Highlights
-
•
Volunteers with larger tympanic membrane pulses have better cognitive performance.
-
•
Tympanic membrane pulsing, easy to measure and influenced by intracranial pressure.
-
•
Relationship seen when supine, not sitting. Significant in the left ear.
-
•
Significance remains after correcting for age, heart rate and height.
Keywords: Intracranial pressure, Tympanic membrane displacement, MOCA, Cognitive assessment, Pulsatility
Abstract
To test the hypothesis that pulsing of intracranial pressure has an association with cognition, we measured cognitive score and pulsing of the tympanic membrane in 290 healthy subjects. This hypothesis was formed on the assumptions that large intracranial pressure pulses impair cognitive performance and tympanic membrane pulses reflect intracranial pressure pulses.
290 healthy subjects, aged 20–80 years, completed the Montreal Cognitive Assessment Test. Spontaneous tympanic membrane displacement during a heart cycle was measured from both ears in the sitting and supine position. We applied multiple linear regression, correcting for age, heart rate, and height, to test for an association between cognitive score and spontaneous tympanic membrane displacement. Significance was set at P < 0.0125 (Bonferroni correction.)
A significant association was seen in the left supine position (p = 0.0076.) The association was not significant in the right ear supine (p = 0.28) or in either ear while sitting. Sub-domains of the cognitive assessment revealed that executive function, language and memory have been primarily responsible for this association.
In conclusion, we have found that spontaneous pulses of the tympanic membrane are associated with cognitive performance and believe this reflects an association between cognitive performance and intracranial pressure pulses.
1. Introduction
Stiffening of arterial walls with age is a well-recognised process and known to be associated with cardiovascular disease. Less well known, though clearly demonstrated, is an association between arterial stiffness and cognitive impairment [2,32,34,[36], [37], [38],43,60,62,65]. This has been shown to exist, independently from age, in several studies from different groups [1,7,12,23,33,39,49,52,56]. Stroke, including sub-clinical microinfarcts, forms an obvious and proven link [6,21,55]. However the relationship between cognitive impairment and arterial wall stiffness remains in subjects without a history of stroke, including healthy young individuals [5,27,31,40,41,48,54]. A complete mechanistic explanation for this association therefore remains to be established.
Elasticity of the aorta and other large arteries absorbs energy from the heartbeat during systole and releases it during diastole. Arterial stiffening reduces the degree to which the pressure pulse is absorbed and therefore increases the pulse pressure transmitted to smaller arteries in distal vascular beds, including in the brain [51,61]. Unlike elsewhere in the body, cerebral arterial pulsing is confined within the rigid volume of the skull. The blood volume increase with each heartbeat therefore generates an increase in the pressure within the rigid skull [63,67]. Cerebral arterial pulsing thus generates a pulsing intracranial pressure (ICP) which is transmitted to the cerebral capillaries and venous system [25,61]. It has been hypothesised that increased intracranial pulsing, due to central arterial stiffening, has a damaging mechanical impact on the cerebral microcirculation and in part explains the relationship between increases in arterial stiffness and cognitive decline with age [17,24,33,45]. This hypothesis is supported by several works that demonstrate an association between measures of cerebral arterial pulsatility and cognition [16,18,50,53,[64], [65], [66],68].
The size of the intracranial pressure pulse cannot be easily measured. Evidence of a link between cerebral pressure pulsing and cognitive decline has been derived from measurements of pulsing of the blood flow rather than the pressure. Pressure pulsing measurements have been principally limited to tonometry from the extracranial carotid artery. Measurement of the pulsing of the intracranial pressure would be a very useful tool to investigate the role of intracranial pulsatility and its impact on the cerebral microcirculation in cognitive decline.
Invasive measures of intracranial pressure are too difficult and hazardous for use in research on large numbers of volunteers [3,13,35]. Several techniques have been proposed for non-invasive assessment of ICP [13,26,46,70]. One of these uses measurements of tympanic membrane displacement [44]. Modelling predicts that ICP pulse pressure waves are transmitted from the posterior fossa to the perilymph in the cochlea [20]. It is therefore likely that ICP pulses are transmitted, via the oval window and ossicles, to the tympanic membrane (TM). Pulsing displacements of the TM can be clearly and easily measured non-invasively from within the auditory canal [28]. A component of this pulse waveform has been demonstrated to originate from the ICP pulse [11,14,15,22,29,58,59] Fig. 1. shows how intracranial pressure pulsing can be transmitted to the tympanic membrane.
Fig. 1.
Illustrating a mechanical link from the intracranial fluids in the posterior fossa via the cochlear aqueduct to the inner ear and from there through the ossicles to the tympanic membrane.
We hypothesise that variations in cognitive function in a healthy population may be associated with intracranial pulsatility and this will be measurable in the TM pulses. To address this hypothesis, tympanic membrane displacement measurements and cognitive assessments were made in a population of healthy volunteers. Multiple linear regression was performed to test for an association between cognitive performance and tympanic membrane displacement.
2. Methods
2.1. Participants
Our target was 360 healthy volunteers in three equal age groups 20–40, 40–60 and 60–80 with equal number of males and females. Volunteers responded to publicity, which was circulated via digital and printed media and through radio, club and society meeting appeals. Subjects were recruited if they had no history of otological or neurological disease, a normal otoscopic examination and normal middle ear pressure (MEP) and compliance (MEC). All participants gave informed consent and ethical approval was given by the Health Research Authority, NRES Committee East of England - Hatfield (14/EE/1126). All research was performed in accordance with relevant guidelines and regulations.
2.2. Data collection
Participants attended a two-hour appointment. Following medical history, health and lifestyle questionnaire, height, weight and blood pressure measurements, all participants undertook the Montreal Cognitive Assessment Test (MOCA)(www.mocatest.org) [47].
Tympanometry was performed to confirm normal middle ear compliance and pressure (0.3–1.6 ml and 0 ± 50 daPa respectively), using a GSI Tympstar Middle Ear Analyser V2 (Grason Stadler, Eden Prairie, MN).
ECG was continually recorded to provide an exact time for each heartbeat (ECG100C, BIOPAC Systems, Goleta, CA )
Spontaneous TMD measurements were then made using the MMS-14 Cerebral and Cochlear Fluid Pressure analyser (Marchbanks Measurement Systems Ltd, Lymington, UK). Data was collected in 20 s time periods with five repeats of good quality data (movement artefact free) giving a total of 100 s of data per measurement.
