Highlights
-
•
Cerebral blood flow (CBF) and spatial coefficient of variation of the arterial transit time (ATT) are less sensitive markers of cognitive impairment and clinical dementia.
-
•
Greater white matter hyperintensity volume (WMHV) was consistently associated with cognitive impairment and dementia, rendering it as a more sensitive longitudinal marker.
Keywords: Cerebral blood flow, Spatial coefficient of variation, White matter hyperintensities, Magnetic resonance imaging, Cognitive decline, Dementia
Abstract
Background
The arterial spin labeling-spatial coefficient of variation (sCoV) is a new vascular magnetic resonance imaging (MRI) parameter that could be a more sensitive marker for dementia-associated cerebral microvascular disease than the commonly used MRI markers cerebral blood flow (CBF) and white matter hyperintensity volume (WMHV).
Methods
195 community-dwelling older people with hypertension were invited to undergo MRI twice, with a three-year interval. Cognition was evaluated every two years for 6-8 years using the mini-mental state examination (MMSE). We assessed relations of sCoV, CBF and WMHV with cognitive decline during follow-up. We also registered dementia diagnoses, up to 9 years after the first scan. In an additional analysis, we compared these MRI parameters between participants that did and did not develop dementia.
Results
136/195 completed the second scan. sCoV and CBF were not associated with MMSE changes during 6-8 years of follow-up. Higher WMHV was associated with declining MMSE scores (-0.02 points/year/ml, 95%CI=-0.03 to -0.00). ScOv and CBF did not differ between participants who did (n=15) and did not (n=180) develop dementia, whereas higher WMHV was reported in participants who developed dementia after the first MRI (13.3 vs 6.1mL, p<0.001). There were no associations between longitudinal change in any of the MRI parameters and cognitive decline or subsequent dementia.
Conclusion
Global sCoV and CBF were less sensitive longitudinal markers of cognitive decline and dementia compared to WMHV in community-dwelling older people with hypertension. Larger longitudinal MRI perfusion studies are needed to identify possible (regional) patterns of cerebral perfusion preceding cognitive decline and dementia diagnosis.
Non-standard Abbreviations and Acronyms
- ASL
Arterial spin labeling
- CBF
Cerebral blood flow
- FLAIR
Fluid-Attenuated Inverse Recovery
- GM
Grey Matter
- MMSE
Mini-mental state examination
- sCoV
Spatial coefficient of variation
- WMHV
White matter hyperintensity volume
- WM
White matter
- GM
Grey matter
Introduction
Dementia is an important health problem, affecting 50 million people worldwide [1]. The pathological mechanisms that cause this condition remain largely unknown, but there is growing evidence that these mechanisms include a cerebrovascular component [2]. Dementia-related cerebral microvascular changes may be detectable years before clinical features become apparent [3]. As potential markers of these microvascular changes white matter hyperintensities (WMH) and dysregulation of cerebral perfusion have been associated with cognitive decline. Cross-sectional studies have demonstrated associations between reductions in CBF and WMH progression as potentially important contributors to cerebral small vessel disease, and thereby to old-age dementia, whereas longitudinal reports are inconsistent [4]. Whether CBF reductions and WMH progression precede or follow cognitive decline is unknown, leaving the circumstances by which these associations occur not fully elucidated [5]. Despite the fact that the direction of the associations between CBF, WMH and cognition is not well understood, both WMH as well as dysregulation of CBF have been associated with cognitive impairment and may be potentially useful for identifying those at risk of cognitive decline and dementia [6], [7], [8], [9], [10]. Magnetic resonance imaging (MRI) can be used for the assessment of WMHs and cerebral perfusion using Fluid-Attenuated Inverse Recovery (FLAIR) and arterial spin labeling (ASL) sequences respectively. In ASL techniques, arterial blood water protons are magnetically labeled [11]. This approach can accurately quantify cerebral perfusion, operationalized as cerebral blood flow (CBF) [12]. However, the time it takes the labeled proton to travel from the labeling plane in the neck to an imaging voxel, the arterial transit time (ATT), ideally requires optimal acquisition timing. Particularly in the presence of increased vascular resistance, for example atherosclerosis, ATT may be prolonged, causing heterogeneity in ASL images and less accurate estimation of CBF [13,14].
The spatial coefficient of variation (sCoV) can be considered a proxy measure of ATT, that estimates ATT indirectly by the distribution of ASL-signal. In the presence of increased vascular resistance, this dynamic vascular parameter has the potential to provide more accurate information on dementia-related cerebral vascular changes, compared to other commonly used MRI markers such as CBF and WMHV [11]. We hypothesize that ASL-sCoV is more strongly related to cognitive decline compared to CBF and WMHV in community-dwelling older adults.
