Highlights
-
•
the adapted tango intervention was very well received as demonstrated by minimal participant attrition, satisfaction questionnaires that indicated high satisfaction, and anecdotal reports.
-
•
Adapted tango intervention may be helpful in controlling or reducing markers of inflammation in AA women with a parental history of AD.
-
•
Participants in tango demonstrated improvements in whole-body spatial cognition and short-term and working memory, and reduced deterioration of executive function.
-
•
Although our tango group did not show large positive effect in cumulative caregiver burden post intervention, the large positive effect in role Captivity, caregiver confidence, and deprivation of intimate exchange displays some of the positive effects of this dance intervention for the caregivers.
Keywords: Alzheimer's disease, African Americans, Female, Biomarkers, Dance therapy, Caregivers, Inflammation, Pilot Projects
Abbreviations: AA, African Americans; AD, Alzheimer's Disease; BP, Blood Pressure; LP, Lumbar Puncture; CRP, C-reactive protein
Abstract
Alzheimer's disease (AD) is a devastating, progressive neurodegenerative disease resulting in memory loss and a severe reduction in the ability to perform activities of daily living. Ethnicity-related genetic factors promoting the development of dementias among African Americans (AA) and increased risk among women for developing AD indicates that AA women with a parental history of AD are at great risk for developing AD. This phase I study assessed the impact of a 12 week, 20-lesson adapted Argentine Tango intervention (n = 24) to a no-contact control group (n = 10) on measures of plasma inflammatory markers, cognition, and motor and psychosocial performance in middle-aged AA woman at increased risk for AD by virtue of parental history. Some woman (n = 16) were also caregivers; thus, the impact of the intervention on caregiving burden was examined in this subset. Preliminary analysis of efficacy was conducted with significance tests on biomarkers and key measures of cognition, including visuospatial and executive function, balance, and strength. After 12 weeks, Tango participants had significantly decreased inflammatory cytokine, including reductions in IL-7 (p = 0.003), IFN-γ (p = 0.011), TNFα (p = 0.011), and MCP-1 (p = 0.042) compared to controls. Large effects were noted for the Tango group on tests of executive functioning (d = 0.89), and inhibition (p = 0.031). Participants in Tango improved in dynamic and static balance (p = 0.018) and functional lower body strength (p = 0.023). Secondary assessment revealed trends favoring the intervention group were noted in spatial cognition and executive function. Moderate effects were noted in caregiving burden measures among the subset of caregivers. These data demonstrate substantial reductions in inflammatory biomarkers along with cognitive and motor improvements through a non-pharmacologic, affordable intervention among a small, well-characterized cohort of AA women with a parental history of AD.
Introduction
Alzheimer's Disease (AD) is the fourth leading cause of death in Black/African Americans (B/AA) and the sixth leading cause of death among Whites in the United States[1], with an estimated 5.7 million Americans of all ages living with AD dementia in 2019[1]. B/AA individuals are 1.6-fold more likely to develop dementia by the age of 85 compared to Whites[2], likely due to genetic AD risk factors, co-morbid cerebrovascular and systemic diseases, socioeconomic status, and psychosocial inequity[3]. Biological sex also impacts risk of developing AD, such that females experience higher incidence and prevalence of AD due to longevity and the loss of estrogen during menopause[4].
Middle-aged B/AA women who are caring for their parent with AD are particularly at risk due the intersectionality of gender, race, menopausal-related hormone changes, caregiver stress and genetics; though, paradoxically, B/AA women caregivers do not self-report high levels of caregiver stress or burden. Research shows that informal family caregivers, most of whom are women, have increased levels of stress, depression, and hypertension, and diminished quality of life (QOL)[5], all of which are independent risk factors for AD[6]. Importantly, B/AA caregivers report worse physical health compared to White caregivers while also receiving less support; this is sometimes compounded by the effects of lower socioeconomic status[7].Our group and others have shown that non-pharmaceutical interventions, including nutrition, exercise, and art-based interventions, have the potential to reduce perceived burden and improve mood and cognition, likely because of improved mood, and physical activity's beneficial effects on reducing inflammation[8], [9], [10], [11], [12].
It has been well documented that peripheral inflammation is implicated in cognitive decline and AD[13], [14], [15], [16], [17]. Recent reports suggest that systemic inflammation correlates to AD biomarkers in the brain, and some inflammatory markers may be distinctly upregulated within B/AAs[3] and AD informal family caregivers[18]. While prior research from our group has shown a positive impact of dance therapy on physical and cognitive function, psychological outcomes, and quality of life in people with AD[19], no studies, to these authors’ knowledge, have evaluated the impact of dance therapies on blood-based inflammatory biomarkers. King et al. demonstrate health and functioning benefits for woman familial caregivers caring for a relative with dementia when initiating in moderate-intensity exercise[20]. Furthermore, work by Canonici et al. suggests that a motor intervention for AD results in a decrease in caregiver burden and improvements in motor functions[21].
With increasing evidence that exercise may be beneficial to both caregivers and those with diagnosed AD[22], [23], a dance intervention represents a uniquely enjoyable program with the potential to provide cognitive, mood, and motor functioning benefits to participants. An adapted form of Argentine Tango dance, Adapted Tango is a mild-moderately challenging aerobic physical activity that promotes social interaction, cognitive engagement, and physical coordination with the rhythm of the music. Research demonstrates that listening to music is effective at decreasing anxiety, depression, and caregiver burden[24], [25]. Furthermore, Adapted Tango has demonstrated benefits for older adults[26] and individuals with Parkinson's disease (PD)[27], [28], [29]: patients with PD demonstrate improvements in mobility and spatial cognition[27], [28], [29] while older adults improve in motor-cognitive integration[26]. Improvements were reported to have lasted up to 1 month post treatment.
In this pilot randomized, placebo-controlled clinical trial, the potential benefit of a 12-week Adapted Tango intervention is investigated in B/AA women with a parental history of AD, half of whom were informal caregivers to parents with AD. To our knowledge, this is the first trial to test the potential benefit of a dance intervention in a cohort at high risk for AD during middle age. Middle age represents the ideal time to stage an intervention. This time is when the irreversible neuropathological AD cascade is only beginning and may have potential to be slowed. This trial enrolled a small but well characterized cohort of middle age, B/AA women with an AD parental history, half of whom were current caregivers, and therefore at risk for developing AD themselves. Primary outcomes included levels of inflammatory blood-based biomarkers, quality of life (QOL), mood, cognitive and physical function in women randomized to the Tango intervention compared to a no-contact control group. The trial also aimed to evaluate the effect sizes of several bio- and behavioral markers of motor and cognitive function post-intervention to power a future phase II trial. These data build a foundation for implementation of rehabilitation approaches in this unique, underserved population.
2. Methods
The study protocol was approved by the Emory University Institutional Review Board and the Atlanta VA Research & Development. All participants gave written informed consent prior to participating. This trial was registered under NCT03269149.
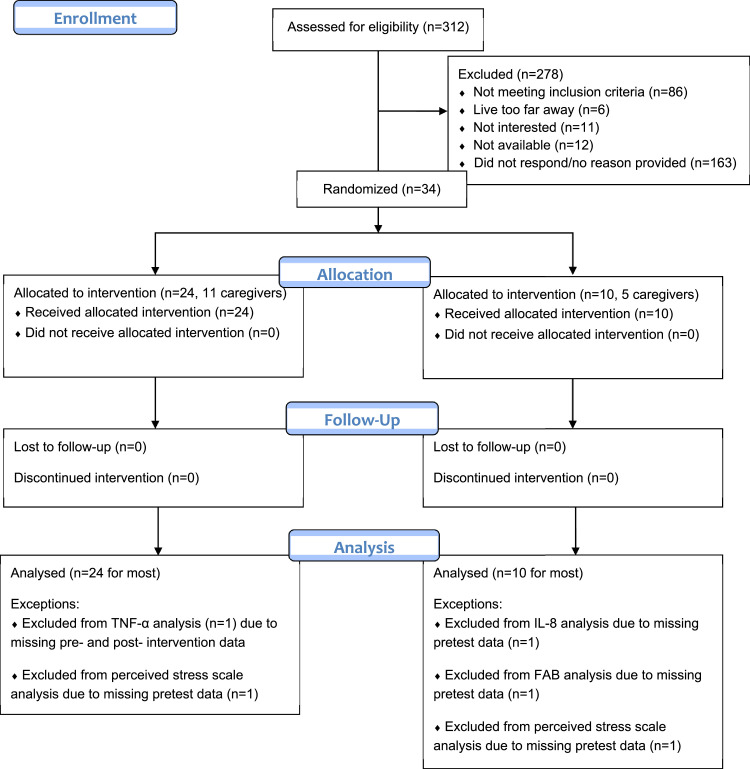
Thirty-four B/AA women, 16 of whom were informal caregivers of their parent with AD, were enrolled for the 12-week, randomized, placebo-controlled Phase I clinical trial. Participants enrolled in Dr. Wharton's ongoing longitudinal studies of AD caregiving were invited to participate in the trial. Recruitment spanned 6/11/2015 through 10/19/2018, ending after achieving target recruitment numbers. Inclusion criteria included 1) a parent diagnosed with probable AD as defined by the National Institute of Neurological Disorders and Stroke-Alzheimer's Disease and Related Disorders Association (NINDS-ARDXA), 2) willingness to undergo all study procedures, and willingness to attend 20 classes over 12 weeks and two clinical visits for blood draw and clinical data collection (height, weight, blood pressure), 3) female sex, 4) self-identification as Black or African American 5) 45+years of age. Verification of the parent's AD diagnosis was obtained using the validated Dementia Questionnaire[30] and medical records, if available. Participants were randomized on a first-come-first-serve basis in a 2:1 ratio to treatment (n = 24) and control (n = 10) conditions with controls frequency matched to cases based on two age stratifications (45–55 years and 65–65 years).