The order of measurements was:- left ear sitting, right sitting, left supine, right supine. Supine measurements were performed lying flat with the head supported on a thin memory-foam pillow. At least five minutes was allowed to elapse after lying down before starting supine recordings. This was to allow ICP to stabilise after the postural change. Where a participant met the inclusion criteria for just one ear, recordings were made from this ear only. ECG and TMD analogue outputs were sampled at 250 Hz (ICM+, Cambridge Enterprise, Cambridge, UK, http://www.neurosurg.cam.ac.uk/icmplus).
2.3. Analysis
Signal processing of spTMD was performed with Matlab (MathWorks, Natick, MA) using the following procedure:-
-
•
All data periods, free from obvious movement or swallowing artefacts, were chosen by visual inspection and included in the analysis.
-
•
The time of every pulse was automatically identified from the QRS peaks in the ECG signal. Accuracy of QRS identification was confirmed by visual inspection.
-
•
The TMD signal corresponding to each beat, from one QRS peak to the next, was then extracted and linearly detrended using the method of least squares.
-
•
The amplitude of each beat was then calculated as: (maximum – minimum).
-
•
spTMD was determined as the median of all measured beats.
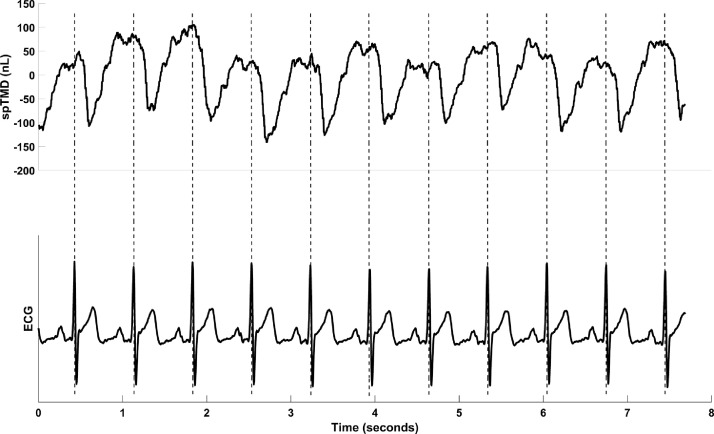
A value for spTMD pulse amplitude was thus determined for the left and right ears in the sitting and supine posture for each subject Fig. 2. illustrates a sample of TMD data and shows how the individual beats were identified from the synchronous ECG trace.
Fig. 2.
Example showing a sample of data and illustrating how the ECG is used to extract heartbeats from a continuous trace of tympanic membrane displacement.
Statistical analysis was performed in MatLab. Linear regression was used to test for a significant association between spTMD and MOCA score. Age, height, pulse pressure, middle ear compliance and heart rate are all parameters that were considered to be potential confounders. The association between each confounder and spTMD was inspected for evidence of a linear association, the significance of which was tested by simple linear regression. If a significant linear association was demonstrated (p < 0.05) they were included as co-variables in the analysis, making it a multiple linear regression analysis. Significance of the association between spTMD and MOCA was determined from the multiple linear regression partial F test. This statistical approach is equivalent to, and gives the same results as, an ANCOVA with spTMD as the dependant variable and MOCA score as the independent variable while correcting for the confounding variables. Sitting and supine position, left and right ears were analysed separately. Significance was set at a p value of < 0.0125 (using Bonferroni correction).
Finally, the individual components of the MOCA test were analysed to determine which if any of these was most strongly associated with spTMD. The analysis was performed on the individual domains, on a composite score made up of the domains associated with executive function, and on the task requiring participants to list as many words as they can beginning with the letter F.
3. Results
3.1. Participants
Altogether 366 volunteers attended between February 2015 and July 2017, 290 of whom satisfied the inclusion criteria and completed the protocol. The mean age was 46.8 years, (stdv 16.6yrs, range 20 – 80). There were 159 female (55%) and 131 male (45%) subjects. In some volunteers either the left or the right ear was excluded, and occasionally a measurement could not be obtained because of an inadequate ear seal with the TMD machine or technical failure. Consequently, differences in the number of data points from each ear and in each position exist. The number of measurements in each configuration were: left ear sitting 247, right ear sitting 237, left ear supine 233 and right ear supine 217.
3.2. The spTMD
For both ears, sitting and supine, the distribution of spTMD pulse amplitude data was visibly skewed to the right and an Anderson-Darling test indicates that it is not normally distributed. After log transformation, with the exception of the right ear sitting, the data becomes consistent with a normal distribution. The statistical analysis has therefore been performed on log transformed spTMD data.
3.3. MOCA scores
A MOCA score of 30 is the maximum possible, 26 or more is considered normal, and a threshold between 24 and 26 is recommended to screen for cognitive impairment [8]. As expected, the majority of our volunteers scored between 25 and 30; a small number scored 24 or less. To ensure sufficient numbers per analysis group, 12 subjects with scores of 24 or less were grouped together. Each of the scores from 25 to 30 had at least 20 subjects which we considered sufficient numbers to be included without further grouping. Thus, seven MOCA score groupings were analysed from 24 (or less), in increments of one, up to 30. The median group size was 33.5, range (12 to 63). One volunteer who scored 15 was considered an outlier and removed from further analysis
(Fig 3) shows dot plots of spTMD results for each MOCA score. The supine plots suggest a trend towards smaller spTMD values in volunteers with lower MOCA scores. No such trend is apparent in the sitting results for either ear.
Fig. 3.
Dot plots showing the association between MOCA score and spTMD, each volunteer in the study is represented as a dot on each of the plots for which they gave data. The trendlines and correlation values (R) are for illustration only, they have been fitted by conventional least squares fit of all data points which assumes that the MOCA score is a continuous linear variable, it is actually an ordinal variable and has been treated as ordinal or categorical in all analysis
3.4. Confounding variables
No non-linear associations were seen by visual inspection between log spTMD and any of the 5 confounding variables. Simple regression analysis is presented in Table 1. A significant association was seen, in at least one ear or posture, with age, height, and heart rate but not with middle ear compliance or pulse pressure. Therefore age, height and heart rate were included as explanatory variables in the multiple regression.
Table 1.
