Highlights
-
•
Glycine mutations in COL4A1/A2 genes increased susceptibility to brain hemorrhage.
-
•
Type IV collagenopathy causes multi-organ damage in adults, children, or fetuses.
-
•
Mutations in the COL4A1 3′UTR non-coding region are responsible for PADMAL.
Keywords: hereditary cerebral small vessel disease, Autosomal dominant, Collagenopathy, Brain hemorrhage, COL4A1, COL4A2
Abstract
COL4A1 and COL4A2 genes encode the alpha1 and the alpha2 chains of type IV collagen, a key component of basement membranes. Mutations located in the coding sequence of COL4A1/COL4A2 genes are responsible for an autosomal dominant (AD) cerebral angiopathy that manifest in either adults, children or fetuses. The most typical among such mutations are missense glycine mutations in the triple helix. They increase the susceptibility to brain hemorrhage but can also promote the occurrence of multiple other types of systemic manifestations that can involve the eyes, kidneys or muscles. This condition is characterized by a very incomplete penetrance, and a wide phenotypic variability even among members of the same family. Recently, mutations in the COL4A1 3′UTR non-coding region that upregulate COL4A1 expression, and COL4A1/COL4A2 duplications, have been shown to cause AD forms of ischemic cerebral small vessel disease in adults.
Herein, we summarize the genetic and pathophysiological aspects of these conditions, detail their clinical and imaging characteristics and discuss some principles in their clinical management.
Introduction
Collagen type IV alpha 1 and 2 chains, respectively encoded by COL4A1 and COL4A2 genes, are key components of basement membranes (BM) in blood vessels and various soft organs in mammals [1]. In 2005, heterozygous mutations affecting the coding sequence of COL4A1 were found responsible for perinatal cerebral hemorrhage and congenital porencephaly, in an autosomal dominant (AD) fashion [2,3]. Simultaneously, identical mutations were involved in the development of a cerebral small vessel disease (cSVD) associated with a high rate of hemorrhagic strokes in adults [3,4]. The implication of COL4A2 mutations in other pedigrees with a similar phenotype was shown few years later, in 2012 [5,6]. In both conditions, brain hemorrhages presumably result from the fragilization of the collagen network within the endothelial BM. Over the past decade, the clinical spectrum related to COL4A1 and COL4A2 mutations was progressively enriched. Multiorgan damages were reported in mutated individuals, especially in eyes, muscles and/or kidneys [4,7]. COL4A1 and COL4A2 mutations were obviously responsible for a widely variable spectrum of manifestations. This huge variability was detected between families but also between close members belonging to the same pedigree. The penetrance was also obviously variable and also appears often reduced [7,8].
More recently, other types of mutations in COL4A1 and COL4A2 genes, and mutations in the functionnally related COLGALT1 gene, have also been involved in AD forms of cSVD in adults, with distinct phenotypes [9], [10], [11].
Herein, we briefly reviewed the pathophysiology of such disorders, their clinical aspects as well as the main aspects of their clinical management.
Haemorrhagic angiopathy due to mutations in the coding regions of COL4A1 and COL4A2 genes
Clinical and radiological phenotype in adults
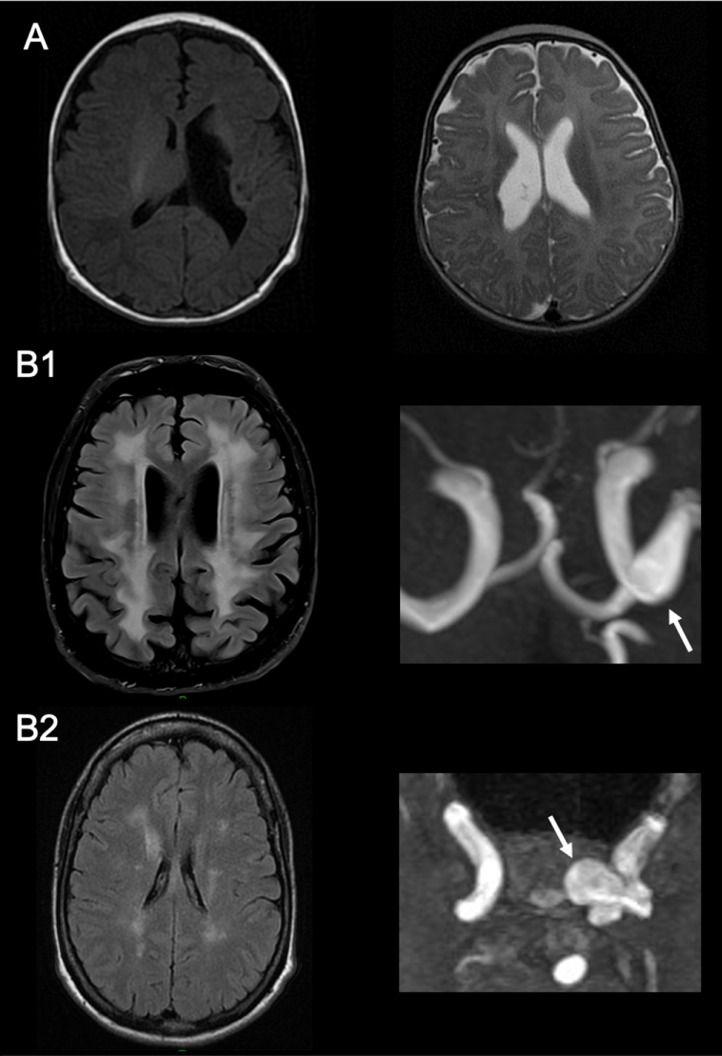
In adults, the clinical presentation widely varies between and within families ranging from asymptomatic COL4A1/COL4A2 carriers to severely disabled individuals. The occurrence of hemorrhagic stroke, even in the absence of any clinical history during childhood, represents a key manifestation and a major contributor to disease severity [12], [13], [14]. COL4A1/COL4A2 mutations are rarely responsible for ischemic strokes or transient ischemic attacks. Some features, such as the preferred sites of focal lesions in the territory of long perforating arteries, the presence of white matter hyperintensities (WMH) in the deepest cerebral areas and accumulating pathological data from COL4A1 mutated mice and humans