Abstract
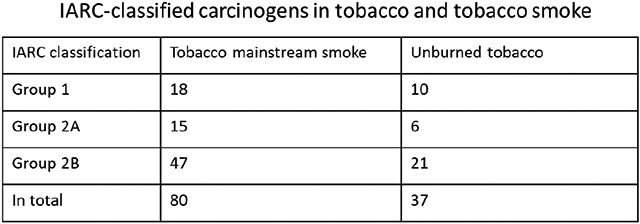
Tobacco and tobacco smoke contain a complex mixture of over 9500 chemical compounds, many of which have been recognized as hazardous to human health by regulatory agencies. In 2012, the U.S. Food and Drug Administration established a list of harmful and potentially harmful constituents in unburned tobacco and tobacco smoke, 79 of which are considered as carcinogens. Over the past 10 years, with advancing analytical technology, significant amounts of new data have been published, increasing our understanding of levels of carcinogens in tobacco products. The International Agency for Research on Cancer (IARC) has released 35 monographs since 2012, with an increasing number of compounds in unburned tobacco and tobacco smoke classified as carcinogens. In this paper, we provide an updated list of IARC-classified carcinogens in unburned tobacco and tobacco mainstream smoke. A total of 83 carcinogens has been identified – 37 in unburned tobacco and 80 in tobacco smoke – with their occurrence levels reported since 2012. No clear decreasing trends were observed for any of these carcinogens in recent years. Surveillance of the levels of tobacco carcinogens as well as regulatory actions are needed to ensure control of their levels so that potential reduced risks of cancer and other diseases may be achieved.
Graphical Abstract:
1. Introduction
Tobacco has been cultivated and used for millennia by humans, with applications ranging from original use in religious ceremonies and medicinal practices to social needs and consequences to addictive tobacco products in modern society (Slade, 1997). Pioneering work from Wynder and Graham (Wynder and Graham, 1950) and Doll and Hill (Doll and Hill, 1954) related cigarette smoking to bronchogenic carcinoma. In 1964, when Dr. Luther L. Terry released the first Surgeon General’s report on smoking and health, the harmful impact of cigarette smoking as a cause of lung cancer and chronic bronchitis became apparent to U.S. society (Centers for Disease Control and Prevention, 2006). Since then, epidemiological studies have demonstrated that tobacco smoking increases the risks of many types of cancer in humans, including cancers of the lung, larynx, esophagus, oral cavity and pharynx, bladder, liver, uterine cervix, kidney, stomach, colorectum, pancreas, and myeloid leukemia (Islami et al., 2018). Chronic diseases such as stroke, chronic obstructive pulmonary disease (COPD), atherosclerotic peripheral vascular disease, and others are also causally linked to tobacco use (American Cancer Society, 2019).
A wide variety of tobacco products is currently available on the market, including cigarettes, cigars, smokeless tobacco and water pipes. Great progress has been made in understanding the chemistry of tobacco products as related to their toxicity and carcinogenicity (International Agency for Research on Cancer, 1986, 2004). Suitable biomarkers for monitoring human exposure and uptake of some tobacco toxicants have also been developed for clinical and epidemiologic studies (Benowitz et al., 2020). This research taken together has significantly contributed to tobacco-associated disease etiology studies, tobacco-caused disease control and prevention, and clinical treatment of addicted tobacco users.
One important aspect of research on tobacco products is the identification of tobacco and tobacco smoke components that are capable of inducing cancer. Beginning with the “Hoffmann list” (Hoffmann and Hoffmann, 2001; International Agency for Research on Cancer, 2004), various authors and groups have published lists of carcinogens in tobacco and tobacco smoke (Hecht, 2003, 2012; Smith et al., 1997; Smith et al., 2000a; Smith et al., 2000b; Talhout et al., 2011; U.S. Food and Drug Administration, 2012), while the book by Rodgman and Perfetti catalogued all known constituents (Rodgman and Perfetti, 2013). Our review in 2012 recognized 72 carcinogens in mainstream smoke (Hecht, 2012). In the same year, the U.S. Food and Drug Administration (FDA) established a list of 93 harmful and potentially harmful constituents in tobacco smoke and tobacco products. That list included carcinogens (79 compounds), respiratory and cardiovascular toxicants, addictive compounds, and others (U.S. Food and Drug Administration, 2012). As of 2022, the International Agency for Research on Cancer (IARC) has provided a list of 533 compounds with confirmed, probable or possible carcinogenicity to humans. Since 2012, 35 monographs (Volumes 100 – 128 and 100A – F) have been released with much new data added to the understanding of tobacco carcinogens (International Agency for Research on Cancer). For example, acrolein – one of the most abundant hazardous components in tobacco smoke – was recently categorized as a Group 2A carcinogen, “probably carcinogenic to humans”. In this paper, we provide an updated review of IARC carcinogens identified in unburned tobacco and tobacco smoke, with a focus on their occurrence in recent reports since 2012.
2. Overview of Tobacco Carcinogens
Tremendous analytical chemistry research over the past decades has identified continually increasing numbers of compounds in tobacco and tobacco smoke. A 1959 review summarized 220 constituents in tobacco and tobacco smoke (Johnstone and Plimmer, 1959). This number was later upgraded to over 1200 in 1968 (Stedman, 1968), 2549 in 1982 (Dube and Green, 1982), 3044 in 1988 (Roberts, 1988) and 8622 in 2008 (Rodgman and Perfetti, 2008). A comprehensive review of chemical components of tobacco and tobacco smoke was published in 2013 by Rodgman and Perfetti (Rodgman and Perfetti, 2013). The total number of compounds identified in tobacco plus tobacco smoke reached 9582 (including 292 partially identified isomers); of which, the chemical components identified in tobacco smoke numbered 6010. The chemical components identified in tobacco numbered 5595, which does not include tobacco ingredients (Baker and Bishop, 2004; Baker et al., 2004a, b) or hundreds of enzymes and other proteinaceous components. With the advance of analytical technologies, and in particular mass spectrometry, the number of compounds to be identified in tobacco and tobacco smoke will probably continue this increasing trend.
