Highlights
-
•
Adrenomedullin (AM) is an endogenous peptide mainly secreted from brain endothelial cells.
-
•
AM has anti-inflammatory, angiogenic and oligodendrogenetic properties.
-
•
AM is currently being tested in clinical trials for ischemic stroke in AMFIS trial.
-
•
AM could be also a therapeutic option for vascular cognitive impairment.
Keywords: Adrenomedullin, Vascular cognitive impairment, Anti-inflammation, Angiogenesis, Clinical trial
Abstract
Adrenomedullin (AM) is an endogenous peptide mainly secreted from endothelial cells, which has multiple physiological actions such as anti-inflammation, vasodilation, vascular permeability regulation and angiogenesis. Blood AM levels are upregulated in a variety of pathological states including sepsis, severe COVID-19, acute ischemic stroke and vascular cognitive impairment with white matter changes, likely serving as a compensatory biological defense response against infection and ischemia. AM is currently being tested in clinical trials for ulcerative colitis, Crohn's disease, severe COVID-19 for its anti-inflammatory properties and in ischemic stroke for its additional angiogenic action. AM has been proposed as a therapeutic option for vascular cognitive impairment as its arteriogenic and angiogenic properties are thought to contribute to a slowing of cognitive decline in mice after chronic cerebral hypoperfusion. As AM promotes differentiation of oligodendrocyte precursor cells into mature oligodendrocytes under hypoxic conditions, AM could also be used in the treatment of CADASIL, where reduced oxygen delivery is thought to lead to the death of hypoxia-prone oligodendrocytes. AM therefore holds potential as an innovative therapeutic drug, which may regenerate blood vessels, while controlling inflammation in cerebrovascular diseases.
Introduction
Advances in acute stroke therapies, such as intravenous tissue plasminogen activator and endovascular treatment, have resulted in improved vessel recanalization rate of 70–80%, benefitting many patients after ischemic stroke. However, according to recent global burden of disease data on stroke, in 2013 there were 25.7 million stroke survivors, 6.5 million deaths from stroke, 113 million DALYs due to stroke, and 10.3 million new strokes. Stroke is also the main cause of becoming bedridden, dementia and death [1]. The treatment of acute stroke poses two major issues; firstly, how to resolve tissue damage resulting from acute stroke and, secondly, how to alleviate long-term stroke-associated effects such as vascular cognitive impairment. Despite considerable enthusiasm and investment from the pharmaceutical industry, clinical trials of many drugs for many cardiovascular diseases, including stroke, have been unsuccessful [2]. Nevertheless, several natural endogenously-occurring peptides or peptidomimetics, synthesized by the human body in the evolutionary process, have been approved and used in clinics, as components of ‘pre-built’ drugs [3]. These include tissue plasminogen activator for ischemic stroke and anti-natriuretic peptide for chronic heart failure. In addition to the model pre-built drug, insulin, glucagon-like peptide-1 has recently been used in the treatment of diabetes mellitus, one of the most important risk factors of stroke [4]. In this review, adrenomedullin (AM), which has shown promising pleiotropic properties and has been suggested as a future possible treatment for a variety of diseases, including ischemic stroke and vascular cognitive impairment, will be examined.
Biochemical features of adrenomedullin
AM is a 52-amino acid vasoactive peptide containing a disulfide bond discovered in human pheochromocytoma tissue and coded by a gene on human chromosome 11 [5,6]. Expression of the AM gene is regulated by hypoxia, inflammatory stimuli and shear stress (Fig. 1) and has consensus sequences for binding sites of transcription factors, which include hypoxia-inducible factor-1, nuclear factor for interleukin-6 expression and shear stress responsive element in its promoter region [6]. AM is widely biosynthesized and secreted, not only in the adrenal medulla, but also the cardiovascular system, including cerebral endothelial cells [7].
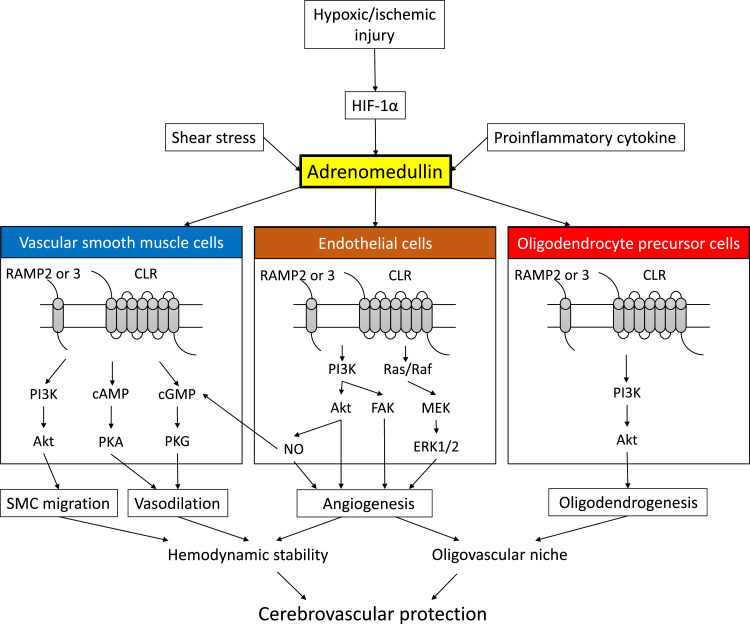
Fig. 1.
