Abstract
Cell-mediated immunity appears to be critical for the prevention and control of varicella-zoster virus (VZV) infection and complications arising from zoster. Current assays of VZV-specific cell-mediated immunity are cumbersome or lack sensitivity. We have developed a gamma interferon ELISPOT assay that provides a direct measure of the number of T cells secreting a cytokine following stimulation with antigen. This assay is extremely sensitive and specific, with the ability to detect gamma interferon spot-forming cells (SFC) in the range of 10 to 1,000 SFC per million peripheral blood mononuclear cells (PBMCs). This assay has been validated by demonstrating the following: (i) the response detected is mediated almost entirely by CD4+ T cells, (ii) ELISPOT responses from fresh-frozen PBMCs are equivalent to those from freshly isolated cells, (iii) frozen PBMCs can be shipped on dry ice for up to 48 h without loss of activity, (iv) frozen PBMC samples can be stored in liquid nitrogen over long periods (>22 months) without any significant change in response, and (v) the numbers of ELISPOTs counted using a computer-based imaging system are equivalent to those counted by humans but have lower variability. The ability to use frozen cells is facilitated by the use of a recombinant nuclease (Benzonase) that can prevent cell clumping when samples are thawed. Frozen PBMC samples can be cycled through multiple changes in storage between liquid nitrogen and dry ice without any change in response being detected. This facilitates collection of samples at one site and testing performed at a remote location. This VZV ELISPOT assay provides a new versatile tool for monitoring cellular immune responses either during a herpes zoster disease outbreak or following vaccination.
The importance of cellular immunity in prevention and control of varicella-zoster virus (VZV) infection has been well documented (1–4, 10, 12). Components of cellular immunity for memory responses following both natural infection and vaccination have been described. This includes detection of both CD4+ (helper)- and CD8+ (cytotoxic)-T-cell responses specific to numerous VZV antigens (7, 11, 13–19, 25, 28, 30, 33, 36–38). Lymphoproliferation assays are not quantitative and measure only CD4 T-cell responses. The limiting-dilution format responder cell frequency (RCF) assay permits some quantitation of response yet is very cumbersome. The cytotoxic-T-cell (CTL) assays can measure CD8 T-cell responses but are also quantitative only in the cumbersome format of limiting-dilution analysis. Intracellular cytokine staining can be used for both CD4 and CD8 T-cell responses, but high background signals can limit detection of low-frequency responses. All of these methods utilize freshly isolated cells for optimum detection of signal. There is a need for new quantitative assays to assess these cellular immune responses both during the course of infection and after vaccination. ELISPOT assays for the detection of cytokine-producing T cells are becoming more widely adopted for these purposes, and the detection of gamma interferon (IFN-γ) production with this method is especially important in monitoring TH1 (helper) and TC1 (cytotoxic) responses.
The IFN-γ ELISPOT assay is a method for determining the number of individual T cells secreting a cytokine after stimulation with a specific antigen or peptide (5, 9, 23, 26, 29, 31). The number of spots increases proportionately with the strength of the immune response. An important advantage of the IFN-γ ELISPOT response is that it is a direct measurement of a TH1 cell-mediated immune response. As such, it is useful for monitoring the effectiveness of a vaccine to induce cell-mediated immunity. The ELISPOT assay utilizes two high-affinity cytokine-specific antibodies directed against different epitopes on the same cytokine molecule. Spots are generated with a colorimetric reaction in which soluble substrate is cleaved, leaving an insoluble precipitate at the site of the reaction. The spot represents a footprint of the original cytokine-producing cell. The number of spots is a direct measurement of the frequency of cytokine-producing T cells. The IFN-γ ELISPOT assay presents novel challenges to validation compared with traditional standard-curve-type assays. The assay endpoint (a spot) is the result of a complex series of events that can be broken down into three categories: (i) the cell culture conditions leading to production of IFN-γ; (ii) the antibody capture, enzyme-mediated detection system; and (iii) the technique for spot counting.
The present report describes both the development of a new IFN-γ ELISPOT assay for the quantitation of cellular responses to VZV antigen and the steps taken to validate the assay. The results indicate that the assay is highly sensitive and specific. Additionally, we show that this assay can be used to evaluate responses with either fresh or frozen peripheral blood mononuclear cell (PBMC) samples, with PBMCs obtained from blood that was stored at 4°C overnight, and that responses can be quantitated with or without sophisticated equipment. This assay provides a new, versatile tool for analysis of cellular immune function following either disease outbreak or vaccination.
MATERIALS AND METHODS
Isolation of PBMCs from whole blood.
Whole blood was collected from donors into either heparin- or EDTA-containing Vacutainer tubes (Becton Dickinson, Franklin Lakes, N.J.). The blood was diluted with Hanks balanced salt solution without calcium and magnesium (Gibco BRL, Gaithersburg, Md.) and layered on top of the frit in Accuspin System Histopaque-1077 cell separation tubes (Sigma, St. Louis, Mo.). The tubes were centrifuged at 1,000 × g for 10 min at 20°C, and the buffy layer containing the PBMCs was removed. The cells were washed, and then the red blood cells were lysed with ACK lysing buffer (Gibco BRL). The cells were washed twice with Hanks balanced salt solution. Cells were counted using a Z1 dual particle counter (Beckman Coulter, Miami, Fla.). The cells were washed and resuspended in complete medium to the desired concentration (see “Preparation of frozen PBMCs” below for a description of complete medium).
If PBMCs were to be frozen, cells were instead resuspended in freezing medium consisting of 90% heat-inactivated fetal bovine serum (HyClone, Logan, Utah) and 10% dimethyl sulfoxide (Sigma). Cells were resuspended to a concentration of 1 × 107 to 2 × 107 cells/ml in the freezing medium and placed into a Nalgene 1°C cryogenic freezing container (Fisher, Bridgewater, N.J.). The freezing container was then stored at −70°C overnight, and frozen cell samples were then transferred to liquid nitrogen (vapor phase) for long-term storage.
Preparation of frozen PBMCs.