Participants underwent clinical data collection as well as cognitive testing, and physical/motor function and mood assessments, both pre- and post-intervention or control, as previously reported[31] and briefly described below. Clinical visits lasted approximately 2 h in which participants completed the following tests: 1) a one-hour cognitive testing battery including a pre/post battery in domains of spatial ability[32], [33], executive function[34], [35], [36], quantitative measures that assess mood, and positive and negative aspects of caregiving[37], [38], [39], [40], [41]; 2) a blood draw for inflammatory markers; 3) height, weight, and blood pressure measurement; and 4) physical/mobility function testing, which included domains of balance[42], [43], walking[44], [45], and motor function[46].
2.1. Consort flowchart
2.2. Intervention
2.2.1. Adapted tango dance intervention (n = 24)
The twenty-four participants randomized to the experimental group participated in twenty, 1.5 hour long Adapted Tango dance sessions over 12 weeks. Participants were encouraged to attend classes two times per week. Dance interventions took place at the Atlanta VA Health Care System in a Movement Studies Laboratory. To increase likelihood of participants completing all 20 classes, three different class times were offered per week. The Adapted Tango classes followed methods outlined in an Adapted Tango manual, which highlights older adult motor impairments and challenges, fall risk and prevention, partnering enhancement exercises, rhythmic entrainment, and a structured syllabus and format[47]. Every class began with a 25-minute standing warm-up followed by partnering and rhythmic exercises. After warming up and learning a new step, participants were taught how to combine new steps with previously learned steps using improvisation. Music was played throughout classes while the participants partnered with other participants, university student volunteers, staff members or volunteer adult members of the community e.g., friends of participants. Participants learned to integrate the new steps and were encouraged to approach the dance artistically while paying attention to aesthetics. Improvisation was encouraged throughout classes.
2.2.2. Non-intervention control group (n = 10)
Ten participants were randomized to the control group. These participants were encouraged not to change their daily health and exercise routine during the twelve weeks between pre- and post-test. Clinical pre- and post-assessments were identical for all participants.
2.3. Outcomes
Testing was conducted by trained research assistants who were blinded to testing condition and study arms.
2.3.1. Pre-test and post-test blood collection and biomarker analysis
Participants underwent a blood draw using well-established research procedures at the Emory Memory Clinic and described elsewhere[3]. Blood was collected after an 8 hour overnight fast by an experienced phlebotomist. Height was taken during the pre-test visit, while weight and two resting blood pressure measures were taken pre- and post-intervention according to established blood pressure guidelines[48].
Blood samples were processed, batched, and stored at −80° until testing. Samples were assayed at the same time by a trained member of the study team. Four panels of biomarkers were measured in plasma using singleplex or multiplex assays in a Luminex 200 platform: interleukin (IL)−7, IL-8, IL-9, IL-10, interferon gamma (IFN-γ), macrophage derived chemokine (MDC), monocyte chemoattractant protein 1 (MCP-1), transforming growth factor alpha (TGF-α), and tumor necrosis factor alpha (TNF- α), C-reactive protein (CRP) and serum amyloid protein (SAP). Markers of inflammation were chosen based on our prior studies showing peripheral and central inflammation influences on AD by race and gender[3], [49].
2.3.2. Cognitive testing
The neuropsychological testing battery included domains of attention, spatial ability, and executive function. Tests and respective functional domains included:
-
•
the Montreal Cognitive Assessment (MoCA; global cognition; pre-test only), a brief cognitive screening tool with high sensitivity and specificity for detecting mild cognitive impairment[50];
-
•
the Delis-Kaplan Executive Function System (D-KEFS) Tower test (executive cognitive functions: organization, planning) which employs the Tower of London to measure the ability of step-by-step reasoning and planning as well as impulsivity[51], [52];
-
•
the D-KEFS Color Word Interference test (inhibition/switching, cognitive flexibility), in which participants are presented with a list of words naming colors that are printed in different colored ink. Participants must shift between color naming and word reading depending on whether the word is listed in a box. Completion time scores and total error scores were converted into scaled values based on age group norms and published guidelines to measures ability to inhibit a dominant and automatic verbal response[51], [53];
-
•
the Trail Making Test (visual attention, cognitive flexibility)[51], [54] to measure flexibility of thinking on a visual-motor sequencing task. The first part of Trail Making Test (TMT) test, TMT-A, requires the subject to rapidly sequence numbers from 1 through 25, with the score being the time to complete the task. The second part, TMT-B, is a more difficult cognitive flexibility task requiring the subject to follow a sequential pattern while shifting cognitive sets, sequencing from 1 to 13 while switching between numbers and letters (i.e., 1-A-2-B-3-C,…), with the score being the time to complete the task.
-
•
the Reverse Corsi Blocks (visuo-spatial memory)[36] is a test of visuospatial function and working memory which required participants to watch the examiner point to a series of blocks on a tray, and then repeat the pattern backwards.
-
•
the Brooks spatial memory test (spatial memory)[55] in which a four by four grid with numbers was shown to participants. The experimenter read out a list of instructions for placing consecutive numbers in the grid at a standard rate. The starting square was the second row of the second column of the grid. After the last instruction, the participants were asked to repeat back the list of instructions; and
-
•
the Body Position Spatial Task (motor-cognitive and spatial-cognitive function)[56] which asks participants to observe and learn a series of multidirectional steps and turns in lengthening sequences, and then execute the movement series.
2.3.3. Physical function tests
A comprehensive battery in motor domains of balance, walking, and lower body strength was used to ascertain effects on mobility. Tests of functional domains, administered per guidelines included: the 30-s chair stand test (lower-body strength)[46], 6 min walk test (aerobic capacity, lower-limb function and mobility)[45], Timed Up and Go Test with simple and Cognitive conditions (TUG; motor-cognitive integration)[57], Fullerton Advanced Balance (FAB; balance)[58] and gait speed tests (preferred, fast, backward)[44].
2.3.4. Mood and stress
Positive and negative aspects of caregiving were assessed by a comprehensive battery including: Positive Aspects of Caregiving Scale (11 item)[40], [41], Pearlin Caregiver Stress Scales[59], The Zarit Burden Interview[39], Center for Epidemiologic Studies Depression Scale (CES-D)[40], Dementia Quality of Life measure (DEMQOL)[41] (Carer v4).
2.4. Statistical methods
Participants with available biomarker data were included in this analysis. Distribution plots and Shapiro's test were conducted to evaluate data normality. Outcome variables, including inflammatory biomarkers and behavioral functional variables, were assessed for both experimental and control groups. Missing data occurred when a participant was not able to finish the assessment or did not respond to the survey questions; missing data at post-test were imputed following the Last Observation Carried Forward (LOCF) method and outcomes that were missing at both time points were considered unavailable for a participant. The number of available data are presented in the result tables. Descriptive analysis was conducted on pre-test demographic characteristic variables and groups were compared with independent t-tests for continuous variables or Fisher's exact test for categorical variables. Change of the outcome variables from before to after the intervention were calculated by subtracting post-test values from pre-test values. Preliminary analyses of efficacy were conducted with significance tests on the biomarkers and one key measure each across important domain of function, i.e., balance (FAB), strength (30 s chair stand), motor-cognitive integration (BPST), visuospatial function (Corsi), set switching (Trails), organization/planning (TOL) and inhibition (Color Word Interference Test). To compare each group's change scores of these key outcome variables, independent t-tests were performed for normally distributed variables (including all the biomarker variables). Mann-Whitney U tests were used to assess non-normally distributed variables. Inhibition Raw Total Errors was transformed into categorical variables and the changes from pre-test to post-test were assessed by Fisher's exact test. All tests were two-tailed and used an alpha=0.05 significance level. The Benjamini Hochberg procedure was used to correct for multiple comparisons[60]. All analyses were performed in R version 3.6.1.
3. Results
3.1. Demographics
Baseline demographics and clinical measures are reported in Table 1. Participants were overall generally healthy and there were minimal between group differences. Average age of the intervention group was 60.3 ± 8.18 years, and 58.1 ± 8.2 years for the control group (p = 0.47). BMI for the intervention group averaged 30.3 ± 5.57, and 31.5 ± 3.61 for the control group (p = 0.53). Years of education was similar between groups, numbering 16 ± 2.17 years for intervention and 17 ± 1.94 years for control (p = 0.22). Participants in the control group had higher diastolic blood pressure than participants randomized to the treatment group, measured at 79 ± 8.18 mmHg and 71.2 ± 8.78 mmHg respectively (p = 0.02). Systolic blood pressure was similar between groups, at 130.1 ± 14.58 mmHg for the intervention group, and 138.6 ± 19.12 mmHg for controls (p = 0.17).
Table 1.