Signaling pathway of adrenomedullin (AM) in cells forming the blood-brain barrier.
AM binds to a heterodimer receptor composed of the calcitonin receptor-like receptor (CLR) and receptor activity-modifying protein type 2 (RAMP2) or 3 (RAMP3), termed AM1 and AM2 receptors, respectively. Upregulation of AM in endothelial cells by hypoxic-ischemic injury, shear stress, or proinflammatory cytokines induces angiogenesis through activation of Akt, MAPK, and FAK in an autocrine manner. AM directly binds to AM receptors on vascular smooth muscle cells (SMCs) and induces SMC migration and vasodilation. AM also promotes differentiation of oligodendrocyte precursor cells into mature oligodendrocytes under hypoxic conditions. These activities may synergistically serve as protective factors, mitigating against cerebrovascular diseases such as ischemic stroke and vascular cognitive impairment.
Abbreviations: cAMP, adenosine 3′,5′-cyclic monophosphate; cGMP, guanosine 3′,5′-cyclic monophosphate; eNOS, endothelial nitric oxide synthase; ERK, extracellular signal-regulated kinase; FAK, focal adhesion kinase; IP3, inositol triphosphate; MEK, mitogen-activated protein kinase; NO, nitric oxide; PI3K, phosphatidylinositol 3-kinase; PKA, protein kinase A; PKG, protein kinase G.
AM exerts bioactivity by binding to a heterodimer receptor composed of the calcitonin receptor-like receptor (CLR) and receptor activity-modifying protein type 2 (RAMP2) or 3 (RAMP3), named AM1 and AM2 receptors, respectively [8]. Upregulation of AM by hypoxia or sheer stress has various physiologically-active effects such as vasodilation, vascular permeability regulation, suppression of vascular endothelium apoptosis / oxidative stress, regulation of vascular smooth muscle growth and angiogenesis [9,10]. These effects are mediated by activation of phosphatidylinositol 3-kinase (PI3K)/Akt, mitogen-activated protein kinase/extracellular signal-regulated kinase 1/2 and focal adhesion kinase in endothelial cells, or by activation of PI3K/Akt and other signaling pathways in vascular smooth muscle cells (Fig. 1). AM also has anti-inflammatory effects by directly stimulating AM receptors in immune cells [11].
AM in infectious disease
Blood AM concentration is markedly increased in patients with sepsis as the AM gene possesses a binding site for nuclear factor for interleukin 6 expression in its promoter region, which mediates inflammatory stimuli [6]. The relationship of AM with infection and inflammation has thus attracted attention in recent years. Administration of AM to septic mice models reduces plasma inflammatory cytokine levels and improves hemodynamics [11]. AM has been shown to play a key role in reducing vascular (hyper) permeability and promoting endothelial stability and integrity following severe infection [12]. Accordingly, AM expression has been found to be higher in more severe respiratory infections, including COVID-19, indicating AM is associated with COVID-19-induced endothelitis [13]. Clinical trials of AM are thus currently undergoing in patients with COVID-19.
AM in inflammatory disease
The strong anti-inflammatory effects of AM has led researchers to study whether AM is effective in intractable ulcerative colitis or Crohn's disease [14], [15], [16]. AM induces anti-inflammatory effects through stimulation of AM receptors in immune cells, which increase intracellular cAMP concentrations and activate of protein kinase A, suppressing pro-inflammatory, and upregulating anti-inflammatory, cytokine transcription [11].
AM as a biomarker of cerebrovascular diseases
Blood concentration of AM is proportional to the severity of cerebrovascular lesions [17]. Indeed, overexpression of the AM gene has been detected in stroke patients and is associated with stroke severity [18]. Because of the short (22-minute) half-life of AM [19], midregional pro-adrenomedullin (MR-proAM), a non-functional precursor of AM, has also been used as an indicator of AM production [20]. Plasma MR-proAM levels were significantly higher in patients with unfavorable, compared to favorable, outcomes after ischemic stroke [21]. MR-proAM levels were significantly increased in patients with acute penetrating artery territory infarction, where symptoms were worsening [22], suggesting AM serves as an indicator of microcirculatory failure. Accordingly, Kuriyama et al. found MR-proAM levels to be associated with progression of deep white matter lesions [23]. The odds ratio for high MR-proAM levels was 3.08 (95% confidence interval: 1.49–5.17) in groups with Fazekas grade 3 on brain MRI, suggesting high MR-proAM levels are an independent risk factor for deep white matter lesions. Moreover, a significant inverse correlation was observed between MR-proAM levels and cognitive test scores, suggesting MR-proAM could act as a serum biomarker for vascular cognitive impairment. Interestingly, the same group reported five SNPs of AM receptor genes, consisting of 1 SNP of RAMP2 and 4 SNPs of CLR, are associated with stroke [24].
