels of these genes. Dendritic spine- and dendrite-related genes show associations with cortical thinning in late childhood (9–12 years) and late adolescence (17–19 years), whereas myelin-specific genes were related to cortical thinning in midadolescence (12–15 years) (82). Moreover, the directionality of the relationship changed over time such that in early maturation, regions with higher expression of those genes showed greater cortical thinning; in later stages, greater cortical thinning was observed in regions with lower expression. Multivariate analyses have further indicated that accelerated cortical thinning during the period from 3 to 21 years is associated with increased expression of gene markers for inhibitory and excitatory neurons, in addition to genes linked to synaptic remodeling and neurodevelopment, mood, and addiction disorders (83). The spatial expression patterns across different gene groups can explain a large proportion of variation in average cortical thickness across regions, accounting for up to 70% early in life (80).
Figure 2.
Transcriptional correlates of neurodevelopment and neurodevelopmental disorders. (A) The relationship between cortical thinning profile (i.e., the degree of cortical thinning per year) throughout life and gene expression in each of 9 cell types. Both in early and late life, significant associations (indicated by circles) were identified for astrocyte, microglia, and CA1 pyramidal cell markers. (B) Regional morphometric similarity change observed in Turner syndrome (characterized by the loss of the X chromosome) and chromosome ranking from a PLS analysis, based on the median rank of gene loadings on a PLS-derived latent variable linking gene expression with regional morphometric similarity change. The chromosomal gene set for the X chromosome shows the lowest rank, which is consistent with the chromosomal deletion known to cause the disorder. (C) Correspondence between brain changes in ADHD and gene expression. Left: differences in cortical thickness, surface area, and subcortical volumes in ADHD as identified by the ENIGMA Consortium, where larger positive Cohen’s d corresponds to decreases in a particular measure in patients with ADHD vs. control subjects. Right: the relationship between regional brain volumes and the expression of genes involved in apoptosis, autophagy, neurodevelopment, neurotransmission, and oxidative stress-related genes. Apoptosis, autophagy, and neurodevelopment-related genes demonstrate significant negative associations such that higher expression of those genes is associated with reductions in brain volume in patients with ADHD. Panel (A) adapted with permission from (81). Panel (B) adapted with permission from (39). Panel (C) adapted with permission from (85). ADHD, attention-deficit/hyperactivity disorder; CA, cornu ammonis; CNV, copy number variant; ENIGMA, Enhancing Neuro Imaging Genetics through Meta Analysis; LCBC, Center for Lifespan Changes in Brain and Cognition; PLS, partial least squares.
A recent study of different neurogenetic conditions has shown the potential power of imaging transcriptomics for identifying pathophysiologically relevant links between IDPs and spatial gene expression patterns. The conditions considered are caused by well-localized genomic copy number variations, including Turner syndrome, velocardiofacial syndrome, Down syndrome, Wilms tumor−aniridia syndrome, and sex chromosome aneuploidies (XXX, XXY, XYY, XXYY) (39). The distinct gene sets defining each of these conditions are known, leading to clear hypotheses about which specific genes should show correlated expression patterns with macroscale imaging measures of brain pathology. In this particular analysis, the authors focused on the average morphometric similarity (MS) of each region to all other brain areas, with MS defined as inter-regional correlations across multiple imaging measures of regional neuroanatomy and microstructure (39). The analysis revealed that regional differences in MS between cases and controls were related to the specific spatial expression of causal genes for each disorder (Figure 2B). Similar approaches have been applied to neurodevelopmental disorders with more complex genetics, such as attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (ASD). Measures of ADHD-associated decreases in subcortical volume, cortical thickness, and surface area, estimated in 2 large samples through analysis of ENIGMA (Enhancing Neuro Imaging Genetics through Meta Analysis) Consortium data, were consistently associated with genes implicated in apoptosis and autophagy pathways, such that higher expression of those genes corresponded to stronger decreases in these brain measures (84,85) (Figure 2C). Moreover, cell deconvolution analysis, which aims to infer cell-type abundance from gene expression levels, identified that smaller regions had elevated levels of astrocytes and oligodendrocyte progenitor cells, implying a compensatory response of glial cells (85). In ASD, two separate studies provide convergent evidence that changes in both cortical thickness (86) and regional volumes (87) are related to the expression of genes that are transcriptionally downregulated in ASD postmortem cortical tissue. In addition, the regional distribution of cortical thickness alterations observed in ASD was also associated with synaptic transmission genes (86), whereas changes in regional volumes were associated with nervous system and ion transport–related genes (87).
Together, this work indicates that developmental alterations in cortical properties are associated with the expression of cell-type marker genes, supporting a link between cellular properties and macroscale phenotypes measured using noninvasive brain imaging. In particular, the analyses of neurogenetic conditions with known genetic origins serve as evidence for the utility of imaging transcriptomics for investigating the genetic correlates of disorder-associated changes.
Psychiatric Disorders
Cell-type marker gene analyses have also been applied to study disorders with onset later in life. Using data from the ENIGMA Consortium, one study found that the spatial patterning of cortical thickness differences between cases and controls for six psychiatric disorders, including ADHD, ASD, major depression, obsessive-compulsive disorder, bipolar disorder, and schizophrenia, is consistently related to the regional expression of transcriptional markers for CA1 pyramidal cells, astrocytes, and microglia (88). These same cell-specific groups of genes have been associated with cortical thickness across the life span (80,81), pointing to shared genetic mechanisms linking neurodevelopmental and adult psychiatric disorders.
More specific investigations into individual psychiatric disorders have identified the involvement of neurobiologically relevant and disorder-associated genes. For example, half of the genes implicated in schizophrenia through GWASs were correlated with deviations in regional gray matter volume between cases and controls (89). Consistent with the hypothesized neurodevelopmental origins of schizophrenia (90), the same set of genes was also enriched for chemical synaptic transmission, central nervous system development, and cell projection, categories that are linked to both connectivity and developmental processes. Interneuron-related dysfunction has been regarded as one of the core features of psychopathology in schizophrenia (91). In line with this hypothesis, polygenic risk for schizophrenia has been shown to be significantly enriched for genes demonstrating spatial coexpression to PVALB genes, one of the core markers for interneurons that demonstrates a characteristic spatial variability correlated with variations in functional MRI signal amplitude across the brain (92). Extending the link to nonclinical populations, schizotypy-related regional MTR variations show associations with genes involved in neuronal affiliation, astrocytes, and microglia, which are transcriptionally dysregulated in postmortem schizophrenia studies (93). These results indicate that genes involved in neurodevelopment and synaptic function are related to macroscale brain changes in schizophrenia.
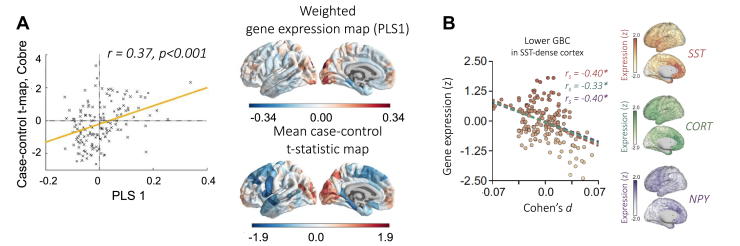
Other work has focused on various aspects of brain connectivity, in line with the dysconnectivity hypothesis of schizophrenia (94,95). Connectivity can be assessed indirectly, using MS networks (96) or structural covariance networks, which quantify inter-regional coupling of morphometric measures such as gray matter volume or cortical thickness (97, 98, 99), or more directly, using diffusion MRI (100,101) [see also (94)]. Regional mean MS differences between cases and controls have been linked to the expression of genes regulating synaptic signaling, nervous system development, and the adenylate cyclase–modulating G protein–coupled receptor signaling pathway that is responsible for changes in the concentration of cAMP (cyclic adenosine monophosphate), a molecule used in intracellular signal transduction (102) (Figure 3A). Moreover, genes that were overexpressed in regions with reduced MS were significantly upregulated in postmortem studies of schizophrenia, suggesting a tight coupling between ex vivo measures and the transcriptional correlates of IDPs measured in vivo. Regional white matter dysconnectivity in schizophrenia, quantified with diffusion MRI, was significantly correlated with the spatial profile of schizophrenia-related genes identified through the same GWAS (38), providing a direct link between structural risk variants, gene expression, and macroscale alterations of connectivity in the disorder. Notably, connectivity changes in bipolar disorder were associated with the expression of risk genes implicated in bipolar disorder, but not schizophrenia, suggesting some level of diagnostic specificity to these associations. There is also some evidence that these genes are specifically linked to diffusion MRI IDPs, given that they have shown only weak associations with other measures (103).
