Abstract
Introduction
Coronavirus disease-2019 (COVID-19) has affected more than 608 million people and has killed 6.5 million people in the world. A few studies showed traditional Chinese medicine can be beneficial for COVID-19 treatment. An herbal preparation Jin Si Herbal Tea (JS) was formulated with herbal extracts known for their potential to decrease spike protein and ACE2 interaction, 3CL, and TRPMSS2 protease activity, and thus aimed to evaluate the clinical course of JS co-treatment along with the usual treatment schedule given for severe COVID-19 patients.
Methods
This retrospective cohort study included patients with severe COVID-19 admitted to Hualien Tzu Chi Hospital between June and July 2021. All the patients were co-treated with JS and the primary outcome was death. The secondary outcomes included laboratory exam, Ct value, clinical course, and hospital stays. There were 10 patients recruited in this study and divided into < 70 years and ≧ 70 years groups (n = 5 in each group).
Results
Older patients (≧70 years) had a higher Charlson Comorbidity Index, VACO index, and lower hemoglobin levels than < 70 years patients. The trend of lymphocyte count, LDH, D-dimer, and Ct value of non-survivors was not consistent with previous studies. The death rate was 20% and the recovery rate to mild illness in 14 days was 40%.
Conclusion
In conclusion, this is the first clinical study of JS co-treatment in severe COVID-19 patients. JS co-treatment might reduce death rate and recovery time. Further large-scale clinical trials would be expected.
Keywords: Chinese medicine, Covid-19, Sars-cov-2, Clinical course, Death rate
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has resulted in 608 million infections and 6.5 million deaths worldwide (World Health Organization, 2022). The estimated mortality rate was 40–60% among severe cases and 5% among mild to moderate cases (Mahendra et al., 2021). To date, only corticosteroids have been confirmed effective against critically ill patients with COVID-19, which is associated with lowered 28-day mortality (WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group et al., 2020). With evidence from the available literature, a symptom-based model has been recently presented by Matricardi et al., (Matricardi et al., 2020) which identifies four different stages of COVID-19, in which the first stage is characterized by upper respiratory tract infection, accompanied by fever, muscle fatigue, and pain is seen 5–6 days after infection. The subsequent second stage is characterized by the development of pneumonia with dyspnea. The third stage is categorized as aggravated clinical scenarios like cytokine storm, serious lung lesions with severe acute respiratory distress syndrome (ARDS), and disseminated intravascular coagulation (DIC). Other notable complications in this worsening stage include acute cardiac and renal damage, sepsis, and secondary infections. The fourth stage is marked by the recovery or death of the patient. The mortality is often associated with advanced age, comorbidities, the severity of the disease, respiratory failure, higher levels of D-Dimer and C-reactive protein, lower lymphocyte counts, and secondary infections (Matricardi et al., 2020, Stasi et al., 2020).
There is no effective treatment to treat SARS-CoV-2 infection, particularly in those with severe infection. During the early stages of SARS-CoV-2 infections, administration of antiviral agents may hinder disease progression, whereas immunomodulatory drugs with antiviral agents are considered to improve clinical outcomes in critically ill COVID-19 patients (Asselah et al., 2021, Stasi et al., 2020). Existing antiviral agents and 49 monoclonal antibodies target viral RNA-dependent RNA polymerase, IL-6 receptor, and viral spike protein. Among various other drugs on trial, Remdesivir - an inhibitor of viral RNA-dependent RNA polymerase, Dexamethasone - that modulates inflammation-mediated lung injury, and Tocilizumab - a human IgG1 monoclonal antibody (mAb) drug that targets soluble as well as membrane-bound IL-6 receptors, have shown modest effects (Asselah et al., 2021). In addition, there is about 50 mAb drugs undergoing clinical trials registered in different countries (Valdez-Cruz et al., 2021), and the recent emergency approval from the FDA for various monoclonal antibodies including bamlavinimab, casirivimab, and imdevimab – that target the spike protein of SARS CoV-2 virus has given small hopes on the treatment options for COVID-19 patients (Deb et al., 2021, Valdez-Cruz et al., 2021). However due to various constraints such as the development of various SARS-CoV-2 variants the application of mAb may still have its limitations (Deb et al., 2021).
In view of such scenarios, several studies have identified traditional Chinese Medicine (TCM) as a potential effective adjuvant treatment for COVID-19. Current TCM used in COVID-19 treatment include Lianhua Qingwen (LHQW) and NRICM101 (Li et al., 2020, Tsai et al., 2021). LHQW is composed of 13 Chinese herbs (Jia and Wu, 2021) and was published in 2020 (Li et al., 2020). LHQW in vitro inhibited SARS-Cov-2 replication, reduced the production of the inflammatory cytokines, and abnormal virion morphology (Li et al., 2020). Another COVID-19 treatment product discovered in Taiwan is NRICM101 (Tsai et al., 2021). NRICM101 is composed of 8 Chinese herbs. Their experiment of NRICM101 in treating SARS-Cov-2 showed reduced spike protein and ACE2 interaction, 3CL protease activity, formation of viral plaque, and cytokine production (IL-6 and TNF-α) (Tsai et al., 2021).
Despite the above TCM formula, another Chinese medicine herbal-based preparation Jin Si Herbal Tea was formulated to add the therapy for mild to moderate COVID-19 patients (Hsieh et al., 2022). The constituents of Jin Si Herbal Tea have shown promising evidence for antiviral effects and protection against comorbidities that forms the rationale for the antiviral preparation (Shibu et al., 2022). The constituents of Jin Si Herbal Tea include leaves of Artemisia argyi, Ohwia caudate, Ophiopogon japonicas, and Perilla frutescens; Roots of Houttuynia cordata, Platycodon grandifloras, and Glycyrrhiza uralensis; and flowers of Chrysanthemum × morifolium (de Almeida-Pititto et al., 2020, Kim et al., 2021, Kwon et al., 2020, Liu et al., 2020, Mahrosh and Mustafa, 2021, Tang et al., 2021, Zhang et al., 2016).
The aim of this study was to evaluate the influence of Jin Si Herbal Tea incorporated treatment strategy on the death and hospital course of severe COVID-19 patients.
2. Materials and methods
This was a retrospective observational study that included patients with severe disease with SARS-CoV-2 infection who were admitted to Hualien Tzu Chi Hospital between May 1 and June 30, 2021. The study protocol was approved by the Research Ethics Committee of Hualien Tzu Chi Hospital (IRB 110–187-B). The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee of the institution. Informed written consent was waived due to low risk and approval by the Research Ethics Committee of Hualien Tzu Chi Hospital.
