Highlights
-
•
RVCL-S is a monogenetic small vessel disease caused by TREX1 mutations.
-
•
RVCL-S should be suspected in middle-aged adults presenting with retinopathy and neurologic deficits.
-
•
Besides neuro-ophthalmological features the syndrome involves internal organ dysfunction.
-
•
Early genetic testing is recommended to prevent systemic complications.
-
•
RVCL-S serves as a unique hereditary neuro-endothelial model for sporadic cerebral small vessel disease (cSVD) and dementia.
Keywords: RVCL-S, Hereditary, Retinopathy, Cerebral small vessel disease
Abbreviations: cSVD, cerebral small vessel disease; CT, computed tomography; MRI, magnetic resonance imaging; OCT, optical coherence tomography; pRNFL, peripapillary retinal nerve fiber layer; RVCL-S, retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations; TMV, total macular volume
Abstract
Retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations (RVCL-S) is a rare, underrecognized, systemic small vessel disease caused by heterozygous C-terminal truncating TREX1 mutations. The disease is characterized by vascular retinopathy, focal neurological complaints, cognitive decline and a wide range of systemic manifestations, including Raynaud's phenomenon, anemia and liver and kidney disease. Eventually, RVCL-S leads to premature death. The underlying pathological finding in RVCL-S is a nonatherosclerotic, amyloid-negative angiopathy involving small arteries and capillaries. However, the exact mechanisms by which the truncated TREX1 protein causes angiopathy remains unknown. Timely recognition of this disease is important to slow down and treat complications of the disorder, but also to prevent unnecessary (invasive) diagnostic or therapeutic procedures. As we move forward, translational research combining basic science advances and clinical findings as well as studies focusing on natural history following RVCL-S patients at different disease stages, will be critical to help elucidate RVCL-S pathophysiology. These studies will also provide the tools to identify appropriate biomarkers and therapeutic agent options for RVCL-S patients.
Introduction
Retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations (RVCL-S) is a rare, autosomal dominant vasculopathy caused by mutations in TREX1. Truncating mutations located in the carboxyl terminus (C-terminus) of three-prime repair exonuclease 1 (TREX1) are pathognomonic for RVCL-S.[1] RVCL-S was first recognized as a hereditary small vessel disease characterized by brain abnormalities and vascular retinopathy.[2] In earlier reports the following terms were used to describe RVCL-S; cerebroretinal vasculopathy (CRV),[2] hereditary vascular retinopathy (HVR),[3,4] Hereditary systemic angiopathy (HSA),[5] hereditary endotheliopathy, retinopathy, nephropathy and stroke (HERNS) [6] and retinal vasculopathy with cerebral leukodystrophy (RVCL). It was recognized that along with the ophthalmological and neurological features of RVCL-S including progressive blindness and focal neurological complaints and vascular dementia, patients develop systemic manifestations, including Raynaud's phenomenon, anemia and liver and kidney disease [7]. The classic onset of the disease is between ages 30 and 50 years. Ultimately, RVCL-S leads to premature death, often due to pneumonia in the setting of general debilitation.
RVCL-S seems an exceedingly rare disorder, with fewer than 30 families described worldwide. However, with already three families originating from the Netherlands, a small country, this number is likely an underestimation. Patients are often misdiagnosed with other conditions (e.g., neurodegenerative disorders, vascular dementia, brain tumor, multiple sclerosis, hypertensive or diabetic retinopathy). With increasing awareness worldwide and new genetic screening techniques, more cases and families are likely to be identified. This review focuses on recent basic science and clinical advances that increase our understanding of RVCL-S as well as recent efforts to identify biomarkers for the disease.
Pathophysiology
Genetics
RVCL-S is caused by heterozygous frameshift or nonsense mutations in the C-terminal of the TREX1 gene (3′−5′ repair exonuclease 1) located on chromosome 3 [1,8]. Up to date, there are less than 15 pathogenic variants of TREX1 known to cause RVCL-S. The frameshift mutation V235Gfs*6 is the most frequently diagnosed pathogenic variant.
TREX1 is the most abundant mammalian 3′−5′ exonuclease with a high affinity for single strand DNA. It is responsible for the removal of nucleoside monophosphates from the 3′-ends of DNA [9,10]. TREX1 consists of a single exon and encodes a protein of 314 amino acids. The protein contains three exonucleases sequence motifs, Exo I, Exo II and Exo III and a hydrophobic c-terminal region containing a transmembrane domain. TREX1 is ubiquitously expressed in most tissues and cell types. The exonuclease domain is required for maintaining innate immune tolerance to cytosolic self-DNA by degrading substrates to prevent initiation of autoimmunity. Whereas the C-terminal domain is necessary for the localization to and interaction with the endoplasmic reticulum (ER) [1,9,11,12]. The pathogenic mutations causing RVCL-S lead to a truncated TREX1 protein. The subcellular localization of TREX1 is shifted, altering its interaction with the endoplasmic reticulum, while the exonuclease function of TREX1 remains unaffected.[11,12]
Biological effects of RVCL-S associated mutations
It remains unclear how this truncated TREX1 protein exactly causes disease. The underlying lesion of RVCL-S is a nonatherosclerotic, amyloid-negative angiopathy involving small arteries and capillaries, primarily in the retina and brain, but also in other organs.[1,7,8] Impaired in vivo endothelial function and elevated markers of chronic endothelial activation (e.g. von Willebrand factor and angiopoietin-2) and impaired cerebrovascular reactivity are found in patients as signs of small vessel pathology [13], [14], [15].
