Abstract
Introduction
Transient ischemic attack (TIA) and minor ischemic stroke (IS) is associated with a increased risk of late life dementia. In this study we aim to study the extent to which the rates of hippocampal atrophy in TIA/IS differ from healthy controls, and how they are correlated to neuropsychological measurements.
Methods
TIA or minor stroke patients were tested with a neuropsychological battery including tests of executive function, and verbal and non-verbal memory at three time points out to 3 years. Annualized rates of hippocampal atrophy in TIA/IS patients were compared to controls. A linear-mixed regression model was used to assess the difference in rates of hippocampal atrophy after adjusting for time and demographic characteristics.
Results
TIA/IS patients demonstrated a higher hippocampal atrophy rate than healthy controls over a 3-year interval: the annual percentage change of the left hippocampal volume was 2.5% (78 mm3 per year (SD 60)) for TIA/IS patients compared to 0.9% (29 mm3 per year (SD 32)) for controls (p < 0.01); and the annual percentage change of the right hippocampal volume was 2.5% (80 mm3 per year (SD 46)) for TIA/IS patients compared to 0.5% (17 mm3 per year (SD 33)) for controls (P < 0.01). Patients with higher annual hippocampal atrophy were more likely to report higher TMT B times, but lower ROC total score, lower California Verbal Learning Test-II total recall, and lower ROC Figure recall scores longitudinally.
Conclusion
TIA/IS patients experience a higher rate of hippocampal atrophy independent of TIA/IS recurrence that are associated with changes in episodic memory and executive function over 3 years.
Keywords: Stroke, MRI, Cognition, Hippocampus, Atrophy
Abbreviations
- AD
Alzheimer's disease
- ADNI
Alzheimer's disease neuroimaging initiative
- BMI
body mass index
- CATCH
CT and MRI in the Triage of TIA and minor cerebrovascular events to identify High risk patients
- CVLT
Californian Verbal Learning Test
- DS
digit symbol coding
- FLAIR
fluid attenuated inversion recovery
- FOV
field of view
- IS
ischemic Stroke
- ICV
intracranial volume
- MRI
magnetic resonance imaging
- NIFTI
neuroimaging informatic technology initiative format
- ROI
region of Interest
- TE
echo time
- TI
inversion time
- TIA
transient ischemic attack
- TR
repetition time
- TMTA/B
trail Making Test A and B
- WM
white matter
- WMH
white matter hyper intensities
Background and purpose
In the absence of cure, prevention has been emphasized as a key element to counteract the dementia epidemic [1]. Brain atrophy is a downstream effect of neurodegeneration and can be detected in early and even pre-symptomatic stages of the disease. Patients presenting with transient ischemic attack (TIA) and minor ischemic stroke (IS) frequently demonstrate mild cognitive deficits, and epidemiological data identifies that TIA/IS patients have a 4–5 × fold increase in the risk of dementia [2]. Therefore, TIA/IS patients are a potential target for early modification of incipient disease processes that precede cognitive impairment through prevention or multi-domain approaches guided by biologically relevant markers.
Cross sectional measurements of brain atrophy patterns are included as core supporting features in many consensus diagnostic guidelines [3]. Longitudinal atrophy rates have the advantage in that they may indicate progression of disease; individuals can be used as internal controls, which may be important when using such an outcome measure in clinical trials determining therapeutic response. We have previously reported that whole brain atrophy rates are higher in TIA and minor stroke patients than healthy controls [4].
The study has two broad aims: (1) to determine whether a validated and robust automated multi atlas-based segmentation method [5] for measuring hippocampal rates of atrophy over three years are greater in TIA/IS than healthy controls, and (2) determine whether increased hippocampal atrophy rates are associated with the decline in cognitive tests assessing verbal and visual memory, and executive function over three years.
