Abstract
Background
Neurobiological measures may inform our understanding of individual differences in adolescents’ general risk for and resilience to depressive symptoms, including during the COVID-19 pandemic. We tested a developmental model linking variation in amygdala–subgenual anterior cingulate cortex (sgACC) resting-state connectivity to perceived parenting experiences earlier in adolescence, to concurrent depressive symptoms before the pandemic, and to subsequent depressive symptoms during the pandemic.
Methods
We used data from a longitudinal study that included three waves (N = 214 adolescents; ages 9–15 years at time 1 [T1], 11–17 years at T2, and 12–19 years during the pandemic at T3). We assessed positive parenting (warm and supportive) (T1), depressive symptoms (T1 to T3), and functional connectivity between the sgACC and basolateral (BLA) and centromedial amygdala (T1 and T2). We modeled associations among earlier positive parenting, amygdala–sgACC connectivity, and depressive symptoms before and during the pandemic.
Results
Less positive parenting at T1 was associated prospectively with stronger BLA–sgACC connectivity at T2 (β = −0.22) over and above the effect of BLA–sgACC connectivity at T1. Stronger BLA–sgACC connectivity, in turn, was associated with heightened depressive symptoms, both before the pandemic (r = 0.21) and during the pandemic (β = 0.19; independent of the effect of pre-pandemic symptoms).
Conclusions
Adolescents who experience less positive parenting may develop a pattern of BLA–sgACC connectivity that increases their risk for mental health problems. BLA–sgACC connectivity may be associated with depressive symptoms in general, including during periods of heightened risk for adolescents, such as the pandemic.
Keywords: Adolescence, Amygdala, COVID-19, Depression, Parenting, Subgenual anterior cingulate cortex
The COVID-19 pandemic is a unique period of disruption. Many individuals are facing multiple challenges related to the pandemic that pose serious threats to mental health, including economic hardship, threats to physical health, and enforced social isolation (1, 2, 3). Adolescents generally are at an increased risk for mental health problems such as depression (4,5), and depressive symptoms in adolescents appear to be increasing during the pandemic (6,7). Some adolescents are more vulnerable than others to experiencing difficulties during the pandemic, differences that have been found to be indexed by neurobiological and physiological measures (8, 9, 10). We recently showed that aspects of neurobiology can moderate risk and resilience to depressive symptoms during the pandemic (8,10) and, therefore, might serve as targets for intervention and prevention. Moreover, identifying developmental factors that are associated with predictors of mental health could inform efforts to promote resilience to psychopathology in general and during future periods of heightened risk for adolescents. In this context, therefore, the present study tests a developmental model linking variation in neural connectivity in adolescents to experiences in the family environment and depressive symptoms before and during the COVID-19 pandemic.
Depression has been found to be characterized by functional abnormalities in the amygdala and subgenual anterior cingulate cortex (sgACC) (11,12). In fact, resting-state functional connectivity (FC) between the amygdala and sgACC has been implicated in both stress and depression (13,14). Broadly, whereas the amygdala supports rapid detection and responding to salient events (15), the sgACC is involved in affective appraisal, sad mood, and rumination (16). Several studies with adolescents have found that stronger positive amygdala–sgACC connectivity is associated with depression (14,17,18), with perceived psychological stress (19), and with increased sensitivity to negative stimuli (20); this latter study also found that increases in amygdala–sgACC connectivity were associated with the onset of depression (20). Similar findings have been reported in adults (13); further, a stress reduction intervention has been found to weaken amygdala–sgACC connectivity (13). Taken together, increased intrinsic amygdala–sgACC connectivity appears to play a role in negative affect and vulnerability to depression. It is unclear, however, whether amygdala–sgACC connectivity is associated with elevated depressive symptoms during periods of heightened risk for mental health problems in adolescents, such as the COVID-19 pandemic.
It is important that a longitudinal predictor of relative risk and resilience be associated with well-being in an enduring manner. For example, it is possible that pre-pandemic amygdala–sgACC connectivity is associated concurrently with depressive symptoms before the pandemic, and that this accounts for prospective associations with symptoms during the pandemic. That is, pre-pandemic amygdala–sgACC connectivity may have a transient rather than an enduring effect on the development of depressive symptoms. If amygdala–sgACC connectivity is a viable marker of individual differences in depressive symptoms in general, including during the pandemic, it should be associated with pre-pandemic symptoms but should also predict symptoms during the pandemic above and beyond the effect of pre-pandemic symptoms.
In addition to considering amygdala–sgACC connectivity as a correlate of functioning both before and during the pandemic, it is important to examine whether variation in the development of this neural connection is related to a history of environmental risk for psychopathology. Holz et al. (21) proposed that the amygdala and ventral regions of the ACC are neural convergence sites for social risk and resilience factors. One such social-environmental factor that has been implicated in adolescent depressive symptoms is parenting practices (22,23), which has also recently been linked to adolescent neurodevelopment (24, 25, 26). Specifically, positive parenting behaviors, including expressions of affection, supportiveness, and sensitivity, have been found to be negatively associated with adolescents’ development of depressed mood (27) and positively associated with adolescents’ effective coping with stress (28). Positive parenting may help contribute to adolescents’ mental health via its influence on neurodevelopment important for emotion regulation (29).
Amygdala–sgACC FC undergoes significant changes during adolescence (30,31) and, therefore, could be sensitive to environmental input such as parenting. A growing body of research is investigating how parenting practices are related to the development and functioning of the amygdala and sgACC. In adolescents with a history of anxiety, parental warmth has been prospectively associated with reduced amygdala and sgACC activation during an emotion processing task and has been linked indirectly with less severe internalizing symptoms via reductions in sgACC activation (32). In other research, adolescents with mothers who directed more negative affect toward them showed blunted amygdala and sgACC response to a social reward (33) and increased resting-state FC between the amygdala and insula, superior temporal gyrus, and temporal cortical regions (34). Thus, although previous studies have reported evidence that parenting is related to amygdala and sgACC activation and FC of the amygdala, researchers have not yet examined parenting practices specifically in relation to amygdala–sgACC FC, despite their broader link with adolescent well-being. In addition, most studies have used cross-sectional samples or longitudinal designs with a single neuroimaging assessment [but see (35) for an exception]. Conducting multiple neuroimaging assessments is critical for examining the effects of parenting on the development of amygdala–sgACC FC during adolescence. Recent work suggests that typical development is characterized by reduced amygdala–sgACC FC in adolescence compared with childhood and adulthood (30). Less positive parenting may impede amygdala–sgACC FC development or, from a functional perspective [e.g., (36)], may influence this connection in a way that is adaptive in a specific context, but that is also developmentally atypical and increases vulnerability to psychopathology (i.e., stronger amygdala–sgACC FC).