Participant Baseline Characteristic (n = 34).
| Total | Tango | Control | P-value | |
| N (%) | 34 | 24 (70.6) | 10 (29.4) | |
| Age (years) | 59.7 ± 8.13 | 60.3 ± 8.18 | 58.1 ± 8.2 | 0.47 |
| BMI | 30.7 ± 5.05 | 30.3 ± 5.57 | 31.5 ± 3.61 | 0.53 |
| Education (years) | 16.3 ± 2.13 | 16 ± 2.17 | 17 ± 1.94 | 0.22 |
| Heart Rate (beats per minute) | 73 ± 13.17 | 74.3 ± 13.05 | 70.1 ± 13.67 | 0.41 |
| Diastolic Blood Pressure (mmHg) | 73.6 ± 9.21 | 71.2 ± 8.78 | 79 ± 8.18 | 0.02 |
| Systolic Blood Pressure (mmHg) | 132.7 ± 16.26 | 130.1 ± 14.58 | 138.6 ± 19.12 | 0.17 |
| Dance experience last 5 years N (%) | 0.13 | |||
| No | 21 (61.8) | 17 (70.8) | 4 (40) | |
| Yes | 13 (38.2) | 7 (29.2) | 6 (60) | |
| Occupational status N (%) | 0.35 | |||
| Disabled | 4 (11.8) | 4 (16.7) | 0 (0) | |
| Retired | 14 (41.2) | 11 (45.8) | 3 (30) | |
| Unemployed/seeking employment | 2 (5.9) | 1 (4.2) | 1 (10) | |
| Work full-time | 10 (29.4) | 5 (20.8) | 5 (50) | |
| Work part-time | 4 (11.8) | 3 (12.5) | 1 (10) | |
| Housing Type N (%) | 1.00 | |||
| House/Apartment/Condominium | 32 (94.1) | 22 (91.7) | 10 (100) | |
| Relative's Home | 2 (5.9) | 2 (8.3) | 0 (0) | |
| Income N (%) | 0.10 | |||
| $19,000 or less | 5 (14.7) | 4 (16.7) | 1 (10) | |
| $20,000-$39,000 | 7 (20.6) | 5 (20.8) | 2 (20) | |
| $40,000-$59,000 | 12 (35.3) | 10 (41.7) | 2 (20) | |
| $60,000-$79,000 | 3 (8.8) | 0 (0) | 3 (30) | |
| $80,000 or more | 7 (20.6) | 5 (20.8) | 2 (20) | |
| Assistive Device N (%) | 0.74 | |||
| Most of the time | 1 (2.9) | 1 (4.2) | 0 (0) | |
| Never | 28 (82.4) | 20 (83.3) | 8 (80) | |
| Some of the time | 5 (14.7) | 3 (12.5) | 2 (20) | |
| Marital Status N (%) | 0.94 | |||
| Married/partnered | 13 (38.2) | 10 (41.7) | 3 (30) | |
| Separated/Divorced | 13 (38.2) | 9 (37.5) | 4 (40) | |
| Single | 5 (14.7) | 3 (12.5) | 2 (20) | |
| Widowed | 3 (8.8) | 2 (8.3) | 1 (10) | |
| Menopause N (%) | 0.41 | |||
| No | 6 (17.6) | 4 (16.7) | 2 (20) | |
| Unsure | 6 (17.6) | 3 (12.5) | 3 (30) | |
| Yes | 22 (64.7) | 17 (70.8) | 5 (50) | |
| Exercise in previous month N (%) | 0.85 | |||
| Greater than once a week | 20 (58.8) | 15 (62.5) | 5 (50) | |
| 1 - 4 times per month | 8 (23.5) | 5 (20.8) | 3 (30) | |
| Once a month | 2 (5.9) | 1 (4.2) | 1 (10) | |
| Never | 3 (8.8) | 2 (8.3) | 1 (10) | |
| Don't know | 1 (2.9) | 1 (4.2) | 0 (0) | |
| Aerobic activity during the past 4 months N (%) | 1.00 | |||
| No | 17 (50) | 12 (50) | 5 (50) | |
| Yes | 17 (50) | 12 (50) | 5 (50) | |
| Fall worry N (%) | 0.41 | |||
| Not at all | 17 (50) | 11 (45.8) | 6 (60) | |
| Slightly | 9 (26.5) | 7 (29.2) | 2 (20) | |
| Somewhat | 4 (11.8) | 4 (16.7) | 0 (0) | |
| Moderately | 3 (8.8) | 2 (8.3) | 1 (10) | |
| Very | 1 (2.9) | 0 (0) | 1 (10) | |
| Single Item Quality of life (/7) N (%) | 0.35 | |||
| Low | 2 (5.9) | 2 (8.3) | 0 (0) | |
| Moderate | 14 (41.2) | 8 (33.3) | 6 (60) | |
| Moderately High | 6 (17.6) | 6 (25) | 0 (0) | |
| High | 9 (26.5) | 6 (25) | 3 (30) | |
| Very High | 3 (8.8) | 2 (8.3) | 1 (10) | |
| Use of Angiotensin Converting Enzyme (ACE) Inhibitors N (%) | 0.20 | |||
| No | 26 (76.5) | 20 (83.3) | 6 (60) | |
| Yes | 8 (23.5) | 4 (16.7) | 4 (40) | |
| Use of Beta blockers N (%) | 0.56 | |||
| No | 30 (88.2) | 22 (91.7) | 8 (80) | |
| Yes | 4 (11.8) | 2 (8.3) | 2 (20) | |
| Alcohol in past month N (%) | 0.68 | |||
| No | 10 (29.4) | 8 (33.3) | 2 (20) | |
| Yes | 24 (70.6) | 16 (66.7) | 8 (80) | |
| High cholesterol N (%) | 0.49 | |||
| No | 14 (41.2) | 9 (37.5) | 5 (50) | |
| Unsure | 2 (5.9) | 1 (4.2) | 1 (10) | |
| Yes | 18 (52.9) | 14 (58.3) | 4 (40) | |
| High blood pressure N (%) | 0.25 | |||
| No | 13 (38.2) | 11 (45.8) | 2 (20) | |
| Yes | 21 (61.8) | 13 (54.2) | 8 (80) | |
| Diabetes N (%) | 0.35 | |||
| No | 27 (79.4) | 18 (75) | 9 (90) | |
| Unsure | 2 (5.9) | 1 (4.2) | 1 (10) | |
| Yes | 5 (14.7) | 5 (20.8) | 0 (0) |
Table 1. Participant Baseline Characteristics. Values are presented as Mean± SD for continuous variables, and N (%) for categorical variables. P values were calculated with a t-test for continuous variables and Fisher's exact test for categorical variables comparing between control and intervention groups.
Occupational status (p = 0.35), housing type (p = 1.00), income (p = 0.10), and marital status (p = 0.94) did not differ significantly between groups. Additionally, distribution of quality of life was not significantly different by group (p = 0.35).
Dance experience in the last 5 years did not differ between groups, with 29.2% of the intervention group and 60% of the control group having recent experience (p = 0.13). Frequency of exercise did not differ between groups for the month prior to the start of the study (p = 0.85) nor four months prior (p = 1.00). The distribution of fall risk was not significantly different by group (p = 0.41).
Medical history evaluations demonstrated that 70.8% and 50% of women in the intervention and control groups, respectively, had undergone menopause (p = 0.41). The percent of participants using an assistive device did not differ between groups, with 83.3% and 80% of the intervention and control groups, respectively, not using a device (p = 0.74). Use of ACE inhibitors (p = 0.20) and beta-blockers (p = 0.56) did not differ between groups. Furthermore, alcohol use in the past month was not significantly different between groups (p = 0.68). High cholesterol and high blood pressure were highly prevalent in our cohort, affecting 52.9% and 61.8% of total participants, but affected both groups similarly (p = 0.49 for high cholesterol; (p = 0.25 for high blood pressure). Lastly, participants with diabetes were equally distributed across groups (p = 0.35).
3.1.1. Attendance
Participants had busy lives and many obligations, which impacted their abilities to attend all the classes. Some individuals had health concerns that ultimately ended dance sessions early for them. 2 participants attended 4 lessons, 1 participant attended 7 lessons, 1 participant attended 11 lessons, 1 participant attended 12 lessons, 1 participant attended 13 lessons, 1 participant attended 14 lessons, 1 participant attended 18 lessons, 1 participant attended 19 lessons, and 16 participants achieved and attended the goal of 20 lessons (n = 24; mean attendance: 17.08 +/- 5.31 lessons). All these participants were included in analyses, using LOCF to account for any posttest data that were not available. As this likely reflects the reality of attendance if this intervention was widely implemented, it was important that we evaluate if non-perfect attendance still translates to improvement for participants, reflecting our reasoning behind the combined analyses.
3.2. Biomarkers
Inflammatory blood-based biomarkers were evaluated in participants at the beginning and end of the intervention or control (Table 2). After correction for multiple comparisons with the Benjamini Hochberg method, Tango participants had significantly decreased levels of IL-7, IFN-γ, TNF-α, and MCP, compared to the control group after the 12-week trial. Intervention was associated with a 0.50 pg/mL reduction in blood IL-7 (p = 0.003), a 1.45 pg/mL reduction in IFN-γ (p = 0.011), a 0.20 pg/mL reduction in TNF-α (p = 0.011), and a 4.05 pg/mL reduction in MCP-1 (p = 0.042). In contrast, the concentrations of all these inflammatory markers in control participants increased. Furthermore, participants in the Tango group had a decline in all 6 additional inflammatory markers measured, although the difference scores were not significantly different from control. Furthermore, both groups experienced increases in serum amyloid protein (SAP). Decreased inflammatory biomarkers post-intervention in the experimental group suggest that Tango may help with increased blood inflammatory biomarkers levels, which is an independent risk factor for developing AD[61].
