Abstract
Background
Although cerebellar morphological involvement has been increasingly recognized in autism spectrum disorder (ASD) and schizophrenia (SZ), the extent to which there are morphological differences between them has not been definitively quantified. Furthermore, although previous studies have demonstrated increased anatomical cerebellocerebral correlations in both conditions, differences between their associations have not been well characterized.
Methods
We compared cerebellar volume between males with ASD (n = 31), males with SZ (n = 28), and typically developing males (n = 49). A total of 31 cerebellar subregions were investigated with the cerebellum segmented into their constituent lobules, in gray matter (GM) and white matter (WM) separately. Additionally, structural correlations with the contralateral cerebrum were analyzed for each cerebellar lobule.
Results
We found significantly larger WM volume in the bilateral lobules VI and Crus I in the ASD group than in other groups. While WM or GM volumes of these right lobules had positive associations with ASD symptoms, there was a negative association between GM volume of the right Crus I and SZ symptoms. We further observed, in the ASD group specifically, significant correlations between WM of the right lobule VI and WM of the left frontal pole (r = 0.67) and between GM of the right lobule VI and the left caudate (r = 0.60).
Conclusions
Our findings support evidence that cerebellar morphology is involved in ASD and SZ with different mechanisms. Furthermore, this study showed that these biological differences require consideration when determining diagnostic criteria and treatment for these disorders.
Keywords: Autism spectrum disorder, Cerebellocerebral structure, Cerebellum, MRI, Schizophrenia, Structural neuroimage
Autism spectrum disorder (ASD) and schizophrenia (SZ) are both neurodevelopmental disorders and share several common features at multiple levels. For example, a family history of SZ is associated with an increased risk for ASD, and vice versa, suggesting that they share genetic factors (1). Additionally, phenomenological studies have identified substantial overlap in clinical symptoms including deficits of social interactions and impairments of cognitive and sensorimotor control (2). From a histological perspective, according to Bleuler's four A's concept of SZ, the autism domain is included as one of the fundamental symptoms of SZ (3). The term “autism” was later redefined by Leo Kanner as a childhood psychiatric condition initially considered as a subset of SZ (4,5). Taking these histological views into consideration, it may not be surprising that these two clinical conditions overlap substantially in pathophysiology and symptomatology. However, the fundamental problem behind the clinical overlap makes it difficult to provide an accurate diagnosis and treatments that are appropriate to the actual condition (6). It has also been pointed out that adults with ASD are frequently misdiagnosed as having SZ (7). In fact, an increasing number of adults with ASD visit psychiatric clinics for treatment of concurrent psychiatric symptoms (8). Consequently, it has become increasingly important to differentiate between them and to understand the pathophysiology of these disorders through biological differences (9).
Many studies using magnetic resonance imaging (MRI) have shown atypical deviations of cerebellar morphology in ASD and SZ. For this reason, the cerebellum is considered to be a key brain structure in these disorders. The most consistent evidence of cerebellar morphological abnormalities in ASD is increased white matter (WM) volume, which has been observed in several developmental stages (10, 11, 12, 13). Correspondingly, increased total cerebellar volume has been reported in a recent literature meta-analysis (14). In addition, other studies have shown hypoplasia in the vermis and a decrease of gray matter (GM) in the lateral superior posterior region (15,16). Regarding SZ, while most early studies have shown either reduced total vermal volume and/or reduced GM volume (17, 18, 19), increased WM volume in the cerebellar vermis was observed in two studies restricted to males (20,21). A recent study using a large multisite sample of individuals with SZ (n = 983) and healthy control subjects (n = 1349) demonstrated that total cerebellar GM volumetric reductions are one of the strongest and most consistent morphological alterations (22). Similar results have been found in other studies that identified GM volume reductions specific to the posterior cerebellar regions (23, 24, 25, 26). However, previous studies have focused on either ASD or SZ exclusively, and as a result, the extent to which there are morphological differences between these two conditions has not been definitively quantified.
The cerebellum is engaged in a wide range of functions, from motor to cognitive and affective processing (27, 28, 29), which are critically associated with clinical symptoms in both ASD and SZ in the reciprocal neural circuit with the cerebrum (30,31). Because the cytoarchitectonic organization is remarkably uniform across the entire cerebellum, functional localization depends on the patterns of input from and output to the cerebral regions. Nonhuman primate studies using anatomical tracers have shown that the cerebellum’s connectivity patterns with the cerebrum are different depending on each lobule. Additionally, human functional MRI studies have shown different functional connectivity patterns with the cerebral cortex based on lobule-wise levels (27,28,32). Given the evidence indicating a lobule-specific function of the cerebellum, understanding the regional pattern of the cerebellar volume is especially important when relating it to the clinical symptoms of ASD and SZ. However, only a limited number of studies have examined cerebellar morphology while taking into account its functional topology. In addition, recent studies have shown significant increases of anatomical correlations between the cerebellum and the cerebrum in ASD and SZ (22,33). More crucially, through studies with animal and human subjects, it has been suggested that the cerebellocerebral connections are associated with phenomenological and psychological processes in both ASD and SZ (34,35). Therefore, although elucidating the cerebellocerebral structual correlation pattern can play a pivotal role in understanding the pathology of ASD and SZ, the difference between these disorders has not been examined.
The first aim of this study was to compare cerebellar volume between individuals with ASD, individuals with SZ, and typically developing (TD) individuals. The cerebellum was divided into constituent lobules, and they were analyzed for GM and WM separately. We hypothesized that individuals with ASD would exhibit increased WM volume compared with individuals with SZ and TD individuals, but we were interested to find out by what degree the difference would be depending on each lobule. The second aim was to determine whether alterations in cerebellocerebral anatomical association for each lobule were present in ASD and SZ. Based on recent evidence that showed significant increases of structural connectivity between the cerebellum and cerebrum in these disorders, we hypothesized a stronger association in cerebellocerebral structure in individuals with ASD and individuals with SZ compared with TD individuals. It was also hypothesized that a unique association pattern in strength and topography would be found between ASD and SZ.