Furthermore, in morphologic studies signs of small vessel disease and endothelial dysfunction have been demonstrated. In patients with RVCL-S, thicker multilaminated basement membranes and fibrous thickening of small vessel walls were seen [8]. TREX1 was found to be expressed in a subset of microglia in the normal human brain, often in close proximity to the microvasculature. Interestingly, in ischemic lesions, the amount of TREX1-positive microglia was increased, indicating a possible role for TREX1 and microglia in vessel homeostasis and response to ischemic injury [16,17]. Moreover, TREX1 was found to be expressed in endothelial cells in the brain of a RVCL-S patient indicating a possible direct effect on endothelium function [17]. Additionally, RVCL-S transgenic knock-in mice show increased mortality, increased infarct volume with experimental stroke and evidence of abnormal vascular function [18].
Auto-inflammation also seems to be a possible disease mechanism. Both erythrocyte-sedimentation rate (ESR) and Fibrinogen levels were elevated in RVCL-S patients.[7] Immune activation in RVCL-S could be due to immunogenic free glycans release demonstrated in lymphoblasts of patients with RVCL-S.[11] This increase in free glycan release is most likely duo to altered oligosaccharyltransferase activity [11]. Furthermore, in a conditional knock-in mice model of RVCL-S (V235fs mutation), a distinct autoantibody profile was found compared with wild type mice [19].
Clinical features of RVCL-S
To date, less than 30 families with RVCL-S have been diagnosed worldwide in different ethnic groups. The disorder is often overlooked and misdiagnosed. In Table 1 a set of features are listed to alert physicians when to consider RVCL-S as a diagnosis. Documentation of a typical pathogenic TREX1 variant by genetic analysis is required to firmly establish the diagnosis.
Table 1.
Diagnosing and suspecting RVCL-S.
| Diagnostic criterium |
| Demonstration of a C-terminal frameshift mutation in TREX1 to genetically confirm the diagnosis. |
| Main features |
| Vascular retinopathy |
| Features of focal and/or global brain dysfunction associated on MRI with |
| punctate T2 hyperintense white matter lesions with nodular enhancement; and/or |
| larger T2 hyperintense white matter mass lesions with rim-enhancement, mass effect, and surrounding edema |
| Family history of autosomal dominant inheritance with middle-age onset of disease manifestations. |
| Supportive features |
| On CT focal white matter calcifications and/or on MRI nonenhancing punctate T2 hyperintense white matter lesions at an age that non-specific age-related white matter hyperintensities are infrequent |
| Microvascular liver disease |
| Microvascular kidney disease |
| Anemia consistent with blood loss and/or chronic disease |
| Microscopic gastro-intestinal bleeding |
| Subclinical hypothyroidism |
| Possibly associated features |
| Raynaud's phenomenon (typically mild) |
| Migraine with or without aura |
| Hypertension |
Adapted from Pelzer et al., 2018.7.
Clinical course and progression
RVCL-S is a progressive disorder, but the rate of progression varies greatly between patients. While symptoms may remain stable for long periods of time, progression may also occur precipitously. Visual impairment is frequently the presenting symptom, while cognitive impairment appears to have a later onset [7]. Neurologic symptoms may be mild until the age of 50, after which symptoms may progress rapidly during the last stage of the disease. Life expectancy in RVCL-S is decreased, with one study demonstrating an average age of death 53 years (standard deviation 9.6, range 32 to 72) [8]. Time from onset to death appears to vary greatly. While there are cases in which death occurs within 5 to 10 years after symptom onset, [20] there are also patients who survived for more than 25 years following the first symptoms. Moreover, this is complicated, as in known mutation carriers, signs and symptoms of the disease are more easily recognized creating a bias that gives the impression that there might be a longer time between onset and death for some patients.
Retinopathy
All RVCL-S patients appear to develop a symptomatic retinopathy, often leading to progressive blindness. While typically presenting around the age of 40, early signs of asymptomatic vascular retinopathy have been demonstrated in patients in their early twenties [3,8]. Therefore, ophthalmologists play an important role in recognizing this underdiagnosed condition.
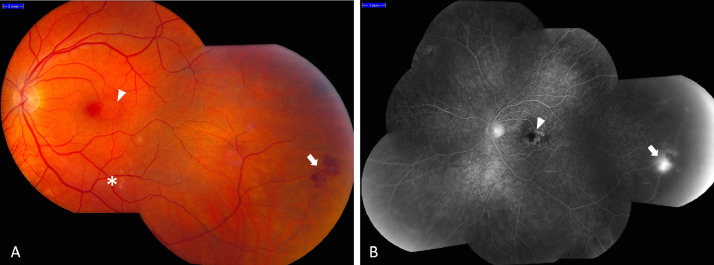
In the early stages, the vascular retinopathy in RVCL-S is characterized by telangiectasia, micro-aneurysms and cotton wool spots. Later, perifoveal capillary obliterations and neovascularizations may occur, predominantly affecting the posterior pole (Fig. 1) [8]. Fluorescein angiography often shows focal and extended areas of ischemia, capillary obliteration and microaneurysms, telangiectasias, neovascularization and fluorescein leaks. Complications of the retinopathy may occur as the disease progresses, such as secondary glaucoma and macula edema.
Fig. 1.
Fundus (fluorescein angiography) photograph of a middle aged RVCL-S patient with vascular retinopathy. A: the fundus photograph shows a micro-aneurysm (arrowhead), cotton-wool spots (asterisk) and an intraretinal hemorrhage with neovascularization (arrow). B: the fluorescein angiography shows capillary ischemia with an enlarged foveal avascular zone (arrowhead) and a neovascularization (arrow).