Table 2.
Pre-test, post-test and change score values for Biomarkers (pg/mL) in Tango and Control groups (N Tango: 24, N Control: 10).
| Pre-test | Post-test | Change | |||||||||
| Tango | Control | Tango | Control | Tango | Control | Mean Difference (CI) | P-value | FDR (i/m) Q* | Cohen's D (95% CI) | N^ | |
| IL-7 | 1.19 ± 1.36 | 1.13 ± 1.58 | 0.94 ± 1.02 | 1.4 ± 1.53 | −0.49 ± 0.83 | 0.55 ± 0.95 | −1.04 (−1.7, −0.4) |
0.003 | 0.005 | −1.2 (−2, −0.4) |
34 |
| IFN-γ | 11.68 ± 7.03 | 11.18 ± 6.17 | 10.23 ± 5.85 | 12.55 ± 5.59 | −1.45 ± 2.99 | 1.37 ± 2.14 | −2.82 (−4.9, −0.7) |
0.011 | 0.009 | −1.02 (−1.8, −0.2) |
34 |
| TNF-α | 10.71 ± 4.67 | 10.66 ± 4.54 | 10.26 ± 4.42 | 10.91 ± 3.63 | −0.2 ± 0.56 | 0.42 ± 0.7 | −0.62 (−1.1, −0.2) |
0.011 | 0.014 | −1.03 (−1.8, −0.2) |
33 |
| MCP-1 | 979.67 ± 355.42 | 843.48 ± 288.69 | 939.62 ± 353.53 | 896.99 ± 310.68 | −4.05 ± 30.3 | 21.57 ± 36.46 | −25.62 (−50.3, −1) |
0.042 | 0.018 | −0.8 (−1.6, 0) |
34 |
| TGF-α | 0.6 ± 1.1 | 0.33 ± 0.56 | 0.46 ± 0.89 | 0.39 ± 0.54 | −0.25 ± 0.66 | 0.27 ± 0.76 | −0.52 (−1, 0) |
0.053 | 0.023 | −0.76 (−1.5, 0) |
34 |
| IL-8 | 4.45 ± 1.19 | 3.99 ± 1.35 | 3.96 ± 1.1 | 4.54 ± 0.93 | −0.64 ± 2.86 | 0.56 ± 1.43 | −1.2 (−2.7, 0.3) |
0.122 | 0.027 | −0.47 (−1.3, 0.3) |
33 |
| MDC | 7.54 ± 12.26 | 4.04 ± 1.89 | 6.9 ± 9.73 | 4.6 ± 1.96 | −40.05 ± 145.55 | 53.51 ± 185.1 | −93.56 (−214.4, 27.3) |
0.125 | 0.032 | −0.59 (−1.4, 0.2) |
34 |
| IL-9 | 170.78 ± 53.22 | 166.26 ± 52.13 | 166.72 ± 48.99 | 187.83 ± 65.05 | −0.13 ± 0.35 | 0.06 ± 0.31 | −0.19 (−0.5, 0.1) |
0.149 | 0.036 | −0.56 (−1.3, 0.2) |
34 |
| IL-10 | 5.46 ± 1.42 | 5.45 ± 1.9 | 5.26 ± 1.24 | 5.87 ± 1.77 | −0.45 ± 2.33 | 0.25 ± 1.65 | −0.7 (−2.4, 1) |
0.397 | 0.041 | −0.32 (−1.1, 0.4) |
34 |
| CRP | 0.35 ± 0.37 | 0.4 ± 0.61 | 0.27 ± 0.32 | 0.41 ± 0.25 | −0.08 ± 0.27 | 0.01 ± 0.44 | −0.09 (−0.4, 0.2) |
0.581 | 0.045 | −0.26 (−1, 0.5) |
34 |
|
SAP |
0.27 ± 0.1 | 0.28 ± 0.08 | 0.28 ± 0.12 | 0.31 ± 0.07 | 0.02 ± 0.06 | 0.02 ± 0.1 | −0.01 (−0.1, 0) |
0.764 | 0.050 | −0.11 (−0.9, 0.7) |
34 |
Table 2. Pre-test, post-test, and change score values for Biomarkers in Tango and Control groups. P-values were generated using an independent t-test comparing difference scores from pre to post-intervention between treatment and control groups. All biomarker values are presented in mean (pg/ml) +/- SD .
* The Benjamini-Hochberg procedure was performed for p-value correction (FDR = 0.05). The overall a level was 0.05, but each individual P value was compared with its Benjamini-Hochberg critical value, (i/m)Q, where i is the rank, m is the total number of tests, and Q is the false discovery rate chosen to be 0.05. The largest P value that has P<(i/m)Q is considered significant. All values above it are considered significant, even if those p-values are lower than the critical values (e.g. IFN-γ has a p-value (p = 003D0.011) larger than its FDR critical value (FDR=0.009), therefore IFN-γ is still considered significant[58].
N^ = Number of data points available for the analysis; A data point was considered unavailable if the data points from both timepoints were missing.
3.3. Key behavioral measures across domains
Table 3 shows cognitive and motor test data. Participants in the intervention group significantly improved on the FAB (p = 0.023), and the 30 second chair stand (p = 0.018). A non-significant effect was noted in the difference score of the Trails Making Test (d = 0.89), favoring the intervention group. More Tango participants improved or maintained (decreased or no change) number of errors performed during the inhibition condition in the Color Word Interference Task compared to controls (p = 0.031). A small-moderate, non-significant effect (d= −0.24) was observed in body position spatial task also favoring the intervention group. Although both groups demonstrated increased Tower of London (TOL) Total Achievement Scores, Tango participants had reduced increases in this score compared to controls demonstrated with a large, non-significant effect (d = 0.64).
Table 3.
Pre-test, Post-test and Change Scores for Cognitive and Behavioral Measures in Tango and Control groups.
| Pre | Post | Change | Mean Difference a | P-value | FDR (i/m) Q* | Cohen's D | N^ | ||||
| Tango | Control | Tango | Control | Tango | Control | (95% CI) | (95% CI) | ||||
| n = 24 | n = 10 | n = 24 | n = 10 | n = 24 | n = 10 | ||||||
| 30 S Chair Stand (no.) ϯ | 12.67 ± 4.59 | 12.7 ± 3.3 | 12.75 ± 4.3 | 11.4 ± 2.76 | 0.08 ± 1.53 | −1.3 ± 1.34 | 1.38 (0.3, 2.5) |
0.018* | 0.017 | 0.94 (0.1, 1.7) |
34 |
| Fullerton Advance Balance Scale (/40) ϯ | 32.83 ± 4.72 | 34.8 ± 5.22 | 33.3 ± 6.06 | 34.2 ± 5.65 | 0.48 ± 2.59 | −0.6 ± 1.71 | 1.08 | 0.023* | 0.033 | 0.46 (−0.3, 1.2) |
33 |
| TOL Total Achievement Score (Scaled) ϯ | 10.12 ± 1.92 | 9 ± 1.63 |
10.67 ± 1.95 | 10.7 ± 1.16 | 0.54 ± 1.56 | 1.7 ± 2.21 | 1.16 (−2.51,0.2) |
0.091 | 0.067 | 0.64 (−0.1, 1.4) |
34 |
| Body position spatial test (product score) ϯ | 17.67 ± 11.06 | 15.6 ± 6.28 | 20.29 ± 9.45 | 15.9 ± 5.86 | 2.6 ± 10.41 | 0.3 ± 7.18 | −2.33 (−5.04,9.69) |
0.525 | 0.083 | −0.24 (−1, 0.5) |
34 |
| Corsi Blocks product Score (product score) ϯ | 25.62 ± 9.45 | 23.7 ± 16.14 | 31.42 ± 14.38 | 30.3 ± 24.67 | 5.79 ± 13.25 | 6.6 ± 13.31 | 0.81 (−10.98,9.36) |
0.872 | 0.100 | 0.06 (−0.7, 0.8) |
34 |
| Trails making difference score (s) ϯ | 44.98 ± 26.03 | 47.87 ± 29.66 | 38.58 ± 20.86 | 63.19 ± 47.89 | −6.4 ± 19.47 | 15.32 ± 32.74 | 21.72 (−45.94,2.5) |
0.074 | 0.050 | 0.89 (0.1, 1.7) |
34 |
| Total | Tango | Control | OR (95% CI)b | P-valuec | |||||||
| n = 34 N (%) | n = 24 N (%) | n = 10 N (%) | |||||||||
| Inhibition Raw Total Errors | 0.14 (0.03, 0.81) | 0.031* | |||||||||
| Decrease in errors/no change | 26 (76.5) | 21 (87.5) | 5 (50) | ||||||||
| Increase in errors | 8 (23.5) | 3 (12.5) | 5 (50) | ||||||||
Table 3. Pre-test, post-test and change score values for Cognitive and Behavioral Measures in Tango and Control groups. Values presented as Mean +/- SD. Independent t tests were used for normally distributed variables, and the Mann-Whitney U test was used for FAB score. CWIT variables were transformed to categorical variables by performing a split between performance that indicated response to intervention versus non-response, post-intervention. "Increase": Change from Pre-test to post-test larger than 0; "Decrease/no change": change from pre-test to post-test lower than or equal to 0. a Difference between the average change of Tango group and the average change of the control group. bOdds ratio for the association between change of outcome variables and treatment group (reference = Control). cP values are obtained from Fisher's exact test. * The Benjamini-Hochberg procedure was performed for p-value correction (FDR = 0.1). The overall FDR level was 0.1, but each individual P value was compared with its Benjamini-Hochberg critical value, (i/m)Q, where i is the rank, m is the total n umber of tests, and Q is the false discovery rate chosen to be 0.1. The largest P value that has P<(i/m)Q is considered significant[62]. N^ = Number of data points available for the analysis. ϯ Variables indicating better performance with a higher value.