Methods and Materials
Participants
A total of 108 Japanese male participants were included in the study. They consisted of 28 individuals with SZ, 31 individuals with ASD, and 49 TD individuals (Table 1). Participants in the clinical groups were recruited from the Department of Neuropsychiatry, University of Tokyo Hospital and Showa University Karasuyama Hospital, Tokyo, Japan. TD individuals were recruited from the same region as the clinical groups by internet referral, message boards in several universities, and voluntary recruitment. Participants with SZ were diagnosed by experienced psychiatrists according to the DSM-IV (36). Psychological symptoms for the SZ group were assessed using the Positive and Negative Syndrome Scale (37). All participants with SZ were confirmed not to have a diagnosis of ASD according to the DSM-IV based on clinical histories and interviews from all participants and their family members. Participants with ASD were diagnosed according to the strict criteria in the DSM-IV-TR (38) confirmed by the Japanese version of the Autism Diagnostic Interview–Revised (39). For one participant who did not reach the threshold on the Autism Diagnostic Interview–Revised social domain, the Autism Diagnostic Observation Schedule (40) was used for the evaluation of symptom severity by an experienced psychologist. All participants with ASD did not take any psychotropic medication. Exclusion criteria for all groups were a current or past neurological comorbidity, a history of electroconvulsive therapy, traumatic brain injury with any known cognitive consequences or loss of consciousness for more than 5 minutes, and any substance abuse or addiction. An additional exclusion criterion for the TD group was any history of neuropsychiatric disorder in the participants or any history of Axis I disorders in first-degree relatives.
Table 1.
Clinical and Demographic Characteristics of Study Participants
| Variable | TD Group (n = 49) | SZ Group (n = 28) | ASD Group (n = 31) | p Value |
|---|---|---|---|---|
| Age, Years, Mean ± SD | 29.3 ± 5.5 | 28.6 ± 9.6 | 28.8 ± 6.3 | .905 |
| Handedness (Right/Mixed/Left), n | 47/2/0 | 27/1/0 | 23/5/3 | .013 |
| Estimated IQ, Mean ± SDa | 108.4 ± 8.3 | 104.8 ± 10.1 | – | |
| Wechsler Adult Intelligence Scale–III, Mean ± SD | ||||
| Full Scale IQ | – | – | 106.3 ± 11.5 | – |
| Verbal IQ | – | – | 112.3 ± 13.3 | – |
| Performance IQ | – | – | 93.7 ± 15.8 | – |
| Positive and Negative Syndrome Scale, Mean ± SD | ||||
| Positive symptoms | – | 16.2 ± 5.3 | – | – |
| Negative symptoms | – | 21.2 ± 5.6 | – | – |
| General psychopathology | – | 38.7 ± 8.8 | – | – |
| Autism Diagnostic Interview–Revised, Mean ± SD | ||||
| Reciprocal social interaction | – | – | 14.8 ± 6.5 | – |
| Communication | – | – | 12.1 ± 4.0 | – |
| Repetitive and stereotypical behaviors | – | – | 4.5 ± 2.1 | – |
ASD, autism spectrum disorder; SZ, schizophrenia; TD, typically developing.
Estimated IQ was determined by the Japanese Adult Reading Test 25 (25-item version of the Japanese Adult Reading Test).
This study was approved by the ethics committee of the University of Tokyo Hospital (Nos. 397 and 2226). After a complete explanation of the experiment, written informed consent was obtained from all participants. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Clinical Assessment and Questionnaire Measures
For all participants, handedness was assessed using the Edinburgh Handedness Inventory (41). Participants were categorized as follows: left-handed from −100 to −71; mixed handed from −70 to +70; and right-handed from +71 to +100 (42). The estimated (premorbid) IQ for the TD and SZ groups was determined using the 25-item version of the Japanese Adult Reading Test (43). IQ for the ASD group was evaluated using the Japanese version of the full-scale Wechsler Adult Intelligence Scale–Revised (44) to judge differing intellectual abilities (45). Medication dosage was calculated using the chlorpromazine equivalent (CPeq) dose (46).
MRI Acquisitions
T1-weighted MRI data were obtained from a GE 3T scanner (GE Signa HDxt; GE Healthcare, Waukesha, WI) with the standard 8-channel head coil with the following parameters: 3-dimensional fast spoiled gradient recalled acquisition in the steady state; echo time, 1.94 ms; repetition time, 6.80 ms; field of view, 240 × 240 mm2; matrix, 256 × 256; flip angle, 20°; slice thickness, 1.0 mm; number of axial slices, 176.
Segmentation
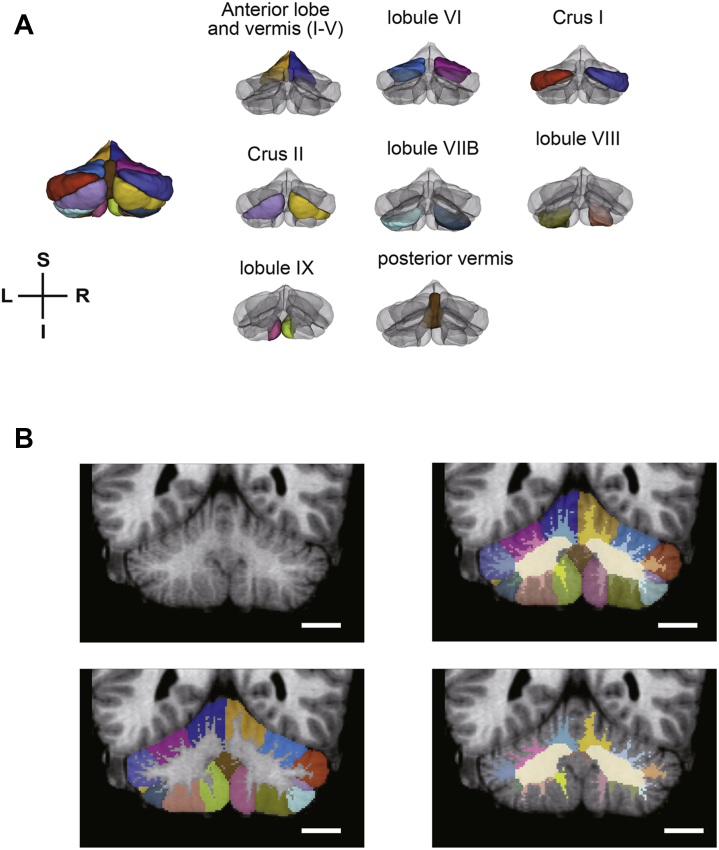
All T1-weighted MR images were first processed using FreeSurfer v6.0 (http://surfer.nmr.mgh.harvard.edu). Our T1-weighted MR images had low signal-to-noise ratios due to the high-resolution T1 image sequence. To improve signal-to-noise ratios, we conducted contrast stretching processing on the intensity-normalized volumes using the FreeSurfer’s process recon-all (47). The norm and aseg files yielded by FreeSurfer recon-all were used in a cerebellar lobule segmentation method proposed by Yang et al. (48), which combined multi-atlas labeling results and tissue/boundary classification on a graph cut segmentation framework. This method helped accurately delineate the cerebellum’s subregional boundaries despite anatomical complexity and variability by integrating features from a wide number of both normal and pathological atlases. Using this method, the cerebellum was divided into its constituent lobules: bilateral lobules I to V, VI, Crus I, Crus II, VIIB, VIII, IX, the corpus medullary, and the posterior vermis (Figure 1). The mean Dice similarity coefficients between the manual and automatic labels for this method were above 0.80 in many lobules, which is comparable with other cerebellar lobule segmentation method (49,50). However, in order to get even more accurate results, manual correction was carried out by a corrector (CM) blinded to subject characteristics (e.g., diagnosis, age) for unbiased removal of noncerebellar tissue, such as blood vessels and meninges. The flocculus and nodulus (hemisphere and vermis X) were excluded because of poor segmentation results. The cerebellum volume was divided into the GM and WM using FSL’s FAST algorithm (51). The threshold of WM partial volume was set at 0.6. The cerebellum was finally divided into 31 subregions through our cerebellum segmentation pipeline.