RPMI 1640 medium was supplemented with 10% heat-inactivated fetal bovine serum (HyClone), 10 mM HEPES buffer (Gibco BRL), 1 mM l-glutamine (Gibco BRL), 100 μg of penicillin per ml, 100 U of streptomycin (Gibco BRL) per ml, and 5 × 10−5 M β-mercaptoethanol (Sigma). The medium, termed complete medium, was warmed to room temperature. Complete medium was supplemented with Benzonase (EM Industries, Hawthorne, N.Y.) to a final concentration of ≥50 U/ml. Frozen cells were thawed at 37°C, and Benzonase-supplemented medium was slowly added. Cells were washed and resuspended in complete medium supplemented with Benzonase. Cells were again washed, resuspended in medium without Benzonase, and quantitated with a Coulter Instruments Z1 dual particle counter. Cells were washed and then resuspended in complete medium without Benzonase at a concentration of 107 cells/ml for assay setup.
IFN-γ ELISPOT assay for VZV responses.
The wells of a 96-well Multiscreen-IP membrane plate (Millipore, Bedford, Mass.) were coated with 100 μl of an anti-human recombinant IFN-γ monoclonal antibody (catalog no. M-700A; Endogen, Woburn, Mass.) at a concentration of 5 μg/ml overnight at 4°C. The wells were washed three times with sterile phosphate-buffered saline (PBS). The plates were then blocked by adding 200 μl of complete medium and incubating at 37°C with 5% CO2 for 1 to 3 h. The wells of the plate were washed once with 100 μl of complete medium. To each well was added 50 μl of complete medium containing the appropriate antigen. The VZV antigen was a UV-inactivated preparation of VZV antigens derived from clarified cell culture supernatants from VZV-infected MRC-5 cells. The control (MRC-5) antigen was produced by the same process as the VZV antigen preparation using uninfected MRC-5 cells. The final VZV antigen dilution used in the assay was selected by titration experiments. The control antigen was diluted to contain approximately the same MRC-5 cell-associated antigen content as in the VZV antigen preparation. Phytohemagglutinin (PHA) M (Sigma) at 5 μg/ml was incorporated as a positive control.
Next, 50 μl of PBMC cell suspension at 107 PBMCs/ml was added to each well. Assay plates were then incubated overnight for 16 to 20 h at 37°C, 5% CO2, and 95% humidity. Plates were washed six times with PBS containing 5% heat-inactivated fetal bovine serum and 0.005% Tween-20 (ELISPOT wash buffer). A biotinylated anti-human recombinant IFN-γ antibody (catalog no. M-701-B; Endogen) was diluted to 1.0 μg/ml in ELISPOT wash buffer and added at 50 μl per well. After overnight incubation at 4°C, the plate was washed six times with ELISPOT wash buffer. Streptavidin-alkaline phosphatase (Pierce, Rockford, Ill.) was diluted appropriately in ELISPOT wash buffer (the specific dilution was determined for each lot of conjugate used). To each well of the assay plate was added 100 μl of diluted conjugate, and the plate was incubated at room temperature for 1.5 to 2.5 h. The plate was washed three times with ELISPOT wash buffer and then three times with PBS. One hundred microliters of 1-Step nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate substrate (Pierce) was added to each well, and spots were developed for 3 to 15 min at room temperature. Substrate was emptied from the wells of the plates, and then the wells were rinsed with water to stop the reaction. The plates were allowed to air dry, and then spots were enumerated by counting under a dissecting microscope or using an ImmunoSpot Image Analyzer system (Resolution Technology Inc., Columbus, Ohio) for automated plate scanning, imaging, and spot counting.
CD4 and CD8 cell depletion.
CD4 or CD8 T-cell populations were depleted from PBMCs using the Dynabead (catalog no. 111.06 or 111.08; Dynal A.S, Oslo, Norway) magnetic bead system according to the manufacturer's recommended procedures. Cells were then resuspended in complete medium at 107 cells/ml for the ELISPOT assay. Depletion was confirmed by fluorescence-activated cell sorter (FACS) analysis.
Statistical analysis of assay validation data.
The variances attributable to the different factors (assay run, reader, and bleed) were estimated using SAS Proc Varcomp. (SAS Institute, Cary, N.C.). For the human readers, the mathematical model compatible with the design of the study used to estimate the three factors (assay run, reader, and bleed) recognized assay run as being nested with donor and reader as being nested with both assay run and donor. The component attributable to bleed was estimated for the residual mean square. For the computer readings, the mathematical model is simplified to one where assay run is nested with donor and bleed was estimated for the residual mean square. The effects of run plus bleed and the sum of the three factors were constructed from the sum of the individual component mean squares. The degrees of freedom were estimated for the individual and sum effects using a Satterthwaite approximation (24). The confidence limits shown in Table 5 were established using chi-square percentiles with these estimated degrees of freedom.
TABLE 5.
Assay variability for testing fresh-frozen PBMC samplesa
| Reading and component | % RSDb
|
||
|---|---|---|---|
| Lower limit | Estimate | Upper limit | |
| Human readers | |||
| Assay run | 11.23 | 17.78 | 47.54 |
| Reader | 19.19 | 23.30 | 30.75 |
| Bleedc | 18.22 | 20.13 | 22.54 |
| Run + bleedd | 25.85 | 30.45 | 37.76 |
| Sum of threee | 32.27 | 38.12 | 47.86 |
| Computer readings | |||
| Assay run | 5.99 | 8.55 | 15.98 |
| Bleedc | 19.18 | 21.03 | 23.31 |
| Run + bleedd | 20.82 | 23.09 | 26.07 |
Fresh-frozen PBMCs were isolated and frozen from blood on same day that blood was collected.
Percent RSD = [exp(SDLn) − 1] × 100, where exp is the natural or Naperian antilogarithm and SDLn is the estimated standard deviation from the variance component analysis performed on the natural logarithm of the plate count. Lower and upper limits are based on the 95% confidence interval.
Use if PBMC samples are tested in the same assay run.
Use if the two samples are tested in different assay runs.
Use if two samples are tested in different assay runs counted by different readers.
RESULTS
Optimization of assay parameters.