Figure 3.
Transcriptional correlates of brain changes in neuropsychiatric disorders. (A) Gene expression profiles are associated with morphometric similarity differences between psychosis cases and controls. Cortical maps represent regional PLS1 scores (right) and case-control differences in morphometric similarity. (B) Associations between the cortical gene expression of SST interneurons, CORT, and NPY and reduced GBC in depression. The scatter plot represents the relationship for SST genes; regression lines represent relationships for all 3 gene markers. Panel (A) adapted with permission from (102). Panel (B) adapted with permission from (105). CORT, cortistatin; GBC, global brain connectivity; NPY, neuropeptide Y; PC1, first principal component; PLS, partial least squares; SST, somatostatin.
Studies of major depressive disorder have consistently identified links between IDPs and the spatial expression pattern of genes that are also transcriptionally dysregulated in postmortem studies. Li et al. (104) used multivariate analysis to identify linear combinations of almost 3000 genes enriched in synapse-related terms and other neurobiologically relevant categories that were related to regional case-control differences in MS. Thirty-four of those genes were also transcriptionally dysregulated in postmortem tissues, mirroring previous findings (93,105). These associations were specific to depression and not evident for genes dysregulated in other psychiatric disorders (104). Functional MRI analysis has revealed that temporal fluctuations in regional signal homogeneity are linked to the expression of several neurobiological gene categories that include synaptic signaling, metabolism, central nervous system development, and neurodegenerative disease (106). Hypotheses including interneuron and glial dysfunction, changes in glutamatergic signaling, and broader dysregulation in apoptotic stress and neuroinflammation-related pathways have been proposed to underlie neurobiological substrates of depression (107, 108, 109). In particular, dysfunction of cortical interneurons and astrocytes, which express the neuropeptide somatostatin, have been suggested to affect the disease onset (107,109,110). One study identified spatial correlations between transcriptomic markers of somatostatin interneurons and regional variations in resting-state functional amplitude, mean regional strength of functional connectivity, and cortical thickness linked to a history of depression and negative affect across 3 large-scale neuroimaging cohorts, supporting a role for cortical somatostatin interneurons in depression-related brain changes (105) (Figure 3B). Moreover, these depression-related changes in anatomical and functional maps were correlated with the spatial expression pattern of genes downregulated in postmortem specimens from patients. Such associations were not found for other psychiatric disorders, again supporting the specificity of these links between imaging and transcriptional measures (105).
In summary, across different psychiatric disorders and imaging modalities, genes that are spatially correlated with disorder-related changes in brain structure or function tend to overlap with genes that are transcriptionally dysregulated in postmortem samples derived from clinical populations and/or genes implicated in disease risk by large-scale GWASs. While there is evidence of diagnostic specificity in some of these associations, some transcriptional correlates are consistent across disorders and may reflect common genetic influences on disease risk (88).
Neurodegeneration and Associated Disorders
Neurodegenerative diseases, such as Huntington’s disease (HD), Alzheimer’s disease (AD), and Parkinson’s disease (PD), are associated with characteristic neurodegenerative patterns (111, 112, 113) that are linked to the accumulation of particular neurochemical agents such as amyloid-β protein and tau in AD and α-synuclein in PD (111). Transcriptional atlas data can be used to identify the molecular correlates of regional vulnerability to disorder-specific neurodegeneration and help understand how the disease spreads across the brain. For instance, HD is caused by a mutation in the single gene, HTT (114), but imaging transcriptomics has begun to shed light on other potential molecular pathways related to brainwide dysfunction in the disorder, given evidence that degeneration of corticostriatal and interhemispheric connectivity in premanifest HD has been associated with the spatial expression patterns of genes related to chemical synaptic transmission and cell projections, with the genes most strongly linked to the IDPs also being transcriptionally dysregulated in postmortem studies of patients (115).
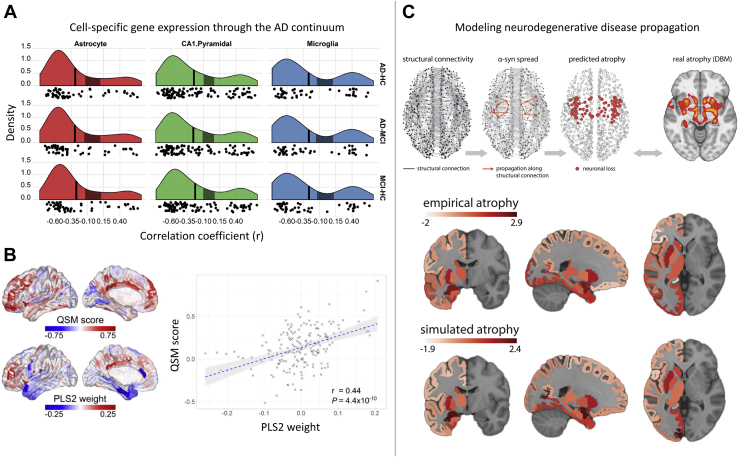
In comparison with HD, the genetic mechanisms underlying risk for AD are more complex (116). Cell-type specific marker analyses indicate that decreases in cortical thickness in patients with AD compared with that in healthy subjects are linked to the expression of the same cell-specific gene groups that are involved in both age-related changes in cortical thickness and psychiatric disorders, namely, CA1 pyramidal cells, microglia, and astrocytes (Figure 4A). Moreover, genes that were coexpressed with microglial genes were significantly enriched for AD risk, providing a direct link to the etiology of the disease and implying a critical role for neuroinflammation (81). One study first identified spatial clusters of voxels that showed reduced volume in people carrying a higher number of AD risk alleles (117). These reductions were then investigated in relation to the expression patterns of genes that are in close proximity to AD risk loci, resulting in a set of 216 genes. Although only 5 of those genes showed significant associations with the volumetric reductions, 3 of them (MEF2C, CLU, and SLC24A4) were previously identified as AD risk genes, linking the spatial patterning of gene expression back to structural risk variants.
Figure 4.
Gene expression is related to neurodegeneration and neurodegenerative disease spread. (A) Cell-specific marker association with cortical thinning in AD. Correlation distributions quantifying the relationship between cell-specific gene expression profiles for CA1 pyramidal, microglia, and astrocyte cell types and cortical thickness reductions in AD (patients with AD vs. HCs; AD vs. MCI; MCI vs. HC). Vertical axis denotes estimated probability density for the correlation coefficients. Vertical black line denotes the average correlation coefficient across all genes; shaded gray box indicates the 95% limits of the empirical null distribution. (B) Gene expression is associated with cortical iron decomposition, quantified using QSM, in Parkinson’s disease. Cortical maps represent QSM scores and regional linearly weighted sum of gene expression scores defined by PLS2. (C) Schematic representation of pathology spread across the brain: misfolded α-synuclein propagates via structural connections; simulated neuronal loss (atrophy) is compared against empirical atrophy, estimated from patients with Parkinson’s disease. Simulated and empirical atrophy patterns show a high level of spatial correspondence (Spearman correlation, r = 0.63, p = 2 × 10−5). Panel (A) adapted with permission from (81). Panel (B) adapted with permission from (121). Panel (C) adapted with permission from (132). α-syn, α-synuclein; AD, Alzheimer’s disease; CA, cornu ammonis; DBM, deformation-based morphometry; HC, healthy control; MCI, mild cognitive impairment; PLS, partial least squares; QSM, quantitative susceptibility mapping.