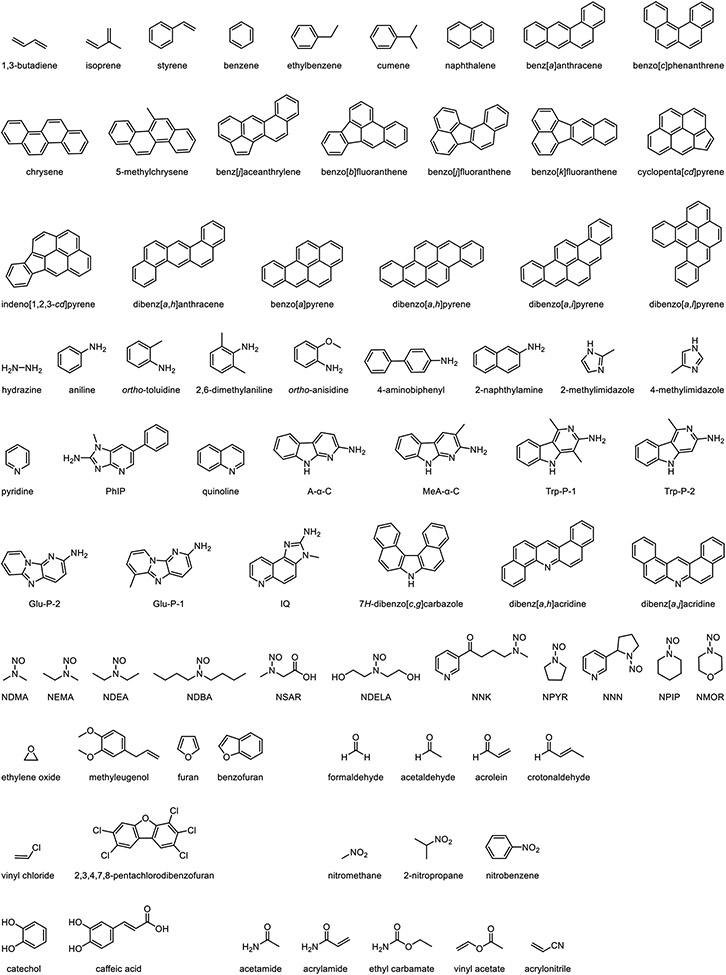
Tobacco and tobacco smoke components can be generally classified as hydrocarbons (0.71%), oxygen-containing components (75.70%), nitrogen-containing components (12.98%), and miscellaneous components (10.61%) (Rodgman and Perfetti, 2013). A more specific classification based on their functional groups has been described by Roberts (Roberts, 1988) and modified by Hoffmann (Hoffmann et al., 2001). Adopted from their work, we have classified all the tobacco carcinogens (with the exception of carcinogens derived from pesticides and growth regulators in tobacco products) into 10 subgroups with minor modifications. As shown in the Table, they are (1) hydrocarbons, (2) amines, (3) N-nitrosamines, (4) ethers, (5) aldehydes, (6) halogenated compounds, (7) nitro compounds, (8) phenolic compounds, (9) miscellaneous compounds, and (10) inorganic compounds. For each subgroup, carcinogens are organized based on increasing structural complexity (Figure).
Table:
A total of 83 tobacco and tobacco smoke components have been classified as carcinogens by IARC as of 2022. Group 1 carcinogens: 18; group 2A carcinogens: 15; group 2B carcinogens: 50.
| Class | Name and CAS number | IARC Group |
Degree of evidence of carcinogenicitya |
Most recent volume |
Year | Hecht listb |
U.S. FDA listc |
Occurrence in tobacco mainstream smoked,e,f | Occurrence in unburned tobacco | |
|---|---|---|---|---|---|---|---|---|---|---|
| Human | Animal | |||||||||
| Hydrocarbons (22 compounds) | ||||||||||
| Alkenes (3) | 1,3-Butadiene (106-99-0) | 1 | Suffic. | Suffic. | 100F | 2012 | × | × | 6.4 – 68.7 μg/cig (Hecht, 2012) | |
| 12.2 – 27.6 (modified ISO) μg/cig in 10 Spanish brands (Marcilla et al., 2012) | ||||||||||
| Present (Oldham et al., 2014) | ||||||||||
| 20.2 – 35.1 (ISO) or 80.9 – 90.5 (CI) μg/cig in 3 commercial brands (Eldridge et al., 2015) | ||||||||||
| 7.6 – 60.4 (ISO) or 67.8 – 118.3 (CI) μg/cig in 50 U.S. brands (Pazo et al., 2016) | ||||||||||
| 50 ± 21 (ISO) or 150 ± 30 (CI) μg/g (mean ± SD) in Dutch commercial cigarettes (Pennings et al., 2020) | ||||||||||
| 94 ± 14 (ISO) or 180 ± 20 (CI) μg/little cigar (mean ± SD) in 60 U.S. brands (Vu et al., 2021) | ||||||||||
| Isoprene (78-79-5) | 2B | ND | Suffic. | 71 | 1999 | × | × | 70 – 586 μg/cig (Hecht, 2012) | <8 – 22.5 ng/g in unheated waterpipe tobacco solid waste (Hsieh et al., 2021) | |
| 1.1 – 2.9 (modified ISO) μg/cig in 10 Spanish brands (Marcilla et al., 2012) | ||||||||||
| Present (Oldham et al., 2014) | ||||||||||
| 207 – 373 (ISO) or 870 – 1013 (CI) μg/cig in 3 commercial brands (Eldridge et al., 2015) | ||||||||||
| 93 ± 39 (ISO) or 290 ± 60 (CI) μg/g (mean ± SD) in Dutch commercial cigarettes (Pennings et al., 2020) | ||||||||||
| 379 ± 57 (ISO) or 644 ± 94 (CI) μg/little cigar (mean ± SD) in 60 U.S. brands (Vu et al., 2021) | ||||||||||
| Styrene (100-42-5) | 2A | Limited | Suffic. | 121 | 2019 | × | × | nd – 48 μg/cig (Hecht, 2012) | ||
| 12.2 – 27.6 (modified ISO) μg/cig in 10 Spanish brands (Marcilla et al., 2012) | ||||||||||
| Present (Oldham et al., 2014) | ||||||||||
| 2.7 – 6.0 (ISO) or 15.0 – 20.0 (CI) μg/cig in 3 commercial brands (Eldridge et al., 2015) | ||||||||||
| 0.18 – 5.36 (ISO) or 10.5 – 22.0 (CI) μg/cig in 50 U.S. brands (Pazo et al., 2016) | ||||||||||
| 7.2 ± 1.2 μg/puff (mean ± SD) in bidi smoke (Oladipupo et al., 2019) | ||||||||||
| 11 ± 6 (ISO) or 45 ± 8 (CI) μg/g (mean ± SD) in Dutch commercial cigarettes (Pennings et al., 2020) | ||||||||||
| Monocyclic Aromatic Hydrocarbons (MAH, 3) | Benzene (71-43-2) | 1 | Suffic. | Suffic. | 120 | 2018 | × | × | 6.1 – 58.9 μg/cig (Hecht, 2012) | |
| 45.7 – 119.5 (modified ISO) μg/cig in 10 Spanish brands (Marcilla et al., 2012) | ||||||||||
| Present (Oldham et al., 2014) | ||||||||||
| 27.9 – 45.5 (ISO) or 83.5 – 98.5 (CI) μg/cig in 3 commercial brands (Eldridge et al., 2015) | ||||||||||
| 6.2 – 48.7 (ISO) or 58.7 – 128.5 (CI) μg/cig in 50 U.S. brands (Pazo et al., 2016) | ||||||||||
| 46 ± 19 (ISO) or 130 ± 20 (CI) μg/g (mean ± SD) in Dutch commercial cigarettes (Pennings et al., 2020) | ||||||||||
| 111 ± 13 (ISO) or 216 ± 18 (CI) μg/little cigar (mean ± SD) in 60 U.S. brands (Vu et al., 2021) | ||||||||||
| Ethylbenzene (100-41-4) | 2B | Inade. | Suffic. | 77 | 2000 | × | × | Present (Hecht, 2012) | ||
| Present (Oldham et al., 2014) | ||||||||||
| 0.41 – 6.13 (ISO) or 9.7 – 21.6 (CI) μg/cig in 50 U.S. brands (Pazo et al., 2016) | ||||||||||
| 12 ± 6 (ISO) or 40 ± 6 (CI) μg/g (mean ± SD) in Dutch commercial cigarettes (Pennings et al., 2020) | ||||||||||