AM for treatment of cerebrovascular diseases
The efficacy of AM administration as a therapeutic agent has been demonstrated in various animal models of cerebrovascular disorders. AM overexpression or administration has been shown to reduce infarct size in a middle cerebral artery (MCA) occlusion model, which recapitulates acute cerebral ischemia [25]. Furthermore, Miyamoto et al. reported a decrease in ischemic tolerance in heterozygous AM knockout (AM +/–) mice [26]. Consistent with these findings, heterozygous RAMP2 knockout mice showed greater reductions in neurons than wild-type mice, together with slower recovery of cerebral blood flow in acute ischemia induced by MCA occlusion [27]. These findings suggest AM is an important endogenous peptide that promotes the biological defense response against cerebral ischemia through the AM-RAMP2 system. In addition, AM suppressed neuronal loss and reactive oxygen species levels in a rat model of transient bilateral common carotid artery occlusion and reperfusion [28]. Brain levels of malondialdehyde, a marker of oxidative stress, were lower, while superoxide dismutase and glutathione peroxidase antioxidant enzyme activity was higher in rats receiving AM, compared to those not. Nevertheless, when AM was conditionally knocked out from endothelial cells, mice exhibited less ischemia-induced brain damage. One plausible mechanism behind this phenomenon is the decreased expression of adhesion molecules, such as Mcam and the proinflammatory mediator Lcn2, in conditional knockout mice [29]. In this context, AM may therefore have a harmful effect in the endothelium but a neuroprotective effect when administered systemically [29]. In addition, intracerebroventricular injection of AM results in an increase in ischemic injury [30]. The effects of AM may thus be context- and/or cell type-dependent.
Importantly, AM has been shown to be effective in chronic cerebral hypoperfusion. AM exerts a tissue-protective effect, through angiogenesis and suppression of microglial activation, in mice with chronic cerebral hypoperfusion induced by bilateral common carotid artery stenosis [31]. In this study, AM was shown to upregulate brain vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) expression, specifically in the hypoperfused, but not normoperfused, brain. Accordingly, increased AM has been shown to trigger a feedback system downregulating RAMP2, in the normoperfused, but not hypoperfused, brain [31,32]. The context-specific properties of AM may thus be useful when applied to cerebrovascular diseases as its signaling operates in hypoperfused tissue only. In addition, heterozygous RAMP2 knockout mice have demonstrated enhanced neuronal loss, along with less compensatory capillary growth, after chronic cerebral hypoperfusion [27]. Taken together, these results suggest AM regulates two key growth factors involved in angiogenesis, VEGF and bFGF, in the AM-RAMP2 signaling pathway, making AM an important peptide in facilitating the biological defense response against cerebral oligemia and ischemia.
Effects of AM in cultured cells
AM promotes capillary tube formation of cultured endothelium in vitro, whereas VEGF neutralization abolishes these effects. The upregulation of VEGF and bFGF by AM is thought to be mediated through the AM receptor and PI3K pathway [32]. PI3K phosphorylates Akt, which then activates endothelial nitric oxide synthase in endothelial cells, subsequently leading to activation of an endothelium-dependent cyclic GMP-dependent kinase pathway in vascular smooth vascular cells (VSMCs) [33]. Alternatively, AM can bind directly to AM receptors on VSMCs and activate the cAMP/protein kinase A or PI3K/Akt pathway, causing vasorelaxation via the endothelium-independent pathway [33]. Activation of such endothelial-dependent and endothelial-independent pathways synergistically improve blood perfusion in ischemic tissue [10].
AM exerts its role in cells other than endothelial and VSMCs, promoting differentiation of oligodendrocyte precursor cells (OPC) into myelin-basic-protein-expressing oligodendrocytes under pathological conditions in vitro. AM treatment increases Akt phosphorylation in OPC cultures and PI3K/Akt inhibitor blocked AM-induced OPC differentiation [34], suggesting that AM preserves white matter integrity through the PI3K/Akt signaling pathway.
Clinical application of AM into cardiovascular diseases
AM safety may become an issue for clinical application in cardiovascular diseases. Blood pressure fluctuations associated with vasodilation in the acute phase of cerebral infarction may lead to worsening of cerebral infarction and complications associated with angiogenesis. However, a Phase 1 clinical trial has demonstrated AM to be safe and clinically-tolerable when administered intravenously [35]. All 24 subjects in the single-dose test with continuous 12-h infusion (placebo, 3 ng/kg/min AM, 9 ng/kg/min AM, or 15 ng/kg/min AM; n = 6 each) completed the study. Of 12 subjects in the 8 h per day for 7 days (placebo or 15 ng/Kg/min) multiple-dosing test, one from the AM group withdrew due to headache. However, no serious AEs were reported. Hemodynamic parameters, such as blood pressure and pulse rate, were well maintained in all subjects.
