Highlights
-
•
Sporadic and genetic small vessel diseases share clinical and radiological features.
-
•
Cerebrovascular dysfunctions are present in both conditions and may overlap.
-
•
INVESTIGATE-SVDs will use a multi-modal, multi-site advanced MRI protocol.
-
•
We will test associations of cerebrovascular dysfuntions and SVD phenotypes.
-
•
We will test feasibility of multi-site MRI to provide outcome measures in trials.
Keywords: Cerebral small vessel disease, CADASIL, Cerebrovascular reactivity, Blood-brain barrier permeability, MRI
Abbreviations: BBB, blood brain barrier; BOLD, blood oxygen level dependent; BP, blood pressure; BPv, blood pressure variability; CADASIL, cerebral autosomal dominant arteriopathy with leukoencephalopathy and subcortical infarcts; CBF, cerebral blood flow; CERAD+, consortium to establish a disease registry for Alzheimer's disease plus battery; CO2, carbon dioxide; CSF, cerebrospinal fluid; CVR, cerebrovascular reactivity; DCE, dynamic contrast enhanced; EtCO2, end-tidal carbon dioxide; GM, grey matter; MMSE, mini-mental state examination; MoCA, Montreal cognitive exam; NIHSS, national institute for health stroke scale; PI, pulsatility index; PVS, perivascular space; RSSI, recent small subcortical infarct; SVDs, small vessel diseases; WM, white matter; WMH, white matter hyperintensity
Abstract
Background
Sporadic cerebral small vessel disease (SVD) and cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) share clinical and neuroimaging features and possibly vascular dysfunction(s). However few studies have included both conditions, assessed more than one vascular dysfunction simultaneously, or included more than one centre. The INVESTIGATE-SVDs study will assess several cerebrovascular dysfunctions with MRI in participants with sporadic SVD or CADASIL at three European centres.
Methods
We will recruit participants with sporadic SVDs (ischaemic stroke or vascular cognitive impairment) and CADASIL in Edinburgh, Maastricht and Munich. We will perform detailed clinical and neuropsychological phenotyping of the participants, and neuroimaging including structural MRI, cerebrovascular reactivity MRI (CVR: using carbon dioxide challenge), phase contrast MRI (arterial, venous and CSF flow and pulsatility), dynamic contrast-enhanced MRI (blood brain barrier (BBB) leakage) and multishell diffusion imaging. Participants will measure their blood pressure (BP) and its variability over seven days using a telemetric device.
Discussion
INVESTIGATE-SVDs will assess the relationships of BBB integrity, CVR, pulsatility and CSF flow in sporadic SVD and CADASIL using a multisite, multimodal MRI protocol. We aim to establish associations between these measures of vascular function, risk factors particularly BP and its variability, and brain parenchymal lesions in these two SVD phenotypes. Additionally we will test feasibility of complex multisite MRI, provide reliable intermediary outcome measures and sample size estimates for future trials.
1. Introduction
Cerebral small vessel diseases (SVDs) cause a quarter of ischaemic strokes, and at least 50% of dementia cases alone or in mixed dementias [1]. Roughly 10% of 65-year-old individuals and many 90 year olds exhibit manifestations of sporadic SVD [2], whilst genetic variants, such as cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), can affect younger individuals. The absence of effective therapies reflects a limited understanding of the disease processes [1].
Our overarching concept is that sporadic and genetic SVDs result from shared molecular defects in vessel wall-glia constituents, including endothelium, pericytes, smooth muscle, basement membrane, extra-cellular matrix, microglia, oligodendrocytes and astrocytes [1]. These defects are thought to lead to compromised blood-brain barrier (BBB) function [3], impaired vasoreactivity [4], altered pulsatility [5] and perivascular interstitial fluid clearance [6]. However, the relation of these dysfunctions to each other, and whether they occur at different stages in the pathogenesis of SVD, is unknown.
Hypertension is the major modifiable risk factor for SVD [1], but only explains a small proportion of the variance in SVD burden [7]. Therefore while there is recent evidence that intensive control of BP may reduce SVD progression [8] the specific relationships of BP to SVD and vascular dysfunctions remain unclear.
Imaging NeuroVascular, Endothelial and STructural InteGrity in prepAration to TrEat Small Vessel Diseases (INVESTIGATE-SVDs) aims to advance knowledge of SVD pathophysiology by assessing several SVD-related cerebrovascular dysfunctions concurrently, in relation to SVD lesions, clinical, neuropsychiatric, and detailed BP measures around the time of MRI. Multicentre trials are usually needed to achieve large-enough sample sizes, therefore INVESTIGATE-SVDs employs a comprehensive, multi-site, quantitative MRI protocol to assess the relationship between BBB integrity, cerebrovascular reactivity (CVR) to CO2, intracranial blood and CSF flow, BP and its variability (BPv) and clinical and structural features of SVD, while also testing the feasibility of using these techniques at multiple centres.
We hypothesise that cerebrovascular dysfunction will be most severe in patients with the worst SVD and that more leakage of gadolinium-based contrast agent across the BBB will be associated with lower CVR and higher pulsatility. Higher BBB permeability may occur without lower CVR if the former occurs at an earlier point in the pathogenesis of SVD than the latter. More peri-vascular space (PVS) visibility on structural imaging will correlate with higher BBB leakage, lower CVR and higher vascular pulsatility. More variable BP will be associated with higher BBB permeability and lower CVR, and this effect will be greater than the effect of elevated BP levels alone.
INVESTIGATE-SVDs is part of the SVDs@Target EU Horizon 2020 project and is designed in collaboration with the TREAT-SVDs study assessing the effects of three antihypertensive medications on CVR and BPv in SVD and the ZOOM-SVDs study assessing SVDs and vascular dysfunction using 7 Tesla MRI.
Greater understanding of cerebrovascular dysfunctions will help identify new treatments for SVD-associated strokes and dementias and intermediary outcomes for use in trials to test their efficacy.