suggest that most cerebrovascular complications in this disorder are related to a diffuse cerebral small vessel disease [15]. Widespread and symmetrical periventricular WMH are often observed on Magnetic Resonance Imaging (MRI) in asymptomatic and symptomatic individuals (Fig. 1) but its absence, even in hemorrhagic stroke cases, doesn't exclude the diagnosis [16]. The association with other markers such as small deep infarcts, microbleeds and dilated perivascular spaces, although not specific, is also suggestive [4,13]. Some patients may have unilateral porencephaly, ventricular dilatation or/and multiple micro-calcifications particularly in the basal ganglia [17,18]. Asymptomatic intracranial aneurysms or dolichoectasia, another hallmark of the disease, can be also detected on magnetic resonance angiography. They are most often localized at the level of internal carotid arteries (and particularly on their C4 and C5 segments). In some cases, they can involve the basilar artery (Fig. 1) [7].
Fig. 1.
Cerebral MRI characteristics in patients with COL4A1 mutation involving a glycine of the triple-helix
A: T1- weighted (left) and T2-weighted (right) axial sequences, showing a ventricle asymmetry due to porencephalic cysts in a one-month-old boy (left) and in a three-months-old girl (right) with a congenital hemiparesis. B: FLAIR sequence (left) and time-of-flight magnetic resonance angiography (right), showing a leukoencephalopathy (left) and a left intracranial internal carotid artery aneurysm (right) in a 70-year-old man (B1) and his 35-year-old daughter (B2) with a history of HANAC syndrome.
Extracranial manifestations are reported in presence or absence of stroke events. The tropism of such clinical manifestations is underpinned by the expression pattern of COL4A1/COL4A2 gene, as the alpha 1 and 2 subunits of collagen type 4, are essential constituents of basement membranes in many tissues, including the vascular and corneal epithelium, conjunctiva of the eye, kidney glomeruli and tubules or muscles. These extra-cranial may be symptomatic or remain totally silent.
The presence of retinal arteriolar tortuosities represents another key manifestation of the disease but is also inconsistent. Abnormal elongation of the central retinal arterioles, preferentially located in the peri-macular area are often detected. These microvascular changes may be asymptomatic or complicated by transient visual loss secondary to retinal hemorrhages. COL4A1 gene mutations can also lead to congenital dysgenesis of the anterior segment of the eyes including the iris and cornea (which can result in the development of the Anxenfeld-Rieger syndrome, a clinically and genetically heterogeneous condition). Ocular manifestations are usually detected during childhood and include congenital or post-traumatic cataract, optic nerve excavation and intraocular hypertension [19–21].
Renal involvement associated with COL4A1 mutations can manifest as microscopic- or gross-hematuria, moderate renal failure, and/or renal cysts. Muscle cramps with CPK elevation and migraine with aura have also been described in some families. Other clinical manifestations appear less frequent such as supraventricular heart rhythm disorders, Raynaud's phenomenon and liver cysts.
A distinct phenotype summarized by the acronym “hereditary angiopathy, nephropathy, aneurysms, and cramps” or HANAC has been related to COL4A1 mutations located in exons 24 and 25 [22]. The cerebrovascular phenotype is characterized by a cerebral small vessel disease with a low risk of hemorrhagic stroke and with aneurysms of the carotid siphon [23]. Bilateral retinal arteriolar tortuosities are constant and often complicated with repeated retinal hemorrhages without other ocular abnormality. Patients with HANAC also present with muscle cramps and kidney lesions that can lead to renal cysts, chronic kidney failure and sometimes to hematuria [24].
Clinical and radiological phenotype in fetuses and children
As in adults, the clinical manifestations related to COL4A1/COL4A2 mutations in children are highly variable both in their type and severity.