Significance of linear association with spTMD for each of the possible confounding variables (MEC = middle ear compliance.).
| Age | Height | Heart Rate | MEC Left | MEC Right | Pulse Pressure | |
|---|---|---|---|---|---|---|
| Left Sitting | p =0.03 | p =0.12 | p =0.02 | p =0.69 | NA | p =0.58 |
| Right Sitting | P =< 0.001 | P =0.008 | P =0.008 | NA | P =0.07 | P =0.16 |
| Left Supine | P =0.54 | P =0.001 | P =0.001 | P =0.28 | NA | P =0.77 |
| Right Supine | P =0.13 | P =<0.001 | P =<0.001 | NA | P = 0.13 | P =0.38 |
3.5. Multiple regression
Our data is from a random sample and thus the observations and the residuals should be independent. The correlation matrix between all predictor variables showed the strongest correlation between age and height (0.27), closely followed by age and MOCA score which was 0.25. As none were highly correlated (r value greater than 0.8) multicollinearity does not present a problem.
The Anderson-Darling test confirmed no violation of the assumption of normality (p < 0.05) and Bartlett's test demonstrated homoscedasticity for all 4 combinations of ear and posture except for the right sitting position which passed the test of homoscedasticity but not normality.
Having satisfied the assumptions, except in the right siting position, multiple regression analysis was used to test if MOCA score significantly contributes to the prediction of spTMD in each of the 4 positions. The results are shown in Table 2.
Table 2.
Showing results from the 4 independently carried out multiple regression analysis, with spTMD as the response variable. We consider a p value less than 0.0125 to be significant.
| SumSq | DF | MeanSq | F | P-value | |
|---|---|---|---|---|---|
| Left Sitting | |||||
| Age | 2.14 | 1 | 2.14 | 5.67 | 0.0181 |
| MOCA | 2.39 | 6 | 0.40 | 1.05 | 0.39 |
| Heart Rate | 2.06 | 1 | 2.06 | 5.45 | 0.020 |
| Height | 1.50 | 1 | 1.50 | 3.97 | 0.047 |
| Error | 89.6 | 237 | 0.378 | 1 | 0.5 |
| Right Sitting | |||||
| Age | 12.76 | 1 | 12.76 | 28.50 | <0.0000 |
| MOCA | 2.644 | 6 | 0.44 | 0.98 | 0.44 |
| Heart Rate | 2.71 | 1 | 2.71 | 6.06 | 0.015 |
| Height | 6.26 | 1 | 6.26 | 13.99 | 0.00023 |
| Error | 101.6 | 227 | 0.45 | 1 | 0.5 |
| Left Supine | |||||
| Age | 0.067 | 1 | 0.067 | 0.223 | 0.64 |
| MOCA | 5.47 | 6 | 0.91 | 3.01 | 0.0076 |
| Heart Rate | 2.59 | 1 | 2.59 | 8.56 | 0.0038 |
| Height | 1.22 | 1 | 1.22 | 4.05 | 0.045 |
| Error | 67.5 | 223 | 0.303 | 1 | 0.5 |
| Right Supine | |||||
| Age | 0.128 | 1 | 0.128 | 0.396 | 0.53 |
| MOCA | 2.44 | 6 | 0.407 | 1.26 | 0.28 |
| Heart Rate | 0.387 | 1 | 0.387 | 1.20 | 0.27 |
| Height | 3.00 | 1 | 3.00 | 9.28 | 0.0026 |
| Error | 66.87 | 207 | 0.32 | 1 | 0.5 |
Approximate normality of residuals was confirmed with QQ plots and the Anderson-Darling test for all analysis with the exception of the right sitting posture. Data from the right sitting posture had also failed the Anderson Darling test of normality. A significant association in this position could not therefore be considered valid.
Our results indicate a significant association between spTMD and MOCA score from the left ear in the supine position (p = 0.0076.) The contribution of MOCA score is not significant in the other 3 positions. The trend seen in the supine position in left and right ears in Fig. 1 has achieved significance for the left ear but not in the right. The co-variables reveal very significant associations of age with spTMD in the sitting position in both ears and yet no association in the supine posture. Height has shown a significant association in the right ear in both postures, the association does not reach significance on the left. Heart rate has shown a significant association in the left supine position but not right supine. In the sitting position neither ear reaches a significant association with heart rate.
3.6. MOCA sub-domain analysis
By analysing individual sub-domains of the MOCA score, with an appropriate change to the number of groups, we determined which cognitive processes were most closely associated with spTMD for the supine data only. We included a composite ‘executive function’ subdomain made up of the alternating number-letter task, the number of F words (N≥11) and performance on abstraction. The total number of words beginning with the letter F in 1 minute was also assessed as a continuous variable. Results are summarised in Table 3.
Table 3.
Showing the significance of the MOCA variable in the multiple regression analysis after substituting the MOCA score with single domains, sub-domains and composite domains to illustrate the components that have been most influential. In the majority of cases the number of groups that have been used in the analysis is less than the maximum possible number to avoid groups with very small or zero numbers. For example the visualspatial/executive domain can be scored from 0 to 5 making 6 possible groups, however only 2 subjects scored 2 or less. They were grouped together with a score of 3 or less and the following 3 groups were used in the analysis, 3 or less, 4 or 5. Nearly all of our volunteers scored full marks for orientation, this domain could not therefore be grouped for analysis.
| Domain | Number of Groups | Left Supine P value | Right Supine P value |
|---|---|---|---|
| Visuospatial/Executive | 3 | 0.213 | 0.230 |
| Naming | 2 | 0.927 | 0.386 |
| Language | 3 | 0.013 | 0.409 |
| Attention | 3 | 0.874 | 0.606 |
| Abstraction | 2 | 0.053 | 0.573 |
| Delayed recall | 4 | 0.013 | 0.085 |
| Orientation | NA | NA | NA |
| Composite Executive | 3 | 0.006 | 0.319 |
| Number of F Words | Continuous variable | 0.007 | 0.816 |
In the left ear, two out of the 7 subdomains analysed (language and delayed recall) produce an association that would have been classed as significant (p < 0.05) had they been the primary research question. The composite executive function variable and the number of F words also produced significant results in the left ear. None of the subdomain variables were significant for the right ear. We have not corrected the sub-domain analysis for multiple comparisons; these results are presented to illustrate which have been most influential in determining the association between MOCA and spTMD. The results clearly point towards the executive function tasks.
