Abstract
Background
Reduced activation of dopamine D1 receptor signaling may be implicated in reward functioning as a potential driver of negative symptoms in schizophrenia. Phosphodiesterase 10A (PDE10A), an enzyme that is highly expressed in the striatum, modulates both dopamine D2- and D1-dependent signaling.
Methods
We assessed whether augmentation of D1 signaling by the PDE10 inhibitor RG7203 enhances imaging and behavioral markers of reward functions in patients with schizophrenia and negative symptoms. In a 3-period, double-blind, crossover study, we investigated the effects of RG7203 (5 mg and 15 mg doses) and placebo as adjunctive treatment to stable background antipsychotic treatment in patients with chronic schizophrenia with moderate levels of negative symptoms. Effects on reward functioning and reward-based effortful behavior were evaluated using the monetary incentive delay task during functional magnetic resonance imaging and the effort-cost-benefit and working memory reinforcement learning tasks.
Results
Patients (N = 33; 30 male, mean age ± SD 36.6 ± 7.0 years; Positive and Negative Syndrome Scale negative symptom factor score 23.0 ± 3.5 at screening) were assessed at three study centers in the United States; 24 patients completed the study. RG7203 at 5 mg significantly increased reward expectation–related activity in the monetary incentive delay task, but in the context of significantly decreased overall activity across all task conditions.
Conclusions
In contrast to our expectations, RG7203 significantly worsened reward-based effortful behavior and indices of reward learning. The results do not support the utility of RG7203 as adjunctive treatment for negative symptoms in patients with schizophrenia.
Keywords: Negative symptoms, PDE10 inhibitor, Proof-of-mechanism study, Reward functioning, RG7203, Schizophrenia
Negative symptoms represent a key symptom domain and an important driver of functional disability in schizophrenia (1) and are present in up to 60% of patients (2). Despite the high medical need, no approved treatment is available for negative symptoms (3). The development of novel drugs has been hampered by the lack of objective measures to test their effects on underlying neurobiological deficits, with the result that companies have conducted—ultimately negative—large and lengthy clinical trials without any prior evidence of beneficial neurobiological effects. As such, it is of the utmost importance from a drug development perspective to deploy biomarkers and functional tests that allow for more rapid testing of potential beneficial treatments and decision making on the basis of fewer patients (4).
Factor analyses of negative symptoms in schizophrenia have demonstrated at least 2 dimensions: 1) avolition (also referred to as apathy, amotivation, asociality, anhedonia, and motivation and pleasure dimension) and 2) expressive deficits (5). The first dimension is a key driver of functional impairment and is related to abnormal reward functions (5). Research inspired by preclinical work has demonstrated specific deficits in learning from positive reward or reinforcement in probabilistic learning paradigms in patients with negative symptoms (6, 7, 8). These deficits are assumed to contribute to the inability of patients with negative symptoms to develop internal representation of rewarding goals, actions, and events, which, in turn, have been implicated in reduced motivation to engage in effortful, value-driven behavior (5).
Consistently, negative symptoms are associated with reduced motivation or willingness to expend high efforts for highly rewarded outcomes in cost-benefit paradigms (10, 11, 9), with reduced activation of the ventral striatum during such tasks, and with reduced expectation of potential monetary rewards (monetary incentive delay [MID]) (12, 13, 14, 15). These studies provide strong evidence for abnormalities in reward functioning and related motivation in patients with schizophrenia experiencing negative symptoms. It is plausible that a treatment expected to treat the avolition dimension of negative symptoms should improve reward functions in patients with these symptoms. Importantly, the above reward paradigms provide an objective means to evaluate potential effects of novel treatments.
The direct and indirect basal ganglia pathways mediate different aspects of reward learning: the direct pathway (dopamine D1 receptor–dependent) is implicated in learning from positive reward (Go learning) and the indirect pathway (dopamine D2 receptor–dependent) from negative reward (NoGo learning) (16). The specific deficit in Go learning in patients with negative symptoms implicates deficient signaling through the D1-dependent direct pathway as a contributing factor in the etiology of negative symptoms (16). Treatments that specifically augment signaling through this pathway may ameliorate the deficit and potentially lead to an improvement in key negative symptoms.
Phosphodiesterase 10 (PDE10) is highly expressed in the striatum. Its inhibition suppresses D2-mediated and enhances D1-dependent signaling (17, 18, 19) and therefore may represent a potential approach to ameliorate the deficits in D1-dependent signaling and reward processing in patients with negative symptoms. In a multiple ascending dose study in healthy volunteers, we demonstrated small, but consistent positive effects across four different paradigms probing reward functioning of the PDE10 inhibitor RG7203 (Figure S1) (20). Here, we report on a randomized, double-blind study exploring the effects of two doses of RG7203 (5 mg and 15 mg; referred to as low and high dose, respectively) versus placebo using a 3-period crossover design in patients with chronic schizophrenia and moderate levels of negative symptoms. We hypothesized that inhibition of PDE10 should enhance functional magnetic resonance imaging (fMRI) and behavioral measures of reward functioning in these subjects. Dose selection was based on results of a positron emission tomography study in healthy volunteers demonstrating mean PDE10 occupancy of approximately 40% with 5 mg RG7203 and approximately 80% occupancy with 15 mg RG7203 (Figure S2). As effects on reward functioning in healthy volunteers were greatest at lower doses, providing occupancy levels of less than 50%, we speculated the lower dose to also be more efficacious in patients.