The first manifestation related COL4A1 mutations to be described was the occurrence of perinatal brain haemorrhages in foetuses and newborns. In 2005, Gould et al observed a high rate of perinatal cerebral hemorrhage in COL4A1 mice mutants. Survivor mutant mice developed porencephalic cavities, fluid-filled cystic cavities within the brain parenchyma communicating sometimes with ventricles. These lesions result from tissue necrosis subsequent to brain haemorrhages [2]. At the same period, in humans, stereotyped COL4A1 mutations were identified in autosomal-dominant familial forms of porencephaly [2,3], and such observations increased in the literature during the following years [25]. (Fig. 1) The involvement of COL4A2 mutations in human porencephaly was established in 2012 [5,6,26]. As in mice models, it was shown that porencephalic cysts in mutated children were also related to brain hemorrhages occurring during the pregnancy or perinatal period. These cysts generally involved the deep periventricular brain regions. Some authors use the terms of “parenchymal hemorrhagic infarctions” to describe such lesions resulting from a deep hemorrhage subsequently leading to impaired venous drainage of medullary veins in the periventricular white matter. These combined mechanisms may lead to the development of such large porencephalic cysts [25]. Both antenatal or perinatal hemorrhages presumably result from endothelial BM weakness. Thus, they should be considered as the earliest manifestations of COL4A1/COL4A2 mutations-related cSVD. In addition to porencephaly, other types of brain lesions related to antenatal hemorrhages have been reported in mutated children, such as schizencephaly, hydranencephaly, hydrocephaly, ventricular asymmetry, periventricular leukomalacia, and brain calcifications [19]. Cortical developmental malformations have also been described, as focal cortical dysplasia. These malformations have been reviewed by Meuwissen et al [8]. In these children, the diagnosis is often made during the neonatal period or during childhood. The clinical spectrum includes the development of infantile hemiparesis, spastic tetraparesis, epilepsy or psychomotor delay of variable severity, sometimes in association with microcephaly or macrocephaly [19,25]. Some children may present milder symptoms, such as isolated minor intellectual disability, or soft motor signs. In the most severe cases, the diagnosis can be made after a late miscarriage, or during pregnancy with ultrasound examination showing a large porencephalic cavity [27]. Fetal MRI may be particularly helpful to detail smaller brain lesions and to lead to the decision of medical abortion [27].
Since the first reports, the very incomplete penetrance of porencephaly observed in the mutated families have led to suspect an important role of environmental stress. The strong influence of birth trauma in triggering perinatal hemorrhages was demonstrated with the drastic reduction of perinatal hemorrhage in mutant mice delivered surgically [3]. In addition to the incomplete penetrance, the high rate of de novo mutations in COL4A1 and COL4A2 genes explain why numbers of affected children present as sporadic cases [27].
Rarely, haemorrhagic stroke may occur during childhood or adolescence, even in the absence of porencephaly. In most cases, cSVD markers are present on cerebral MRI [19,28].
With the brain, the eyes are the sites the most frequently altered in newborns and young children carrying COL4A1 or COL4A2 mutations. Developmental malformations involving the anterior segment (congenital or juvenile cataract, microcornea, Axenfeldt Rieger syndrome), or the posterior segment of the eye (congenital or juvenile glaucoma, optic nerve dysgenesis), or both compartments are described. In extreme cases, microphthalmy and congenital blindness are possible. A strabismus is frequent. [8,20] As in adults, damages in other soft organs may also occur in children. Kidneys (cysts, hematuria), muscles (creatine kinase elevation in serum, cramps) especially can be detected in isolation [22,24]. In children's HANAC cases, as in adults, retinal artery tortuosities are frequent but usually asymptomatic [24].
Genetics and pathophysiology
Type IV collagen is a key component of BM of the vascular endothelium and many soft tissues. Collagen IV α1 and α2 chains are, respectively encoded by COL4A1 and COL4A2 genes, which are both located on the 13q34 chromosome in a head-to-head conformation, and share a same bi-directional promotor. Both chains share around 45% identity. Two α1 and one α2 chains are assembled within the endoplasmic reticulum into an heterotrimeric helix before to be excreted in the extracellular matrix [1]. Each chain of collagen is divided into three main domains: a large central triple-helical domain, a C-terminal non-collagenous (NC1) domain, and an amino-terminal 7S domain [1,29]. The triple-helical domain is compounded by amino-acid repeated sequences Gly-X-Y, where Gly represent a glycine residue, and X and Y variable amino-acids, Y being most often a proline residue. In the extracellular matrix, heterotrimers are assembled together through their 7S and NC1 domains to form a macromolecular network. This collagen network also interact with non-collagenous matrix proteins, such as integrins [1,29].
Pathogenic mutations are found in both COL4A1 and COL4A2 genes, but have been reported 10 times more frequently in COL4A1 [8,30]. More than 100 distinct heterozygous pathogenic mutations have been reported in humans. Around half of mutations are inherited in an autosomal dominant fashion, the other being de novo mutations [30].
Most of pathogenic mutations are missense mutations involving highly conserved glycine residues belonging to the triple-helical domain [8,30]. Missense mutations involving non-glycine residues of the triple-helix, splice-site mutations and small in-frame deletions that lead to the loss of one or several residues of the triple-helix are also observed [8,30]. Through a dominant negative effect, the substitution or the loss of highly conserved residues in the triple-helical domain is assumed to impact the whole heterotrimer structure. These changes alter the secretion of heterotrimers in the matrix and lead to their accumulation into the cells [2,29,31,32]. In the vascular wall, the depletion of collagen in the extracellular matrix presumably contributes to endothelial BM weakness, which will prone the rupture of arterioles [32,33]. At the tissue level, this is corroborated by the observation of irregularities and focal disruption of the BM in the cerebral and renal vessels of COL4A1 mutant mice [2,3]. In humans, histologic analysis of skin and kidney biopsy sampled from mutated patients also showed BM defects [22]. However, some studies suggest that arteriolar fragility is rather due to the deleterious effect of the accumulation of collagen into the parietal vascular cells [32,33].