Methods
Study design and participants
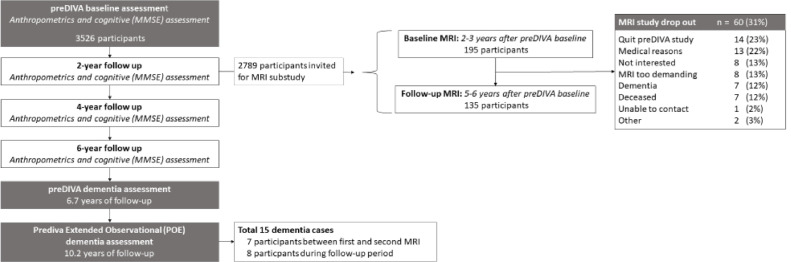
Fig. 1 provides a brief overview of the study. We performed a post-hoc analysis on the MRI substudy (preDIVA-M) of the Prevention of Dementia by Intensive Vascular Care (preDIVA) Trial. The preDIVA trial and the preDIVA-M substudy have been described in detail previously [15,16]. In brief, preDIVA was a cluster-randomized controlled trial in community-dwelling older adults, studying the efficacy of nurse-led cardiovascular risk management compared to usual care at the general practitioner setting [15].
Fig. 1.
Study Flowchart
For the preDIVA-M substudy, a subset of 195 preDIVA participants, equally distributed between intervention arms, with systolic hypertension (>140 mmHg) and without dementia at the two-year follow-up visit, were invited to undergo MRI (scan 1) and again three years later (scan 2) [15]. For the current study, we analysed the preDIVA-M population as a single cohort irrespective of treatment allocation, because there were no differences between the preDIVA trial arms in WMHV, ASL measurements, or dementia incidence [15], nor was it likely that the preDIVA intervention affected the relation of WMHs or ASL measurements with cognitive decline or incident dementia.
Cognitive decline and dementia
Clinical parameters were evaluated by trained research nurses at baseline and every following 2 years for a total of 6-8 years. Assessments included anthropometrics, medical history, medication use, risk factors for cardiovascular disease and dementia, and assessment of cognitive function using the Mini-Mental State Examination (MMSE) [17]. Information was cross-checked with the general practitioner's electronic health records. In the current study, we operationalized cognitive decline as the course of the MMSE-scores during the 6–8-years of follow-up.
For dementia and mortality, follow-up extended up to 10-12 years after preDIVA trial baseline [15]. All potentially relevant information on (suspected) dementia during this time was gathered from participants’ electronic health records. Incident dementia was defined as a clinical diagnosis according to the Diagnostic and Statistical Manual of Mental Disorders-IV [16], confirmed by an independent outcome adjudication committee, consisting of geriatricians, old-age psychiatrists, general practitioners, cardiologists, and neurologists, who evaluated all available clinical data, including reports on hospital admissions, outpatient diagnostic evaluations, neuropsychological examinations, and reports by the general practitioner. All dementia diagnoses were re-evaluated on the basis of the clinical course after 1 year to minimize the risk of false-positive diagnoses.
MR Imaging measures
MRI acquisition details have been described previously [16]. Studied MRI measures included WMHV, and CBF and sCoV in the grey matter (GM). CBF and sCoV were evaluated both with use of vascular crushing gradients in ASL (crushed; more sensitive for microvasculature perfusion) and without vascular crushing (non-crushed; including both micro- and macrovascular perfusion). The most relevant value for CBF would be the crushed measure, providing the best proxy for cerebral perfusion, and for sCoV the non-crushed value, providing the best proxy for distribution of ASL signal. However, for completeness both crushed and non-crushed are reported in this paper. Brain volumes were calculated from 3DT1 scans using Statistical Parametric Mapping (SPM12) [18].
Image processing for the structural and ASL images was performed with ExploreASL [19]. Structural processing employed Lesion Segmentation Toolbox (LST) (WMH segmentation and T1w lesion-filling before GM/WM segmentation) [20,21] and CAT12 (T1w segmentation and spatial normalization).[22] GM masks were defined as pGM>0.7 [23]. ASL image processing included motion correction, motion outlier detection and rigid-body registration of CBF to the GM map [19]. CBF was quantified from ASL images using a single compartment model [14]. The sCoV was defined as the standard deviation of CBF divided by the mean CBF in the total GM [24]. To affirm the validity of our CBF estimations, we compared the associations of CBF with age and sex in our study, which are both well-documented modifiers of CBF, to those found in other studies in similar populations of community-dwelling older people [8,25,26].
Statistical analysis
In our primary analysis, we assessed the relation of CBF, sCoV, and WMHV with cognitive decline, by using repeated measurement models of the change in MMSE score during the preDIVA trial relative to the preDIVA baseline. In these models, we used the preDIVA two-yearly follow-up visits’ MMSE scores prior to MRI as outcomes, predicted by each individual MRI measure, adjusted for baseline MMSE score, sex, age at MMSE assessment, age at MRI, antihypertension medication use, and MRI visit number (scan 1/scan 2), with a random intercept per participant to account for multiple measurements within individuals. These models included both MRI measures collected at scan 1 and scan 2 for the main model. WHMV and sCoV were logistically transformed to approach a normal distribution [27,28].
To affirm the robustness of our findings, we repeated analyses assessing MRI measures at scan 1 and 2 separately. We also assessed the relation between change in MMSE score and change in MRI measures in similar models, with the change per year in MRI measure from scan 1 to scan 2 as predictor, additionally adjusted for the MRI value at scan 1 for WMHV. Two previous studies from the preDIVA-M cohort did not report an association between blood pressure levels and global WMH progression, which is why we did not adjust for blood pressure levels in the current study [27,29].