Methods
Study population
Patients were recruited from the Extended-CATCH (CT And MRI in the Triage of TIA and minor Cerebrovascular events to identify High risk patients) study at the Foothills Medical center or Calgary Stroke Prevention Clinic from March 2009 to December 2012, while healthy controls were drawn from the Alzheimer's Disease Neuroimaging Initiative (ADNI) study database, (adni.loni.usc.edu), made permissible by the approval of ADNI collaborators. TIA was defined clinically; patients were eligible if they had experienced disturbance of speech and motor function that lasted longer than five minutes but less than 24 h. Minor stroke was defined as persistent focal neurological deficit lasting greater than 24 h with objective severity determined by an NIHSS score of less than four. Demographic and relevant medical information was collected, which included vascular risk factors such as blood pressure, body mass index (BMI), history of hypertension (determined by past medical history or concurrent anti-hypertensive treatment), and diabetes.
Inclusion criteria for Extended CATCH TIA/minor stroke cohort included: (1) age 50–80 years; (2) established vascular risk factors; and (3) the acquisition of serial brain MRI scans at baseline and three years (+/- six months). Exclusion criteria for Extended CATCH TIA/minor stroke cohort included: (1) dementia as defined by the National Institute of Aging-Alzheimer Association Criteria [6]; (2) other central nervous system diseases (e.g., MS), alcoholism, substance abuse; (3) comorbidities that could significantly interfere with cognitive performance and functional outcome (e.g., recent coronary bypass surgery, comorbidities), (4) English as a second language, and (5) the inability to complete neuropsychological testing. The University of Calgary Conjoint Health Research Ethics Board formally approved the study, and all patients provided written informed consent prior to participation.
ADNI inclusion criteria for healthy controls included a Mini Mental State Examination score between 24 and 30; a Clinical Dementia Rating of 0, no indication of depression, Mild Cognitive Impairment, or dementia, cognitively normal; low vascular risk with modified Hachinski score ≤ 4; Geriatric Depression Scale < 6, and normal memory function.
Clinical data collection
At study entry, baseline evaluation included clinical review, fasting cholesterol, glucose, and renal function. Blood pressure (BP) measurement was recorded from average of three sitting BPs at baseline and each subsequent visit.
Image acquisition
Extended CATCH TIA/minor stroke cohort
To evaluate whole brain atrophy rates, TIA/IS patients underwent a T1-weighted volumetric acquisition using a 3T scanner (Signa NV/i or Discovery 750; General Electric Healthcare, Waukesha, WI). High-resolution T1 images were acquired at baseline (within 48 h of symptoms) and at follow-up (TE/TR= 2.73 ms/7.0 ms, flip angle = 8°, TI=650 ms, acquisition matrix = 256 × 256). 3D FLAIR (TE/TR/flip angle 140 ms/ 9000 ms/ 90°, acquisition matrix = 256 × 256) were acquired at the same time points.
The segmentation of white matter hyperintensities (WMHs) volume (corrected for total intracranial volume) were calculated using Cerebra-WML software, a semi-automated software measured the volume (ml) of white matter lesions segmentation is based on the global threshold and contrast between the different anatomical regions of the brain as previously described [4], following international recommendations for the reporting, image acquisition and analysis of small vessel disease [7].
ADNI healthy controls
ADNI healthy controls were imaged using the standardized ADNI protocol (adni.loni.usc.edu/methods/documents/MRI-protocols). Initial inspection of the ADN1-and ADNI GO database revealed 417 healthy controls, and then randomly identified 41 healthy controls that met the age inclusion criteria, and had undergone 3T MRI at baseline, and follow up at three years ± 6 months (N = 183). Subjects were not age matched as the average age was 75 and there were too few ADNI controls to match with the Ex-CATCH subjects who were on average much younger. Controls were arranged in the ascending order by their research ID and a random number generator was used to select 1 in 4 control subjects. As a result, 41 ADNI subjects were identified and included in the study. 3T MRI data was download from the ADNI website and included MRIs obtained on instruments from three scanner vendors (Siemens Medical Solutions, Phillips, and General Electric Healthcare). MR protocols included the acquisition of sagittal high-resolution volumetric T1-weighted, inversion recovery prepared, structural images, and 3D FLAIR. MPRAGE (or vendor equivalent) high resolution T1 sequences were acquired (TE=min full echo, TR=2300, TI=900 ms, acquisition matrix = 256 × 256 @1 × 1 × 1 mm)) and 3D FLAIR (Effective TE=119, TR=4800, T1=1650, acquisition matrix=256 × 256 @ 1.2 × 1 × 1 mm).