| Cumene (98-82-8) | 2B | ND | Suffic. | 101 | 2012 | 7 – 14 μg/cig (International Agency for Research on Cancer, 2012d) | ||||
| Polycyclic Aromatic Hydrocarbons (PAH, 16) | Naphthalene (NAP, 91-20-3) | 2B | Inade. | Suffic. | 82 | 2002 | × | × | 65 – 868 ng/cig (Hecht, 2012) | ~360 ng/g in Alaskan lq’mik smokeless tobacco and 360 ng/g in Copenhagen snuff (Hearn et al., 2013) |
| 0.8 – 3.3 (modified ISO) μg/cig in 10 Spanish brands (Marcilla et al., 2012) | Present (Oldham et al., 2014) | |||||||||
| Present (Oldham et al., 2014) | nd – 351.2 ng/g in 18 Chinese smokeless tobacco products (Wang et al., 2021) | |||||||||
| 0.1 – 324.0 ng/cig in 13 Chinese brands (Gao et al., 2015) | ||||||||||
| 14 – 804 (ISO) or 608 – 2120 (CI) ng/cig in 35 top-selling U.S. brands (Vu et al., 2015) | ||||||||||
| 0.02 ± 0.10 (ISO) or 0.8 ± 0.6 (CI) μg/g (mean ± SD) in Dutch commercial cigarettes (Pennings et al., 2020) | ||||||||||
| Benz[a]anthracene (B[a]A, 56-55-3) | 2B | ND | Suffic. | 92 | 2010 | × | × | 2.6 – 26.8 ng/cig (Hecht, 2012) | 36 – 292 ng/g in Alaskan lq’mik smokeless tobacco and 81 ng/g in Copenhagen snuff (Hearn et al., 2013) | |
| Present (Oldham et al., 2014) | Present (Oldham et al., 2014) | |||||||||
| 2.0 – 56.4 ng/cig in 13 Chinese brands (Gao et al., 2015) | nd – 769.0 ng/g in 18 Chinese smokeless tobacco products (Wang et al., 2021) | |||||||||
| 3.7 – 39 (ISO) or 21 – 59 (CI) ng/cig in 35 top-selling U.S. brands (Vu et al., 2015) | ||||||||||
| 15 – 74 (ISO) or 35 – 100 (CI) ng/cig in 13 Natural American Spirit cigarettes and 17 – 34 (ISO) or 33 – 53 (CI) ng/cig in 5 U.S. popular cigarette brands (Jain et al., 2019) | ||||||||||
| Benzo[c]phenanthrene (B[c]P, 195-19-7) | 2B | ND | Limited | 92 | 2010 | × | × | Present (Hecht, 2012) | ||
| Present (Oldham et al., 2014) | ||||||||||
| nd – 18.6 ng/cig in 13 Chinese brands (Gao et al., 2015) | ||||||||||
| Chrysene (CHR, 218-01-9) | 2B | ND | Suffic. | 92 | 2010 | × | × | 2.6 – 24.7 ng/cig (Hecht, 2012) | 37 – 359 ng/g in Alaskan lq’mik smokeless tobacco) and 97 ng/g in Copenhagen snuff (Hearn et al., 2013) | |
| Present (Oldham et al., 2014) | Present (Oldham et al., 2014) | |||||||||
| 2.5 – 64.4 ng/cig in 13 Chinese brands (Gao et al., 2015) | <1.42 – 211 ng/g wet weight in Rapé tobacco products (Stanfill et al., 2015) | |||||||||
| 4.5 – 61 (ISO) or 30 – 97 (CI) ng/cig in 35 top-selling U.S. brands (Vu et al., 2015) | nd – 936.7 ng/g in 18 Chinese smokeless tobacco products (Wang et al., 2021) | |||||||||
| 23 – 103 (ISO) or 52 – 145 (CI) ng/cig in 13 Natural American Spirit cigarettes and 24 – 43 (ISO) or 51 – 69 (CI) ng/cig in 5 U.S. popular cigarette brands (Jain et al., 2019) | ||||||||||
| 5-Methylchrysene (3697-24-3) | 2B | ND | Suffic. | 92 | 2010 | × | × | nd – 2 ng/cig (Hecht, 2012) | nd – 18 ng/g in Alaskan lq’mik smokeless tobacco and 8.4 ng/g in Copenhagen snuff (Hearn et al., 2013) | |
| Present (Oldham et al., 2014) | ||||||||||
| <1.5 ng/cig in 3R4F cigarette (Jeffery et al., 2018) | ||||||||||
| Benz[j]aceanthrylene (B[j]A, 202-33-5) | 2B | ND | Limited | 92 | 2010 | × | × | Present (Hecht, 2012) | ||
| Present (Oldham et al., 2014) | ||||||||||
| <2.5 ng/cig in 3R4F cigarette (Jeffery et al., 2018) | ||||||||||
| Benzo[b]fluoranthene (B[b]F, 205-99-2) | 2B | ND | Suffic. | 92 | 2010 | × | × | 1.3 – 17.0 ng/cig (Hecht, 2012) | 19.4 – 68 ng/g in Alaskan lq’mik smokeless tobacco and 31 ng/g in Copenhagen snuff ng/g (Hearn et al., 2013) | |
| Present (Oldham et al., 2014) | Present (Oldham et al., 2014) | |||||||||
| 0.5 – 17.7 ng/cig in 13 Chinese brands (Gao et al., 2015) | <0.79 – 23.2 ng/g wet weight in Rapé tobacco products (Stanfill et al., 2015) | |||||||||
| 1.9 – 22 (ISO) or 15 – 37 (CI) ng/cig in 35 top-selling U.S. brands (Vu et al., 2015) | nd – 203.9 ng/g in 18 Chinese smokeless tobacco products (Wang et al., 2021) | |||||||||
| Benzo[j]fluoranthene (B[j]F, 205-82-3) | 2B | ND | Suffic. | 92 | 2010 | × | 1.8 – 24 ng/cig (Hecht, 2012) | 18 – 63 ng/g in Alaskan lq’mik smokeless tobacco and 30 ng/g in Copenhagen snuff (Hearn et al., 2013) | ||
| B[b]F plus B[j]F: 10 – 41 (ISO) or 26 – 50 (CI) ng/cig in 13 Natural American Spirit cigarettes and 12 – 22 (ISO) or 27 – 37 (CI) ng/cig in 5 U.S. popular cigarette brands (Jain et al., 2019) | nd – 1052.8 ng/g in 18 Chinese smokeless tobacco products (Wang et al., 2021) | |||||||||
| Benzo[k]fluoranthene (B[k]F, 207-08-9) | 2B | ND | Suffic. | 92 | 2010 | × | × | 0.5 – 3.3 ng/cig (Hecht, 2012) | 5.4 – 39 (Alaskan lq’mik smokeless tobacco) and 10 (Copenhagen snuff) ng/g (Hearn et al., 2013) | |
