Highlights
-
•
This is the first study providing longitudinal data regarding the effect of orthostatic hypotension (OH) on small vessel disease (SVD) progression over a 9-year follow-up with a large sample size of SVD patients. By performing serial extensive MRI sequences at three time points, we were able to examine the progression of WMH by location, over time.
-
•
Cross-sectional analysis of the baseline data showed that OH was associated with higher white matter hyperintensity volume, higher mean diffusivity, and with presence of microbleeds.
-
•
Longitudinally, OH was however not associated with a progression of total WMH volume or with higher MD.
-
•
There was no association between OH and cognitive performance, both at baseline and over time.
Keywords: Small vessel disease, Orthostatic hypotension, Progression, Cognitive decline, Mri, Cerebral hypoperfusion
Abstract
Introduction
Cerebral hypoperfusion is thought to play an important role in the etiology of cerebral small vessel disease (SVD). Orthostatic hypotension (OH) is assumed to be a cause of cerebral hypoperfusion by causing recurrent hypoperfusion episodes, and might thus be related to progression of SVD. Here, we investigated whether presence of OH is associated with the progression of SVD MRI-markers and cognitive decline over a time period of 9 years in a cohort of sporadic SVD patients.
Methods
This study included SVD patients from the RUN DMC study, a prospective longitudinal single-center cohort study. In total, 503 patients were included at baseline (2006), from whom 351 participated at first follow-up (2011), and 293 at second follow-up (2015). During all visits, patients underwent MRI and cognitive testing. Association between presence of OH at baseline and progression of SVD-markers on MRI and cognitive decline over time was estimated using linear mixed-effects models.
Results
Of the 503 patients who participated at baseline, 46 patients (9.1%) had OH. Cross-sectional analysis of the baseline data showed that OH was associated with higher white matter hyperintensity (WMH) volume (β = 0.18, p = 0.03), higher mean diffusivity (MD; β = 0.02, p = 0.002), and with presence of microbleeds (OR 2.37 95% CI 1.16–4.68). Longitudinally, OH was however not associated with a progression of total WMH volume (β = -0.17, p = 0.96) or with higher MD (β = -0.001, p = 0.49). There was no association between OH and cognitive performance, both at baseline and over time.
Conclusion
In this longitudinal observational study, there was no evidence that presence of OH is associated with progression of SVD-markers or cognitive decline over time. Our findings indicate that OH may not be causally related to SVD progression over time
Introduction
Cerebral small vessel disease (SVD) is a heterogeneous disease with high inter-individual variability in progression and clinical symptoms over time [1, 2]. Unraveling risk factors contributing to SVD-progression provides a better understanding of the pathophysiology of SVD. Current knowledge regarding risk factors of SVD is predominantly based on longitudinal cohort studies and includes traditional vascular risk factors (e.g. hypertension and diabetes). However, the variation in clinical course over time raises the possibility that other still unverified mechanisms beyond traditional risk factors may also play an eminent role in SVD progression.
Previous studies suggest that cerebral hypoperfusion play an important role in the etiology of SVD [3, 4]. Orthostatic hypotension (OH), characterized by a significant drop of blood pressure after postural change, occurs commonly in the elderly and has been proposed as a risk factor for cognitive decline and dementia [5], [6], [7], [8]. OH is assumed to be a clinical marker for cerebral hypoperfusion, whenever the cerebral autoregulation is impaired, by causing recurrent transient hypoperfusion episodes [9]. We therefore hypothesized that OH is causally related to progression of white matter hyperintensities (WMH) [10, 11], more specifically of those in the periventricular regions. Especially those areas are thought to be particularly vulnerable for hypoperfusion [12], supporting the hypothesis that spatial distribution of WMH may reflect differential etiologies [12, 13].
Current knowledge regarding relation between OH and SVD is limited to inconclusive cross-sectional studies [14], [15], [16], [17], while longitudinal studies investigating progression of SVD over a long time-period are completely lacking. In this study, we aimed to investigate the effect of OH on progression of SVD-markers on MRI and cognitive decline over time over a time-period of 9 years.
Methods
Study population
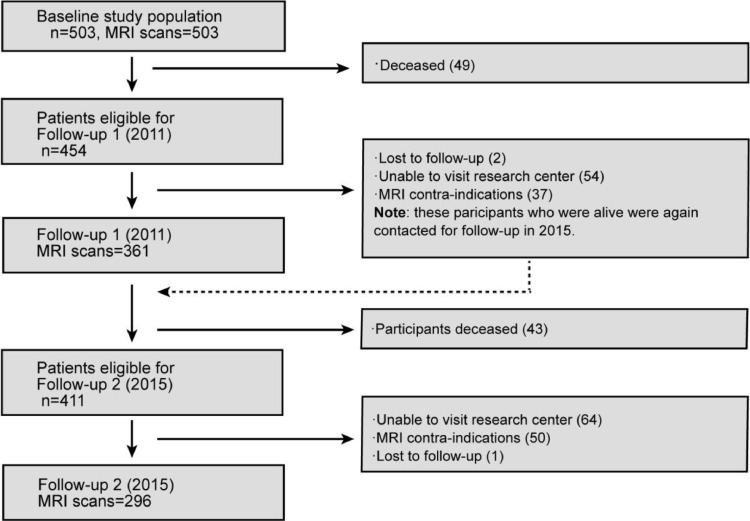
This study is part of the RUN DMC study, a prospective cohort study among 503 individuals with SVD, that investigates risk factors and clinical consequences of SVD. The detailed study protocol has been published previously [18]. In short, we included community-dwelling consecutive patients, who were referred to our outpatient clinic and who underwent diagnostic cerebral imaging for different clinical reasons (e.g. TIA, cognitive/motor disturbances). Inclusion criteria were age between 50 and 85 years and presence of SVD on neuroimaging (i.e., WMH or lacunes). Of the 503 patients who participated at baseline in 2006, 361 underwent repeated MRI assessment at first follow‐up in 2011, and 296 patients at second follow‐up in 2015 (Fig. 1). Of those, 10 patients were excluded because of insufficient scan quality at first follow‐up, and three at second follow‐up, yielding a sample of 503 patients for neuroimaging analyses at baseline, 351 at first follow‐up, and 293 at second follow‐up. In total, 273 subjects underwent repeated cognitive and neuroimaging assessments at all three time‐points and could be used for longitudinal analyses.