Neurologic manifestations and neuroimaging
Neurological manifestations
Among the primary manifestations of RVCL-S are symptoms of focal and global brain dysfunction, such as (sub)acute neurological deficits, cognitive decline, migraine and psychiatric disturbances. Focal neurological symptoms are described in 68% of symptomatic patients, increasing to 97% at time of death, and cognitive impairment is described in 56% of symptomatic patients, increasing to 75% at time of death [8].
Focal neurological symptoms can be caused by intracerebral mass lesions, called tumefactive lesions or pseudotumors, by ischemic events or specific RVCL-S lesions (see neuro-imaging) [8]. Ischemic episodes often present as classic lacunar syndromes (e.g. pure motor stroke, pure sensory stroke, dysarthria-clumsy hand syndrome, ataxic hemiparesis), but other lacunar syndromes are also observed. It can be difficult to distinguish ischemic events from nonischemic causes of focal neurological symptoms in RVCL-S.
The most frequently described complaints of global brain dysfunction and cognitive decline in RVCL-S are bradyphrenia, apathy, irritability, and complaints of memory and judgement [4,8]. The cognitive decline seems to be slowly progressive, with additional stepwise deterioration superimposed, similar to other hereditary small vessel disorders. Neuropsychologic testing usually shows deficits in multiple cognitive domains including working memory, executive function, and cognitive processing speed [8,21].
Other neurological complaints seen in RVCL-S are migraine and epilepsy. In cross-sectional studies, up to 42% of the patients suffered from migraine (with and without aura) and 17% suffered from epileptic seizures [7,8,12,22]. Migraine onset in the general population typically occurs in childhood or adolescence, while in patients with RVCL-S it often occurs later in adulthood (e.g. age 40 years), suggesting that migraine might occur as a consequence of vasculopathy.[7]
Furthermore, psychiatric disturbances are described frequently in RVCL-S patients. Many patients develop adjustment disorder or moderate depression, but major depression, psychosis, anxiety, and other psychiatric complaints have all been reported [8,12,22]. Apathy, defined as a primary loss of motivation, has been reported in clinical practice, and although apathy frequently occurs with depression, it may develop in the absence of depression [8,12,22].
Neuro-imaging
All abnormalities on CT- and MRI-imaging that have been described so far in RVCL-S patients are restricted to the white matter, sparing the cortex (Fig. 2) [8,21,23]. From an early age on, RVCL-S mutation carriers have more focal white matter lesions than non-mutation carriers of similar age. These lesions are non-specific, but remarkable for the patients relatively young age [24].
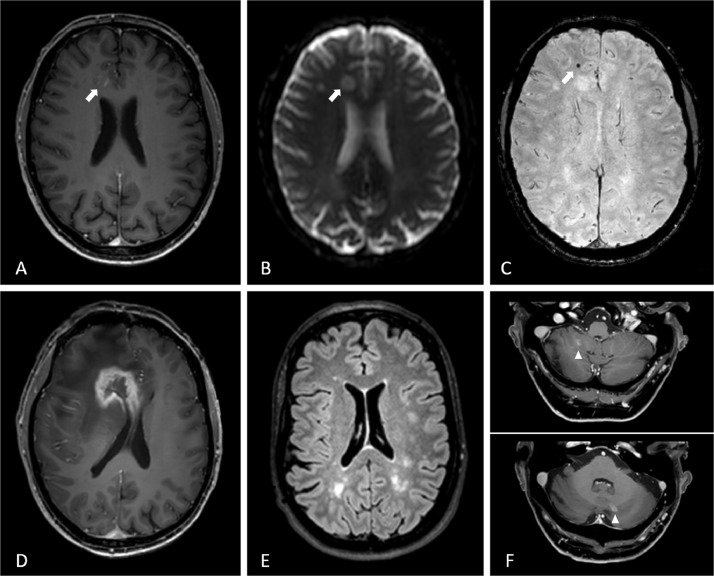
Fig. 2.
Typical RVCL-S lesions in patients on MR-imaging. A-C: 3D-T1-weighted gadolinium enhanced, diffusion weighted and susceptibility weighted images of a 47-year old male show a small lesion (arrow) with contrast enhancement (A), diffusion restriction (B) and a susceptibility artefact (C). D: four months later the same patient had developed a tumefactive lesion with ring contrast enhancement and surrounding edema with mass effect and midline shift. E: FLAIR image of a 44 year old woman with several white matter hyperintensities. F: 3D-T1-weighted gadolinium enhanced images of a 68-year old male with cerebellar lesions with contrast enhancement (arrowhead).
Additionally, almost all patients develop T2-hyperintense lesions with long-lasting nodular or rim enhancement after gadolinium contrast. These lesions are predominantly seen in the periventricular and deep white matter, and in the basal ganglia [8,[23], [24], [25]] Sometimes, they are associated with diffusion restriction. The presence of these contrast-enhanced lesions indicate a local disruption of the blood brain barrier (BBB). Typically, the lesions shrink over time, eventually leading to ischemic defects often with persistent central punctate contrast enhancement, perifocal T2 hyperintense gliosis and enlargement of adjacent ventricles [23].