Pairs of coating and detecting antibodies were compared to qualitatively select those giving the lowest background (with no stimulation) and the highest signal with positive control (mitogen) stimulation (data not shown). The antibody pair selected for assay use was then tested in a cross titration over a range of concentrations to select appropriate levels for incorporation into a standardized assay protocol. The capture antibody was effective over the range of concentrations tested from 2.5 up to 10 μg/ml. The detecting antibody produced a good signal with no reduction in spot count over the range from 0.1 to 2.5 μg/ml in combination with all capture antibody levels. There was a slight reduction in both spot count and signal quality when the capture antibody was used at 2.5 μg/ml in combination with the detecting antibody at 0.1 μg/ml.
A titration of VZV and MRC-5 antigen dilution levels from 1:40 down to 1:640 was performed. Responses were similar for most VZV antigen dilutions, with no decrease in response observed even when the VZV antigen was tested at a 1:320 dilution (data not shown). Additionally, background responses to the MRC-5 cell lysate control antigen were similar over the dilution range tested.
The kinetics of antigen incubation required for spot formation was analyzed with the VZV antigen at a 1:80 dilution. There was an increase in the number of spot-forming cells (SFC) detected at 16 h compared with 6 h of incubation. The total number and quality of spots in the assay were similar over an incubation range of 16 to 21 h. Beyond 21 h of incubation there was no increase in the number of spots detected, while there was a reduction in the qualitative measures (more diffuse and overlapping spots, making enumeration more difficult).
For the final standardized assay protocol, the capture antibody was applied at 5 μg/ml and detecting antibody was added at 1 μg/ml. The VZV antigen was added at a dilution of 1:80, MRC-5 control antigen was added at a dilution of 1:160 (providing MRC-5 protein levels equivalent to those of the VZV antigen preparation), and an incubation time of 16 to 20 h was used. Responses were evaluated in terms of adjusted VZV spot counts. The adjusted VZV spot count represents the mean spot count for VZV antigen-stimulated wells minus the mean spot count for MRC-5 control-stimulated wells.
Titration of cells per assay well.
The PBMC numbers in each assay well were titrated over a range from 1 × 105 to 6 × 105 cells/well. The results are shown in Fig. 1. When responses were adjusted to SFC per 106 PBMCs, the responses for individual PBMC samples were similar over the range of cell concentrations from 2 × 105 to 6 × 105 cells/well. Responses for donor PBMCs with the highest SFC numbers overall remained fairly linear over the range of 2 × 105 up to 6 × 105 cells per well. As the donor response decreased there was a reduction in the normalized response (SFC/106 PBMC) when the assay well concentration was at or below 2 × 105 cells/well. This was especially noted when samples from an individual having fewer than 100 SFC per million PBMCs were analyzed.
FIG. 1.
Effect of cell concentration on IFN-γ VZV ELISPOT response. SFC represent the adjusted VZV ELISPOT response, i.e., the mean response to VZV antigen (n = 3) minus the mean response to MRC-5 control antigen (n = 3).
The final, standardized operating procedure for the VZV ELISPOT assay was designed with the addition of 5 × 105 cells per assay well.
Prevention of cell clumping using a recombinant nuclease (Benzonase).
Testing freshly isolated PBMCs is cumbersome when monitoring large clinical trials or when monitoring patient responses to natural infection over time. Testing frozen PBMCs offers a more practical alternative; however, when frozen PBMCs were initially utilized, problems with cell clumping upon thawing were sometimes encountered. This appears to be related to both the donor and the blood handling. PBMCs from certain donors were found to clump more than cells from other donors, even for bleeds separated by time. In addition, clumping occurred more frequently when blood was stored (e.g., overnight) before isolation of PBMCs. To address this problem, we added a recombinant nuclease (Benzonase) to the assay medium for the first two wash steps during the thawing procedure for frozen cells. As a demonstration of the utility of this method, an experiment was performed in which blood was collected from donors and then frozen on the day it was collected (fresh) or stored overnight at 4°C before processing and freezing the PBMCs. At a later time, frozen PBMCs were thawed with and without Benzonase and tested in the ELISPOT assay. As shown in Fig. 2, the results demonstrate no difference in response for frozen PBMCs isolated from fresh blood when processed either with or without Benzonase. However, the spot count results for overnight-stored blood processed without Benzonase showed a dramatic decrease in three of the four donors compared with the VZV responses for the corresponding PBMCs isolated from fresh blood. The greatest decreases were detected for samples in which the highest degree of cell clumping was observed. The SFC results from the overnight blood PBMCs processed with Benzonase more closely approximate the results obtained with cells isolated from fresh blood. These increases in response for overnight blood PBMCs were greatest for the two samples (donors 1015 and 4963) that had the highest degree of cell clumping when processed without Benzonase. However, these responses were still lower than those detected with the PBMCs frozen from freshly collected blood. The incorporation of Benzonase did not result in detectable changes either in cell viability upon thawing or in the expression of cell surface markers for CD4, CD8, CD38, or CD62L (data not shown).
FIG. 2.
Comparison of results with frozen PBMCs isolated from freshly collected blood versus blood stored overnight (4°C). SFC represent the adjusted VZV ELISPOT response, i.e., the mean response to VZV antigen for replicate wells minus the mean response to MRC-5 control antigen for replicate wells.
Comparison of fresh versus frozen PBMC samples.
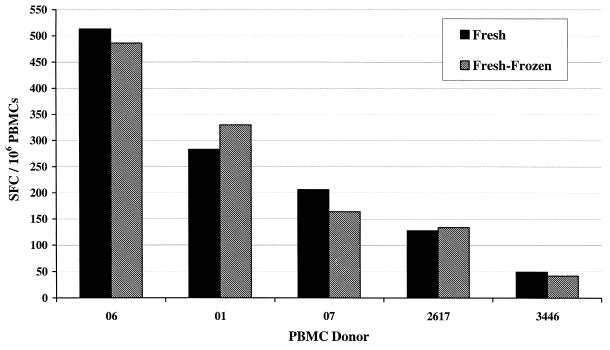
The response of freshly isolated (not frozen) PBMCs was compared with the response of frozen PBMCs, isolated from the fresh blood (fresh frozen), in the VZV ELISPOT assay. The blood was collected into EDTA anticoagulant, PBMCs were isolated and aliquoted, and some aliquots were frozen. Frozen PBMCs were thawed following the procedure with incorporation of Benzonase-supplemented medium. As shown in Fig. 3, the adjusted VZV responses of frozen PBMCs were comparable to the responses observed with the freshly isolated PBMCs. This was consistent for both low- and high-responding donors.