Methods and Materials
Participants and Study Design
Eligible patients had a DSM-5 diagnosis of schizophrenia, were 15–50 years old, had a score ≥18 on the Positive and Negative Syndrome Scale (PANSS) negative symptom factor score (21) at screening, were symptomatically stable, and were on antipsychotic treatment not exceeding a dose equivalent to 6 mg risperidone. Additional inclusion criteria included Clinical Global Impression Severity scale score ≥ 3 (at least mildly ill); PANSS depression score (G6) ≤ 4 (moderate or less); and Calgary Depression Rating Scale for Schizophrenia ≤ 8. A score of > 2 (mild) for any of the four Clinical Global Impression Severity scale items of the Extrapyramidal Symptom Rating Scale and treatment with olanzapine or clozapine within 3 months of screening were exclusionary (for all eligibility criteria, see Supplement).
Patients were randomized to receive placebo, 5 mg, or 15 mg RG7203 (matching oral capsules), once daily in one of six different treatment sequences (approximately 8 patients per sequence) (see Figure S3). For the 15-mg dose, treatment was uptitrated to the target dose during the first week. Each treatment period lasted 3 weeks, followed by a 2-week washout, with fMRI and behavioral tasks at the end of each treatment period (day 22). Patients were assessed weekly for safety, tolerability, and psychopathology, and approximately 2 weeks after the last dose of study medication for follow-up. Compliance was monitored with a smartphone application.
Reward-based effortful behavior, probabilistic learning, and working memory were evaluated using fMRI (MID and n-back) and behavioral tasks (working memory reinforcement learning task [WMRLT] and effort-cost-benefit task [ECBT]) (14,22, 23, 24).
The study was registered on ClinicalTrials.gov (NCT02824055) and approved by central and local site-specific institutional review boards.
MRI Acquisition
MRI images were acquired on three 3T scanners (GE 3T Discover 750w 25.0; GE Healthcare, North Richland Hills, TX; Siemens 3T MAGNETOM Trio; Siemens Healthineers, Erlangen, Germany; Siemens 3T Verio; Siemens Healthineers]). At all sites, blood oxygen level–dependent (BOLD) fMRI data were collected using a T2∗-weighted echo-planar imaging sequence (repetition time 2000 ms, echo time 27 ms, flip angle 90°, 39 slices, voxel size 3 × 3 × 3 mm, 1 mm gap). In addition, a standard structural T1-weighted scan (1 × 1 × 1 mm) was acquired for coregistration purposes for each patient using standard stock sequences available at each imaging center. Acquisition of fMRI data for the MID task comprised 363 volumes. Four scanner discarded volumes were acquired before task onset to allow for stabilization of the magnetic field.
fMRI Tasks
Monetary Incentive Delay
The MID task was adopted from Knutson et al. (14,25). Patients were asked to respond as quickly as possible to a white box on the screen, which was preceded by a stimulus that informed the patient about the consequences of their response. Three conditions were presented: 1) win-high: the patient wins a higher amount of money ($2) if the response is sufficiently fast, 2) win-low: the patient wins a lower amount of money ($0.2) if the response is sufficiently fast, and 3) neutral control: the patient does not win or lose money but is still asked to respond as fast as possible. At the end of each trial, feedback on the total amount of money won, as well as the amount won in the last trial, was presented. Accuracy, reaction times, and amount of money gained were recorded. The hit reaction time window was adaptively tailored to the individual response times of the patient to have comparable winnings across patients and sessions.
n-back Task
This task required patients to constantly update their mental set while recalling previous stimuli (26). Numbers from 1 to 4 were presented at set points of a diamond, one at a time, every 2 seconds for 500 ms. Patients were instructed to press a response button corresponding to the number seen in “current trial − n” and were tested for 0- and 2-back memory loads (Figure S4). Therefore, each number was a probe as well as a target. A block design was used, in which the 0-back (a sensory-motor control condition not involving working memory) alternated with the 2-back task for 30 seconds each, over 8 repetitions. Performance was measured as percent accuracy and reaction time.