Overall, the Tango group demonstrated improvement or maintenance in all functional measures except the TOL, and showed no deterioration in the FAB and 30 s chair stand; therefore, these behavioral tests should further be investigated in future studies to support evidence of the benefits following an Adapted Tango intervention to B/AA individuals at risk for AD.
Although the key measures across important domains of functions discussed above were selected for hypothesis testing, additional evaluations not subject to hypothesis testing were performed to expand our previous battery of tests to include cognitive, motor and psychosocial function measures not previously explored in a dance intervention with the stated purpose. Table 4 shows means, variability and effect sizes of performance on these additional motor and cognitive tests at pre-test and post-test and results from the administration of psychosocial questionnaires. Little change was observed from pre to post in several measures of motor and cognitive function in both groups, including gait speed and distance walked in 6 min in both groups. Trends (d = 0.39–0.61) were observed in depression and stress with the control group showing less stress after 12 weeks. Among the 16 caregivers, the control group had a large positive effect in Caregiver Burden (d = 0.69). Large positive effects in Role Captivity, Caregiver confidence, and Deprivation of Intimate Exchange were observed in participants in the intervention group.
Table 4.
Additional Motor and Cognitive Testing.
| Pre | Post | Change | Mean Difference b | Cohen's D | N | ||||
| Tango | Control | Tango | Control | Tango | Control | (95% CI) | N^ | ||
| n = 24 | n = 10 | n = 24 | n = 10 | n = 24 | n = 10 | ||||
| Cognitive Tests | |||||||||
| Brooks Spatial Memory Percent Correct (%) ϯ | 71 ± 18.57 | 64.8 ± 15.92 | 71.08 ± 21.09 | 66.6 ± 15.41 | 0.08 ± 8.78 | 1.8 ± 10.13 | −1.72 (−5.32,8.75) |
−0.19 (−0.96, 0.58) |
34 |
| Tower of London | |||||||||
| TOL Move Accuracy Ratio Scaled ϯ | 8.08 ± 3.11 | 7.3 ± 2.41 | 7.5 ± 2.59 | 7.4 ± 2.67 | −0.58 ± 2.36 | 0.1 ± 2.08 | −0.68 (−1.07,2.43) |
−0.29 (−1,0.5) |
34 |
| Motor Tests | |||||||||
| Backward Gait Speeda ϯ (m/s) | 0.81 ± 0.25 | 0.82 ± 0.22 | 0.77 ± 0.22 | 0.85 ± 0.21 | −0.04 ± 0.15 | 0.03 ± 0.16 | −0.07 (−0.19, 0.05) | −0.46 (−1.23 , 0.32) |
34 |
| Forward Gait Speed (m/s) ϯ | 1.06 ± 0.19 | 1.07 ± 0.16 | 1.05 ± 0.21 | 1.12 ± 0.16 | −0.01 ± 0.11 | 0.05 ± 0.12 | −0.06 (−0.14, 0.03) |
−0.54 (−1.32, 0.24) |
34 |
| Fast as Possible Gait Speed (m/s) ϯ | 1.63 ± 0.34 | 1.63 ± 0.41 | 1.52 ± 0.39 | 1.64 ± 0.35 | −0.11 ± 0.27 | 0.02 ± 0.27 | −0.13 (−0.33, 0.08) |
−0.47 (−1.24, 0.31) |
34 |
| 6 Min Walk Test (m) ϯ | 456 ± 88 | 464 ± 75 | 457 ± 96 | 470 ± 83 | 1.1 ± 55.56 | 5.79 ± 53.78 | −4.69 (−46.9, 37.53) |
−0.09 (−0.85, 0.68) |
34 |
| Timed Up and Go (TUG) | |||||||||
| TUG Simple (s) | 8.13 ± 3.14 | 8.56 ± 1.46 | 8.42 ± 3.11 | 8.65 ± 1.64 | 0.29 ± 0.8 | 0.08 ± 0.84 | 0.21 (−0.83,0.41) |
0.26 (−0.51, 1.03) |
34 |
| TUG Cognitive (s) | 11.44 ± 4.2 | 12.89 ± 5.08 | 11.43 ± 3.3 | 11.56 ± 3.99 | −0.01 ± 2.27 | −1.33 ± 5.71 | 1.32 (−5.47, 2.82) |
0.37 (−0.4, 1.14) |
34 |
| Questionnaires | |||||||||
| Center for Epidemiological Studies Depression (CESD) | 16.71 ± 8.90 | 20.40 ± 6.35 | 16.71 ± 8.22 | 18.00 ± 4.78 | −0.71 ± 6.09 | 0.00 ± 4.65 | 2.4 (−4.02, 8.82) |
0.39 (−0.39, 1.17) |
34 |
| Perceived Stress Scale Score | 5.32 ± 3.66 | 6.3 ± 4.32 | 5.32 ± 2.68 | 4.8 ± 3.36 | 0 ± 1.93 | −1.5 ± 3.37 | 1.5 (−1, 4) |
0.61 (−0.18, 1.41) |
32 |
| Caregiver Questionnaires | |||||||||
| The Zarit Burden Interview (ZBI)* | 43.73 ± 18.29 | 33 ± 17.31 | 42.73 ± 19.11 | 28.2 ± 11.19 | −1 ± 2.28 | −4.8 ± 9.63 | 3.8 (−8.08, 15.68) |
0.69 (−0.5, 1.88) |
16 |
| Quality of Life Score (QOL)* | 25.64 ± 4.13 | 28.8 ± 5.36 | 26 ± 4.63 | 28.4 ± 3.91 | 0.36 ± 1.12 | −0.4 ± 2.88 | 0.76 (−2.76, 4.29) |
0.42 (−0.75, 1.59) |
16 |
| Pearlin Caregiver Stress Scales * | |||||||||
| Deprivation of Intimate Exchange Score | 6.46 ± 1.86 | 5.8 ± 1.92 | 6.36 ± 1.63 | 6.2 ± 2.28 | −0.09 ± 1.3 | 0.4 ± 0.55 | −0.49 (−1.81, 0.83) |
−0.43 (−1.6, 0.74) |
16 |
| Deprivation of Goals and Activities Score | 4.82 ± 1.60 | 4.4 ± 2.88 | 4.64 ± 1.43 | 4.2 ± 1.48 | −0.18 ± 0.98 | −0.2 ± 1.92 | 0.02 (−1.51, 1.55) |
0.01 (−1.14, 1.17) |
16 |
| Role Captivity Score | 4.64 ± 2.01 | 4.8 ± 1.92 | 4.46 ± 2.02 | 6 ± 1.41 | −0.18 ± 0.4 | 1.2 ± 1.3 | −1.38 (−2.98, 0.22) |
−1.78 (−3.12, −0.44) |
16 |
| Loss of Self Score | 5.8 ± 1.94 | 5.8 ± 2.28 | 6.1 ± 2.21 | 5.8 ± 1.1 | 0.27 ± 0.79 | 0 ± 1.41 | 0.27 (−0.89, 1.44) |
0.27 (−0.89, 1.43) |
16 |
| Caregiving Competence Score ϯ | 6.73 ± 2.15 | 5.4 ± 1.95 | 7.09 ± 2.21 | 4.8 ± 2.39 | 0.36 ± 0.67 | −0.6 ± 1.95 | 0.96 (−1.43, 3.36) |
0.81 (−0.39, 2.01) |
16 |
| Management of Situation Score ϯ | 8.2 ± 1.99 | 6 ± 1.58 | 7 ± 2.14 | 6.4 ± 1.14 | −1.18 ± 1.47 | 0.4 ± 1.52 | −1.58 (−3.3, 0.14) |
−1.07 (−2.29, 0.16) |
16 |
| Management of Meaning Score ϯ | 16.36 ± 5.32 | 15.6 ± 4.1 | 16.91 ± 5.3 | 16.6 ± 4.51 | 0.55 ± 1.37 | 1 ± 2.35 | −0.46 (−2.43, 1.52) |
−0.27 (−1.43, 0.9) |
16 |
Table 4. Pre-test, post-test and change score values for Behavioral Measures in Tango and Control groups. Values are presented are Mean +/- SD except as noted. a Values presented are the average speed of three trials for gait variables. b Difference between the change on average of the tango and control groups. N^ Number of data points available for the analysis; a data point was considered unavailable if the data points from both timepoints were missing. *Surveys completed only by caregivers in the study (n = 16). ϯ Variables indicating better performance with a higher value. Those variables without notation indicate a worse performance with a higher value.
3.4. Satisfaction with tango
16 participants completed the exit questionnaire regarding satisfaction with the Tango classes. Most participants (n = 13) strongly agreed that they enjoyed participating in the program. 14 participants strongly agreed they would continue participating if possible. Most participants somewhat or strongly agreed that their balance, walking, mood, coordination, and endurance had improved. Half of the participants somewhat or strongly agreed that their strength had improved. Most participants somewhat or strongly agreed that they were more mentally and physically active, post-program (Table 5).
Table 5.