2. Materials and methods
2.1. Design
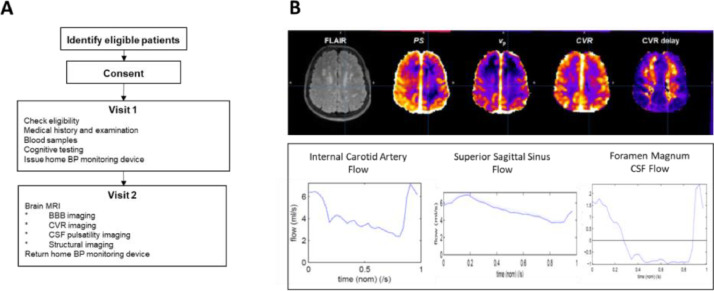
INVESTIGATE-SVDs is a multi-centre, cross-sectional, observational study. Participants with SVDs are recruited from stroke, memory or specialist genetic SVDs clinics in three centres (Edinburgh, Munich, Maastricht). Participants attend for two visits over an eight-day period for clinical, cognitive, physiological and brain imaging assessments (Fig. 1). Telemetric BP is performed between the two visits. Patients who travel for a longer distance to the specialist genetic SVDs clinic in Munich attend their visits on two consecutive days followed by a period of BP monitoring.
Fig. 1.
A – Study flowchart. B – Example output of quantitative brain imaging parameters. Top row shows FLAIR image with corresponding voxelwise maps of BBB PS, vp and CVR parameters. Brighter colours indicate higher parameter values. Bottom row shows flow curves across the cardiac cycle of blood in the internal carotid artery, superior sagittal sinus and CSF motion at the foramen magnum. FLAIR = Fluid Attenuated Inversion Recovery; PS = permeability-surface area product; vp = plasma volume; CVR = cerebrovascular reactivity magnitude (%/mmHg change in end-tidal CO2); CVR delay (seconds).
2.2. Ethical approvals
Each centre obtained research ethics approvals locally (Edinburgh – South East Scotland Research Ethics Committee, Reference 16/SS/0123; Maastricht- Medical Ethical Committee of Maastricht University Medical Centre, Reference 16-2044; Munich – Ethics Committee of the LMU Munich, Reference 658-16). All participants provide written informed consent. INVESTIGATE-SVDs is registered (ISRCTN 10514229).
2.3. Objectives
-
(i)
To assess BBB integrity and the relationship to CVR, pulsatility and CSF flow in a range of severities of sporadic and monogenic SVDs.
-
(ii)
To identify factors that contribute to the malfunction of BBB, CVR, pulsatility and CSF flow such as BP and its variability, as potential therapeutic targets.
-
(iii)
To establish associations between BBB malfunction, CVR, pulsatility and CSF flow and the profile of brain parenchymal lesions in different SVDs.
-
(iv)
To test feasibility of a multisite protocol to measure the different cerebrovascular dysfunctions contemporaneously on MRI.
-
(v)
To derive data to provide reliable intermediary outcome measures to estimate sample sizes for future randomised clinical trials.
2.4. Patient population
We recruit patients with ‘symptomatic SVD’ defined as one of:
-
a
a clinical lacunar stroke in the last 5 years with a recent small subcortical infarct (RSSI) visible on MRI or CT scan compatible with the clinical syndrome at the time of stroke presentation.
-
b
vascular cognitive impairment with SVD.
-
c
a diagnosis of CADASIL.
Table 1 lists the full inclusion and exclusion criteria.
Table 1.
Inclusion and exclusion criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Symptomatic SVD defined as a history compatible with clinical lacunar stroke in the last 5 years with a recent small subcortical infarct visible on contemporaneous MRI scan or CT scan* compatible with the clinical syndrome. *On MRI, a recent infarct is defined as a DWI lesion on the acute MRI scan. On CT, recent infarct is defined as a novel small low attenuation <2 cm diam +/-swelling on CT within 3 weeks after the event that was not visible on the admission CT and is consistent with the symptoms. or SVD associated vascular cognitive impairment defined as visiting a memory clinic and a clinical dementia rating score of ≥ 0.5, and capacity to consent, with confluent deep WMH on MRI (defined on the Fazekas scale as deep WMH score ≥ 2). or a diagnosis of CADASIL established by molecular genetic testing of the NOTCH3 gene (presence of an archetypical, cysteine-affecting mutation) or the presence of granular osmiophilic material in ultrastructural, electron microscopy analysis of skin biopsy. Age 18 years or older Ability to undergo MRI Capacity to give written informed consent Independent in activities of daily living (Modified Rankin score <3) |
Inclusion criteria are not met Unwillingness or inability to give written consent Pregnant or breastfeeding women, women of childbearing age not taking contraception. As defined by the Heads of Medicines Agencies’ Clinical Trials Facilitation Groups Contraindications to MRI (pacemaker, aneurysm clip, cochlear implant etc.) Contraindications to gadolinium contrast agent used for MRI Contraindication to CO2 challenge eg severe respiratory disease Other major neurological or psychiatric conditions affecting the brain and interfering with the study design (e.g. multiple sclerosis) In case of clinical lacunar stroke other causes of stroke identified by local site's standard post-stroke investigations such as ≥50% luminal stenosis (NASCET) in large arteries supplying the area of ischaemia major-risk cardioembolic source of embolism (permanent or paroxysmal atrial fibrillation, sustained atrial flutter, intracardiac thrombus, prosthetic cardiac valve, atrial myxoma or other cardiac tumours, mitral stenosis, recent (<4 weeks) myocardial infarction, left ventricular ejection fraction less than 30%, valvular vegetations, or infective endocarditis) other specific causes of stroke identified (e.g. haemorrhage, arteritis, dissection, migraine/vasospasm, drug misuse) Other stroke risk factor requiring immediate intervention that would preclude involvement in the study Renal impairment (eGFR <30 ml/min) |
We will include 45 participants with sporadic SVD and 30 participants with CADASIL (all CADASIL participants will be recruited in Munich). We will not include healthy controls, primarily because it would be difficult to justify ethically the intravenous injection of gadolinium. Additionally, there is already a body of knowledge from prior use of each MRI method individually in quite varied populations and the differing risk factors, SVD burdens and medication use between controls and SVD participants would confound comparisons. Furthermore, scientific and ethics discussion leading up to the study did not consider that, in this situation, there would be sufficient added scientific value from healthy controls in this situation to justify the high burden to the volunteers of participating in the research. However, volunteers and specific test phantoms are scanned with the study sequences without gadolinium or CO2 at regular intervals throughout the project to monitor MRI stability and minimise between-scanner differences.