As a secondary analysis, we investigated whether the evaluated MRI parameters differed between individuals who developed dementia during the 6-9 years follow-up following MRI, using a case-cohort design, and performing Student's t tests and Wilcoxon rank-sum tests comparing MRI measures. In addition, we compared mean and median annual change in imaging measures between these groups, operationalized as the difference between MRI measures at scan 1 and 2 divided by the time between measurements. To evaluate the influence of potential confounders, repeated measurement models were performed with MRI measures on scan 1 and scan 2 as outcomes and subsequent dementia as predictor. Again, WHMV and sCoV were logistically transformed to approach a normal distribution [27,28]. All repeated measurement analyses were adjusted for age, sex, and antihypertension medication use. WMHV was additionally adjusted for total brain volume. We verified the findings of our repeated measurement models using linear regression models for the baseline and follow-up MRI separately. All analyses were carried out using R statistical software v3.6.1 [30].
Results
Population characteristics
In total, 195 participants underwent the first MRI scan of whom 136 (69%) also attended the follow-up scan, on average 34 (SD1.2) months later (Fig. 1). Table 1 lists the population characteristics. The mean age of the population at the first scan was 77.3±2.5 years.
Table 1.
Population characteristics of participants.
| Scan 1n=195 | Scan 2n=136 | |
|---|---|---|
| Women, % | 103 (52.8) | 72 (52.9) |
| Age in years, mean (± SD) | 77.3 (2.5) | 80.0 (2.6) |
| History stroke (incl. cortical infarctions), % | 13 (6.8) | 10 (7.5) |
| History DM2, % | 20 (10.3) | 14 (10.3) |
| History CVD, % | 40 (21.3) | 26 (19.8) |
| MMSE, mean (±SD) | 28.6 (1.5) | 28.9 (1.3) |
|
Smoking status, Never / Former/ Current % |
14 (7.2) /3 (1.5) /178 (91.3) | 6 (4.4) /3 (2.2)/119 (87.5) |
| Antihypertension medication use, % | 79 (40.5) | 75 (55.2) |
| Antiplatelet use, % | 52 (36.3) | 35 (27.3) |
| Antithrombotic use, % | 60 (30.8) | 43 (33.6) |
| SBP, mean (±SD) | 151.2 (21.5) | 156.4 (18.3) |
| DBP, mean (±SD) | 81.8 (11.6) | 79.5 (11.0) |
| Cholesterol, mean (±SD) | 5.4 (1.2) | 5.2 (1.1) |
| LDL, mean (±SD) | 3.3 (1.1) | 2.8 (1.0) |
| HDL, mean(±SD) | 1.6 (0.4) | 1.6 (0.5) |
Abbreviations: SD= standard deviation, DM2=diabetes mellitus type 2, CVD = cardiovascular disease, MMSE=mini mental state examination, SBP=systolic blood pressure, DBP=diastolic blood pressure, LDL=low-density lipoprotein, HDL=high-density lipoprotein.
Missings scan 1/2: Sex 0/0, age: 0/0, history of stroke 3/2, DM2 0/0, CVD 7/5, MMSE 1/2, smoking status 0/8, antiplatelet use 0/8, antithrombotic use 0/8, SBP 1/9, DBP 1/9, cholesterol 35/19, LDL 40/19, HDL 37/19.
Cognitive decline
CBF and sCoV were not associated with annual MMSE change during the preDIVA trial (-0.00 points per mL/100g/min, 95%CI=-0.01-0.00, p=0.46 and 0.04 points/SD, 95%CI=-0.08-0.16, p=0.55, respectively) (Table 2). These relations were also absent when looking at scan 1 and 2 separately (Supplementary Table 1). Higher volume of WMH was associated with greater annual decline in MMSE (-0.02 points/mL, 95%CI=-0.03-0.00, p=0.03) during preDIVA (Table 2), with comparable effect sizes for scan 1 (-0.02 points/mL, 95%CI=-0.03-0.00, p=0.03) and scan 2 (-0.01 points/mL, 95%CI=-0.02-0.01, p=0.28, Supplementary Table 1). There were no significant associations between annual change in MMSE during the study and change in MRI measures between scan 1 and scan 2 (Supplementary Table 2).
Table 2.
Association between MRI parameters and annual change in MMSE during the study. Results of repeated measurements mixed models with each individual MRI parameter as predictor and annual MMSE change per 1.0 point during the study as outcome, adjusted for age, sex, antihypertension medication use, and MMSE at baseline.
| MRIparameter | n MMSE /n individuals | ES | 95%CI | p-value |
|---|---|---|---|---|
| WMH volume (mL) | 833/179 | -0.02 | -0.03 to -0.00 | 0.03 |
| CBF GM (mL/100g/min) | 840/186 | -0.00 | -0.01 to 0.00 | 0.40 |
| CBF GM, crushed (mL/100g/min) | 840/186 | -0.00 | -0.01 to 0.00 | 0.46 |
| CBF spatial CoV (SD) a | 840/186 | 0.04 | -0.08 to 0.16 | 0.55 |
| CBF spatial CoV, crushed (SD) a | 840/186 | 0.02 | -0.10 to 0.13 | 0.79 |
Abbreviations: ES= Effect Size, CI= confidence interval GM = grey matter, WMH = white matter hyperintensity, CBF = cerebral blood flow, CoV = coefficient of variation
These variables were introduced on a log scale.