More rarely, disruptive mutations (i.e. splice-site mutations or small insertion-deletions that disrupt the open-reading-frame) that may lead to haploinsufficiency through a nonsense mediated mRNA decay are found in patients [8,30]. This suggests that, in addition to a dominant negative mechanism, a quantitative mechanism is also involved in the pathophysiology. Mutations in the NC1 domain are also reported in a small number of patients. As disruptive mutations, mutations involving the NC1 are expected to reduce the amount of heterotrimers without impairing the structure of the triple-helix [30].
The phenotypic spectrum of patients carrying a pathogenic mutation in COL4A1/COL4A2 genes is highly heterogeneous and the penetrance very incomplete, suggesting the intervention of modifying factors [25]. Families in which a children born with a severe porencephaly have inherited the mutation from an asymptomatic or paucisymptomatic parent are not rare [25,19]. Of note, COL4A2 mutations seem to be associated with an even more reduced penetrance and a globally milder phenotype than COL4A1 mutations [30]. The potential impact of factors such as head traumas, organic stress, high blood pressure, and anticoagulant therapy as triggers for brain haemorrhages, contributes to this phenotypic heterogeneity [32]. But these factors cannot sum up the whole intrafamilial phenotypic heterogeneity. Additional modifying factors, either environmental or genetics, are likely involved and remain to be identified.
The allelic heterogeneity is another factor that may contribute to the phenotypic heterogeneity observed between families. Indeed, mutations involving glycine residues that belong to exons 24-25 of COL4A1 are involved in the HANAC syndrome, described above. These mutations cluster in a CB3 (IV) region of the triple-helical domain, which encompasses major integrin-binding sites [24]. This suggests that some functional subdomains might be differentially involved according the phenotype considered. Moreover, genotype-phenotype correlation studies conducted in patients mutated in a triple-helix glycine residue suggest a position-dependent effect of the mutation on the phenotype. Substitutions involving a glycine located in the C-terminal part the triple-helix tend to be associated with brain haemorrhage that occur at a younger age and are more severe that those located in the N-terminal part [30,29]. Regarding the ocular manifestations, the presence of retinal artery hypertortuosities is highly penetrant in Glycine substitutions involving the first N-terminal third of the helix domain, as compared to substitutions near to the C-terminus that are more prone to the development of eye congenital malformations [30]. At last, mice experiments suggest that the biological impact of quantitative mutations is milder than dominant negative mutations. However, genotype-phenotype correlation analyses conducted in patients failed to demonstrate that quantitative mutations are associated with a milder phenotype [30]. These observations still need to be replicated and refined by additional studies.
Diagnosis and therapeutic strategy
Diagnostic guidance in COL4A1/COL4A2 angiopathies requires a complete clinical examination and careful questioning, looking for any personal neonatal and any familial history of neurological, ophthalmological or renal disorders.
Molecular analysis of the COL4A1/COL4A2 genes is justified in several circumstances. During infancy, the identification of porencephaly, peri- or post-natal cerebral hemorrhage without identified cause, especially if associated with intrauterine growth retardation, microcephaly at birth, congenital anomalies of the anterior segment of eye, cataract, glaucoma, optic nerve atrophy, cerebral cortex abnormality, retinal arterial tortuosity or/and leukoencephalopathy, requires molecular analysis of the COL4A1/COL4A2 genes. Later in life, molecular screening should be discussed in cases of HANAC syndrome, cerebral hemorrhage or confluent leukoencephalopathy without other identified cause particularly if in presence of features such as intracranial aneurysms, porencephaly, retinal arteriolar tortuosities, and any alteration of the anterior segment of the eye, premature cataract or renal cysts.
No specific preventive or curative treatment of proven efficacy is available to date. However, the patients and their physicians should be informed that the underlying mechanisms of cerebral or retinal hemorrhages may be linked to the fragility of the vascular wall predisposing to rupture under certain conditions such as trauma (shocks, practice of certain sports, vaginal delivery) or the use of anticoagulants. Consequently, it is recommended to avoid intense physical activities, and anticoagulants in these patients [34]. However, in case of high risk of thrombosis or embolism (eg, atrial fibrillation, mechanical valves), anticoagulant drugs should be discussed after a careful examination of risk/benefit balance on a case-by-case basis, and under strict blood pressure control [35]. Antiplatelet therapy is usually not recommended in COL4A1/2 angiopathy [34]. However, their use may be discussed after ischemic stroke, in the absence of previous intracranial hemorrhage, or in presence of a strong indication (eg, coronary artery disease) [35]. The use of fibrinolysis is not recommended in acute small deep ischemic lesion in patients with COL4A1/COL4A2 mutations, but must be discussed on a case-by-case basis in context of large artery occlusion, in patients with no history of intracranial hemorrhage [35].
In fetuses who carry a COL4A1/COL4A2 mutation, a Caesarean section must be considered aiming to reduce the risk of perinatal brain hemorrhage [34].
Finally, patients with COL4A1 or COL4A2 mutations should be regularly followed clinically and using cerebral and visceral arteries imaging. A multisystem workup should be performed, including cerebral MRI, intracranial and cervical MR angiography, cardiac echography, ocular and retinal vessel examination, renal echography, and a blood test with renal function and CPK dosage, in agreement with recent European guidelines [34]. Any recommendation is available concerning the frequency of this work-up.