Since AM is an endogenous substance, its antigenicity is low and its safety guaranteed. In addition, AM has already been administered in clinical trials in inflammatory bowel disease [14], congestive heart failure [9], acute myocardial infarction [36] and old cerebral infarction (personal communications with Prof. Kitamura), with no major adverse events recorded. Based on this evidence, an investigator-initiated, double-blind, placebo-controlled, randomized clinical trial, the AMFIS trial (JRCT ID: jRCT2051190092) for acute cerebral infarction, has commenced at a dose of 9 ng/kg/min AM [37]. AM is expected to be an innovative therapeutic drug in the regeneration blood vessels, while controlling inflammation, in the acute phase of cerebral infarction.
Clinical application of AM into vascular cognitive impairment
The effects of AM may also be felt in an important stroke-related comorbidity, vascular cognitive impairment (VCI). Neuronal atrophy resulting from vascular damage in both the temporal and frontal lobes may underlie VCI [38]; therefore, a strategy restoring cerebral blood flow to such regions may hold promise. AM could prove an ideal drug for VCI as it promotes arteriogenesis through arterial dilatation and angiogenesis through capillary outgrowth [31]. In addition, AM may inhibit microglial activation and inflammation observed in VCI [39], as inflammation underlies blood brain barrier disruption and white matter damage in VCI [40]. Such pleiotropic actions of AM may warrant further examination in clinical trials in VCI.
Another target disease of AM could be a hereditary type of VCI caused by NOTCH3 mutations, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). Sporadic VCI and CADASIL share underlying mechanisms such as blood-brain barrier disruption and white matter changes [41]. In addition, genetic and clinical studies have shown that CADASIL may be underdiagnosed in patients with mild clinical or radiological phenotypes [42,43]. In CADASIL, increased NOTCH3 activity mediates pathological changes in the structure of cerebral arteries, leading to a reduction in maximal dilator capacity of cerebral arteries and cerebral blood flow [44]. Since NOTCH3 is specifically expressed in mural cells among mature somatic cells, impaired cerebral autoregulation in CADASIL is thought to arise from dysfunctional mural cells (e.g. vascular smooth muscle cells and pericytes). However, a recent study has shown pericyte coverage loss and blood brain barrier leakage is not a consistent feature of white matter lesions in CADASIL [45]. Instead, this study suggests reduced oxygen delivery in CADASIL may lead to the death of hypoxia-sensitive oligodendrocytes. Consistent with this notion, NOTCH3 has been shown to be essential for oligodendrocyte development in zebrafish, and NOTCH3 mutants with abnormal myelination show transient reduction in OPCs [46]. In other words, impaired oligodendrogenesis and myelination may be a primary and direct effect of the NOTCH3 mutation in CADASIL. Given AM promotes in vitro differentiation of OPCs, even under hypoxic conditions [34], it may compensate for the death of oligodendrocytes through its direct effect on OPCs and resultant increase in the phosphorylated Akt cell survival signal [34] (Fig. 1). Thus, AM may have at least triple sites of action on components of the neuro-glial-vascular unit consisting of vessels, microglia and oligodendrocytes [47,48] or, more specifically, on the white matter oligovascular unit [49] (Fig. 2).
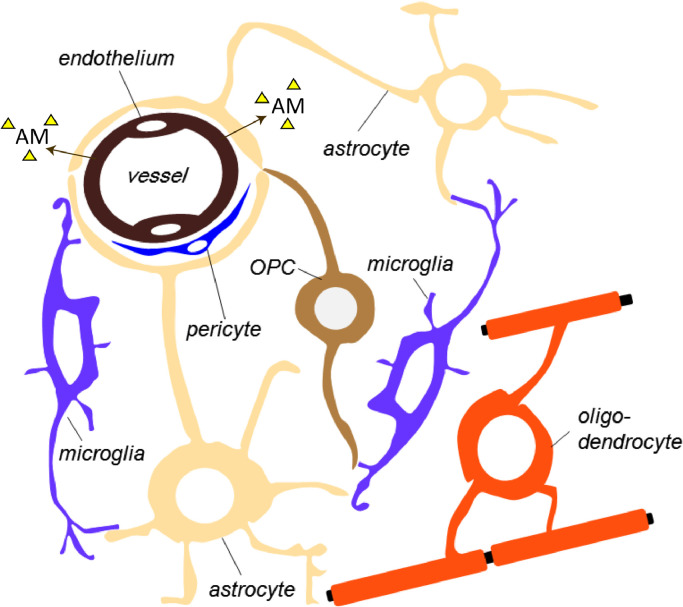
Fig. 2.
Schematic diagram of the interactions between major cellular components within the brain white matter (WM).
WM microvessels are associated with astrocytes, oligodendrocyte precursor cells (OPCs), microglia/macrophages and pericytes. The gliovascular unit of the WM is formed by astrocyte end feet juxtaposed to the WM endothelium. Glial and endothelial cells functionally interact with each other in a paracrine manner. Together with astrocytes, pericytes are important players within the gliovascular unit and modulate vessel diameter. The oligovascular niche is formed by OPCs and the endothelium, where an exchange of soluble signals (e.g. trophic factors such as adrenomedullin (AM) or chemical messengers) plays an important role in sustaining oligodendrocyte homeostasis and WM integrity. Microglial processes are closely associated with the brain endothelium in a fraction of cerebral microvessels and patrol vasculature in the central nervous system.