The effect sizes for the associations of CBF with age and sex were similar to those in previous studies in similar populations, with CBF being lower with higher age (standardized beta: -0.21, 95%CI=-0.27 to -0.15, p<0.001), and lower in women (standardized beta: -0.41, 95%CI=-0.65- -0.16, p=0.001) (Supplementary Table 3) [8,25,26].
Dementia
Fifteen (7.7%) participants developed dementia during a median follow-up of 7.0 years (interquartile range (IQR) 6.4-8.3) since the first scan, eight of whom also underwent a second scan before developing dementia.
Mean and longitudinal change in CBF and sCoV (non-crushed and crushed) were similar for individuals who developed dementia versus those who did not in the years following MRI at either scan (Table 3).
Table 3.
Imaging characteristics for participants who did and did not develop dementia
| Imaging variable | Radiological assessment | No DementiaScan 1 n=180,Scan 2 n=128 | DementiaScan 1 n=15,Scan 2 n=8 | p-value |
|---|---|---|---|---|
|
WMH Volume (mL), Median (IQR) |
Scan 1 | 6.1 (3.4 – 10.5) | 13.3 (9.4 – 20.0) | < 0.001 |
| Scan 2 | 7.9 (4.4 – 12.3) | 15.0 (9.5 – 28.0) | 0.14 | |
| ∆ /year (SD) | 0.5 (0.7) | 1.2 (1.0) | 0.10 | |
|
CBF GM (mL/100g/min), Mean (±SD) |
Scan 1 | 67.89 (22.11) | 60.49 (17.97) | 0.17 |
| Scan 2 | 64.45 (24.74) | 67.83 (16.18) | 0.68 | |
| ∆/year (SD) | -1.78 (7.9) | 1.57 (8.1) | 0.41 | |
|
CBF GM crushed (mL/100gr/minute), Mean (±SD) |
Scan 1 | 79.43 (18.24) | 78.99 (19.80) | 0.94 |
| Scan 2 | 80.39 (24.33) | 79.32 (27.39) | 0.94 | |
| ∆/year (SD) | -0.02 (8.3) | 0.72 (10.55) | 0.88 | |
|
CBF spatial CoV (SD), Median (IQR) |
Scan 1 | 0.46 (0.40 – 0.57) | 0.51 (0.48 – 0.60) | 0.24 |
| Scan 2 | 0.45 (0.39 – 0.54) | 0.46 (0.42 – 0.48) | 0.72 | |
| ∆/year (SD) | - 0.01 (0.0) | 0.00 (0.1) | 0.93 | |
| Scan 2 | 0.46 (0.42 – 0.55) | 0.48 (0.46 – 0.52) | 0.76 | |
| ∆/year (SD) | 0.00 (0.1) | -0.01 (0.1) | 0.49 |
Abbreviations: GM = grey matter, WMH = white matter hyperintensity, CBF = cerebral blood flow, CoV = coefficient of variation, IQR=interquartile range, SD= standard deviation, crushed = measure for cerebral microvasculature, ∆ = scan 2-scan 1/number of years between scan 1 and scan 2.
Missings no dementia scan 1/2: WMH Volume 10/8, CBF GM 8/8, CBF GM crushed 8/8, CBF spatial CoV 8/8, CBF spatial CoV crushed 8/8.
Missings dementia scan 1/2: WMH Volume 0/2, CBF GM 1/3, CBF GM crushed 1/3, CBF spatial CoV 1/3, CBF spatial CoV crushed 1/3.
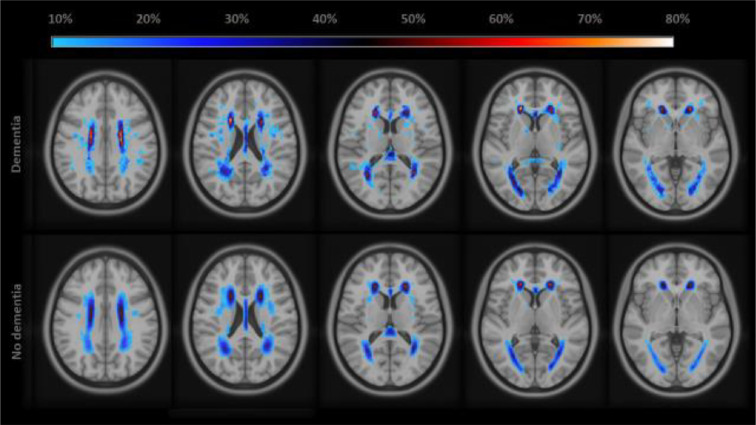
WHMV at scan 1 was higher in participants who developed dementia following MRI (Table 3; Fig. 2) compared to those who did (13.3 vs 6.1 mL, p<0.001). This difference was similar at scan 2, albeit not significant due to less power (15.0 vs 7.9 mL, p=0.14). Annual WHMV progression did not significantly differ between dementia vs no dementia groups (1.2 vs 0.5 ml/year, p=0.10). Results were similar when only including participants who attended both scan waves, but not significant due to less power (Supplementary table 4).