Image analysis
Measurement of baseline brain, cortical gray matter and white matter volume
SIENAX (Structural Imaging Evaluation Using Normalization of Atrophy, Cross-sectional) was used to measure the brain volume, normalized for head size, cortical gray and white matter volume. Image analysis using SIENAX consists of four main steps: brain extraction, registration to MNI152 standard template, standard-space masking, tissue-type segmentation and calculation of total brain volume.
Image quality assurance and analysis
Quality assurance was performed to detect image distortion to assess the adequacy of key image properties including image uniformity, image contrast and signal-to-noise. Image quality was graded according to neck and head movement, quality of registration, and intensity inhomogeneity.
The FSL BET algorithm was first applied to all T1 MR datasets to remove the skull (FSL, FMRIB, Oxford, UK). All subjects with longitudinal data available had his/her follow-up T1 MRIs at three years linearly registered to the corresponding baseline MRI (FSL's “FLIRT” 6 ° of freedom registration algorithm). Next, to reduce bias between baseline and follow-up MRIs, we computed halfway forward and backward transformations for each image pair, using the FLIRT “avscale” algorithm. These intermediate transformations represented half the distance between a subject's baseline and follow-up MRI. The forward and backward intermediate transformations were then applied to the baseline and follow-up MRIs, respectively to reconstruct an intermediate image for each time point. This step ensured that all follow-up scans were linearly aligned.
Hippocampal volume was computed using the Sunny Brook Hippocampal Volumetry (SBHV) tool, which is a fully automated, multi-atlas and registration-based segmentation technique [5]. The SBHV tool compares a given MRI to a series of MRI templates (library) with manually traced hippocampal labels, and based on an image similarity metric, selects the optimal templates/labels for propagation to the individual T1 image. This technique uses rigorous criteria for developing the atlas-based segmentation by considering several minimum requirements. These included a common pipeline for label generation. A single expert operator (SMN) labeled all template MRIs for consistency and according to standard hippocampal segmentation protocols. These images and corresponding labels are then nonlinearly registered to the individual T1 MRI in intermediate space and fused using a voting strategy. SBHV has been previously validated using the ADNI dataset, and achieved high accuracy compared to manual labels in healthy elders and persons with dementia [5].
Neuropsychological assessment
A modified version of the Canadian Stroke Network and National Institute on Neurological Disorders and Stroke Vascular Cognitive Impairment battery was used [8] 90-days post ictus, and then at three years. Specifically, the tests used were: Trail Making Test (Parts A and B), WAIS-III Digit Symbol Coding, Controlled Oral Word Association Task (COWAT), Boston Naming Test second edition – short form, California Verbal Learning Test – II (CVLT), Rey Osterrieth Complex Figure Task, Boston Diagnostic Aphasia Examination third edition – Complex Ideational Material subtest, and CLOX1 clock drawing task. The cognitive tests were measured at baseline, year 1, and year 3 follow-ups.
Statistical analysis
Sample characteristics at baseline were described using descriptive statistics and frequency distributions. Differences between TIA/IS patients and ADNI controls were compared using independent t-test or Wilcoxon rank-sum test (if data were skewed) and Chi-square (or Fisher's exact if the number of cells was < 5) tests. Our regression analysis aimed to determine whether Extended CATCH patients had increased hippocampal atrophy rates. A linear mixed-effects (LME) regression model with randomly varying intercepts that allow for different variances between TIA and control groups was employed to compare atrophy rates between Extended-CATCH and ADNI data. The model incorporated potential confounders as fixed factors, which included age (years), sex (female), time since the first MRI (years), brain volume, and vascular risk factors including history of hypertension, WMH volume (mL) and BMI. Moreover, in order to examine if annualized hippocampal atrophy rate is associated with cognitive functioning (defined by the selected cognitive tests) changes over time, LME models with randomly varying intercepts was used, with consideration of years at cognitive measures (baseline, year 1, and year 3), age (years), sex (female), brain volume, baseline hippocampal volume, and vascular risk factors including history of hypertension, white-matter hyperintensity volume (mL) and BMI. These analyses were conducted in STATA 13.0.