The current longitudinal study examined whether amygdala–sgACC resting-state FC in adolescents 1) is related to parental warmth and supportiveness (i.e., positive parenting) earlier in adolescence and 2) is related to depressive symptoms both concurrently (i.e., pre-pandemic) and prospectively during the early phase of the pandemic. We hypothesized that less positive parenting would be linked to patterns of amygdala–sgACC FC that heighten risk for adolescent depressive symptoms and that potentially reflect aberrant development of this neural connection. Thus, we expected that less positive parenting earlier in adolescence would be associated both concurrently and prospectively with stronger amygdala–sgACC connectivity which, in turn, would be associated with heightened depressive symptoms, both before and during the pandemic. Finally, we expected that the prospective association between positive parenting and amygdala–sgACC connectivity would be independent of the effect of earlier amygdala–sgACC connectivity on later connectivity, and that the prospective association between amygdala–sgACC connectivity and depressive symptoms during the pandemic would be independent of the effect of pre-pandemic depressive symptoms.
Methods and Materials
Participants and Procedures
Participants were adolescents from the San Francisco Bay Area from an ongoing longitudinal study of the effects of early-life stress on neurodevelopment during puberty (N = 214; 121 females; mean age at baseline = 11.40, SD = 1.0; 10.3% Asian, 20.1% biracial, 8.4% Black, 8.4% Hispanic/Latinx, 6.5% Other, 44.4% White, 1.9% did not report; median family income = $100K–$125K, range ≤$5K–≥$150K). Families were recruited from the community using flyers, media, and online advertisements. Study exclusion criteria at baseline included inability to undergo magnetic resonance imaging (MRI) (e.g., had metal implants, braces), a history of neurological disorder or major medical illness, cognitive or physical challenges that might interfere with the ability to understand or complete procedures, nonfluent English speakers, and, for females, the onset of menses. Males and females were matched on self-reported pubertal stage at the baseline assessment; thus, females were, on average, younger than males in our sample (see the Supplement). The current analysis included adolescents who completed up to three assessments that were, on average, 2 years apart (N = 214; interval between assessments = 1−4 years) (see the Supplement for additional information about attrition and study design). At time 1 (T1) (n = 208; 117 females; mean age = 12.02 years, SD = 1.45, range = 9.17−15.82), adolescents reported on parenting behaviors of their primary caregiver, their own depressive symptoms, and completed a resting-state functional MRI scan from which we measured FC between the amygdala and sgACC. At T2 (n = 144; 78 females; mean age = 14.29 years, SD = 1.59, range = 11.08−17.91), adolescents again completed a resting-state functional MRI scan and assessment of their own depressive symptoms. At T3 (n = 109; 65 females; mean age = 16.30 years, SD = 1.45, range = 12.82–19.98), approximately 3 weeks after shelter-in-place was implemented in the Bay Area (April 2020), adolescents reported on their depressive symptoms over the preceding 1 week using a measure administered online (see the Supplement for details about the state of the pandemic during our assessment and some of the effects on our sample). The current analyses included participants who had usable data on any variable of interest at any study time point. All families signed assent and consent forms and were compensated for their participation. The study protocol was approved by the Stanford University Institutional Review Board.
Positive Parenting at T1
At T1, adolescents reported on positive parenting using the 11-item warmth and supportiveness subscale of the Parenting Styles and Dimensions Questionnaire (e.g., “my parent gives comfort and understanding when I am upset,” “my parent gives me praise when I am good”) (37). Adolescents rated items on a 5-point scale ranging from 1 (“almost never”) to 5 (“very often”). We averaged responses to yield a total score for parental warmth at T1 (α = 0.89).
Resting-State Functional MRI at T1 and T2
At T1 and T2 (1.28–3.85 years after the T1 assessment; mean interval = 2.03 years, SD = 0.34), neuroimaging data were acquired using a GE Discovery MR750 3T scanner (GE Medical Systems) with a 32-channel head coil (Nova Medical). Detailed scan parameters, described previously (38), as well as our approach to preprocessing and motion correction, are presented in the Supplement.
As described in Miller et al. (39), we focused on two major subdivisions of the amygdala: the basolateral amygdala (BLA) and centromedial amygdala (CMA). The BLA is involved in associative learning and evaluation of affective significance of environmental stimuli (15). The CMA is involved in allocation of attentional resources and increasing arousal via regulation of autonomic, endocrine, and behavioral responses to evocative stimuli (40). We defined BLA and CMA subdivisions as region of interest (see Figure 1A) using the Juelich anatomic atlas of probabilistic cytoarchitectonic maps provided in FSL (41,42). We defined the sgACC as Brodmann area 25 in the Mackey atlas of probabilistic cytoarchitectonic maps provided in AFNI (see Figure 1B) (43).
Figure 1.
Regions of interest including (left) the basolateral amygdala (yellow) and centromedial amygdala (red) and (right) the subgenual anterior cingulate cortex (red), used in the examination of amygdala–subgenual anterior cingulate cortex resting-state functional connectivity.
Adolescent Depressive Symptoms at T1, T2, and T3 (During the Pandemic)
At T1 and T2, adolescents reported on their depressive symptoms over the preceding 2 weeks using the 10-item version of the Children’s Depression Inventory (CDI) (44). For each item, adolescents endorsed one of three statements reflecting graded severity of symptoms. We summed responses to yield a total score for pre-pandemic depressive symptoms at T1 (α = 0.78) and T2 (α = 0.90).