fMRI Preprocessing
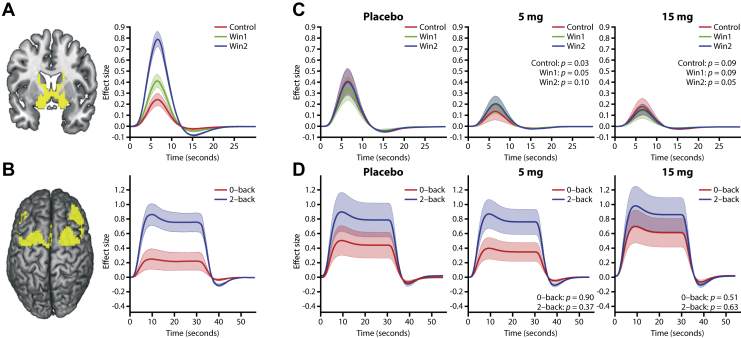
Preprocessing of fMRI data was performed using Statistical Parametric Mapping software (The Wellcome Centre for Human Neuroimaging, UCL Queen Square Institute of Neurology, London, UK) (27) and MATLAB (R2013b; The MathWorks, Inc., Natick, MA). Preprocessing comprised motion correction, distortion correction, spatial registration to a structural scan with a subsequent normalization into the Montreal Neurological Institute space, masking of non–gray matter voxels, and smoothing with a Gaussian kernel of 6 mm full width at half maximum. To determine task-dependent activation, (first-level) t contrasts of active versus control condition (win > control) were computed per patient and session. For the n-back task, the contrast 2-back > 0-back was evaluated; a quality control procedure was adapted comprising exclusion of sessions where patients were evidently performing the 0- or 1-back instead of 2-back or had a high miss rate or low accuracy in the 0-back condition. Effects of motion were controlled for in all tasks by including six motion parameters (translation and rotation) in all models. Eigenvariates adjusting for the effects of motion were extracted for the above contrast for all patients using an a priori–defined ventral striatal (MID) and dorsolateral prefrontal (n-back) activation mask obtained from the same task in a previous study in healthy volunteers (28) (Figure 1A, B). In addition, the rfxplot toolbox (29) was used to extract the fitted hemodynamic response function (HRF) and its amplitude for each of the three MID task conditions and for the two n-back task conditions per patient and session. At the end of the session, patients received the actual amount of money earned.
Figure 1.
Deployed masks and results of hemodynamic response function (HRF) analyses. (A) Ventral striatal monetary incentive delay activation mask extracted from a previous study in healthy volunteers alongside the corresponding HRF for the different task conditions (control = $0, win1 = $0.2, win2 = $2) (28). (B) n-back dorsolateral prefrontal activation mask extracted from a previous study in healthy volunteers alongside the corresponding HRF for the different task conditions (28). (C) HRF for the monetary incentive delay task per treatment and task condition. p values from the paired t test for each task condition vs. placebo are reported. (D) HRF for the n-back task per treatment and task condition. p values from the paired t test for each task condition vs. placebo are reported. The shaded area for all HRFs represents the standard error of mean.
Behavioral Tasks
A full description of the behavioral tasks is provided in the Supplement.
Working Memory Reinforcement Learning Task
Learning phase (LP): The WMRLT was modified from a classic conditional associative learning paradigm (30,31). In the LP, patients learned to select one correct out of three possible button presses for a given stimulus (one stimulus presented at a time) by receiving feedback about the correctness of their action. Stimuli were presented in 12 blocks, with block sizes ranging from 2 to 5 stimuli and stimuli in each block corresponding to a different category of images (e.g., sports, fruits, places) leading to a total of (4 blocks of 2 stimuli) + (3 blocks of 3) + (2 blocks of 4) + (3 blocks of 5) = 40 different stimuli. Correct button choices were rewarded with 1 or 2 points with preassigned probabilities of receiving the reward (0.25, 0.5, 0.75) for each stimulus. Because patients with schizophrenia display an impaired reinforcement learning performance with increased working memory load in this task (31), the proportion of correct choices in late trials (trials 11, 12, and 13) for blocks of size 4 and 5 (high working memory load) was used for the analyses described below.
Test phase (TP): After LP, patients underwent a TP, in which they were presented with pairs of images they previously encountered and asked to choose the image they perceived to have given them most points in LP. No reward was given for the choices made. TP included 115 pairs of images that were selected among all possible pairs and based on the actual responses of the patient in LP, ensuring the inclusion of sufficient pairs with a range of value differences and means. For TP, a general linear model was fitted for each patient and session. As suggested by previous research using this task in patients with schizophrenia, the beta coefficient (2) describing the modulation of the value difference by the value mean was used for evaluating treatment effects (23). This coefficient is considered to capture the deficit observed in patients with schizophrenia with negative symptoms related to the “choose A, avoid B” paradigm in which patients learn to choose a highly rewarded stimulus and avoid a less valuable stimulus.
Effort-Cost-Benefit Task
In the ECBT, adapted from Gold et al. (22), patients chose between a low-effort, low-reward option (20 pumps; 1 point) and a high-effort, high-reward option task (100, 120, or 150 pumps) where the reward varied from 3, 5, or 7 points with a prespecified certainty of 50 or 100% of actually receiving the reward. Each set of accumulated 20 points converted to a $1 bonus. A total of 72 experiments per session were presented. The percentage of high-effort choices under deterministic reward condition (100% reward) for reward magnitudes 5 and 7 was used for evaluating treatment effects.
Statistical Analyses
Primary Analyses
Before unblinding, a prespecified statistical analysis plan was created. Only the primary analyses and additional exploratory analyses are reported. The primary analyses for MID and WMRLT TP were performed using a general linear model implemented in SAS (www.sas.com). The model included fixed effects for treatment (placebo, 5 mg, 15 mg) and visit (1, 2, 3) and an error term assumed to be correlated across visits within each patient. Each dose was compared with placebo. Primary analyses for WMRLT LP examined the effect of treatment on the proportion of correct choices in late trials with high working memory load. Within-patient differences were derived for each dose versus placebo and were analyzed using a one-sample t test.