The management of an intracranial aneurysm associated with COL4A1/COL4A2 angiopathy is identical to that of sporadic intracranial aneurysms and based on a case-by-case multidisciplinary approach. Vascular risk factors, especially high blood pressure, should be systematically investigated and treated if needed [[7], [34], [35]].
The existence of preventive measures for carriers of COL4A1/COL4A2 mutations and the importance of genetic counselling due to the risk for the offspring make it necessary to inform relatives at risk [36]. This should be done during a genetic consultation and/or a multidisciplinary consultation with a neurologist, a geneticist and a psychologist. In families in which the disease is revealed early, during childhood, and/or in the neonatal or antenatal period, the parents sometimes express a request for antenatal diagnosis. This request must be processed in a prenatal diagnosis centre, in compliance with the ethical rules and legal framework. In practice, the decision for antenatal diagnosis should be always made on a case-by-case basis in agreement with the local ethical rules and according to the legal context.
Other mutations involving COL4A1 and COL4A2 genes: phenotype and genetics
COL4A1 and COL4A2 coding mutations leading to a loss-of-function effect, either by a dominant-negative effect or by haploinsufficiency, are not the only mutations to be involved in human pathology. Since the mid-2010s, mutations leading to overexpression of these genes have been shown to be involved in AD forms of adult-onset ischemic cSVD.
In a few patients, either sporadic or belonging to multiplex AD families of cSVD, large duplications or triplications involving the 13q33-34 chromosomal region and encompassing COL4A1 and COL4A2 genes have been reported [9,37,38]. The size of the interval duplicated was variable among patients, the minimal interval reported encompassing a total of 6 genes including COL4A1 and COL4A2 [37]. In all cases, COL4A1 was entirely duplicated, however, in one family, only the 4 first exons of COL4A2 were included in the duplication [37]. The detection of these large chromosomal rearrangements required the use of quantitative methods such as CGH arrays or quantitative PCR, since classical sequencing methods failed to detect them. In mutated patients, the first symptoms appeared after 30 years of age, and consisted in transient focal deficit or recurrent small subcortical ischemic stroke [9,37,38]. In one patient, progressive walking difficulties were noticed with ageing [38]. All patients showed the classical markers of ischemic cSVD on brain MRI including a white-matter lesions increasing with age and/or old deep or subcortical small infarcts. These lesions were reported even in the absence of cardiovascular risk factors. Microbleeds were inconsistent. There was no brain hemorrhage. Patients presented frequently with vertebrobasilar dolichoectasia [9,37,38].
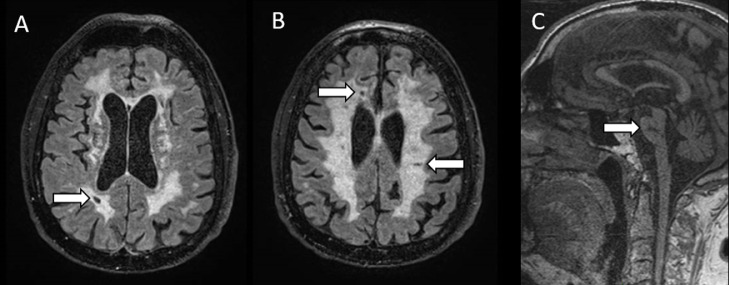
In 2016, mutations located in a small 3’ untranslated region (UTR) of COL4A1 were shown to cause an autosomal dominant form of adult onset cSVD called PADMAL (Pontine Autosomal Dominant Microangiopathy with Leukoencephalopathy) [10]. PADMAL is characterized by recurrent small infarcts beginning around 40 years of age and leading to a progressive or stepwise cognitive decline and motor disability [10,39,40]. The cognitive profile of patients is marked by altered executive functions, processing speed and flexibility. Such dysexecutive profile is consistent with the subcortical profile usually encountered in cSVDs [10]. All patients showed a severe and diffuse leukoencephalopathy worsening with age, involving subcortical and deep white matter, and lacunes always present in the pons of symptomatic patients but also frequent in brain hemispheres (Fig. 2). In some patients, WMH involving the anterior temporal lobes and external capsules have been noticed, as observed in other genetic conditions such as CADASIL or CARASIL. As in duplications, microbleeds are inconsistent or scarce, and brain hemorrhage was not previously reported in PADMAL patients [10,39,40].
Fig. 2.
Cerebral MRI characteristics in a patient with PADMAL
T2-weighted axial sequences (A,B) and T1- weighted sagittal sequence (C), showing a diffuse leukopathy in centrum semi-ovale and periventricular white matter (A,B), and lacunes in pons (C, arrows) and subcortical white matter (A,B, arrows) in a 57 years-old man with history of transient focal deficits, progressive walking and cognitive disability.
PADMAL mutations are rare substitutions involving a 7 base-pair region located in the COL4A1 non-coding 3’UTR region, which constitute a binding site for miR-29 microRNA. Mutations in this region alter the fixation of miR-29 to the binding site, and lead to an upregulation of COL4A1 expression [10]. According the sequencing data from a large French cohort, the prevalence of PADMAL mutations could account for around 2.5% of familial cSVD of unknown origin [10].
In duplications of collagen genes as observed in PADMAL, the phenotype seems to be restricted to cerebrovascular lesions. Today, data remain too scarce to establish the exact penetrance in these disorders.