2.1. Inclusion criteria
We used ICD-10 code U07.1 to search the patients within the electrical history system. Patients aged > 20 years with severe COVID-19 disease admitted for medical care were included. All the patients co-treated with Jin Si Herbal Tea were included.
Jin Si Herbal Tea was a standardized manufactured product (manufactured by XIE LI PRODUCTS CO., LTD, Hualien, Taiwan, batch number 21566001), which was the aqueous extract from herbs and concentrated to 15 mL in one pack (Artemisia Argyi 3.4%, Kalabhangra 3.4%, Ophiopogonis Radix 2.3%, Dokudami 2.3%, Platycodon grandiflorus 2.3%, Glycyrrhizae Radix, 1.1%, Perilla, 1.1%, and Chrysanthemi Flos 0.1%). The administration frequency and duration of Jin Si Herbal Tea were 15 mL twice daily for the whole hospital course.
2.2. Exclusion criteria
Patients without Jin-Si Herbal Tea co-treatment and loss to follow-up were excluded.
2.3. Data collection
Demographic variables, clinical course, laboratory data, medications, and hospital stay were collected (between May 1 and Aug 5, 2021). We divided the patients into < 70 years and ≧ 70 years.
2.4. Primary outcome
The primary outcome was death.
2.5. Secondary outcome
The secondary outcomes included laboratory change, viral load, clinical course, and hospital stays.
2.6. Statistical analysis
Continuous variables were expressed as mean±SD. Categorical variables were expressed as numbers and percentages. Compared with continuous variables, we used Student’s t-test and the Mann-Whitney-Wilcoxon test were used. Compared with categorical variables, we used Pearson’s chi-square and Fisher’s exact test. The Shapiro-Wilks test was adopted to detect the departure from normality. Logistic regression analysis was used to find the association factor for severe-COVID-19 illness. The analysis was performed by the statistical package SPSS (version 22, IBM, New York, NY, USA). We considered p < 0.05 as statistical significance.
3. Results
There were 10 patients registered in this study. Five in the < 70 years and 5 in the ≧ 70 years group.
3.1. Baseline clinical characteristics, comorbidities, and laboratory findings
Table 1 shows the demographics, comorbidities, and clinical characteristics of ten patients. The mean age was 68.1 ± 9.88 years, 80% were male, and BMI was 24.98 ± 3.17. Among ten patients, 5 and 5 were in the < 70 years and ≧ 70 years group, respectively. The prevalence of hypertension, diabetes mellitus, coronary heart disease, chronic obstructive pulmonary disease, cancer, chronic kidney disease, and hyperlipidemia were no significant differences.
Table 1.
Demographics (n = 10).
| Item | Age< 70 y/o (n = 5) |
Age≧ 70 y/o (n = 5) |
Total (n = 10) |
P-value |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age | 60.4 ± 7.7 | 75.8 ± 3.49 | 68.1 ± 9.88 | 0.004 * |
| Sex | 1.000 | |||
| Male | 4(80.0%) | 4(80.0%) | 8(80.0%) | |
| Female | 1(20.0%) | 1(20.0%) | 2(20.0%) | |
| BMI, kg/m2 | 25.76 ± 3.94 | 24.20 ± 2.37 | 24.98 ± 3.17 | 0.470 |
| Temperature, °C | 37.04 ± 0.87 | 36.76 ± 0.84 | 36.90 ± 0.82 | 0.618 |
| Heart rate, bpm | 91.00 ± 14.16 | 84.60 ± 16.88 | 87.80 ± 15.07 | 0.534 |
| Respiratory rate, tpm | 23.60 ± 5.50 | 21.40 ± 3.65 | 22.50 ± 4.55 | 0.478 |
| SpO2, % | 90.20 ± 5.02 | 96.00 ± 1.58 | 93.10 ± 4.65 | 0.039 * |
| SBP, mmHg | 114.40 ± 15.81 | 144.00 ± 27.18 | 129.20 ± 26.13 | 0.068 |
| Smoker(%) | 4(80.0%) | 2(20.0%) | 5(50.0%) | 0.206 |
| Charlson comorbidity index (CCI) | 2.4 ± 1.52 | 4.6 ± 1.34 | 3.5 ± 1.78 | 0.041 * |
| Comorbidity | ||||
| HTN | 3(60.0%) | 5(100.0%) | 8(80.0%) | 0.444 |
| DM | 2(40.0%) | 4(80.0%) | 6(60.0%) | 0.524 |
| CAD | 1(20.0%) | 0(0.0%) | 1(10.0%) | 1.000 |
| COPD | 1(20.0%) | 0(0.0%) | 1(10.0%) | 1.000 |
| Cancer | 0(0.0%) | 1(20.0%) | 1(10.0%) | 1.000 |
| CKD | 0(0.0%) | 0(0.0%) | 0(0.0%) | 1.000 |
| Hyperlipidemia | 0(0.0%) | 3(60.0%) | 3(30.0%) | 0.167 |
| APACHE-II | 13.80 ± 6.72 | 14.00 ± 6.36 | 13.90 ± 6.17 | 0.963 |
| APACHE-II: Estimated mortality | 0.21 ± 0.12 | 0.19 ± 0.14 | 0.20 ± 0.12 | 0.869 |
| NIAID ordinal scale (admission) | 6.40 ± 0.55 | 6.00 ± 0.71 | 6.20 ± 0.63 | 0.347 |
| CURB-65 score | 2.00 ± 1.00 | 2.40 ± 0.55 | 2.20 ± 0.79 | 0.455 |
| SOFA score | 4.40 ± 2.79 | 3.60 ± 1.34 | 4.00 ± 2.11 | 0.580 |
| VACO index (risk of 30-day mortality) | 0.08 ± 0.06 | 0.16 ± 0.05 | 0.12 ± 0.07 | 0.041 * |
| COVID-GRAM Critical Illness Risk Score | 166.00 ± 58.63 | 164.20 ± 13.24 | 165.10 ± 40.08 | 0.948 |
| COVID-GRAM: Risk of ICU, invasive ventilation or death | 0.56 ± 0.33 | 0.67 ± 0.12 | 0.62 ± 0.24 | 0.507 |
| MuLBSTA Score | 12.60 ± 2.19 | 12.20 ± 1.79 | 12.40 ± 1.90 | 0.760 |
| MuLBSTA Score:90-day mortality | 0.20 ± 0.11 | 0.18 ± 0.06 | 0.19 ± 0.08 | 0.667 |
| WBC, 10^3/uL | 8.21 ± 2.76 | 9.68 ± 3.39 | 8.94 ± 3.01 | 0.473 |