For the ECBT, the general linear model included fixed effects of treatment and period, as well as continuous effects for effort, reward, side, and their interaction with treatment. Repeated observations within patients were modeled using autoregressive correlations across the ordered results within each patient and session. Each dose was compared with placebo.
For all primary analyses, a directional hypothesis assuming beneficial effects of treatment was prespecified (for MID: increases in differential activation in the win vs. control conditions; for WMRLT LP: an increase in the proportion of correct choices; for WMRLT TP: a higher beta coefficient; for ECBT: the proportion of high effort choices). For the directional hypothesis test related to MID, a one-sided p value of .05 (two-sided .1) was the criterion for a statistically significant positive treatment effect. For directional hypotheses related to ECBT and WMRLT, the corresponding values were .1 (one-sided) and .2 (two-sided). These choices were considered adequate for this exploratory signal-seeking proof-of-mechanism study.
Secondary and Exploratory Analyses
We evaluated the effects of treatment with RG7203 on working memory activation (n-back fMRI task; contrast 2-back > 0-back) and behavioral performance in the n-back (accuracy in the 2-back condition) and the MID (reaction times in the high win condition) tasks. Before unblinding, a review of behavioral n-back data was performed to ensure that patients were able to perform the task, resulting in the exclusion of 28% (n = 22 sessions) of all sessions from analyses (reasons: high omission rate: n = 6; performing the 2-back as 0-back: n = 10; performing 2-back as 1-back: n = 4; high error rate in 0-back condition: n = 2).
For all three analyses, the model described above for MID and WMRLT TP was fitted to the data comparing each dose with placebo. An increased differential activation and a higher accuracy in the n-back and faster reaction times in the MID task were considered beneficial.
We explored the underlying HRF changes leading to differential activation in the MID and n-back tasks across active and placebo treatment conditions by extracting the HRF effect sizes (the amplitude of the BOLD response) for each task condition per patient and session and submitting them to paired t tests comparing each RG7203 dose versus placebo.
Effects on scores on the PANSS (total, positive, and negative subscales) and the Brief Negative Symptom Scale were tested using model M1 described above.
Two-sided p values are reported throughout and were considered statistically significant if < .1. No corrections for multiplicity were performed.
Results
Demographics
In total, 33 patients with schizophrenia (30 male; 21 African American, 9 Caucasian, 3 Asian; mean age ± SD, 36.6 ± 7.0 years) were recruited at three study centers in the United States. At baseline, mean PANSS total score was 68.6 ± 9.3, PANSS negative symptom factor score was 23.0 ± 3.5, and Clinical Global Impression Severity scale score was 3.7 ± 0.5 (Table 1). A total of 24 patients completed the study (Figure S3); 2 patients discontinued owing to adverse events (dystonic reactions) and 7 owing to nonsafety issues. Because both patients who discontinued owing to safety reasons completed two of the three treatment periods, including placebo and one of the active treatments, their data were included in the analyses.
Table 1.
Patient Characteristics at Baseline (N = 33)
| Characteristics | Mean (SD) or n (%) | Scale Range |
|---|---|---|
| Age, Years | 36.6 (7.0) | |
| Sex, Male | 30 (91%) | |
| Race | ||
| African American | 21 (64%) | |
| Caucasian | 9 (27%) | |
| Asian | 3 (9%) | |
| CGI-S | 3.7 (0.5) | [1−7] |
| CGI-S-N | 4.0 (0.4) | [1−7] |
| PANSS Total Score | 68.6 (9.3) | [30−210] |
| Negative symptom factor score | 23.0 (3.5) | [7−49] |
| Positive symptom factor score | 19.2 (4.8) | [8−56] |
| BNSS Total Score | 36.0 (11.5) | [0−78] |
| Blunted affect subscale | 8.5 (3.3) | [0−18] |
| Alogia subscale | 4.5 (3.1) | [0−12] |
| Asociality subscale | 6.2 (2.6) | [0−12] |
| Anhedonia subscale | 8.8 (3.8) | [0−18] |
| Avolition subscale | 6.1 (2.2) | [0−12] |
| Distress subscale | 2.2 (1.5) | [0−6] |
BNSS, Brief Negative Symptom Scale; CGI-S, Clinical Global Impression Severity scale; CGI-S-N, CGI-S for negative symptoms; PANSS, Positive and Negative Syndrome Scale.