Disruptions in cell-cell interactions in the oligovascular unit may lead to vascular cognitive impairment [50]. One example is an abnormal interactions between pericytes and endothelial cells in CADASIL [51]. Direct effects of AM on endothelial and mural cells, the two major components of the oligovascular unit, may lead to hemodynamic stability through facilitated angiogenesis and vasodilation (Fig. 1). OPCs and endothelium contribute to the oligovascular unit, where an exchange of soluble signals plays an important role in sustaining oligodendrocyte homeostasis and WM integrity [49]. In addition, OPCs and pericytes directly adhere to, and communicate with each other, via basal lamina in their proliferation and differentiation [52]. Such effects may be facilitated via the AM-induced PI3K-Akt signaling cascade (Fig. 1) [34]. Clinical trials are planned for AM in CADASIL, which currently has no treatment to prevent clinical deterioration. Lomerizine hydrochloride, a calcium channel blocker with smooth muscle relaxing activity, has been suggested to be effective in CADASIL through its vasodilatory effect [53], with similar expectations for AM. However, the above-mentioned direct effects of AM on endothelial cells and OPCs may distinguish AM from vasodilators in the treatment of CADASIL.
Limitations of AM, when applied to chronic diseases, such as VCI, include a short half-life and hypotensive effects. Hypotensive effects are generally mild: even high doses of AM only induce approximately a 10% reduction of systolic blood pressure [31]. As a non-hypotensive dose of AM has been shown to be effective in animal models of acute stroke [25] and chronic cerebral hypoperfusion [31], low doses (9 ng/Kg/min) have been used in clinical trials for acute ischemic stroke [37]. In addition, PEGylated AM has been reported to have a longer half-life and significantly smaller blood pressure-lowering effect in rats, compared with native AM [54]. Though AM should only be administered intravenously, PEGylated AM may be injected subcutaneously [55]. Future research on this promising endogenous peptide is imperative to meet the needs for effective treatment of VCI.
Conclusions
Based on its angiogenic and anti-inflammatory properties, clinical trials of AM are now taking place in patients with inflammatory bowel disease, COVID-19 and acute ischemic stroke, with more in VCI planned. Furthermore, the oligodendrogenetic properties of AM may offer additional benefits in CADASIL, which features inherent impairments in oligo-vascular unit function.
Declaration of Competing Interest
Dr. Ihara reports personal fees from Daiichi Sankyo, personal fees from Eisai and Bayer, grants from Panasonic, Bristol-Myers Squibb and Otsuka Pharmaceutical, outside the submitted work; Dr. Washida has nothing to disclose; Dr. Yoshimoto reports other from Takeda Pharmaceutical Company Limited, outside the submitted work; Dr. Saito has nothing to disclose.
Acknowledgments
Acknowledgment
We would like to thank Dr. Ahmad Khundakar for critical editing of this manuscript.
Source of funding
This study is funded by the Japan Agency for Medical Research and Development (19lk0201096h0001 and 20lk0201096h0002).
References
- 1.Donkor E.S. Stroke in the 21st century: a snapshot of the burden, epidemiology, and quality of life. Stroke Res. Treat. 2018;2018:3238165. doi: 10.1155/2018/3238165. Hindawi Limited. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shuaib A., Lees K.R., Lyden P., Grotta J., Davalos A., Davis S.M., et al. NXY-059 for the treatment of acute ischemic stroke. N. Engl. J. Med. 2007;357:562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- 3.Qvit N., Rubin S.J.S., Urban T.J., Mochly-Rosen D., Gross E.R. Peptidomimetic therapeutics: scientific approaches and opportunities. Drug Discov. Today. 2017;22:454–462. doi: 10.1016/j.drudis.2016.11.003. Elsevier Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malhotra K., Katsanos A.H., Lambadiari V., Goyal N., Palaiodimou L., Kosmidou M., et al. GLP-1 receptor agonists in diabetes for stroke prevention: a systematic review and meta-analysis. J. Neurol. 2020;267:2117–2122. doi: 10.1007/s00415-020-09813-4. [DOI] [PubMed] [Google Scholar]
- 5.Kitamura K., kenji Kangawa, Kawamoto M., Ichiki Y., Nakamura S., Matsuo H., et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem. Biophys. Res. Commun. 1993;192:553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- 6.Ishimitsu T., Kojima M., Kangawa K., Hino J., Matsuoka H., Kitamura K., et al. Genomic structure of human adrenomedullin gene. Biochem. Biophys. Res. Commun. 1994;203:631–639. doi: 10.1006/bbrc.1994.2229. [DOI] [PubMed] [Google Scholar]