Results
Patient and ADNI control subject characteristics
A total of 90 patients were recruited to the Extended-CATCH study of which 77 had two serial MRI scans (baseline and at approximately three years). These TIA/IS participants were compared to 41 ADNI healthy controls randomly selected according to the inclusion criteria of the study. TIA/IS and healthy volunteer demographics and vascular risk factor data are summarized in Table 1. TIA/IS on average were significantly younger than the healthy controls (P < 0.01), proportionally had more males (P < 0.01), and diabetes (P = 0.01). Neuropsychological testing was performed for the patient group on average 111 ± 24 days post TIA/IS and then after 3 years (mean 1130 ± 43 days). During the study period none of the Extended-CATCH patients had documented recurrent stroke or TIA.
Table 1.
Demographic, vascular risk factors, and hippocampal atrophy rates for TIA/IS patients and healthy controls (SD, standard deviation).
| Characteristics | Patient Group | Healthy Volunteer Group | P | |
|---|---|---|---|---|
| Number of subjects, n | 77 | 41 | ||
| Age, years (mean ± SD) | 65.0 ± 12.4 | 75.0 ± 5.7 | <0.01 | |
| Gender, males (%) | 62 (80.5%) | 18 (43.9%) | <0.01 | |
| Hypertension, n (%) | 39 (50.7%) | 21 (51.2%) | 0.95 | |
| Diabetes, n (%) | 12 (15.6%) | 0 (0%) | 0.01 | |
| BMI (kg/m2) (mean ± SD) | 27.0 ± 4.0 | 26.0 ± 4.3 | 0.11 | |
| WMH*1000 (mm3, mean ± SD) | 10.7 ± 9.2 | 3.2 ± 1.9 | <0.01 | |
| Baseline volume of left hippocampus (mm3, mean ± SD) | 3205.4 ± 420.0 | 3144.8 ± 416.4 | 0.31 | |
| Baseline volume of right hippocampus (mm3, mean ± SD) | 3341.9 ± 460.5 | 3240.1 ± 414.2 | 0.18 | |
| Baseline volume of total hippocampus (mm3, mean ± SD) | 6547.2 ± 855.2 | 6384.9 ± 809.0 | 0.17 | |
| Total (mean ± SD) | Absolute Annual Volumetric change (mm3) | 158.4 ± 86.9 | 45.4 ± 54.9 | <0.01 |
| Relative change annually | 4.9% ± 2.7% | 1.4% ± 1.7% | <0.01 | |
| Left (mean ± SD) | Absolute Annual Volumetric change (mm3) | 78.2 ± 60.2 | 28.6 ± 31.5 | <0.01 |
| Relative change annually | 2.5% ± 1.8% | 0.9% ± 1.0% | <0.01 | |
| Right (mean ± SD) | Absolute Annual Volumetric change (mm3) | 80.2 ± 46.2 | 16.8 ± 32.5 | <0.01 |
| Relative change annually | 2.5% ± 1.5% | 0.5% ± 1.0% | <0.01 |
Hippocampal rates of atrophy
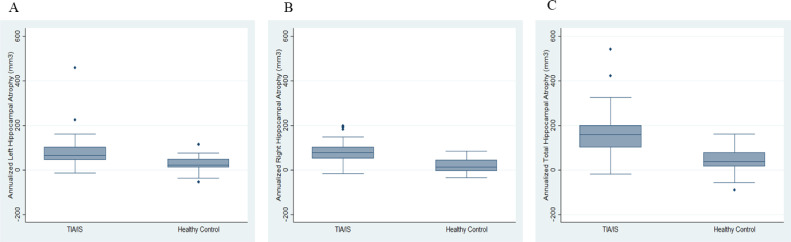
TIA/IS patients demonstrated higher annualized atrophy rates for both the left and right hippocampi compared to healthy controls over a 3-year interval (Fig. 1). The annual percentage change of the left hippocampal volume was 2.5% (78 mm3 per year (SD = 60) for TIA/IS patients compared to 0.9% (28 mm3 per year (SD =32)) for healthy controls was statistically different (P < 0.01); and the annual percentage change of the right hippocampal volume was 2.5% (80 mm3 per year (SD = 46)) for stroke/TIA patients compared to 0.5% (17 mm3 per year (SD =33) for healthy controls was statistically different (P < 0.01) . The linear mixed effect model showed that the change of total hippocampal volume over time were different between TIA/IS group and volunteer groups, after adjusting for age, sex, WMH, brain volume, BMI, and hypertension (Table 2). Specifically, on average at Year 3 the total hippocampal volume for the TIA/minor stroke group was 329 mm3 lower with 95% confidence interval (CI) = [−679, 20], compared with the total hippocampal volume for the healthy volunteer group.