Figure 1.
Cerebellar atlas and segmentation results. (A) Atlas of each lobular in the cerebellum. (B) Results of lobular segmentation. Scale bar = 20 mm. I, inferior; L, left; R, right; S, superior.
Cortical and subcortical segmentations were obtained by FreeSurfer recon-all results. Cortical thickness and cerebral WM volume were produced using the default atlas (Desikan-Killiany) (52).
Statistical Analyses
All statistical analyses were performed using R statistical software version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria). Demographic characteristics were compared between groups using Fisher’s exact test or Welch’s t test or analysis of variance, as appropriate, with significance levels set at .05. Group comparisons for the volume of cerebellar subregions were conducted with analysis of covariance (ANCOVA), controlling for age and estimated total intracranial volume (eTIV) calculated by FreeSurfer preprocessing. Before cerebellar volumetric analysis, we confirmed that CPeq was not associated with any cerebellar region-of-interest volumes in the SZ group using multiple regression analysis adjusted for age and eTIV. The α level was adjusted for the 32 ANCOVA tests using Bonferroni correction (equivalent to p < .0016). Holm’s multiple comparison tests were applied after ANCOVA analysis. The effect size was reported using generalized η2 (ηG2) for ANCOVA and Hedges’ g for multiple comparisons by Holm’s post hoc test. Multiple regression analysis was used to determine the association between cerebellar lobular volumes and Autism Diagnostic Interview–Revised subscales (social interaction, communication, repetitive and stereotypical behaviors [RBs]), adjusting for age and eTIV in the ASD group, as well as the association between cerebellar lobular volumes and Positive and Negative Syndrome Scale subscales (positive symptoms, negative symptoms, general psychopathology) adjusting for age, eTIV, and CPeq in the SZ group, respectively. The α level was adjusted for the 30 ANCOVA tests using Bonferroni correction (equivalent to p < .0017).
To examine correlations between cerebellar lobular volumes and cerebral thicknesses and cerebral WM/subcortical volume (75 regions per hemisphere), Pearson partial correlation analysis was performed, adjusting for age and eTIV. Because the cerebellum is anatomically interconnected with the contralateral cerebrum, correlations were analyzed based on this connectivity (right cerebellum–left cerebrum, and vice versa) within each matter type. Regarding the cortical regions, a thickness was used according to the previous study (22). The significance level was set to p < .001, uncorrected in reference to multiple corrections in previous studies. This method of multiple correction was conventionally used for whole-brain analysis in the MRI study (53, 54, 55). The significance of the differences in correlation coefficients in the cerebellocerebral structural connectivity between ASD and SZ was analyzed using Fisher’s r-to-z transformation with the psych package (56). The α level was adjusted for the two tests by Bonferroni correction (equivalent to p < .025).
Results
Total Volume
At first, we confirmed whether volumes of the whole cerebellum and corpus medullary were different between groups. No statistically significant group differences were found in these volumes (total cerebellum [F2,103 = 1.45, ηG2 = 0.03, p = .240, Bonferroni corrected], corpus medullary [F2,103 = 1.70, ηG2 = 0.03, p = .188, Bonferroni corrected]).
Lobules-wise Cerebellar Volumes
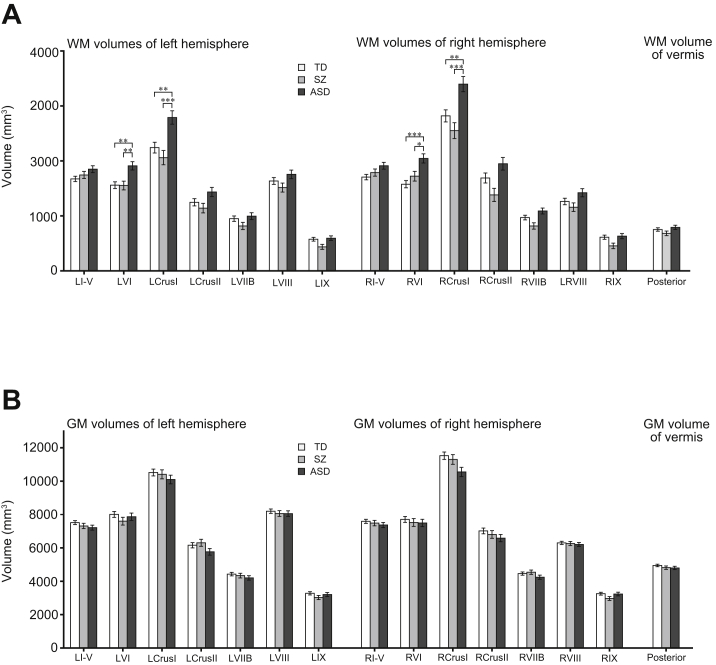
There were significant differences between groups in WM volume of the bilateral lobule VI (left [F2,103 = 7.69, ηG2 = 0.11, p < .001], right [F2,103 = 9.75, ηG2 = 0.15, p < .001]) (Figure 2A) and the bilateral Crus I (left [F2,103 = 9.62, ηG2 = 0.16, p < .001, Bonferroni corrected], right [F2,103 = 9.65, ηG2 = 0.15, p < .001, Bonferroni corrected]). Holm’s post hoc multiple comparison tests showed significantly larger WM volumes of the bilateral lobule VI in the ASD group than in the TD group (left [t103 = 3.61, g = 0.82, p = .001, Holm’s corrected], right [t103 = 4.40, g = 1.00, p < .001, Holm’s corrected]) and in the SZ group (left [t103 = 3.23, g = 0.83, p = .003, Holm’s corrected], right [t103 = 2.67, g = 0.69, p = .018, Holm’s corrected]). Additionally, the ASD group also exhibited significant increased WM volumes in the bilateral Crus I compared with the TD group (left [t103 = 3.51, g = 0.80, p = .001, Holm’s corrected], right [t103 = 3.28, g = 0.75, p = .003, Holm’s corrected]) and SZ group (left [t103 = 4.11, g = 1.06, p < .001, Holm’s corrected], right [t103 = 4.23, g = 1.09, p < .001, Holm’s corrected]). In contrast, significant group differences were not found in any lobular GM volumes (Figure 2B). The statistical results of all cerebellar lobular volumes are shown in Table S1.