2.5. Study assessments
2.5.1. Clinical
Participants are assessed for details of their SVD subtype, including date of diagnosis, presenting symptoms and relevant investigations. Details of vascular risk factors, other medical history and medication history are also recorded. Participant's BP, heart rate, respiratory rate, oxygen saturations, height and weight are measured. Additionally, participants with an inclusion illness of stroke have a National Institute of Health Stroke Scale (NIHSS) examination.
2.5.2. Cognitive
All participants undergo cognitive testing, in their first language, with the Consortium to Establish a Disease Registry for Alzheimer's Disease Plus battery (CERAD+). This comprises the following tests: verbal fluency (number of animal names generated within 60 s), Boston Naming Test (naming of 15 items presented as pictures), Mini-Mental State Exam (MMSE), word list learning (memorising a list of 10 words presented in three consecutive trials), constructional praxis (copying of four geometric figures), word list delayed recall (recalling the 10 words of the word list after 30 min), word list recognition (recognition of the 10 words of the word list amongst distracters), and constructional praxis delayed recall (redrawing the four geometric figures after 30 min). Additionally the Trail Making Test A and B timed (connecting 25 targets in a sequential order as numbers (TMT-A) and alternating between numbers and letters (TMT-B) with recording of time taken to complete each test), phonemic fluency (number of words beginning with the letter ‘s’ generated within 60 s), digit span, forward and back, National Adult Reading Test (estimates peak adult cognitive ability), Montreal Cognitive Assessment (MoCA) and Centre for Epidemiologic Studies Depression Scale (CES-D) are obtained. Educational attainment (highest level, years of education) is also recorded.
The tests are performed in a fixed order as described above to ensure standardisation across sites. The individual test results will be standardised into z-scores based on the average score and standard deviation. The individual tests are then grouped into cognitive domains (executive function, language, memory, visuo-spatial, attention and processing speed) with the z-scores of the individual tests averaged to give a domain z-score.
2.5.3. Electronic data recording
All clinical and cognitive data and study procedures are recorded in a secure, password-protected anonymised proprietary electronic case record form (Castor EDC, Amsterdam, the Netherlands).
2.5.4. Brain imaging
All sites use a Siemens Prisma 3T MRI scanner, with the exception of the first 3 participants in Munich who were scanned on a Siemens Skyra 3T MRI scanner. At Edinburgh and Maastricht, participants are scanned with a 32-channel head coil except for the phase contrast scans of the carotids and subarachnoid CSF which use a 20-channel head/neck coil. At Munich, all scans are acquired using a 64-channel head/neck coil except for the CVR scan where a 20-channel head/neck and 32-channel head coil is used for scans on the Skyra and Prisma respectively. Total imaging time is around 90 min, with variation due to some sequences being pulse gated.
All sites are experienced in neuroimaging of patients with SVD. Nonetheless, all sites receive in person training in the cerebrovascular function MRI procedures, to ensure consistent performance of MRI and ancillary procedures such as CO2 administration. All imaging sequences below had been used in previous studies in one of the three centres prior to INVESTIGATE-SVDs, and consequently had already undergone refinement and streamlining in previous studies. The final imaging protocol was agreed after piloting and minor adaptations to further facilitate implementation on the participating scanners, to reduce scan duration and increase participant comfort. The sequences are tested on phantoms and volunteers (excluding iv injections) during set up and regularly thereafter to ensure scanner stability throughout the study. Image data transfer methods were tested, and image quality assessed, prior to scanning the first participants.
Structural Imaging: 3D structural imaging sequences include essential structural sequences for assessment of SVD including T1-weighted (T1w), T2-weighted (T2w), fluid-attenuated inversion recovery (FLAIR) (0.9 – 1.0-mm isotropic resolution), and susceptibility-weighted imaging (SWI). These assess structural features of SVD and generate tissue masks for quantitative MRI analysis [9]. Full parameters are given in Table 2.
Table 2.
Acquisition parameters for the imaging protocol. Voxel size in mm. TR/TE/TI all in milliseconds. Acquisition time in minutes and seconds. Flip angle in degrees. CVR =Cerebrovascular reactivity; MPRAGE =Magnetisation-prepared rapid acquisition with gradient echo; FLAIR =Fluid-attenuated inversion recovery; PD =Proton density; SPACE =Sampling perfection with application-optimised contrast using different flip-angle evolution; SWI =Susceptibility weighted imaging; dMRI =Diffusion imaging; pcASL =Pseudo-continuous arterial spin labelling; TOF =Time-of-flight; PC =Phase-contrast; SACSF =Subarachnoid cerebrospinal fluid; IR =Inversion recovery; sGRE =Spoiled gradient recalled echo; TI =Inversion time; FA =Flip angle; DCE =Dynamic contrast-enhanced; TR =Repetition time; TE =Echo time; R=parallel imaging acceleration factor; MB=multiband acceleration factor; NA = number of averages.