Fig. 1.
Box plot showing annualized hippocampal atrophy (A-left; B-right; C-total in TIA/minor stroke patients and healthy controls.
Table 2.
A linear mixed effects model comparing longitudinal change in total hippocampal volume (HV) between TIA/IS patients and ADNI healthy controls, adjusting for demographic characteristics and vascular risk factors.
| Left HV change (mm3) | Right HV change (mm3) | Total HV change (mm3) | ||||
|---|---|---|---|---|---|---|
| Effect | Estimate [95% CI] | p values | Estimate [95% CI] | p values | Estimate [95% CI] | p values |
| TIA/IS vs control | −17.9 [−194.9, 159.1] | 0.84 | 23.9 [−165.0, 212.8] | 0.80 | 6.2 [−343.8, 356.2] | 0.972 |
| Years from the first scan | −28.7 [−38.1, −19.2] | <0.01 | −16.8 [−26.5, −7.2] | <0.01 | −45.5 [−61.8, −29.2] | <0.001 |
| TIA * years from the first scan | −49.3 [−65.7, −33.0] | <0.01 | −62.5 [−76.6, −48.4] | <0.01 | −111.8 [−137.2, −86.5] | <0.001 |
| Age | −7.8 [−15.3, −0.3] | 0.04 | −7.3 [−15.4, 0.7] | 0.07 | −15.1 [−29.9, −0.3] | 0.047 |
| Female vs male | −179.8 [−329.0, −30.6] | 0.02 | −146.8 [−306.6, 13.0] | 0.07 | −326.2 [−622.4, −30.0] | 0.031 |
| WMH volume (mm3) | −12.7 [−21.6, - 3.7] | <0.01 | −12.6 [−22.2, −3.1] | 0.01 | −25.2 [−42.9, −7.5] | 0.005 |
| Brain volume (mm3) | 0.9 [−0.1, 1.9] | 0.19 | 1.2 [−0.1, 2.2] | 0.14 | 2.1 [0.1, 4.1] | 0.042 |
| BMI | 10.8 [−5.3, 26.9] | 0.19 | 16.8 [−0.5, 34.0] | 0.06 | 27.6 [−4.4, 59.6] | 0.091 |
| Hypertension (Yes vs no) | −87.6 [−218.3, 43.2] | 0.01 | −101.7 [−241.5, 38.1] | 0.15 | −189.1 [−448.3, 70.2] | 0.153 |
Neuropsychological correlates of atrophy
The relationship between neuropsychological test scores and atrophy rates over 3 years are shown in Table 3. Patients with higher left annual hippocampal atrophy were more likely to have lower ROC total score, California Verbal Learning Test-II (CVLT) total recall, and ROC Figure recall scores longitudinally, after adjusting for age, sex, hippocampal volume at baseline, brain volume, hypertension, BMI, and WMH. Specifically, for every 1 mm3 increase of the annual left hippocampal atrophy, the estimated average of ROC Figure Recall score would decrease 0.04 units (95% CI −0.05 to −0.02]. We found that higher left and right annual hippocampal atrophy were associated with greater increases in Trail Making times, indicting worse performance, after adjusting for age, sex, hippocampal volume at baseline, brain volume, hypertension, BMI, and WMH. For example, for every 1 mm3 each increase of the annual left hippocampal atrophy was associated with an increase in Trail Making time of 0.23 s (95% CI 0.13 to 0.33).