Figure 2.
Cerebellar morphometric group differences in each cerebellar lobule. (A, B) Bar plots represent estimated marginal means, standard error, and significance for post hoc analysis after analysis of covariance. (A) Significant group differences were found in white matter (WM) volumes of the bilateral lobules VI and Crus I. (B) On the other hand, no statistically significant group differences were found in gray matter (GM) volumes of any cerebellar lobules. ∗p < .05, ∗∗p < .01, ∗∗∗p < .001. ASD, autism spectrum disorder; L, left; R, right; SZ, schizophrenia; TD, typically developing.
Relationship Between Cerebellar Volumes and Clinical Symptoms in ASD and SZ
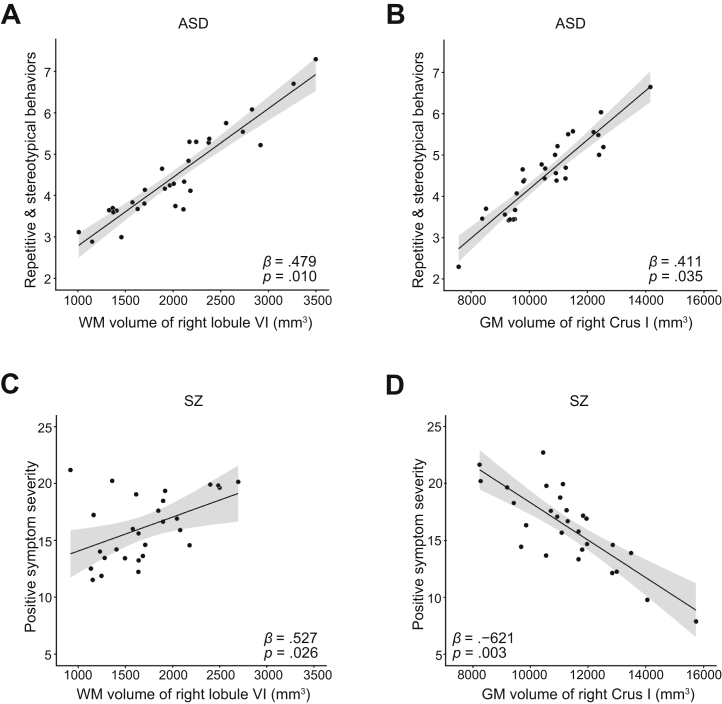
Next, we examined the association between volumes of cerebellar lobules and clinical symptoms in the ASD and SZ groups. Though not significant after multiple comparisons, in the right lobules of VI and Crus I, which had significant group differences in the above analysis, the correlations were observed between brain volumes and clinical symptoms in these two clinical conditions. For the ASD group, there was a positive association between WM of the right VI, as well as the GM volume of the right Crus I, and RBs (WM of right VI [adjusted R2 = 0.18, β = 0.479, p = .010, uncorrected], GM of right Crus I [adjusted R2 = 0.11, β = 0.411, p = .035, uncorrected]) (Figure 3A, B). In the SZ group, while WM of the right VI had a positive association with positive symptoms severity (adjusted R2 = 0.19, β = 0.527, p = .026, uncorrected) (Figure 3C), a negative association was found in GM volumes of the right Crus I (adjusted R2 = 0.32, β = −0.621, p = .003, uncorrected) (Figure 3D). The results of all associations are summarized in Table S2. Associations with clinical symptoms of diagnostic groups were also found in lobules VIII and IX (p < .05 but did not survive Bonferroni correction) (Table S2).
Figure 3.
Results of multiple regression analysis between cerebellar lobular volumes and clinical symptoms in autism spectrum disorder (ASD) and schizophrenia (SZ). (A, B) Results of the ASD group. Positive associations were found between repetitive and stereotypical behaviors and (A) white matter (WM) of the right lobule VI and (B) gray matter (GM) volume of the right Crus I. (C, D) Results of the SZ group. (C) WM of the right lobule VI had a positive association with positive symptom severity. (D) On the other hand, GM volumes of the right Crus I were negatively associated with positive symptoms.
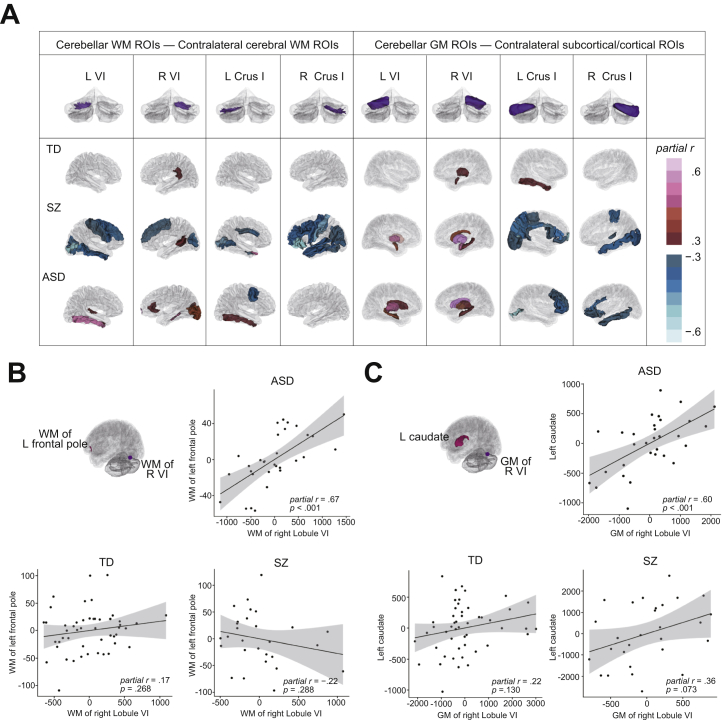
Cerebellocerebal Structural Correlations
As a whole, increased cerebellocerebral structural correlations were found in ASD and SZ groups compared with the TD group (Figures S1–S4). In cerebellar lobular volumes that had a significant group effect, significant positive correlations were identified between WM of the right lobule VI and WM of the frontal pole (partial r = 0.67, p < .001, uncorrected), as well as between GM of the right lobule VI and caudate (partial r = 0.60, p < .001, uncorrected) in the ASD group (Figure 4). Although no significant difference in the magnitude of correlation coefficient between clinical conditions was found between the left caudate and GM of the right lobule VI, a higher correlation coefficient was observed in the ASD group between WM of the left frontal pole and WM of the right VI in the SZ group (z = 2.13, 2-tailed p = .030 but did not survive Bonferroni correction). In other cerebellar lobular volumes, significant increased correlations were found in several regions, especially in the ASD group (Table S3).