| Lym count | 7.98 ± 5.02 | 11.94 ± 10.58 | 9.96 ± 8.08 | 0.471 |
| Hb, g/dL | 15.30 ± 2.55 | 12.32 ± 0.58 | 13.81 ± 2.35 | 0.034 * |
| PLT, 10^3/uL | 125.60 ± 58.78 | 189.40 ± 67.02 | 157.50 ± 68.28 | 0.148 |
| Albumin, g/dL | 2.96 ± 0.72 | 3.40 ± 0.16 | 3.18 ± 0.55 | 0.220 |
| Lactate, mmol/L | 1.15 ± 0.53 | 2.80 ± 1.41 | 1.70 ± 1.14 | 0.087 |
| ALT, U/L | 39.80 ± 10.08 | 83.60 ± 142.3 | 61.70 ± 97.87 | 0.512 |
| BUN, mg/dL | 20.80 ± 7.26 | 18.60 ± 7.99 | 19.70 ± 7.29 | 0.661 |
| CRE, mg/dL | 0.90 ± 0.47 | 0.92 ± 0.40 | 0.91 ± 0.41 | 0.927 |
| CK, U/L | 216.20 ± 195.62 | 76.80 ± 42.65 | 146.50 ± 152.36 | 0.158 |
| hsTnTI, pg/mL | 22.60 ± 14.45 | 38.86 ± 64.71 | 30.73 ± 45.03 | 0.598 |
| PT, sec | 10.74 ± 0.94 | 10.46 ± 0.43 | 10.60 ± 0.70 | 0.560 |
| D-dimer, ng/mL | 1843.45 ± 1896.56 | 1208.55 ± 441.18 | 1526 ± 1340.57 | 0.487 |
| Ferritin, ng/mL | 2972.24 ± 3903.56 | 1662.74 ± 2002.79 | 2317.49 ± 3005.23 | 0.523 |
| IL-6, pg/mL | 67.98 ± 42.33 | 102.30 ± 41.77 | 85.14 ± 43.04 | 0.292 |
| CRP, mg/dL | 8.48 ± 7.47 | 6.02 ± 5.57 | 7.25 ± 6.34 | 0.571 |
| Procalcitonin, ng/mL | 0.16 ± 0.13 | 0.27 ± 0.26 | 0.21 ± 0.20 | 0.424 |
| ESR, mm/h | 29.50 ± 10.61 | 48.40 ± 20.82 | 43.00 ± 19.82 | 0.293 |
| E gene Ct value (admission) | 26.26 ± 4.30 | 24.32 ± 7.29 | 25.29 ± 5.74 | 0.622 |
| N2 gene Ct value (admission) | 27.72 ± 4.30 | 25.28 ± 6.63 | 26.50 ± 5.42 | 0.509 |
| Brixia CXR score at ICU admission | 14.40 ± 2.61 | 10.00 ± 4.18 | 12.20 ± 4.02 | 0.081 |
| CXR severity score | 18.40 ± 2.61 | 14.60 ± 4.56 | 16.50 ± 4.03 | 0.144 |
| Duration of symptom onset to admission, days | 10.80 ± 5.97 | 6.80 ± 4.02 | 8.80 ± 5.25 | 0.250 |
| Other hospital stay, days | 1.40 ± 1.95 | 4.00 ± 4.64 | 2.70 ± 3.62 | 0.281 |
Data are presented as n or mean ± standard deviation.
*p-value< 0.05 was considered statistically significant after test
BMI: body mass index, SpO2: saturation of peripheral oxygen, SBP: systolic blood pressure, HTN: hypertension, DM: diabetes mellitus, CAD: coronary artery disease, COPD: chronic obstructive pulmonary disease, CKD: chronic kidney disease, apache-II: acute physiology and chronic health evaluation II, NIAID: National Institute of Allergy and Infectious Diseases, SOF: Sequential Organ Failure Assessment, VACO: The Veterans Health Administration COVID-19, WBC: white blood cell, lym: lypmhocyte, Hb: hemoglobin, PLT: platelet, ALT: alanine transaminase, BUN: blood urea nitrogen, CRE: creatinine, CK: creatine kinase, hsTnTI: high-sensitivity troponin I, PT: prothrombin time, IL-6: interleukin 6, CRP: C-reactive protein, ESR: erythrocyte sedimentation rate, CXR: chest X ray,
The duration of symptoms onset to admission, other hospital stays, body temperature, heart rate, respiratory rate, and systolic blood pressure at admission were not significant between the two groups. The age was different between the two groups (p = 0.004). The SpO2 was significantly different between the two groups (90.20 ± 5.02 vs. 96.00 ± 1.58 in the <70 years and ≧70 years, respectively, p = 0.039).
3.2. Comorbidities
CCI was significantly different between the two groups (2.4 ± 1.52 vs. 4.6 ± 1.34 in the <70 years and ≧ 70 years, respectively, p = 0.041). VACO index (risk of 30-day mortality) was significantly higher in the ≧ 70 years group (0.16 ± 0.05) than in the < 70 years group (0.08 ± 0.06, p = 0.041). Other scores such as APACHE-II, APACHE-II: Estimated mortality, NIAID ordinal scale (admission), CURB-65 score, SOFA score, COVID-GRAM Critical Illness Risk Score, COVID-GRAM (Risk of intensive care unit [ICU], invasive ventilation or death), and MuLBSTA Score, MuLBSTA Score (90-day mortality) were not different between the two groups.
3.3. Laboratory findings
There was no difference in WBC, lymphocyte count, platelet, albumin, lactate, liver enzyme (ALT), renal function (creatinine), heart function (CK, hsTnTI), thrombosis markers (PT, D-dimer), inflammation markers (ferritin, IL-6, CRP, procalcitonin, and ESR), Ct value of E and N2 genes, Brixia CXR score at ICU admission, and CXR severity score between the two groups. There was a significant difference in hemoglobin between the two groups (15.30 ± 2.55 vs. 12.32 ± 0.58 in the <70 years and ≧70 years, respectively, p = 0.034).
3.4. Current treatment protocol plus Jin Si Herbal Tea
Table 2 shows current treatments for COVID-19 include antibiotics, antiviral treatment, corticosteroids, anti-thrombosis therapy, high-flow nasal cannula oxygen therapy, invasive mechanical ventilation, ECMO, and renal replacement therapy with co-treatment with Jin Si Herbal Tea. There was no patient receiving ECMO and renal replacement therapy in this study. There was no significant difference between the two groups.
Table 2.