Type 2 diabetes (T2D) is associated with increased risk for AD (118). Nugent et al. (119) investigated the expression of genes that are close to 46 T2D risk single nucleotide polymorphisms and found that the spatial expression patterns of just 5 of these genes could explain more than 70% of variance in regional glucose metabolism uptake in both healthy and probable AD groups, thus demonstrating a link between risk factors for T2D and glucose metabolism in both normal aging and AD. Similar findings were demonstrated in PD, in which regional expression of only 17 genes that were previously implicated in the disease explained 42% of variance in regional disease–related atrophy patterns (120). Regional gene expression could also explain 20% of variance in quantitative susceptibility mapping measures, which index iron deposition (121) (Figure 4B). Genes associated with iron deposition were transcriptionally dysregulated in postmortem specimens of patients with PD, PD with dementia, and dementia with Lewy bodies, pointing to common mechanisms that drive regional susceptibility to neurodegeneration across diagnostic categories.
Other hypothesis-driven work has supported the general involvement of the tau protein in different neurodegenerative conditions. Regional expression of the MAPT gene, which codes for the tau protein, is associated with the severity of neurodegeneration in AD (122) and has been linked to reduced functional connectivity in patients with PD (123). At the same time, the expression of APP, a gene that encodes amyloid precursor protein, was associated with regional amyloid burden but not neurodegeneration (122). Together, the results from these 2 studies suggest that the spread of neurodegeneration in both AD and PD is primarily mediated by tau pathology.
Multiple lines of evidence suggest that neurodegeneration across disorders originates in a relatively small number of susceptible regions and then propagates along axonal pathways (124, 125, 126, 127). These spreading processes can be modeled mathematically using various diffusion models simulated on an underlying brain-network architecture (128, 129, 130). One such model was used to predict the pathological seed regions and pattern of disease spread in PD and then test the assumption that transcriptional profiles of 67 PD risk genes derived from a GWAS are associated with disease origination and progression (131). The results indicated that the expression patterns of those genes are related to the likelihood of a region being identified as the initiation site for the pathology and are related to early disease spread, but not the resulting regional atrophy pattern. More specifically, the expression of several immune-related (HLA-DQA1, HLA-DRB6, IL1R2) and lysosomal (GBA, TMEM175) PD risk genes was most strongly associated with seed regions consistent with a causal role of lysosomal dysfunction and neuroinflammation in PD pathogenesis (131). Another study proposed an agent-based S-I-R model in which the agents are individual proteins that spread to affect different regions in the network, leading to regions having the status of either susceptible (S, yet to be infected), infected (I, capable of transmitting the infection), or removed (R, no longer active in disease spread), as shown in Figure 4C (132). Incorporating the regional expression of SNCA and GBA genes, which are involved in the synthesis and clearance of α-synuclein, respectively, into the model improved the correspondence between modeled and empirically observed atrophy patterns in PD (132). Extending this agent-based model to include a factor responsible for the transmission probability of pathological agents, by incorporating the expression patterns of the LRRK2 gene implicated in the development of PD through the transmembrane transport, further improved model accuracy, supporting a role of LRRK2 in modulating the propagation of misfolded proteins (133). Transcriptional atlas data thus provide a principled way of constraining and parameterizing models of dynamic processes in both health and disease [see also (134)].
Together, studies of neurodegeneration highlight the role of cell-specific and disorder-related genes in regional vulnerability to neurodegeneration. Specifically, the involvement of CA1 pyramidal cells, microglia, and astrocytes is evident across the life span, suggesting common mechanisms associated with healthy development, aging, and clinical neurodegeneration. Differences in regional vulnerability to neurodegeneration and subsequent spread of the disease are also linked to the expression patterns of genes involved in disease pathology. The integration of transcriptional atlas data with models of network dynamics appears to be a particularly fruitful avenue for testing different hypotheses about disease evolution.
Challenges for the Field
The findings considered here highlight the potential of imaging transcriptomics to identify the molecular correlates of clinically relevant IDPs. However, the field is nascent, and several methodological challenges must be overcome to ensure the robustness of the findings. One such challenge is that the transcriptional atlas data often require a degree of processing before they are ready for analysis. The processing involves several steps, including mapping of tissue samples to regions of interest defined using a specified parcellation, selecting representative gene probes to quantify the expression of a gene, and accounting for gene expression differences between donor brains. Some of these steps entail choices that can alter resulting spatial expression maps and affect the final results (50,60,61). Just as variations in the processing of neuroimaging data can affect final outcomes (135, 136, 137, 138, 139), different choices in the processing of expression data can influence one’s findings. Some of these choices have been investigated in detail, leading to the proposal of recommended workflows and open-source toolboxes (60,61).
A second challenge is related to the intrinsic spatial autocorrelation of both gene expression and neuroimaging data. This autocorrelation is such that regions that are in close proximity to each other often exhibit more similar gene expression profiles and IDP values (46,47,60,140,141). Spatially autocorrelated patterns are prominent across species, including in mouse (41,47) and human (42,60,142, 143, 144), with gene expression correlations decaying approximately exponentially with separation distance. Moreover, the correlation length scales with brain size across mouse development (47). The spatial autocorrelation of gene expression patterns results in nonindependent spatial samples and thus requires more careful statistical analysis than applying traditional statistical methods that assume independence. Accordingly, studies that have used parametric statistical methods or randomization approaches that do not preserve the intrinsic spatial autocorrelation of the data will yield lenient estimates of statistical significance and inflate the false positive rates (53). Alternative null models, such as permutation-based procedures that preserve the spatial structure of the data (48,51), model-based techniques that generate surrogate data that preserve spatial autocorrelation structure (46,49), or regression-based measures that account for distance effects (41) are required for valid significance testing between spatial maps. Notably, the performance of different spatially constrained randomization techniques can depend on the research context (53).
A third challenge relates to characterizing the specificity of any identified associations. Certain categories of genes may be more likely to be identified as significantly enriched because they have a spatial expression pattern that is conserved across individuals. Although analyses of the AHBA indicate that the variation in gene expression across regions is greater than across individuals (28), the limited number of specimens comprising the AHBA precludes any opportunity for a detailed study of individual differences in gene expression. Thus, while imaging transcriptomics can offer a first approximation of molecular correlates of anatomically varying IDPs, further investigation is required to understand how such correlates vary across individual brains. The specificity of findings in hypothesis-driven studies should also be appropriately addressed to ensure that any observed associations are not a by-product of correlations with other genes. At a minimum, gene specificity should be examined relative to randomly selected subsets of random genes of the same size as the hypothesized set (52). One recent study has demonstrated that even among the associations (identified through linear regression) that survive spatial null hypothesis testing, 42% are not specific to selected genes but can be found for a wide range of random genes, and only 5% of associations could surpass both spatial and random gene nulls, suggesting that gene and spatial specificity should be evaluated in tandem for appropriate significance testing (52). Moreover, other properties of spatial gene expression, such as their tissue specificity and the strength of coexpression between genes in a tested set, need to be considered to provide a null distribution that is adequate for evaluating the specificity of the relationships between IDPs and brain-specific variations in gene expression (52,59).
A fourth challenge concerns statistical inference in GCEA. Traditional GCEA workflows were developed for the investigation of individual tissue samples or structural DNA variation and were not designed to deal with spatially embedded atlas data. The approaches have nonetheless been used in virtually all enrichment analyses in imaging transcriptomics studies thus far. Under the traditional workflow, the statistical significance of a gene category is computed with respect to a null distribution generated by randomizing gene-to-category assignments. Fulcher et al. (59) showed that this approach, when used to test for enrichment of correlations with random brainwide phenotypic maps, can lead to a >500-fold average inflation in the false positive rate across all GO categories. The false positive bias is largely driven by gene-gene coexpression within a category, such that categories containing genes with more consistent expression patterns are more susceptible to this bias. This effect is additionally modulated by gene-specific variations in spatial autocorrelation properties. These issues raise concerns regarding the validity of many enrichment analyses that apply GCEA to transcriptional atlas data, considering that GO categories with the highest false positive rates tend to be related to brain function and, as shown by Fulcher et al. (59), are among the most frequently reported in the literature. One way to address this problem is to randomize the IDP rather than the gene-expression data. Depending on the hypothesis, it may be appropriate to randomize the IDP in a way that accounts for its intrinsic spatial autocorrelation [e.g., (49,53)]. With this approach, the number of significantly enriched categories observed for several neural phenotypes measured in human and mouse dropped considerably when compared with the standard implementation; for example, 9 of 14 GCEA analyses performed by Fulcher et al. (59) yielded significantly enriched GO categories under the conventional null, whereas only 1 analysis revealed significant enrichment under a random phenotype null, and none under a null using random spatially autocorrelated phenotypes null. These results highlight the importance of performing enrichment analyses with respect to carefully designed null models and indicate the need for caution when interpreting GCEA reports in imaging transcriptomics.