At T3, 1.08–4.35 years after T2 (mean interval = 2.32, SD = 0.83), adolescents reported on their depressive symptoms over the preceding week using the Center for Epidemiological Studies Depression Scale for Children (CES-DC) (45). We used the CES-DC at T3 because some participants at this assessment were older than the validated upper age limit for the CDI. The CES-DC has 20 items, each of which adolescents rated on a 4-point scale ranging from 0 (not at all) to 3 (a lot). We summed responses to yield a total score for depressive symptoms during the pandemic (α = 0.76).
Finally, to make depressive symptom scores comparable across the three time points, we converted CDI scores at T1 and T2 and CES-DC scores at T3 to proportion of maximum scale scores (46).
Statistical Analyses
We used path analysis to examine hypothesized relations among parenting at T1, amygdala–sgACC FC and depressive symptoms at T1 and T2 (both pre-pandemic) and depressive symptoms at T1, T2 (both pre-pandemic), and T3 (during the pandemic). We tested separate models using bilateral average BLA–sgACC and CMA–sgACC as the neural connection of interest. We conducted secondary analyses testing amygdala–sgACC FC as a mediator between positive parenting and depressive symptoms. Model fit was assessed using χ2 tests, the comparative fit index, and the root mean square error of approximation with a 90% confidence interval (47). Good fit was indicated by nonsignificant χ2 values, comparative fit index values ≥ 0.95, and root mean square error of approximation values ≤ 0.05. Full information maximum likelihood estimation was used to produce model parameter estimates and to account for missing data. Little’s test suggested that data were missing completely at random (χ2177 = 197.32, p = .141). All models were tested using the lavaan package in R software (48). We used false discovery rate–adjusted p values to correct for multiple tests (i.e., all modeled paths and covariances) within each model.
Results
Descriptive statistics and zero-order correlations are presented in Table 1. Adolescents reported higher levels of depressive symptoms during the pandemic (T3) than before the pandemic (T2) (mean difference = 0.21, SD = 0.18, p < .001). During the pandemic, 62% of adolescents reported depressive symptoms in the clinically significant range (CES-DC proportion of maximum score >0.25). Depressive symptoms were not significantly different across T1 and T2 (before the pandemic) (mean difference = 0.01, SD = 0.13, p = .334). BLA–sgACC connectivity was significantly stronger than CMA–sgACC connectivity at both time points (all ps < .001).
Table 1.
Descriptive Statistics and Zero-Order Correlations
| Characteristic | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Sex (Female = 1) | 1 | |||||||||||
| 2 T1 Age | −0.19a | 1 | ||||||||||
| 3 T2 Age | −0.25b | 0.98a | 1 | |||||||||
| 4 T3 Age | −0.21c | 0.86a | 1 | 1 | ||||||||
| 5 T1 Positive Parenting | 0.10 | −0.15c | −0.11 | −0.00 | 1 | |||||||
| 6 T1 BLA–sgACC | 0.07 | −0.21b | −0.15 | −0.20c | 0.13 | 1 | ||||||
| 7 T2 BLA–sgACC | 0.00 | −0.03 | −0.00 | 0.02 | −0.20c | 0.14 | 1 | |||||
| 8 T1 CMA–sgACC | 0.00 | −0.19c | −0.20c | −0.07 | 0.03 | 0.47a | 0.03 | 1 | ||||
| 9 T2 CMA–sgACC | 0.01 | 0.10 | 0.11 | 0.08 | −0.12 | 0.12 | 0.44a | 0.18 | 1 | |||
| 10 T1 Depressive Symptoms | 0.07 | 0.10 | 0.09 | 0.06 | −0.25a | −0.04 | 0.05 | −0.02 | 0.05 | 1 | ||
| 11 T2 Depressive Symptoms | 0.18c | 0.21c | 0.25b | 0.18 | −0.26a | −0.07 | 0.19c | −0.12 | 0.11 | 0.56a | 1 | |
| 12 T3 Depressive Symptoms (During Pandemic) | 0.33a | 0.03 | 0.07 | 0.13 | −0.13 | 0.02 | 0.26c | 0.06 | 0.06 | 0.34b | 0.46a | 1 |
| n | 214 | 210 | 137 | 180 | 127 | 170 | 142 | 170 | 142 | 208 | 136 | 101 |
| Mean | 2.37 | 3.88 | 4.03 | 3.87 | 0.38 | 0.37 | 0.22 | 0.24 | 0.12 | 0.13 | 0.35 | |
| SD | 1.04 | 0.96 | 0.72 | 0.83 | 0.21 | 0.19 | 0.19 | 0.18 | 0.13 | 0.15 | 0.19 | |
| Range | 1.00 to 5.00 | 1.00 to 5.00 | 1.91 to 5.00 | 1.27 to 5.00 | −0.13 to 0.82 | −0.15 to 0.88 | −0.43 to 0.76 | −0.20 to 0.73 | 0.00 to 0.65 | 0.00 to 0.65 | 0.00 to 0.85 |
Depressive symptoms at all time points presented as proportion of maximum scale scores. All p values are unadjusted.
BLA, basolateral amygdala; CMA, centromedial amygdala; sgACC, subgenual anterior cingulate cortex; T, time.
p < .001.
p < .01.
p < .05.
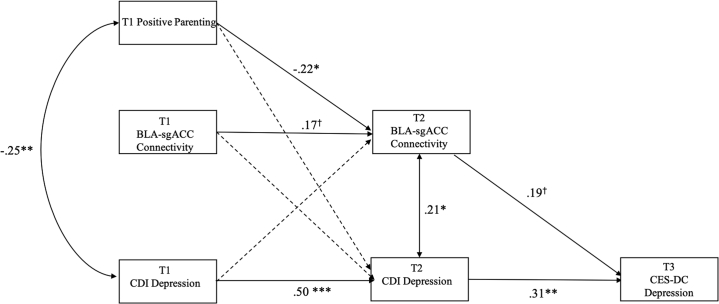
The model that included bilateral T2 BLA–sgACC as the connection of interest is presented in Figure 2. To increase model parsimony, we excluded covariances among T1 variables that were unrelated based on zero-order correlations, as well as paths from sex and age at T2 to BLA–sgACC connectivity at T2 (both βs < −0.05, unadjusted ps > .554). Excluding these covariances and paths did not diminish model fit (χ25 = 2.63, p = .757). We retained other paths and covariances involving sex and age in the final model but have omitted them from Figure 2 to focus on the primary variables of interest. The estimates for all modeled paths and covariances are presented in Table 2.