3.7. spTMD waveforms
To further illustrate the spTMD pulse waveform association with cognitive score, a coherently averaged TMD heartbeat was produced for each subject and then further averaging of these was performed, grouped by MOCA score. Thus, producing the average shape of the TMD pulse for each of the MOCA groups. This was done for the left and right ears in the supine posture. Data for the averaging was extracted from 1 second before up to 1.5 s after each ECG trigger. The extracted beats were linearly detrended before incorporation into the average. Shown in Fig. 4 it clearly illustrates the increase in pulse amplitude with increasing MOCA score in the left supine data. The only exception to the trend is subjects with a MOCA score of 24 or less. This was the smallest group, statistically less reliable, and arguably containing some un-diagnosed pathologies. Data from the right ear shows a similar though less distinct trend.
Fig. 4.
Pulse waveforms of tympanic membrane displacement during a heartbeat, showing the average for each cognitive assessment score.
4. Discussion
We have observed a significant association between spTMD and MOCA score in healthy volunteers. This is seen in the supine position, but not in the sitting position, and after correction for multiple comparisons, reaches significance in the left ear only. We believe that these results are important as they show a novel measurement, likely related to intracranial pressure pulsing, to be associated with subtle compromises in brain performance within a seemingly healthy population, and across a broad range of ages. An association with developing cognitive impairment seems likely.
Our assumption, prior to analysis, was that higher ICP pulses would generate larger spTMD pulses and be associated with poorer cognitive performance. The results are the opposite of this prediction. We discuss here some possible explanations for this unexpected result. We based our assumption on known links between cognitive impairment and vascular measurements including arterial stiffening, increased pulsatility of the extracranial carotid artery and increased pulsatility of the intracranial blood flow velocity. While it would seem intuitive that intracranial pressure pulsing would increase under each of these conditions it is not necessarily the case. This overlooks the complex association between intracranial flow pulsatility and intracranial pressure pulsatility [63]. Increasing arterial stiffness does increase flow pulsatility and intraluminal pressure pulsing however it decreases the vessel wall expansion. This therefore can reduce the transmission of arterial pulse pressure to the intracranial pressure despite an increase in arterial pressure pulsing. It may therefore be the case that a reduced intracranial pressure pulsatility is associated with cognitive decline, although this counters the argument that increased intracranial pulsatility is responsible for mechanical damage associated with cognitive impairments.
We also made the assumption that an increase in ICP pulse amplitude will generate an increased spTMD. This assumption may not always hold true. ICP is one of several factors that contribute to the spTMD [11,15] and it is possible that some of these contributions are in opposition with one another in the force they apply to the TM and either an inward or an outward movement of the TM is produced depending upon which of the driving forces dominates. If ICP is the weaker of two opposing drivers then an increasing ICP pulse will act to reduce rather than increase the size of the spTMD.
These arguments show that the factors that predict the direction of any association between spTMD and cognitive performance are unclear and require further research. What we feel is important is the demonstration of an association. Our statistical analysis was two-tailed and therefore valid regardless of the direction of the association.
As always in regression analysis a significant association requires all confounding variables to have been taken into account. This is particularly difficult in complex biological systems. We have considered the key variables, that we felt might have an influence on the association between spTMD and MOCA score. They have been accounted for and do not therefore explain the results. It is still possible, however, that other factors, or interactions between factors, that we have overlooked are confounding our result.
We have observed a difference between sitting and supine results. The difference in ICP between sitting and lying is typically 11 mmHg [9,42]. An associated change in ICP pulsatility might also to be expected, however the physiology is not so well understood. Intracranial compliance is reduced which will increase ICP pulsatility, this however will be countered by vasoconstriction which occurs in response to increased cerebral perfusion pressure to maintain cerebral blood flow and will reduce ICP pulsing. The normal change in ICP pulsatility with posture, in healthy subjects, remains to be established. Similar changes with posture are anticipated in the inner ear fluid pressures, and all vascular pressures within the inner, middle and outer ear. It is not a surprise therefore that a move to the supine position is a profound enough disturbance to have exposed an association between spTMD and cognitive performance that is not seen in the sitting position.
Age has shown a strong association with spTMD in the sitting position and yet no clear association in the supine position. We had not predicted this, and while we might speculate, we are not sure of the reasons why aging would have a more profound impact in the sitting position than supine. Given the high strength of this observation and the potential significance to our understanding of the vascular physiology in the aging brain, we feel this should be further researched.
Less predictable than a postural difference is the difference between left and right ears that has been observed. The study was not designed to determine, and we have not therefore tested, the significance of this observed difference. It is possible therefore that the difference is within the bounds of statistical variations. It does however support a similar left to right difference observed in evoked TM displacement in the supine position [4]. The results suggest that an anatomical difference may exist between left and right ears. One anatomical difference known to exist is in the size of the jugular vein:- the right side being generally larger [57]. An alternative explanation is related to the sequence of our measurements. Left supine measurements were made sooner after lying down than those from the right (typically 5 min and 10 min respectively).
The domains of the MOCA test that show the strongest association with spTMD in the left supine position are primarily related to executive function, language and memory. These are left hemisphere domains in the majority of the population, which is intriguing. The strongest association was seen with executive function tasks, particularly letter fluency, again, strongly influenced by left frontal, temporal and parietal structures. In a healthy population, greater left-sided pulsatility may reflect improved perfusion, and therefore function. Where this fails, these brain regions may be disproportionately affected, explaining the relationships with impaired cognition seen in disease populations reported previously.
Studies using Doppler ultrasound or MR imaging techniques consistently demonstrate abnormal cerebral flow pulsatility in patients with cognitive impairment [10,16,18,19,50,66]. Further evidence of an association between vascular pulsatility and cognitive performance has been provided by measurements from the systemic arterial system, which are reasonably assumed to be influencing the intracranial vascular pulsatility [30,69]. These reports of an association between intracranial pulsatility (whether flow or pressure) and impaired cognition, in predominantly elderly patient populations, are increasing in number and beginning to gain acceptance. While intracranial flow pulsatility measurements can be made relatively easily in research participants, intracranial pressure pulsatility measurements are much more challenging. The relationship between pressure and flow pulsatility is complex and very likely to differ between normal and pathological conditions [63].