Conclusions
Imaging transcriptomics has been applied in diverse clinical settings across the lifespan. IDPs of neurodevelopmental conditions have been linked to gene groups involved in cell death and neuronal communication or those known to play a causal role in the disorder. For disorders with later onset, specific transcriptional correlates of IDPs are related to transcriptionally dysregulated genes in clinical postmortem samples and genes implicated in disease risk by GWASs. Transcriptional correlates of connectivity-related phenotypes show some evidence of disorder specificity, whereas those related to cortical thickness may be disorder general. In neurodegeneration, the expression of pathology-related genes for specific diseases is related to regional vulnerability and spread of the disease.
As the field develops, we anticipate that the refinement of existing methodologies will enable more robust and rigorous inference. Functional genomics data derived from consortia initiatives such as PsychENCODE have already provided valuable information that can be incorporated into imaging transcriptomics research, allowing the identification of brain-expressed genes and cell-specific expression profiles (145). Rapid developments in single-cell RNA sequencing have already produced single-cell transcriptomic atlases of early human brain development (146,147) and later life (148) with increasing spatial coverage. Comparative transcriptomics datasets further extend the scope for cross-species investigations identifying conserved and human-specific transcriptional signatures (149). When coupled with increasingly more comprehensive and precise transcriptional atlas data, measured across more individuals and multiple points across the life span, these methods will offer a powerful framework for identifying the molecular correlates of disease-related brain changes observed in vivo and thus for linking microscale models of disease pathophysiology with macroscale measures of brain dysfunction.
Acknowledgments and Disclosures
This study was supported by the National Health and Medical Research Council of Australia (Grant Nos. 1146292 and 1197431 [to AF]), Sylvia and Charles Viertel Charitable Foundation, and a National Health and Medical Research Council of Australia Senior Research Fellowship (to MAB).
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Arnatkeviciute A., Fulcher B.D., Bellgrove M.A., Fornito A. Where the genome meets the connectome: Understanding how genes shape human brain connectivity. Neuroimage. 2021;244:118570. doi: 10.1016/j.neuroimage.2021.118570. [DOI] [PubMed] [Google Scholar]
- 2.Bigos K.L., Weinberger D.R. Imaging genetics—Days of future past. Neuroimage. 2010;53:804–809. doi: 10.1016/j.neuroimage.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 3.Fornito A., Arnatkevičiūtė A., Fulcher B.D. Bridging the gap between connectome and transcriptome. Trends Cogn Sci. 2019;23:34–50. doi: 10.1016/j.tics.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto R., Ohi K., Yamamori H., Yasuda Y., Fujimoto M., Umeda-Yano S., et al. Imaging genetics and psychiatric disorders. Curr Mol Med. 2015;15:168–175. doi: 10.2174/1566524015666150303104159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson P.M., Cannon T.D., Narr K.L., van Erp T., Poutanen V.P., Huttunen M., et al. Genetic influences on brain structure. Nat Neurosci. 2001;4:1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- 6.Thompson P.M., Ge T., Glahn D.C., Jahanshad N., Nichols T.E. Genetics of the connectome. Neuroimage. 2013;80:475–488. doi: 10.1016/j.neuroimage.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnatkeviciute A., Fulcher B.D., Bellgrove M.A., Fornito A. Where the genome meets the connectome: Understanding how genes shape human brain connectivity. Neuroimage. 2021;244:118570. doi: 10.1016/j.neuroimage.2021.118570. [DOI] [PubMed] [Google Scholar]
- 8.Arnatkeviciute A., Fulcher B.D., Oldham S., Tiego J., Paquola C., Gerring Z., et al. Genetic influences on hub connectivity of the human connectome. Nat Commun. 2021;12:4237. doi: 10.1038/s41467-021-24306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baaré W.F.C., Hulshoff Pol H.E., Boomsma D.I., Posthuma D., de Geus E.J., Schnack H.G., et al. Quantitative genetic modeling of variation in human brain morphology. Cereb Cortex. 2001;11:816–824. doi: 10.1093/cercor/11.9.816. [DOI] [PubMed] [Google Scholar]
- 10.Ge T., Holmes A.J., Buckner R.L., Smoller J.W., Sabuncu M.R. Heritability analysis with repeat measurements and its application to resting-state functional connectivity. Proc Natl Acad Sci U S A. 2017;114:5521–5526. doi: 10.1073/pnas.1700765114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pizzagalli F., Auzias G., Yang Q., Mathias S.R., Faskowitz J., Boyd J.D., et al. The reliability and heritability of cortical folds and their genetic correlations across hemispheres. Commun Biol. 2020;3:510. doi: 10.1038/s42003-020-01163-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen K.K., Rose S., Fripp J., McMahon K.L., de Zubicaray G.I., Martin N.G., et al. Investigating brain connectivity heritability in a twin study using diffusion imaging data. Neuroimage. 2014;100:628–641. doi: 10.1016/j.neuroimage.2014.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elliott L.T., Sharp K., Alfaro-Almagro F., Shi S., Miller K.L., Douaud G., et al. Genome-wide association studies of brain imaging phenotypes in UK Biobank. Nature. 2018;562:210–216. doi: 10.1038/s41586-018-0571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hibar D.P., Stein J.L., Renteria M.E., Arias-Vasquez A., Desrivières S., Jahanshad N., et al. Common genetic variants influence human subcortical brain structures. Nature. 2015;520:224–229. doi: 10.1038/nature14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith S.M., Douaud G., Chen W., Hanayik T., Alfaro-Almagro F., Sharp K., Elliott L.T. An expanded set of genome-wide association studies of brain imaging phenotypes in UK Biobank. Nat Neurosci. 2021;24:737–745. doi: 10.1038/s41593-021-00826-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao B., Luo T., Li T., Li Y., Zhang J., Shan Y., et al. Genome-wide association analysis of 19,629 individuals identifies variants influencing regional brain volumes and refines their genetic co-architecture with cognitive and mental health traits. Nat Genet. 2019;51:1637–1644. doi: 10.1038/s41588-019-0516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hibar D.P., Adams H.H.H., Jahanshad N., Chauhan G., Stein J.L., Hofer E., et al. Novel genetic loci associated with hippocampal volume. Nat Commun. 2017;8:13624. doi: 10.1038/ncomms13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satizabal C.L., Adams H.H.H., Hibar D.P., White C.C., Knol M.J., Stein J.L., et al. Genetic architecture of subcortical brain structures in 38,851 individuals. Nat Genet. 2019;51:1624–1636. doi: 10.1038/s41588-019-0511-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi J.K., Kim S.C. Environmental effects on gene expression phenotype have regional biases in the human genome. Genetics. 2007;175:1607–1613. doi: 10.1534/genetics.106.069047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cole S.W. Social regulation of human gene expression. Curr Dir Psychol Sci. 2009;18:132–137. doi: 10.1111/j.1467-8721.2009.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fraser H.B., Khaitovich P., Plotkin J.B., Pääbo S., Eisen M.B. Aging and gene expression in the primate brain. PLoS Biol. 2005;3:e274. doi: 10.1371/journal.pbio.0030274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawrylycz M.J., Lein E.S., Guillozet-Bongaarts A.L., Shen E.H., Ng L., Miller J.A., et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–399. doi: 10.1038/nature11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lein E.S., Hawrylycz M.J., Ao N., Ayres M., Bensinger A., Bernard A., et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 24.Schulze A., Downward J. Navigating gene expression using microarrays—A technology review. Nat Cell Biol. 2001;3:E190–E195. doi: 10.1038/35087138. [DOI] [PubMed] [Google Scholar]