Treatment of COVI-19 patients (n = 10).
| Item | Age< 70 y/o (n = 5) |
Age≧ 70 y/o (n = 5) |
Total (n = 10) |
P-value |
|---|---|---|---|---|
| Treatment | ||||
| Jing-Si herbal tea | 5(100.0%) | 5(100.0%) | 10(100.0%) | 1.000 |
| Antibiotics | 5(100.0%) | 5(100.0%) | 10(100.0%) | 1.000 |
| Sterioid | 5(100.0%) | 5(100.0%) | 10(100.0%) | 1.000 |
| Remdesivir | 5(100.0%) | 4(80.0%) | 9(90.0%) | 1.000 |
| Tocilizumab | 3(60.0%) | 3(60.0%) | 6(60.0%) | 1.000 |
| Enoxaparin | 4(80.0%) | 1(20.0%) | 5(50.0%) | 0.206 |
| Low flow oxygen | 5(100.0%) | 5(100.0%) | 10(100.0%) | 1.000 |
| High-flow nasal cannula oxygen therapy | 4(80.0%) | 3(60.0%) | 7(70.0%) | 1.000 |
| Invasive mechanical ventilation | 4(80.0%) | 1(20.0%) | 5(50.0%) | 0.206 |
| ECMO | 0(0.0%) | 0(0.0%) | 0(0.0%) | 1.000 |
| Renal replacement therapy | 0(0.0%) | 0(0.0%) | 0(0.0%) | 1.000 |
| Outcomes | ||||
| Sepsis with secondary infection | 2(40.0%) | 1(20.0%) | 3(30.0%) | 1.000 |
| Respiratory failure | 5(100.0%) | 5(100.0%) | 10(100.0%) | 1.000 |
| ARDS | 5(100.0%) | 5(100.0%) | 10(100.0%) | 1.000 |
| Heart failure | 2(40.0%) | 2(40.0%) | 4(40.0%) | 1.000 |
| Embolic event | 1(20.0%) | 0(0.0%) | 1(10.0%) | 1.000 |
| Acute cardiac injury | 3(60.0%) | 2(40.0%) | 5(50.0%) | 1.000 |
| Acute kidney injury | 1(20.0%) | 3(60.0%) | 4(40.0%) | 0.524 |
| Hypoproteinaemia | 3(60.0%) | 4(80.0%) | 7(70.0%) | 1.000 |
| ICU admission | 5(100.0%) | 5(100.0%) | 10(100.0%) | 1.000 |
| Lengh of hospital stay, days | 33.00 ± 16.05 | 28.60 ± 5.90 | 30.80 ± 11.63 | 0.565 |
| ICU length of stay, days | 23.20 ± 15.64 | 13.20 ± 6.46 | 23.20 ± 15.64 | 0.223 |
| Mortality | 1(20.0%) | 1(20.0%) | 2(20.0%) | 1.000 |
| Brixia CXR score (day 7) | 8.60 ± 3.85 | 13.40 ± 2.30 | 11.00 ± 3.92 | 0.022 |
| CXR severity score (day 7) | 12.00 ± 4.18 | 17.00 ± 2.00 | 14.50 ± 4.06 | 0.021 |
| Brixia CXR score (day 14) | 9.40 ± 4.28 | 13.20 ± 3.90 | 11.30 ± 4.35 | 0.090 |
| CXR severity score (day 14) | 13.60 ± 3.78 | 15.20 ± 4.02 | 14.40 ± 3.78 | 0.268 |
Data are presented as n or mean ± standard deviation.
*p-value< 0.05 was considered statistically significant after test
ECMO: extracorporeal membrane oxygenation, ARDS: adult respiratory distress syndrome, ICU: intensive care unit, CXR: chest X ray
The outcomes were also listed in Table 2. The outcomes included sepsis, respiratory failure, ARDS, heart failure, embolic event, acute cardiac injury, acute kidney injury, hypoproteinemia, ICU admission, and mortality. There was no significant difference between the two groups.
The length of hospital stay (33.00 ± 16.05 vs. 28.60 ± 5.90 days in the <70 years and ≧70 years, respectively) and ICU stay (23.20 ± 15.64 vs. 13.20 ± 6.46 days in the <70 years and ≧70 years, respectively) was not significantly different between the two groups (Table 2).
The death rate was 20% and recovery to mild illness in 14 days rate was 40%.
Two patients died during this registry. By this period, COVID vaccination was not widely available in Taiwan; thus, none of these patients were vaccinated. The detailed information on death cases, including the cause of death, comorbidities, and combined treatments were listed in Table 3.
Table 3.
Detailed information of death cases.
| Death Case 1 | Death Case 2 | |
|---|---|---|
| Age group | < 70 y/o | ≧70 y/o |
| Sex | Male | Male |
| Smoker | Yes | No |
| Comorbidities | Chronic obstructive pulmonary disease, diabetes mellitus, and hypertension | Hypertension |
| Combined treatment | • Antiviral treatment: Remdesivir (5-day course) Tocilizumab Acyclovir • Anti-inflammatory drug: Dexamethasone (10-day course) • Antibiotics and antifungal drugs: Ertapenam Meropenem Ceftazidime-avibactam Amikacin Colistin Linezolid Anidulafungin Voriconazole Liposomal-amphotericin • Anticoagulant: Enoxaparin |
• Antiviral treatment: Remdesivir (5-day course) Tocilizumab • Anti-inflammatory drug: Dexamethasone (10-day course) • Antibiotics and antifungal drugs: Levofloxacin Meropenem Trimethoprim/ Sulfamethoxazole Isavuconazole Liposomal Amphotericin • Anticoagulant: Enoxaparin |
| Cause of death | ARDS due to nosocomial pneumonia caused by MDR pathogens | Nosocomial infection, sepsis, and multiple organ failure |
ARDS: acute respiratory distress syndrome, MDR: multidrug-resistant
Table 4 shows the ordinal scale and Ct values before and after treatment. In the < 70 years group, the ordinal scale change was no different. But the Ct value of E and N2 genes were significantly higher after treatment compared with before treatment. There was no difference in ordinal scale and Ct value before and after treatment in the ≧ 70 years group.
Table 4.
Comparison of clinical characteristics before and after treatment (n = 10).
| Group | N | Item | Baseline | After treatment | Diff. (Post-Pre) | P-value |
|---|---|---|---|---|---|---|
| Age< 70 y/o | 5 | NIAID ordinal scale | 6.40 ± 0.55 | 5.40 ± 2.07 | -1.00 ± 2.12 | 0.351 |
| 5 | E gene Ct value | 23.77 ± 2.74 | 27.97 ± 2.27 | 4.20 ± 0.90 | 0.015 * | |
| 5 | N2 gene Ct value | 27.72 ± 4.30 | 33.12 ± 5.55 | 5.40 ± 3.11 | 0.018 * | |
| Age≧ 70 y/o | 5 | NIAID ordinal scale | 6.00 ± 0.71 | 4.20 ± 2.17 | -1.80 ± 1.64 | 0.070 |
| 5 | E gene Ct value | 23.75 ± 8.29 | 30.25 ± 6.80 | 6.50 ± 12.92 | 0.388 | |
| 5 | N2 gene Ct value | 25.28 ± 6.63 | 33.00 ± 6.38 | 7.72 ± 10.57 | 0.178 | |
| Total | 10 | NIAID ordinal scale | 6.20 ± 0.63 | 4.80 ± 2.10 | -1.40 ± 1.84 | 0.039 * |
| 10 | E gene Ct value | 23.76 ± 6.07 | 29.27 ± 5.13 | 5.51 ± 9.23 | 0.165 | |
| 10 | N2 gene Ct value | 26.50 ± 5.42 | 33.06 ± 5.64 | 6.56 ± 7.44 | 0.021 * |
Data are presented as mean ± standard deviation.