Conclusion
Cerebrovascular manifestations encountered in COL4A1/COL4A2 mutations are highly heterogeneous. They vary from a preponderant hemorrhagic phenotype related to COL4A1/COL4A2 glycine mutations up to an ischemic phenotype in PADMAL andCOL4A1/COL4A2 duplications. Heterogeneity is also observed regarding the age of onset, which is not limited in cases with glycine mutations contrasting with the adult-onset of PADMAL and in presence of COL4A1/COL4A2 duplications. In line, the clinical manifestations related to COL4A1/COL4A2 glycine mutations are of incomplete penetrance and widely vary. In contrast, the clinical phenotype in PADMAL and COL4A1/COL4A2 duplications appears restricted to an ischemic form of cSVD. The exact pathophysiological mechanisms of such conditions and the sources of this large heterogeneity remain incompletely understood. For these conditions, prospective cohort studies are now crucial to identify the key predictors of clinical worsening and modifying factors involved in their clinical heterogeneity. These studies in addition to lessons from preclinical models should help to develop prevention and to refine guidance for clinical care and follow up of patients with COL4A1/COL4A2 mutations.
References
- 1.Khoshnoodi J., Pedchenko V., Hudson B.G. Mammalian collagen IV. Microsc. Res. Tech. 2008;71:357–370. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gould D.B., Phalan F.C., Breedveld G.J., van Mil S.E., Smith R.S., Schimenti J.C., Aguglia U., van der Knaap M.S., Heutink P., John S.W.M. Mutations in COL4A1 cause perinatal cerebral hemorrhage and porencephaly. Science. 2005;308:1167–1171. doi: 10.1126/science.1109418. [DOI] [PubMed] [Google Scholar]
- 3.Gould D.B., Phalan F.C., van Mil S.E., Sundberg J.P., Vahedi K., Massin P., Bousser M.G., Heutink P., Miner J.H., Tournier-Lasserve E., et al. Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N. Engl. J. Med. 2006;354:1489–1496. doi: 10.1056/NEJMoa053727. [DOI] [PubMed] [Google Scholar]
- 4.Lanfranconi S., Markus H.S. COL4A1 mutations as a monogenic cause of cerebral small vessel disease: a systematic review. Stroke J. Cereb. Circ. 2010;41:e513–e518. doi: 10.1161/STROKEAHA.110.581918. [DOI] [PubMed] [Google Scholar]
- 5.Jeanne M., Labelle-Dumais C., Jorgensen J., Kauffman W.B., Mancini G.M., Favor J., Valant V., Greenberg S.M., Rosand J., Gould D.B. COL4A2 mutations impair COL4A1 and COL4A2 secretion and cause hemorrhagic stroke. Am. J. Hum. Genet. 2012;90:91–101. doi: 10.1016/j.ajhg.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoneda Y., Haginoya K., Arai H., Yamaoka S., Tsurusaki Y., Doi H., Miyake N., Yokochi K., Osaka H., Kato M., et al. De novo and inherited mutations in COL4A2, encoding the type IV collagen α2 chain cause porencephaly. Am. J. Hum. Genet. 2012;90:86–90. doi: 10.1016/j.ajhg.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vahedi K., Alamowitch S. Clinical spectrum of type IV collagen (COL4A1) mutations: a novel genetic multisystem disease. Curr. Opin. Neurol. 2011;24:63–68. doi: 10.1097/WCO.0b013e32834232c6. [DOI] [PubMed] [Google Scholar]
- 8.Meuwissen M.E.C., Halley D.J.J., Smit L.S., Lequin M.H., Cobben J.M., de Coo R., van Harssel J., Sallevelt S., Woldringh G., van der Knaap M.S., et al. The expanding phenotype of COL4A1 and COL4A2 mutations: clinical data on 13 newly identified families and a review of the literature. Genet. Med. Off. J. Am. Coll. Med. Genet. 2015;17:843–853. doi: 10.1038/gim.2014.210. [DOI] [PubMed] [Google Scholar]
- 9.Renard D., Miné M., Pipiras E., Labauge P., Delahaye A., Benzacken B., Tournier-Lasserve E. Cerebral small-vessel disease associated with COL4A1 and COL4A2 gene duplications. Neurology. 2014;83:1029–1031. doi: 10.1212/WNL.0000000000000769. [DOI] [PubMed] [Google Scholar]
- 10.Verdura E., Hervé D., Bergametti F., Jacquet C., Morvan T., Prieto-Morin C., Mackowiak A., Manchon E., Hosseini H., Cordonnier C., et al. Disruption of a miR-29 binding site leading to COL4A1 upregulation causes pontine autosomal dominant microangiopathy with leukoencephalopathy. Ann. Neurol. 2016;80:741–753. doi: 10.1002/ana.24782. [DOI] [PubMed] [Google Scholar]
- 11.Miyatake S., Schneeberger S., Koyama N., Yokochi K., Ohmura K., Shiina M., Mori H., Koshimizu E., Imagawa E., Uchiyama Y., et al. Biallelic COLGALT1 variants are associated with cerebral small vessel disease. Ann. Neurol. 2018;84:843–853. doi: 10.1002/ana.25367. [DOI] [PubMed] [Google Scholar]