Fig. 2.
Density map on white matter hyperintensity location and volume of participants with and without dementia.
Repeated measurement models combining scan 1 and scan 2, showed that participants who developed dementia had higher WMHVs than those who did not (8.59 mL, 95%CI 4.62 – 13.1; Table 4). These results were consistent in separate regression analyses of scan 1 and scan 2 (Supplementary table 5).
Table 4.
Difference in radiological measures in individuals with and without incipient dementia.
| Imaging variable | ß (95% CI) | p-value |
|---|---|---|
| WMH volume (mL) | 8.59 (4.62– 13.1) | <0.001 |
| CBF GM (mL/100gr/minute) | -3.04 (-14.25 – 8.17) | 0.60 |
| CBF GM crushed (mL/100gr/minute) | -0.05 (-10.51 – 10.41) | 0.99 |
| CBF spatial CoV (SD) a | 0.17 (-0.34 – 0.67) | 0.52 |
| CBF spatial CoV, crushed (SD) a | 0.12 (-0.37 - 0.62) | 0.64 |
Results of repeated measurements mixed models.
These variables were introduced on a log scale. All measures adjusted for age, antihypertension medication use, and sex. WMH additionally adjusted for TBV. Abbreviations: CI=confidence interval. GM = grey matter, WMH = white matter hyperintensity, CBF = cerebral blood flow, CoV = coefficient of variation, crushed = measure for cerebral microvasculature ∆ = scan 2-scan 1/number of years between scan 1 and scan 2.
Discussion
This study has three main findings. First, both sCoV and CBF were not associated with changes in MMSE score during 6-8 year of follow-up, whereas higher WMHV was associated with declining MMSE scores during the trial. Second, sCoV and CBF did not differ between participants who did and did not develop dementia in the 8 years follow-up period, whereas WMHV was significantly higher in those who developed dementia. Finally, there were no associations between longitudinal change from the first to second MRI and cognitive decline nor subsequent dementia for any of the investigated MRI measures.
Our findings from the repeated measurement models imply that, compared to WMHV, CBF and sCoV were not sensitive markers to detect longitudinal vascular or perfusion correlates of cognitive decline or dementia in community-dwelling older adults. This finding may have several explanations. First, our study might have been underpowered to detect differences in the ASL-outcomes between the group, especially given the low incidence of dementia in our cohort. Despite this relatively small sample size, we did observe significant associations between higher WMHV and more rapid cognitive decline, and that WMHV was significantly higher in individuals who later developed dementia. Although the difference was not significant, WMHV progression was higher in those who developed dementia compared to those who did not. Next to the relatively low number of individuals who developed dementia, the relatively short time-interval between scans (mean: 2.8 years) may have also contributed to the lack of an association with CBF and sCoV, although WMHV was not affected by this [28].
The macrovascular assessment of cerebral hemodynamic status, using CBF and sCoV, seems less sensitive for identifying individuals at risk of cognitive decline and possibly incipient dementia than WMHV. These findings are in line with previous reports [31], [32], [33], [34].
CBF heterogeneity may also occur at the microvascular level of cerebral capillaries as the result of progressive disturbances in CBF patterns caused by (incipient) dementia [35]. Local, rather than global, dysregulation of CBF has particularly been associated with tissue damage – manifesting as WMH [36,37]. Ultimately, the micro- and macrovascular properties of CBF heterogeneity may help improve our understanding on the pathological mechanisms that underlie cerebral small vessel disease and dementia.
A second explanation to our findings lies in the large physiological variability that CBF and, to a lesser extent, sCoV are prone to throughout the day. This large intra-individual variability may negatively affect the suitability of these markers to study between group differences, both cross-sectionally and longitudinally. WMHV is a parameter that is less subject to this variability [38].
Third, CBF estimations might have been insufficiently accurate, but cross-checking the associations of CBF and established CBF modifiers (i.e., age, sex) with findings from previous studies, confirmed the validity of our measurements in comparison with previous cohorts, making this less plausible [8,25,26].
Fourth, although the association of CBF and CBF velocity with cognitive deterioration has frequently been described in midlife [8,39], the null findings in this study could reflect the absence of an association of CBF and sCoV with cognitive decline and incipient dementia in older populations. A possible explanation is that CBF loses its discriminative properties in older age due to age-related changes in CBF and sCoV occurring with normal ageing, which might reflect microvascular changes such as decreased elasticity and increased tortuosity of the small vessels [40,41]. In addition, most studies investigated CBF associations in participants with advanced disease, whereas our study sample included relatively healthy participants [42]. It is possible that certain correlations may be stronger and easier to detect in study populations with (advanced) cerebrovascular pathology.