*p-value< 0.05 was considered statistically significant after test
NIAID: National Institute of Allergy and Infectious Diseases
3.5. The trend of lymphocyte count, LDH, D-dimer, and Ct values of the survivors and non-survivors
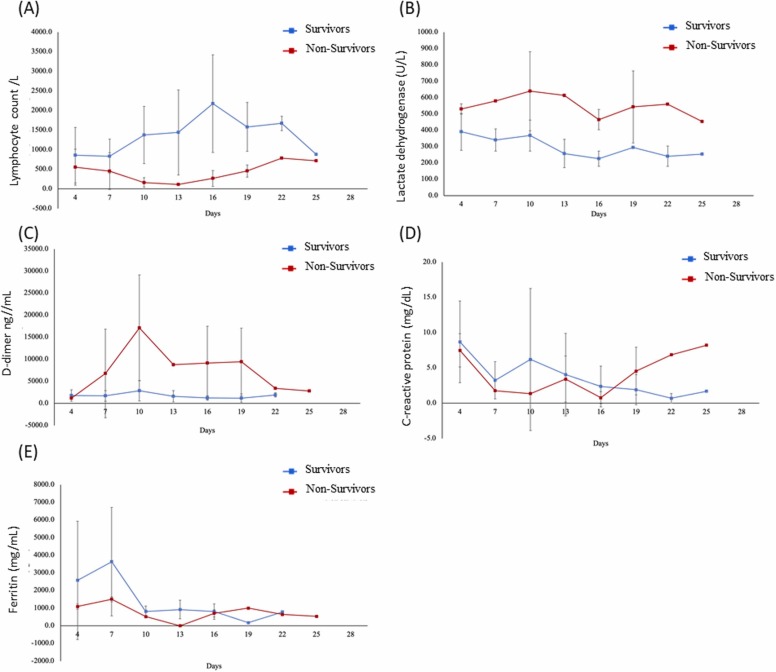
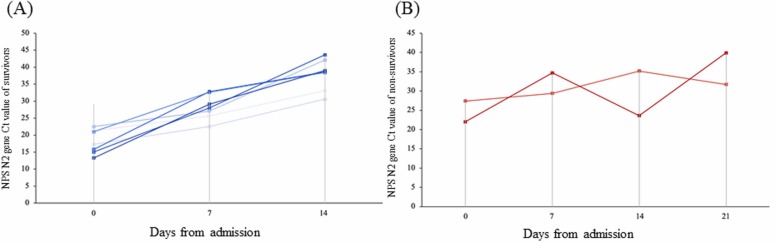
Non-survivors showed lymphopenia from admission to death. However, the lymphocyte number rose to near the normal value at the end. LDH and D-dimer rose at admission but declined at the end ( Fig. 1). The Ct value of the survivor rose gradually, but the Ct value of the non-survivor fluctuated ( Fig. 2) signifying the correlation between viral load and mortality.
Fig. 1.
Changes in laboratory markers from illness onset in patients hospitalized with COVID-19: Temporal changes in the lymphocyte count (A), lactate dehydrogenase (B), D-dimer (C), C-reactive protein (D), and ferritin (E).
Fig. 2.
Detection of SARS-CoV-2 RNA in patient samples by using a specific RT-PCR test: The Ct values of the RT-PCR test for the SARS-CoV-N2 region were used to assess viral RNA levels in patient samples. The nasopharyngeal swab specimens were used for analysis. The weekly assessment of the NPS N2 gene Ct value showed a gradual reduction in the detectable range among survivors but the Ct values were fluctuating among eventual non-survivors.
3.6. Relationship between the commencement of Jing Si Herbal Tea and Viral load
Analyzing the relationship between time from illness to Jing Si Herbal Tea and NIAID ordinal scale shows that there was a little trend to show the earlier use of Herbal Tea, the lower scale ( Fig. 3) with the r value was 0.187.
Fig. 3.
The relationship between time from illness to Jing Si Herbal Tea and NIAID ordinal scale at day 14: The patient recovery showed only a little correlation with the treatment onset. The r-value was 0.187.
4. Discussion
This retrospective cohort study was the first study showing hospital courses for severe COVID-19 patients co-treatment with Jing Si Herbal Tea. Older patients (≧70 years) had higher CCI, VACO index, and lower hemoglobin levels than < 70 years patients. The trend of lymphocyte count, LDH, D-dimer, and Ct value of non-survivors was not consistent with previous studies. The death rate was 20% and recovery to mild illness in 14 days rate was 40%.
Current traditional Chinese medicines used in COVID-19 treatment include Lianhua Qingwen (LHQW) and NRICM101 (Li et al., 2020, Tsai et al., 2021). LHQW is composed of 13 Chinese herbs (Jia and Wu, 2021) and was published in 2020 (Li et al., 2020). Another COVID-19 treatment product discovered in Taiwan is NRICM101 (Tsai et al., 2021). NRICM101 is composed of 8 Chinese herbs. The authors study using co-treatment of Jing Si Herbal Tea is composed of 8 Chinese herbs different from NRICM101 (Hsieh et al., 2022, Shibu et al., 2022). In the preliminary pharmaceutical study, Jing Si Herbal Tea decreased spike protein and ACE2 interaction, 3CL protease activity, and TRPMSS2 protease activity (Kwon et al., 2020, Lau et al., 2008, Mahendra et al., 2021, Shibu et al., 2022, Tang et al., 2021). In addition, the herbal tea also contains constituents that provide pulmonary and cardiovascular protection and those that provide anti-inflammatory effects (Chen et al., 2017, Liu et al., 2020, Zhang et al., 2016). Due to the presence of such proven herbal constituents, the Jing Si Herbal Tea may provide a multi-thronged therapeutic enhancement in patients under pharmacological treatment.