Figure 4.
Structural correlations between the cerebellum and cerebrum. (A) Structural correlations between the cerebellum and cerebrum in each group. (Upper row) Cerebellar region-of-interest (ROI) volumes. (Lower row) Color map of correlations with cerebellar ROIs. Correlations with the absolute value of partial r higher than 0.3 are demonstrated. The top, middle, and bottom present the typically developing (TD), schizophrenia (SZ), and autism spectrum disorder (ASD) groups, respectively. (B, C) Partial regression plot between cerebellar ROI volumes and cerebral ROI volumes (age and estimated total intracranial volume controlled). Both variables in the partial regression plot are residuals. (B) A significantly positive correlation was found between white matter (WM) of the right lobule VI and WM of the left frontal pole in the ASD group. (C) A significantly positive correlation was found between gray matter (GM) of the right Crus I and the left caudate nucleus in the ASD group. L, left; R, right.
Discussion
To our knowledge, this is the first study to examine the differences in detailed morphological features of cerebellum structures in individuals with ASD and individuals with SZ. Our structural analysis yielded three main findings. First, the ASD group exhibited significantly increased WM volumes of the bilateral lobule VI and Crus I. Second, posterior cerebellar lobules including lobules VI and Crus I were associated with clinical symptoms of the ASD and SZ groups; however, their patterns were different in each clinical condition. Third, atypical correlations between cerebellar and cerebral structural features were more pronounced in the ASD group compared with the TD and SZ groups. These findings of the present study support the existing evidence that the morphology of the cerebellum is involved in the pathogenesis of ASD and SZ. More crucially, the present study provides preliminary evidence of a disease-specific cerebellar morphometric profile in these two clinical conditions.
In line with previous research that demonstrated substantial increased WM volume of the cerebellum in ASD (10,11), our lobule-wise structural analysis in GM and WM separately identified specific subregions that had crucially increased WM volumes; the bilateral lobule VI and Crus I was significantly increased in the ASD group compared with the TD and SZ groups. Herbert et al. (57) also reported increased WM volume in several brain regions in ASD: more interestingly, they also showed that these brain regions were late- or longer-myelinating regions, such as the prefrontal cortex. Similar to the prefrontal cortex, the cerebellum is considered to have a prolonged developmental trajectory including myelination (31). The protracted developmental course of the cerebellum was also observed in morphological maturation during childhood and adolescence. Notably, lobule VI and Crus I were later in reaching their peak volumes than other cerebellar lobules (58). Therefore, our findings not only support evidence that cerebellar structural abnormalities persist until adulthood, but also raise the possibility that WM volumetric alterations occur in the developmental context.
In contrast to some previous studies that showed reduced GM volume in SZ, we did not find a statistically significant difference in GM volumes between groups. This discrepancy can be partly explained by the difference of the sample size between previous studies and the present study. Namely, although previous studies included a large number of participants from multisites (22,24), participants in the present study were recruited from a single site, and their number was modest. Consequently, our sample size was possibly insufficient to detect the same effect as observed in the previous studies.
Our correlation analysis between cerebellar lobular volumes and clinical symptoms presented that the right lobules VI and Crus I, which had a significant group effect, were related to RBs in ASD and positive symptoms in SZ, respectively. According to functional topological studies of the cerebellum, the part of lobule VI adjacent to lobule V is engaged in motor processing; in contrast, the posterior part of lobule VI and Crus I are engaged in complex cognitive functions such as language, working memory, executive function, and emotional processing (28,59,60). RBs in ASD are broadly ranged from repetitive movements of the body to more cognitive-mediated behaviors. Regarding positive symptoms of SZ, it is assumed that disturbances of cognitive and emotional processes are related to developing positive symptoms (61). We also found associations between lobules VIII/IX and clinical symptoms in ASD and SZ. These regions also play an important role in motor and cognitive processing (59). Taking these factors into consideration, associations may be found because each clinical symptom of ASD and SZ includes the factors related to these cerebellar lobules.
Interestingly, the association pattern was different in the ASD and SZ groups. This may be partially explained by an immune-mediated mechanism. Previous studies have reported that aberrant neuroinflammation can lead to abnormalities in neuroanatomy and behavioral phenotype in ASD and SZ (62,63). For instance, interleukin (IL)-6, which plays an important role in immune responses, is increased in the cerebellum of individuals with ASD (64). The study with the IL-6–overexpressing mice showed that elevated IL-6 is associated with increased total brain volume (65), and additionally, findings of other studies have suggested that elevation of IL-6 mediates behavioral abnormalities of ASD (66). Given these findings, positive associations may reflect elevated neuroinflammation in ASD. On the other hand, SZ is considered to be associated with progressive brain change. In diseases with progressive brain change such as Alzheimer’s disease, activation of microglia was found before neural loss and subsequent clinical signs of disease (67). Therefore, a dissociated association between GM and WM in the SZ group may be a reflection of different microstructural involvement in each tissue type. Taken together, these results suggest that the cerebellar morphology underlies the clinical symptoms of ASD and SZ, but the underpinning mechanism of its influence was different for ASD and SZ.