| Present (Oldham et al., 2014) | Present (Oldham et al., 2014) | |||||||||
| 0.6 – 17.1 ng/cig in 13 Chinese brands (Gao et al., 2015) | <0.53 – 9.83 ng/g wet weight in Rapé tobacco products (Stanfill et al., 2015) | |||||||||
| 0.7 – 3.7 (ISO) or 2.0 – 7.0 (CI) ng/cig in 35 top-selling U.S. brands (Vu et al., 2015) | nd – 921.4 ng/g in 18 Chinese smokeless tobacco products (Wang et al., 2021) | |||||||||
| 1.4 – 7.7 (ISO) or 2.0 – 3.8 (CI) ng/cig in 13 Natural American Spirit cigarettes and 12 – 22 (ISO) or 4.1 – 6.5 (CI) ng/cig in 5 U.S. popular cigarette brands (Jain et al., 2019) | ||||||||||
| Cyclopenta[cd]pyrene (CP[cd]P, 27208-37-3) | 2A | ND | Suffic. | 92 | 2010 | × | × | Present (Hecht, 2012) | ||
| Present (Oldham et al., 2014) | ||||||||||
| 0.5 – 12.1 ng/cig in 13 Chinese brands (Gao et al., 2015) | ||||||||||
| Indeno[1,2,3-cd]pyrene (I[cd]P, 193-39-5) | 2B | ND | Suffic. | 92 | 2010 | × | × | 0.65 – 11.2 ng/cig (Hecht, 2012) | 30 – 77 ng/g in Alaskan lq’mik smokeless tobacco and 36 ng/g in Copenhagen snuff (Hearn et al., 2013) | |
| Present (Oldham et al., 2014) | Present (Oldham et al., 2014) | |||||||||
| 0.3 – 18.1 ng/cig in 13 Chinese brands (Gao et al., 2015) | nd – 446.8 ng/g in 18 Chinese smokeless tobacco products (Wang et al., 2021) | |||||||||
| 2.6 – 17.3 (ISO) or 7.4 – 23 (CI) ng/cig in 13 Natural American Spirit cigarettes and 8.8 – 15.9 (ISO) or 8.4 – 13 (CI) ng/cig in 5 U.S. popular cigarette brands (Jain et al., 2019) | ||||||||||
| Dibenz[a,h]anthracene (DB[ah]A, 53-70-3) | 2A | ND | Suffic. | 92 | 2010 | × | × | nd – 6 ng/cig (Hecht, 2012) | 5.7 – 19 (Alaskan lq’mik smokeless tobacco) and 6.3 (Copenhagen snuff) ng/g (Hearn et al., 2013) | |
| Present (Oldham et al., 2014) | Detectable (Oldham et al., 2014) | |||||||||
| 0.1 – 3.3 ng/cig in 13 Chinese brands (Gao et al., 2015) | nd – 97.3 ng/g in 18 Chinese smokeless tobacco products (Wang et al., 2021) | |||||||||
| 3.8 – 8.6 (ISO) or 6.1 – 10 (CI) ng/cig in 13 Natural American Spirit cigarettes and 3.0 – 4.8 (ISO) or 5.0 – 6.9 (CI) ng/cig in 5 U.S. popular cigarette brands (Jain et al., 2019) | ||||||||||
| Benzo[a]pyrene (B[a]P, 50-32-8) | 1 | ND | Suffic. | 100F | 2012 | × | × | 1.0 – 15.2 ng/cig (Hecht, 2012) | 13 – 104 (Alaskan lq’mik smokeless tobacco) and 27 (Copenhagen snuff) ng/g (Hearn et al., 2013) | |
| Present (Oldham et al., 2014) | Present (Oldham et al., 2014) | |||||||||
| 1.1 – 41.6 ng/cig in 13 Chinese brands (Gao et al., 2015) | 3.51 – 24.3 ng/g wet weight in Rapé tobacco products (Stanfill et al., 2015) | |||||||||
| 2.1 – 22 (ISO) or 13 – 38 (CI) ng/cig in 35 top-selling U.S. brands (Vu et al., 2015) | <0.5 – 1.9 ng/g unheated waterpipe tobacco solid waste (Hsieh et al., 2021) | |||||||||
| 4.43 – 8.03 (ISO) or 13.04 – 17.27 (CI) ng/cig in 3 commercial brands (Eldridge et al., 2015) | nd – 366.8 ng/g in 18 Chinese smokeless tobacco products (Wang et al., 2021) | |||||||||
| 4 – 44 ng/cig in 43 Chinese brands (Yershova et al., 2016) | ||||||||||
| 2.0 – 8.7 (ISO) or 6.3 – 17.8 (CI) ng/cig in SPECTRUM research cigarettes (Ding et al., 2017) | ||||||||||
| 8.0 – 34.8 (ISO) or 15 – 38 (CI) ng/cig in 13 Natural American Spirit cigarettes and 8.8 – 15.9 (ISO) or 18 – 23 (CI) ng/cig in 5 U.S. popular cigarette brands (Jain et al., 2019) | ||||||||||
| Dibenzo[a,h]pyrene (DB[ah]P, 189-64-0) | 2B | ND | Suffic. | 92 | 2010 | × | × | 5 – 9.5 ng/cig (Hecht, 2012) | ||
| Detectable (Oldham et al., 2014) | ||||||||||
| 0.1 – 1.1 ng/cig in 3R4F cigarette (Jeffery et al., 2018) | ||||||||||
| Dibenzo[a,i]pyrene (DB[ai]P, 189-55-9) | 2B | ND | Suffic. | 92 | 2010 | × | × | 0.7 – 1.2 ng/cig (Hecht, 2012) | 1.4 – 2.2 ng/g in Alaskan lq’mik smokeless tobacco) and 1.5 ng/g in Copenhagen snuff (Hearn et al., 2013) | |
| Present (Oldham et al., 2014) | ||||||||||
| 0.1 – 0.8 ng/cig in 3R4F cigarette (Jeffery et al., 2018) | ||||||||||
| Dibenzo[a,l]pyrene (DB[al]P, 191-30-0) | 2A | ND | Suffic. | 92 | 2010 | × | × | 0.1 ng (Hecht, 2012) | ||
| Barely detectable (Oldham et al., 2014) | ||||||||||
| 0.03 or 0.1 ng/cig in 3R4F cigarette (Jeffery et al., 2018) | ||||||||||
| Amines (22 compounds) | ||||||||||
| Aliphatic Amine (1) | Hydrazine (302-01-2) | 2A | Limited | Suffic. | 115 | 2018 | × | × | 24 – 57 ng/cig (Hecht, 2012) | |
| Detectable (Oldham et al., 2014) | ||||||||||
| Aromatic Amines (AA, 6) | Aniline (62-53-3) | 2A | Inade. | Suffic. | 127 | 2021 | 430 – 9570 or 120 – 809 or 129 – 838 ng/cig (International Agency for Research on Cancer, 2021b) | |||