- 12.Vahedi K., Kubis N., Boukobza M., Arnoult M., Massin P., Tournier-Lasserve E., Bousser M.G. COL4A1 mutation in a patient with sporadic, recurrent intracerebral hemorrhage. Stroke. 2007;38:1461–1464. doi: 10.1161/STROKEAHA.106.475194. [DOI] [PubMed] [Google Scholar]
- 13.Vahedi K., Boukobza M., Massin P., Gould D.B., Tournier-Lasserve E., Bousser M.G. Clinical and brain MRI follow-up study of a family with COL4A1 mutation. Neurology. 2007;69:1564–1568. doi: 10.1212/01.wnl.0000295994.46586.e7. [DOI] [PubMed] [Google Scholar]
- 14.Takenouchi T., Ohyagi M., Torii C., Kosaki R., Takahashi T., Kosaki K. Porencephaly in a fetus and HANAC in her father: variable expression of COL4A1 mutation. Am. J. Med. Genet. A. 2015;167A:156–158. doi: 10.1002/ajmg.a.36823. [DOI] [PubMed] [Google Scholar]
- 15.Nandeesh B.N., Bindu P.S., Narayanappa G., Chickabasaviah Yasha T., Mahadevan A., Kulanthaivelu K., Santosh V. Cerebral small vessel disease with hemorrhagic stroke related to COL4A1 mutation: a case report. Neuropathol. Off. J. Jpn. Soc. Neuropathol. 2020;40:93–98. doi: 10.1111/neup.12607. [DOI] [PubMed] [Google Scholar]
- 16.Corlobe A., Tournier-Lasserve E., Mine M., Menjot de Champfleur N., Carra Dalliere C., Ayrignac X., Labauge P., Arquizan C. COL4A1 mutation revealed by an isolated brain hemorrhage. Cerebrovasc. Dis. Basel Switz. 2013;35:593–594. doi: 10.1159/000351520. [DOI] [PubMed] [Google Scholar]
- 17.Kinoshita K., Ishizaki Y., Yamamoto H., Sonoda M., Yonemoto K., Kira R., Sanefuji M., Ueda A., Matsui H., Ando Y., et al. De novo p.G696S mutation in COL4A1 causes intracranial calcification and late-onset cerebral hemorrhage: a case report and review of the literature. Eur. J. Med. Genet. 2020;63 doi: 10.1016/j.ejmg.2019.103825. [DOI] [PubMed] [Google Scholar]
- 18.Ayrignac X., Nicolas G., Carra-Dallière C., Hannequin D., Labauge P. Brain calcifications in adult-onset genetic leukoencephalopathies: a review. JAMA Neurol. 2017;74:1000–1008. doi: 10.1001/jamaneurol.2017.1062. [DOI] [PubMed] [Google Scholar]
- 19.Shah S., Ellard S., Kneen R., Lim M., Osborne N., Rankin J., Stoodley N., van der Knaap M., Whitney A., Jardine P. Childhood presentation of COL4A1 mutations. Dev. Med. Child Neurol. 2012;54:569–574. doi: 10.1111/j.1469-8749.2011.04198.x. [DOI] [PubMed] [Google Scholar]
- 20.Sibon I., Coupry I., Menegon P., Bouchet J.P., Gorry P., Burgelin I., Calvas P., Orignac I., Dousset V., Lacombe D., et al. COL4A1 mutation in axenfeld-rieger anomaly with leukoencephalopathy and stroke. Ann. Neurol. 2007;62:177–184. doi: 10.1002/ana.21191. [DOI] [PubMed] [Google Scholar]
- 21.Coupry I., Sibon I., Mortemousque B., Rouanet F., Mine M., Goizet C. Ophthalmological features associated with COL4A1 mutations. Arch. Ophthalmol. Chic. Ill. 2010;128:483–489. doi: 10.1001/archophthalmol.2010.42. 1960. [DOI] [PubMed] [Google Scholar]
- 22.Plaisier E., Gribouval O., Alamowitch S., Mougenot B., Prost C., Verpont M.C., Marro B., Desmettre T., Cohen S.Y., Roullet E., et al. COL4A1 mutations and hereditary angiopathy, nephropathy, aneurysms, and muscle cramps. N. Engl. J. Med. 2007;357:2687–2695. doi: 10.1056/NEJMoa071906. [DOI] [PubMed] [Google Scholar]
- 23.Alamowitch S., Plaisier E., Favrole P., Prost C., Chen Z., Van Agtmael T., Marro B., Ronco P. Cerebrovascular disease related to COL4A1 mutations in HANAC syndrome. Neurology. 2009;73:1873–1882. doi: 10.1212/WNL.0b013e3181c3fd12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plaisier E., Chen Z., Gekeler F., Benhassine S., Dahan K., Marro B., Alamowitch S., Paques M., Ronco P. Novel COL4A1 mutations associated with HANAC syndrome: a role for the triple helical CB3[IV] domain. Am. J. Med. Genet. A. 2010;152A:2550–2555. doi: 10.1002/ajmg.a.33659. [DOI] [PubMed] [Google Scholar]
- 25.de Vries L.S., Koopman C., Groenendaal F., Van Schooneveld M., Verheijen F.W., Verbeek E., Witkamp T.D., van der Worp H.B., Mancini G. COL4A1 mutation in two preterm siblings with antenatal onset of parenchymal hemorrhage. Ann. Neurol. 2009;65:12–18. doi: 10.1002/ana.21525. [DOI] [PubMed] [Google Scholar]