Fig. 1.
Flowchart of the RUN DMC Study.
The Medical Review Ethics Committee region Arnhem‐Nijmegen approved the study and all participants gave written informed consent.
Orthostatic hypotension
Systolic and diastolic blood pressures (SBP/DBP) were measured three times (separated by 1–2 min) after 5 min of supine rest. Then, after one minute of standing, the blood pressure was measured again, with the BP cuff positioned at heart level. Orthostatic hypotension was defined as either a SBP decrease of at least 20 mmHg and/or DBP decrease of at least 10 mmHg after one minute of standing up (difference in blood pressure measurements between the mean supine and the standing position) according to accepted criteria [19].
Vascular risk factors
Hypertension was defined as a SBP of ≥ 140 mmHg and/or DBP of ≥ 90 mmHg and/or the use of blood pressure lowering agents. Diabetes mellitus and hypercholesterolemia were considered to be present if the patient was taking oral glucose-lowering drugs or insulin or lipid-lowering drugs, respectively.
Use of OH-inducing drugs was defined as use of the following group of drugs: anti-depressants, alpha-blockers, beta-blockers, calcium-blockers, diuretics and angiotensin-system-blockers.
Cognition
Cognitive function was assessed by a standardized neuropsychological test battery during all waves of data collection and has been described in detail elsewhere [20]. Briefly, global cognitive function was evaluated by MMSE and a cognitive index was calculated covering the following cognitive domains: verbal and visuospatial memory, executive function, psychomotor speed, fluency and attention. The cognitive index is a compound score that was calculated as the mean of the z-scores of the 1-letter subtask of the Verbal Series Attention Test, the Paper–Pencil Memory Scanning Task, the Verbal fluency Tasks, the mean of the reading subtask of the Stroop test, the mean of the Symbol–Digit Substitution Task, and the mean of the added score on the three learning trials of the Rey Auditory verbal Learning Test and the delayed recall of this last test.
The executive function was calculated as the interference score of the Stroop Test by dividing SAT-scores of the color-word task by the mean SAT-scores of the reading and color naming tasks of the Stroop Test, the verbal fluency task, and the SAT-scores of the Verbal Series Attention Test. Psychomotor speed was calculated as the mean of the z-scores of the 1-letter subtask of the Paper-Pencil Memory Scanning Task, the reading and color naming tasks of the Stroop Test, and the Symbol Digit Substitution Task.
MRI protocol
Images were acquired at three time points on 1.5-Tesla MRI (2006: Siemen, Magnetom Sonata; 2011 and 2015: Siemens, Magnetom Avanto) and included the following whole brain scans: 3D T1 MPRAGE imaging (isotropic voxel size 1.0 mm3); FLAIR sequences (2006: voxel size 0.5 × 0.5 × 5.0 mm, interslice gap 1.0 mm; 2011 and 2015: voxel size 0.5 × 0.5 × 2.5 mm; interslice gap 0.5 mm); and a transversal T2*-weighted gradient echo sequence (2006: isotropic voxel size 2.5 mm3, 4 unweighted scans, 30 diffusion weighted scans at b = 900 s/mm2; 2011 and 2015: 8 unweighted scans, 60 diffusion weighted scans at b = 900 s/mm2. The same head coil was used at all 3 time points. Full acquisition details have been described previously [18].
Brain volumetry
Gray matter volume (GMV), white matter volume (WMV) and CSF volumes were assessed by using Statistical Parametric Mapping, SPM12 (https://www.fil.ion.ucl.ac.uk/spm) unified segmentation routines on the T1 MPRAGE images corrected for WMH, as has been described in detail previously [21]. Intracranial volume (ICV) was calculated by summing GMV, WMV, and CSF volumes, and total brain volume. To correct for inter-scan effects, we corrected for differences in ICV between baseline and follow-up. We normalized all volumes to baseline ICV to account for head size.
MRI-markers of small vessel disease
MRI-markers of SVD were rated according to the Standards for Reporting Vascular Changes on Neuroimaging (STRIVE) criteria [22]. WMH volumes were calculated by a semiautomatic WMH segmentation method, which has been described in detail elsewhere [23]. Segmentations were visually checked for segmentation errors by one trained rater, blinded for clinical data. In addition, we distinguished periventricular WMH (PWMH) and deep WMH (DWMH) using FMRIB Software Library (FSL; www.fsl.fmrib.ox.ac.uk/fsl). More specifically, we generated ventricle masks and calculated the geometrical distance from this mask in each voxel using Brain Intensity AbNormality Classification Algorithm (BIANCA) and the command distancemap [12, 24, 25]. Based on previously validated criteria in literature [12], we considered voxels within 10 mm distance of the ventricles as PWMH and calculated the corresponding volumes. WMH volumes were corrected for interscan differences in ICV and then normalized to baseline ICV.