In this cohort study, the trend of lymphocyte count, LDH, D-dimer, and Ct value of non-survivors was not consistent with previous studies, which might be related to the effect of Jing Si Herbal Tea. Anemia was reported associated with poor outcomes in COVID-19 patients. Anemia COVID-19 patients are usually older and have a higher frequency of comorbidities, higher frequency of death, ICU admission, and ventilator requirement (Faghih Dinevari et al., 2021). Another report showed anemia was independently associated with mortality, especially when Hb < 11 g/dL (Oh et al., 2021). However, another report revealed anemia in COVID-19 patients was not associated with mortality but influenced the quality of life (Bergamaschi et al., 2021). In our study, anemia was a significantly higher percentage in the ≧ 70 years group than in the < 70 years group, which means the prognosis might be poor in the ≧ 70 years group.
Lymphopenia and increased levels of IL-6 have been reported associated with the severity of COVID-19 disease (Tavakolpour et al., 2020). T cells are important for shaping immune responses in the body. In COVID-19 patients, decreasing lymphocytes were noted after admission to ICU. Th1 cells and cellular immunity are the main mechanisms for the control of coronavirus infection (Janice Oh et al., 2012). Lymphopenia severity level may be associated with poor outcomes in COVID-19 patients (Lee et al., 2021). Another report also revealed lymphopenia was a reliable factor for predicting the severity and hospitalization of COVID-19 patients (Tan et al., 2020). Despite predicting the outcome of COVID-19 patients, lymphocyte count also could be used for identifying who will benefit from corticosteroid therapy (Lu et al., 2021). In this study, lymphopenia was noted in non-survivor patients. The decreasing trend of lymphocyte count was noted in most non-survivor patients (Zhou et al., 2020). However, in our cohort, the decreasing trend of lymphocyte count was not observed which might be the effect of Jin Si Herbal Tea.
The trend of the D-dimer test was increasing in the non-survivor group than in the survivor group (Zhou et al., 2020). D-dimer > 2000 ng/mL at admission was associated with increased mortality chance (OR: 10.17, 95% CI: 1.10–94.38, p = 0.041) (Yao et al., 2020). The systematic review also reported the increment of D-dimer value at 3–4 folds compared to the admission's value was linked to the poor prognosis of COVID-19 patients (Rostami and Mansouritorghabeh, 2020). 2025 ng/mL D-dimer could be used for predicting the death risk (He et al., 2021). D-dimer levels were associated with the outcome of COVID-19 patients (Ozen et al., 2021). In our study, the mean value of D-dimer was 1843.45 ± 1896.56 and 1208.55 ± 441.18 ng/mL in the < 70 years and ≧ 70 years groups, respectively. There was no significant difference between the two groups. The trend of D-dimer change was not increasing in the non-survivor group. Jin Si Herbal Tea might play a role in this phenomenon.
The trend of LDH was increasing in the non-survivor group than in the survivor group (Zhou et al., 2020). LDH reflects the inflammation status of the body. Therefore, LDH could be used for predicting the prognosis and chance of mortality (Bartziokas and Kostikas, 2021). Elevated LDH levels were associated with an increased chance of developing the severe disease (6-fold) and mortality (16-fold) in COVID-19 patients (Henry et al., 2020). Another study showed that LDH was higher in the non-survivor group (Zhu et al., 2020). A study using LDH, lymphocytes, and CRP could predict the survival of COVID-19 patients 10 days in advance using computer calculation (Yan et al., 2020). In this study, the trend of LDH was increasing levels and then decreasing levels in the non-survivor group which was different from the previous report. Jin Si Herbal Tea might play a role in the phenomenon.
Ct values are lowest as symptoms begin, then gradually rise as time elapses (Salvatore et al., 2021). The median Ct value within 7 days after symptoms onset was 26.5 and rose to 35.0 on 21 days after onset (Salvatore et al., 2021). Another study also showed Ct value was significantly higher in the ≥ 80 years patients and negative association with survival (Zhao et al., 2021). Lower Ct values at diagnosis were associated with higher mortality among COVID-19 patients (Miller et al., 2021). In this study, the Ct values of survivors increased gradually when the time elapsed. However, Ct values of non-survivors fluctuated.
The death rate of severe COVID-19 patients decreased from 41.4% to 29.3% after dexamethasone intervention (RECOVERY Collaborative Group et al., 2021). A US study showed the in-hospital mortality rate of COVID-19 patients was 20.3% (Rosenthal et al., 2020). In this study, the death rate was 20%. Jin Si Herbal Tea might play a role in the low death rate.
This is the first clinical study of Jin Si Herbal Tea in severe COVID-19 patients. However, there is no control group to test the pure effect of Jin Si Herbal Tea. How to exclude the use of drugs with similar efficacy to Jin Si herbal tea is the limitation of this study. A comparison of the previous report of COVID-19 patient's treatment was done in this study to exclude the use of drugs with similar efficacy. Second, the patient numbers were small. Therefore, the interpretation of the results needs to be cautious, it can only show that some outcomes may be related to the treatment. Further large-scale clinical trials are needed to confirm the results.
5. Conclusion
In conclusion, this is the first clinical study of Jin Si Herbal Tea co-treatment in severe COVID-19 patients. The death rate was 20% and recovery to mild illness in 14 days rate was 40%. Further large-scale clinical trials should be expected.
Conflict of interest
The authors declare that the Jin Si Herbal Tea is an herbal decoction used on patients admitted to Hualien Tzu Chi hospital and will be further continued on patients.
Author contribution
T-J.H, C-Y.H, and S-Z.L designed the experiments, H-S.W, H.H, J-H.W, Ji-H.W, H-C.C, H-Y.Y, S-H.S, Y-F.C, and C-H.T performed experiments, M.A.S, T-J.H, C-Y.H, C-Y.C, and Y-J.L, C-Y.S prepared the formula, P-C.L and D-C.D analyzed the data, P-C.L, M.A.S and D-C.D prepared the draft. All the authors approve the final version of the manuscript.