Primary Analyses
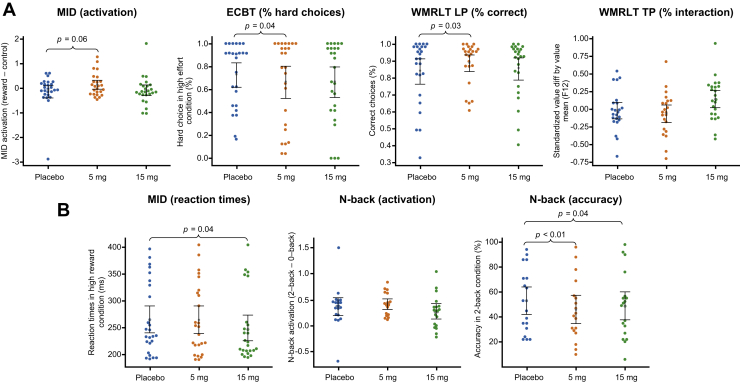
In line with our primary hypothesis for the MID, a significant increase in differential activation in the reward versus control conditions was observed with low-dose RG7203 versus placebo (p = .06, two-sided) (Figure 2A and Table 2). There was no such effect with the high dose (p = .36). Similarly, during the LP of the WMRLT, a significant improvement in the proportions of correct choices in late trials with high working memory load—thought to index incremental reinforcement learning contributions—was observed with the low dose (p = .03) but not the high dose (p = .98) of RG7203 versus placebo (Figure S5). However, during the TP of the WMRLT, there was no significant difference in the key outcome variable (beta coefficients for modulation of value difference by value mean, with high coefficients indicating an improved performance) between the low dose and placebo (p = .17) (Figure 2A and Figure S6) or the high dose and placebo (p = .13) (Table 2). Contrary to our primary hypothesis for the ECBT, the percentage of high-effort choices was lower with both doses of RG7203 (Figures 2A and 3; Figure S4). The difference between the low dose versus placebo reached significance (p = .04; high dose vs. placebo p = .36) (Table 2).
Figure 2.
Results of primary and exploratory analyses. (A) Results for the four primary outcomes are displayed per treatment condition. (B) Results for the three secondary end points are displayed. p values are two-sided. Error bars are 95% boot-strapped confidence intervals of the mean. ECBT, effort-cost-benefit task; LP, learning phase; MID, monetary incentive delay; TP, test phase; WMRLT, working memory reinforcement learning task.
Table 2.
Summary Results for Primary End Points
| Test | End Point | RG7203 Dose | Effect Estimate for Active vs. Placebo (90% CI) | p Value |
|---|---|---|---|---|
| fMRI | Ventral striatal activity during reward expectation in the monetary incentive delay task | 5 mg | 0.24 (0.03 to 0.45) | .06 |
| 15 mg | −0.12 (−0.35 to 0.10) | .36 | ||
| ECBT | % correct high effort choices under deterministic reward values 5 or 7 | 5 mg | −0.39 (−0.63 to −0.15) | .04 |
| 15 mg | −0.29 (−0.66 to 0.11) | .36 | ||
| WMRLT LP | % correct choices in late trials and blocks of size 4 or 5 | 5 mg | 0.05 (0.02 to 0.08)a | .03 |
| 15 mg | 0.00 (−0.04 to 0.05)a | .98 | ||
| WMRLT TP | Value difference modulated by value mean | 5 mg | −0.10 (−0.19 to −0.01)a | .17 |
| 15 mg | 0.12 (0.02 to 0.21)a | .13 |
CI, confidence interval; ECBT, effort-cost-benefit task; fMRI, functional magnetic resonance imaging; LP, learning phase; TP, test phase; WMRLT, working memory reinforcement learning task.
80% CI.
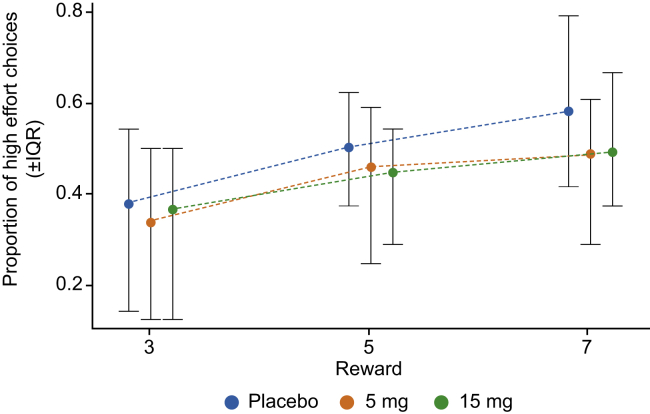
Figure 3.
Proportion of patients choosing the high effort/high reward option by magnitude of the reward value when receiving the reward was certain. During placebo treatment, patients show only a small increase in high effort choices with increasing reward—a profile typical of patients with negative symptoms. During treatment with either dose of RG7203, patients became less inclined to choose the high effort option—an effect predictive of worsening of negative symptoms. IQR, interquartile range.
Secondary and Exploratory Analyses
Reaction times in the MID task in the high-reward condition were significantly faster with the high dose (p = .04) but not the low dose (p = .68) of RG7203 versus placebo (Figure 2B). In the working memory n-back fMRI task, there was no significant differential activation in 2-back versus 0-back with the low dose (p = .51) or the high dose of RG7203 (p = .36) versus placebo. We found significantly lower accuracy in the n-back task with both the high (p = .04) and the low (p < .01) dose of RG7203 versus placebo (Figure 2B).
To interpret the observed effects of RG7203 on ventral striatal activation during reward anticipation and the seemingly contradictory effects in the ECBT, we extracted the HRF and its amplitude (effect size) for each MID and n-back task condition using the MATLAB toolbox rfxplot (29) per patient and treatment condition. The amplitude of the BOLD responses for the MID task was significantly or marginally significantly reduced in all task conditions at the high dose (control: p = .09; win1: p = .09; win2: p = .05) and the low dose (control: p = .03; win1: p = .05; win2: p = .10) of RG7203 versus placebo (Figure 1C). We performed logistic regression analysis to evaluate how far the performance in the ECBT was driven by the significant increase in differential BOLD response between reward expectation and the control condition, and the overall mean decrease. This demonstrated a significant association between the reduction in effortful behavior and the overall mean decrease in striatal activation (p = .02). For the n-back task, there was no significant difference in BOLD amplitude in any of the task conditions (Figure 1D).