Figure 2.
Path analysis model of the concurrent and longitudinal associations among time 1 (T1) parenting; T1 and T2 basolateral amygdala (BLA)–subgenual anterior cingulate cortex (sgACC) connectivity; and T1, T2, and T3 depressive symptoms (during the pandemic). False discovery rate–adjusted ∗∗∗p < .001, ∗∗p < .01, ∗p < .05, †p = .072 for path from T1 BLA–sgACC connectivity to T2 BLA–sgACC connectivity (unadjusted p = .055) and †p = .055 for path from T2 BLA–sgACC connectivity to T3 Center for Epidemiological Studies Depression Scale for Children (CES-DC) depression (unadjusted p = .039). CDI, Children’s Depression Inventory.
Table 2.
Path Model Estimates
| Paths | B | SE | 95% CI | β | p | FDR p |
|---|---|---|---|---|---|---|
| Outcome: T2 BLA–sgACC | ||||||
| T1 positive parenting | −0.06 | 0.02 | −0.10 to −0.01 | −0.22 | .015 | .028 |
| T1 BLA–sgACC | 0.15 | 0.08 | −0.00 to 0.31 | 0.17 | .055 | .072 |
| T1 depressive symptoms | −0.01 | 0.12 | −0.26 to 0.23 | −0.01 | .934 | .934 |
| Outcome: T2 Depressive Symptoms | ||||||
| Sex | 0.04 | 0.02 | 0.00 to 0.08 | 0.16 | .029 | .044 |
| T2 age | 0.02 | 0.01 | 0.01 to 0.03 | 0.23 | .001 | .003 |
| T1 positive parenting | −0.01 | 0.02 | −0.04 to 0.02 | −0.06 | .472 | .535 |
| T1 BLA–sgACC | −0.02 | 0.05 | −0.12 to 0.08 | −0.03 | .706 | .750 |
| T1 depressive symptoms | 0.55 | 0.08 | 0.39 to 0.70 | 0.50 | <.001 | <.001 |
| Outcome: T3 Depressive Symptoms | ||||||
| Sex | 0.10 | 0.04 | 0.04 to 0.17 | 0.27 | .003 | .008 |
| T3 age | 0.02 | 0.01 | −0.01 to 0.05 | 0.14 | .127 | .154 |
| T2 BLA–sgACC | 0.20 | 0.09 | 0.01 to 0.38 | 0.19 | .039 | .055 |
| T2 depressive symptoms | 0.41 | 0.13 | 0.15 to 0.67 | 0.31 | .002 | .005 |
| Outcome: T2 Age | ||||||
| T1 age | 1.04 | 0.02 | 1.00 to 1.07 | 0.98 | <.001 | <.001 |
| Outcome: T3 Age | ||||||
| T2 age | 0.74 | 0.05 | 0.65 to 0.83 | 0.86 | <.001 | <.001 |
| Covariances | cov | SE | 95% CI | r | p | FDR p |
| T1 Positive Parenting ↔ T1 Depressive Symptoms | −0.02 | 0.01 | −0.04 to −0.01 | −0.25 | .001 | .003 |
| T1 BLA–sgACC ↔ T1 Age | −0.06 | 0.02 | −0.11 to −0.01 | −0.20 | .010 | .022 |
| T2 BLA–sgACC ↔ T2 Depressive Symptoms | 0.004 | 0.001 | 0.000 to 0.007 | 0.21 | .017 | .029 |
BLA, basolateral amygdala; cov, covariance; FDR p, false discovery rate–adjusted p value; sgACC, subgenual anterior cingulate cortex; T, time.
Our final model that included bilateral T2 BLA–sgACC as the connection of interest showed acceptable fit with the data (χ228 = 56.54, p = .001, comparative fit index = 0.96, root mean square error of approximation = 0.07 [90% CI, 0.04–0.10]). Less positive parenting at T1 was associated concurrently with more severe depressive symptoms (r = −0.25, p = .003). This pattern of parenting at T1 was also associated prospectively with stronger BLA–sgACC connectivity at T2 (β = −0.22, p = .028) over and above the longitudinal effect of BLA–sgACC connectivity at T1 (β = 0.17, p = .072). Stronger BLA–sgACC connectivity at T2, in turn, was associated concurrently with more severe depressive symptoms (r = 0.21, p = .029) and prospectively with more severe depressive symptoms during the pandemic (although this effect was not statistically significant after false discovery rate (FDR) adjustment; β = 0.19, p = .055). This longitudinal association was independent of the effect of pre-pandemic (T2) depressive symptoms on depressive symptoms during the pandemic (T3) (β = 0.31, p = .005).
In the Supplement, we present an analysis showing that BLA–sgACC connectivity did not mediate parenting effects on depressive symptoms during the COVID-19 pandemic. The Supplement also includes a multiple-group comparison of our model in males and females, models that focused on either right or left BLA–sgACC connectivity, a model adjusting for motion, and a model adjusting for pubertal stage at T1 and T2 instead of for age. These analyses yielded similar results to our primary model. Finally, the Supplement includes analyses showing that BLA–sgACC connectivity at T2 did not moderate the association between COVID-19-related stress and depressive symptoms during the pandemic.
We also fit a model that included bilateral CMA–sgACC instead of BLA–sgACC as the connection of interest. CMA–sgACC connectivity at T1 and at T2 were not significantly associated with parenting or depressive symptoms at any assessment (all unadjusted ps > .147). The longitudinal stability path of CMA–sgACC connectivity at T1 to CMA–sgACC connectivity at T2 was not statistically significant after FDR adjustment (β = 0.19, p = .070). Finally, to test the specificity of our findings to BLA–sgACC connectivity, we measured FC between the BLA and perigenual ACC (pgACC), defined as Brodmann area 32 in the Mackey atlas (43). Positive parenting at T1 was positively associated with BLA–perigenual ACC connectivity at T1 (r = 0.20, p = .026). No other path linking BLA–perigenual ACC connectivity to parenting or depressive symptoms was statistically significant (all unadjusted ps > .131).