- 25.Kang H.J., Kawasawa Y.I., Cheng F., Zhu Y., Xu X., Li M., et al. Spatio-temporal transcriptome of the human brain. Nature. 2011;478:483–489. doi: 10.1038/nature10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sousa A.M.M., Zhu Y., Raghanti M.A., Kitchen R.R., Onorati M., Tebbenkamp A.T.N., et al. Molecular and cellular reorganization of neural circuits in the human lineage. Science. 2017;358:1027–1032. doi: 10.1126/science.aan3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keil J.M., Qalieh A., Kwan K.Y. Brain transcriptome databases: A user’s guide. J Neurosci. 2018;38:2399–2412. doi: 10.1523/JNEUROSCI.1930-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawrylycz M., Miller J.A., Menon V., Feng D., Dolbeare T., Guillozet-Bongaarts A.L., et al. Canonical genetic signatures of the adult human brain. Nat Neurosci. 2015;18:1832–1844. doi: 10.1038/nn.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanherkar R.R., Bhatia-Dey N., Csoka A.B. Epigenetics across the human lifespan. Front Cell Dev Biol. 2014;2:49. doi: 10.3389/fcell.2014.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goyal M.S., Hawrylycz M., Miller J.A., Snyder A.Z., Raichle M.E. Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell Metab. 2014;19:49–57. doi: 10.1016/j.cmet.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Işıldak U., Somel M., Thornton J.M., Dönertaş H.M. Temporal changes in the gene expression heterogeneity during brain development and aging. Sci Rep. 2020;10:4080. doi: 10.1038/s41598-020-60998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller J.A., Ding S.L., Sunkin S.M., Smith K.A., Ng L., Szafer A., et al. Transcriptional landscape of the prenatal human brain. Nature. 2014;508:199–206. doi: 10.1038/nature13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris K.D., Hochgerner H., Skene N.G., Magno L., Katona L., Bengtsson Gonzales C., et al. Classes and continua of hippocampal CA1 inhibitory neurons revealed by single-cell transcriptomics. PLoS Biol. 2018;16 doi: 10.1371/journal.pbio.2006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lake B.B., Ai R., Kaeser G.E., Salathia N.S., Yung Y.C., Liu R., et al. Neuronal subtypes and diversity revealed by single-nucleus RNA sequencing of the human brain. Science. 2016;352:1586–1590. doi: 10.1126/science.aaf1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao Z., van Velthoven C.T.J., Nguyen T.N., Goldy J., Sedeno-Cortes A.E., Baftizadeh F., et al. A taxonomy of transcriptomic cell types across the isocortex and hippocampal formation. Cell. 2021;184:3222–3241.e26. doi: 10.1016/j.cell.2021.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenbaum D., Colangelo C., Williams K., Gerstein M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003;4:117. doi: 10.1186/gb-2003-4-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwanhäusser B., Busse D., Li N., Dittmar G., Schuchhardt J., Wolf J., et al. Global quantification of mammalian gene expression control. Nature. 2011;473:337–342. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- 38.Romme I.A.C., de Reus M.A., Ophoff R.A., Kahn R.S., van den Heuvel M.P. Connectome disconnectivity and cortical gene expression in patients with schizophrenia. Biol Psychiatry. 2017;81:495–502. doi: 10.1016/j.biopsych.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 39.Seidlitz J., Nadig A., Liu S., Bethlehem R.A.I., Vértes P.E., Morgan S.E., et al. Transcriptomic and cellular decoding of regional brain vulnerability to neurogenetic disorders. Nat Commun. 2020;11:3358. doi: 10.1038/s41467-020-17051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnatkevičiūtė A., Fulcher B.D., Pocock R., Fornito A. Hub connectivity, neuronal diversity, and gene expression in the Caenorhabditis elegans connectome. PLoS Comput Biol. 2018;14 doi: 10.1371/journal.pcbi.1005989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fulcher B.D., Fornito A. A transcriptional signature of hub connectivity in the mouse connectome. Proc Natl Acad Sci U S A. 2016;113:1435–1440. doi: 10.1073/pnas.1513302113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richiardi J., Altmann A., Milazzo A-C., Chang C., Chakravarty M.M., Banaschewski T., et al. Correlated gene expression supports synchronous activity in brain networks. Science. 2015;348:1241–1244. doi: 10.1126/science.1255905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Forest M., Iturria-Medina Y., Goldman J.S., Kleinman C.L., Lovato A., Oros Klein K., et al. Gene networks show associations with seed region connectivity. Hum Brain Mapp. 2017;38:3126–3140. doi: 10.1002/hbm.23579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oldham M.C., Konopka G., Iwamoto K., Langfelder P., Kato T., Horvath S., Geschwind D.H. Functional organization of the transcriptome in human brain. Nat Neurosci. 2008;11(Art. No. 11) doi: 10.1038/nn.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romero-Garcia R., Whitaker K.J., Váša F., Seidlitz J., Shinn M., Fonagy P., et al. Structural covariance networks are coupled to expression of genes enriched in supragranular layers of the human cortex. Neuroimage. 2018;171:256–267. doi: 10.1016/j.neuroimage.2017.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burt J.B., Demirtaş M., Eckner W.J., Navejar N.M., Ji J.L., Martin W.J., et al. Hierarchy of transcriptomic specialization across human cortex captured by structural neuroimaging topography. Nat Neurosci. 2018;21:1251–1259. doi: 10.1038/s41593-018-0195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lau H.Y.G., Fornito A., Fulcher B.D. Scaling of gene transcriptional gradients with brain size across mouse development. Neuroimage. 2021;224:117395. doi: 10.1016/j.neuroimage.2020.117395. [DOI] [PubMed] [Google Scholar]
- 48.Alexander-Bloch A.F., Mathias S.R., Fox P.T., Olvera R.L., Göring H.H.H., Duggirala R., et al. Human cortical thickness organized into genetically determined communities across spatial resolutions. Cereb Cortex. 2019;29:106–118. doi: 10.1093/cercor/bhx309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burt J.B., Helmer M., Shinn M., Anticevic A., Murray J.D. Generative modeling of brain maps with spatial autocorrelation. Neuroimage. 2020;220:117038. doi: 10.1016/j.neuroimage.2020.117038. [DOI] [PubMed] [Google Scholar]
- 50.Selvaggi P., Rizzo G., Mehta M.A., Turkheimer F.E., Veronese M. Integration of human whole-brain transcriptome and neuroimaging data: Practical considerations of current available methods. J Neurosci Methods. 2021;355:109128. doi: 10.1016/j.jneumeth.2021.109128. [DOI] [PubMed] [Google Scholar]
- 51.Váša F., Seidlitz J., Romero-Garcia R., Whitaker K.J., Rosenthal G., Vértes P.E., et al. Adolescent tuning of association cortex in human structural brain networks. Cereb Cortex. 2018;28:281–294. doi: 10.1093/cercor/bhx249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei Y., de Lange S.C., Pijnenburg R., Scholtens L.H., Ardesch D.J., Watanabe K., et al. Statistical testing in transcriptomic-neuroimaging studies: A how-to and evaluation of methods assessing spatial and gene specificity. Hum Brain Mapp. 2022;43:885–901. doi: 10.1002/hbm.25711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Markello R.D., Misic B. Comparing spatial null models for brain maps. NeuroImage. 2021;236:118052. doi: 10.1016/j.neuroimage.2021.118052. [DOI] [PubMed] [Google Scholar]
- 54.Hansen J.Y., Markello R.D., Vogel J.W., Seidlitz J., Bzdok D., Misic B. Mapping gene transcription and neurocognition across human neocortex. Nat Hum Behav. 2021;5:1240–1250. doi: 10.1038/s41562-021-01082-z. [DOI] [PubMed] [Google Scholar]