- 7.Kis B., Kaiya H., Nishi R., Deli M.A., Ábrahám C.S., Yanagita T., et al. Cerebral endothelial cells are a major source of adrenomedullin. J. Neuroendocrinol. 2002;14:283–293. doi: 10.1046/j.1365-2826.2002.00778.x. [DOI] [PubMed] [Google Scholar]
- 8.Husmann K., Born W., Fischer J.A., Muff R. Three receptor-activity-modifying proteins define calcitonin gene-related peptide or adrenomedullin selectivity of the mouse calcitonin-like receptor in COS-7 cells. Biochem. Pharmacol. 2003;66:2107–2115. doi: 10.1016/j.bcp.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Nagaya N., Satoh T., Nishikimi T., Uematsu M., Furuichi S., Sakamaki F., et al. Hemodynamic, renal, and hormonal effects of adrenomedullin infusion in patients with congestive heart failure. Circulation. 2000;101:498–503. doi: 10.1161/01.CIR.101.5.498. [DOI] [PubMed] [Google Scholar]
- 10.Nagaya N., Mori H., Murakami S., Kangawa K., Adrenomedullin K.S. Angiogenesis and gene therapy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:1432–1437. doi: 10.1152/ajpregu.00662.2004. [DOI] [PubMed] [Google Scholar]
- 11.Geven C., Kox M., Pickkers P. Adrenomedullin and adrenomedullin-targeted therapy as treatment strategies relevant for sepsis. Front. Immunol. 2018;9:292. doi: 10.3389/fimmu.2018.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elke G., Bloos F., Wilson D.C., Brunkhorst F.M., Briegel J., Reinhart K., et al. The use of mid-regional proadrenomedullin to identify disease severity and treatment response to sepsis - a secondary analysis of a large randomised controlled trial. Crit. Care. 2018;22:79. doi: 10.1186/s13054-018-2001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hupf J., Mustroph J., Hanses F., Evert K., Maier L.S., Jungbauer C.G. RNA-expression of adrenomedullin is increased in patients with severe COVID-19. Crit. Care. 2020;24:527. doi: 10.1186/s13054-020-03246-1. BioMed Central Ltd; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashizuka S., Inatsu H., Kita T., Kitamura K. Adrenomedullin therapy in patients with refractory ulcerative colitis: a case series. Dig. Dis. Sci. 2016;61:872–880. doi: 10.1007/s10620-015-3917-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kita T., Ashizuka S., Ohmiya N., Yamamoto T., Kanai T., Motoya S., et al. Adrenomedullin for steroid-resistant ulcerative colitis: a randomized, double-blind, placebo-controlled phase-2a clinical trial. J. Gastroenterol. 2021;56:147–157. doi: 10.1007/s00535-020-01741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashizuka S., Kuroishi N., Nakashima K., Inatsu H., Kita T., Kitamura K. Adrenomedullin: a novel therapy for intractable Crohn's disease with a loss of response to infliximab. Intern. Med. 2019;58:1573–1576. doi: 10.2169/internalmedicine.1791-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serrano-Ponz M., Rodrigo-Gasqué C., Siles E., Martínez-Lara E., Ochoa-Callejero L., Martínez A. Temporal profiles of blood pressure, circulating nitric oxide, and adrenomedullin as predictors of clinical outcome in acute ischemic stroke patients. Mol. Med. Rep. 2016;13:3724–3734. doi: 10.3892/mmr.2016.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J., Yan J., Greer J.M., Read S.J., Henderson R.D., Rose S.E., et al. Correlation of Adrenomedullin gene expression in peripheral blood leukocytes with severity of ischemic stroke. Int. J. Neurosci. 2014;124:271–280. doi: 10.3109/00207454.2013.837462. [DOI] [PubMed] [Google Scholar]
- 19.Meeran K., O’Shea D., Upton P.D., Small C.J., Ghatei M.A., Byfield P.H., et al. Circulating adrenomedullin does not regulate systemic blood pressure but increases plasma prolactin after intravenous infusion in humans: a pharmacokinetic study. J. Clin. Endocrinol. Metab. 1997;82:95–100. doi: 10.1210/jcem.82.1.3656. [DOI] [PubMed] [Google Scholar]
- 20.Gumusel B., Chang J.K., Hyman A., Lippton H. Adrenotensin: an ADM gene product with the opposite effects of ADM. Life Sci. 1995;57:PL87–PL90. doi: 10.1016/0024-3205(95)02012-8. [DOI] [PubMed] [Google Scholar]
- 21.Seifert-Held T., Pekar T., Gattringer T., Simmet N.E., Scharnagl H., Bocksrucker C., et al. Plasma midregional pro-adrenomedullin improves prediction of functional outcome in ischemic stroke. PLoS ONE. 2013;8:e68768. doi: 10.1371/journal.pone.0068768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawano T., Miyashita K., Takeuchi M., Nagakane Y., Yamamoto Y., Kamiyama K., et al. Blood biomarkers associated with neurological deterioration in patients with acute penetrating artery territory infarction: a multicenter prospective observational study. Int. J. Stroke. 2018;13:207–216. doi: 10.1177/1747493016677982. [DOI] [PubMed] [Google Scholar]