The ring-enhancing lesions may progress into space occupying lesions, often surrounded by extensive edema, displacing adjacent structures and leading to sulcal effacement. These mass lesions are often referred to as pseudo-tumors, but tumefactive lesions might be a better characterization. Tumefactive lesions are hyperintense on T2-weighted images, hypointense on T1-weighted images, show ring enhancement with gadolinium contrast, and are mostly localized in the frontoparietal lobe. [8,23,25,26]. Mass lesions have been reported in up to 75% of RVCL-S patients, most often in later stages of disease [8,27]. Susceptibility artefacts are frequently seen in these lesions. As calcifications often occur in RVCL-S (as seen on CT images), not all susceptibility artefacts should be interpreted as microbleeds.
Patients with RVCL-S can also have (multiple) contrast enhancing cerebellar lesions, often bilaterally present and punctiform [23,24]. Cerebellar lesions seem to start to develop with more advanced disease, and often increase in number over the years. Progression in size with rim enhancement is very rare for cerebellar lesions [24].
Neuroimaging findings in RVCL-S have been mistaken for vasculitis, multiple sclerosis or neoplasms in the past, leading to unnecessary brain biopsies [8,22,28,29]. Although, some of the neuroimaging characteristics of RVCL-S may be observed in other small-vessel diseases, the pattern and evolution of the findings are typical for RVCL-S. Particularly, the long-term contrast enhancement with long-term diffusion restriction characterize this disease [23,24]. These findings, together with the clinical features, should alert radiologists to consider the diagnosis of RVCL-S.
Systemic features
A wide range of systemic features are part of the clinical spectrum of RVCL-S (Table 2, Fig. 3). Typically, the disease starts from the age of 20 with Raynaud's phenomenon and (pre-symptomatic) vascular retinopathy. From age of 35–40 onwards, patients may develop multiple internal organ disease, justifying regular screening [7].
Table 2.
Overview of imaging and pathology findings of RVCL-S.
| System | Imaging findings | Pathology findings |
|---|---|---|
| Eyes | ||
| Fundoscopy/fluorescein angiography:- Telangiectasia- Micro-aneurysms- Cotton wool spots- Perifoveal capillary obliterations- Neovascularization's- Fluorescein leaks- Ischemia Optical coherence tomography:- Peripapillary retinal nerve fiber layer thinning- Reduced total macular volume |
Scattered microinfarcts Thickened hyalinized retinal arterial walls Focal areas of disruption of the ganglion cell layer and inner nuclear layer |
|
| Brain | ||
| MRI:- White Matter Hyperintensities- T2 hyperintense lesions with long-lasting contrast enhancement- Tumefactive lesions surrounded by edema CT:- Calcifications |
Multiple (often confluent) foci of ischemic necrosis of white matter Vessel wall thickening, luminal stenosis and telangiectasias A modest chronic inflammatory cell infiltrate Focal calcifications and reactive astrocytosis Myelin loss |
|
| Kidney | ||
| – | Renal arteriolosclerosis Focal or diffuse glomerulosclerosis |
|
| Liver | ||
| Ultrasound:- Liver cirrhosis |
Nodular regenerative hyperplasia Micro and macro vesicular steatosis Periportal inflammation Bridging and portal fibrosis |
|
| Gastrointestinal | ||
| Gastroscopy:- Telangiectasia- Angiodysplasia Colonoscopy:- Blotchy, erythematous mucosa- Ulcerations- Telangiectasia- Angiodysplasia |
Telangiectasias Thin-walled vasculopathy Focal or diffuse (chronic) inflammation |
|
CT = computed tomography; MRI = magnetic resonance imaging.
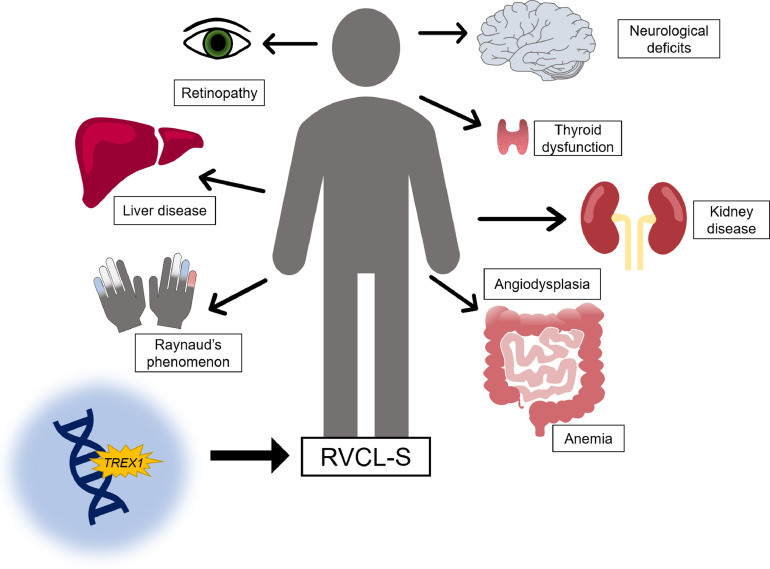
Fig. 3.
Schematic display of the main features of RVCL-S. RVCL-S is a systemic endotheliopathy caused by C-terminal truncating TREX1 mutations. The main features are vascular retinopathy and focal and global neurological deficits. In addition, a wide range of systemic manifestations can occur, most notably liver and kidney disease, anemia, thyroid dysfunction, gastro-intestinal angiodysplasia and Raynaud's phenomenon.
Microvascular renal disease occurs in approximately 30 to 50% of the patients [7,8]. Commonly, the renal involvement has a mild phenotype, however, it may progress to severe kidney disease, and occasionally fatal stage 4 kidney disease occurs.[8,12,20]
Evidence of liver dysfunction was found in 78% of RVCL-S patients [8]. In the majority of the cases, there were (mildly) elevated levels of alkaline phosphatase and gamma-glutamyltransferase.