| ortho-Toluidine (95-53-4) | 1 | Suffic. | Suffic. | 100F | 2012 | × | × | 8.6 – 144 ng/cig (Hecht, 2012) | ||
| Present (Oldham et al., 2014) | ||||||||||
| 21.2 – 99.9 (ISO) or 115.7 – 206.9 (CI) ng/cig in SPECTRUM research cigarettes (Ding et al., 2017) | ||||||||||
| 6.89 – 46.75 (ISO) or 53.18 – 149.70 (CI) ng/cig in 16 top-selling Chinese cigarettes (Zhang et al., 2017) | ||||||||||
| 2,6-Dimethylaniline (87-62-7) | 2B | ND | Suffic. | 57 | 1993 | × | × | 3.6 – 18 ng/cig (Hecht, 2012) | ||
| Present (Oldham et al., 2014) | ||||||||||
| 7.1 – 37.4 (ISO) or 34.4 – 74.7 (CI) ng/cig in SPECTRUM research cigarettes (Ding et al., 2017) | ||||||||||
| 0.38 – 1.63 (ISO) or 0.64 – 2.67 (CI) ng/cig in 16 top-selling Chinese cigarettes (Zhang et al., 2017) | ||||||||||
| ortho-Anisidine (90-04-0) | 2A | Inade. | Suffic. | 127 | 2021 | × | × | Present (Hecht, 2012) | ||
| Present (Oldham et al., 2014) | ||||||||||
| 0.7 – 2.6 (ISO) or 1.7 – 5.7 (CI) ng/cig in SPECTRUM research cigarettes (Ding et al., 2017) | ||||||||||
| 0.23 – 1.52 (ISO) or 1.02 – 3.98 (CI) ng/cig in 16 top-selling Chinese cigarettes (Zhang et al., 2017) | ||||||||||
| 4-Aminobiphenyl (4-ABP, 92-67-1) | 1 | Suffic. | Suffic. | 100F | 2012 | × | × | 0.3 – 3.3 ng/cig (Hecht, 2012) | ||
| Present (Oldham et al., 2014) | ||||||||||
| 0.83 – 1.61 (ISO) or 2.60 – 3.64 (CI) ng/cig in 3 commercial brands (Eldridge et al., 2015) | ||||||||||
| 1.2 – 4.6 (ISO) or 5.6 – 11.1 (CI) ng/cig in SPECTRUM research cigarettes (Ding et al., 2017) | ||||||||||
| 0.16 – 1.36 (ISO) or 0.38 – 5.95 (CI) ng/cig in 16 top-selling Chinese cigarettes (Zhang et al., 2017) | ||||||||||
| 2-Naphthylamine (2-NA, 91-59-8) | 1 | Suffic. | Suffic. | 100F | 2012 | × | × | 1.47 – 17.2 ng/cig (Hecht, 2012) | ||
| Present (Oldham et al., 2014) | ||||||||||
| 4.7 – 9.9 (ISO) or 10.9 – 16.6 (CI) ng/cig in 3 commercial brands (Eldridge et al., 2015) | ||||||||||
| 3.6 – 15.1 (ISO) or 17.1 – 36.3 (CI) ng/cig in SPECTRUM research cigarettes (Ding et al., 2017) | ||||||||||
| 0.35 – 1.88 (ISO) or 0.89 – 4.60 (CI) ng/cig in 16 top-selling Chinese cigarettes (Zhang et al., 2017) | ||||||||||
| Heterocyclic Aromatic Amines (HAA, 15) | 2-Methylimidazole (693-98-1) | 2B | ND | Suffic. | 101 | 2012 | Possibly present (International Agency for Research on Cancer, 2012a) | |||
| 4-Methylimidazole (822-36-6) | 2B | ND | Suffic. | 101 | 2012 | 2.3 – 15 μg/cig (International Agency for Research on Cancer, 2012b) | ||||
| Pyridine (110-86-1) | 2B | Inade. | Suffic. | 119 | 2019 | 31.1 ± 1.7 (ISO) or 59 ± 4.9 (CI) μg/cig (mean ± SD) in a Canadian cigarette brand (Moir et al., 2008) | ||||
| 2.00 – 13.10 μg/cig in 10 Indian popular cigarettes (Saha et al., 2010) | ||||||||||
| 3 – 28 μg/cig (International Agency for Research on Cancer, 2019a) | ||||||||||
| 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP, 105650-23-5) | 2B | Inade. | Suffic. | 56 | 1993 | × | × | 11 – 23 ng/cig (Hecht, 2012) | ||
| Barely detectable (Oldham et al., 2014) | ||||||||||
| Quinoline (91-22-5) | 2B | Inade. | Suffic. | 121 | 2019 | 1.31 ± 0.08 (ISO) μg/cig (mean ± SD) in a Canadian cigarette brand (Moir et al., 2008) | ||||
| 0.07 – 1.19 μg/cig in 10 Indian popular cigarettes (Saha et al., 2010) | ||||||||||
| 0.23 – 0.30 μg/cig (International Agency for Research on Cancer, 2019b) | ||||||||||
| 2-Amino-9H-pyrido[2,3-b]indole (A-α-C, 26148-68-5) | 2B | ND | Suffic. | Sup 7 | 1987 | × | × | nd – 260 ng/cig (Hecht, 2012) | ||
| Present (Oldham et al., 2014) | ||||||||||
| 49 – 160 ng/cig in U.S. commercial cigarettes (Zhang et al., 2011) | ||||||||||
| 2-Amino-3-methyl-9H-pyrido[2,3-b]indole (MeA-α-C, 68006-83-7) | 2B | ND | Suffic. | Sup 7 | 1987 | × | × | 2 – 37 ng/cig (Hecht, 2012) | ||
| Present (Oldham et al., 2014) | ||||||||||
| 3.1 – 9.7 ng/cig in U.S. commercial cigarettes (Zhang et al., 2011) | ||||||||||
| 3-Amino-1,4-dimethyl-5H-pyrido[4,3-b]indole (Trp-P-1, 62450-06-0) | 2B | ND | Suffic. | Sup 7 | 1987 | × | × | 0.2 – 0.3 ng/cig (Hecht, 2012) | ||
| Barely detectable (Oldham et al., 2014) | ||||||||||
| 0.6 – 2.4 ng/cig in U.S. commercial cigarettes (Zhang et al., 2011) | ||||||||||
| 3-Amino-1-methyl-5H-pyrido[4,3-b]indole (Trp-P-2, 62450-07-1) | 2B | ND | Suffic. | Sup 7 | 1987 | × | × | nd – 0.2 ng/cig (Hecht, 2012) | ||
| Barely detectable (Oldham et al., 2014) | ||||||||||
| 1.8 – 9.2 ng/cig in U.S. commercial cigarettes (Zhang et al., 2011) | ||||||||||
| 2-Aminodipyrido[1,2-a:3',2'-d]imidazole (Glu-P-2, 67730-10-3) | 2B | ND | Suffic. | Sup 7 | 1987 | × | × | 0.25 – 0.88 ng/cig (Hecht, 2012) | ||