| CVR | T1w | FLAIR | PD | T2w | SWI | dMRI | MRA | Flow | Quantitative T1 | DCE-MRI | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sequence | 2D GE-EPI | MPRAGE (3D IR-sGRE) | SPACE (3D RARE) | 3D sGRE | SPACE(3D RARE) | 3D sGRE | 2D GE-EPI | TOF | 2D PC (carotids) | 2D PC (SACSF) | 2D PC(veins) | 3D IR-sGRE | 3D IR-sGRE | 3D sGRE | T1w 3D sGRE |
| Voxel Size | 2.5 × 2.5 × 2.5 | 1 × 1 × 1 | 1 × 1 × 1 | 1 × 1 × 1 | 0.9 × 0.9 × 0.9 | 0.6 × 0.6 × 3 | 2.0 × 2.0 × 2 | 0.5 × 0.7 × 1.6 | 1.0 × 1.0 × 5 | 0.8 × 0.8 × 5 | 0.7 × 0.7 × 5 | 1.2 × 1.2 × 1.2 | 1.2 × 1.2 × 1.2 | 1.2 × 1.2 × 1.2 | 2 × 2 × 2 |
| TR | 3000 | 2500 | 5000 | 6.04 | 3200 | 28 | 4300 | 20.0 | 19.60 | 25.18 | 21.70 | 1040 | 1940 | 5.4 | 3.44 |
| TE | 30.0 | 4.37 | 388 | 2.44 | 408 | 20 | 74.0 | 3.51 | 5.82 | 8.45 | 6.59 | 1.82 | 1.82 | 1.82 | 1.68 |
| TI | – | 1100 | 1800 | – | – | – | – | – | – | – | – | 600 | 1500 | – | – |
| Flip Angle | 90 | 7 | – | 2.0 | – | 9 | – | 20 | 12 | 12 | 12 | 5 | 5 | 2, 5, 12 | 15 |
| Acquisition Time | 12:30 | 3:45 | 5:57 | 1:57 | 3:42 | 4:02 | 11:16 | 2:45 | 1.39 approx. | 1:55 approx. | 2:11 approx. | 1:55 | 3:35 | 1:36 × 3 | 21:08 |
| Other | R = 2 | R = 3 | R = 3 | R = 3 | R = 2 × 2 | R = 2 | R = 2, MB=2, 15 x b = 0 s/mm2, 3 x b = 200 s/mm2, 6 xb=500 s/mm2, 64 x b = 1000s/mm2, 64 x b = 2000s/mm2 (3 x b0 acquired with reversed phase encoding | R = 2 venc=70 cm/s NA=2 | R = 2 venc=6 cm/s | R = 2 venc=50 cm/s | R = 2 | R = 2 | R = 2 | vol | |
Diffusion imaging: Multi-shell diffusion imaging with optimal angular coverage is obtained using a simultaneous multi-slice single-shot spin-echo echo planar-imaging sequence. An additional 3 b0 volumes were obtained with reversed phase encoding direction to facilitate correction of geometric distortions.
CVR imaging: We assess CVR using BOLD MRI with CO2 challenge [4,10]. A multi-slice single-shot gradient echo echo planar-imaging sequence (2.5 mm isotropic resolution) covering the entire brain acquires images every 3 s during a 12 min breathing paradigm (alternating 2 min of medical air and 3 min of breathing 6% CO2) [10]. Gases are administered via a unidirectional anaesthetic breathing circuit (Intersurgical, Wokingham, UK). Participants’ end tidal CO2 (ETCO2), pulse rate and peripheral oxygen saturation during scanning are recorded using MRI-conditional monitors. A physician is present during CVR scans to monitor the procedure. In total, participants are inside the scanner wearing the facemask for 15–20 min.
Phase-contrast MRI: To assess blood and CSF flow and pulsatility, we acquire velocity- sensitive phase (and magnitude) images using a 2-dimensional cine phase-contrast pulse sequence with retrospective peripheral pulse gating [5]. In four separate acquisitions, each approximately 2,3 min duration, we measure (a) CSF flow through the cerebral aqueduct, (b) venous blood flow through the straight, transverse and superior sagittal venous sinuses, (c) blood flow through the internal carotid arteries, vertebral arteries and internal jugular veins and (d) CSF flow through the cervical subarachnoid space at the foramen magnum. Velocity is measured at 32 phases of the cardiac cycle.
Quantitative T1 measurement: As a quantitative marker of tissue integrity and for quantitative analysis of DCE-MRI, T1 and flip angle are measured using the DESPOT1-HIFI method (1.2-mm isotropic resolution) [11,12].
Dynamic contrast-enhanced MRI (DCE-MRI): BBB leakage is assessed using DCE-MRI [13,14]. Participants are cannulated and given an intravenous bolus injection of Gadobuterol (Gadovist, Bayer, Germany) at 0.1 mmol/kg body weight (usual clinical dose). Injection time and delivery rate are determined based on participant weight to begin contrast delivery 130 s after starting the scan following acquisition of 3 pre-contrast volumes. Contrast delivery is followed by a saline injection at the same rate to deliver the remaining contrast volume. Participants are scanned using a T1-weighted spoiled gradient echo sequence (2-mm isotropic resolution, 40 s temporal resolution) for 21 min. Fig. 1 gives example quantitative data.
2.5.5. Telemetric BP
Participants undergo BP monitoring for up to seven days using a telemetric device, starting at the first study visit. BP monitoring is performed at home. The device (Tel-O-Graph® GSM Plus) has been validated according to the European standard ISO 81060–2:2009 and is graded A/A by the British Hypertension Society. Data are transferred via mobile phone networks anonymously to a central database for analysis. Participants are asked to measure their BP at the same three time points each day (after waking, middle of the day, before bed), and to take at least two consecutive readings at each timepoint.
2.5.6. Blood sampling
A fresh non-fasting venous blood sample will be drawn to measure basic haematology and biochemistry laboratory parameters.
In addition, blood is obtained in two 8 ml cell preparation tubes with sodium citrate (BD, 362,782). Directly after collection, peripheral blood mononuclear cells will be isolated and stored at −80° Celsius [15], with the aim of exploring the contribution of immune cells to SVDs.
2.6. Image analyses
MRI data are anonymised locally and transferred to the Brain Research Imaging Centre, University of Edinburgh, in DICOM or NIFTI formats for quality checking and analysis. A scan housekeeping system (SMARTIS, https://www.ed.ac.uk/clinical-sciences/edinburgh-imaging/research/services-and-collaboration/smartis) facilitates efficient electronic scan transfer while accurately tracking scan completeness and distributing different sequences to different platforms for image analysis.