Frequency table for Exit questions for participants in Tango group. .
| Exit Questions (n = 16) |
Strongly Agree n (%) |
Somewhat Agree n (%) |
Neither Agree nor Disagree n (%) |
Somewhat Disagree n (%) |
Strongly Disagree n (%) |
| I enjoyed participating in this program | 13 (81.2) | 3 (18.8) | 0 (0) | 0 (0) | 0 (0) |
| My balance has improved | 7 (43.8) | 6 (37.5) | 3 (18.8) | 0 (0) | 0 (0) |
| My walking has improved | 5 (31.2) | 5 (31.2) | 6 (37.5) | 0 (0) | 0 (0) |
| My mood has improved | 8 (50) | 2 (12.5) | 6 (37.5) | 0 (0) | 0 (0) |
| My coordination has improved | 5 (31.2) | 7 (43.8) | 4 (25) | 0 (0) | 0 (0) |
| My strength has improved | 4 (25) | 4 (25) | 8 (50) | 0 (0) | 0 (0) |
| My endurance has improved | 6 (37.5) | 3 (18.8) | 7 (43.8) | 0 (0) | 0 (0) |
| If I could, I would continue participating | 9 (56.2) | 5 (31.2) | 1 (6.2) | 0 (0) | 1 (6.2) |
| I have been more physically active | 8 (50) | 4 (25) | 4 (25) | 0 (0) | 0 (0) |
| I have been more mentally active | 9 (56.2) | 6 (37.5) | 1 (6.2) | 0 (0) | 0 (0) |
4. Discussion
4.1. Conclusions
This Phase I randomized placebo controlled clinical trial was able to evaluate biomarkers of inflammation as well as behavioral and motor function in participants at high risk of AD due to family history and social risk factors before and after an Adapted Tango dance intervention. A significant reduction in inflammatory cytokines was observed, including IL-7, TNF-α, IFN-γ, and MCP-1, as well as intervention-associated improvement or maintenance of participant cognition, strength (as measured by the 30 Second Chair Stand), and balance (as measured by the FAB test), which may represent useful candidates for primary measures in our upcoming phase II clinical trials. These results extend data obtained in other studies evaluating dance and exercise-based interventions. Although little change was noted in motor and cognitive function in both the Tango and control group for secondary assessments, large positive effects were found in the Tango group for some of the caregiver measures. These secondary and exploratory outcomes of caregiver burden suggest potentially positive effects of this dance intervention for the caregiver. However, these findings are not definitive, and a follow up clinical trial with a larger group of caregivers is needed to thoroughly evaluate the effects of a dance intervention on mood and caregiver burden. The significant reductions in blood inflammatory biomarkers and improvements/maintenance seen in cognitive and motor domains despite the short intervention period, in combination with high patient satisfaction with the intervention implies potential for an Adapted Tango based intervention for this underserved population.
4.2. Reception of adapted tango and attrition
While the sample size was small, the Adapted Tango intervention was very well received as demonstrated by reasonably low participant attrition, satisfaction questionnaires that indicated high satisfaction and anecdotal reports. As more than 50% of the participants completed 15 or more lessons, these high satisfaction rates support the acceptability of the intervention. These busy caregivers had many demands on their time and some individuals found it tough to fit in therapy, even enjoyable therapy. Issues related to time of day or other circumstances related to perceived or real barriers for caregivers to attend these therapies must be investigated in future iterations of this research. However, those who could attend and who completed the Exit questionnaire indicated they strongly enjoyed the program, would continue if given the opportunity, reported being more mentally and physically active and suggested they had noticed several benefits to physical health.
4.3. Blood biomarker measures
Recent studies[13], [14], [15], [16], [17], [63], [64] demonstrate that increased blood-based inflammatory biomarkers are correlated to the development of AD, and that chronic inflammation imparts a risk of cognitive decline. As blood-based biomarkers represent a low-invasive and low-cost screen tool to monitor cerebral inflammatory activity, these analytes are ideal for evaluation in an asymptomatic population. However, these outcomes have not been evaluated in cohorts at high risk of AD due to caregiver burden and/or family history following a dance-based intervention. In this pilot clinical trial of AA women with a family history of AD, Tango participants showed decreased levels of inflammatory cytokines - IL-7, TNF-α, IFN-γ, and MCP-1 - at 12 weeks post-test, compared to the control group. These results suggest that an Adapted Tango intervention may be helpful in controlling or reducing markers of inflammation in AA women with a parental history of AD. Our trial highlights the potential cognitive, functional and physiological benefit of dance-based intervention in larger cohorts of B/AA and White middle age women at risk for AD.
Research has shown that disease-aggravating inflammation begins decades prior to the appearance of cognitive decline or AD[65], [66], [67]. Indeed, results presented here suggest the modulation of a key AD-associated neuroinflammatory pathway by Adapted Tango: specifically, microglial activation induced by amyloid-β through the TNF-α signaling pathway has been demonstrated to induce patterns of nerve cell death found in human disease[68]. The role of TNF-α in early neural proinflammatory processes is supported by animal models[69], [70], [71], [72] and human longitudinal studies of AD[71], [72], [73], [74]. Although pharmacologic interventions in late phase CNS inflammation have broadly shown no effectiveness or been detrimental to patients[61], recent studies suggest targeting inflammation earlier in the disease process may prevent AD related inflammatory induced pathology[66]. Therein, programs such as Adapted Tango, which are both well received by participants and may reduce inflammatory burden, represent viable options for early intervention prior to the onset of AD symptoms.
4.4. Effects on cognitive and motor measures
Prior research has shown improved cognitive performance and motor function for AD patients and caregivers after participating in dance therapies and exercise-induced interventions[19], [20], [21]. Within the cognitive domain, our primary outcomes demonstrated promising, albeit underpowered, results: participants in Tango demonstrated improvements in whole-body spatial cognition and short-term and working memory, and reduced deterioration of executive function. Furthermore, more participants in Tango improved or maintained (decreased or no change) number of errors performed during the inhibition condition in the Stroop Color Word Interference Task compared to controls. Indeed, improvements in attention and processing speed, as well as executive function and memory improvement, are a common feature of exercise-based interventions[75], and duration of exercise is essential for benefit. Improvements in spatial cognition and executive function have previously been reported for individuals with PD following Adapted Tango[29]. Therefore, interventions such as Adapted Tango, which are well enjoyed by participants, have low rate of discontinuation, and demonstrate effects within cognitive domains present an important opportunity for risk reduction. Indeed, other dance interventions for older sedentary adults such as BAILAMOS[76] suggest improvements in memory, attention, and focus[77] while individuals with MCI enrolled in a 10-month ballroom dancing program improved in thinking and memory. Additional dance-based interventions are being evaluated[78].
With more studies showing the benefits of motor interventions for familial caregivers caring for a relative with dementia[21], this is the first clinical study to conduct a dance intervention in B/AA woman familial AD caregivers who are at risk of developing AD due to numerous factors. These pilot data suggest improvements or maintenance of motor function within the functional domains of strength and balance, as demonstrated by the FAB and 30 second chair stand tests. Within our secondary outcome measures, including gait speed and distance, our results showed little change from pre to post intervention in measures of motor and cognitive function in both the Tango and control groups. These data mirror motor function results obtained following Adapted Tango intervention in individuals with PD; individuals completing an Adapted Tango program experience improvements in balance and motor experiences of daily living as evaluated by the Unified Parkinson's Disease Rating Scale score[79], and these outcomes are maintained for at least one month[80]. Indeed, as compared to other dance interventions such as ballroom dancing, which have described benefits for cognitive function, Adapted Tango may provide additional motor benefits[27].
4.5. Effects on psychosocial measures and measures of caregiving burden
Sixteen participants were current caregivers, limiting the scope of analyses for this population but allowing exploratory analysis of this subgroup. Posthoc tests were assessed to determine the potential impact of Adapted Tango on caregiver burden and quality of life. The sample size was small and therefore these results require further investigation. The control group reported decreased stress and depression, as well as a large positive effect in Caregiver Burden per the Zarit Burden Interview after 12 weeks. However, the Tango group showed large positive effects in Role Captivity, Caregiver confidence, and Deprivation of Intimate Exchange. Previous literature has supported that dance interventions are a therapeutic treatment in improving the quality of life of patients of AD and their caregivers[19]. Furthermore, exercise interventions have shown beneficial impacts on perceived caregiver burden[21]. Although our Tango group did not show large positive effect in cumulative caregiver burden post intervention, the large positive effect in Role Captivity, Caregiver confidence, and Deprivation of Intimate Exchange displays some of the positive effects of this dance intervention for the caregivers. To more thoroughly investigate caregiver burden, stress, and quality of life, future studies should be conducted using a large sample size of caregivers.
4.6. Limitations
This pilot's sample size was small and therefore underpowered; however, these effects will be used to power a future Phase II trial with a larger sample. Additionally, compared to other exercise-based interventions, our Adapted Tango intervention was relatively short; although similar to durations for other populations that have been investigated in the context of dance rehabilitation. Future studies should look at longer durations of Adapted Tango, e.g., 6 months, or one year, in this population. Lastly, prior studies of dance therapies[26], [27], [28], [29] have identified lasting effects following cessation of intervention; future evaluations of Adapted Tango in B/AA at risk for AD and/or caregivers should implement testing post-intervention to identify the length of effect.