Discussion
Depressive symptoms in adolescents have increased during the COVID-19 pandemic (6). We previously described in this sample that adolescents reported experiencing more internalizing symptoms during the pandemic than they did in the 3 months before the pandemic (10). In the current analysis, we found that adolescents reported significantly more depressive symptoms during the early phase of the pandemic than they did in years before the pandemic. In fact, during the pandemic, the majority of adolescents in our sample reported experiencing depressive symptoms in the clinically significant range (45), a higher rate than what has been reported in adolescents before the pandemic (49, 50, 51). Taken together, we believe that the elevated depressive symptoms documented at our T3 assessment were related, at least in part, to the pandemic. Not surprisingly, however, the pandemic has been more consequential for some adolescents than for others (6,7,52). In the current study, we examined whether amygdala–sgACC resting-state FC assessed 1–4 years before the pandemic indicated risk for depressive symptoms in adolescents both before and during the pandemic. Further, we tested whether connections between the sgACC and specific subcomponents of the amygdala were related to positive parenting and depressive symptoms. We found that increased BLA–sgACC resting-state connectivity at T2, but not earlier, was associated concurrently with higher levels of pre-pandemic depressive symptoms and prospectively with higher levels of depressive symptoms during the pandemic (although this relationship was reduced to a trend after FDR adjustment). The prospective association was independent of the longitudinal stability of individual differences in depressive symptoms. Our findings build on previous studies linking stronger BLA–sgACC connectivity with stress and depression (13,14,17,19,20) by demonstrating that this neurophenotype is associated with depressive symptoms both before and during the pandemic. To our knowledge, this study is among the first to report on the utility of resting-state FC as a longitudinal predictor of mental health in adolescents during the COVID-19 pandemic (10).
We found that the development of BLA–sgACC resting-state FC was related to positive parenting. Specifically, higher positive parenting earlier in adolescence was associated prospectively with the development of weaker BLA–sgACC connectivity; furthermore, this was independent of the longitudinal stability of BLA–sgACC connectivity. Prior research suggests that BLA–sgACC connectivity is weaker during adolescence than during childhood and adulthood (30). Thus, our findings suggest that positive parenting is associated with the development of a neurophenotype that may be normative during adolescence and that may confer reduced risk for depressive symptoms. Prior research suggests that the amygdala and the ventral regions of the ACC are sensitive to social-environmental protective and risk factors (21). Positive versus aversive parenting practices have been identified as social-environmental factors that are related to resilience and risk for depressive symptoms related to adversity and development in adolescents (22,23,27,53,54); these types of parenting practices have also been associated with neurophenotypes implicated in risk for depressive symptoms, including altered amygdala and sgACC functioning (32, 33, 34). Our study extends this literature by examining longitudinally the link between positive parenting and intrinsic connectivity between the amygdala and sgACC. It is noteworthy, however, that the indirect effect of positive parenting on depressive symptoms during the pandemic through pre-pandemic BLA–sgACC connectivity did not reach statistical significance; we may have needed more statistical power to adequately test this indirect path (55). It is also possible that other neural connections act as a more robust link between parenting and the development of increased versus decreased risk for experiencing depressive symptoms during the pandemic. For example, aversive parenting has been associated with positive connectivity between the amygdala and medial prefrontal cortex during socioemotional processing in adolescents (56), a pattern that has been linked to internalizing symptoms (57,58). It is also noteworthy that in a model controlling for pubertal status, the concurrent association between BLA–sgACC connectivity and pre-pandemic symptoms was reduced to a trend after FDR adjustment; furthermore, in our primary model, the prospective association between BLA–sgACC connectivity and depressive symptoms during the pandemic was reduced to a trend after FDR adjustment. The relations between BLA–sgACC connectivity and symptoms appear to be modest in size. Thus, greater statistical power may have been necessary to detect effects that were robust to analyses that adjusted for advanced pubertal stage and for multiple tests. Longitudinal studies with larger samples are needed to test these possibilities.
Notably, our findings linking amygdala–sgACC FC to positive parenting and depressive symptoms were specific to the BLA. Prior research suggests that the BLA plays a critical role in associative learning and evaluation and regulation of emotionally evocative stimuli (15,40), that stronger BLA–sgACC connectivity is related to stronger encoding of negative-emotion related information in individuals with higher internalizing symptoms (59), and that disruptions of these psychological processes are related to depression (60). Thus, it may be that adolescents who exhibit stronger BLA–sgACC resting-state connectivity engage in psychological processes that confer vulnerability for experiencing depressive symptoms and that are sensitive to parenting influences. It is also possible that the BLA, compared with other amygdala subdivisions, is involved in coordinating activity with the sgACC. Nonhuman animal research shows that amygdala projections to the ACC, including the sgACC region, originate primarily from the BLA (61,62). Adolescents in our sample had significantly stronger BLA–sgACC connectivity than CMA–sgACC connectivity.
We should note several limitations of this study. First, we used adolescents’ reports of parenting practices. Thus, as a neurophenotype linked to depressive symptoms, BLA–sgACC connectivity may reflect negative perceptions that adolescents have of their parents, not necessarily parenting behaviors per se. A few neuroimaging studies have now integrated observational measures of laboratory-based parent-adolescent interactions (24,34,35). Similarly, researchers have recently used ecological momentary assessment to gain a more comprehensive understanding of daily interactions, behaviors, and emotions outside of the laboratory in relation to adolescent neurobiology (63). In this context, it is important that investigators conduct multimethod assessments to further elucidate the role of parenting and parent-child relationships in adolescent brain development in general and, specifically, during periods of risk. Second, our resting-state scan was 6 minutes in duration; longer resting-state scans improve the reliability of connectivity estimates, which is important for biomarker research (64), although estimates have been found to be reliable in scans as short as 5 minutes (65). Third, our model did not include potential mechanisms linking BLA–sgACC connectivity to earlier parenting practices and subsequent depressive symptoms. Thus, it is not clear precisely how parenting practices earlier in adolescence might lead to altered BLA–sgACC connectivity, or precisely how this connectivity could influence later depressive symptoms during the pandemic in an enduring manner. Fourth, researchers have documented health and social inequalities in the effects of the COVID-19 pandemic (2). While our sample may not have spanned the full range of financial and family problems that have been implicated in adolescent mental health during the pandemic, it is important to note that almost one-third of our sample reported that a family member earned less money during the early phase of the pandemic than before the pandemic (8). Finally, we used a different measure of depressive symptoms at T3 (CES-DC) than at T1 and T2 (CDI), which may have contributed to some of our findings. It is worth reiterating, however, that symptoms at T3 were higher than what has been reported in pre-pandemic studies that administered the CES-DC. Thus, it is unlikely that the higher levels of depressive symptoms at T3 can be fully explained by differences in how adolescents respond to the two measures.