Pulsatility measures of both pressure and flow are key to understanding cerebral haemodynamics and CSF dynamics. The spTMD measurement described in this paper is non-invasive, safe, quick and easy to perform and we believe influenced by intracranial pressure pulsatility [11]. It forms a valuable addition to the tools available for the investigation of cerebral fluid dynamics and its role in cognitive performance and cognitive decline.
In conclusion we have found, in 290 healthy volunteers, that larger spontaneous pulses of the TM are associated with better cognitive performance. This was shown in the left ear in the supine posture. It was not replicated in the right ear. Despite this we believe that this study has raised the probability of an association between cognitive performance and TM displacement measurements from very speculative to likely. Further work to assess spTMD in patients with different degrees of cognitive impairment is an important next step in the development of biomarkers for cognitive decline based upon CSF flow dynamics
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Author contributions
A.A.B.:- Designed and conceptualised the study; analysed the data; drafted the manuscript for intellectual content.; W.K.E.B.:- Analysed and interpreted the data; revised the manuscript for intellectual content.; R.J.M.:- Designed and conceptualised the study; interpreted the data; revised the manuscript for intellectual content.; L.A.M.:- Major role in analysis of the data; revised the manuscript for intellectual content.; C.M.C-B:- Major role in the acquisition of data; interpreted the data; revised the manuscript for intellectual content.; C.M.K.:- Interpreted the data; Revised the manuscript for intellectual content.; D.O.B. Designed and conceptualised the study; interpreted the data; revised the manuscript for intellectual content.
Conflicts of Interest
Dr. Marchbanks is the majority shareholder in Marchbanks Measurement Systems Ltd, a spinout from Southampton University that manufactures the TMD Analyser for clinical evaluation purposes. None of the other authors have any competing interest to declare.
Acknowledgements
The authors would like to thank Steve Burnage, Gabriella Howard, Chloe Cox, Shannon Cawte and Tom Bower for their work in conducting the measurements. It was supported by the NIHR/Wellcome Trust Southampton Clinical Research Facility.
References
- 1.Alvarez-Bueno C., Cunha P.G., Martinez-Vizcaino V., Pozuelo-Carrascosa D.P., Visier-Alfonso M.E., Jimenez-Lopez E., Cavero-Redondo I. Arterial stiffness and cognition among adults: a systematic review and meta-analysis of observational and longitudinal studies. J. Am. Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.014621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araghi M., Shipley M.J., Wilkinson I.B., McEniery C.M., Valencia-Hernández C.A., Kivimaki M., Sabia S., Singh-Manoux A., Brunner E.J. Association of aortic stiffness with cognitive decline: Whitehall II longitudinal cohort study. Eur. J. Epidemiol. 2020;35:861–869. doi: 10.1007/s10654-019-00586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bekar A., Doğan S., Abaş F., Caner B., Korfali G., Kocaeli H., Yilmazlar S., Korfali E. Risk factors and complications of intracranial pressure monitoring with a fiberoptic device. J. Clin. Neurosci. 2009;16:236–240. doi: 10.1016/j.jocn.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Campbell-Bell C.M., Birch A.A., Vignali D., Bulters D., Marchbanks R.J. Reference intervals for the evoked tympanic membrane displacement measurement: a non-invasive measure of intracranial pressure. Physiol. Meas. 2018;39 doi: 10.1088/1361-6579/aaa1d3. [DOI] [PubMed] [Google Scholar]
- 5.Cooper L.L., Himali J.J., Torjesen A., Tsao C.W., Beiser A., Hamburg N.M., DeCarli C., Vasan R.S., Seshadri S., Pase M.P., Mitchell G.F. Inter-relations of orthostatic blood pressure change, aortic stiffness, and brain structure and function in young adults. J. Amer. Heart Assoc. 2017 doi: 10.1161/JAHA.117.006206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coutinho T., Turner S.T., Kullo I.J. Aortic pulse wave velocity is associated with measures of subclinical target organ damage. JACC Cardiovasc. Imaging. 2011;4 doi: 10.1016/j.jcmg.2011.04.011. 10.1016/j.jcmg.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui C., Sekikawa A., Kuller L.H., Lopez O.L., Newman A.B., Kuipers A.L., Mackey R.H. Aortic stiffness is associated with increased risk of incident dementia in older adults. J. Alzheimers Dis. 2018;66:297–306. doi: 10.3233/JAD-180449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damian A.M., Jacobson S.A., Hentz J.G., Belden C.M., Shill H.A., Sabbagh M.N., Caviness J.N., Adler C.H. The Montreal cognitive assessment and the mini-mental state examination as screening instruments for cognitive impairment: item analyses and threshold scores. Dement. Geriatr. Cogn. Disord. 2011;31:126–131. doi: 10.1159/000323867. [DOI] [PubMed] [Google Scholar]
- 9.Eklund A., Jóhannesson G., Johansson E., Holmlund P., Qvarlander S., Ambarki K., Wåhlin A., Koskinen L.-O.D., Malm J. The pressure difference between eye and brain changes with posture. Ann. Neurol. 2016;80:269–276. doi: 10.1002/ana.24713. [DOI] [PubMed] [Google Scholar]
- 10.El Sankari S., Gondry-Jouet C., Fichten A., Godefroy O., Serot J.M., Deramond H., Meyer M.E., Balédent O. Cerebrospinal fluid and blood flow in mild cognitive impairment and Alzheimer's disease: a differential diagnosis from idiopathic normal pressure hydrocephalus. Fluids Barriers CNS. 2011;8:12. doi: 10.1186/2045-8118-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Bouri W.K., Vignali D., Iliadi K., Bulters D., Marchbanks R.J., Birch A.A., Simpson D.M. Quantifying the contribution of intracranial pressure and arterial blood pressure to spontaneous tympanic membrane displacement. Physiol. Meas. 2018;39 doi: 10.1088/1361-6579/aad308. [DOI] [PubMed] [Google Scholar]