All image analysis will be performed by an analyst not involved in the clinical assessments, and blind to all other data, using established protocols. Structural images will be analysed to measure intracranial volume, whole brain volume, grey matter, white matter and white matter hyperintensity (WMH) volumes using established validated methods. Visual scores for WMH, index and old infarcts, PVS, microbleeds and brain atrophy are performed using validated scores [9,16,17] on radiological quality image viewing platform (Carestream Health Inc, Rochester, United States).
T1 maps are generated from DESPOT1-HIFI images as previously described [12]. BBB leakage and CVR will be assessed in brain tissue regions (subcortical grey, normal appearing white matter and WMH). Parallel analyses will be conducted using manually defined regions sampling representative areas of subcortical grey and white matter, building on previous work [10], and computational subcortical grey and white matter segmentation before excluding WMH, eroding to limit partial volume effect and manual correction. BBB leakage is estimated as the permeability-surface area product PS by fitting concentration-time curves to the Patlak pharmacokinetic model in line with recent recommendations [14] and as described in [13]. CVR is calculated by regression of the percentage signal change (ΔS/S0) to the ETCO2 response, which will be time-shifted to maximise the cross-correlation in each voxel or region as previously described [10].
Blood and CSF flow and pulsatility variables will be determined from phase-contrast velocity images as described previously [5].
2.7. Primary outcomes
-
1
BBB leakage rate (PS) in normal-appearing white matter, cortical and deep grey matter and WMH.
-
2
CVR in white matter, deep grey matter and WMH.
-
3
Arterial and venous pulsatility and CSF flow.
2.8. Secondary outcomes
-
1
Structural markers of SVD – WMH, enlarged PVS, lacunes, microbleeds, individually and by SVD score.
-
2
PVS score, count, and volume assessed using validated methods.
-
3
Diffusion tensor imaging metrics of microstructural integrity.
-
4
BP parameters (peak systolic, mean, diastolic arterial pressures, pulse pressures, and variability).
-
5
Systemic measures of vascular stiffness derived from the telemetric BP readings.
-
6
Immune cell activity and cerebral endothelial function.
-
7
Feasibility of performing complex MRI to assess cerebrovascular function at several sites in one study.
2.9. Blinding
All clinical including cognitive and neuropsychological assessments are performed blind to neuroimaging and BP findings and vice versa.
2.10. Sample size and statistical analysis
Sample size estimate is based on BBB PS of 3.8 ± 1.7 × 10–4 min-1 in WMH and 2.9 ± 1.65 × 10–4 min-1 in white matter as observed recently [13]: a sample of 65 patients would have 80% power (α=0.05) to detect a difference in PS of 1 ± 1.6 × 10–4 min-1 in subcortical tissues between patients with low and high WMH scores. BBB PS signal is of lower magnitude than the range of signals derived from CVR, hence a power calculation based on CVR would underestimate the sample required for the BBB PS analyses. Our primary analysis will be of all participants, with pre-specified sensitivity analyses on the following variables: sporadic vs monogenic SVDs, by low vs. high SVD burden score, between hypertensive and normotensive patients, and by mode of presentation (cognitive vs. stroke). We will include 75 patients in order to allow for drop-outs and anticipating 10% image data failures.
The primary analysis will use linear regression modelling, testing whether BBB leakage varies with vasoreactivity to CO2 in this exploratory analysis. We will also assess whether BBB leakage varies with measures of cerebral and systemic vascular compliance, severity of SVD features (WMH, PVS, total burden score) and averaged BP parameters recorded over the week prior to MRI. BP analysis will use the second reading at each time point and BP variability will be defined as the co-efficient of variation.
We will also test whether CVR to CO2 varies with measures of cerebral and systemic vascular compliance and averaged BP parameters recorded over one week, and with SVD features as above.
All analyses will be adjusted for key covariates including age, hypertension diagnosis, smoker status, WMH volume and SVD type. We will also analyse the association of cognitive test scores with vascular dysfunctions, SVD lesions and SVD type.
We considered including site as a covariate, however as all CADASIL participants are at one site this would cause problems of collinearity.
3. Discussion
INVESTIGATE-SVDs features unique approaches to advance the understanding of key pathogenic roles of dynamic cerebral vascular dysfunctions in both sporadic and monogenic SVDs.
3.1. Different SVD presentations
Most studies of SVD focus on a single clinical sporadic or monogenic phenotype, with different protocols making direct comparisons very difficult. INVESTIGATE-SVDs will study participants who present with stroke or cognitive impairment presentations of sporadic SVD or the CADASIL monogenetic variant of SVD.
The NOTCH3 mutation in CADASIL results in impaired cerebral autoregulation and increased vascular smooth muscle cell myogenic tone in animal models [18]. Impaired cerebrovascular reactivity is also seen in patients with sporadic SVDs. INVESTIGATE-SVDs will assess these changes in humans, relating several key measures of cerebrovascular dysfunction to clinical and radiological disease stage and comparing CADASIL with sporadic SVD patients. The results will enhance translational work between pre-clinical and clinical studies of monogenic and sporadic SVD models.
We anticipate the differing age, risk factor profiles and disease burden between sporadic and CADASIL participants may make interpretation challenging, however investigating cerebrovascular malfunctions that are common to different SVDs in the same protocol will potentially identify new targets for intervention.
We discussed including a healthy control group at some length, but felt on balance that such a group would not aid the interpretation of results beyond data already available given the expected differences in risk factor profiles and SVD burden, and ethical difficulty of using intravenous gadolinium in healthy subjects. Instead, we focus on variation in cerebrovascular function with SVD burden, type and BP, and associations between different cerebrovascular dysfunctions, and feasibility of multicentre studies in SVD using complex MRI, while monitoring MRI stability with volunteer and phantom data.
We also considered including patients with intracerebral haemorrhage presentations of SVD however we felt this would make the patient population too heterogenous and would present image analysis difficulties due to large residual haemorrhagic lesions.
3.2. Multisite approach
Most advanced neuroimaging studies, particularly those testing dynamic cerebrovascular functions, occur in a single specialist centre using a single MRI scanner. INVESTIGATE-SVDs will attempt to perform a complex neuroimaging study across three specialist centres.