Author contributions
Study design and conceptualization were performed by WW, MEH, and LEM. WW, MEH, AAB, RJS, HS, and ARH contributed to participant recruitment. RJS performed clinical study visits, WH performed biospecimen processing and analysis, MEH, AAB and ARH (and team members) conducted motor and cognitive measures, and MEH, ARH and AAB administered the dance intervention. Data analysis was performed by LJ and RJS, with statistical consultation by LN. Manuscript preparation was completed by LJ and DS. All authors read and approved the final manuscript. The authors declare that they have no potential conflict of interest in relation to the study in this paper.
Acknowledgments
A Department of Veterans Affairs Career Development award supported ME Hackney (IK2RX000870). This trial was supported by the Emory Goizueta Alzheimer's Disease Research Center and the Atlanta VA Center for Visual and Neurocognitive Rehabilitation. We gratefully acknowledge the participants in this study for their time and energy. The Emory Center for Health in Aging provided space for interventions and assessments.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cccb.2021.100018.
Appendix. Supplementary materials
References
- 1.Alzheimer's disease facts and figures. 2019. 15(3): p. 321–387.
- 2.Green R.C., et al. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA. 2002;287(3):329–336. doi: 10.1001/jama.287.3.329. [DOI] [PubMed] [Google Scholar]
- 3.Wharton W., et al. Interleukin 9 alterations linked to alzheimer disease in african americans. Ann. Neurol. 2019;86(3):407–418. doi: 10.1002/ana.25543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paganini-Hill A., Henderson V.W. Estrogen deficiency and risk of Alzheimer's disease in women. Am. J. Epidemiol. 1994;140(3):256–261. doi: 10.1093/oxfordjournals.aje.a117244. [DOI] [PubMed] [Google Scholar]
- 5.Goren A., et al. Impact of caring for persons with Alzheimer's disease or dementia on caregivers' health outcomes: findings from a community based survey in Japan. BMC Geriatr. 2016;16:122. doi: 10.1186/s12877-016-0298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulz, R. and S.R. Beach, Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. 1999. 282(23): p. 2215–2219. [DOI] [PubMed]
- 7.Pinquart M., Sörensen S. Ethnic differences in stressors, resources, and psychological outcomes of family caregiving: a meta-analysis. Gerontologist. 2005;45(1):90–106. doi: 10.1093/geront/45.1.90. [DOI] [PubMed] [Google Scholar]
- 8.Zheng G., et al. Effect of Aerobic Exercise on Inflammatory Markers in Healthy Middle-Aged and Older Adults: a Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front Aging Neurosci. 2019;11:98. doi: 10.3389/fnagi.2019.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui M.Y., et al. Exercise Intervention Associated with Cognitive Improvement in Alzheimer's Disease. Neural Plast. 2018 doi: 10.1155/2018/9234105. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irons J.Y., et al. An integrative systematic review of creative arts interventions for older informal caregivers of people with neurological conditions. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0243461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wharton W., et al. Photojournalism-Based Intervention Reduces Caregiver Burden and Depression in Alzheimer's Disease Family Caregivers. J. Holist. Nurs. 2018;37(3):214–224. doi: 10.1177/0898010118801636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nutaitis A.C., et al. Diet as a Risk Factor for Cognitive Decline in African Americans and Caucasians with a Parental History of Alzheimer's Disease: a Cross-Sectional Pilot Study Dietary Patterns. J Prev Alzheimers Dis. 2019;6(1):50–55. doi: 10.14283/jpad.2018.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan A.R., et al. Inflammatory biomarkers in Alzheimer's disease plasma. Alzheimer's & dementia: the journal of the Alzheimer's Association. 2019;15(6):776–787. doi: 10.1016/j.jalz.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorelick P.B. Role of inflammation in cognitive impairment: results of observational epidemiological studies and clinical trials. Ann. N. Y. Acad. Sci. 2010;1207:155–162. doi: 10.1111/j.1749-6632.2010.05726.x. [DOI] [PubMed] [Google Scholar]
- 15.Schneider P., Hampel H., Buerger K. Biological marker candidates of Alzheimer's disease in blood, plasma, and serum. CNS Neurosci. Ther. 2009;15(4):358–374. doi: 10.1111/j.1755-5949.2009.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai K.S.P., et al. Peripheral inflammatory markers in Alzheimer's disease: a systematic review and meta-analysis of 175 studies. J. Neurol. Neurosurg. Psychiatry. 2017;88(10):876–882. doi: 10.1136/jnnp-2017-316201. [DOI] [PubMed] [Google Scholar]
- 17.Patel R.A., et al. Association between anti-inflammatory interleukin-10 and executive function in African American women at risk for Alzheimer's disease. J. Clin. Exp. Neuropsychol. 2020:1–13. doi: 10.1080/13803395.2020.1798879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gouin J.-.P., et al. Chronic stress, daily stressors, and circulating inflammatory markers. Health Psychol. 2012;31(2):264. doi: 10.1037/a0025536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruiz-Muelle A., López-Rodríguez M.M. Dance for People with Alzheimer's Disease: a Systematic Review. Curr Alzheimer Res. 2019;16(10):919–933. doi: 10.2174/1567205016666190725151614. [DOI] [PubMed] [Google Scholar]
- 20.King A.C., et al. Effects of Moderate-Intensity Exercise on Physiological, Behavioral, and Emotional Responses to Family Caregiving: a Randomized Controlled Trial. The Journals of Gerontology: Series A. 2002;57(1):M26–M36. doi: 10.1093/gerona/57.1.m26. [DOI] [PubMed] [Google Scholar]
- 21.Canonici A.P., et al. Functional dependence and caregiver burden in Alzheimer's disease: a controlled trial on the benefits of motor intervention. Psychogeriatrics. 2012;12(3):186–192. doi: 10.1111/j.1479-8301.2012.00407.x. [DOI] [PubMed] [Google Scholar]
- 22.Hernández S.S., et al. What are the Benefits of Exercise for Alzheimer's Disease? A Systematic Review of the Past 10 Years. J. Aging Phys. Act. 2015;23(4):659–668. doi: 10.1123/japa.2014-0180. [DOI] [PubMed] [Google Scholar]
- 23.Lange-Asschenfeldt C., Kojda G. Alzheimer's disease, cerebrovascular dysfunction and the benefits of exercise: from vessels to neurons. Exp. Gerontol. 2008;43(6):499–504. doi: 10.1016/j.exger.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Thoma M.V., et al. The effect of music on the human stress response. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0070156. e70156-e70156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gok Ugur H., et al. Effects of Music Therapy on the Care Burden of In-Home Caregivers and Physiological Parameters of Their In-Home Dementia Patients: a Randomized Controlled Trial. Complementary Medicine Research. 2019;26(1):22–30. doi: 10.1159/000490348. [DOI] [PubMed] [Google Scholar]
- 26.Hackney M.E., et al. Adapted Tango improves mobility, motor–cognitive function, and gait but not cognition in older adults in independent living. J. Am. Geriatr. Soc. 2015;63(10):2105–2113. doi: 10.1111/jgs.13650. [DOI] [PubMed] [Google Scholar]
- 27.Hackney M.E., Earhart G.M. Effects of dance on movement control in Parkinson's disease: a comparison of Argentine tango and American ballroom. J. Rehabil. Med. 2009;41(6):475–481. doi: 10.2340/16501977-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hackney M.E., et al. Multimodal exercise benefits mobility in older adults with visual impairment: a preliminary study. Journal of Aging Physical Activity. 2015;23(4):630–639. doi: 10.1123/japa.2014-0008. [DOI] [PubMed] [Google Scholar]
- 29.McKee K.E., Hackney M.E. The effects of adapted tango on spatial cognition and disease severity in Parkinson's disease. J. Mot. Behav. 2013;45(6):519–529. doi: 10.1080/00222895.2013.834288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Center F.H.C.R. Women's Health Initiative; 2001. Dementia Questionnaire V2.0. [Google Scholar]
- 31.Hackney M.E., et al. Rationale and Design of a Clinical Trial of Adapted Tango to Improve Negative Health Impacts in Middle Aged African-American Female Caregivers of Persons with Alzheimer's Disease (ACT Trial) J. Alzheimers Dis. 2019;68(2):767–775. doi: 10.3233/JAD-181130. [DOI] [PubMed] [Google Scholar]
- 32.Vandenberg S.G., Kuse A.R. Mental rotations, a group test of three-dimensional spatial visualization. Perceptual motor skills. 1978;47(2):599–604. doi: 10.2466/pms.1978.47.2.599. [DOI] [PubMed] [Google Scholar]
- 33.Kessels R.P., et al. The backward span of the Corsi Block-Tapping Task and its association with the WAIS-III Digit Span. Assessment. 2008;15(4):426–434. doi: 10.1177/1073191108315611. [DOI] [PubMed] [Google Scholar]
- 34.Krueger C.E., et al. Know thyself: real-world behavioral correlates of self-appraisal accuracy. Clin. Neuropsychol. 2011;25(5):741–756. doi: 10.1080/13854046.2011.569759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krueger C., et al. Conflict monitoring in early frontotemporal dementia. Neurology. 2009;73(5):349–355. doi: 10.1212/WNL.0b013e3181b04b24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kramer, J. Domain Specific Tasks of Executive Function. [cited 2011; Available from: http://examiner.ucsf.edu/index.htm.