| Barely detectable (Oldham et al., 2014) | ||||||||||
| 2-Amino-6-methyldipyrido[1,2-a:3',2'-d]imidazole (Glu-P-1, 67730-11-4) | 2B | ND | Suffic. | Sup 7 | 1987 | × | × | nd – 0.89 ng/cig (Hecht, 2012) | ||
| Barely detectable (Oldham et al., 2014) | ||||||||||
| 2-Amino-3-methylimidazo[4,5-f]quinoline (IQ, 76180-96-6) | 2A | Inade. | Suffic. | 56 | 1993 | × | × | 0.3 ng/cig (Hecht, 2012) | ||
| Barely detectable (Oldham et al., 2014) | ||||||||||
| 7H-Dibenzo[c,g]carbazole (194-59-2) | 2B | ND | Suffic. | 103 | 2013 | × | nd – 0.7 ng/cig (Hecht, 2012) | |||
| Barely detectable (Oldham et al., 2014) | ||||||||||
| Dibenz[a,h]acridine (226-36-8) | 2B | ND | Suffic. | 103 | 2013 | × | nd – 0.1 ng/cig (Hecht, 2012) | |||
| Barely detectable (Oldham et al., 2014) | ||||||||||
| Dibenz[a,j]acridine (224-42-0) | 2A | ND | Suffic. | 103 | 2013 | × | nd – 10 ng/cig (Hecht, 2012) | |||
| Barely detectable (Oldham et al., 2014) | ||||||||||
| N-Nitrosamines (11 compounds) | ||||||||||
| Acylic N-Nitrosamines (7) | N-Nitrosodimethylamine (NDMA) (62-75-9) | 2A | ND | Suffic. | Sup 7 | 1987 | × | × | nd – 7.9 ng/cig (Hecht, 2012) | <LOQ – 39.8 and <LOQ – 24.5 ng/g dry weight in U.S. and Swedish smokeless tobacco, respectively. (Borgerding et al., 2012) |
| Present (Oldham et al., 2014) | Detectable (Oldham et al., 2014) | |||||||||
| <LOQ – 8.7 ng/g in 7 different Chinese tobacco samples (Lv et al., 2016) | ||||||||||
| nd – 8.9 ng/g in 18 Chinese smokeless tobacco products (Wang et al., 2021) | ||||||||||
| N-Nitrosoethylmethylamine (NEMA, 10595-95-6) | 2B | ND | Suffic. | Sup 7 | 1987 | × | × | nd – 0.2 ng/cig (Hecht, 2012) | ||
| Detectable (Oldham et al., 2014) | ||||||||||
| N-Nitrosodiethylamine (NDEA, 55-18-5) | 2A | ND | Suffic. | Sup 7 | 1987 | × | × | nd – 7.6 ng/cig (Hecht, 2012) | 0.5 ng/g in one out of 7 different Chinese tobacco samples (Lv et al., 2016) | |
| Detectable (Oldham et al., 2014) | ||||||||||
| N-Nitrosodi-n-butylamine (NDBA, 924-16-3) | 2B | ND | Suffic. | Sup 7 | 1987 | nd – 1.4 ng/g in 7 different Chinese tobacco samples (Lv et al., 2016) | ||||
| N-Nitrososarcosine (NSAR, 13256-22-9) | 2B | ND | Suffic. | Sup 7 | 1987 | × | 22 – 460 ng/cig (National Toxicology Program, 2016) | 550.5 ± 43.7 ng/g in one U.S. dry snuff (Wu et al., 2012b) | ||
| 29.9 and 61.9 ng/g in 2 U.S. smokeless tobacco products (Werneth et al., 2021) | ||||||||||
| N-Nitrosodiethanolamine (NDELA, 1116-54-7) | 2B | Inade. | Suffic. | 77 | 2000 | × | × | nd – 290 ng/cig (Hecht, 2012) | Detectable (Oldham et al., 2014) | |
| Present (Oldham et al., 2014) | ||||||||||
| 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK, 64091-91-4) | 1 | Inade. | Suffic. | 100E | 2012 | × | × | 25.6 – 146.1 ng/cig in 17 U.S. cigarette brands (Stepanov et al., 2012) | <LOQ – 6761 and <LOQ – 319 ng/g dry weight in U.S. and Swedish smokeless tobacco, respectively (Borgerding et al., 2012) | |
| 21.4 – 50.5 (ISO) or 78.1 – 151.3 (CI) ng/cig in 3 commercial brands (Eldridge et al., 2015) | 0.34 – 0.91 μg/g wet weight in the tobacco filler of 17 U.S. cigarette brands (Stepanov et al., 2012) | |||||||||
| 3.3 – 63.9 ng/cig in 43 Chinese cigarette brands (Yershova et al., 2016) | 0.109 – 4.57 μg/g dry weight in selected Indian smokeless tobacco products (Stepanov et al., 2017b) | |||||||||
| 13 – 122.4 (ISO) or 39.9 – 245.9 (CI) ng/cig in 50 U.S. cigarette brands (Edwards et al., 2017) | 0.185 – 1.13 μg/g in 216 U.S. snus and dissolvable tobacco products (Stepanov et al., 2014a) | |||||||||
| 9.2 – 39 (ISO) or 38 – 128 (CI) ng/cig in 13 Natural American Spirit cigarettes and 71 – 121 (ISO) or 129 – 198 (CI) ng/cig in 5 U.S. popular cigarette brands (Jain et al., 2019) | 0.19 – 3.44 μg/g wet weight in 31 U.S. smokeless tobacco brands (Hatsukami et al., 2015) | |||||||||
| 89 – 879 (ISO) or 138 – 1570 (CI) ng/cigar in 60 U.S. little cigars (Edwards et al., 2021) | 1.2 – 5.0 μg/g wet weight in Chaini Khaini from Indian market (Stepanov et al., 2015) | |||||||||
| 0.032 – 1.35 μg/g in the tobacco filler of 43 Chinese cigarette brands (Yershova et al., 2016) | ||||||||||
| 192 ± 21 (prepackaged) and 71 ± 4 (bulk) ng/g wet weight (mean ± SD) in nasvai from Kyrgyzstan (Stepanov et al., 2017a) | ||||||||||
| 193.9 – 1092.9 ng/g in cigarette filler of 50 U.S. brands (Edwards et al., 2017) | ||||||||||
| 0.11 – 0.35 μg/g in cigarette filler of 13 Natural American Spirit cigarette varieties and 0.11 – 0.35 μg/g in cigarette filler of 5 U.S. popular cigarette brands (Jain et al., 2019) | ||||||||||