Additionally, RVCL-S patients appear to suffer frequently from hypertension [7,8]. Raynaud's phenomenon is present in approximately 40% and is usually mild without causing ischemic injury [7,8]. Subclinical hypothyroidism was present in 37% of RVCL-S patients over age 40 [7]. Mild to moderate anemia occurs frequently, this may be due to microscopic gastrointestinal bleeding that occurs in RVCL-S [7,8]. There are some reports of avascular necrosis of the femoral head, cardiomyopathy and skin lesions also being part of the clinical spectrum [12,20,28].
Potential biomarkers for disease monitoring
Biomarker discovery is particularly important in RVCL-S as a tool to predict onset, track progression and measure therapeutic effectiveness. Several promising candidate markers have been identified.
Ophthalmological markers
While retinal imaging techniques could potentially serve as biomarkers for the retinopathy in RVCL-S, their use is most likely not limited to this. Since the retina is an extension of the brain and shares a common embryological origin with the central nervous system, there is an intense interest in using retinal imaging technology to diagnose and monitor neurological diseases. In vivo optical coherence tomography (OCT) imaging of the retina provides near histologic level resolution of the different retinal layers, including neurosensory tissue.
Recently, the potential of using OCT findings as early biomarker for RVCL-S was shown [30]. A decreased peripapillary retinal nerve fiber layer (pRNFL) thickness and total macular volume (TMV) was demonstrated in TREX1 mutation carriers compared with controls. The pRNFL contains unmyelinated axons of ganglion cells found in the ganglion cell layer, thus showing loss of intraocular axonal integrity. Additionally, thinning of the inner plexiform layer was found, where dendrites from inner nuclear layer neurons are located. Indicating an effect of RVCL-S on retinal neurons [30]. Notably, this decreased pRNFL and TMV were already present in 9 out of 12 eyes with normal funduscopic examination.[30] As OCT is a noninvasive, safe and precise diagnostic tool, OCT measurements may be useful as a (pre)clinical biomarker in RVCL-S.
Neuroradiological markers
In RVCL-S, white matter volumes declined linearly and declined faster in RVCL-S patients compared to controls [21]. Its role as a biomarker for disease progression deserves further investigating.
Cerebrovascular reactivity (CVR) is known as the cerebrovascular reserve capacity and is a marker for cerebrovascular health. It can be measured as change in cerebral blood flow (CBF) to a hypercapnic stimulus. CVR was decreased in both gray and white matter of RVCL-S patients compared with controls [15]. Interestingly, CVR in white matter was already lower in RVCL-S patients aged < 40 years [15].
Endothelial markers
Von Willebrand factor (VWF) is considered a reliable circulating marker of endothelial dysfunction, and its level is increased after endothelial damage and during acute phase responses. Another marker associated with endothelial damage or activation is angiopoietin-2. Elevated levels of VWF and angiopoietin-2 have been found in RVCL-S patients compared with healthy controls. Levels of VWF and angiopoietin-2 were also elevated in mutation carriers aged <40 years, indicating that they may serve as early biomarkers of disease activity [14]. Moreover, a correlation with endothelial markers and markers of kidney and liver disease and inflammation (i.e., systemic symptoms of RVCL-S) was demonstrated [14].
Clinical recommendations
Diagnosis
The diagnosis of RVCL-S should be suspected when middle-aged adults present with vascular retinopathy with focal or global neurologic deficits and/or signs of internal organ disease (Table 1). Especially if there is a positive family history of retinopathy or neurological disease. Still, a negative family history does not exclude RVCL-S because de novo TREX1 mutations have also been described [12]. Furthermore, family members with RVCL-S may be misdiagnosed with other conditions. Demonstration of a frameshift or nonsense pathogenic variant in the C-terminus of TREX1 confirms the diagnosis.
Treatment
As of now, there is no specific disease modifying treatment for RVCL-S and given the rarity of the disease, there are limited data available on the disease management. Many disease modifying treatments have been tried so far, among which are immunosuppressors such as corticosteroids, cyclophosphamide, methotrexate, chloroquine and azathioprine [2,6,12,20,26,31,32]. Moreover, intravenous immunoglobins and plasmapheresis have also been tried [22]. Unfortunately, there have been no clear benefits to starting such treatments as of today. Research is hampered by the rareness of the disorder and our limited understanding of its pathophysiology. One clinical trial studied aclarubicin as it may correct glycan and immune defects caused by dysregulation of oligosaccharyltransferase (For further information about the rational see Section 2.2 Biological effects of RVCL-S associated mutations). This trial was discontinued after only four participants were included (NCT02723448). There is an ongoing trial studying the effectiveness of crizanlizumab (NCT04611880) for treating RVCL-S of which there are no results yet. Further research is needed to shed light on the pathophysiology of RVCL-S so that more targeted treatment can be evaluated. Another promising avenue of research includes gene therapy. In the future, physicians might employ gene editing technologies that will fully correct disease-causing mutations in the TREX1 gene.
We advise to monitor mutation carriers yearly to detect treatable complications and symptoms. Recommended tests include ophthalmological evaluation, brain MRI with gadolinium, neurological examination, assessment of blood pressure and assessment of renal function, liver enzymes, thyroid function and complete blood count and hemoglobin levels (Table 3). More frequent monitoring may be necessary, depending on the extent of the symptoms.
Table 3.