- 26.Verbeek E., Meuwissen M.E.C., Verheijen F.W., Govaert P.P., Licht D.J., Kuo D.S., Poulton C.J., Schot R., Lequin M.H., Dudink J., et al. COL4A2 mutation associated with familial porencephaly and small-vessel disease. Eur. J. Hum. Genet. EJHG. 2012;20:844–851. doi: 10.1038/ejhg.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maurice P., Guilbaud L., Garel J., Mine M., Dugas A., Friszer S., Maisonneuve E., Moutard M.L., Coste T., Héron D., et al. Prevalence of COL4A1 and COL4A2 mutations in severe fetal multifocal hemorrhagic and/or ischemic cerebral lesions. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2021;57:783–789. doi: 10.1002/uog.22106. [DOI] [PubMed] [Google Scholar]
- 28.Shah S., Kumar Y., McLean B., Churchill A., Stoodley N., Rankin J., Rizzu P., van der Knaap M., Jardine P. A dominantly inherited mutation in collagen IV A1 (COL4A1) causing childhood onset stroke without porencephaly. Eur. J. Paediatr. Neurol. EJPN Off. J. Eur. Paediatr. Neurol. Soc. 2010;14:182–187. doi: 10.1016/j.ejpn.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Kuo D.S., Labelle-Dumais C., Gould D.B. COL4A1 and COL4A2 mutations and disease: insights into pathogenic mechanisms and potential therapeutic targets. Hum. Mol. Genet. 2012;21:R97–110. doi: 10.1093/hmg/dds346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeanne M., Gould DB. Genotype-phenotype correlations in pathology caused by collagen type IV alpha 1 and 2 mutations. Matrix Biol. J. Int. Soc. Matrix Biol. 2017;57–58:29–44. doi: 10.1016/j.matbio.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuo D.S., Labelle-Dumais C., Mao M., Jeanne M., Kauffman W.B., Allen J., Favor J., Gould D.B. Allelic heterogeneity contributes to variability in ocular dysgenesis, myopathy and brain malformations caused by COL4A1 and COL4A2 mutations. Hum. Mol. Genet. 2014;23:1709–1722. doi: 10.1093/hmg/ddt560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeanne M., Jorgensen J., Gould DB. Molecular and genetic analyses of collagen type IV mutant mouse models of spontaneous intracerebral hemorrhage identify mechanisms for stroke prevention. Circulation. 2015;131:1555–1565. doi: 10.1161/CIRCULATIONAHA.114.013395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ratelade J., Mezouar N., Domenga-Denier V., Rochey A., Plaisier E., Joutel A. Severity of arterial defects in the retina correlates with the burden of intracerebral haemorrhage in COL4A1-related stroke. J. Pathol. 2018;244:408–420. doi: 10.1002/path.5023. [DOI] [PubMed] [Google Scholar]
- 34.Mancuso M., Arnold M., Bersano A., Burlina A., Chabriat H., Debette S., Enzinger C., Federico A., Filla A., Finsterer J., et al. Monogenic cerebral small-vessel diseases: diagnosis and therapy. Consensus recommendations of the European academy of neurology. Eur. J. Neurol. 2020;27:909–927. doi: 10.1111/ene.14183. [DOI] [PubMed] [Google Scholar]
- 35.Guey S., Lesnik Oberstein S.A.J., Tournier-Lasserve E., Chabriat H. Hereditary cerebral small vessel diseases and stroke: a guide for diagnosis and management. Stroke. 2021;52:3025–3032. doi: 10.1161/STROKEAHA.121.032620. [DOI] [PubMed] [Google Scholar]
- 36.Mine M., Tournier-Lasserve E. Intracerebral hemorrhage and COL4A1 mutations, from preterm infants to adult patients. Ann. Neurol. 2009;65:1–2. doi: 10.1002/ana.21607. [DOI] [PubMed] [Google Scholar]
- 37.Saskin A., Sillon G., Palfreeman N., Buhas D. COL4A1/2 CNVs and cerebral small vessel disease: narrowing in on the critical chromosomal region. Neurology. 2018;90:1026–1028. doi: 10.1212/WNL.0000000000005601. [DOI] [PubMed] [Google Scholar]
- 38.Kuuluvainen L., Mönkäre S., Kokkonen H., Zhao F., Verkkoniemi-Ahola A., Schleutker J., Hakonen A.H., Hartikainen P., Pöyhönen M., Myllykangas L. COL4A1 and COL4A2 duplication causes cerebral small vessel disease with recurrent early onset ischemic strokes. Stroke. 2021;52:e624–e625. doi: 10.1161/STROKEAHA.120.033864. [DOI] [PubMed] [Google Scholar]
- 39.Ding X.Q., Hagel C., Ringelstein E.B., Buchheit S., Zeumer H., Kuhlenbäumer G., Appenzeller S., Fiehler J. MRI features of pontine autosomal dominant microangiopathy and leukoencephalopathy (PADMAL) J. Neuroimaging Off. J. Am. Soc. Neuroimaging. 2010;20:134–140. doi: 10.1111/j.1552-6569.2008.00336.x. [DOI] [PubMed] [Google Scholar]
- 40.Grobe-Einsler M., Urbach H., Paus S. Recurrent pontine strokes in a young male. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2020;29 doi: 10.1016/j.jstrokecerebrovasdis.2020.105386. [DOI] [PubMed] [Google Scholar]