- 23.Kuriyama N., Ihara M., Mizuno T., Ozaki E., Matsui D., Watanabe I., et al. Association between mid-regional proadrenomedullin levels and progression of deep white matter lesions in the brain accompanying cognitive decline. J. Alzheimers Dis. 2017;56:1253–1262. doi: 10.3233/JAD-160901. [DOI] [PubMed] [Google Scholar]
- 24.Koyama T., Kuriyama N., Ozaki E., Matsui D., Watanabe I., Takeshita W., et al. Genetic variants of RAMP2 and CLR are associated with stroke. J. Atheroscler. Thromb. 2017;24:1267–1281. doi: 10.5551/jat.41517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyashita K., Itoh H., Arai H., Suganami T., Sawada N., Fukunaga Y., et al. The neuroprotective and vasculo-neuro-regenerative roles of adrenomedullin in ischemic brain and its therapeutic potential. Endocrinology. 2006;147:1642–1653. doi: 10.1210/en.2005-1038. [DOI] [PubMed] [Google Scholar]
- 26.Miyamoto N., Tanaka R., Shimosawa T., Yatomi Y., Fujita T., Hattori N., et al. Protein kinase A-dependent suppression of reactive oxygen species in transient focal ischemia in adrenomedullin-deficient mice. J. Cereb. Blood Flow Metab. 2009;29:1769–1779. doi: 10.1038/jcbfm.2009.92. [DOI] [PubMed] [Google Scholar]
- 27.Igarashi K., Sakurai T., Kamiyoshi A., Ichikawa-Shindo Y., Kawate H., Yamauchi A., et al. Pathophysiological roles of adrenomedullin-RAMP2 system in acute and chronic cerebral ischemia. Peptides. 2014;62:21–31. doi: 10.1016/j.peptides.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Kirisci M., Gunes H., Kocarslan A., Metin T.O., Aykan D.A., Seyithanoglu M., et al. Protective effects of adrenomedullin on rat cerebral tissue after transient bilateral common carotid artery occlusion and reperfusion. Brazil. J. Cardiovasc. Surg. 2020;35:314–322. doi: 10.21470/1678-9741-2019-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ochoa-Callejero L., Pozo-Rodrigálvarez A., Martínez-Murillo R., Martínez A. Lack of adrenomedullin in mouse endothelial cells results in defective angiogenesis, enhanced vascular permeability, less metastasis, and more brain damage. Sci. Rep. 2016;6:33495. doi: 10.1038/srep33495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X., Yue T.L., Barone F.C., White R.F., Clark R.K., Willette R.N., et al. Discovery of adrenomedullin in rat ischemic cortex and evidence for its role in exacerbating focal brain ischemic damage. Proc. Natl. Acad. Sci. U.S.A. 1995;92:11480–11484. doi: 10.1073/pnas.92.25.11480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maki T., Ihara M., Fujita Y., Nambu T., Miyashita K., Yamada M., et al. Angiogenic and vasoprotective effects of adrenomedullin on prevention of cognitive decline after chronic cerebral hypoperfusion in mice. Stroke. 2011;42:1122–1128. doi: 10.1161/STROKEAHA.110.603399. [DOI] [PubMed] [Google Scholar]
- 32.Maki T., Ihara M., Fujita Y., Nambu T., Harada H., Ito H., et al. Angiogenic roles of adrenomedullin through vascular endothelial growth factor induction. Neuroreport. 2011;22:442–447. doi: 10.1097/WNR.0b013e32834757e4. [DOI] [PubMed] [Google Scholar]
- 33.Shimekake Y., Nagata K., Ohta S., Kambayashi Y., Teraoka H., Kitamura K., et al. Adrenomedullin stimulates two signal transduction pathways, cAMP accumulation and Ca2+ mobilization, in bovine aortic endothelial cells. J. Biol. Chem. 1995;270:4412–4417. doi: 10.1074/jbc.270.9.4412. [DOI] [PubMed] [Google Scholar]
- 34.Maki T., Takahashi Y., Miyamoto N., Liang A.C., Ihara M., Lo E.H., et al. Adrenomedullin promotes differentiation of oligodendrocyte precursor cells into myelin-basic-protein expressing oligodendrocytes under pathological conditions in vitro. Stem. Cell Res. 2015;15:68–74. doi: 10.1016/j.scr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kita T., Kaji Y., Kitamura K. Safety, tolerability, and pharmacokinetics of adrenomedullin in healthy males: a randomized, double-blind, phase 1 clinical trial. Drug Des. Devel. Ther. 2020;14:1–11. doi: 10.2147/DDDT.S225220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagaya N., Goto Y., Satoh T., Sumida H., Kojima S., Miyatake K., et al. Intravenous adrenomedullin in myocardial function and energy metabolism in patients after myocardial infarction. J. Cardiovasc. Pharmacol. 2002;39:754–760. doi: 10.1097/00005344-200205000-00017. [DOI] [PubMed] [Google Scholar]
- 37.Japan Registry of Clinical Trials (jRCT) 2020. Adrenomedullin for Ischemic Stroke Study.https://rctportal.niph.go.jp/en/detail?trial_id=jRCT2051190092 [cited 30 Oct 2020]. Available: [Google Scholar]