Recommended surveillance and treatment options of manifestations of RVCL-S.
| Concern | Evaluation1 | Treatment |
|---|---|---|
| Eyes | ||
| Retinopathy | Fundoscopy & FA | Retinal laser and/or anti-VEGF therapy |
| Macular edema | Fundoscopy & FA & OCT | Anti-VEGF therapy |
| Elevated eye pressure/glaucoma |
Fundoscopy & FA & IOP | Standard treatment |
| Neurologic | ||
| Focal neurological complaints | - Neurologic examination - MRI brain with Gd |
IVT therapy is not recommended |
| Tumefactive lesions | MRI brain with Gd | Consider corticosteroid therapy in case of tumefactive lesions with surrounding edema |
| Seizures | EEG | Standard treatment with anti-epileptic drugs |
| Cognitive decline | Assessment of cognition | Consider referral to neuropsychologist |
| Migraine | Thorough headache interview | Standard treatment (CGRP antagonists are contraindicated) |
| Cardiovascular | ||
| Blood pressure evaluation | Standard treatment for hypertension | |
| Renal disease | ||
| Renal function tests2 | - Standard treatment for kidney disease - Renal replacement therapy (incl. transplantation) may be considered for those with end-stage renal disease |
|
| Liver disease | ||
| - Liver enzymes3 & serum albumin - Ultrasound of liver |
- There is no treatment for liver enzyme abnormalities - Monitor to prevent complications associated with liver fibrosis and to exclude other causes |
|
| Anemia | ||
| - Hb, MCV, complete blood count, iron status - Gastroscopy and/or colonoscopy to screen for gastro-intestinal bleeding |
- Standard treatment - Intravenous iron therapy or blood transfusion - Coagulation of gastro-intestinal malformations |
|
| Hypothyroidism | ||
| TSH and free T4 | Thyroid replacement therapy | |
| Raynaud phenomenon | ||
| – | Standard treatment | |
| Other | ||
| Genetic counselling | – | Consider consultation with clinical geneticist and/or genetic counsellor |
| Psychiatric complaints | Assessment of psychiatric symptoms | - Standard treatment - Refer to psychiatrist or neuropsychologist |
CGRP = calcitonin gene-related peptide; EEG = electro-encephalography; FA = fluorescein angiography; Gd = gadolinium; Hb = hemoglobin; IOP = intra-ocular pressure; IVT = intravenous thrombolysis; MCV = mean corpuscular volume; MRI = magnetic resonance imaging; OCT = optical coherence tomography; TSH = thyroid stimulating hormone; VEGF = vascular endothelial growth factor.
Annual monitoring is advised starting in the 4th decade or as appropriate based on symptoms, more frequent monitoring may be necessary for individual patients.
Serum creatinine, blood urea nitrogen (BUN), urinanalysis.
Aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase, gamma-glutamyltransferase (GGT).
Most symptoms of RVCL-S can be treated with standard care (Table 3). Retinopathy and macular edema should be treated with retinal laser photocoagulation and intravitreal anti-VEGF therapy. Treatment of secondary glaucoma may also be necessary. Control of hypertension is important, especially in the case of renal disease. Anemia can be treated with iron supplementation and in severe cases blood transfusions may be required. We recommend treating symptomatic tumefactive white matter lesions (pseudotumors) with surrounding vasogenic cerebral edema with a course of intravenous methylprednisolone followed by oral glucocorticoids. This seems a reasonable intervention, however, evidence of efficacy is lacking.
Of note, we recommend to not treat RVCL-S patients with acute ischemic stroke with intravenous thrombolytic (IVT) therapy. There is no proof that IVT is effective in RVCL-S and the bleeding risk may be increased, as RVCL-S is an angiopathy. Otherwise we advise to manage acute ischemic events in RVCL-S according to the general principles of stroke medicine. For secondary stroke prevention in patients with RVCL-S who have a MRI confirmed symptomatic ischemic event, available risk reduction strategies should be considered, including antiplatelet therapy, statin treatment, lifestyle modifications, and if present treatment for hypertension and diabetes mellitus. However, there is no specific evidence that these measures are effective in reducing stroke risk in RVCL-S.
Discussion
While research into RVCL-S is still in its infancy, over the last two decades, our understanding has increased as more patients have been identified and research and clinical techniques have evolved. With whole exome and genome testing, entire families can be sequenced and pre-symptomatic individuals can be identified. Research has opened the door to the critical needed possibility of identifying biomarkers and potential therapeutic targets. Yet, RVCL-S remains a complex condition, of which the mechanisms still have to be elucidated.
Learning about disease mechanisms in RVCL-S will likely contribute to our understanding of more prevalent forms of sporadic cerebral small vessel diseases (cSVD). RVCL-S can serve as a unique hereditary neurovascular model for stroke, cSVD and dementia, as cSVD is the second leading cause of dementia [33]. In genetic forms of cSVD such as RVCL-S and CADASIL similar but more severe microvascular pathologic findings have been demonstrated compared to sporadic cSVD. Due to their clearly defined pre-symptomatic stage, they provide an unique opportunity to study pre-symptomatic stages of sporadic cSVD [34]. Therefore, studying RVCL-S may facilitate further research into the pathophysiology of sporadic cSVD, and may lead to novel therapies which may be implicated in common forms of cSVD.
RVCL-S is a rare, underrecognized and fatal systemic small vessel disease. Translational research combining basic science advances and clinical findings are needed to identify novel treatment options. Natural history studies enrolling RVCL-S patients at different disease stages are critically needed to help elucidate its pathophysiology and to identify predictive biomarkers and therapeutic treatment possibilities for RVCL-S patients and associated cSVD diseases.