FIG. 3.
Comparison of IFN-γ VZV ELISPOT responses with fresh versus frozen PBMCs. SFC represent the adjusted VZV ELISPOT response, i.e., the mean response to VZV antigen for replicate wells minus the mean response to MRC-5 control antigen for replicate wells.
Characterization of T-cell response in ELISPOT assay.
Fresh-frozen PBMC samples were tested in the ELISPOT assay after depletion of CD4+- or CD8+-T-cell subsets. Following subset depletion, PBMCs were tested in the ELISPOT assay along with the undepleted cell population for responses to both VZV antigen and tetanus toxoid. As shown in Table 1, depletion of the CD4+ T cells resulted in a complete loss of response to both VZV and tetanus toxoid antigens in the assay. The response to mitogen (PHA) remained very strong with these samples (scored as too numerous to count [data not shown]). The loss of response signal was seen even in the absence of complete depletion of the CD4+-T-cell population (approximately 10% CD4+ T cells remained following depletion as assessed by FACS staining analysis [data not shown]). When the CD8+-T-cell population was depleted, there was no observed reduction in VZV- or tetanus toxoid-specific response. In fact, there was a slight increase in the measured response. The adjusted VZV responses went from 227 to 277 SFC for donor 1481 and from 191 to 207 for donor 2515, when comparing the undepleted response with response following CD8+-T-cell depletion. The CD8+ depletion was complete, with <1% CD8+ T cells detected by FACS analysis (data not shown). The increase in detected response for these samples most likely resulted from enrichment of the CD4 population concomitant with the depletion of CD8 T cells.
TABLE 1.
IFN-γ ELISPOT response following CD4 and CD8 T-cell depletion
| Antigen and donor | Adjusted SFC/106 PBMCsa
|
||
|---|---|---|---|
| Unseparated | CD4 depleted | CD8 depleted | |
| VZV | |||
| 1481 | 227 | 1 | 277 |
| 2515 | 191 | 1 | 207 |
| Tetanus toxoid | |||
| 1481 | 35 | 4 | 66 |
| 2515 | 112 | 0 | 128 |
Adjusted SFC counts represent the mean VZV or tetanus toxoid counts minus the mean counts for the appropriate control (MRC-5 antigen for VZV and medium for tetanus). All spot counts were determined from the means for replicate assay wells.
Effect of storage conditions on frozen PBMCs.
Aliquots of frozen PBMCs were exposed to different combinations of liquid nitrogen (vapor phase) and dry ice storage. The samples were then tested in an ELISPOT assay (Table 2). There were no significant differences in the adjusted VZV SFC for PBMC aliquots following any of the storage conditions tested, with the exception of the 2515 sample, which was cycled from liquid nitrogen to dry ice for 48 h and then back to liquid nitrogen. This PBMC sample had a response of 108 SFC, compared with 185 SFC for the sample that was stored only in liquid nitrogen. This decrease in response was not observed with PBMC samples from other donors exposed to the same storage conditions.
TABLE 2.
Comparison of IFN-γ VZV ELISPOT responses of frozen PBMCs exposed to different storage conditions
| Donor | Adjusted SFCa/106 PBMCs with the following storage conditions for the frozen PBMC aliquot prior to assay testingb:
|
||||
|---|---|---|---|---|---|
| LN2 | LN2, DI 24 h | LN2, DI 24 h, LN2 | LN2, DI 48 h, LN2 | LN2, DI 24 h, LN2, DI 24 h, LN2 | |
| 01 | 195 | 180 | 168 | 193 | 197 |
| 06 | 232 | 226 | 215 | 210 | 231 |
| 1481 | 244 | 218 | 179 | 171 | NTc |
| 2515 | 185 | 185 | 176 | 108 | NT |
See Table 1, footnote a.
LN2, liquid nitrogen, vapor phase storage; DI, samples encased in dry ice.
NT, not tested.
We have also tested frozen PBMC samples over an extended period (>22 months) of liquid nitrogen storage. A large number (>100) of PBMC aliquots were prepared and frozen at a single time from two different donors. These PBMC aliquots were then monitored over time for cell yields, viability on thawing, and ELISPOT response. As shown in Fig. 4, ELISPOT responses from these donor cells remained relatively consistent for up to 22 months of storage. Table 3 contains a summary of the mean cell yields and adjusted VZV spot counts for assays over this 22-month period. The cell yield and spot count results were determined from a total of 68 events for donor 1481 and >40 events for donor 2515. There were no changes in either cell yield (per frozen aliquot) on thawing or cell viability (data not shown) detected over this storage period. The cell yields per vial had standard deviations that were less than 10% of the total cells obtained on thawing. The viability of the frozen aliquots is consistently around 95% (by trypan blue dye exclusion) with up to 22 months of frozen storage. The response (SFC) variability for PBMC aliquots from these donors (percent relative standard deviations [RSDs] of 20.27 and 31.20) falls within the assay variability attributable to the combination of reader (23.3%) and run (17.8%) variabilities for testing fresh-frozen PBMC samples in the ELISPOT assay (see below).
FIG. 4.
IFN-γ ELISPOT response of frozen cells from a positive control cell bank over a 22-month period. The dates shown for the open and closed circles indicate when PBMCs were collected and frozen. Aliquots were stored in vapor phase liquid nitrogen.
TABLE 3.