| 0.72 – 34 and 3.7 – 9.7 μg/g wet weight in two Bangladeshi smokeless tobacco products Zarda and Gul, respectively (Nasrin et al., 2020) | ||||||||||
| 321 ± 68.1 ng/g wet weight (mean ± SD) in 8 U.S. snus products (Lawler et al., 2020) | ||||||||||
| 26 – 2950 ng/g in the tobacco filler of 60 U.S. little cigars (Edwards et al., 2021) | ||||||||||
| Cyclic N-Nitrosamines (4) | N-Nitrosopyrrolidine (NPYR, 930-55-2) | 2B | ND | Suffic. | Sup 7 | 1987 | × | × | nd – 19.7 ng/cig (Hecht, 2012) | Present (Oldham et al., 2014) |
| Present (Oldham et al., 2014) | nd – 30.7 ng/g in 7 different Chinese tobacco samples (Lv et al., 2016) | |||||||||
| 1.1 – 13.5 ng/g in 18 Chinese smokeless tobacco products (Wang et al., 2021) | ||||||||||
| N′-Nitrosonornicotine (NNN, 16543-55-8) | 1 | Inade. | Suffic. | 100E | 2012 | × | × | 19.5 – 232.1 ng/cig in 17 U.S. cigarette brands (Stepanov et al., 2012) | <LOQ – 14424 and <LOQ – 1318 ng/g dry weight in U.S. and Swedish smokeless tobacco products, respectively (Borgerding et al., 2012) | |
| 60.4 – 138.7 (ISO) or 163.5 – 329.1 (CI) ng/cig in 3 commercial brands (Eldridge et al., 2015) | 0.33 – 4.03 μg/g wet weight in tobacco filler of 17 U.S. cigarette brands (Stepanov et al., 2012) | |||||||||
| 1.8 – 135.3 ng/cig in 43 Chinese cigarette brands (Yershova et al., 2016) | 0.457 – 37.0 μg/g dry weight in selected Indian smokeless tobacco products (Stepanov et al., 2017b) | |||||||||
| 17.2 – 171.1 (ISO) or 32.8 – 323.3 (CI) ng/cig in 50 U.S. cigarette brands (Edwards et al., 2017) | 0.267 – 2.66 μg/g in 216 U.S. snus and dissolvable tobacco products (Stepanov et al., 2014a) | |||||||||
| 7.1 – 113 (ISO) or 32 – 323 (CI) ng/cig in 13 Natural American Spirit cigarettes and 117 – 165 (ISO) or 237 – 382 (CI) ng/cig in 5 U.S. popular cigarette brands (Jain et al., 2019) | 0.48 – 11.11 μg/g wet weight in 31 U.S. smokeless tobacco brands (Hatsukami et al., 2015) | |||||||||
| 201 – 1450 (ISO) or 445 – 2780 (CI) ng/cigar ng/cigar in 60 U.S. little cigars (Edwards et al., 2021) | 13.2 – 29.4 μg/g wet weight in Chaini Khaini from Indian market (Stepanov et al., 2015) | |||||||||
| 0.020 – 4.67 μg/g in tobacco filler of 43 Chinese cigarette brands (Yershova et al., 2016) | ||||||||||
| 1189 ± 68 (prepackaged) and 642 ± 53 (bulk) ng/g wet weight (mean ± SD) in nasvai from Kyrgyzstan (Stepanov et al., 2017a) | ||||||||||
| 0.14 – 1.76 μg/g in cigarette filler of 13 Natural American Spirit cigarette varieties and 0.99 – 2.06 μg/g in cigarette filler of 5 U.S. popular cigarette brands (Jain et al., 2019) | ||||||||||
| 305.7 – 2970 ng/g in the tobacco filler of 50 U.S. cigarette brands (Edwards et al., 2017) | ||||||||||
| 2.8 – 59 and 13 – 25 μg/g wet weight in two Bangladeshi smokeless tobacco products Zarda and Gul, respectively (Nasrin et al., 2020) | ||||||||||
| 1050 ± 168 ng/g wet weight (mean ± SD) in 8 U.S. snus products (Lawler et al., 2020) | ||||||||||
| 1440 – 12100 ng/g in the tobacco filler of 60 U.S. little cigars (Edwards et al., 2021) | ||||||||||
| N-Nitrosopiperidine (NPIP, 100-75-4) | 2B | ND | Suffic. | Sup 7 | 1987 | × | × | nd – 231 ng/cig (Hecht, 2012) | Barely detectable (Oldham et al., 2014) | |
| Barely detectable (Oldham et al., 2014) | nd – 9.1 ng/g in 7 different Chinese tobacco samples (Lv et al., 2016) | |||||||||
| nd – 5.2 ng/g in 18 Chinese smokeless tobacco products (Wang et al., 2021) | ||||||||||
| N-Nitrosomorpholine (NMOR, 59-89-2) | 2B | ND | Suffic. | Sup 7 | 1987 | × | Detectable (Oldham et al., 2014) | |||
| nd – 2.7 ng/g in 7 different Chinese tobacco samples (Lv et al., 2016) | ||||||||||
| nd – 4.4 ng/g in 18 Chinese smokeless tobacco products (Wang et al., 2021) | ||||||||||
| Ethers (4 compounds) | ||||||||||
| Ethylene oxide (75-21-8) | 1 | Limited | Suffic. | 100F | 2012 | × | × | Present (Hecht, 2012) | ||
| Present (Oldham et al., 2014) | ||||||||||
| Methyleugenol (93-15-2) | 2B | ND | Suffic. | 101 | 2012 | 0.018 – 0.021 μg/g in U.S. cigarettes and 0.49 – 61 μg/g in U.S. and Indian bidi cigarettes (International Agency for Research on Cancer, 2012c) | ||||
| Furan (110-00-9) | 2B | Inade. | Suffic. | 63 | 1995 | × | × | 18 – 65 μg/cig (Hecht, 2012) | ||
| 7.2 – 16.1 (modified ISO) μg/cig in 10 Spanish brands (Marcilla et al., 2012) | ||||||||||
| Present (Oldham et al., 2014) | ||||||||||
| 4.7 – 34.1 (ISO) or 32.4 – 68.3 μg/cig (CI) in 50 U.S. brands (Pazo et al., 2016) | ||||||||||
| Benzofuran (271-89-6) | 2B | Inade. | Suffic. | 63 | 1995 | × | × | Present (Hecht, 2012) | ||
| Present (Oldham et al., 2014) | ||||||||||