To enable this, we established face-to-face training for all study sites, based on several years of experience in one site, to ensure all sites perform the imaging procedures accurately and consistently. We established a programme of test scans prior to study commencement, coupled with an ongoing programme of regular quality assurance MR phantom and volunteer scanning. The lead image processing site (Edinburgh) reviews all imaging data after upload, enabling errors to be identified early and rectified. Additionally, all sites have access to expert help when acquiring scans and regular teleconferences and in person meetings are used to reiterate key points of the techniques.
INVESTIGATE-SVDs will establish the feasibility of multisite scanning, including imaging success rates, both in terms of patients completing a long scanning paradigm involving breathing apparatus, and obtaining technically complete and analysable data across different scanners with different staff. The multisite approach allows a larger sample than in any individual centre, using complex MRI techniques which have typically been applied in relatively small populations to date.
3.3. Multimodal imaging in single session
The combination of CVR, BBB permeability, phase contrast flow and pulsatility, diffusion imaging and high-resolution structural imaging will provide an extensive dataset of neurovascular integrity and function, perhaps the most comprehensive obtained in a single imaging session to date. Only a few studies have utilised multimodal cerebrovascular function imaging [4,5,19] and none have used the combination we propose.
Aside from the technical challenges, the time to obtain the images presents a challenge to participants. Prioritising participant comfort in the scanner is key to success. This includes allowing participants to trial breathing equipment prior to the scan and keeping them well informed of what to expect during each scan segment. Prioritising the order of scanning is also important – more uncomfortable and movement sensitive scans such as the CVR will have higher success if performed early, before the patient fatigues. Incorporating breaks into the scanning paradigm, e.g. comfort breaks and to allow participants to move between sequences helps prevent fatigue and ensures participants know they need to lie still only for short segments. Whilst this adds additional scan time for re-localisation, we find this a worthy investment in terms of scan completion and image quality.
In terms of prior knowledge, CVR is reduced in patients with sporadic SVD [4,20] and CADASIL [21]. One study suggested CVR impairment precedes SVD lesion development [22]. In CADASIL, higher disease burden and peripheral and carotid vascular dysfunction are associated with impaired CVR [21]. In sporadic SVD, CVR impairment is associated with more pulsatile venous sinus blood flow and abnormal CSF flow [4,5]. However there was heterogeneity in the methods and results [23]. Studies of CVR and SVD have typically been performed in a single centre and reproducibility between sites and different scanners remains unknown [23,24].
Increased BBB permeability occurs in patients with lacunar stroke [25], WMH [[26], [27], [28]] and vascular cognitive impairment [29]. High field MRI has suggested that BBB permeability increases with age, declining cognitive function [30], pericyte dysfunction [31], and interstitial fluid accumulation in older subjects with cognitive decline [32].However BBB permeability measurement and analysis measures vary, with some being potentially unreliable [14]. Fortunately, there are now recommendations to standardise BBB imaging (used in INVESTIGATE-SVDs) [14] and test-retest reproducibility has been assessed [33].
3.4. BP and BP variability
INVESTIGATE-SVDs will use telemetric BP devices to monitor participants’ BP over one week of normal activities around the MRI. As well as BP, the devices assess arterial stiffness by measuring the pulse waveform and pulse wave velocity. Data will therefore be available for standard BP metrics (systolic, diastolic, mean arterial pressure and pulse pressure) with additional data on BPv, augmentation index, central BP and pulse wave velocity. The longer-term monitoring of participants’ BP and arterial stiffness characteristics will provide a profile of participants BP during their normal activities and allow us to test previous findings based on one-off clinic BP readings and examine whether BP on its own, BPv, or arterial stiffness have the strongest associations with SVD features.
3.5. Study progress
The study is ongoing, recruitment has now completed (n = 77), but data analyses are ongoing. The study expects to report its final results in 2022. The study protocol as described here has not changed since regulatory approvals were obtained and the study registered (ISRCTN: 10514229).
4. Conclusion
Advanced multimodal MRI will improve our knowledge of the mechanisms driving sporadic and genetic SVDs. Our multi-site, multi-modal imaging approach will provide proof of concept for clinical trials using advanced MRI measures as outcomes.
CRediT authorship contribution statement
Gordon W. Blair: Data curation, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. Michael S. Stringer: Data curation, Investigation, Methodology, Project administration, Software, Visualization, Writing – review & editing. Michael J. Thrippleton: Data curation, Investigation, Methodology, Project administration, Software, Visualization, Writing – review & editing. Francesca M. Chappell: Data curation, Methodology, Project administration, Software, Writing – review & editing. Kirsten Shuler: Data curation, Investigation, Project administration, Writing – review & editing. Iona Hamilton: Data curation, Investigation, Project administration, Writing – review & editing. Daniela Jaime Garcia: Data curation, Investigation, Project administration, Writing – review & editing. Fergus N. Doubal: Data curation, Investigation, Methodology, Supervision, Writing – review & editing. Anna Kopczak: Data curation, Investigation, Methodology, Project administration, Writing – review & editing. Marco Duering: Data curation, Investigation, Methodology, Project administration, Writing – review & editing. Michael Ingrisch: Data curation, Investigation, Methodology, Project administration, Writing – review & editing. Danielle Kerkhofs: Data curation, Investigation, Methodology, Project administration, Writing – review & editing. Julie Staals: Data curation, Investigation, Methodology, Supervision, Writing – review & editing. Hilde van den Brink: Data curation, Investigation, Methodology, Project administration, Writing – review & editing. Tine Arts: Data curation, Investigation, Methodology, Project administration, Writing – review & editing. Walter H Backes: Data curation, Investigation, Methodology, Software, Writing – review & editing. Robert van Oostenbrugge: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. Geert Jan Biessels: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. Martin Dichgans: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. Joanna M. Wardlaw: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
None declared.