Stronger structural associations between cerebellar and cerebral regions have been reported in individuals with ASD and individuals with SZ relative to TD individuals in each related previous study (22,34). Our cerebellocerebral structural correlation analysis also observed increased correlations in the two clinical groups supporting these findings. Regarding cerebellar lobular volumes that had a significant group effect, there were significant correlations between WM volumes of the right lobule VI and WM of the left frontal pole and between GM volumes of the right lobule VI and caudate volume in ASD. Of note, previous studies have demonstrated structural and functional deviations of the frontal pole and the caudate nucleus in ASD. For example, functional MRI studies have shown aberrant activation in the frontal pole in response to social anticipation (68). Also, consistent with our findings, substantial increased WM volume of the frontal pole has been reported in ASD (69). Correspondingly, enlargement of the caudate volume has also been reported in ASD. More importantly, several studies have found that caudate volume was associated with RBs in ASD (70). Considering all these findings together, significant structural correlation with regions of the cerebrum that are associated with ASD traits suggest a link with the cerebellocerebral structure in the process of the pathology of ASD (71). In addition to lobules VI and Crus I, significant increased correlations with the cerebrum were found in several cerebellar subregions. This finding possibly indicates that cerebellar subregional volumes have a responsibility for the pathophysiology of ASD in relationship with the cerebrum, regardless of whether an aberrant cerebellar volume exists or not.
The design of our study has several limitations. First, the participants in the present study were limited to men and the number of participants was relatively small. Given the sexual dimorphism and heterogeneity in ASD and SZ, our findings need to be confirmed in a future study with a larger sample including both sexes. Second, although different clinical assessments were used for each clinical group in the present study, evaluating multidimensional assessments including cognitive and behavioral symptoms with the same battery seems to be more useful to clarify differences in the nature and extent of the effect of cerebellar morphology on their symptomatology. Third, in the SZ group, although CPeq was statistically considered, its complex and variable effects on brain volume and psychotic symptoms cannot be completely excluded (72). Finally, our findings do not allow us to deduce the pathological mechanism that caused the distinct morphometric features of the cerebellum between ASD and SZ. However, given the differing neurodevelopmental profiles in these disorders, the longitudinal morphometric differences between them should be revealed in a future study to bring insight into the nature of their underlying pathophysiology.
In conclusion, our findings provide evidence that cerebellar morphology is involved in both ASD and SZ with different mechanisms. Consequently, the findings of our study present the need to consider these biological differences in determining diagnostic criteria and treatment for these disorders.
Acknowledgments and Disclosures
This work was supported by the Japan Agency for Medical Research and Development under Grant Nos. JP20dm0307001 (to OA, KK) and JP20dm0207069 (to KK, SK), Japan Society for the Promotion of Science KAKENHI Grant No. 16H06399 to (KK), the University of Tokyo Center for Integrative Science of Human Behaviour, the International Research Center for Neurointelligence at the University of Tokyo Institutes for Advanced Study, and the Joint Usage/Research Program of Medical Institute of Developmental Disabilities Research at Showa University.
CM and SK designed the study. CM analyzed the data and wrote the article. YN performed technical support for analyzing MRI data. KK, HY, and SK recruited and assessed participants with schizophrenia. HY recruited and HY and HK assessed participants with autism spectrum disorder. HK, OA, KK and HY conducted magnetic resonance imaging scans.
We gratefully acknowledge the cooperation of all participants. We thank K. Nemoto for magnetic resonance imaging technical advice and M. Takada for helpful discussion.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2021.05.010.
Supplementary Material
References
- 1.Sullivan P.F., Magnusson C., Reichenberg A., Boman M., Dalman C., Davidson M., et al. Family history of schizophrenia and bipolar disorder as risk factors for autism. Arch Gen Psychiatry. 2012;69:1099–1103. doi: 10.1001/archgenpsychiatry.2012.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eack S.M., Bahorik A.L., McKnight S.A., Hogarty S.S., Greenwald D.P., Newhill C.E., et al. Commonalities in social and non-social cognitive impairments in adults with autism spectrum disorder and schizophrenia. Schizophr Res. 2013;148:24–28. doi: 10.1016/j.schres.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peralta V., Cuesta M.J. Eugen Bleuler and the schizophrenias: 100 years after. Schizophr Bull. 2011;37:1118–1120. doi: 10.1093/schbul/sbr126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- 5.Crespi B.J. Revisiting Bleuler: Relationship between autism and schizophrenia. Br J Psychiatry. 2010;196:495. doi: 10.1192/bjp.196.6.495. author reply 495-496. [DOI] [PubMed] [Google Scholar]
- 6.Raja M., Azzoni A. Autistic spectrum disorders and schizophrenia in the adult psychiatric setting: Diagnosis and comorbidity. Psychiatr Danub. 2010;22:514–521. [PubMed] [Google Scholar]
- 7.King B.H., Lord C. Is schizophrenia on the autism spectrum? Brain Res. 2011;1380:34–41. doi: 10.1016/j.brainres.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 8.Takei R., Matsuo J., Takahashi H., Uchiyama T., Kunugi H., Kamio Y. Verification of the utility of the social responsiveness scale for adults in non-clinical and clinical adult populations in Japan. BMC Psychiatry. 2014;14:302. doi: 10.1186/s12888-014-0302-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshihara Y., Lisi G., Yahata N., Fujino J., Matsumoto Y., Miyata J., et al. Overlapping but asymmetrical relationships between schizophrenia and autism revealed by brain connectivity. Schizophr Bull. 2020;46:1210–1218. doi: 10.1093/schbul/sbaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Courchesne E., Karns C.M., Davis H.R., Ziccardi R., Carper R.A., Tigue Z.D., et al. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- 11.Akshoomoff N., Lord C., Lincoln A.J., Courchesne R.Y., Carper R.A., Townsend J., et al. Outcome classification of preschool children with autism spectrum disorders using MRI brain measures. J Am Acad Child Adolesc Psychiatry. 2004;43:349–357. doi: 10.1097/00004583-200403000-00018. [DOI] [PubMed] [Google Scholar]