Historical IFN-γ VZV ELISPOT responses and frozen-aliquot cell yields for long-term storage control PBMC samples
| Donor | Adjusted VZV SFCa
|
Cell yield/vial (107)
|
||||
|---|---|---|---|---|---|---|
| Mean | SD | % RSDb | Mean | SD | % RSD | |
| 1481c | 254 | 53.29 | 21.00 | 1.39 | 0.17 | 11.90 |
| 2515d | 192 | 44.67 | 23.24 | 1.54 | 0.15 | 9.95 |
SFC/106 PBMCs determined by the mean spot count for replicate VZV wells minus the mean spot count for replicate MRC-5 control wells.
Coefficient of variation (standard deviation/mean) × 100.
n > 60 for both spot count and cell yield.
n = 49 for spot count and n = 42 for cell yield.
ELISPOT assay variability using fresh-frozen PBMCs.
A validation study was performed with the final, standardized VZV ELISPOT protocol testing fresh-frozen PBMC samples. This protocol was designed to assess the assay repeatability and variability for monitoring patient responses over time. PBMCs were collected from four donors on at least four different days. The PBMCs were isolated and frozen from blood on the day it was collected (fresh-frozen cells). Aliquots of frozen PBMCs from four different bleeds for each donor were assayed together within a run. This was repeated for three separate assay runs. The spots on the assay plates were then counted by five different human readers and were also imaged and counted five times with the ImmunoSpot image analysis computer system. The variability of the ELISPOT assay was assessed for determination of significant differences between bleeds from the same individual. The adjusted VZV spot count (SFC) results for the individual blood donors, determined by both human and computer readings, are presented in Table 4. The assay variability (percent RSD) determined by the statistical analysis of these validation data is presented in Table 5. The overall variability for the assay has been broken down into three areas: assay run, bleed, and (in the case of human readers) reader. The spot counts obtained by the computer are similar in magnitude, and without change in rank order, to those obtained with the human readers (Table 4). The counts obtained with the ImmunoSpot computer system are at least as reproducible as the human counts, with percent RSDs that are either equivalent to or smaller than those associated with the comparable human counts. The assay variabilities (percent RSD), shown in Table 5, associated with bleed (PBMCs collected at different times) were similar for human readers (20.13) and the computer (21.03). The variability for assay run (run to run) was determined to be higher when the assay was quantified with human readers (17.78) than when counted with the computer-assisted imaging system (8.55). The combined run-plus-bleed variability of the assay, indicating overall assay variability when counted by the same reader, was higher for human readers (30.45) than for the computer-read assays (23.09). The largest single factor for variability associated with the VZV ELISPOT assay was attributed to the human reader (23.30).
TABLE 4.
Comparison of adjusted VZV spot counts performed by human readers versus the ImmunoSpot Image Analyzera
| Donor and bleed no. | Human readers
|
ImmunoSpot computer
|
||
|---|---|---|---|---|
| Mean SFC | % RSD | Mean SFC | % RSD | |
| 2617 | ||||
| 1 | 63 | 25.8 | 51 | 8.8 |
| 2 | 57 | 25.7 | 43 | 6.4 |
| 3 | 134 | 14.1 | 116 | 15.1 |
| 4 | 150 | 17.9 | 124 | 12.7 |
| 3446 | ||||
| 1 | 35 | 26.6 | 29 | 12.4 |
| 2 | 38 | 24.2 | 33 | 25.2 |
| 3 | 30 | 31.2 | 27 | 17.7 |
| 4 | 35 | 32.2 | 27 | 17.0 |
| 3802 | ||||
| 1 | 195 | 29.7 | 184 | 5.5 |
| 2 | 272 | 19.9 | 252 | 6.9 |
| 3 | 259 | 23.7 | 234 | 13.3 |
| 4 | 328 | 30.4 | 287 | 16.5 |
| 5 | 241 | 27.7 | 205 | 4.2 |
| 4574 | ||||
| 1 | 421 | 18.7 | 434 | 3.8 |
| 2 | 323 | 44.8 | 319 | 6.2 |
| 3 | 313 | 18.3 | 319 | 6.8 |
| 4 | 340 | 20.3 | 351 | 4.4 |
| 5 | 444 | 23.8 | 513 | 11.1 |
Results are for frozen PBMCs collected at four or five different time points for each donor. Mean values and percent RSDs (coefficient of variation [standard deviation/mean] × 100) are based on counts obtained from five human readings and five ImmunoSpot computer readings for each donor bleed. SFC are per 106 PBMCs and represent the mean SFC for VZV antigen minus the mean SFC for MRC-5 control antigen.
DISCUSSION
We have developed a VZV IFN-γ ELISPOT assay applicable for assessing cellular immune responses following either natural infection or vaccination. The traditional methods used for assessment of cellular responses to VZV have proven to be both cumbersome and labor-intensive. In general, they also lack desired levels of sensitivity and are not very versatile. The use of a lymphoproliferation assay, the RCF assay, has been described and extensively employed for quantifying T-cell responses (8, 14–16, 21, 33). However, this assay has disadvantages in that it requires the use of freshly isolated PBMCs, is very labor-intensive, requires clonal expansion of responding cell populations over an extended incubation period, and incorporates labeling and detection with radionucleotide. CTL assays have also been previously employed to look at CD8+-T-cell responses (13, 30). These assays have many of the same encumbrances as the RCF assay and have the additional constraint of not being quantitative without incorporation of the even more laborious limiting-dilution format. The limited data for CTL responses following VZV immunization have indicated low frequencies for these populations, and the specific role of CD8+ T cells in the control of varicella infections is not well described to date (13, 28, 30). More recently, the method of intracellular cytokine staining for detection of antigen-specific IFN-γ production has been employed (6). This method permits monitoring of both CD4+ and CD8+ responding cells within the same assay. Additionally, this assay allows a quantitative analysis of responding cell frequencies without the need for clonal expansion and large numbers of cells being analyzed with limiting dilution. The limitations with intracellular cytokine staining may lie with the background responses detected. The signal-to-noise ratios presented for VZV antigen stimulation may not allow adequate resolution of changes in low-frequency responses of samples and individuals. Additionally, this assay utilizes either fresh blood or freshly isolated cells and requires expensive instrumentation and trained personnel for sample analysis. However, this is a new methodology with much promise and the opportunity for further refinement.