- 12.Elias M., Robbins M., Budge M., Abhayaratna W., Dore G., Elias P. Arterial pulse wave velocity and cognition with advancing age. Hypertension. 2009;53 doi: 10.1161/HYPERTENSIONAHA.108.126342. (Dallas, Tex. : 1979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evensen K.B., Eide P.K. Measuring intracranial pressure by invasive, less invasive or non-invasive means: limitations and avenues for improvement. Fluids Barriers CNS. 2020;17:34. doi: 10.1186/s12987-020-00195-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evensen K.B., Paulat K., Prieur F., Holm S., Eide P.K. Utility of the tympanic membrane pressure waveform for non-invasive estimation of the intracranial pressure waveform. Sci. Rep. 2018;8:15776. doi: 10.1038/s41598-018-34083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finch L.C., Marchbanks R.J., Bulters D., Birch A.A. Refining non-invasive techniques to measure intracranial pressure: comparing evoked and spontaneous tympanic membrane displacements. Physiol. Meas. 2018;39 doi: 10.1088/1361-6579/aaa9f8. [DOI] [PubMed] [Google Scholar]
- 16.Fleysher R., Lipton M.L., Noskin O., Rundek T., Lipton R., Derby C.A. White matter structural integrity and transcranial Doppler blood flow pulsatility in normal aging. Magn. Reson. Imaging. 2018;47:97–102. doi: 10.1016/j.mri.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fulop G.A., Tarantini S., Yabluchanskiy A., Molnar A., Prodan C.I., Kiss T., Csipo T., Lipecz A., Balasubramanian P., Farkas E., Toth P., Sorond F., Csiszar A., Ungvari Z. Role of age-related alterations of the cerebral venous circulation in the pathogenesis of vascular cognitive impairment. Am. J. Physiol. Heart Circ. Physiol. 2019;316:H1124–H1140. doi: 10.1152/ajpheart.00776.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzales J.U., James C.R., Yang H.S., Jensen D., Atkins L., Al-Khalil K., O'Boyle M. Carotid flow pulsatility is higher in women with greater decrement in gait speed during multi-tasking. Gait Posture. 2017;54:271–276. doi: 10.1016/j.gaitpost.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 19.González-Suárez I., Arpa J., Ríos-Blanco J.J. Brain microvasculature involvement in ANCA positive vasculitis. Cerebrovasc. Dis. 2016;41:313–321. doi: 10.1159/000443750. [DOI] [PubMed] [Google Scholar]
- 20.Gopen Q., Rosowski J.J., Merchant S.N. Anatomy of the normal human cochlear aqueduct with functional implications. Hear Res. 1997;107:9–22. doi: 10.1016/s0378-5955(97)00017-8. [DOI] [PubMed] [Google Scholar]
- 21.Gorelick P.B., Scuteri A., Black S.E., Decarli C., Greenberg S.M., Iadecola C., Launer L.J., Laurent S., Lopez O.L., Nyenhuis D., Petersen R.C., Schneider J.A., Tzourio C., Arnett D.K., Bennett D.A., Chui H.C., Higashida R.T., Lindquist R., Nilsson P.M., Roman G.C., Sellke F.W., Seshadri S., American Heart Association Stroke Council. Council on Epidemiology and Prevention. Council on Cardiovascular Nursing. Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American heart association/american stroke association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gwer S., Kazungu M., Chengo E., Ohuma E.O., Idro R., Birch T., Marchbanks R., Kirkham F.J., Newton C.R. Abnormal intra-aural pressure waves associated with death in African children with acute nontraumatic coma. Pediatr Res. 2015;78:38–43. doi: 10.1038/pr.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han F., Zhai F.-F., Li M.-L., Zhou L.-X., Ni J., Yao M., Jin Z.-Y., Cui L.-Y., Zhang S.-Y., Zhu Y.-C. Arterial stiffness is associated with white matter disruption and cognitive impairment: a community-based cohort study. J. Alzheimers Dis. 2021;80:567–576. doi: 10.3233/JAD-201424. [DOI] [PubMed] [Google Scholar]
- 24.Henry-Feugeas M.C., Koskas P. Cerebral vascular aging: extending the concept of pulse wave encephalopathy through capillaries to the cerebral veins. Curr. Aging Sci. 2012;5:157–167. doi: 10.2174/1874609811205020157. [DOI] [PubMed] [Google Scholar]
- 25.Hu X., Alwan A.A., Rubinstein E.H., Bergsneider M. Reduction of compartment compliance increases venous flow pulsatility and lowers apparent vascular compliance: implications for cerebral blood flow hemodynamics. Med. Eng. Phys. 2006;28:304–314. doi: 10.1016/j.medengphy.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Khan M.N., Shallwani H., Khan M.U., Shamim M.S. Noninvasive monitoring intracranial pressure - A review of available modalities. Surg. Neurol. Int. 2017;8:51. doi: 10.4103/sni.sni_403_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamballais S., Sajjad A., Leening M.J.G., Gaillard R., Franco O.H., Mattace-Raso F.U.S., Jaddoe V.W.V., Roza S.J., Tiemeier H., Ikram M.A. Association of blood pressure and arterial stiffness with cognition in 2 population-based child and adult cohorts. J. Am. Heart Assoc. 2018 doi: 10.1161/JAHA.118.009847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang E.W., Paulat K., Witte C., Zolondz J., Mehdorn H.M. Noninvasive intracranial compliance monitoring. Technical note and clinical results. J. Neurosurg. 2003;98:214–218. doi: 10.3171/jns.2003.98.1.0214. [DOI] [PubMed] [Google Scholar]
- 29.Lehrer J., Ogunlusi A., Knutsen J., Marchbanks R. The value of transcranial cerebral sonography in diagnosing neurootological disorders. Int. Tinnitus J. 2009;15 [PubMed] [Google Scholar]
- 30.Lilamand M., Vidal J.-S., Plichart M., De Jong L.W., Duron E., Hanon O. Arterial stiffness and medial temporal lobe atrophy in elders with memory disorders. J. Hypertens. 2016;34:1331–1337. doi: 10.1097/HJH.0000000000000954. [DOI] [PubMed] [Google Scholar]