Number of lacunes and microbleeds were rated manually on FLAIR/T1-weighted and T2*-weighted MRI scans according to the STRIVE criteria by two trained raters blinded for clinical data. Follow-up FLAIR images were resliced to match the baseline scans to prevent differences in partial volume effects between baseline and follow-up scans. Interrater and interrater reliability were excellent [24].
Diffusion tensor imaging (DTI) analysis has been described elsewhere [26]. Diffusion data were first preprocessed by using local algorithm [27] to correct for cardiac and head motion as well as eddy currents. Using DTIFit within the Functional MRI of the Brain diffusion toolbox, we created fractional anisotropy (FA) and mean diffusivity (MD) images, which were imported into TBSS pipeline [28]. We then calculated the mean MD within the skeleton for each subjects.
Statistical analysis
First, we identified differences in baseline clinical and MRI-characteristics between patients with and without OH, by using chi-square test or Mann-Whitney test, where appropriate.
Second, we investigated the association between presence of OH and cognitive index, executive function, psychomotor speed, as well as MRI-characteristics of SVD, by using multivariable linear regression for the continuous outcome variables and logistic regression for dichotomous outcome variables, adjusting for age, sex, presence of hypertension, hypercholesterolemia and use of any OH-inducing drugs.
Finally, we studied the effect of baseline presence of OH on the change of cognition (hereby again focusing on cognitive index, executive function, and psychomotor speed) and progression of WMH (stratified by its location: total WMH, DWMH and PWMH) over time. We fitted linear-mixed effects (LME) models via R package “lme4”. LME models allow for the simultaneous modeling of fixed (population-average) and random (subject-specific) effects, allowing us to investigate change in outcome variables in the entire population while accounting for individual differences in rates of WMH progression. We specified the following fixed-effects: presence of OH, baseline age, sex, hypertension, usage of any OH-inducing drugs, an interaction term between follow-up time and presence of OH/absolute SBP changes/absolute DBP changes. We included random intercepts and slopes of time per subject. To evaluate a possible quadratic relationship, indicating nonlinear progression of SVD, we compared model fit between the full model and the full model with a quadratic term during follow-up. We evaluated change in Akaike information criterion (AIC) by one-way ANOVA. Correction for multiple comparisons was done by FDR correction. All statistical analyses were carried out in R Programming Language version 4.1.1.
Results
Of the 503 patients who participated at baseline, 46 patients (9.1%) had OH. Differences in baseline characteristics between the two groups are presented in Table 1. Patients with OH were older than patients without OH (mean age at baseline: 68.9 ± SD 7.6 years vs 65.3 years ± SD 8.9, p = 0.01) and showed worse cognitive performance at baseline compared to non-OH patients (median cognitive index: 0.01 vs −0.20, p = 0.04). Patients with OH had also a significant higher prevalence of hypertension (p = 0.02) and hypercholesterolemia (p = 0.002).
Table 1.
Baseline characteristics of the patients with and without OH.
| Clinical Characteristics | Total sample n = 503 | Without OH n = 457 | With OH n = 46 | p value |
|---|---|---|---|---|
| Demographics | ||||
| Sex (male,%) | 284 (56.5) | 257 (56.2) | 27 (58.7) | 0.87 |
| Age, mean ± SD | 65.6 ± 8.8 | 65.3 ± 8.6 | 68.9 ± 7.6 | 0.01 |
| Cognition | ||||
| MMSE | 28 | 29 | 28 | 0.11 |
| (IQR) | (27–29) | (27–29) | (26–29) | |
| Cognitive index | −0.04 | −0.01 | −0.20 | 0.04 |
| (IQR) | (−0.6–0.5) | (−0.2–0.6) | (−0.8–0.2) | |
| Executive function, mean ± SD | −0.01 ± 0.8 | 0.01 ± 0.8 | −0.18 ± 0.7 | 0.11 |
| Psychomotor speed, mean ± SD | −0.002 ± 0.9 | 0.02 ± 0.9 | −0.23 ± 0.9 | 0.04 |
| OH-inducing drugs | ||||
| Anti-depressants, n (%) | 62 (12.3) | 55 (12.0) | 7 (15.2) | 0,70 |
| Alpha-blockers, n (%) | 2 (0.4) | 2 (0.4) | 0 (0.0) | 1.00 |
| Beta-blockers, n (%) | 181 (36.0) | 158 (34.6) | 23 (50.0) | 0.06 |
| Calcium-blockers, n (%) | 51 (10.1) | 43 (9.4) | 8 (17.4) | 0.15 |