References
- de Almeida-Pititto B., Dualib P.M., Zajdenverg L., Dantas J.R., de Souza F.D., Rodacki M., Bertoluci M.C. Severity and mortality of COVID 19 in patients with diabetes, hypertension and cardiovascular disease: a meta-analysis. Diabetol. Metab. Syndr. 2020;12:75. doi: 10.1186/s13098-020-00586-4. (Brazilian Diabetes Society Study Group (SBD)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselah T., Durantel D., Pasmant E., Lau G., Schinazi R.F. COVID-19: discovery, diagnostics and drug development. J. Hepatol. 2021;74:168–184. doi: 10.1016/j.jhep.2020.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartziokas K., Kostikas K. Lactate dehydrogenase, COVID-19 and mortality. Med. Clin. 2021;156:37. doi: 10.1016/j.medcle.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi G., Borrelli de Andreis F., Aronico N., Lenti M.V., Barteselli C., Merli S., Pellegrino I., Coppola L., Cremonte E.M., Croce G., Mordà F., Lapia F., Ferrari S., Ballesio A., Parodi A., Calabretta F., Ferrari M.G., Fumoso F., Gentile A., Melazzini F., Di Sabatino A. Anemia in patients with Covid-19: pathogenesis and clinical significance. Clin. Exp. Med. 2021;21:239–246. doi: 10.1007/s10238-020-00679-4. Internal Medicine Covid-19 Collaborators. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.-L., Zhang H.-J., Chao J., Liu J.-F. Essential oil of Artemisia argyi suppresses inflammatory responses by inhibiting JAK/STATs activation. J. Ethnopharmacol. 2017;204:107–117. doi: 10.1016/j.jep.2017.04.017. [DOI] [PubMed] [Google Scholar]
- Deb P., Molla M.M.A., Saif-Ur-Rahman K.M. An update to monoclonal antibody as therapeutic option against COVID-19. Biosaf. Health. 2021;3:87–91. doi: 10.1016/j.bsheal.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faghih Dinevari M., Somi M.H., Sadeghi Majd E., Abbasalizad Farhangi M., Nikniaz Z. Anemia predicts poor outcomes of COVID-19 in hospitalized patients: a prospective study in Iran. BMC Infect. Dis. 2021;21:170. doi: 10.1186/s12879-021-05868-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Yao F., Chen J., Wang Y., Fang X., Lin X., Long H., Wang Q., Wu Q. The poor prognosis and influencing factors of high D-dimer levels for COVID-19 patients. Sci. Rep. 2021;11:1830. doi: 10.1038/s41598-021-81300-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B.M., Aggarwal G., Wong J., Benoit S., Vikse J., Plebani M., Lippi G. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: a pooled analysis. Am. J. Emerg. Med. 2020;38:1722–1726. doi: 10.1016/j.ajem.2020.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J., RECOVERY Collaborative Group Dexamethasone in hospitalized patients with covid-19. New Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh P.-C., Chao Y.-C., Tsai K.-W., Li C.-H., Tzeng I.-S., Wu Y.-K., Shih C.Y. Efficacy and safety of complementary therapy with jing si herbal tea in patients with mild-to-moderate COVID-19: a prospective cohort study. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.832321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janice Oh H.-L., Ken-En Gan S., Bertoletti A., Tan Y.-J. Understanding the T cell immune response in SARS coronavirus infection. Emerg. Microbes Infect. 2012;1 doi: 10.1038/emi.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z., Wu Y. Clinical applications and pharmacological research progress of Lianhua Qingwen capsules/granules. J. Trad. Chin. Med Sci. 2021;8:101–109. [Google Scholar]
- Kim T.Y., Jeon S., Jang Y., Gotina L., Won J., Ju Y.H., Kim S., Jang M.W., Won W., Park M.G., Pae A.N., Han S., Kim S., Lee C.J. Platycodin D, a natural component of Platycodon grandiflorum, prevents both lysosome- and TMPRSS2-driven SARS-CoV-2 infection by hindering membrane fusion. Exp. Mol. Med. 2021;53:956–972. doi: 10.1038/s12276-021-00624-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon E.B., Yang H.J., Choi J.-G., Li W. Protective effect of flavonoids from Ohwia caudata against influenza a virus infection. Molecules. 2020;25:4387. doi: 10.3390/molecules25194387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau K.-M., Lee K.-M., Koon C.-M., Cheung C.S.-F., Lau C.-P., Ho H.-M., Lee M.Y.-H., Au S.W.-N., Cheng C.H.-K., Lau C.B.-S., Tsui S.K.-W., Wan D.C.-C., Waye M.M.-Y., Wong K.-B., Wong C.-K., Lam C.W.-K., Leung P.-C., Fung K.-P. Immunomodulatory and anti-SARS activities of Houttuynia cordata. J. Ethnopharmacol. 2008;118:79–85. doi: 10.1016/j.jep.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Park S.-S., Kim T.Y., Lee D.-G., Kim D.-W. Lymphopenia as a biological predictor of outcomes in COVID-19 patients: a nationwide cohort study. Cancers. 2021;13:471. doi: 10.3390/cancers13030471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Hou Y., Huang J., Pan W., Ma Q., Shi Y., Li C., Zhao J., Jia Z., Jiang H., Zheng K., Huang S., Dai J., Li X., Hou X., Wang L., Zhong N., Yang Z. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol. Res. 2020;156 doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Zheng Q., Pan K., Xu X. Protective effect of Chrysanthemum morifolium Ramat. ethanol extract on lipopolysaccharide induced acute lung injury in mice. BMC Complement Med Ther. 2020;20:235. doi: 10.1186/s12906-020-03017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C., Liu Y., Chen B., Yang H., Hu H., Liu Y., Zhao Y. Prognostic value of lymphocyte count in severe COVID-19 patients with corticosteroid treatment. Signal Transduct. Target Ther. 2021;6:106. doi: 10.1038/s41392-021-00517-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahendra M., Nuchin A., Kumar R., Shreedhar S., Mahesh P.A. Predictors of mortality in patients with severe COVID-19 pneumonia - a retrospective study. Adv. Respir. Med. 2021;89:135–144. doi: 10.5603/ARM.a2021.0036. [DOI] [PubMed] [Google Scholar]