- 31.Maillard P., Mitchell G.F., Himali J.J., Beiser A., Tsao C.W., Pase M.P., Satizabal C.L., Vasan R.S., Seshadri S., DeCarli C. Effects of arterial stiffness on brain integrity in young adults from the framingham heart study. Stroke. 2016;47:1030–1036. doi: 10.1161/STROKEAHA.116.012949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer M.L., Palta P., Tanaka H., Deal J.A., Wright J., Knopman D.S., Griswold M.E., Mosley T.H., Heiss G. Association of central arterial stiffness and pressure pulsatility with mild cognitive impairment and dementia: the atherosclerosis risk in communities study-neurocognitive study (ARIC-NCS) J. Alzheimers Dis. 2017;57:195–204. doi: 10.3233/JAD-161041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell G., van Buchem M., Sigurdsson S., Gotal J., Jonsdottir M., Kjartansson Ó., Garcia M., Aspelund T., Harris T., Gudnason V., Launer L. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility–Reykjavik study. Brain. 2011;134 doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muela H.C.S., Costa-Hong V.A., Yassuda M.S., Machado M.F., Nogueira R.de C., Moraes N.C., Memória C.M., Macedo T.A., Bor-Seng-Shu E., Massaro A.R., Nitrini R., Bortolotto L.A. Impact of hypertension severity on arterial stiffness, cerebral vasoreactivity, and cognitive performance. Dement. Neuropsychol. 2017;11:389–397. doi: 10.1590/1980-57642016dn11-040008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nag D.S., Sahu S., Swain A., Kant S. Intracranial pressure monitoring: Gold standard and recent innovations. World J. Clin. Cases. 2019;7:1535–1553. doi: 10.12998/wjcc.v7.i13.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nilsson E.D., Elmståhl S., Minthon L., Nilsson P.M., Pihlsgård M., Tufvesson E., Nägga K. Nonlinear association between pulse wave velocity and cognitive function: a population-based study. J. Hypertens. 2014;32:2152–2157. doi: 10.1097/HJH.0000000000000329. discussion 2157. [DOI] [PubMed] [Google Scholar]
- 37.Palta P., Sharrett A.R., Wei J., Meyer M.L., Kucharska-Newton A., Power M.C., Deal J.A., Jack C.R., Knopman D., Wright J., Griswold M., Tanaka H., Mosley T.H., Heiss G. Central arterial stiffness is associated with structural brain damage and poorer cognitive performance: the ARIC study. J. Am. Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.011045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pase M.P., Herbert A., Grima N.A., Pipingas A., O'Rourke M.F. Arterial stiffness as a cause of cognitive decline and dementia: a systematic review and meta-analysis. Intern. Med. J. 2012;42:808–815. doi: 10.1111/j.1445-5994.2011.02645.x. [DOI] [PubMed] [Google Scholar]
- 39.Pase M.P., Herbert A., Grima N.A., Pipingas A., O'Rourke M.F. Arterial stiffness as a cause of cognitive decline and dementia: a systematic review and meta-analysis. Intern. Med. J. 2012;42:808–815. doi: 10.1111/j.1445-5994.2011.02645.x. [DOI] [PubMed] [Google Scholar]
- 40.Pase M.P., Himali J.J., Mitchell G.F., Beiser A., Maillard P., Tsao C., Larson M.G., DeCarli C., Vasan R.S., Seshadri S. Association of aortic stiffness with cognition and brain aging in young and middle-aged adults: the framingham third generation cohort study. Hypertension. 2016;67:513–519. doi: 10.1161/HYPERTENSIONAHA.115.06610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pase M.P., Pipingas A., Kras M., Nolidin K., Gibbs A.L., Wesnes K.A., Scholey A.B., Stough C. Healthy middle-aged individuals are vulnerable to cognitive deficits as a result of increased arterial stiffness. J. Hypertens. 2010;28:1724–1729. doi: 10.1097/HJH.0b013e32833b1ee7. [DOI] [PubMed] [Google Scholar]
- 42.Poca M.A., Sahuquillo J., Topczewski T., Lastra R., Font M.L., Corral E. Posture-induced changes in intracranial pressure: a comparative study in patients with and without a cerebrospinal fluid block at the craniovertebral junction. Neurosurgery. 2006;58:899–906. doi: 10.1227/01.NEU.0000209915.16235.6D. discussion 899-906. [DOI] [PubMed] [Google Scholar]
- 43.Rabkin S.W., Jarvie G. Comparison of vascular stiffness in vascular dementia, Alzheimer dementia and cognitive impairment. Blood Press. 2011;20:274–283. doi: 10.3109/08037051.2011.566246. [DOI] [PubMed] [Google Scholar]
- 44.Reid A., Marchbanks R.J., Burge D.M., Martin A.M., Bateman D.E., Pickard J.D., Brightwell A.P. The relationship between intracranial pressure and tympanic membrane displacement. Br. J. Audiol. 1990;24:123–129. doi: 10.3109/03005369009077853. [DOI] [PubMed] [Google Scholar]
- 45.Rensma S.P., Stehouwer C.D.A., Boxtel M.P.J.V., Houben A.J.H.M., Berendschot T.T.J.M., Jansen J.F.A., Schalkwijk C.G., Verhey F.R.J., Kroon A.A., Henry R.M.A., Backes W.H., Dagnelie P.C., Dongen M.C.J.M.van, Eussen S.J.P.M., Bosma H., Köhler S., Reesink K.D., Schram M.T., Sloten T.T.van. Associations of arterial stiffness with cognitive performance, and the role of microvascular dysfunction. Hypertension. 2020 doi: 10.1161/HYPERTENSIONAHA.119.14307. [DOI] [PubMed] [Google Scholar]
- 46.Rosenberg J.B., Shiloh A.L., Savel R.H., Eisen L.A. Non-invasive methods of estimating intracranial pressure. Neurocrit. Care. 2011;15:599–608. doi: 10.1007/s12028-011-9545-4. [DOI] [PubMed] [Google Scholar]
- 47.Rossetti H.C., Lacritz L.H., Cullum C.M., Weiner M.F. Normative data for the montreal cognitive assessment (MoCA) in a population-based sample. Neurology. 2011;77:1272–1275. doi: 10.1212/WNL.0b013e318230208a. [DOI] [PubMed] [Google Scholar]
- 48.Scuteri A., Brancati A.M., Gianni W., Assisi A., Volpe M. Arterial stiffness is an independent risk factor for cognitive impairment in the elderly: a pilot study. J. Hypertens. 2005;23:1211–1216. doi: 10.1097/01.hjh.0000170384.38708.b7. [DOI] [PubMed] [Google Scholar]
- 49.Scuteri A., Tesauro M., Appolloni S., Preziosi F., Brancati A.M., Volpe M. Arterial stiffness as an independent predictor of longitudinal changes in cognitive function in the older individual. J. Hypertens. 2007;25:1035–1040. doi: 10.1097/HJH.0b013e3280895b55. [DOI] [PubMed] [Google Scholar]