- 12.D'Mello A.M., Stoodley C.J. Cerebro-cerebellar circuits in autism spectrum disorder. Front Neurosci. 2015;9:408. doi: 10.3389/fnins.2015.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodge S.M., Makris N., Kennedy D.N., Caviness V.S., Jr., Howard J., McGrath L., et al. Cerebellum, language, and cognition in autism and specific language impairment. J Autism Dev Disord. 2010;40:300–316. doi: 10.1007/s10803-009-0872-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Traut N., Beggiato A., Bourgeron T., Delorme R., Rondi-Reig L., Paradis A.L., et al. Cerebellar volume in autism: Literature meta-analysis and analysis of the Autism Brain Imaging Data Exchange Cohort. Biol Psychiatry. 2018;83:579–588. doi: 10.1016/j.biopsych.2017.09.029. [DOI] [PubMed] [Google Scholar]
- 15.D'Mello A.M., Crocetti D., Mostofsky S.H., Stoodley C.J. Cerebellar gray matter and lobular volumes correlate with core autism symptoms. Neuroimage Clin. 2015;7:631–639. doi: 10.1016/j.nicl.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webb S.J., Sparks B.F., Friedman S.D., Shaw D.W., Giedd J., Dawson G., et al. Cerebellar vermal volumes and behavioral correlates in children with autism spectrum disorder. Psychiatry Res. 2009;172:61–67. doi: 10.1016/j.pscychresns.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ichimiya T., Okubo Y., Suhara T., Sudo Y. Reduced volume of the cerebellar vermis in neuroleptic-naive schizophrenia. Biol Psychiatry. 2001;49:20–27. doi: 10.1016/s0006-3223(00)01081-7. [DOI] [PubMed] [Google Scholar]
- 18.Joyal C.C., Pennanen C., Tiihonen E., Laakso M.P., Tiihonen J., Aronen H.J. MRI volumetry of the vermis and the cerebellar hemispheres in men with schizophrenia. Psychiatry Res. 2004;131:115–124. doi: 10.1016/j.pscychresns.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Loeber R.T., Cintron C.M., Yurgelun-Todd D.A. Morphometry of individual cerebellar lobules in schizophrenia. Am J Psychiatry. 2001;158:952–954. doi: 10.1176/appi.ajp.158.6.952. [DOI] [PubMed] [Google Scholar]
- 20.Lee K.H., Farrow T.F., Parks R.W., Newton L.D., Mir N.U., Egleston P.N., et al. Increased cerebellar vermis white-matter volume in men with schizophrenia. J Psychiatr Res. 2007;41:645–651. doi: 10.1016/j.jpsychires.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Levitt J.J., McCarley R.W., Nestor P.G., Petrescu C., Donnino R., Hirayasu Y., et al. Quantitative volumetric MRI study of the cerebellum and vermis in schizophrenia: Clinical and cognitive correlates. Am J Psychiatry. 1999;156:1105–1107. doi: 10.1176/ajp.156.7.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moberget T., Doan N.T., Alnæs D., Kaufmann T., Córdova-Palomera A., Lagerberg T.V., et al. Cerebellar volume and cerebellocerebral structural covariance in schizophrenia: A multisite mega-analysis of 983 patients and 1349 healthy controls. Mol Psychiatry. 2018;23:1512–1520. doi: 10.1038/mp.2017.106. [DOI] [PubMed] [Google Scholar]
- 23.Ding Y., Ou Y., Pan P., Shan X., Chen J., Liu F., et al. Cerebellar structural and functional abnormalities in first-episode and drug-naive patients with schizophrenia: A meta-analysis. Psychiatry Res Neuroimaging. 2019;283:24–33. doi: 10.1016/j.pscychresns.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Laidi C., Hajek T., Spaniel F., Kolenic M., d'Albis M.A., Sarrazin S., et al. Cerebellar parcellation in schizophrenia and bipolar disorder. Acta Psychiatr Scand. 2019;140:468–476. doi: 10.1111/acps.13087. [DOI] [PubMed] [Google Scholar]
- 25.Kim T., Lee K.H., Oh H., Lee T.Y., Cho K.I.K., Lee J., et al. Cerebellar structural abnormalities associated with cognitive function in patients with first-episode psychosis. Front Psychiatry. 2018;9:286. doi: 10.3389/fpsyt.2018.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kühn S., Romanowski A., Schubert F., Gallinat J. Reduction of cerebellar grey matter in Crus I and II in schizophrenia. Brain Struct Funct. 2012;217:523–529. doi: 10.1007/s00429-011-0365-2. [DOI] [PubMed] [Google Scholar]
- 27.Stoodley C.J., Valera E.M., Schmahmann J.D. An fMRI study of intra-individual functional topography in the human cerebellum. Behav Neurol. 2010;23:65–79. doi: 10.3233/BEN-2010-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoodley C.J. The cerebellum and cognition: Evidence from functional imaging studies. Cerebellum. 2012;11:352–365. doi: 10.1007/s12311-011-0260-7. [DOI] [PubMed] [Google Scholar]
- 29.Grimaldi G., Manto M. Topography of cerebellar deficits in humans. Cerebellum. 2012;11:336–351. doi: 10.1007/s12311-011-0247-4. [DOI] [PubMed] [Google Scholar]
- 30.Wang S.S., Kloth A.D., Badura A. The cerebellum, sensitive periods, and autism. Neuron. 2014;83:518–532. doi: 10.1016/j.neuron.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andreasen N.C., Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64:81–88. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stoodley C.J., Schmahmann J.D. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 33.Stoodley C.J., D'Mello A.M., Ellegood J., Jakkamsetti V., Liu P., Nebel M.B., et al. Author correction: Altered cerebellar connectivity in autism and cerebellar-mediated rescue of autism-related behaviors in mice. Nat Neurosci. 2018;21:1016. doi: 10.1038/s41593-018-0096-2. [DOI] [PubMed] [Google Scholar]
- 34.Stoodley C.J., D'Mello A.M., Ellegood J., Jakkamsetti V., Liu P., Nebel M.B., et al. Altered cerebellar connectivity in autism and cerebellar-mediated rescue of autism-related behaviors in mice. Nat Neurosci. 2017;20:1744–1751. doi: 10.1038/s41593-017-0004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brady R.O., Jr., Gonsalvez I., Lee I., Ongur D., Seidman L.J., Schmahmann J.D., et al. Cerebellar-prefrontal network connectivity and negative symptoms in schizophrenia. Am J Psychiatry. 2019;176:512–520. doi: 10.1176/appi.ajp.2018.18040429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.American Psychiatric Association . 4th ed. American Psychiatric Press; Washington DC: 1994. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 37.Kay S.R., Fiszbein A., Opler L.A. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 38.American Psychiatric Association . American Psychiatric Press; Washington DC: 2000. Diagnostic Criteria From DSM-IV-TR. [Google Scholar]