Despite these limitations, the results of the current study are important in indicating that the development of BLA–sgACC resting-state connectivity in adolescents is related to the quality of parenting practices and, furthermore, is associated with concurrent depressive symptoms and prospective risk for experiencing depressive symptoms during the pandemic. Adolescents who perceive experiencing less parental warmth and support may develop a pattern of BLA–sgACC connectivity that is implicated in risk for mental health difficulties broadly, including during periods of heightened risk for depression, such as the COVID-19 pandemic.
Acknowledgments and Disclosures
This research was supported by the National Institutes of Health (Grant Nos. R37MH101495 [to IHG], T32MH019908 [to Allan Reiss, funding JGM], F32MH120975 [to RC], and K01MH117442 [to TCH]), the Stanford University Precision Mental Health and Integrated Diagnostics Center (to IHG, JSK, and TCH), and the Fonds de Recherche du Québec–Santé (Resident Physician Health Research Career Training Program) (to AJG).
We thank Rachel Weisenburger and Johanna Walker for their assistance with data collection and organization, as well as the families who participated in this study.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2021.07.005.
Supplementary Material
References
- 1.Galea S., Merchant R.M., Lurie N. The mental health consequences of COVID-19 and physical distancing: The need for prevention and early intervention. JAMA Intern Med. 2020;180:817–818. doi: 10.1001/jamainternmed.2020.1562. [DOI] [PubMed] [Google Scholar]
- 2.Holmes E.A., O’Connor R.C., Perry V.H., Tracey I., Wessely S., Arseneault L., et al. Multidisciplinary research priorities for the COVID-19 pandemic: A call for action for mental health science. Lancet Psychiatry. 2020;7:547–560. doi: 10.1016/S2215-0366(20)30168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prime H., Wade M., Browne D.T. Risk and resilience in family well-being during the COVID-19 pandemic. Am Psychol. 2020;75:631–643. doi: 10.1037/amp0000660. [DOI] [PubMed] [Google Scholar]
- 4.Thapar A., Collishaw S., Pine D.S., Thapar A.K. Depression in adolescence. Lancet. 2012;379:1056–1067. doi: 10.1016/S0140-6736(11)60871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costello E.J., Copeland W., Angold A. Trends in psychopathology across the adolescent years: What changes when children become adolescents, and when adolescents become adults? J Child Psychol Psychiatry. 2011;52:1015–1025. doi: 10.1111/j.1469-7610.2011.02446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barendse M., Flannery J., Cavanagh C., Aristizabal M., Becker S.P., Berger E., et al. Longitudinal change in adolescent depression and anxiety symptoms from before to during the COVID-19 pandemic: A collaborative of 12 samples from 3 countries. PsyArXiv. 2021 doi: 10.31234/osf.io/hn7us. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magson N.R., Freeman J.Y.A., Rapee R.M., Richardson C.E., Oar E.L., Fardouly J. Risk and protective factors for prospective changes in adolescent mental health during the COVID-19 pandemic. J Youth Adolesc. 2021;50:44–57. doi: 10.1007/s10964-020-01332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller J.G., Chahal R., Kirshenbaum J.S., Ho T.C., Gifuni A.J., Gotlib I.H. Heart rate variability moderates the effects of COVID-19-related stress and family adversity on emotional problems in adolescents: Testing models of differential susceptibility and diathesis stress [published online ahead of print Jun 8] Dev Psychopathol. 2021 doi: 10.1017/S095457942100033X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weissman D.G., Rodman A.M., Rosen M.L., Kasparek S.W., Mayes M., Sheridan M., et al. Contributions of emotion regulation and brain structure and function to adolescent internalizing problems and stress vulnerability during the COVID-19 pandemic: A longitudinal study. Biol Psychiatry Global Open Sci. 2021;1:272–282. doi: 10.1016/j.bpsgos.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chahal R., Kirshenbaum J.S., Miller J.G., Ho T.C., Gotlib I.H. Higher executive control network coherence buffers against puberty-related increases in internalizing symptoms during the COVID-19 pandemic. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6:79–88. doi: 10.1016/j.bpsc.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drevets W.C., Savitz J., Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gotlib I.H., Hamilton J.P. Neuroimaging and depression: Current status and unresolved issues. Curr Dir Psychol Sci. 2008;17:159–163. [Google Scholar]
- 13.Taren A.A., Gianaros P.J., Greco C.M., Lindsay E.K., Fairgrieve A., Brown K.W., et al. Mindfulness meditation training alters stress-related amygdala resting state functional connectivity: A randomized controlled trial. Soc Cogn Affect Neurosci. 2015;10:1758–1768. doi: 10.1093/scan/nsv066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connolly C.G., Wu J., Ho T.C., Hoeft F., Wolkowitz O., Eisendrath S., et al. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol Psychiatry. 2013;74:898–907. doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis M., Whalen P.J. The amygdala: Vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton J.P., Farmer M., Fogelman P., Gotlib I.H. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol Psychiatry. 2015;78:224–230. doi: 10.1016/j.biopsych.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho T.C., Yang G., Wu J., Cassey P., Brown S.D., Hoang N., et al. Functional connectivity of negative emotional processing in adolescent depression. J Affect Disord. 2014;155:65–74. doi: 10.1016/j.jad.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perlman G., Simmons A.N., Wu J., Hahn K.S., Tapert S.F., Max J.E., et al. Amygdala response and functional connectivity during emotion regulation: A study of 14 depressed adolescents. J Affect Disord. 2012;139:75–84. doi: 10.1016/j.jad.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu J., Geng X., Shao R., Wong N.M.L., Tao J., Chen L., et al. Neurodevelopmental changes in the relationship between stress perception and prefrontal-amygdala functional circuitry. Neuroimage Clin. 2018;20:267–274. doi: 10.1016/j.nicl.2018.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davey C.G., Whittle S., Harrison B.J., Simmons J.G., Byrne M.L., Schwartz O.S., Allen N.B. Functional brain-imaging correlates of negative affectivity and the onset of first-episode depression. Psychol Med. 2015;45:1001–1009. doi: 10.1017/S0033291714002001. [DOI] [PubMed] [Google Scholar]