Fifth, previous studies mainly suggest an association between specific regional cerebral differences (e.g., cingulate, precuneus, parietal lobes and inferior frontal regions among others) in perfusion measures in participants with and without dementia [43]. These regional decreases in CBF have also been reported in the preclinical phase of dementia, predicting cognitive decline and progression to dementia in patients with mild cognitive impairment [44], [45], [46], [47], [48].
Our study has several strengths, including a very complete follow-up of cognition and dementia over a long period of time. In addition, both longitudinal and cross-sectional data were available for a large cohort of older participants with ASL, allowing to study the associations of MRI measures and dementia both at a certain point and over time.
This study also has limitations. First, we had a limited number of dementia cases available for analysis. However, these analyses on dementia only served as secondary analyses to confirm our findings regarding cognitive decline. Furthermore, although the number of dementia cases was limited, we did clearly identify differences in WMHV between those with and without incipient dementia, suggesting that CBF and sCoV in our study were clearly not superior markers of late life cognitive deterioration compared to WMHV.
Second, our longitudinal time-interval was relatively short, reducing statistical power to detect potential longitudinal between-group differences. As opposed to WMHV, the perfusion parameters CBF and sCoV are greatly susceptible to physiological variability. Prolonged longitudinal investigation of these parameters may reveal specific patterns of long-term longitudinal changes in sCOV and CBF preceding dementia that may have more value for the identification of individuals with cognitive decline and dementia.
Third, the reliability of estimating sCoV in WM is limited compared to GM, because of the longer WM ATT and low WM CBF, which will lead to WM sCoV being dominated by noise [24].
In addition, similar to normal CBF calculations, ASL-sCoV can be affected by WM contamination of GM signal and potentially biased by cortical thinning in certain subjects, until methods for partial volume correction in ASL-sCoV are developed.
Fourth, besides use of antihypertension medication, other medications with vasoactive properties that may influence CBF (e.g. nitroglycerine, benzodiazepines) were not considered, because these data were not available.
Finally, as a more general limitation, CBF is measured with participants in a supine position, potentially concealing impaired cerebral autoregulation that may become apparent when the participant is upright [49,50].
Conclusion
HMs Therefore, further research into white matter hyperintensities and the exact role in the pathophysiology of dementia is required and should involve imaging- neuropathology studies, and in-vivo research with advanced MRI sequences, such as diffusion tensor imaging to quantify the microstructural damage of the white matter Therefore, further research into white matter hyperintensities and the exact role in the pathophysiology of dementia is required and should involve imaging- neuropathology studies, and in-vivo research with advanced MRI sequences, such as diffusion tensor imaging to quantify the microstructural damage of the white matter Therefore, further research into white matter hyperintensities and the exact role in the pathophysiology of dementia is required and should involve imaging- neuropathology studies, and in-vivo research with advanced MRI sequences, such as diffusion tensor imaging to quantify the microstructural damage of the white matter Therefore, further research into white matter hyperintensities and the exact role in the pathophysiology of dementia is required and should involve imaging- neuropathology studies, and in-vivo research with advanced MRI sequences, such as diffusion tens
In this cohort of community dwelling hypertensive older adults, global sCoV and CBF were not associated with cognitive decline or dementia onset, whereas WMHV was. This suggests that sCoV and CBF are not superior markers of late life cognitive decline and incipient dementia compared to WMHV. Larger and longer longitudinal MRI perfusion studies may be needed to identify possible patterns of cerebral perfusion preceding cognitive decline and dementia diagnosis, and whether this would add information over and above the predictive information of changes in WMHV.
Author's contributions
* H. Abdulrahman: analysis and interpretation of data, drafting of the manuscript
* M. Hafdi: analysis and interpretation of data, drafting of the manuscript
H.J.M.M. Mutsaerts: analysis and interpretation of data, and critical revision of the manuscript
J. Petr: analysis and interpretation of data, and critical revision of the manuscript
E. Richard: concept and design, acquisition of data, and critical revision of the manuscript
W.A. van Gool: concept and design, acquisition of data, and critical revision of the manuscript
J.W. van Dalen: analysis and interpretation of data, concept and design, drafting and critical revision of the manuscript.
*These authors contributed equally
Sponsor's role
This project is funded by The Netherlands Organization for Health Research and Development (ZonMw) VIDI grant 91718303 to E. Richard. The funder did not play a role in any part (such as initiation, execution, or interpretation of the results) of this brief report. The corresponding author affirms that she has listed everyone who contributed significantly to the work. All authors meet the criteria for authorship stated in the Uniform Requirements for Manuscripts Submitted to Biomedical Journals.
Declaration of Competing Interest
The authors have no conflicts of interest.
Acknowledgments
None.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cccb.2022.100125.
Appendix. Supplementary materials
References
- 1.Prince, M., World Alzheimer Report 2015. The global impact of dementia. An analysis of prevalence, incidence, cost and trends. 2015.