The ELISPOT assay that we have developed is extremely sensitive, with the ability to detect fewer than 10 SFC per million PBMCs. Additionally, background responses to medium or MRC-5 cell lysate controls in this ELISPOT assay are very low, generally less than 10% of the antigen-specific responses. The assay is very sensitive for measuring changes in VZV-specific responses between two bleed points for an individual donor. The ability to use a computer-assisted image analysis for spot quantitation also reduces the most labor-intensive and variable aspect of the assay. The ImmunoSpot image analysis system utilized in this study produced spot counts statistically similar to the counts obtained by human readers, with reduced variability attributed both to reader and total assay (reader and run variabilities combined).
Only CD4+-T-cell responses were detected with this assay. Evidence for low-frequency CD8+-T-cell responses has been presented for immunized individuals (13, 30). However, assessment of memory responses to VZV with this ELISPOT assay has not detected any CD8+-T-cell component with the antigens tested to date, consistent with the observations recently published for intracellular cytokine staining (6). It may be that CD8+ memory populations are undetectable without clonal expansion. It remains to be determined if this assay can be adapted (i.e., by using peptide antigens, testing cells during acute infection, or longer incubation) for detection of any CD8+-T-cell responses to VZV that may exist.
The VZV antigen response was shown to be linear over a narrow range of input cell numbers (from 2 × 105 to 6 × 105 cells/assay well). Accessory cells play an important role in antigen processing and presentation in association with major histocompatibility complex class II molecules for CD4+-T-cell responses. Dilution of cell numbers in the assay well reduces both responding cells and antigen-presenting cells, which also may affect contact between the two populations. It has been shown in a mouse ELISPOT assay system that the ratio of spots (response) to primed cells remained linear only when feeder cells were added to keep the total number of cells per well constant (26). There is a critical level reached where the concentration of antigen-presenting cells and CD4+ T cells is inadequate for detection of a response. This presents an even greater detection problem for donors whose responses are low, even when tested at higher cell concentrations. These lower responses became more difficult to detect when seeding only 2 × 105 cells/assay well.
The ability to use cryopreserved PBMCs in this IFN-γ ELISPOT lends itself to monitoring patient responses over time. The ability to cryopreserve PBMCs for long periods without a significant reduction over time in cell recovery percentage, viability, and CD4+ lymphocyte levels has previously been demonstrated (20). Cryopreserved PBMCs have been shown to respond to mitogen (PHA) in an ELISPOT assay similarly to fresh PBMCs (32). We have demonstrated that the VZV antigen-specific responses detected with cells frozen at the time of blood collection (fresh blood) accurately represent responses measured with the freshly isolated cells. These cryopreserved cell aliquots can be stored over long periods in liquid nitrogen without any significant change in the antigen-specific response being detected. Additionally, the cryopreserved samples can be exposed to certain changes in storage conditions without reduction in the assay signal. This allows sample collection at one site with testing performed at a remote site without compromising the responses detected.
Cryopreserved PBMCs isolated from blood that was stored overnight can also be tested with this assay. However, in most cases the responses detected are substantially reduced compared with those from cryopreserved PBMCs isolated from fresh blood. Cell clumping was observed during the thawing of many frozen PBMC samples isolated from blood stored overnight. Incorporation of DNase has been utilized to minimize cell clumping in other systems (22, 27). Extraction of lymphocytes from tissue using high DNase levels (1 mg/ml) over a prolonged incubation period (3 h) has been shown to have no effect on levels of CD4, CD25, or CD38 cell surface markers, but did reduce detection of CD8 and adhesion molecules such as L-selectin, by FACS analysis (34). We have chosen to incorporate an ultrapure nuclease (Benzonase), to eliminate the concern about any contaminating proteinases, at very low levels (50 U/ml) with short exposure periods. Incorporation of Benzonase in the thawing procedure eliminated the cell clumping. In some cases, responses detected for overnight blood PBMC samples processed with Benzonase were similar to those detected with the PBMCs isolated from fresh blood. However, the ELISPOT responses for most PBMC samples from overnight blood were still not representative of responses detected with fresh blood. We observed no differences in cell surface detection by FACS of CD4, CD8, CD38, or CD62L with or without the incorporation of Benzonase. Whereas most cryopreserved PBMCs from fresh blood did not exhibit cell clumping, infrequently there was a sample that did clump on thawing. Benzonase prevented cell clumping without any negative effects on assay results for cryopreserved PBMCs isolated from fresh blood. Based on all of these results, we have adopted the use of Benzonase in all thawing procedures for cryopreserved PBMCs from either fresh or overnight blood.
The use of cryopreserved cells presents an additional advantage for the ELISPOT assay over the RCF assay. RCF assays require testing of PBMCs immediately after they are isolated from freshly collected blood (14, 35). The use of cryopreserved PBMC samples allows direct comparison, within a single assay run, of PBMC samples from multiple time points during the course of patient study. This reduces the variability associated with sample testing, as only intra-assay variability need be applied and the components of interassay variability (run-to-run or reader variability) in response comparisons can be eliminated. The use of the computer-assisted ImmunoSpot image analyzer can also reduce the assay variability associated with readers and assay runs, while providing sample response values and variability estimates that are consistent with those of human readers. This new, validated VZV IFN-γ ELISPOT assay provides a sensitive, versatile, and quantitative tool for assessing the cellular immune responses to VZV.
ACKNOWLEDGMENTS
We thank Paul Keller for providing the VZV and control antigens used in these studies, and we thank Rupert Vessey for helpful comments on the manuscript.