- 55.Whitaker K.J., Vértes P.E., Romero-Garcia R., Váša F., Moutoussis M., Prabhu G., et al. Adolescence is associated with genomically patterned consolidation of the hubs of the human brain connectome. Proc Natl Acad Sci U S A. 2016;113:9105–9110. doi: 10.1073/pnas.1601745113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ashburner M., Ball C.A., Blake J.A., Botstein D., Butler H., Cherry J.M., et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanehisa M., Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fulcher B.D., Arnatkeviciute A., Fornito A. Overcoming false-positive gene-category enrichment in the analysis of spatially resolved transcriptomic brain atlas data. Nat Commun. 2021;12:2669. doi: 10.1038/s41467-021-22862-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arnatkeviciute A., Fulcher B.D., Fornito A. A practical guide to linking brain-wide gene expression and neuroimaging data. Neuroimage. 2019;189:353–367. doi: 10.1016/j.neuroimage.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 61.Markello R.D., Arnatkevičiūtė A., Poline J.-B., Fulcher B.D., Fornito A., Misic B. Standardizing workflows in imaging transcriptomics with the abagen toolbox. Elife. 2021;10:e72129. doi: 10.7554/eLife.72129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Giedd J.N., Rapoport J.L. Structural MRI of pediatric brain development: What have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gilmore J.H., Knickmeyer R.C., Gao W. Imaging structural and functional brain development in early childhood. Nat Rev Neurosci. 2018;19:123–137. doi: 10.1038/nrn.2018.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Knickmeyer R.C., Gouttard S., Kang C., Evans D., Wilber K., Smith J.K., et al. A structural MRI study of human brain development from birth to 2 years. J Neurosci. 2008;28:12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amlien I.K., Fjell A.M., Tamnes C.K., Grydeland H., Krogsrud S.K., Chaplin T.A., et al. Organizing principles of human cortical development—Thickness and area from 4 to 30 years: Insights from comparative primate neuroanatomy. Cereb Cortex. 2016;26:257–267. doi: 10.1093/cercor/bhu214. [DOI] [PubMed] [Google Scholar]
- 66.Tamnes C.K., Herting M.M., Goddings A.L., Meuwese R., Blakemore S.J., Dahl R.E., et al. Development of the cerebral cortex across adolescence: A multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. J Neurosci. 2017;37:3402–3412. doi: 10.1523/JNEUROSCI.3302-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khundrakpam B.S., Lewis J.D., Kostopoulos P., Carbonell F., Evans A.C. Cortical thickness abnormalities in autism spectrum disorders through late childhood, adolescence, and adulthood: A large-scale MRI study. Cereb Cortex. 2017;27:1721–1731. doi: 10.1093/cercor/bhx038. [DOI] [PubMed] [Google Scholar]
- 68.Niu M., Wang Y., Jia Y., Wang J., Zhong S., Lin J., et al. Common and specific abnormalities in cortical thickness in patients with major depressive and bipolar disorders. EBiomedicine. 2017;16:162–171. doi: 10.1016/j.ebiom.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suh J.S., Schneider M.A., Minuzzi L., MacQueen G.M., Strother S.C., Kennedy S.H., Frey B.N. Cortical thickness in major depressive disorder: A systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2019;88:287–302. doi: 10.1016/j.pnpbp.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 70.van Haren N.E.M., Schnack H.G., Cahn W., van den Heuvel M.P., Lepage C., Collins L., et al. Changes in cortical thickness during the course of illness in schizophrenia. Arch Gen Psychiatry. 2011;68:871–880. doi: 10.1001/archgenpsychiatry.2011.88. [DOI] [PubMed] [Google Scholar]
- 71.Gogtay N., Vyas N.S., Testa R., Wood S.J., Pantelis C. Age of onset of schizophrenia: Perspectives from structural neuroimaging studies. Schizophr Bull. 2011;37:504–513. doi: 10.1093/schbul/sbr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Häfner H., Maurer K., Löffler W., Fätkenheuer B., an der Heiden W., Riecher-Rössler A., et al. The epidemiology of early schizophrenia. Influence of age and gender on onset and early course. Br J Psychiatry Suppl. 1994;23:29–38. [PubMed] [Google Scholar]
- 73.Natu V.S., Gomez J., Barnett M., Jeska B., Kirilina E., Jaeger C., et al. Apparent thinning of human visual cortex during childhood is associated with myelination. Proc Natl Acad Sci U S A. 2019;116:20750–20759. doi: 10.1073/pnas.1904931116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mancarci B.O., Toker L., Tripathy S.J., Li B., Rocco B., Sibille E., Pavlidis P. Cross-laboratory analysis of brain cell type transcriptomes with applications to interpretation of bulk tissue data. eNeuro. 2017;4 doi: 10.1523/ENEURO.0212-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zeisel A., Muñoz-Manchado A.B., Codeluppi S., Lönnerberg P., La Manno G., Juréus A., et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015;347:1138–1142. doi: 10.1126/science.aaa1934. [DOI] [PubMed] [Google Scholar]
- 76.Fulcher B.D. Discovering conserved properties of brain organization through multimodal integration and interspecies comparison. J Exp Neurosci. 2019;13 doi: 10.1177/1179069519862047. 1179069519862047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schmierer K., Scaravilli F., Altmann D.R., Barker G.J., Miller D.H. Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann Neurol. 2004;56:407–415. doi: 10.1002/ana.20202. [DOI] [PubMed] [Google Scholar]
- 78.Patel Y., Shin J., Gowland P.A., Pausova Z., Paus T., IMAGEN consortium Maturation of the human cerebral cortex during adolescence: Myelin or dendritic arbor? Cereb Cortex. 2019;29:3351–3362. doi: 10.1093/cercor/bhy204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paquola C., Bethlehem R.A., Seidlitz J., Wagstyl K., Romero-Garcia R., Whitaker K.J., et al. Shifts in myeloarchitecture characterise adolescent development of cortical gradients. eLife. 2019;8 doi: 10.7554/eLife.50482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shin J., French L., Xu T., Leonard G., Perron M., Pike G.B., et al. Cell-specific gene-expression profiles and cortical thickness in the human brain. Cereb Cortex. 2018;28:3267–3277. doi: 10.1093/cercor/bhx197. [DOI] [PubMed] [Google Scholar]
- 81.Vidal-Pineiro D., Parker N., Shin J., French L., Grydeland H., Jackowski A.P., et al. Cellular correlates of cortical thinning throughout the lifespan. Sci Rep. 2020;10:21803. doi: 10.1038/s41598-020-78471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Parker N., Patel Y., Jackowski A.P., Pan P.M., Salum G.A., Pausova Z., et al. Assessment of neurobiological mechanisms of cortical thinning during childhood and adolescence and their implications for psychiatric disorders. JAMA Psychiatry. 2020;77:1127–1136. doi: 10.1001/jamapsychiatry.2020.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ball G., Seidlitz J., Beare R., Seal M.L. Cortical remodelling in childhood is associated with genes enriched for neurodevelopmental disorders. Neuroimage. 2020;215:116803. doi: 10.1016/j.neuroimage.2020.116803. [DOI] [PubMed] [Google Scholar]
- 84.Hess J.L., Akutagava-Martins G.C., Patak J.D., Glatt S.J., Faraone S.V. Why is there selective subcortical vulnerability in ADHD? Clues from postmortem brain gene expression data. Mol Psychiatry. 2018;23:1787–1793. doi: 10.1038/mp.2017.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hess J.L., Radonjić N.V., Patak J., Glatt S.J., Faraone S.V. Autophagy, apoptosis, and neurodevelopmental genes might underlie selective brain region vulnerability in attention-deficit/hyperactivity disorder. Mol Psychiatry. 2021;26:6643–6654. doi: 10.1038/s41380-020-00974-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Romero-Garcia R., Warrier V., Bullmore E.T., Baron-Cohen S., Bethlehem R.A.I. Synaptic and transcriptionally downregulated genes are associated with cortical thickness differences in autism. Mol Psychiatry. 2019;24:1053–1064. doi: 10.1038/s41380-018-0023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xie Y., Zhang X., Liu F., Qin W., Fu J., Xue K., Yu C. Brain mRNA expression associated with cortical volume alterations in autism spectrum disorder. Cell Rep. 2020;32:108137. doi: 10.1016/j.celrep.2020.108137. [DOI] [PubMed] [Google Scholar]