- 37.Tarlowe M.H., et al. Prospective study of neutrophil chemokine responses in trauma patients at risk for pneumonia. American journal of respiratory critical care medicine. 2005;171(7):753–759. doi: 10.1164/rccm.200307-917OC. [DOI] [PubMed] [Google Scholar]
- 38.Roth D.L., et al. Positive aspects of family caregiving for dementia: differential item functioning by race. Journals of Gerontology Series B: Psychological Sciences Social Sciences. 2015;70(6):813–819. doi: 10.1093/geronb/gbv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zarit S.H., Reever K.E., Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20(6):649–655. doi: 10.1093/geront/20.6.649. [DOI] [PubMed] [Google Scholar]
- 40.Radloff L.S. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 41.Smith S., et al. Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology. Health Technol. Assess. (Rockv) 2005;9(10):1–93. doi: 10.3310/hta9100. [DOI] [PubMed] [Google Scholar]
- 42.Moore M., Barker K. The validity and reliability of the four square step test in different adult populations: a systematic review. Syst Rev. 2017;6(1):187. doi: 10.1186/s13643-017-0577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamb S.E., Keene D.J. Measuring physical capacity and performance in older people. Best Practice Research Clinical Rheumatology. 2017;31(2):243–254. doi: 10.1016/j.berh.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 44.Laufer Y. Effect of age on characteristics of forward and backward gait at preferred and accelerated walking speed. The Journals of Gerontology Series A: Biological Sciences Medical Sciences. 2005;60(5):627–632. doi: 10.1093/gerona/60.5.627. [DOI] [PubMed] [Google Scholar]
- 45.Benavent-Caballer V., et al. Factors associated with the 6-minute walk test in nursing home residents and community-dwelling older adults. J Phys Ther Sci. 2015;27(11):3571–3578. doi: 10.1589/jpts.27.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones C.J., Rikli R.E., Beam W.C. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Research quarterly for exercise sport. 1999;70(2):113–119. doi: 10.1080/02701367.1999.10608028. [DOI] [PubMed] [Google Scholar]
- 47.McKee K.E., Hackney M.E. The effects of adapted tango on spatial cognition and disease severity in Parkinson's disease. J. Mot. Behav. 2013;45(6):519–529. doi: 10.1080/00222895.2013.834288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muntner P., et al. Measurement of Blood Pressure in Humans: a Scientific Statement From the American Heart Association. Hypertension. 2019;73(5):e35–e66. doi: 10.1161/HYP.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar V.V., et al. Baseline Results: the Association Between Cardiovascular Risk and Preclinical Alzheimer's Disease Pathology (ASCEND) Study. J. Alzheimers Dis. 2020;75(1):109–117. doi: 10.3233/JAD-191103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nasreddine Z.S., et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 51.Delis D.C., Kaplan E., Kramer J.H. San Antonio; PearsonTX: 2001. Delis-Kaplan Executive Function System (D-Kefs): Examiners manual: Flexibility of thinking, Concept formation, Problem solving, planning, creativity, Impluse control, Inhibition. [Google Scholar]
- 52.Rainville C., et al. Executive function deficits in patients with dementia of the Alzheimer's type A study with a Tower of London task. Arch. Clin. Neuropsychol. 2002;17(6):513–530. [PubMed] [Google Scholar]
- 53.Golden, C.J. and S.M. Freshwater, Stroop color and word test. 1978.
- 54.Bowie C.R., Harvey P.D. Administration and interpretation of the Trail Making Test. Nat. Protoc. 2006;1(5):2277. doi: 10.1038/nprot.2006.390. [DOI] [PubMed] [Google Scholar]
- 55.Brooks L.R. The suppression of visualization by reading. Q. J. Exp. Psychol. 1967;19(4):289–299. doi: 10.1080/14640746708400105. [DOI] [PubMed] [Google Scholar]
- 56.Hackney M.E., et al. Dancing for balance: feasibility and efficacy in oldest-old adults with visual impairment. Nurs. Res. 2013;62(2):138–143. doi: 10.1097/NNR.0b013e318283f68e. [DOI] [PubMed] [Google Scholar]
- 57.Hofheinz M., Schusterschitz C. Dual task interference in estimating the risk of falls and measuring change: a comparative, psychometric study of four measurements. Clin. Rehabil. 2010;24(9):831–842. doi: 10.1177/0269215510367993. [DOI] [PubMed] [Google Scholar]
- 58.Klein P.J., Fiedler R.C., Rose D.J. Rasch analysis of the Fullerton Advanced Balance (FAB) scale. Physiother. Can. 2011;63(1):115–125. doi: 10.3138/ptc.2009-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pearlin L.I., et al. Caregiving and the stress process: an overview of concepts and their measures. Gerontologist. 1990;30(5):583–594. doi: 10.1093/geront/30.5.583. [DOI] [PubMed] [Google Scholar]
- 60.Benjamini Y., Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B-Methodological. 1995;57(1):289–300. [Google Scholar]
- 61.Hampel H., et al. A Path Toward Precision Medicine for Neuroinflammatory Mechanisms in Alzheimer's Disease. Front. Immunol. 2020;11:456. doi: 10.3389/fimmu.2020.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Storey J.D. The positive false discovery rate: a Bayesian interpretation and the q -value. Ann. Statist. 2003;31(6):2013–2035. [Google Scholar]
- 63.Sartori A.C., et al. The impact of inflammation on cognitive function in older adults: implications for healthcare practice and research. J. Neurosci. Nurs. 2012;44(4):206–217. doi: 10.1097/JNN.0b013e3182527690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.López-Valdés H.E., Martínez-Coria H. The Role of Neuroinflammation in Age-Related Dementias. Rev. Invest. Clin. 2016;68(1):40–48. [PubMed] [Google Scholar]
- 65.Iulita M.F., et al. Identification and Preliminary Validation of a Plasma Profile Associated with Cognitive Decline in Dementia and At-Risk Individuals: a Retrospective Cohort Analysis. J. Alzheimers Dis. 2019;67(1):327–341. doi: 10.3233/JAD-180970. [DOI] [PubMed] [Google Scholar]
- 66.Cuello A.C. Early and Late CNS Inflammation in Alzheimer's Disease: two Extremes of a Continuum? Trends Pharmacol. Sci. 2017;38(11):956–966. doi: 10.1016/j.tips.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 67.Rogers J. Principles for central nervous system inflammation research: a call for a consortium approach. Alzheimers Dement. 2018;14(11):1553–1559. doi: 10.1016/j.jalz.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 68.Bhaskar K., et al. Microglial derived tumor necrosis factor-α drives Alzheimer's disease-related neuronal cell cycle events. Neurobiol. Dis. 2014;62:273–285. doi: 10.1016/j.nbd.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McAlpine F.E., Tansey M.G. Neuroinflammation and tumor necrosis factor signaling in the pathophysiology of Alzheimer's disease. J Inflamm Res. 2008;1:29–39. doi: 10.2147/jir.s4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cantarella G., et al. Neutralization of TNFSF10 ameliorates functional outcome in a murine model of Alzheimer's disease. Brain. 2015;138(Pt 1):203–216. doi: 10.1093/brain/awu318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Decourt B., Lahiri D.K., Sabbagh M.N. Targeting Tumor Necrosis Factor Alpha for Alzheimer's Disease. Curr Alzheimer Res. 2017;14(4):412–425. doi: 10.2174/1567205013666160930110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ekert J.O., et al. TNF alpha inhibitors in Alzheimer's disease: a systematic review. Int. J. Geriatr. Psychiatry. 2018;33(5):688–694. doi: 10.1002/gps.4871. [DOI] [PubMed] [Google Scholar]
- 73.Brosseron F., et al. Body fluid cytokine levels in mild cognitive impairment and Alzheimer's disease: a comparative overview. Mol. Neurobiol. 2014;50(2):534–544. doi: 10.1007/s12035-014-8657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iulita M.F., Caraci F., Cuello A.C. A Link Between Nerve Growth Factor Metabolic Deregulation and Amyloid-β-Driven Inflammation in Down Syndrome. CNS Neurol Disord Drug Targets. 2016;15(4):434–447. doi: 10.2174/1871527315666160321104916. [DOI] [PubMed] [Google Scholar]
- 75.Smith P.J., et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom. Med. 2010;72(3):239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marquez D.X., et al. Regular Latin Dancing and Health Education May Improve Cognition of Late Middle-Aged and Older Latinos. J. Aging Phys. Act. 2017;25(3):482–489. doi: 10.1123/japa.2016-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lazarou I., et al. International Ballroom Dancing Against Neurodegeneration: a Randomized Controlled Trial in Greek Community-Dwelling Elders With Mild Cognitive impairment. Am. J. Alzheimers Dis. Other Demen. 2017;32(8):489–499. doi: 10.1177/1533317517725813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guzmán-García A., Mukaetova-Ladinska E., James I. Introducing a Latin ballroom dance class to people with dementia living in care homes, benefits and concerns: a pilot study. Dementia (London) 2013;12(5):523–535. doi: 10.1177/1471301211429753. [DOI] [PubMed] [Google Scholar]
- 79.Hackney M.E., et al. Effects of tango on functional mobility in Parkinson's disease: a preliminary study. J. Neurol. Phys. Ther. 2007;31(4):173–179. doi: 10.1097/NPT.0b013e31815ce78b. [DOI] [PubMed] [Google Scholar]
- 80.Hackney M.E., Earhart G.M. Effects of dance on gait and balance in Parkinson's disease: a comparison of partnered and nonpartnered dance movement. Neurorehabil. Neural Repair. 2010;24(4):384–392. doi: 10.1177/1545968309353329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.