There was no significant difference between the RG7203 treatment groups and placebo in PANSS total, negative, and positive symptoms scores or mean Brief Negative Symptom Scale score (Figures S7 and S8; Tables S1 and S2).
Discussion
Consistent with our primary hypotheses, an improvement in task-based activation and performance was observed in three of four prespecified primary end points with low-dose RG7203. These findings were supported by faster reaction times in the MID task (high dose). We also replicated reductions in differential fMRI activation in the MID task previously reported in patients with schizophrenia and negative symptoms (14,32). Effects were seen mostly at the low dose, consistent with expectations from our earlier study in healthy volunteers (Figure S1) (20).
However, these effects occurred in the setting of an overall blunted BOLD response in all MID task conditions with both doses of RG7203 versus placebo and a reduction of the percentage of hard choices in the ECBT by RG7203, suggesting a negative effect of PDE10 inhibition on reward functioning. Indeed, a post hoc logistic regression demonstrated that the decrease in the percentage of hard choices was primarily driven by the overall blunting of the BOLD response in the ventral striatum during reward anticipation, and not by the differential activation between the reward and control conditions. The additional D2 blockade, exerted by PDE10 inhibition on top of the background antipsychotic medication, may explain this deleterious effect, masking the potentially beneficial effects of enhanced D1-dependent signaling assumed to be shown by the enhancement of differential activation and reinforcement learning. Therefore, it would be of interest to examine the effect of RG7203 on reward functioning in antipsychotic-free patients.
Our results on the differential activation and the absolute BOLD amplitude for the MID task underscore the necessity of considering both of these outcome measures in fMRI studies evaluating pharmacologic interventions. Most fMRI studies still exclusively report the differential contrasts of interest. Had we only considered this differential activation, we might have concluded that inhibition of PDE10 with RG7203 improves overall reward functioning. However, the increase in differential activation was only achieved in the context of the overall diminished ventral striatal activation, thus moving patients even further from the normal striatal response observed in healthy volunteers.
Similar to most previous studies evaluating the n-back task in all-comer patients with schizophrenia (33, 34, 35, 36), we observed a visually reduced differential activation of the dorsolateral prefrontal cortex activation during the performance of the n-back task. The significant reduction in accuracy in the 2-back condition at both doses tested supports a negative impact of RG7203 on working memory.
While our findings do not support the utility of a PDE10 inhibitor as adjunctive treatment for negative symptoms of schizophrenia, this study achieved several methodology advances. To our knowledge, it was the first 3-way crossover imaging study in patients with schizophrenia to test a pharmacologic compound, avoiding the drawbacks of a conventional parallel group, randomized controlled trial (which would be large, lengthy, and costly). As each patient acts as his or her own control, a crossover design allows for a smaller sample size and a shorter time frame. This meant that a Go/NoGo decision for product development could be reached in less than a year, thereby greatly reducing the financial and societal burdens imposed by the study, compared with a typical phase 2 study lasting approximately 3 years.
Results of this study contrast with findings in a previous study in healthy volunteers where RG7203 exerted positive effects in tests of reward functioning. The reasons for this discrepancy may include the additional treatment with a D2 antagonist or, more speculatively, that abnormalities in dopamine signaling may be associated with aberrant wiring of basal ganglia pathways resulting in paradoxical pharmacologic effects (37).
The study has limitations. First, the sample consisted mostly of male subjects; thus, our findings may not apply to female subjects. However, as negative symptoms are much more prominent in male subjects, findings may still be valid for the majority of patients with negative symptoms. Second, the relatively small sample size did not allow for meaningful analysis of subgroups defined by behavioral profiles. Third, a crossover design carries the risk of carryover effects. Given the terminal half-life of about 14 hours, we believed a washout period of 14 days was sufficient from a pharmacokinetic point of view. Although we cannot exclude persistent pharmacodynamics or tachyphylactic effect, the fact that key negative symptoms were very similar on day 1 of each treatment period speaks against such effects (Tables S1 and S2).
In conclusion, using a novel and cost-effective study design, we have identified consistent psychoactive modulation of reward and cognitive functioning on administration of the PDE10 inhibitor RG7203. However, the results are in the opposite direction of what would be considered a positive treatment effect on reward functioning in schizophrenia. Overall, our findings do not support the utility of RG7203 as an adjunctive treatment for negative symptoms of schizophrenia. The usefulness of this agent as monotherapy requires exploration in future studies.
Acknowledgments and Disclosures
This study was funded and supported by F. Hoffmann-La Roche Ltd.
DU, PT, and JD conceived, designed, implemented, and supervised the study; MA planned and conducted the statistical analyses; JD and ŠH conducted the imaging data analysis; CC supervised the collection and quality control of the behavioral data; MJF and AGEC programmed and deployed the behavioral tests, implemented quality control measures, and processed the behavioral data; DPW, RM, DG, and LG executed the study and recruited the patients; JS and SK supervised the overall execution of the study; all authors reviewed and edited the manuscript.