Acknowledgments
Funding
European Union Horizon 2020 (project No 666881, ‘SVDs@Target’). Additional support is provided by The Stroke Association Princess Margaret Research Development Fellowship scheme (GWB), Alzheimer's Society (Ref: 252(AS-PG-14-033), GWB), the Stroke Association Garfield Weston Foundation Senior Clinical Lectureship (FND), NHS Research Scotland (FND), NHS Lothian Research and Development Office (MJT), the Scottish Funding Council through the Scottish Imaging Network, A Platform for Scientific Excellence (SINAPSE) Collaboration. Funding is gratefully acknowledged from the Fondation Leducq (ref no. 16 CVD 05), Edinburgh and Lothians Health Foundation and the MRC UK Dementia Research Institute (funded by the MRC, Alzheimer's Society and Alzheimer's Research UK). Further support is provided by the Vascular Dementia Research Foundation and the German Research Foundation (DFG) as part of the Munich Cluster for Systems Neurology (EXC 2145 SyNergy).
Informed consent
All participants give written informed consent prior to inclusion in the study.
Ethical approval
Each site obtained ethical approvals. Edinburgh: South East Scotland Research Ethics Committee, Reference 16/SS/0123; Maastricht: Medical Ethical Committee of Maastricht University Medical Centre, Reference 16-2044; Munich: Ethics Committee of the LMU Munich, Reference 658-16.
Acknowledgments
In Munich we thank Sandra Hein for patient care and data entry, Mathias Hübner and Susan Habash for technical assistance. Finally, we thank all patients for study participation.
Contributor Information
Gordon W. Blair, Email: gordon.blair@ed.ac.uk.
Joanna M. Wardlaw, Email: joanna.wardlaw@ed.ac.uk.
Reference
- 1.Wardlaw J.M., Smith C., Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 2019;18(7):684–696. doi: 10.1016/S1474-4422(19)30079-1. 2019/07/01/ [DOI] [PubMed] [Google Scholar]
- 2.Debette S., Schilling S., Duperron M.-.G., Larsson S.C., Markus H.S. Clinical significance of magnetic resonance imaging markers of vascular brain injury: a systematic review and meta-analysis. JAMA Neurol. 2019;76(1):81–94. doi: 10.1001/jamaneurol.2018.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wardlaw J.M., Makin S.J., Valdés Hernández M.C., Armitage P.A., Heye A.K., Chappell F.M., et al. Blood-brain barrier failure as a core mechanism in cerebral small vessel disease and dementia: evidence from a cohort study. Alzheimers Dement. 2017;13(6):634–643. 2017/06/01/ [Google Scholar]
- 4.Blair G.W., Thrippleton M.J., Shi Y., Hamilton I., Stringer M., Chappell F., et al. Intracranial hemodynamic relationships in patients with cerebral small vessel disease. Neurology. 2020;94(21):e2258. doi: 10.1212/WNL.0000000000009483. -e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi Y., Thrippleton M.J., Blair G.W., Dickie D.A., Marshall I., Hamilton I., et al. Small vessel disease is associated with altered cerebrovascular pulsatility but not resting cerebral blood flow. J. Cereb. Blood Flow Metab. 2020;40(1):85–99. doi: 10.1177/0271678X18803956. PubMed PMID: 30295558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iliff J.J., Wang M., Liao Y., Plogg B.A., Peng W., Gundersen G.A., et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 2012;4(147):147ra11. doi: 10.1126/scitranslmed.3003748. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wardlaw J.M., Allerhand M., Doubal F.N., Valdes Hernandez M., Morris Z., Gow A.J., et al. Vascular risk factors, large-artery atheroma, and brain white matter hyperintensities. Neurology. 2014;82(15):1331–1338. doi: 10.1212/WNL.0000000000000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Group TSMIftSR Association of intensive vs standard blood pressure control with cerebral white matter lesions. JAMA. 2019;322(6):524–534. doi: 10.1001/jama.2019.10551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wardlaw J.M., Smith E.E., Biessels G.J., Cordonnier C., Fazekas F., Frayne R., et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–838. doi: 10.1016/S1474-4422(13)70124-8. 2013/08/01/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thrippleton M.J., Shi Y., Blair G., Hamilton I., Waiter G., Schwarzbauer C., et al. Cerebrovascular reactivity measurement in cerebral small vessel disease: rationale and reproducibility of a protocol for MRI acquisition and image processing. Int. J. Stroke. 2018;13(2):195–206. doi: 10.1177/1747493017730740. PubMed PMID: 28933655. [DOI] [PubMed] [Google Scholar]
- 11.Deoni S.C. High-resolution T1 mapping of the brain at 3T with driven equilibrium single pulse observation of T1 with high-speed incorporation of RF field inhomogeneities (DESPOT1-HIFI) J. Magn. Reson. Imaging. 2007;26(4):1106–1111. doi: 10.1002/jmri.21130. OctPubMed PMID: 17896356Epub 2007/09/27. eng. [DOI] [PubMed] [Google Scholar]
- 12.Thrippleton M.J., Blair G.W., Valdes-Hernandez M.C., Glatz A., Semple S.I.K., Doubal F., et al. MRI relaxometry for quantitative analysis of USPIO uptake in cerebral small vessel disease. Int. J. Mol. Sci. 2019;20(3) doi: 10.3390/ijms20030776. Feb 12PubMed PMID: 30759756PMCID: PMC6387454. Epub 2019/02/15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heye A.K., Thrippleton M.J., Armitage P.A., Valdes Hernandez M.D.C., Makin S.D., Glatz A., et al. Tracer kinetic modelling for DCE-MRI quantification of subtle blood-brain barrier permeability. Neuroimage. 2016;125:446–455. doi: 10.1016/j.neuroimage.2015.10.018. Jan 15PubMed PMID: 26477653PMCID: PMC4692516. Epub 2015/10/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thrippleton M.J., Backes W.H., Sourbron S., Ingrisch M., van Osch M.J.P., Dichgans M., et al. Quantifying blood-brain barrier leakage in small vessel disease: review and consensus recommendations. Alzheimers Dement. 2019;15(6):840–858. doi: 10.1016/j.jalz.2019.01.013. JunPubMed PMID: 31031101PMCID: PMC6565805. Epub 2019/04/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamot G., Ammerlaan W., Mathay C., Kofanova O., Betsou F. Method validation for automated isolation of viable peripheral blood mononuclear cells. Biopreserv. Biobank. 2015;13(3):152–163. doi: 10.1089/bio.2014.0054. 2015/06/01. [DOI] [PubMed] [Google Scholar]