Table 3.
Rates of hippocampal atrophy (mm3 per annum) is associated with a decline in neuropsychological tests for verbal and visual memory, and executive function.
| Trail Making B | ROC Total Score | CVLT Delayed Recall | ROC Figure Recall | |||||
|---|---|---|---|---|---|---|---|---|
| Effects | Estimate (95% CI) | p values | Estimate (95% CI) | p values | Estimate (95% CI) | p values | Estimate (95% CI) | p values |
| Left annual atrophy |
0.23 [0.13, 0.33] |
<0.01 |
−0.01 [−0.02, −0.003] |
0.01 |
−0.04 [−0.08, −0.01] |
0.02 |
−0.04 [−0.05, −0.02] |
<0.01 |
| Right annual atrophy | 0.16 [0.01, 0.32] |
0.04 |
−0.01 [−0.02, 0.003] |
0.14 |
−0.04 [−0.09, 0.01] |
0.11 |
−0.03 [−0.05, 0.001] |
0.06 |
| Total annual atrophy | 0.16 [0.08, 0.23] |
<0.01 |
−0.01 [−0.02, −0.002] |
0.01 |
−0.03 [−0.05, −0.01] |
0.01 |
−0.02 [−0.04, −0.01] |
<0.01 |
| Trail Making B | ROC Total Score | CVLT Delayed Recall | ROC Figure Recall | |||||
| Effects | Estimate (95% CI) | p values | Estimate (95% CI) | p values | Estimate (95% CI) | p values | Estimate (95% CI) | p values |
| Left annual atrophy |
0.23 [0.13, 0.33] |
<0.01 |
−0.01 [−0.02, −0.003] |
0.01 |
−0.04 [−0.08, −0.01] |
0.02 |
−0.04 [−0.05, −0.02] |
<0.01 |
| Right annual atrophy | 0.16 [0.01, 0.32] |
0.04 |
−0.01 [−0.02, 0.003] |
0.14 |
−0.04 [−0.09, 0.01] |
0.11 |
−0.03 [−0.05, 0.001] |
0.06 |
| Total annual atrophy | 0.16 [0.08, 0.23] |
<0.01 |
−0.01 [−0.02, −0.002] |
0.01 |
−0.03 [−0.05, −0.01] |
0.01 |
−0.02 [−0.04, −0.01] |
<0.01 |
Conclusions
In this study, our data show that patients with TIA/IS had higher rates of hippocampal atrophy over three years compared with healthy controls. In patients with TIA/IS, we found that hippocampal rates of atrophy predicted the change neuropsychological cognitive performance affecting episodic memory, specifically verbal and visual memory, and executive function over 3 years. Moreover, progression of hippocampal atrophy in the left and right hippocampus predicted further decline in neuropsychological variables, and were associated with a change in the delayed responses on verbal and visual memory tests, and an increase in timed response on the Trail Making Test B test (executive function). We were able to demonstrate a relationship with hypertension and BMI (but statistically significant) with increased rates of left hippocampal atrophy, but not with diabetes. To our knowledge, this study is the first to identify increased hippocampal atrophy rates in non-demented patients following TIA and minor stroke, and concur with a post mortem study which showed reduced hippocampal neuronal density measurements in post stroke dementia [9].