| Diuretics, n (%) | 124 (24.7) | 111 (24.3) | 13 (28.3) | 0.68 |
| Angiotensin-system blocker, n (%) | 160 (31.8) | 140 (30.6) | 20 (43.5) | 0.11 |
| Any OH-inducing drugs, n (%) | 303 (60.2) | 269 (58.9) | 34 (73.9) | 0.07 |
| Risk factors SVD | ||||
| Ever smoking, n (%) | 353 (70.2) | 317 (69.4) | 36 (78.3) | 0.28 |
| Hypertension, n (%) | 369 (73.4) | 328 (71.8) | 41 (89.1) | 0.02 |
| Diabetes, n (%) | 66 (13.1) | 58 (12.7) | 8 (17.4) | 0.50 |
| Hypercholesterolemia,n (%) | 237 (47.1) | 205 (44.9) | 32 (69.6) | 0.002 |
| BMI, kg/m2, mean ± SD | 27.2 ± 4.1 | 27.2 ± 4.1 | 27.0 ± 4.2 | 0.53 |
| MRI Characteristics | ||||
| WMH (ml; IQR) | 8.6 | 3.3 | 8.9 | <0.001 |
| (1.2–11.5) | (1.2–10.5) | (3.3–17.6) | ||
| DWMH (ml; IQR) | 2.9 | 0.9 | 2.5 | 0.002 |
| (0.3–2.9) | (0.3–2.6) | (0.6–6.8) | ||
| PWMH (ml; IQR) | 5.7 | 2.27 | 6.02 | <0.001 |
| (0.8–8.0) | (0.8–7.1) | (2.6–13.0) | ||
| GMV (ml; ± SD) | 606.2 ± 52.6 | 607.5 ± 52.7 | 594.0 ± 50.2 | 0.09 |
| WMV (ml; ± SD) | 454.7 ± 46.0 | 456.2 ± 46.7 | 439.4 ± 34.6 | 0.07 |
| MD white matter | 0.9 | 0.9 | 0.9 | 0.66 |
| (10−3 mm2/s; IQR)# | (0.9–0.9) | (0.9–0.9) | (0.9–1.0) | |
| Presence of microbleeds, n (%) | 83 (13.6) | 68 (15.0) | 15 (32.6) | 0.002 |
| Presence of lacunes, n (%) | 132 (26.2) | 111 (24.3) | 21 (45.7) | 0.002 |
Data represent numbers (%), medians (IQR), or means ± SD. MMSE: Mini Mental State Examination, OH: Orthostatic Hypotension, BMI: Body Mass Index, WMH: White Matter Hyperintensities, DWMH: Deep White Matter Hyperintensities, PWMH: Periventricular White Matter Hyperintensities, GMV: gray Matter Volume, WMV: White Matter Volume, MD: Mean Diffusivity, SD: Standard Deviation.
OH and small vessel disease burden
OH was cross-sectionally associated with higher WMH (β = 0.18, p = 0.03), DMWH (β = 0.24, p = 0.02) and PWMH (β = 0.17, p = 0.04) volumes at baseline, adjusted for sex, age, hypertension, hypercholesterolemia and OH-inducing drugs. Patients with OH had a higher MD (β = 0.02, p = 0.002) and were more likely to have microbleeds compared to patients without OH (OR 2.37 95% CI 1.16–4.68). Table 2 shows the associations between having OH and MRI-characteristics of SVD.
Table 2.
Multivariable cross-sectional association between presence of OH and cognitive and radiological correlates in SVD.
| Cognition | β (95% CI) | p value |
|---|---|---|
| Cognitive index | −0.056 (−0.25–0.14) | 0.57 |
| Executive function | −0.002 (−0.21–0.21) | 0.99 |
| Psychomotor speed | −0.04 (−0.27–0.19) | 0.75 |
| MRI Characteristics | ||
| WMH* | 0.18 (0.02–0.34) | 0.03 |
| DWMH* | 0.24 (0.04–0.43) | 0.02 |
| PWMH* | 0.17 (0.01–0.33) | 0.04 |
| GMV | 3.59 (−8.34–15.52) | 0.56 |
| WMV | −0.01 (−0.02–0.01) | 0.41 |
| MD white matter# | 0.02 (0.01 – 0.03) | 0.002 |
| OR (95% CI) | ||
| Presence of microbleeds^ | 2.37 (1.16– 4.68) | 0.02 |
| Presence of lacunes | 1.88 (0.97–3.61) | 0.06 |
Data represents β estimates (95% CI), OR values (95% CI) or p-values. *Log transformed. All models were adjusted for sex, age, hypertension, hypercholesterolemia, and use of any OH inducing drugs. #8 non-OH patients and 1 OH-case were excluded due to insufficient scan quality. ^4 patients without OH were excluded for missing data regarding microbleeds. OH: Orthostatic Hypotension, WMH: White Matter Hyperintensities, DWMH: Deep White Matter Hyperintensities, PWMH: Periventricular White Matter Hyperintensities, GMV: gray Matter Volume, WMV: White Matter Volume, MD: Mean Diffusivity.
OH and progression of SVD
Of the 46 OH-patients at baseline, 28 patients participated at first follow-up and 19 at second follow-up. Mean follow-up duration was 5.3 (SD 0.2) years until first follow-up, and 8.7 (SD 0.2) years until the second follow-up.
We studied the effect of presence of OH at baseline on the progression of WMH (by location) over time. Including a quadratic term for follow-up time, allowing to model non-linear progression of WMH, significantly improved all models (all p < 0.001). When adjusting for sex, age, hypertension and OH-inducing drugs, presence of OH was not associated with progression of total WMH volume (β = −0.17, p = 0.96), DWMH volume (β = −0.06, p = 0.96), PWMH volume (β = 0.04, p = 0.96) (Table 3), or MD over time (β = −0.001, p #x003D; 0.49).