Acknowledgments
Financial disclosure statement
GMT reports consultancy support from Novartis, Allergan/Abbvie, Lilly, and Teva, and Lundbeck and independent support from the Dutch Research Council, the Dutch Heart & Brain Foundations, International Retinal Research Foundation (IRRF) and Dioraphte (20010407). IdB reports independent support from the Dutch Heart Foundation (2020T065) and IRRF. AEW reports no disclosures.
Funding acknowledgement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
The authors thank M. Al-Nofal (Department of Ophthalmology, Leiden University Medical centre, Leiden, the Netherlands) for her contribution to Fig. 1.
References
- 1.Richards A., van den Maagdenberg A.M., Jen J.C., et al. C-terminal truncations in human 3′-5′ DNA exonuclease TREX1 cause autosomal dominant retinal vasculopathy with cerebral leukodystrophy. Nat. Genet. 2007;39(9):1068–1070. doi: 10.1038/ng2082. 9/2007. [DOI] [PubMed] [Google Scholar]
- 2.Grand M.G., Kaine J., Fulling K., et al. Cerebroretinal vasculopathy. A new hereditary syndrome. Ophthalmology. 1988;95(5):649–659. doi: 10.1016/s0161-6420(88)33131-3. [DOI] [PubMed] [Google Scholar]
- 3.Storimans C.W., Schooneveld v M.J., Oosterhuis J.A., Bos P.J. A new autosomal dominant vascular retinopathy syndrome. Eur J Ophthalmol. 1991;1(2):73–78. doi: 10.1177/112067219100100204. [DOI] [PubMed] [Google Scholar]
- 4.Terwindt G.M., Haan J., Ophoff R.A., et al. Clinical and genetic analysis of a large Dutch family with autosomal dominant vascular retinopathy, migraine and Raynaud's phenomenon. Brain. 1998;121(2):303–316. doi: 10.1093/brain/121.2.303. Feb 1998. [DOI] [PubMed] [Google Scholar]
- 5.Winkler D.T., Lyrer P., Probst A., et al. Hereditary systemic angiopathy (HSA) with cerebral calcifications, retinopathy, progressive nephropathy, and hepatopathy. J Neurol. Jan 2008;255(1):77–88. doi: 10.1007/s00415-008-0675-3. [DOI] [PubMed] [Google Scholar]
- 6.Jen J., Cohen A.H., Yue Q., et al. Hereditary endotheliopathy with retinopathy, nephropathy, and stroke (HERNS). Case Reports. Neurology. 1997;49(5):1322–1330. doi: 10.1212/wnl.49.5.1322. Nov 1997. [DOI] [PubMed] [Google Scholar]
- 7.Pelzer N., Hoogeveen E.S., Haan J., et al. Systemic features of retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations: a monogenic small vessel disease. J. Intern. Med. 2019;285(3):317–332. doi: 10.1111/joim.12848. [DOI] [PubMed] [Google Scholar]
- 8.Stam A.H., Kothari P.H., Shaikh A., et al. Retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations. Brain. 2016;139(11):2909–2922. doi: 10.1093/brain/aww217. 11/1/2016Not in File. doi:aww217 [pii];10.1093/brain/aww217 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazur D.J., Perrino F.W. Identification and expression of the TREX1 and TREX2 cDNA sequences encoding mammalian 3′–>5′ exonucleases. J Biol Chem. 1999;274(28):19655–19660. doi: 10.1074/jbc.274.28.19655. [DOI] [PubMed] [Google Scholar]
- 10.Höss M., Robins P., Naven T.J., Pappin D.J., Sgouros J., Lindahl T. A human DNA editing enzyme homologous to the Escherichia coli DnaQ/MutD protein. EMBO J. 1999;18(13):3868–3875. doi: 10.1093/emboj/18.13.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasan M., Fermaintt C.S., Gao N., et al. Cytosolic nuclease TREX1 regulates oligosaccharyltransferase activity independent of nuclease activity to suppress immune activation. Immunity. 2015;43(3):463–474. doi: 10.1016/j.immuni.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiFrancesco J.C., Novara F., Zuffardi O., et al. TREX1 C-terminal frameshift mutations in the systemic variant of retinal vasculopathy with cerebral leukodystrophy. Neurol Sci. 2015;36:323–330. doi: 10.1007/s10072-014-1944-9. [DOI] [PubMed] [Google Scholar]
- 13.de Boer I., Stam A.H., Buntinx L., et al. RVCL-S and CADASIL display distinct impaired vascular function. Neurology. 2018;91(10):e956–e963. doi: 10.1212/WNL.0000000000006119. 9/4/2018Not in File. doi:WNL.0000000000006119 [pii];10.1212/WNL.0000000000006119 [doi] [DOI] [PubMed] [Google Scholar]
- 14.Pelzer N., Bijkerk R., Reinders M.E.J., et al. Circulating endothelial markers in retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations. Stroke. 2017;48(12):3301–3307. doi: 10.1161/STROKEAHA.117.018556. [DOI] [PubMed] [Google Scholar]
- 15.Hoogeveen E.S., Pelzer N., Ghariq E., et al. Cerebrovascular reactivity in retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations. J. Cereb. Blood Flow Metab. 2021;41(4):831–840. doi: 10.1177/0271678X20929430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kothari P.H., Kolar G.R., Jen J.C., et al. TREX1 is expressed by microglia in normal human brain and increases in regions affected by ischemia. Brain Pathol. 2018;28(6):806–821. doi: 10.1111/bpa.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saito R., Nozaki H., Kato T., et al. Retinal vasculopathy with cerebral leukodystrophy: clinicopathologic features of an autopsied patient with a heterozygous TREX 1 mutation. J Neuropathol Exp Neurol. 2019;78(2):181–186. doi: 10.1093/jnen/nly115. [DOI] [PubMed] [Google Scholar]