- 21.Holz N.E., Tost H., Meyer-Lindenberg A. Resilience and the brain: A key role for regulatory circuits linked to social stress and support. Mol Psychiatry. 2020;25:379–396. doi: 10.1038/s41380-019-0551-9. [DOI] [PubMed] [Google Scholar]
- 22.Ge X., Best K.M., Conger R.D., Simons R.L. Parenting behaviors and the occurrence and co-occurrence of adolescent depressive symptoms and conduct problems. Dev Psychol. 1996;32:717–731. [Google Scholar]
- 23.Schwartz O.S., Dudgeon P., Sheeber L.B., Yap M.B.H., Simmons J.G., Allen N.B. Parental behaviors during family interactions predict changes in depression and anxiety symptoms during adolescence. J Abnorm Child Psychol. 2012;40:59–71. doi: 10.1007/s10802-011-9542-2. [DOI] [PubMed] [Google Scholar]
- 24.Whittle S., Simmons J.G., Dennison M., Vijayakumar N., Schwartz O., Yap M.B.H., et al. Positive parenting predicts the development of adolescent brain structure: A longitudinal study. Dev Cogn Neurosci. 2014;8:7–17. doi: 10.1016/j.dcn.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan P.Z., Oppenheimer C.W., Ladouceur C.D., Butterfield R.D., Silk J.S. A review of associations between parental emotion socialization behaviors and the neural substrates of emotional reactivity and regulation in youth. Dev Psychol. 2020;56:516–527. doi: 10.1037/dev0000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X., McCormick E.M., Ravindran N., McElwain N.L., Telzer E.H. Maternal emotion socialization in early childhood predicts adolescents’ amygdala-vmPFC functional connectivity to emotion faces. Dev Psychol. 2020;56:503–515. doi: 10.1037/dev0000852. [DOI] [PubMed] [Google Scholar]
- 27.Hipwell A., Keenan K., Kasza K., Loeber R., Stouthamer-Loeber M., Bean T. Reciprocal Influences Between Girls’ Conduct Problems and Depression, and Parental Punishment and Warmth: A six year Prospective Analysis. J Abnorm Child Psychol. 2008;36:663–677. doi: 10.1007/s10802-007-9206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolfradt U., Hempel S., Miles J.N.V. Perceived parenting styles, depersonalisation, anxiety and coping behaviour in adolescents. Pers Individ Dif. 2003;34:521–532. [Google Scholar]
- 29.Morris A.S., Criss M.M., Silk J.S., Houltberg B.J. The impact of parenting on emotion regulation during childhood and adolescence. Child Dev Perspect. 2017;11:233–238. [Google Scholar]
- 30.Odriozola P., Dajani D.R., Burrows C.A., Gabard-Durnam L.J., Goodman E., Baez A.C., et al. Atypical frontoamygdala functional connectivity in youth with autism. Dev Cogn Neurosci. 2019;37:100603. doi: 10.1016/j.dcn.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jalbrzikowski M., Larsen B., Hallquist M.N., Foran W., Calabro F., Luna B. Development of white matter microstructure and intrinsic functional connectivity between the amygdala and ventromedial prefrontal cortex: Associations with anxiety and depression. Biol Psychiatry. 2017;82:511–521. doi: 10.1016/j.biopsych.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butterfield R.D., Silk J.S., Lee K.H., Siegle G.S., Dahl R.E., Forbes E.E., et al. Parents still matter! Parental warmth predicts adolescent brain function and anxiety and depressive symptoms 2 years later. Dev Psychopathol. 2021;33:226–239. doi: 10.1017/S0954579419001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan P.Z., Lee K.H., Dahl R.E., Nelson E.E., Stroud L.J., Siegle G.J., et al. Associations between maternal negative affect and adolescent’s neural response to peer evaluation. Dev Cogn Neurosci. 2014;8:28–39. doi: 10.1016/j.dcn.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Callaghan B.L., Dandash O., Simmons J.G., Schwartz O., Byrne M.L., Sheeber L., et al. Amygdala resting connectivity mediates association between maternal aggression and adolescent major depression: A 7-year longitudinal study. J Am Acad Child Adolesc Psychiatry. 2017;56:983–991.e3. doi: 10.1016/j.jaac.2017.09.415. [DOI] [PubMed] [Google Scholar]
- 35.Pozzi E., Vijayakumar N., Byrne M.L., Bray K.O., Seal M., Richmond S., et al. Maternal parenting behavior and functional connectivity development in children: A longitudinal fMRI study. Dev Cogn Neurosci. 2021;48:100946. doi: 10.1016/j.dcn.2021.100946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Callaghan B.L., Tottenham N. The Stress Acceleration Hypothesis: Effects of early-life adversity on emotion circuits and behavior. Curr Opin Behav Sci. 2016;7:76–81. doi: 10.1016/j.cobeha.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson C.C., Mandleco B., Olsen S.F., Hart C.H. Authoritative, authoritarian, and permissive parenting practices: Development of a new measure. Psychol Rep. 1995;77:819–830. [Google Scholar]
- 38.Ordaz S.J., LeMoult J., Colich N.L., Prasad G., Pollak M., Popolizio M., et al. Ruminative brooding is associated with salience network coherence in early pubertal youth. Soc Cogn Affect Neurosci. 2017;12:298–310. doi: 10.1093/scan/nsw133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller J.G., Ho T.C., Humphreys K.L., King L.S., Foland-Ross L.C., Colich N.L., et al. Early life stress, frontoamygdala connectivity, and biological aging in adolescence: A longitudinal investigation. Cereb Cortex. 2020;30:4269–4280. doi: 10.1093/cercor/bhaa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LeDoux J. The amygdala. Curr Biol. 2007;17:R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 42.Amunts K., Kedo O., Kindler M., Pieperhoff P., Mohlberg H., Shah N.J., et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anat Embryol (Berl) 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- 43.Mackey S., Petrides M. Quantitative demonstration of comparable architectonic areas within the ventromedial and lateral orbital frontal cortex in the human and the macaque monkey brains. Eur J Neurosci. 2010;32:1940–1950. doi: 10.1111/j.1460-9568.2010.07465.x. [DOI] [PubMed] [Google Scholar]