Table 3.
Longitudinal association between OH and progression of white matter hyperintensities, stratified by location.
| Total WMH | p value | DWMH | p value | PWMH | p value | MD white matter | p value | |
|---|---|---|---|---|---|---|---|---|
| Baseline age (years) | 0.458 (0.346–0.571) |
<0.001 | 0.128 (0.074–0.183) |
<0.001 | 0.330 (0.259–0.400) |
<0.001 | 0.002 (0.002–0.003) |
<0.001 |
| Sex (male) |
−0.078 (−1.662–1.506) |
0.99 | −0.240 (−1.155–0.675) |
0.75 | 0.433 (−0.712–1.578) |
0.69 |
−0.003 (−0.010–0.003) |
0.69 |
| Hypertension | 1.479 (−0.725–3.684) |
0.60 | 0.320 (−0.968–1.608) |
0.82 | 0.739 (−0.864–2.341) |
0.80 |
0.006 (−0.003–0.014) |
0.60 |
| OH | 3.633 (0.289–6.978) |
0.98 | 1.368 (−0.237–2.973) |
0.98 | 2.290 (0.211–4.369) |
0.98 | 0.018 (0.006–0.029) |
0.98 |
| FUP-time*age | 0.041 (0.031–0.051) |
<0.001 | 0.002 (−0.003–0.006) |
0.46 | 0.027 (0.018–0.036) |
<0.001 | 5.172e05 (−2.604e05–1.4e04) |
0.26 |
| FUP-time*OH | −0.061 (−0.375–0.256) |
0.97 | −0.035 (−0.172–0.101) |
0.97 | −0.101 (−0.374–0.174) |
0.97 | −0.001 (−0.004–0.002) |
0.97 |
| FUP-time*∆SBP | 0.002 (−0.005–0.008) |
0.66 | 0.001 (−0.002–0.004) |
0.66 | −0.002 (−0.007–0.004) |
0.58 | 1.181e05 (−4.077e5–6.440e05) |
0.66 |
| FUP-time*∆DBP | 0.004 (−0.009–0.017) |
0.97 | −0.001 (−0.005–0.005) |
0.97 | 0.002 (−0.009–0.012) |
0.97 | 8.554e06 (−9.255e05–1.095e4) |
0.97 |
Fixed effects results from linear mixed-effects models explaining progression of total WMH, DWMH, PWMH and MD. Data represents β-estimates (95% CI) or p-values. We studied the interaction between FUP time and OH and postural SBP/DBP changes, in the different models. In all models a quadratic term was included. WMH: white matter hyperintensities; DWMH: deep white matter hyperintensities; PWMH: periventricular white matter hyperintensities; MD: Mean Diffusivity; FUP: Follow-Up; OH: Orthostatic Hypotension; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure.
The absolute changes in SBP and DBP after 1 min standing up were not associated with an increase of WMH, DMWH, PWMH, or MD over time (Table 3).
OH and cognition
At cross-sectional level, OH was not associated with cognitive index, executive function nor psychomotor speed in the multivariable analysis (Table 2). Furthermore, no differences in change of cognitive index, executive function or psychomotor speed was observed over time between patients with and without OH (Table 4).
Table 4.
Longitudinal association between OH and change of cognitive index, executive function and psychomotor speed.
| Cognitive index | p value | Executive function | p value | Psychomotor speed | p value | |
|---|---|---|---|---|---|---|
| Baseline age (years) | −0.040 (−0.047–0.034) |
<0.001 | −0.037 (−0.043- −0.030) |
<0.001 | −0.043 (−0.051- −0.036) |
<0.001 |
|
Sex (male) |
−0.037 (−0.144–0.069) |
0.69 |
0.047 (−0.068–0.161) |
0.69 |
−0.077 (−0.201–0.046) |
0.69 |
| Hypertension | 0.004 (−0.145–0.152) |
0.99 | −0.050 (−0.145–0.152) |
0.82 | −0.017 (−0.190–0.156) |
0.95 |
| OH | −0.074 (−0.264–0.117) |
0.98 | −0.041 (−0.207–0.108) |
0.98 | −0.054 (−0.281–0.173) |
0.98 |
| FUP-time*age | −0.003 (−0.004—0.002) |
<0.001 | −0.003 (−0.003 - −0.002) |
<0.001 | −0.002 (−0.003- −0.002) |
<0.001 |
| FUP-time*OH | 0.022 (0.002–0.041) |
0.47 | 0.005 (−0.019–0.029) |
0.97 | 0.030 (0.004–0.055) |
0.47 |
| FUP-time*∆SBP | 3.23e04 (−7.798e05–7.219e04) |
0.58 | 3.68e06 (−4.757e4–4.818e04) |
0.66 | 3.987e04 (−1.136e04– 9.10e04) |
0.58 |
| FUP-time*∆DBP | 5.840e04 (−1.771e04–0.001) |
0.97 | −4.322e04 (−9.578e04–8.680e04) |
0.97 | 8.859e04 (−8.787e05–0.002 |
0.97 |
Fixed effects results from linear mixed-effects models explaining change of cognitive index, executive function and psychomotor speed over time. Data represents β-estimates with (95% CI) or p-values. We studied the interaction between FUP time and OH and postural SBP/DBP changes, in the different models. In all models a quadratic term was included. WMH: white matter hyperintensities; DWMH: deep white matter hyperintensities; PWMH: periventricular white matter hyperintensities; MD: Mean Diffusivity; FUP: Follow-Up; OH: Orthostatic Hypotension; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure.