We found a relationship between WMH, a surrogate of small vessel cerebrovascular disease, and symmetrical hippocampal atrophy. Hippocampal atrophy in small vessel disease is well described in patients with and without evidence of amyloid plaques [10], and up to 50% of post-stroke patients will develop dementia [11]. We have demonstrated that there was a significant change in hippocampal volume over 3 years that was temporally related to the incident stroke, perhaps implicating both humoral or inflammatory mechanisms [12], as well as linking stroke with incipient neurodegenerative disease [13]. This observation is in line with current evidence that both the burden of WMH and amyloid positively independently contribute to disproportionate hippocampal atrophy in non-demented individuals [14]. A previous study investigating subfield hippocampal atrophy showed similar patterns of atrophy in cerebrovascular disease and early AD [15]. Our data also supports other studies that have concluded that neuronal cell loss exemplified by hippocampal atrophy is specific to cognitive decline independent of etiology (i.e., AD versus vascular disease) [16]. Future studies such as the PREVENT Study, will aim to explore temporal patterns of disease using markers of early neurodegenerative and vascular disease (structural and functional MRI, fluid biomarkers including amyloid abeta 1–42 and tau) with phenotypic expression of cognitive profiles in minor stroke and transient ischemic attack patients [17].
This study has several limitations. The use of ADNI healthy controls is a potential source of bias; however, there is precedence for their use in similar comparison studies [18,19]. We attempted to mitigate selection bias by randomly selecting ADNI healthy controls. Additionally, we were not able to adequately match the minor stroke/TIA group and ADNI healthy controls for age and gender because ADNI healthy controls are considerably older than those patients in the Extended-CATCH cohort had a propensity of females. Therefore, age matching Extended-CATCH patients ADNI healthy controls was not possible considering the CATCH cohort were much younger and we were also constrained by the availability of serial MRIs that were acquired at similar times apart. For this reason, the ADNI healthy controls were on average 10-years older than the TIA/IS group. Needless to say, despite the ADNI healthy controls being older, their baseline hippocampal volume was not significantly different from the TIA/IS cohort. This raises the interesting phenomenon that the hippocampal atrophy rate possibly increased after stroke. Additionally, the percentage annualized change in hippocampal volume in our ADNI healthy control group conforms with previously published data [20].
In summary, this study demonstrates that patients presenting with TIA/IS have higher rates of hippocampal atrophy than healthy controls over a 3-year period, allowing for adjustments of age, time, and vascular risk factors. We also demonstrated that hippocampal atrophy was positively associated with a decline in cognition, most profoundly observed in visual memory, following TIA/IS. Future work should investigate the processes by which distributed and specific tissue loss may occur in regions beyond the infarction, which may offer new targets for drug development. The early detection and precise measurement of hippocampal atrophy will inform future clinical dementia prevention trial design, including sample size calculations which have traditionally depended on cognitive outcome measures.
Disclosures
None
Acknowledgments
The CATCH study and the baseline imaging was funded by the Canadian Institute of Health Research Operating Grant.
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
References
- 1.Prince M.A.E, Guerchet M., et al. World Alzheimer report 2014. Dementia and risk reduction: an analysis of protective and modifiable risk factors.