REFERENCES
- 1.Abendroth A, Arvin A. Varicella-zoster virus immune evasion. Immunol Rev. 1999;168:143–156. doi: 10.1111/j.1600-065x.1999.tb01289.x. [DOI] [PubMed] [Google Scholar]
- 2.Arvin A M. Cell-mediated immunity to varicella-zoster virus. J Infect Dis. 1992;166:S35–S41. doi: 10.1093/infdis/166.supplement_1.s35. [DOI] [PubMed] [Google Scholar]
- 3.Arvin A M. Clinical manifestations of varicella and herpes zoster and the immune response to varicella-zoster virus. In: Hyman R, editor. The natural history of varicella-zoster virus. New York, N.Y: CRC Press; 1987. pp. 67–130. [Google Scholar]
- 4.Arvin A M, Koropchak C M, Williams B R G, Grumet F C, Foung S K H. The early immune response in healthy and immunocompromised subjects with primary varicella-zoster virus infection. J Infect Dis. 1986;154:422–429. doi: 10.1093/infdis/154.3.422. [DOI] [PubMed] [Google Scholar]
- 5.Asai T, Storkus W J, Whiteside T L. Evaluation of the modified ELISPOT assay for gamma interferon production in cancer patients receiving antitumor vaccines. Clin Diagn Lab Immunol. 2000;7:145–154. doi: 10.1128/cdli.7.2.145-154.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asanuma H, Sharp M, Maecker H T, Maino V C, Arvin A M. Frequencies of memory T cells specific for varicella-zoster virus, herpes simplex virus, and cytomegalovirus by intracellular detection of cytokine expression. J Infect Dis. 2000;181:859–866. doi: 10.1086/315347. [DOI] [PubMed] [Google Scholar]
- 7.Bergen R E, Sharp M, Sanchez A, Judd A K, Arvin A M. Human T cells recognize multiple epitopes of an immediate early/tegument protein (IE62) and glycoprotein I of varicella zoster virus. Viral Immunol. 1991;4:151–166. doi: 10.1089/vim.1991.4.151. [DOI] [PubMed] [Google Scholar]
- 8.Berger R, Trannoy E, Hollander G, Bailleux F, Rudin C, Creusvaux H. A dose-response study of a live attenuated varicella-zoster virus (Oka strain) vaccine administered to adults 55 years of age and older. J Infect Dis. 1998;178:S99–103. doi: 10.1086/514265. [DOI] [PubMed] [Google Scholar]
- 9.ElGhazali G E B, Paulie S, Andersson G, Hansson Y, Holmquist G, Sun J, Olsson T, Ekre H P, Troy-Blomberg M. Number of interleukin-4 and interferon-γ-secreting human T cells reactive with tetanus toxoid and the mycobacterial antigen PPD or phytohemagglutinin: distinct response profiles depending on the type of antigen used for activation. Eur J Immunol. 1993;23:2740–2745. doi: 10.1002/eji.1830231103. [DOI] [PubMed] [Google Scholar]
- 10.Giller R H, Winistorfer S, Grose C. Cellular and humoral immunity to varicella zoster virus glycoproteins in immune and susceptible human subjects. J Infect Dis. 1989;160:919–928. doi: 10.1093/infdis/160.6.919. [DOI] [PubMed] [Google Scholar]
- 11.Habermehl P, Lignitz A, Knuf M, Schmitt H, Slaoui M, Zepp F. Cellular immune response of a varicella vaccine following simultaneous DTaP and VZV vaccination. Vaccine. 1999;17:669–674. doi: 10.1016/s0264-410x(98)00249-7. [DOI] [PubMed] [Google Scholar]
- 12.Hayes F A, Feldman S. Cell-mediated immunity to varicella-zoster virus in children being treated for cancer. Cancer. 1978;42:159–163. doi: 10.1002/1097-0142(197807)42:1<159::aid-cncr2820420126>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 13.Hayward A, Villanueba E, Cosyns M, Levin M. Varicella-zoster virus (VZV)-specific cytotoxicity after immunization of nonimmune adults with Oka strain attenuated VZV vaccine. J Infect Dis. 1992;166:260–264. doi: 10.1093/infdis/166.2.260. [DOI] [PubMed] [Google Scholar]
- 14.Hayward A R, Buda K, Levin M J. Immune response to secondary immunization with live or inactivated VZV vaccine in elderly adults. Viral Immunol. 1994;7:31–36. doi: 10.1089/vim.1994.7.31. [DOI] [PubMed] [Google Scholar]
- 15.Hayward A R, Cosyns M, Jones M, Levin M J, Villaneuba E, Weinberg A, Chan C Y. Cytokine production in varicella-zoster virus-stimulated cultures of human blood lymphocytes. J Infect Dis. 1998;178:S95–S98. doi: 10.1086/514266. [DOI] [PubMed] [Google Scholar]
- 16.Hayward A R, Zerbe G O, Levin M J. Clinical application of responder cell frequency estimates with four years of follow up. J Immunol Methods. 1994;170:27–36. doi: 10.1016/0022-1759(94)90242-9. [DOI] [PubMed] [Google Scholar]
- 17.Jenkins D E, Redman R L, Lam E M, Liu C, Lin I, Arvin A M. Interleukin (IL)-10, IL-12, and interferon-γ production in primary and memory immune responses to varicella-zoster virus. J Infect Dis. 1998;178:940–948. doi: 10.1086/515702. [DOI] [PubMed] [Google Scholar]
- 18.Jenkins D E, Yasukawa L L, Benike C J, Engleman E G, Arvin A M. Isolation and utilization of human dendritic cells from peripheral blood to assay an in vitro primary immune response to varicella-zoster virus peptides. J Infect Dis. 1998;178:S39–42. doi: 10.1086/514258. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins D E, Yasukawa L L, Bergen R, Benike C, Engleman E G, Arvin A M. Comparison of primary sensitization of naive human T cells to varicella-zoster virus peptides by dendritic cells in vitro with responses elicited in vivo by varicella vaccination. J Immunol. 1999;162:560–567. [PubMed] [Google Scholar]