- 88.Writing Committee for the Attention-Deficit/Hyperactivity Disorder, Autism Spectrum Disorder, Bipolar Disorder, Major Depressive Disorder, Obsessive-Compulsive Disorder, and Schizophrenia ENIGMA Working Groups, et al. Virtual histology of cortical thickness and shared neurobiology in 6 psychiatric disorders. JAMA Psychiatry. 2021;78:47–63. doi: 10.1001/jamapsychiatry.2020.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ji Y., Zhang X., Wang Z., Qin W., Liu H., Xue K., et al. Genes associated with gray matter volume alterations in schizophrenia. Neuroimage. 2021;225:117526. doi: 10.1016/j.neuroimage.2020.117526. [DOI] [PubMed] [Google Scholar]
- 90.Owen M.J., O’Donovan M.C., Thapar A., Craddock N. Neurodevelopmental hypothesis of schizophrenia. Br J Psychiatry. 2011;198:173–175. doi: 10.1192/bjp.bp.110.084384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lewis D.A., Curley A.A., Glausier J.R., Volk D.W. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Anderson K.M., Collins M.A., Chin R., Ge T., Rosenberg M.D., Holmes A.J. Transcriptional and imaging-genetic association of cortical interneurons, brain function, and schizophrenia risk. Nat Commun. 2020;11:2889. doi: 10.1038/s41467-020-16710-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Romero-Garcia R., Seidlitz J., Whitaker K.J., Morgan S.E., Fonagy P., Dolan R.J., et al. Schizotypy-related magnetization of cortex in healthy adolescence is colocated with expression of schizophrenia-related genes. Biol Psychiatry. 2020;88:248–259. doi: 10.1016/j.biopsych.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fornito A., Zalesky A., Pantelis C., Bullmore E.T. Schizophrenia, neuroimaging and connectomics. Neuroimage. 2012;62:2296–2314. doi: 10.1016/j.neuroimage.2011.12.090. [DOI] [PubMed] [Google Scholar]
- 95.Stephan K.E., Friston K.J., Frith C.D. Dysconnection in schizophrenia: From abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seidlitz J., Váša F., Shinn M., Romero-Garcia R., Whitaker K.J., Vértes P.E., et al. Morphometric similarity networks detect microscale cortical organization and predict inter-individual cognitive variation. Neuron. 2018;97:231–247.e7. doi: 10.1016/j.neuron.2017.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alexander-Bloch A.F., Vértes P.E., Stidd R., Lalonde F., Clasen L., Rapoport J., et al. The anatomical distance of functional connections predicts brain network topology in health and schizophrenia. Cereb Cortex. 2013;23:127–138. doi: 10.1093/cercor/bhr388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gong G., He Y., Chen Z.J., Evans A.C. Convergence and divergence of thickness correlations with diffusion connections across the human cerebral cortex. Neuroimage. 2012;59:1239–1248. doi: 10.1016/j.neuroimage.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 99.Goulas A., Uylings H.B.M., Hilgetag C.C. Principles of ipsilateral and contralateral cortico-cortical connectivity in the mouse. Brain Struct Funct. 2017;222:1281–1295. doi: 10.1007/s00429-016-1277-y. [DOI] [PubMed] [Google Scholar]
- 100.van den Heuvel M.P., Mandl R.C.W., Stam C.J., Kahn R.S., Hulshoff Pol H.E.H. Aberrant frontal and temporal complex network structure in schizophrenia: A graph theoretical analysis. J Neurosci. 2010;30:15915–15926. doi: 10.1523/JNEUROSCI.2874-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zalesky A., Fornito A., Seal M.L., Cocchi L., Westin C.F., Bullmore E.T., et al. Disrupted axonal fiber connectivity in schizophrenia. Biol Psychiatry. 2011;69:80–89. doi: 10.1016/j.biopsych.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morgan S.E., Seidlitz J., Whitaker K.J., Romero-Garcia R., Clifton N.E., Scarpazza C., et al. Cortical patterning of abnormal morphometric similarity in psychosis is associated with brain expression of schizophrenia-related genes. Proc Natl Acad Sci U S A. 2019;116:9604–9609. doi: 10.1073/pnas.1820754116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu F., Tian H., Li J., Li S., Zhuo C. Altered voxel-wise gray matter structural brain networks in schizophrenia: Association with brain genetic expression pattern. Brain Imaging Behav. 2019;13:493–502. doi: 10.1007/s11682-018-9880-6. [DOI] [PubMed] [Google Scholar]
- 104.Li J., Seidlitz J., Suckling J., Fan F., Ji G.J., Meng Y., et al. Cortical structural differences in major depressive disorder correlate with cell type-specific transcriptional signatures. Nat Commun. 2021;12:1647. doi: 10.1038/s41467-021-21943-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Anderson K.M., Collins M.A., Kong R., Fang K., Li J., He T., et al. Convergent molecular, cellular, and cortical neuroimaging signatures of major depressive disorder. Proc Natl Acad Sci U S A. 2020;117:25138–25149. doi: 10.1073/pnas.2008004117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xue K., Liang S., Yang B., Zhu D., Xie Y., Qin W., et al. Local dynamic spontaneous brain activity changes in first-episode, treatment-naïve patients with major depressive disorder and their associated gene expression profiles [published online ahead of print Oct 30] Psychol Med. 2020 doi: 10.1017/S0033291720003876. [DOI] [PubMed] [Google Scholar]
- 107.Fee C., Banasr M., Sibille E. Somatostatin-positive gamma-aminobutyric acid interneuron deficits in depression: Cortical microcircuit and therapeutic perspectives. Biol Psychiatry. 2017;82:549–559. doi: 10.1016/j.biopsych.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Murrough J.W., Abdallah C.G., Mathew S.J. Targeting glutamate signalling in depression: Progress and prospects. Nat Rev Drug Discov. 2017;16:472–486. doi: 10.1038/nrd.2017.16. [DOI] [PubMed] [Google Scholar]
- 109.Rajkowska G., Stockmeier C.A. Astrocyte pathology in major depressive disorder: Insights from human postmortem brain tissue. Curr Drug Targets. 2013;14:1225–1236. doi: 10.2174/13894501113149990156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tripp A., Kota R.S., Lewis D.A., Sibille E. Reduced somatostatin in subgenual anterior cingulate cortex in major depression. Neurobiol Dis. 2011;42:116–124. doi: 10.1016/j.nbd.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ballatore C., Lee V.M.-Y., Trojanowski J.Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 112.Johnson E.B., Ziegler G., Penny W., Rees G., Tabrizi S.J., Scahill R.I., Gregory S. Dynamics of cortical degeneration over a decade in Huntington’s disease. Biol Psychiatry. 2021;89:807–816. doi: 10.1016/j.biopsych.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Seeley W.W. Mapping neurodegenerative disease onset and progression. Cold Spring Harb Perspect Biol. 2017;9:a023622. doi: 10.1101/cshperspect.a023622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Myers R.H. Huntington’s disease genetics. NeuroRx. 2004;1:255–262. doi: 10.1602/neurorx.1.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McColgan P., Gregory S., Seunarine K.K., Razi A., Papoutsi M., Johnson E., et al. Brain regions showing white matter loss in Huntington’s disease are enriched for synaptic and metabolic genes. Biol Psychiatry. 2018;83:456–465. doi: 10.1016/j.biopsych.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jansen I.E., Savage J.E., Watanabe K., Bryois J., Williams D.M., Steinberg S., et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat Genet. 2019;51:404–413. doi: 10.1038/s41588-018-0311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Roshchupkin G.V., Adams H.H., van der Lee S.J., Vernooij M.W., van Duijn C.M., Uitterlinden A.G., et al. Fine-mapping the effects of Alzheimer’s disease risk loci on brain morphology. Neurobiol Aging. 2016;48:204–211. doi: 10.1016/j.neurobiolaging.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 118.Lu F.P., Lin K.P., Kuo H.K. Diabetes and the risk of multi-system aging phenotypes: A systematic review and meta-analysis. PLoS One. 2009;4:e4144. doi: 10.1371/journal.pone.0004144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nugent S., Potvin O., Cunnane S.C., Chen T.H., Duchesne S. Associating type 2 diabetes risk factor genes and FDG-PET brain metabolism in normal aging and Alzheimer’s disease. Front Aging Neurosci. 