We thank Ashfield Healthcare Communications Ltd., part of UDG Healthcare plc for editorial assistance, funded by F. Hoffmann-La Roche Ltd.
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche’s criteria for eligible studies are available here: https://vivli.org/members/ourmembers/.
For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
DU, MA, CC, and ŠH are full-time employees of F. Hoffmann-La Roche Ltd. DU, MA, and PT are stockholders of F. Hoffmann-La Roche Ltd; JS, SK, and JD were full-time employees of F. Hoffmann-La Roche Ltd. during the time of the study; AGEC, DPW, and MJF have acted as consultants for F. Hoffmann-La Roche Ltd. All other authors report no biomedical financial interests or potential conflicts of interest.
ClinicalTrials.gov: A Study to Evaluate The Effects of RO5545965 in Participants With Negative Symptoms of Schizophrenia Treated With Antipsychotics; https://clinicaltrials.gov/ct2/show/NCT02824055; NCT02824055.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2021.03.001.
Supplementary Material
References
- 1.Harvey P.D., Strassnig M.T., Silberstein J. Prediction of disability in schizophrenia: Symptoms, cognition, and self-assessment. J Exp Psychopathol. 2019;10:1–20. [Google Scholar]
- 2.Bobes J., Arango C., Garcia-Garcia M., Rejas J., CLAMORS Study Collaborative Group Prevalence of negative symptoms in outpatients with schizophrenia spectrum disorders treated with antipsychotics in routine clinical practice: Findings from the CLAMORS study. J Clin Psychiatry. 2010;71:280–286. doi: 10.4088/JCP.08m04250yel. [DOI] [PubMed] [Google Scholar]
- 3.Cerveri G., Gesi C., Mencacci C. Pharmacological treatment of negative symptoms in schizophrenia: Update and proposal of a clinical algorithm. Neuropsychiatr Dis Treat. 2019;15:1525–1535. doi: 10.2147/NDT.S201726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hyman S.E. Psychiatric drug development: Diagnosing a crisis. Cerebrum. 2013;2013:5. [PMC free article] [PubMed] [Google Scholar]
- 5.Foussias G., Remington G. Negative symptoms in schizophrenia: Avolition and Occam’s razor. Schizophr Bull. 2010;36:359–369. doi: 10.1093/schbul/sbn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gold J.M., Waltz J.A., Matveeva T.M., Kasanova Z., Strauss G.P., Herbener E.S., et al. Negative symptoms and the failure to represent the expected reward value of actions: Behavioral and computational modeling evidence. Arch Gen Psychiatry. 2012;69:129–138. doi: 10.1001/archgenpsychiatry.2011.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strauss G.P., Frank M.J., Waltz J.A., Kasanova Z., Herbener E.S., Gold J.M. Deficits in positive reinforcement learning and uncertainty-driven exploration are associated with distinct aspects of negative symptoms in schizophrenia. Biol Psychiatry. 2011;69:424–431. doi: 10.1016/j.biopsych.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waltz J.A., Gold J.M. Probabilistic reversal learning impairments in schizophrenia: Further evidence of orbitofrontal dysfunction. Schizophr Res. 2007;93:296–303. doi: 10.1016/j.schres.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barch D.M., Treadway M.T., Schoen N. Effort, anhedonia, and function in schizophrenia: Reduced effort allocation predicts amotivation and functional impairment. J Abnorm Psychol. 2014;123:387–397. doi: 10.1037/a0036299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCarthy J.M., Treadway M.T., Bennett M.E., Blanchard J.J. Inefficient effort allocation and negative symptoms in individuals with schizophrenia. Schizophr Res. 2016;170:278–284. doi: 10.1016/j.schres.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Treadway M.T., Peterman J.S., Zald D.H., Park S. Impaired effort allocation in patients with schizophrenia. Schizophr Res. 2015;161:382–385. doi: 10.1016/j.schres.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirschner M., Hager O.M., Bischof M., Hartmann M.N., Kluge A., Seifritz E., et al. Ventral striatal hypoactivation is associated with apathy but not diminished expression in patients with schizophrenia. J Psychiatry Neurosci. 2016;41:152–161. doi: 10.1503/jpn.140383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hägele C., Schlagenhauf F., Rapp M., Sterzer P., Beck A., Bermpohl F., et al. Dimensional psychiatry: Reward dysfunction and depressive mood across psychiatric disorders. Psychopharmacology (Berl) 2015;232:331–341. doi: 10.1007/s00213-014-3662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juckel G., Schlagenhauf F., Koslowski M., Wüstenberg T., Villringer A., Knutson B., et al. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2006;29:409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 15.Simon J.J., Biller A., Walther S., Roesch-Ely D., Stippich C., Weisbrod M., Kaiser S. Neural correlates of reward processing in schizophrenia--Relationship to apathy and depression. Schizophr Res. 2010;118:154–161. doi: 10.1016/j.schres.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Maia T.V., Frank M.J. An integrative perspective on the role of dopamine in schizophrenia. Biol Psychiatry. 2017;81:52–66. doi: 10.1016/j.biopsych.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishi A., Kuroiwa M., Miller D.B., O’Callaghan J.P., Bateup H.S., Shuto T., et al. Distinct roles of PDE4 and PDE10A in the regulation of cAMP/PKA signaling in the striatum. J Neurosci. 2008;28:10460–10471. doi: 10.1523/JNEUROSCI.2518-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishi A., Kuroiwa M., Shuto T. Mechanisms for the modulation of dopamine d(1) receptor signaling in striatal neurons. Front Neuroanat. 2011;5:43. doi: 10.3389/fnana.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siuciak J.A., McCarthy S.A., Chapin D.S., Fujiwara R.A., James L.C., Williams R.D., et al. Genetic deletion of the striatum-enriched phosphodiesterase PDE10A: Evidence for altered striatal function. Neuropharmacology. 2006;51:374–385. doi: 10.1016/j.neuropharm.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Umbricht D, Dukart J, Abt M, Tamburri P, Chatham C, Frank M, et al. (2017): T187. A proof-of-mechanism study of the PDE10A inhibitor RG7203 in patients with schizophrenia and negative symptoms probing reward functions with imaging and behavioral approaches. Presented at the ACNP 56th Annual Meeting, December 3–7, Palm Springs, California. Neuropsychopharmacology, Supplement, S415.