Discussion
We found that the presence of OH was associated with higher WMH volume, impaired structural integrity and higher prevalence of microbleeds at the cross sectional level. However, OH was not associated with progression of MRI-markers of SVD over time or with cognitive decline in individuals with sporadic small vessel disease. Similar longitudinal findings were found when analysing the effect of absolute changes in SBP and DBP, between supine and standing position, rather than the dichotomous definition of OH.
The vascular hypothesis of autonomic dysfunction proposes that recurrent episodic cerebral hypoperfusion causes vascular oxidative stress, which results in alterations in cerebral vasculature (e.g. impaired vasodilation) [29]. This could lead to chronic ischaemia that on its turn may partly explain the occurrence and progression of WMH lesions [30, 31]. Although blood pressure variability is increasingly recognized to be associated with SVD-burden [31, 32], and is even seen as predictor of dementia [6], it is still unknown whether postural blood pressure changes (OH) truly induces harmful levels of cerebral hypoperfusion and may thus be causally related to SVD.
The cross-sectional associations between presence of OH and MRI-markers of SVD found in our study are consistent with several previous studies [15, 17, 33], though other studies have failed to find these associations [14, 16].
However, we found OH not to be associated with WMH progression over time nor with cognitive decline. This might be explained by the assumption that OH does not lead to harmful (episodic) reductions in cerebral perfusion, due to compensatory cerebral autoregulation [14, 29]. OH is defined by a blood pressure reduction that persists after 1 min (or more) of standing. Within that timeframe, there is sufficient time for cerebral autoregulation to adapt to this reduction in blood pressure and return CBF to normal [34 14, 29]. Indeed, it is only the initial phase of the postural response where blood pressure changes occur too fast for autoregulation to adapt [5]. Therefore, a likely possibility is that OH is only detrimental in case of impaired cerebral autoregulation [14]. Another explanation could be that not per se the severity of drop in blood pressure that matters, but rather the duration of delayed recovery from OH that mediates the pathologic effect of OH [17].
In contrast to our results, there is some support for clinical evidence of OH earlier in life and SVD pathological changes in the brain vasculature in a postmortem study [30]. However, ‘reversed causality’ may have played a role in this study as they included only dementia and/or Parkinson patients. The neuronal loss observed during these diseases may affect brain structures involved in blood pressure control leading to autonomic dysfunction. In addition, recent evidence suggests that incomplete recovery of BP at 1 min of standing was related to cognitive decline and mortality in people with clinical Alzheimer's disease [35]. These patients however did not meet criteria for OH, but impaired BP recovery was defined at failure to recover to 100% of baseline. Therefore, mechanisms other than OH likely play a role.
Regional separation of WMH is recently reported to be an imported avenue in SVD research, given that PWMH and DWMH have not only been associated with different cardiovascular risk factors, but also with different underlying microstructural and neuropathological properties, suggesting at least partially distinct pathogenic mechanisms [12, 13, 30]. DMWH has been more attributed to SVD related hypoxic/ischemic damage, while PWMH is thought to be more related to hemodynamic factors (e.g. hypoperfusion) with an accompanying inflammatory component [12, 13]. However, we found no significant differences of the effect of OH on the progression of these two distinct regional white matter lesions.
This is the first study providing longitudinal data regarding the effect of OH on SVD progression over a 9-year follow-up with a large sample size of SVD patients. By performing serial extensive MRI sequences at three time points, we were able to examine the progression of WMH by location, over time. Furthermore, the 10 mm distance rule we used for WMH stratification gives most comparable results to the current literature in terms of showing the best separation in risk factors and pathological features between PWMH and DWMH [12].
Nevertheless, some limitations need to be addressed when interpreting the results of this study. First, we had a relatively small number of patients with OH. This attrition bias due to loss of participants may have influenced our results. Second, we were not able to disentangle patients with possibly more severe (symptomatic) OH from those with (asymptomatic) OH only based on blood pressure decrease during our research assessment. OH accompanied by symptoms as dizziness, syncope and falls may indicate more severe OH with, given the symptoms, possible cerebral hypoperfusion. However, for the present study these clinical data were not available. Patients with more severe OH could also be identified by applying a more strict definition of OH, like often applied in multiple system atrophy (SBP drop by 30 mmHg or DBP drop by 15 mmHg within 3 min) [36]. Unfortunately, we would then reach even smaller numbers of patients with OH for statistical analyses.
In conclusion, in this longitudinal observational study, there was no evidence that presence of OH or SBP/DBP postural variability is associated with more severe WMH progression over time. Our data indicate that OH may not be causally related to SVD progression over time. Future studies should implement techniques that are reliably able to measure cerebral perfusion in longitudinal design to assess its relation with small vessel disease.
Declaration of Competing Interest
Non reported.
Acknowledgments
Author Contributions: M.A. Jacob and A.M. Tuladhar had full access to all the data in the study and take full responsibility for the integrity of the data and accuracy of the data analysis.
Study concept and design: M.A. Jacob, FE de Leeuw, A.M. Tuladhar.
Analysis or interpretation of the data: M.A. Jacob, M. Cai, FE de Leeuw, A.M. Tuladhar.