- 39.Lord C., Rutter M., Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 40.Lord C., Risi S., Lambrecht L., Cook E.H., Jr., Leventhal B.L., DiLavore P.C., et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- 41.Oldfield R.C. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 42.Deep-Soboslay A., Hyde T.M., Callicott J.P., Lener M.S., Verchinski B.A., Apud J.A., et al. Handedness, heritability, neurocognition and brain asymmetry in schizophrenia. Brain. 2010;133:3113–3122. doi: 10.1093/brain/awq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsuoka K., Uno M., Kasai K., Koyama K., Kim Y. Estimation of premorbid IQ in individuals with Alzheimer's disease using Japanese ideographic script (Kanji) compound words: Japanese version of National Adult Reading Test. Psychiatry Clin Neurosci. 2006;60:332–339. doi: 10.1111/j.1440-1819.2006.01510.x. [DOI] [PubMed] [Google Scholar]
- 44.Wechsler D. Psychological Corporation; San Antonio, TX: 1981. Wechsler Adult Intelligence Scale-Revised (WAIS-R) [Google Scholar]
- 45.American Psychiatric Association . 5th ed. American Psychiatric Press; Washington, DC: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 46.Inada T., Inagaki A. Psychotropic dose equivalence in Japan. Psychiatry Clin Neurosci. 2015;69:440–447. doi: 10.1111/pcn.12275. [DOI] [PubMed] [Google Scholar]
- 47.Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 48.Yang Z., Ye C., Bogovic J.A., Carass A., Jedynak B.M., Ying S.H., et al. Automated cerebellar lobule segmentation with application to cerebellar structural analysis in cerebellar disease. Neuroimage. 2016;127:435–444. doi: 10.1016/j.neuroimage.2015.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han S., Carass A., He Y., Prince J.L. Automatic cerebellum anatomical parcellation using U-Net with locally constrained optimization. Neuroimage. 2020;218:116819. doi: 10.1016/j.neuroimage.2020.116819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carass A., Cuzzocreo J.L., Han S., Hernandez-Castillo C.R., Rasser P.E., Ganz M., et al. Comparing fully automated state-of-the-art cerebellum parcellation from magnetic resonance images. Neuroimage. 2018;183:150–172. doi: 10.1016/j.neuroimage.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y., Brady M., Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 52.Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 53.Du A.T., Schuff N., Kramer J.H., Rosen H.J., Gorno-Tempini M.L., Rankin K., et al. Different regional patterns of cortical thinning in Alzheimer's disease and frontotemporal dementia. Brain. 2007;130:1159–1166. doi: 10.1093/brain/awm016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Righart R., Duering M., Gonik M., Jouvent E., Reyes S., Hervé D., et al. Impact of regional cortical and subcortical changes on processing speed in cerebral small vessel disease. Neuroimage Clin. 2013;2:854–861. doi: 10.1016/j.nicl.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh S., Khushu S., Kumar P., Goyal S., Bhatia T., Deshpande S.N. Evidence for regional hippocampal damage in patients with schizophrenia. Neuroradiology. 2018;60:199–205. doi: 10.1007/s00234-017-1954-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Revelle W.R. psych: Procedures for personality and psychological research. 2017. https://cran.r-project.org/web/packages/psych/index.html Available at: Accessed May 5, 2019.
- 57.Herbert M.R., Ziegler D.A., Makris N., Filipek P.A., Kemper T.L., Normandin J.J., et al. Localization of white matter volume increase in autism and developmental language disorder. Ann Neurol. 2004;55:530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- 58.Tiemeier H., Lenroot R.K., Greenstein D.K., Tran L., Pierson R., Giedd J.N. Cerebellum development during childhood and adolescence: A longitudinal morphometric MRI study. Neuroimage. 2010;49:63–70. doi: 10.1016/j.neuroimage.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.King M., Hernandez-Castillo C.R., Poldrack R.A., Ivry R.B., Diedrichsen J. Functional boundaries in the human cerebellum revealed by a multi-domain task battery. Nat Neurosci. 2019;22:1371–1378. doi: 10.1038/s41593-019-0436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmahmann J.D. The cerebellum and cognition. Neurosci Lett. 2019;688:62–75. doi: 10.1016/j.neulet.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 61.Garety P.A., Kuipers E., Fowler D., Freeman D., Bebbington P.E. A cognitive model of the positive symptoms of psychosis. Psychol Med. 2001;31:189–195. doi: 10.1017/s0033291701003312. [DOI] [PubMed] [Google Scholar]
- 62.Monji A., Kato T.A., Mizoguchi Y., Horikawa H., Seki Y., Kasai M., et al. Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:115–121. doi: 10.1016/j.pnpbp.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 63.Wei H., Alberts I., Li X. Brain IL-6 and autism. Neuroscience. 2013;252:320–325. doi: 10.1016/j.neuroscience.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 64.Wei H., Zou H., Sheikh A.M., Malik M., Dobkin C., Brown W.T., et al. IL-6 is increased in the cerebellum of autistic brain and alters neural cell adhesion, migration and synaptic formation. J Neuroinflammation. 2011;8:52. doi: 10.1186/1742-2094-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wei H., Mori S., Hua K., Li X. Alteration of brain volume in IL-6 overexpressing mice related to autism. Int J Dev Neurosci. 2012;30:554–559. doi: 10.1016/j.ijdevneu.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 66.Wei H., Chadman K.K., McCloskey D.P., Sheikh A.M., Malik M., Brown W.T., et al. Brain IL-6 elevation causes neuronal circuitry imbalances and mediates autism-like behaviors. Biochim Biophys Acta. 2012;1822:831–842. doi: 10.1016/j.bbadis.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 67.Eikelenboom P., Bate C., Van Gool W.A., Hoozemans J.J., Rozemuller J.M., Veerhuis R., et al. Neuroinflammation in Alzheimer's disease and prion disease. Glia. 2002;40:232–239. doi: 10.1002/glia.10146. [DOI] [PubMed] [Google Scholar]
- 68.Dichter G.S., Richey J.A., Rittenberg A.M., Sabatino A., Bodfish J.W. Reward circuitry function in autism during face anticipation and outcomes. J Autism Dev Disord. 2012;42:147–160. doi: 10.1007/s10803-011-1221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carper R.A., Moses P., Tigue Z.D., Courchesne E. Cerebral lobes in autism: Early hyperplasia and abnormal age effects. Neuroimage. 2002;16:1038–1051. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- 70.Langen M., Schnack H.G., Nederveen H., Bos D., Lahuis B.E., de Jonge M.V., et al. Changes in the developmental trajectories of striatum in autism. Biol Psychiatry. 2009;66:327–333. doi: 10.1016/j.biopsych.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 71.Alexander-Bloch A., Giedd J.N., Bullmore E. Imaging structural co-variance between human brain regions. Nat Rev Neurosci. 2013;14:322–336. doi: 10.1038/nrn3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moncrieff J., Leo J. A systematic review of the effects of antipsychotic drugs on brain volume. Psychol Med. 2010;40:1409–1422. doi: 10.1017/S0033291709992297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.