- 44.Kovács M. Multi-Health Systems, Inc; Tonawanda: 2003. Children’s Depression Inventory (CDI): Technical Manual Update. [Google Scholar]
- 45.Fendrich M., Weissman M.M., Warner V. Screening for depressive disorder in children and adolescents: Validating the Center for Epidemiologic Studies Depression Scale for Children. Am J Epidemiol. 1990;131:538–551. doi: 10.1093/oxfordjournals.aje.a115529. [DOI] [PubMed] [Google Scholar]
- 46.Little T.D. Guilford Press; New York: 2013. Longitudinal Structural Equation Modeling. [Google Scholar]
- 47.Kline R.B. 3rd ed. Guilford Press; New York: 2011. Principles and Practice of Structural Equation Modeling; p. 427. [Google Scholar]
- 48.Rosseel Y. lavaan: An R package for Structural Equation Modeling. J Stat Softw. 2012;48:1–36. [Google Scholar]
- 49.Olsson G., von Knorring A.L. Depression among Swedish adolescents measured by the self-rating scale Center for Epidemiology Studies-Depression Child (CES-DC) Eur Child Adolesc Psychiatry. 1997;6:81–87. doi: 10.1007/BF00566670. [DOI] [PubMed] [Google Scholar]
- 50.Klasen F., Otto C., Kriston L., Patalay P., Schlack R., Ravens-Sieberer U., BELLA study group Risk and protective factors for the development of depressive symptoms in children and adolescents: results of the longitudinal BELLA study. Eur Child Adolesc Psychiatry. 2015;24:695–703. doi: 10.1007/s00787-014-0637-5. [DOI] [PubMed] [Google Scholar]
- 51.Finan L.J., Ohannessian C.M., Gordon M.S. Trajectories of depressive symptoms from adolescence to emerging adulthood: The influence of parents, peers, and siblings. Dev Psychol. 2018;54:1555–1567. doi: 10.1037/dev0000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zeytinoglu S., Morales S., Lorenzo N.E., Chronis-Tuscano A., Degnan K.A., Almas A.N., et al. A developmental pathway from early behavioral inhibition to young adults’ anxiety during the COVID-19 pandemic. J Am Acad Child Adolesc Psychiatry. 2021;60:1300–1308. doi: 10.1016/j.jaac.2021.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yap M.B.H., Pilkington P.D., Ryan S.M., Jorm A.F. Parental factors associated with depression and anxiety in young people: A systematic review and meta-analysis. J Affect Disord. 2014;156:8–23. doi: 10.1016/j.jad.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 54.Sandler I., Ingram A., Wolchik S., Tein J.Y., Winslow E. Long-term effects of parenting-focused preventive interventions to promote resilience of children and adolescents. Child Dev Perspect. 2015;9:164–171. doi: 10.1111/cdep.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fritz M.S., MacKinnon D.P. Required sample size to detect the mediated effect. Psychol Sci. 2007;18:233–239. doi: 10.1111/j.1467-9280.2007.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gard A.M., Hein T.C., Mitchell C., Brooks-Gunn J., McLanahan S.S., Monk C.S., Hyde L.W. Prospective longitudinal associations between harsh parenting and corticolimbic function during adolescence [published online ahead of print Jan 25] Dev Psychopathol. 2021 doi: 10.1017/S0954579420001583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Demenescu L.R., Kortekaas R., Cremers H.R., Renken R.J., van Tol M.J., van der Wee N.J.A., et al. Amygdala activation and its functional connectivity during perception of emotional faces in social phobia and panic disorder. J Psychiatr Res. 2013;47:1024–1031. doi: 10.1016/j.jpsychires.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 58.Gee D.G., Gabard-Durnam L.J., Flannery J., Goff B., Humphreys K.L., Telzer E.H., et al. Early developmental emergence of human amygdala–prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A. 2013;110:15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hakamata Y., Mizukami S., Izawa S., Moriguchi Y., Hori H., Kim Y., et al. Basolateral amygdala connectivity with subgenual anterior cingulate cortex represents enhanced fear-related memory encoding in anxious humans. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:301–310. doi: 10.1016/j.bpsc.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 60.Joormann J., Gotlib I.H. Emotion regulation in depression: Relation to cognitive inhibition. Cogn Emot. 2010;24:281–298. doi: 10.1080/02699930903407948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharma K.K., Kelly E.A., Pfeifer C.W., Fudge J.L. Translating fear circuitry: Amygdala projections to subgenual and perigenual anterior cingulate in the macaque. Cereb Cortex. 2020;30:550–562. doi: 10.1093/cercor/bhz106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoover W.B., Vertes R.P. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- 63.Sequeira S.L., Silk J.S., Edershile E.A., Jones N.P., Hanson J.L., Forbes E.E., Ladouceur C.D. From scanners to cell phones: Neural and real-world responses to social evaluation in adolescent girls. Soc Cogn Affect Neurosci. 2021;16:657–669. doi: 10.1093/scan/nsab038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Birn R.M., Molloy E.K., Patriat R., Parker T., Meier T.B., Kirk G.R., et al. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. Neuroimage. 2013;83:550–558. doi: 10.1016/j.neuroimage.2013.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Dijk K.R.A., Hedden T., Venkataraman A., Evans K.C., Lazar S.W., Buckner R.L. Intrinsic functional connectivity as a tool for human connectomics: Theory, properties, and optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.