- 20.Kleeberger C A, Lyles R H, Margolick J B, Rinaldo C R, Phair J P, Giorgi J V. Viability and recovery of peripheral blood mononuclear cells cryopreserved for up to 12 years in a multicenter study. Clin Diagn Lab Immunol. 1999;6:14–19. doi: 10.1128/cdli.6.1.14-19.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levin M J, Barber D, Goldblatt E, Jones M, LaFleur B, Chan C, Stinson D, Zerbe G, Hayward A R. Use of a live attenuated varicella vaccine to boost varicella-specific immune responses in seropositive people 55 years of age and older: duration of booster effect. J Infect Dis. 1998;178:S109–12. doi: 10.1086/514264. [DOI] [PubMed] [Google Scholar]
- 22.Machalinski B, Szolomicka P, Kijowski J, Baskiewicz M, Karbicka A, Byra E, Majka M, Giedrys-Kalemba S, Ratajczak M Z. Short-term storage of human haematopoietic cells. Influence of air and deoxyribonuclease I. Ann Transplant. 1999;4:29–36. [PubMed] [Google Scholar]
- 23.McCutcheon M, Wehner N, Wensky A, Kushner M, Doan S, Hsiao L, Calabresi P, Ha T, Tran T V, Tate K M, Winkelhake J, Spack E G. A sensitive ELISPOT assay to detect low-frequency human T lymphocytes. J Immunol Methods. 1997;210:149–166. doi: 10.1016/s0022-1759(97)00182-8. [DOI] [PubMed] [Google Scholar]
- 24.Milliken G, Johnson D. Analysis of messy data. 1. Designed experiments. Belmont, Calif: Wadsworth Inc.; 1984. [Google Scholar]
- 25.Nader S, Bergen R, Sharp M, Arvin A M. Age-related differences in cell-mediated immunity to varicella-zoster virus among children and adults immunized with live attenuated varicella vaccine. J Infect Dis. 1995;171:13–17. doi: 10.1093/infdis/171.1.13. [DOI] [PubMed] [Google Scholar]
- 26.Power C A, Grand C L, Ismail N, Peters N C, Yurkowski D P, Bretscher P A. A valid ELISPOT assay for enumeration of ex vivo, antigen-specific, IFN-γ-producing cells. J Immunol Methods. 1999;227:99–107. doi: 10.1016/s0022-1759(99)00074-5. [DOI] [PubMed] [Google Scholar]
- 27.Querol S, Capmany G, Azqueta C, Gabarro M, Fornas O, Martin-Henao G A, Garcia J. Direct immunomagnetic method for CD34+ cell selection from cryopreserved cord blood grafts for ex vivo expansion protocols. Transfusion. 2000;40:625–631. doi: 10.1046/j.1537-2995.2000.40060625.x. [DOI] [PubMed] [Google Scholar]
- 28.Sadzot-Delvaux C, Arvin A M, Rentier B. Varicella-zoster virus IE63, a virion component expressed during latency and acute infection, elicits humoral and cellular immunity. J Infect Dis. 1998;178:S43–7. doi: 10.1086/514259. [DOI] [PubMed] [Google Scholar]
- 29.Schmittel A, Keilholz U, Scheibenbogen C. Evaluation of the interferon-γ ELISPOT-assay for quantification of peptide specific T lymphocytes from peripheral blood. J Immunol Methods. 1997;210:167–174. doi: 10.1016/s0022-1759(97)00184-1. [DOI] [PubMed] [Google Scholar]
- 30.Sharp M, Terada K, Wilson A, Nader S, Kinchington P E, Ruyechan W T, Hay J, Arvin A M. Kinetics and viral protein specificity of the cytotoxic T lymphocyte response in healthy adults immunized with live attenuated varicella vaccine. J Infect Dis. 1992;165:852–858. doi: 10.1093/infdis/165.5.852. [DOI] [PubMed] [Google Scholar]
- 31.Skeie G O, Bentsen P T, Freiburg A, Arli J A A, Gilhus N E. Cell-mediated immune response against titin in myasthenia gravis: evidence for the involvement of Th1 and Th2 cells. Scand J Immunol. 1998;47:76–81. doi: 10.1046/j.1365-3083.1998.00260.x. [DOI] [PubMed] [Google Scholar]
- 32.Sobota V, Bubenik J, Indrova M, Vlk V, Jakoubkova J. Use of cryopreserved lymphocyte for assessment of the immunological effects of interferon therapy in renal carcinoma patients. J Immunol Methods. 1997;203:1–10. doi: 10.1016/s0022-1759(97)00020-3. [DOI] [PubMed] [Google Scholar]
- 33.Trannoy E, Berger R, Hollander G, Bailleux F, Heimendinger P, Vuillier D, Creusvaux H. Vaccination of immunocompetent elderly subjects with a live attenuated Oka strain of varicella zoster virus: a randomized, controlled, dose-response trial. Vaccine. 2000;18:1700–1706. doi: 10.1016/s0264-410x(99)00510-1. [DOI] [PubMed] [Google Scholar]
- 34.Van Damme N, Baeten D, De Vos M, Demetter P, Elewaut D, Mielants H, Verbruggen G, Cuvelier C, Veys E, De Keyser F. Chemical agents and enzymes used for the extraction of gut lymphocytes influence flow cytometric detection of T cell surface markers. J Immunol Methods. 2000;236:27–35. doi: 10.1016/s0022-1759(99)00243-4. [DOI] [PubMed] [Google Scholar]
- 35.Weinberg A, Betensky R A, Zhang L, Ray G. Effect of shipment, storage, anticoagulant, and cell separation on lymphocyte proliferation assays for human immunodeficiency virus-infected patients. Clin Diagn Lab Immunol. 1998;5:804–807. doi: 10.1128/cdli.5.6.804-807.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zerboni L, Nader S, Aoki K, Arvin A M. Analysis of the persistence of humoral and cellular immunity in children and adults immunized with varicella vaccine. J Infect Dis. 1998;177:1701–1704. doi: 10.1086/517426. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Cosyns M, Levin M J, Hayward A R. Cytokine production in varicella zoster virus-stimulated limiting dilution lymphocyte cultures. Clin Exp Immunol. 1994;98:128–133. doi: 10.1111/j.1365-2249.1994.tb06618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, White C J, Levin M, Hayward A R. Cytokine production in varicella-zoster virus-stimulated lymphocyte cultures. Neurology. 1995;45:S38–S40. doi: 10.1212/wnl.45.12_suppl_8.s38. [DOI] [PubMed] [Google Scholar]