Drafting of the manuscript: M.A. Jacob.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical Analysis: M.A. Jacob, M. Cai.
Role of the Funder/Sponsor: The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Contributor Information
Mina A. Jacob, Email: mina.a.jacob@radboudumc.nl.
Mengfei Cai, Email: mengfei.cai@radboudumc.nl.
Michelle G. Jansen, Email: m.jansen@donders.ru.nl.
Noortje van Elderen, Email: noortje.vanelderen@radboudumc.nl.
Mayra Bergkamp, Email: mayra.bergkamp@radboudumc.nl.
Jurgen A.H.R. Claassen, Email: Jurgen.Claassen@radboudumc.nl.
Frank-Erik de Leeuw, Email: frankerik.deleeuw@radboudumc.nl.
Anil M. Tuladhar, Email: anil.tuladhar@radboudumc.nl.
References
- 1.van Leijsen E.M.C., van Uden I.W.M., Ghafoorian M., et al. Nonlinear temporal dynamics of cerebral small vessel disease: the RUN DMC study. Neurology. 2017;89:1569–1577. doi: 10.1212/WNL.0000000000004490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amin Al Olama A., Wason J.M.S., Tuladhar A.M., et al. Simple MRI score aids prediction of dementia in cerebral small vessel disease. Neurology. 2020;94:e1294–e1302. doi: 10.1212/WNL.0000000000009141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Promjunyakul N., Lahna D., Kaye J.A., et al. Characterizing the white matter hyperintensity penumbra with cerebral blood flow measures. Neuroimage Clin. 2015;8:224–229. doi: 10.1016/j.nicl.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi Y., Thrippleton M.J., Makin S.D., et al. Cerebral blood flow in small vessel disease: a systematic review and meta-analysis. J. Cereb. Blood Flow Metab. 2016;36:1653–1667. doi: 10.1177/0271678X16662891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finucane C., O’Connell M.D., Fan C.W., et al. Age-related normative changes in phasic orthostatic blood pressure in a large population study: findings from The Irish Longitudinal Study on Ageing (TILDA) Circulation. 2014;130:1780–1789. doi: 10.1161/CIRCULATIONAHA.114.009831. [DOI] [PubMed] [Google Scholar]
- 6.Alpérovitch A., Blachier M., Soumaré A., et al. Blood pressure variability and risk of dementia in an elderly cohort, the Three-City Study. Alzheimers Dement. 2014;10:S330–S337. doi: 10.1016/j.jalz.2013.05.1777. [DOI] [PubMed] [Google Scholar]
- 7.Cremer A., Soumaré A., Berr C., et al. Orthostatic Hypotension and Risk of Incident Dementia: results From a 12-Year Follow-Up of the Three-City Study Cohort. Hypertension. 2017;70:44–49. doi: 10.1161/HYPERTENSIONAHA.117.09048. [DOI] [PubMed] [Google Scholar]
- 8.McNicholas T., Tobin K., Carey D., O’Callaghan S., Kenny R.A. Is Baseline Orthostatic Hypotension Associated With a Decline in Global Cognitive Performance at 4-Year Follow-Up? Data From TILDA (The Irish Longitudinal Study on Ageing) J. Am. Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.008976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehagnoul-Schipper D.J., Vloet L.C., Colier W.N., Hoefnagels W.H., Jansen R.W. Cerebral oxygenation declines in healthy elderly subjects in response to assuming the upright position. Stroke. 2000;31:1615–1620. doi: 10.1161/01.str.31.7.1615. [DOI] [PubMed] [Google Scholar]
- 10.Sambati L., Calandra-Buonaura G., Poda R., Guaraldi P., Cortelli P. Orthostatic hypotension and cognitive impairment: a dangerous association? Neurol. Sci. 2014;35:951–957. doi: 10.1007/s10072-014-1686-8. [DOI] [PubMed] [Google Scholar]
- 11.Zuccalà G., Onder G., Pedone C., et al. Hypotension and cognitive impairment: selective association in patients with heart failure. Neurology. 2001;57:1986–1992. doi: 10.1212/wnl.57.11.1986. [DOI] [PubMed] [Google Scholar]
- 12.Griffanti L., Jenkinson M., Suri S., et al. Classification and characterization of periventricular and deep white matter hyperintensities on MRI: a study in older adults. Neuroimage. 2018;170:174–181. doi: 10.1016/j.neuroimage.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Kim K.W., MacFall J.R., Payne M.E. Classification of white matter lesions on magnetic resonance imaging in elderly persons. Biol. Psychiatry. 2008;64:273–280. doi: 10.1016/j.biopsych.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster-Dingley J.C., Moonen J.E.F., de Ruijter W., van der Mast R.C., van der Grond J. Orthostatic hypotension in older persons is not associated with cognitive functioning, features of cerebral damage or cerebral blood flow. J. Hypertens. 2018;36:1201–1206. doi: 10.1097/HJH.0000000000001681. [DOI] [PubMed] [Google Scholar]
- 15.Kario K., Eguchi K., Hoshide S., et al. U-curve relationship between orthostatic blood pressure change and silent cerebrovascular disease in elderly hypertensives: orthostatic hypertension as a new cardiovascular risk factor. J. Am. Coll. Cardiol. 2002;40:133–141. doi: 10.1016/s0735-1097(02)01923-x. [DOI] [PubMed] [Google Scholar]