- 18.Mulder I.A., Rubio-Beltran E., Ibrahimi K., et al. Increased mortality and vascular phenotype in a knock-in mouse model of retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations. Stroke. 2020;51(1):300–307. doi: 10.1161/strokeaha.119.025176. [DOI] [PubMed] [Google Scholar]
- 19.Sakai T., Miyazaki T., Shin D., et al. DNase-active TREX1 frame-shift mutants induce serologic autoimmunity in mice. J. Autoimmun. 2017;81:13–23. doi: 10.1016/j.jaut.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vodopivec I., Oakley D.H., Cory A., Perugino C.A., Venna N., Hedley-Whyte E.T., Stone J.H. A 44-year-old man with eye, kidney, and brain dysfunction. Ann. Neurol. 2016;79(4):507–519. doi: 10.1002/ana.24583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ford A.L., Chin V.W., Fellah S., et al. Lesion evolution and neurodegeneration in RVCL-S: a monogenic microvasculopathy. Neurology. 2020;95(14):1918–1931. doi: 10.1212/WNL.0000000000010659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mateen F.J., Krecke K., Younge B.R., et al. Evolution of a tumor-like lesion in cerebroretinal vasculopathy and TREX1 mutation. Neurology. 2010;75(13):1211–1213. doi: 10.1212/WNL.0b013e3181f4d7ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hedderich D.M., Lummel N., Deschauer M., et al. Magnetic resonance imaging characteristics of retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations. Clin Neuroradiol. 2020;30(2):229–236. doi: 10.1007/s00062-018-0755-4. [DOI] [PubMed] [Google Scholar]
- 24.Hoogeveen E.S., Pelzer N., de Boer I., van Buchem M.A., Terwindt G.M., Kruit M.C. Neuroimaging findings in retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations. Am. J. Neuroradiol. 2021;42(9):1604–1609. doi: 10.3174/ajnr.A7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhamija R., Schiff D., Lopes M.B.S., Jen J.C., Lin D.D., Worrall B.B. Evolution of brain lesions in a patient with TREX1 cerebroretinal vasculopathy. Case Report. Neurology. 2015;85(18):1633–1634. doi: 10.1212/WNL.0000000000002092. [DOI] [PubMed] [Google Scholar]
- 26.Schuh E., Ertl-Wagner B., Lohse P., et al. Multiple sclerosis–like lesions and type I interferon signature in a patient with RVCL. Neurol Neuroimmunol Neuroinflamm. Feb 2015;2(1):e55. doi: 10.1212/NXI.0000000000000055. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raynowska J., Miskin D.P., Pramanik B., et al. Retinal vasculopathy with cerebral leukoencephalopathy (RVCL): a rare mimic of tumefactive MS. Neurology. 2018;91(15):1423–1428. doi: 10.1212/WNL.0000000000006329. [DOI] [PubMed] [Google Scholar]
- 28.Hardy T.A., Young S., Sy S.S., et al. Tumefactive lesions in retinal vasculopathy with cerebral leucoencephalopathy and systemic manifestations (RVCL-S): a role for neuroinflammation? J. Neurol. Neurosurg. Psychiatr. 2017 doi: 10.1136/jnnp-2017-316142. [DOI] [PubMed] [Google Scholar]
- 29.Weil S., Reifenberger G., Dudel C., Yousry T.A., Schriever S., Noachtar S. Cerebroretinal vasculopathy mimicking a brain tumor: a case of a rare hereditary syndrome. Neurology. 1999;53(3):629–631. doi: 10.1212/wnl.53.3.629. [DOI] [PubMed] [Google Scholar]
- 30.de Boer I., Steenmeijer S.R., Pelzer N., et al. Spectral domain optical coherence tomography in retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations: a monogenic small vessel disease. J Neuroophthalmol. 2021, doi: 10.1097/WNO.0000000000001336. [DOI] [PubMed] [Google Scholar]
- 31.Monroy-Jaramillo N., Cerón A., León E., et al. Phenotypic variability in a Mexican mestizo family with retinal vasculopathy with cerebral leukodystrophy and TREX1 Mutation p.V235Gfs*6. Rev. Invest. Clin. 2018;70(2):68–75. doi: 10.24875/ric.18002492. [DOI] [PubMed] [Google Scholar]
- 32.Gruver A.M., Schoenfield L., Coleman J.F., Hajj-Ali R., Rodriguez E.R., Tan C.D. Novel ophthalmic pathology in an autopsy case of autosomal dominant retinal vasculopathy with cerebral leukodystrophy. J. Neuroophthalmol. 2011;31(1):20–24. doi: 10.1097/WNO.0b013e3181f45dba. [DOI] [PubMed] [Google Scholar]
- 33.Gorelick P.B., Scuteri A., Black S.E., et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Craggs L.J.L., Yamamoto Y., Deramecourt V., Kalaria R.N. Microvascular pathology and morphometrics of sporadic and hereditary small vessel diseases of the brain. Brain Pathol. 2014;24(5):495–509. doi: 10.1111/bpa.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]