- 50.Shi Y., Thrippleton M.J., Marshall I., Wardlaw J.M. Intracranial pulsatility in patients with cerebral small vessel disease: a systematic review. Clin. Sci. (Lond) 2018;132:157–171. doi: 10.1042/CS20171280. [DOI] [PubMed] [Google Scholar]
- 51.Shirwany N.A., Zou M. Arterial stiffness: a brief review. Acta Pharmacol. Sin. 2010;31:1267–1276. doi: 10.1038/aps.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singer J., Trollor J.N., Baune B.T., Sachdev P.S., Smith E. Arterial stiffness, the brain and cognition: a systematic review. Ageing Res. Rev. 2014;15:16–27. doi: 10.1016/j.arr.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 53.Stone J., Johnstone D.M., Mitrofanis J., O'Rourke M. The mechanical cause of age-related dementia (Alzheimer's disease): the brain is destroyed by the pulse. J. Alzheimers Dis. 2015;44:355–373. doi: 10.3233/JAD-141884. [DOI] [PubMed] [Google Scholar]
- 54.Suri S., Chiesa S.T., Zsoldos E., Mackay C.E., Filippini N., Griffanti L., Mahmood A., Singh-Manoux A., Shipley M.J., Brunner E.J., Kivimäki M., Deanfield J.E., Ebmeier K.P. Associations between arterial stiffening and brain structure, perfusion, and cognition in the Whitehall II Imaging Sub-study: A retrospective cohort study. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tabata N., Sueta D., Yamashita T., Utsunomiya D., Arima Y., Yamamoto E., Tsujita K., Kojima S., Kaikita K., Hokimoto S. Relationship between asymptomatic intra-cranial lesions and brachial-ankle pulse wave velocity in coronary artery disease patients without stroke. Hypertens. Res. 2017;40:392–398. doi: 10.1038/hr.2016.159. [DOI] [PubMed] [Google Scholar]
- 56.Taniguchi Y., Fujiwara Y., Nofuji Y., Nishi M., Murayama H., Seino S., Tajima R., Matsuyama Y., Shinkai S. Prospective study of arterial stiffness and subsequent cognitive decline among community-dwelling older Japanese. J. Epidemiol. 2015;25:592–599. doi: 10.2188/jea.JE20140250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tartière D., Seguin P., Juhel C., Laviolle B., Mallédant Y. Estimation of the diameter and cross-sectional area of the internal jugular veins in adult patients. Crit. Care. 2009;13:1–4. doi: 10.1186/cc8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Traboulsi R., Avan P. Transmission of infrasonic pressure waves from cerebrospinal to intralabyrinthine fluids through the human cochlear aqueduct: Non-invasive measurements with otoacoustic emissions. Hear Res. 2007;233:30–39. doi: 10.1016/j.heares.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 59.Traboulsi R., Poumarat G., Chazal J., Avan P., Mom T., Ronchan-Cole I., Traboulsi S. The estimation of the time constant of the human inner ear pressure change by noninvasive technique. Modell. Simul. Eng. 2009;2009 doi: 10.1155/2009/570124. [DOI] [Google Scholar]
- 60.Tsao C.W., Himali J.J., Beiser A.S., Larson M.G., DeCarli C., Vasan R.S., Mitchell G.F., Seshadri S. Association of arterial stiffness with progression of subclinical brain and cognitive disease. Neurology. 2016;86:619–626. doi: 10.1212/WNL.0000000000002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tucker T. Arterial stiffness as a vascular contribution to cognitive impairment: a fluid dynamics perspective. Biomed. Phys. Eng. Express. 2021 doi: 10.1088/2057-1976/abdf36. [DOI] [PubMed] [Google Scholar]
- 62.van Sloten T.T., Protogerou A.D., Henry R.M.A., Schram M.T., Launer L.J., Stehouwer C.D.A. Association between arterial stiffness, cerebral small vessel disease and cognitive impairment: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2015;53:121–130. doi: 10.1016/j.neubiorev.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wagshul M.E., Eide P.K., Madsen J.R. The pulsating brain: a review of experimental and clinical studies of intracranial pulsatility. Fluids Barriers CNS. 2011;8:5. doi: 10.1186/2045-8118-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wåhlin A., Ambarki K., Birgander R., Malm J., Eklund A. Intracranial pulsatility is associated with regional brain volume in elderly individuals. Neurobiol Aging. 2014;35:365–372. doi: 10.1016/j.neurobiolaging.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 65.Waldstein S.R., Rice S.C., Thayer J.F., Najjar S.S., Scuteri A., Zonderman A.B. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore longitudinal study of aging. Hypertension. 2008;51:99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- 66.Webb A.J.S., Simoni M., Mazzucco S., Kuker W., Schulz U., Rothwell P.M. Increased cerebral arterial pulsatility in patients with leukoaraiosis: arterial stiffness enhances transmission of aortic pulsatility. Stroke. 2012;43:2631–2636. doi: 10.1161/STROKEAHA.112.655837. [DOI] [PubMed] [Google Scholar]
- 67.Wilson M.H. Monro-Kellie 2.0: the dynamic vascular and venous pathophysiological components of intracranial pressure. J. Cereb. Blood Flow Metab. 2016;36:1338–1350. doi: 10.1177/0271678X16648711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yaneva-Sirakova T., Tarnovska-Kadreva R., Traykov L. Pulse pressure and mild cognitive impairment. J. Cardiovasc. Med. (Hagerstown) 2012;13:735–740. doi: 10.2459/JCM.0b013e328357ba78. [DOI] [PubMed] [Google Scholar]
- 69.Yaneva-Sirakova T., Tarnovska-Kadreva R., Traykov L. Pulse pressure and mild cognitive impairment. J. Cardiovasc. Med. (Hagerstown) 2012;13:735–740. doi: 10.2459/JCM.0b013e328357ba78. [DOI] [PubMed] [Google Scholar]
- 70.Zhang X., Medow J.E., Iskandar B.J., Wang F., Shokoueinejad M., Koueik J., Webster J.G. Invasive and noninvasive means of measuring intracranial pressure: a review. Physiol. Meas. 2017;38:R143–R182. doi: 10.1088/1361-6579/aa7256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.