- 16.Soennesyn H., Nilsen D.W., Oppedal K., Greve O.J., Beyer M.K., Aarsland D. Relationship between orthostatic hypotension and white matter hyperintensity load in older patients with mild dementia. PLoS ONE. 2012;7:e52196. doi: 10.1371/journal.pone.0052196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckley A., Carey D., Meaney J.M., Kenny R., Harbison J. Is there an association between orthostatic hypotension and cerebral white matter hyperintensities in older people? The Irish longitudinal study on ageing. JRSM Cardiovasc. Dis. 2020;9 doi: 10.1177/2048004020954628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Norden A.G., de Laat K.F., Gons R.A., et al. Causes and consequences of cerebral small vessel disease. The RUN DMC study: a prospective cohort study. Study rationale and protocol. BMC Neurol. 2011;11:29. doi: 10.1186/1471-2377-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Neurology. 1996;46:1470. doi: 10.1212/wnl.46.5.1470. [DOI] [PubMed] [Google Scholar]
- 20.van Uden I.W., van der Holst H.M., Schaapsmeerders P., et al. Baseline white matter microstructural integrity is not related to cognitive decline after 5 years: the RUN DMC study. BBA Clin. 2015;4:108–114. doi: 10.1016/j.bbacli.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuladhar A.M., van Uden I.W., Rutten-Jacobs L.C., et al. Structural network efficiency predicts conversion to dementia. Neurology. 2016;86:1112–1119. doi: 10.1212/WNL.0000000000002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wardlaw J.M., Smith E.E., Biessels G.J., et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghafoorian M., Karssemeijer N., van Uden I.W., et al. Automated detection of white matter hyperintensities of all sizes in cerebral small vessel disease. Med. Phys. 2016;43:6246. doi: 10.1118/1.4966029. [DOI] [PubMed] [Google Scholar]
- 24.van Uden I.W., Tuladhar A.M., van der Holst H.M., et al. Diffusion tensor imaging of the hippocampus predicts the risk of dementia; the RUN DMC study. Hum. Brain Mapp. 2016;37:327–337. doi: 10.1002/hbm.23029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffanti L., Zamboni G., Khan A., et al. BIANCA (Brain Intensity AbNormality Classification Algorithm): a new tool for automated segmentation of white matter hyperintensities. Neuroimage. 2016;141:191–205. doi: 10.1016/j.neuroimage.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Laat K.F., Tuladhar A.M., van Norden A.G., Norris D.G., Zwiers M.P., de Leeuw F.E. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain J. Neurol. 2011;134:73–83. doi: 10.1093/brain/awq343. [DOI] [PubMed] [Google Scholar]
- 27.Zwiers M.P. Patching cardiac and head motion artefacts in diffusion-weighted images. Neuroimage. 2010;53:565–575. doi: 10.1016/j.neuroimage.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Smith S.M., Johansen-Berg H., Jenkinson M., et al. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat. Protoc. 2007;2:499–503. doi: 10.1038/nprot.2007.45. [DOI] [PubMed] [Google Scholar]
- 29.Liu H., Zhang J. Cerebral hypoperfusion and cognitive impairment: the pathogenic role of vascular oxidative stress. Int. J. Neurosci. 2012;122:494–499. doi: 10.3109/00207454.2012.686543. [DOI] [PubMed] [Google Scholar]
- 30.Hase Y., Polvikoski T.M., Firbank M.J., et al. Small vessel disease pathological changes in neurodegenerative and vascular dementias concomitant with autonomic dysfunction. Brain Pathol. 2020;30:191–202. doi: 10.1111/bpa.12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tully P.J., Yano Y., Launer L.J., et al. Association Between Blood Pressure Variability and Cerebral Small-Vessel Disease: a Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Heus R.A.A., Reumers S.F.I., van der Have A., Tumelaire M., Tully P.J., Claassen J. Day-to-Day Home Blood Pressure Variability is Associated with Cerebral Small Vessel Disease Burden in a Memory Clinic Population. J. Alzheimer’s Dis. JAD. 2020;74:463–472. doi: 10.3233/JAD-191134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsubayashi K., Okumiya K., Wada T., et al. Postural dysregulation in systolic blood pressure is associated with worsened scoring on neurobehavioral function tests and leukoaraiosis in the older elderly living in a community. Stroke. 1997;28:2169–2173. doi: 10.1161/01.str.28.11.2169. [DOI] [PubMed] [Google Scholar]
- 34.de Heus R.A.A., de Jong D.L.K., Sanders M.L., et al. Dynamic Regulation of Cerebral Blood Flow in Patients With Alzheimer Disease. Hypertension. 2018;72:139–150. doi: 10.1161/HYPERTENSIONAHA.118.10900. [DOI] [PubMed] [Google Scholar]
- 35.de Heus R.A.A., de Jong D.L.K., Rijpma A., Lawlor B.A., Olde Rikkert M.G.M., Claassen J. Orthostatic Blood Pressure Recovery Is Associated With the Rate of Cognitive Decline and Mortality in Clinical Alzheimer’s Disease. J. Gerontol. A Biol. Sci. Med. Sci. 2020;75:2169–2176. doi: 10.1093/gerona/glaa129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun Z., Jia D., Shi Y., et al. Prediction of orthostatic hypotension in multiple system atrophy and Parkinson disease. Sci. Rep. 2016;6:21649. doi: 10.1038/srep21649. [DOI] [PMC free article] [PubMed] [Google Scholar]