- 2.Kalaria R.N., Ballard C. Overlap between pathology of Alzheimer disease and vascular dementia. Alzheimer Dis. Assoc. Disord. 1999;13(3):S115–S123. doi: 10.1097/00002093-199912003-00017. Suppl. [DOI] [PubMed] [Google Scholar]
- 3.de la Torre J. The vascular hypothesis of Alzheimer's disease: a key to preclinical prediction of dementia using neuroimaging. J. Alzheimers Dis. 2018;63(1):35–52. doi: 10.3233/JAD-180004. [DOI] [PubMed] [Google Scholar]
- 4.Stewart C.R., et al. Associations between white matter hyperintensity burden, cerebral blood flow and transit time in small vessel disease: an updated meta-analysis. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.647848. 647848-647848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iturria-Medina Y., et al. Early role of vascular dysregulation on late-onset Alzheimer's disease based on multifactorial data-driven analysis. Nat. Commun. 2016;7:11934. doi: 10.1038/ncomms11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mortamais M., Artero S., Ritchie K. White matter hyperintensities as early and independent predictors of Alzheimer's disease risk. J. Alzheimers Dis. 2014;42(4):S393–S400. doi: 10.3233/JAD-141473. Suppl. [DOI] [PubMed] [Google Scholar]
- 7.Prins N.D., Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat. Rev. Neurol. 2015;11(3):157–165. doi: 10.1038/nrneurol.2015.10. [DOI] [PubMed] [Google Scholar]
- 8.Wolters F.J., et al. Cerebral Perfusion and the Risk of Dementia. Circulation. 2017;136(8):719–728. doi: 10.1161/CIRCULATIONAHA.117.027448. [DOI] [PubMed] [Google Scholar]
- 9.Chao L.L., et al. ASL perfusion MRI predicts cognitive decline and conversion from MCI to dementia. Alzheimer Dis. Assoc. Disord. 2010;24(1):19–27. doi: 10.1097/WAD.0b013e3181b4f736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celsis P., et al. Age related cognitive decline: a clinical entity? A longitudinal study of cerebral blood flow and memory performance. J. Neurol. Neurosurg. Psychiatry. 1997;62(6):601–608. doi: 10.1136/jnnp.62.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grade M., et al. A neuroradiologist's guide to arterial spin labeling MRI in clinical practice. Neuroradiology. 2015;57(12):1181–1202. doi: 10.1007/s00234-015-1571-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heijtel D.F., et al. Accuracy and precision of pseudo-continuous arterial spin labeling perfusion during baseline and hypercapnia: a head-to-head comparison with ¹⁵O H₂O positron emission tomography. Neuroimage. 2014;92:182–192. doi: 10.1016/j.neuroimage.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Dai W., et al. Effects of arterial transit delay on cerebral blood flow quantification using arterial spin labeling in an elderly cohort. J. Magn. Reson. Imaging. 2017;45(2):472–481. doi: 10.1002/jmri.25367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alsop D.C., et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn. Reson. Med. 2015;73(1):102–116. doi: 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moll van Charante E.P., et al. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet. 2016;388(10046):797–805. doi: 10.1016/S0140-6736(16)30950-3. [DOI] [PubMed] [Google Scholar]
- 16.Dalen J.W.v., et al. Effect of long-term vascular care on progression of cerebrovascular lesions. Stroke. 2017;48(7):1842–1848. doi: 10.1161/STROKEAHA.117.017207. [DOI] [PubMed] [Google Scholar]
- 17.Folstein M.F., Robins L.N., Helzer J.E. The mini-mental state examination. Arch. Gen. Psychiatry. 1983;40(7) doi: 10.1001/archpsyc.1983.01790060110016. 812-812. [DOI] [PubMed] [Google Scholar]
- 18.Ashburner J., Friston K.J. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Mutsaerts H., et al. Comparison of arterial spin labeling registration strategies in the multi-center GENetic frontotemporal dementia initiative (GENFI) J. Magn. Reson. Imaging. 2018;47(1):131–140. doi: 10.1002/jmri.25751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steenwijk M.D., et al. Accurate white matter lesion segmentation by k nearest neighbor classification with tissue type priors (kNN-TTPs) NeuroImage: Clinical. 2013;(3):462–469. doi: 10.1016/j.nicl.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt P., et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. Neuroimage. 2012;59(4):3774–3783. doi: 10.1016/j.neuroimage.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 22.Franke K., et al. Estimating the age of healthy subjects from T1-weighted MRI scans using kernel methods: exploring the influence of various parameters. Neuroimage. 2010;50(3):883–892. doi: 10.1016/j.neuroimage.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Mutsaerts H.J.M.M., et al. Gray matter contamination in arterial spin labeling white matter perfusion measurements in patients with dementia. NeuroImage: Clinical. 2014;4:139–144. doi: 10.1016/j.nicl.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mutsaerts H.J., et al. The spatial coefficient of variation in arterial spin labeling cerebral blood flow images. J. Cereb. Blood Flow Metabol. 2017;37(9):3184–3192. doi: 10.1177/0271678X16683690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y., et al. Arterial spin labeling MRI study of age and gender effects on brain perfusion hemodynamics. Magn. Reson. Med. 2012;68(3):912–922. doi: 10.1002/mrm.23286. [DOI] [PubMed] [Google Scholar]