2020;12:580633. doi: 10.3389/fnagi.2020.580633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Freeze B., Acosta D., Pandya S., Zhao Y., Raj A. Regional expression of genes mediating trans-synaptic alpha-synuclein transfer predicts regional atrophy in Parkinson disease. Neuroimage Clin. 2018;18:456–466. doi: 10.1016/j.nicl.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thomas G.E.C., Zarkali A., Ryten M., Shmueli K., Gil-Martinez A.L., Leyland L.A., et al. Regional brain iron and gene expression provide insights into neurodegeneration in Parkinson’s disease. Brain. 2021;144:1787–1798. doi: 10.1093/brain/awab084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Grothe M.J., Sepulcre J., Gonzalez-Escamilla G., Jelistratova I., Schöll M., Hansson O., et al. Molecular properties underlying regional vulnerability to Alzheimer’s disease pathology. Brain. 2018;141:2755–2771. doi: 10.1093/brain/awy189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rittman T., Rubinov M., Vértes P.E., Patel A.X., Ginestet C.E., Ghosh B.C.P., et al. Regional expression of the MAPT gene is associated with loss of hubs in brain networks and cognitive impairment in Parkinson disease and progressive supranuclear palsy. Neurobiol Aging. 2016;48:153–160. doi: 10.1016/j.neurobiolaging.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brettschneider J., Del Tredici K.D., Lee V.M.-Y., Trojanowski J.Q. Spreading of pathology in neurodegenerative diseases: A focus on human studies. Nat Rev Neurosci. 2015;16:109–120. doi: 10.1038/nrn3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Desplats P., Lee H.J., Bae E.J., Patrick C., Rockenstein E., Crews L., et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of α-synuclein. Proc Natl Acad Sci U S A. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fornito A., Zalesky A., Breakspear M. The connectomics of brain disorders. Nat Rev Neurosci. 2015;16:159–172. doi: 10.1038/nrn3901. [DOI] [PubMed] [Google Scholar]
- 127.Jucker M., Walker L.C. Propagation and spread of pathogenic protein assemblies in neurodegenerative diseases. Nat Neurosci. 2018;21:1341–1349. doi: 10.1038/s41593-018-0238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cornblath E.J., Li H.L., Changolkar L., Zhang B., Brown H.J., Gathagan R.J., et al. Computational modeling of tau pathology spread reveals patterns of regional vulnerability and the impact of a genetic risk factor. Sci Adv. 2021;7 doi: 10.1126/sciadv.abg6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pandya S., Zeighami Y., Freeze B., Dadar M., Collins D.L., Dagher A., Raj A. Predictive model of spread of Parkinson’s pathology using network diffusion. Neuroimage. 2019;192:178–194. doi: 10.1016/j.neuroimage.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Raj A., Kuceyeski A., Weiner M. A network diffusion model of disease progression in dementia. Neuron. 2012;73:1204–1215. doi: 10.1016/j.neuron.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Freeze B., Pandya S., Zeighami Y., Raj A. Regional transcriptional architecture of Parkinson’s disease pathogenesis and network spread. Brain. 2019;142:3072–3085. doi: 10.1093/brain/awz223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zheng Y.Q., Zhang Y., Yau Y., Zeighami Y., Larcher K., Misic B., Dagher A. Local vulnerability and global connectivity jointly shape neurodegenerative disease propagation. PLoS Biol. 2019;17 doi: 10.1371/journal.pbio.3000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yan W., Ye C., Wang T., Sun J., Wu T., Ma T. Misfolded protein propagation in an integrated computational model of structural network and LRRK2 gene expression. Annu Int Conf IEEE Eng Med Biol Soc. 2020;2020:2368–2371. doi: 10.1109/EMBC44109.2020.9178266. [DOI] [PubMed] [Google Scholar]
- 134.Deco G., Kringelbach M.L., Arnatkeviciute A., Oldham S., Sabaroedin K., Rogasch N.C., et al. Dynamical consequences of regional heterogeneity in the brain’s transcriptional landscape. Sci Adv. 2021;7 doi: 10.1126/sciadv.abf4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Aquino K.M., Fulcher B.D., Parkes L., Sabaroedin K., Fornito A. Identifying and removing widespread signal deflections from fMRI data: Rethinking the global signal regression problem. Neuroimage. 2020;212:116614. doi: 10.1016/j.neuroimage.2020.116614. [DOI] [PubMed] [Google Scholar]
- 136.Botvinik-Nezer R., Holzmeister F., Camerer C.F., Dreber A., Huber J., Johannesson M., et al. Variability in the analysis of a single neuroimaging dataset by many teams. Nature. 2020;582:84–88. doi: 10.1038/s41586-020-2314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ciric R., Wolf D.H., Power J.D., Roalf D.R., Baum G.L., Ruparel K., et al. Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage. 2017;154:174–187. doi: 10.1016/j.neuroimage.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Oldham S., Arnatkevičiūtė A., Smith R.E., Tiego J., Bellgrove M.A., Fornito A. The efficacy of different preprocessing steps in reducing motion-related confounds in diffusion MRI connectomics. Neuroimage. 2020;222:117252. doi: 10.1016/j.neuroimage.2020.117252. [DOI] [PubMed] [Google Scholar]
- 139.Parkes L., Fulcher B., Yücel M., Fornito A. An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI. Neuroimage. 2018;171:415–436. doi: 10.1016/j.neuroimage.2017.12.073. [DOI] [PubMed] [Google Scholar]
- 140.Roberts J.A., Perry A., Lord A.R., Roberts G., Mitchell P.B., Smith R.E., et al. The contribution of geometry to the human connectome. Neuroimage. 2016;124:379–393. doi: 10.1016/j.neuroimage.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 141.Salvador R., Suckling J., Coleman M.R., Pickard J.D., Menon D., Bullmore E. Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb Cortex. 2005;15:1332–1342. doi: 10.1093/cercor/bhi016. [DOI] [PubMed] [Google Scholar]
- 142.Krienen F.M., Yeo B.T.T., Ge T., Buckner R.L., Sherwood C.C. Transcriptional profiles of supragranular-enriched genes associate with corticocortical network architecture in the human brain. Proc Natl Acad Sci U S A. 2016;113:E469–E478. doi: 10.1073/pnas.1510903113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pantazatos S.P., Li X. Commentary: BRAIN NETWORKS. Correlated gene expression supports synchronous activity in brain networks. Science 348, 1241-1244. Front Neurosci. 2017;11:412. doi: 10.3389/fnins.2017.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Richiardi J., Altmann A., Greicius M. Distance is not everything in imaging genomics of functional networks: Reply to a commentary on Correlated gene expression supports synchronous activity in brain networks. bioRxiv. 2017 doi: 10.1101/132746. [DOI] [Google Scholar]
- 145.Wang D., Liu S., Warrell J., Won H., Shi X., Navarro F.C.P., et al. Comprehensive functional genomic resource and integrative model for the human brain. Science. 2018;362 doi: 10.1126/science.aat8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Eze U.C., Bhaduri A., Haeussler M., Nowakowski T.J., Kriegstein A.R. Single-cell atlas of early human brain development highlights heterogeneity of human neuroepithelial cells and early radial glia. Nat Neurosci. 2021;24:584–594. doi: 10.1038/s41593-020-00794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Polioudakis D., de la Torre-Ubieta L., Langerman J., Elkins A.G., Shi X., Stein J.L., et al. A single-cell transcriptomic atlas of human neocortical development during mid-gestation. Neuron. 2019;103:785–801.e8. doi: 10.1016/j.neuron.2019.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Song L., Pan S., Zhang Z., Jia L., Chen W.H., Zhao X.M. STAB: A spatio-temporal cell atlas of the human brain. Nucleic Acids Res. 2021;49:D1029–D1037. doi: 10.1093/nar/gkaa762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bakken T.E., van Velthoven C.T., Menon V., Hodge R.D., Yao Z., Nguyen T.N., et al. Single-cell and single-nucleus RNA-seq uncovers shared and distinct axes of variation in dorsal LGN neurons in mice, non-human primates, and humans. eLife. 2021;10 doi: 10.7554/eLife.64875. [DOI] [PMC free article] [PubMed] [Google Scholar]