- 2.Pendlebury S.T., Rothwell P.M. Risk of recurrent stroke, other vascular events and dementia after transient ischaemic attack and stroke. Cerebrovasc. Dis. 2009;27(Suppl 3):1–11. doi: 10.1159/000209260. [DOI] [PubMed] [Google Scholar]
- 3.Cash D.M., Rohrer J.D., Ryan N.S., Ourselin S., Fox N.C. Imaging endpoints for clinical trials in Alzheimer's disease. Alzheimers Res. Ther. 2014;6:87. doi: 10.1186/s13195-014-0087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munir M., Ursenbach J., Reid M., Gupta Sah R., Wang M., Sitaram A., et al. Longitudinal brain atrophy rates in transient ischemic attack and minor ischemic stroke patients and cognitive profiles. Front. Neurol. 2019;10:18. doi: 10.3389/fneur.2019.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nestor S.M., Gibson E., Gao F.Q., Kiss A., Black S.E. A direct morphometric comparison of five labeling protocols for multi-atlas driven automatic segmentation of the hippocampus in Alzheimer's disease. Neuroimage. 2013;66:50–70. doi: 10.1016/j.neuroimage.2012.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jack C.R., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B., et al. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimer's Dement. J. Alzheimer's Assoc. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wardlaw J.M., Smith E.E., Biessels G.J., Cordonnier C., Fazekas F., Frayne R., et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hachinski V., Iadecola C., Petersen R.C., Breteler M.M., Nyenhuis D.L., Black S.E., et al. National institute of neurological disorders and stroke-Canadian stroke network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 9.Gemmell E., Bosomworth H., Allan L., Hall R., Khundakar A., Oakley A.E., et al. Hippocampal neuronal atrophy and cognitive function in delayed poststroke and aging-related dementias. Stroke. 2012;43:808–814. doi: 10.1161/STROKEAHA.111.636498. [DOI] [PubMed] [Google Scholar]
- 10.Lee J.H., Kim S.H., Kim G.H., Seo S.W., Park H.K., Oh S.J., et al. Identification of pure subcortical vascular dementia using 11c-pittsburgh compound b. Neurology. 2011;77:18–25. doi: 10.1212/WNL.0b013e318221acee. [DOI] [PubMed] [Google Scholar]
- 11.Pendlebury S.T., Rothwell P.M. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 12.Shen Z., Bao X., Wang R. Clinical pet imaging of microglial activation: implications for microglial therapeutics in Alzheimer's disease. Front. Aging Neurosci. 2018;10:314. doi: 10.3389/fnagi.2018.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akinyemi R.O., Allan L.M., Oakley A., Kalaria R.N. Hippocampal neurodegenerative pathology in post-stroke dementia compared to other dementias and aging controls. Front. Neurosci. 2017;11 doi: 10.3389/fnins.2017.00717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiford C.M., Manning E.N., Bartlett J.W., Cash D.M., Malone I.B., Ridgway G.R., et al. White matter hyperintensities are associated with disproportionate progressive hippocampal atrophy. Hippocampus. 2017;27:249–262. doi: 10.1002/hipo.22690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selnes P., Grambaite R., Rincon M., Bjornerud A., Gjerstad L., Hessen E., et al. Hippocampal complex atrophy in post stroke and mild cognitive impairment. J. Cereb. Blood Flow Metab. 2015;35:1729–1737. doi: 10.1038/jcbfm.2015.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arvanitakis Z., Capuano A.W., Leurgans S.E., Bennett D.A., Schneider J.A. Relation of cerebral vessel disease to Alzheimer's disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol. 2016;15:934–943. doi: 10.1016/S1474-4422(16)30029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tariq S., d'Esterre C.D., Sajobi T.T., Smith E.E., Longman R.S., Frayne R., et al. A longitudinal magnetic resonance imaging study of neurodegenerative and small vessel disease, and clinical cognitive trajectories in non-demented patients with transient ischemic attack: the prevent study. BMC Geriatr. 2018;18:163. doi: 10.1186/s12877-018-0858-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickerson B.C., Fenstermacher E., Salat D.H., Wolk D.A., Maguire R.P., Desikan R., et al. Detection of cortical thickness correlates of cognitive performance: reliability across MRI scan sessions, scanners, and field strengths. Neuroimage. 2008;39:10–18. doi: 10.1016/j.neuroimage.2007.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scorzin J.E., Kaaden S., Quesada C.M., Muller C.A., Fimmers R., Urbach H., et al. Volume determination of amygdala and hippocampus at 1.5 and 3.0t MRI in temporal lobe epilepsy. Epilepsy Res. 2008;82:29–37. doi: 10.1016/j.eplepsyres.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Henneman W.J., Sluimer J.D., Barnes J., van de Flier W.M., Sluimer I.C., Fox N.C., Scheltens P., Vrenken H., Barkhoff M.D. Hippocampal atrohpy rates in Alzheimer disease. Neurology. 2009;72:999–1007. doi: 10.1212/01.wnl.0000344568.09360.31. [DOI] [PMC free article] [PubMed] [Google Scholar]