- 16.Potter G.M., Chappell F.M., Morris Z., Wardlaw J.M. Cerebral perivascular spaces visible on magnetic resonance imaging: development of a qualitative rating scale and its observer reliability. Cerebrovasc. Dis. 2015;39(3–4):224–231. doi: 10.1159/000375153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cordonnier C., Potter G.M., Jackson C.A., Doubal F., Keir S., Sudlow C.L.M., et al. Improving interrater agreement about brain microbleeds. Stroke. 2009;40(1):94–99. doi: 10.1161/STROKEAHA.108.526996. [DOI] [PubMed] [Google Scholar]
- 18.Chabriat H., Joutel A., Dichgans M., Tournier-Lasserve E., Bousser M.G. Cadasil. Lancet Neurol. 2009;8(7):643–653. doi: 10.1016/S1474-4422(09)70127-9. 2009/07/01/ [DOI] [PubMed] [Google Scholar]
- 19.Wong S.M., Jansen J.F.A., Zhang C.E., Hoff E.I., Staals J., van Oostenbrugge R.J., et al. Blood-brain barrier impairment and hypoperfusion are linked in cerebral small vessel disease. Neurology. 2019;92(15):e1669–e1e77. doi: 10.1212/WNL.0000000000007263. [DOI] [PubMed] [Google Scholar]
- 20.Blair G.W., Hernandez M.V., Thrippleton M.J., Doubal F.N., Wardlaw J.M. Advanced neuroimaging of cerebral small vessel disease. Curr. Treat. Options Cardiovasc. Med. 2017;19(7):56. doi: 10.1007/s11936-017-0555-1. 2017/06/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreton F.C., Cullen B., Delles C., Santosh C., Gonzalez R.L., Dani K., et al. Vasoreactivity in CADASIL: comparison to structural MRI and neuropsychology. J. Cereb. Blood Flow Metab. 2018;38(6):1085–1095. doi: 10.1177/0271678X17710375. PubMed PMID: 28537106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sam K., Crawley A.P., Conklin J., Poublanc J., Sobczyk O., Mandell D.M., et al. Development of white matter hyperintensity is preceded by reduced cerebrovascular reactivity. Ann. Neurol. 2016;80(2):277–285. doi: 10.1002/ana.24712. 01 AugPubMed PMID: 611647487. [DOI] [PubMed] [Google Scholar]
- 23.Blair G.W., Doubal F.N., Thrippleton M.J., Marshall I., Wardlaw J.M. Magnetic resonance imaging for assessment of cerebrovascular reactivity in cerebral small vessel disease: a systematic review. J. Cereb. Blood Flow Metab. 2016;36(5):833–841. doi: 10.1177/0271678X16631756. PubMed PMID: 26884471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Guio F., Jouvent E., Biessels G.J., Black S.E., Brayne C., Chen C., et al. Reproducibility and variability of quantitative magnetic resonance imaging markers in cerebral small vessel disease. J. Cereb. Blood Flow Metab. 2016;36(8):1319–1337. doi: 10.1177/0271678X16647396. PubMed PMID: 27170700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wardlaw J.M., Doubal F., Armitage P., Chappell F., Carpenter T., Maniega S.M., et al. Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Ann. Neurol. 2009;65(2):194–202. doi: 10.1002/ana.21549. 2009. [DOI] [PubMed] [Google Scholar]
- 26.Topakian R., Barrick T.R., Howe F.A., Markus H.S. Blood-brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leucoaraiosis. J. Neurol. Neurosurg. Psychiatry. 2010;81(2):192–197. doi: 10.1136/jnnp.2009.172072. 2010. [DOI] [PubMed] [Google Scholar]
- 27.Uh J., Yezhuvath U., Cheng Y., Lu H. In vivo vascular hallmarks of diffuse leukoaraiosis. J. Magn. Reson. Imaging. 2010;32(1):184–190. doi: 10.1002/jmri.22209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang C.E., Wong S.M., van de Haar H.J., Staals J., Jansen J.F.A., Jeukens C.R.L.P.N., et al. Blood–brain barrier leakage is more widespread in patients with cerebral small vessel disease. Neurology. 2017;88(5):426–432. doi: 10.1212/WNL.0000000000003556. [DOI] [PubMed] [Google Scholar]
- 29.Taheri S., Gasparovic C., Huisa B.N., Adair J.C., Edmonds E., Prestopnik J., et al. Blood-brain barrier permeability abnormalities in vascular cognitive impairment. Stroke. 2011;42(8):2158–2163. doi: 10.1161/STROKEAHA.110.611731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang C.E., Wong S.M., Uiterwijk R., Backes W.H., Jansen J.F.A., Jeukens C.R.L.P.N., et al. Blood–brain barrier leakage in relation to white matter hyperintensity volume and cognition in small vessel disease and normal aging. Brain Imaging Behav. 2019;13(2):389–395. doi: 10.1007/s11682-018-9855-7. 2019/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montagne A., Barnes S.R., Sweeney M.D., Halliday M.R., Sagare A.P., Zhao Z., et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85(2):296–302. doi: 10.1016/j.neuron.2014.12.032. 1/21/2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aribisala B.S., Royle N.A., Munoz Maniega S., Valdes Hernandez M.C., Murray C., Penke L., et al. Quantitative multi-modal MRI of the hippocampus and cognitive ability in community-dwelling older subjects. Cortex. 2014;53:34–44. doi: 10.1016/j.cortex.2013.12.012. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong S.M., Jansen J.F.A., Zhang C.E., Staals J., Hofman P.A.M., van Oostenbrugge R.J., et al. Measuring subtle leakage of the blood–brain barrier in cerebrovascular disease with DCE-MRI: test–retest reproducibility and its influencing factors. J. Magn. Reson. Imaging. 2017;46(1):159–166. doi: 10.1002/jmri.25540. [DOI] [PubMed] [Google Scholar]