- 21.Marder S.R., Davis J.M., Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: Combined results of the North American trials. J Clin Psychiatry. 1997;58:538–546. doi: 10.4088/jcp.v58n1205. [DOI] [PubMed] [Google Scholar]
- 22.Gold J.M., Strauss G.P., Waltz J.A., Robinson B.M., Brown J.K., Frank M.J. Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biol Psychiatry. 2013;74:130–136. doi: 10.1016/j.biopsych.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins A.G.E., Albrecht M.A., Waltz J.A., Gold J.M., Frank M.J. Interactions among working memory, reinforcement learning, and effort in value-based choice: A new paradigm and selective deficits in schizophrenia. Biol Psychiatry. 2017;82:431–439. doi: 10.1016/j.biopsych.2017.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Callicott J.H., Ramsey N.F., Tallent K., Bertolino A., Knable M.B., Coppola R., et al. Functional magnetic resonance imaging brain mapping in psychiatry: Methodological issues illustrated in a study of working memory in schizophrenia. Neuropsychopharmacology. 1998;18:186–196. doi: 10.1016/S0893-133X(97)00096-1. [DOI] [PubMed] [Google Scholar]
- 25.Knutson B., Adams C.M., Fong G.W., Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Callicott J.H., Mattay V.S., Bertolino A., Finn K., Coppola R., Frank J.A., et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9:20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- 27.Friston K.J., Holmes A.P., Worsley K.J., Poline J.P., Frith C.D., Frackowiak R.S.J. Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp. 1994;2:189–210. [Google Scholar]
- 28.Holiga Š., Sambataro F., Luzy C., Greig G., Sarkar N., Renken R.J., et al. Test-retest reliability of task-based and resting-state blood oxygen level dependence and cerebral blood flow measures. PLoS One. 2018;13 doi: 10.1371/journal.pone.0206583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gläscher J. Visualization of group inference data in functional neuroimaging. Neuroinformatics. 2009;7:73–82. doi: 10.1007/s12021-008-9042-x. [DOI] [PubMed] [Google Scholar]
- 30.Petrides M. Deficits on conditional associative-learning tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia. 1985;23:601–614. doi: 10.1016/0028-3932(85)90062-4. [DOI] [PubMed] [Google Scholar]
- 31.Collins A.G., Brown J.K., Gold J.M., Waltz J.A., Frank M.J. Working memory contributions to reinforcement learning impairments in schizophrenia. J Neurosci. 2014;34:13747–13756. doi: 10.1523/JNEUROSCI.0989-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Snellenberg J.X., Girgis R.R., Horga G., van de Giessen E., Slifstein M., Ojeil N., et al. Mechanisms of working memory impairment in schizophrenia. Biol Psychiatry. 2016;80:617–626. doi: 10.1016/j.biopsych.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minzenberg M.J., Laird A.R., Thelen S., Carter C.S., Glahn D.C. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manoach D.S. Prefrontal cortex dysfunction during working memory performance in schizophrenia: Reconciling discrepant findings. Schizophr Res. 2003;60:285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- 35.Deserno L., Sterzer P., Wüstenberg T., Heinz A., Schlagenhauf F. Reduced prefrontal-parietal effective connectivity and working memory deficits in schizophrenia. J Neurosci. 2012;32:12–20. doi: 10.1523/JNEUROSCI.3405-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kyriakopoulos M., Dima D., Roiser J.P., Corrigall R., Barker G.J., Frangou S. Abnormal functional activation and connectivity in the working memory network in early-onset schizophrenia. J Am Acad Child Adolesc Psychiatry. 2012;51:911–920.e2. doi: 10.1016/j.jaac.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 37.Cazorla M., de Carvalho F.D., Chohan M.O., Shegda M., Chuhma N., Rayport S., et al. Dopamine D2 receptors regulate the anatomical and functional balance of basal ganglia circuitry. Neuron. 2014;81:153–164. doi: 10.1016/j.neuron.2013.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.