- 26.Liu W., Lou X., Ma L. Use of 3D pseudo-continuous arterial spin labeling to characterize sex and age differences in cerebral blood flow. Neuroradiology. 2016;58(9):943–948. doi: 10.1007/s00234-016-1713-y. [DOI] [PubMed] [Google Scholar]
- 27.van Dalen J.W., et al. Longitudinal relation between blood pressure, antihypertensive use, and cerebral blood flow, using arterial spin labelling MRI. J. Cereb. Blood Flow Metabol. 2020 doi: 10.1177/0271678X20966975. p. 0271678X20966975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Dalen J.W., et al. White matter hyperintensity volume and cerebral perfusion in older individuals with hypertension using arterial spin-labeling. AJNR. 2016;37(10):1824–1830. doi: 10.3174/ajnr.A4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Dalen J.W., et al. Cortical microinfarcts detected in vivo on 3 Tesla MRI: clinical and radiological correlates. Stroke. 2015;46(1):255–257. doi: 10.1161/STROKEAHA.114.007568. [DOI] [PubMed] [Google Scholar]
- 30.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2019. R: A language and environment for statistical computing. [Google Scholar]
- 31.Wang Y.L., et al. Associations of white matter hyperintensities with cognitive decline: a longitudinal study. J. Alzheimers Dis. 2020;73(2):759–768. doi: 10.3233/JAD-191005. [DOI] [PubMed] [Google Scholar]
- 32.Qi X., et al. White matter hyperintensities predict cognitive decline: a community-based study. Can. J. Neurol. Sci. 2019;46(4):383–388. doi: 10.1017/cjn.2019.47. [DOI] [PubMed] [Google Scholar]
- 33.Legdeur N., et al. White matter hyperintensities and hippocampal atrophy in relation to cognition: the 90+ study. J. Am. Geriatr. Soc. 2019;67(9):1827–1834. doi: 10.1111/jgs.15990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye S., et al. White-matter hyperintensities and lacunar infarcts are associated with an increased risk of Alzheimer’s disease in the elderly in China. J. Clin. Neurol. 2019;15(1):46–53. doi: 10.3988/jcn.2019.15.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Østergaard L., et al. The capillary dysfunction hypothesis of Alzheimer's disease. Neurobiol. Aging. 2013;34(4):1018–1031. doi: 10.1016/j.neurobiolaging.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9(7):689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 37.Brown W.R., Thore C.R. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol. Appl. Neurobiol. 2011;37(1):56–74. doi: 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wardlaw J.M., et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruitenberg A., et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam study. Ann. Neurol. 2005;57(6):789–794. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- 40.Xu X., et al. Age-related impairment of vascular structure and functions. Aging Dis. 2017;8(5):590–610. doi: 10.14336/AD.2017.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zimmerman B., et al. Age-related changes in cerebrovascular health and their effects on neural function and cognition: A comprehensive review. Psychophysiology. 2021;58(7):e13796. doi: 10.1111/psyp.13796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wierenga C.E., Hays C.C., Zlatar Z.Z. Cerebral blood flow measured by arterial spin labeling MRI as a preclinical marker of Alzheimer's disease. J. Alzheimers Dis. 2014;4(4):S411–S419. doi: 10.3233/JAD-141467. 42 SupplSuppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang N., Gordon M.L., Goldberg T.E. Cerebral blood flow measured by arterial spin labeling MRI at resting state in normal aging and Alzheimer's disease. Neurosci. Biobehav. Rev. 2017;72:168–175. doi: 10.1016/j.neubiorev.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 44.Xekardaki A., et al. Arterial spin labeling may contribute to the prediction of cognitive deterioration in healthy elderly individuals. Radiology. 2015;274(2):490–499. doi: 10.1148/radiol.14140680. [DOI] [PubMed] [Google Scholar]
- 45.Ikeda E., et al. Total Mini-Mental State Examination score and regional cerebral blood flow using Z score imaging and automated ROI analysis software in subjects with memory impairment. Ann. Nucl. Med. 2008;22(6):539–542. doi: 10.1007/s12149-007-0148-2. [DOI] [PubMed] [Google Scholar]
- 46.Binnewijzend M.A., et al. Cerebral perfusion in the predementia stages of Alzheimer's disease. Eur. Radiol. 2016;26(2):506–514. doi: 10.1007/s00330-015-3834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saito H., et al. Association between regional cerebral blood flow and mini-mental state examination score in patients with Alzheimer’s disease. Curr. Med. Imaging. 2020;16(10):1290–1299. doi: 10.2174/1573405616666200124125130. [DOI] [PubMed] [Google Scholar]
- 48.Benedictus M.R., et al. Lower cerebral blood flow is associated with faster cognitive decline in Alzheimer's disease. Eur. Radiol. 2017;27(3):1169–1175. doi: 10.1007/s00330-016-4450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ouchi Y., et al. Absolute changes in regional cerebral blood flow in association with upright posture in humans: an orthostatic PET study. J. Nucl. Med. 2001;42(5):707–712. [PubMed] [Google Scholar]
- 50.van Osch M.J.P., et al. Association between supine cerebral perfusion and symptomatic orthostatic hypotension. Neuroimage. 2005;27(4):789–794. doi: 10.1016/j.neuroimage.2005.05.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.