- Mahrosh H.S., Mustafa G. An in silico approach to target RNA-dependent RNA polymerase of COVID-19 with naturally occurring phytochemicals. Environ. Dev. Sustain. 2021:1–14. doi: 10.1007/s10668-021-01373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matricardi P.M., Dal Negro R.W., Nisini R. The first, holistic immunological model of COVID-19: Implications for prevention, diagnosis, and public health measures. Pediatr. Allergy Immunol. 2020;31:454–470. doi: 10.1111/pai.13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E.H., Zucker J., Castor D., Annavajhala M.K., Sepulveda J.L., Green D.A., Whittier S., Scherer M., Medrano N., Sobieszczyk M.E., Yin M.T., Kuhn L., Uhlemann A.-C. Pretest symptom duration and cycle threshold values for severe acute respiratory syndrome coronavirus 2 reverse-transcription polymerase chain reaction predict coronavirus disease 2019 mortality. Open Forum Infect. Dis. 2021;8 doi: 10.1093/ofid/ofab003. ofab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S.M., Skendelas J.P., Macdonald E., Bergamini M., Goel S., Choi J., Segal K.R., Vivek K., Nair S., Leff J. On-admission anemia predicts mortality in COVID-19 patients: a single center, retrospective cohort study. Am. J. Emerg. Med. 2021;48:140–147. doi: 10.1016/j.ajem.2021.03.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozen M., Yilmaz A., Cakmak V., Beyoglu R., Oskay A., Seyit M., Senol H. D-Dimer as a potential biomarker for disease severity in COVID-19. Am. J. Emerg. Med. 2021;40:55–59. doi: 10.1016/j.ajem.2020.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal N., Cao Z., Gundrum J., Sianis J., Safo S. Risk factors associated with in-hospital mortality in a us national sample of patients with COVID-19. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.29058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami M., Mansouritorghabeh H. D-dimer level in COVID-19 infection: a systematic review. Expert Rev. Hematol. 2020;13:1265–1275. doi: 10.1080/17474086.2020.1831383. [DOI] [PubMed] [Google Scholar]
- Salvatore P.P., Dawson P., Wadhwa A., Rabold E.M., Buono S., Dietrich E.A., Reses H.E., Vuong J., Pawloski L., Dasu T., Bhattacharyya S., Pevzner E., Hall A.J., Tate J.E., Kirking H.L. Epidemiological correlates of polymerase chain reaction cycle threshold values in the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2021;72:e761–e767. doi: 10.1093/cid/ciaa1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibu M.A., Lin Y.-J., Chiang C.-Y., Lu C.-Y., Goswami D., Sundhar N., Agarwal S., Islam M.N., Lin P.-Y., Lin S.-Z., Ho T.-J., Tsai W.-T., Kuo W.-W., Huang C.-Y. Novel anti-aging herbal formulation Jing Si displays pleiotropic effects against aging associated disorders. Biomed. Pharmacother. 2022;146 doi: 10.1016/j.biopha.2021.112427. [DOI] [PubMed] [Google Scholar]
- Stasi C., Fallani S., Voller F., Silvestri C. Treatment for COVID-19: an overview. Eur. J. Pharmacol. 2020;889 doi: 10.1016/j.ejphar.2020.173644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne J.A.C., Murthy S., Diaz J.V., Slutsky A.S., Villar J., Angus D.C., Annane D., Azevedo L.C.P., Berwanger O., Cavalcanti A.B., Dequin P.-F., Du B., Emberson J., Fisher D., Giraudeau B., Gordon A.C., Granholm A., Green C., Haynes R., Heming N., Higgins J.P.T., Horby P., Jüni P., Landray M.J., Le Gouge A., Leclerc M., Lim W.S., Machado F.R., McArthur C., Meziani F., Møller M.H., Perner A., Petersen M.W., Savovic J., Tomazini B., Veiga V.C., Webb S., Marshall J.C. Association between administration of systemic corticosteroids and mortality among critically Ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L., Wang Q., Zhang D., Ding J., Huang Q., Tang Y.-Q., Wang Q., Miao H. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct. Target Ther. 2020;5:33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W.-F., Tsai H.-P., Chang Y.-H., Chang T.-Y., Hsieh C.-F., Lin C.-Y., Lin G.-H., Chen Y.-L., Jheng J.-R., Liu P.-C., Yang C.-M., Chin Y.-F., Chen C.C., Kau J.-H., Hung Y.-J., Hsieh P.-S., Horng J.-T. Perilla (Perilla frutescens) leaf extract inhibits SARS-CoV-2 via direct virus inactivation. Biomed. J. 2021;44:293–303. doi: 10.1016/j.bj.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakolpour S., Rakhshandehroo T., Wei E.X., Rashidian M. Lymphopenia during the COVID-19 infection: What it shows and what can be learned. Immunol. Lett. 2020;225:31–32. doi: 10.1016/j.imlet.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai K.-C., Huang Y.-C., Liaw C.-C., Tsai C.-I., Chiou C.-T., Lin C.-J., Wei W.-C., Lin S.J.-S., Tseng Y.-H., Yeh K.-M., Lin Y.-L., Jan J.-T., Liang J.-J., Liao C.-C., Chiou W.-F., Kuo Y.-H., Lee S.-M., Lee M.-Y., Su Y.-C. A traditional Chinese medicine formula NRICM101 to target COVID-19 through multiple pathways: a bedside-to-bench study. Biomed. Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.111037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez-Cruz N.A., García-Hernández E., Espitia C., Cobos-Marín L., Altamirano C., Bando-Campos C.G., Cofas-Vargas L.F., Coronado-Aceves E.W., González-Hernández R.A., Hernández-Peralta P., Juárez-López D., Ortega-Portilla P.A., Restrepo-Pineda S., Zelada-Cordero P., Trujillo-Roldán M.A. Integrative overview of antibodies against SARS-CoV-2 and their possible applications in COVID-19 prophylaxis and treatment. Microb. Cell Fact. 2021;20:88. doi: 10.1186/s12934-021-01576-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2022. WHO Coronavirus (COVID-19) Dashboard [WWW Document]. World Health Organization. URL https://covid19.who.int/ (accessed 5.17.22).
- Yan L., Zhang H.-T., Goncalves J., Xiao Y., Wang M., Guo Y., Sun C., Tang X., Jing L., Zhang M., Huang X., Xiao Y., Cao H., Chen Y., Ren T., Wang F., Xiao Y., Huang S., Tan X., Huang N., Jiao B., Cheng C., Zhang Y., Luo A., Mombaerts L., Jin J., Cao Z., Li S., Xu H., Yuan Y. An interpretable mortality prediction model for COVID-19 patients. Nat. Mach. Intell. 2020;2:283–288. [Google Scholar]
- Yao Y., Cao J., Wang Q., Shi Q., Liu K., Luo Z., Chen X., Chen S., Yu K., Huang Z., Hu B. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study. J. Intensive Care Med. 2020;8:49. doi: 10.1186/s40560-020-00466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Fan S., Mao Y., Ji Y., Jin L., Lu J., Chen X. Cardiovascular protective effect of polysaccharide from Ophiopogon japonicus in diabetic rats. Int. J. Biol. Macromol. 2016;82:505–513. doi: 10.1016/j.ijbiomac.2015.09.069. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Cunningham M.H., Mediavilla J.R., Park S., Fitzgerald S., Ahn H.S., Li X., Zhan C., Hong T., Munk G., Chow K.F., Perlin D.S. Diagnosis, clinical characteristics, and outcomes of COVID-19 patients from a large healthcare system in northern New Jersey. Sci. Rep. 2021;11:4389. doi: 10.1038/s41598-021-83959-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Du Z., Zhu Y., Li W., Miao H., Li Z. Evaluation of organ function in patients with severe COVID-19 infections. Med. Clin. 2020;155:191–196. doi: 10.1016/j.medcli.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]