- 38.Kalaria R.N. Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for Alzheimer’s disease. Acta Neuropathologica. 2016;131:659–685. doi: 10.1007/s00401-016-1571-z. Springer Verlag. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Consonni A., Morara S., Codazzi F., Grohovaz F., Zacchetti D. Inhibition of lipopolysaccharide-induced microglia activation by calcitonin gene related peptide and adrenomedullin. Mol. Cell Neurosci. 2011;48:151–160. doi: 10.1016/j.mcn.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenberg G.A. Inflammation and white matter damage in vascular cognitive impairment. Stroke. 2009;40:S20–S23. doi: 10.1161/STROKEAHA.108.533133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saito S., Yamamoto Y., Ihara M. Development of a multicomponent intervention to prevent Alzheimer’s disease. Front. Neurol. 2019;10:490. doi: 10.3389/fneur.2019.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okada T., Washida K., Irie K., Saito S., Noguchi M., Tomita T., et al. Prevalence and atypical clinical characteristics of NOTCH3 mutations among patients admitted for acute lacunar infarctions. Front. Aging Neurosci. 2020;12:130. doi: 10.3389/fnagi.2020.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rutten J.W., Dauwerse H.G., Gravesteijn G., van Belzen M.J., van der Grond J., Polke J.M., et al. Archetypal NOTCH3 mutations frequent in public exome: implications for CADASIL. Ann. Clin. Transl. Neurol. 2016;3:844–853. doi: 10.1002/acn3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baron-Menguy C., Domenga-Denier V., Ghezali L., Faraci F.M., Joutel A. Increased NOTCH3 activity mediates pathological changes in structure of cerebral arteries. Hypertension. 2017;69:60–70. doi: 10.1161/HYPERTENSIONAHA.116.08015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rajani R.M., Ratelade J., Domenga-Denier V., Hase Y., Kalimo H., Kalaria R.N., et al. Blood brain barrier leakage is not a consistent feature of white matter lesions in CADASIL. Acta Neuropathol. Commun. 2019;7:187. doi: 10.1186/s40478-019-0844-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zaucker A., Mercurio S., Sternheim N., Talbot W.S., Marlow F.L. NOTCH3 is essential for oligodendrocyte development and vascular integrity in zebrafish. Dis. Model Mech. 2013;6:1246–1259. doi: 10.1242/dmm.012005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hase Y., Horsburgh K., Ihara M., Kalaria R.N. White matter degeneration in vascular and other ageing-related dementias. J. Neurochem. 2018;144:617–633. doi: 10.1111/jnc.14271. [DOI] [PubMed] [Google Scholar]
- 48.Duncombe J., Kitamura A., Hase Y., Ihara M., Kalaria R.N., Horsburgh K. Chronic cerebral hypoperfusion: a key mechanism leading to vascular cognitive impairment and dementia. Closing the translational gap between rodent models and human vascular cognitive impairment and dementia. Clin. Sci. 2017;131:2451–2468. doi: 10.1042/CS20160727. [DOI] [PubMed] [Google Scholar]
- 49.Arai K., Lo E.H. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J. Neurosci. 2009;29:4351–4355. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ihara M., Yamamoto Y. Emerging evidence for pathogenesis of sporadic cerebral small vessel disease. Stroke. 2016;47:554–560. doi: 10.1161/STROKEAHA.115.009627. [DOI] [PubMed] [Google Scholar]
- 51.Armulik A., Abramsson A., Betsholtz C. Endothelial/pericyte interactions. Circ. Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 52.Maki T., Maeda M., Uemura M., Lo E.K., Terasaki Y., Liang A.C., et al. Potential interactions between pericytes and oligodendrocyte precursor cells in perivascular regions of cerebral white matter. Neurosci. Lett. 2015;597:164–169. doi: 10.1016/j.neulet.2015.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watanabe-Hosomi A., Mizuta I., Koizumi T., Yokota I., Mukai M., Hamano A., et al. Effect of lomerizine hydrochloride on preventing strokes in patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Clin. Neuropharmacol. 2020;43:146–150. doi: 10.1097/WNF.0000000000000402. [DOI] [PubMed] [Google Scholar]
- 54.Kubo K., Tokashiki M., Kuwasako K., Tamura M., Tsuda S., Kubo S., et al. Biological properties of adrenomedullin conjugated with polyethylene glycol. Peptides. 2014;57:118–121. doi: 10.1016/j.peptides.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 55.Nagata S., Yamasaki M., Kitamura K. Anti-inflammatory effects of PEGylated human adrenomedullin in a mouse DSS-induced colitis model. Drug Dev. Res. 2017;78:129–134. doi: 10.1002/ddr.21383. [DOI] [PubMed] [Google Scholar]