| Aldehydes (4 compounds) | ||||||||||
| Formaldehyde (50-00-0) | 1 | Suffic. | Suffic. | 100F | 2012 | × | × | 1.6 – 75.5 μg/cig (Hecht, 2012) | Present (Oldham et al., 2014) | |
| Present (Oldham et al., 2014) | 0.2 – 5.4 μg/g in unheated waterpipe tobacco solid waste (Hsieh et al., 2021) | |||||||||
| 9.0 – 34.3 (ISO) or 46.8 – 92.7 (CI) μg/cig in 3 commercial brands (Eldridge et al., 2015) | ||||||||||
| 55 – 108 (CI) μg/cig in U.S. commercial cigarettes (Ding et al., 2016) | ||||||||||
| ND – 24.5 (ISO) or 3.4 – 45.6 (CI) μg/cig in SPECTRUM research cigarettes (Ding et al., 2017) | ||||||||||
| 20 ± 13 (ISO) or 67 ± 20 (CI) μg/g (mean ± SD) in Dutch commercial cigarettes (Pennings et al., 2020) | ||||||||||
| 9.0 – 75 (ISO) or 61 – 267 (CI) μg/cig in 13 Natural American Spirit cigarettes and 22 – 62 (ISO) or 65 – 149 (CI) μg/cig in 5 U.S. popular cigarette brands (Jain et al., 2019) | ||||||||||
| Acetaldehyde (75-07-0) | 2B | Inade. | Suffic. | 71 | 1999 | × | × | 32 – 828 μg/cig (Hecht, 2012) | Present (Oldham et al., 2014) | |
| 249.5 – 528.3 (modified ISO) μg/cig in 10 Spanish brands (Marcilla et al., 2012) | ||||||||||
| Present (Oldham et al., 2014) | ||||||||||
| 243 – 428 (ISO) or 973 – 1103 (CI) μg/cig in 3 commercial brands (Eldridge et al., 2015) | ||||||||||
| 1198 – 1947 (CI) μg/cig in U.S. commercial cigarettes (Ding et al., 2016) | ||||||||||
| 126 – 1143 (ISO) or 1098 – 2244 (CI) μg/cig in 50 U.S. brands (Pazo et al., 2016) | ||||||||||
| 434 – 1129 (ISO) or 1658 – 2170 (CI) μg/cig in SPECTRUM research cigarettes (Ding et al., 2017) | ||||||||||
| 477 – 921 (ISO) or 1245 – 1523 (CI) μg/cig in 13 Natural American Spirit cigarettes and 631 – 941 (ISO) or 1250 – 1521 (CI) μg/cig in 5 U.S. popular cigarette brands (Jain et al., 2019) | ||||||||||
| 470 ± 280 (ISO) or 1400 ± 200 (CI) μg/g (mean ± SD) in Dutch commercial cigarettes (Pennings et al., 2020) | ||||||||||
| Acrolein (107-02-8) | 2A | Inade. | Suffic. | 128 | 2021 | × | 7.8 – 20.0 (modified ISO) μg/cig in 10 Spanish brands (Marcilla et al., 2012) | <56 ng/g in unheated waterpipe tobacco solid waste (Hsieh et al., 2021) | ||
| Present (Oldham et al., 2014) | ||||||||||
| 24.9 – 48.5 (ISO) or 100.0 – 120.7 (CI) μg/cig in 3 commercial brands (Eldridge et al., 2015) | ||||||||||
| 106 – 144 (CI) μg/cig in U.S. commercial cigarettes (Ding et al., 2016) | ||||||||||
| 8.6 – 62.7 (ISO) or 82.9 – 110 (CI) μg/cig in SPECTRUM research cigarettes (Ding et al., 2017) | ||||||||||
| 30.8 – 105 (ISO) or 105 – 213 (CI) μg/unit in 15 sheet-wrapped cigars and 35 cigarettes from the U.S. market (Cecil et al., 2017) | ||||||||||
| 21 – 64 (ISO) or 103 – 158 (CI) μg/cig in 13 Natural American Spirit cigarettes and 49 – 60 (ISO) or 100 – 162 (CI) μg/cig in 5 U.S. popular cigarette brands (Jain et al., 2019) | ||||||||||
| 55 ± 37 (ISO) or 230 ± 40 (CI) μg/g (mean ± SD) in Dutch commercial cigarettes (Pennings et al., 2020) | ||||||||||
| 24.9 – 223 μg/cig (International Agency for Research on Cancer, 2021a) | ||||||||||
| Crotonaldehyde ((E/Z), 4170-30-3) | 2B | Inade. | Limited | 128 | 2021 | × | 2.0 – 4.7 (modified ISO) μg/cig in 10 Spanish brands (Marcilla et al., 2012) | Present (Oldham et al., 2014) | ||
| Present (Oldham et al., 2014) | ||||||||||
| 4.8 – 12.1 (ISO) or 37.9 – 47.1 (CI) μg/cig in 3 commercial brands (Eldridge et al., 2015) | ||||||||||
| 0.86 – 17.45 (ISO) or 29.2 – 52.7 (CI) μg/cig in 50 U.S. brands (Pazo et al., 2016) | ||||||||||
| 25 – 72 (CI) μg/cig in U.S. commercial cigarettes (Ding et al., 2016) | ||||||||||
| 3.9 – 20.3 (ISO) or 34.0 – 99.5 (CI) μg/cig in SPECTRUM research cigarettes (Ding et al., 2017) | ||||||||||
| 11 – 36 (ISO) or 20 – 52 (CI) μg/cig in 13 Natural American Spirit cigarettes and 13 – 31 (ISO) or 41 – 63 (CI) μg/cig in 5 U.S. popular cigarette brands (Jain et al., 2019) | ||||||||||
| 19.79 μg/cig (mean value) in Chinese cigarettes (Cai et al., 2019) | ||||||||||
| Halogenated compounds (2 compounds) | ||||||||||
| Vinyl chloride (75-01-4) | 1 | Suffic. | Suffic. | 100F | 2012 | × | × | nd – 36.6 ng/cig (Hecht, 2012) | ||
| <0.005 – 0.042 (ISO) or 0.046 – 0.098 (CI) μg/cig in 50 U.S. brands (Pazo et al., 2016) | ||||||||||
| 2,3,4,7,8-Pentachlorodibenzofuran (2,3,4,7,8-PeCDF, 57117-31-4) | 1 | ND | Suffic. | 100F | 2012 | × | Detectable (Oldham et al., 2014) | |||
| Nitro compounds (3 compounds) | ||||||||||
| Nitromethane (75-52-5) | 2B | ND | Suffic. | 77 | 2000 | × | × | 0.13 – 2.3 (ISO) μg/cig in 10 Chinese cigarette brands (Wang et al., 2015) | ||
| 0.27 – 3.26 (ISO) or 2.13 – 7.66 (CI) μg/cig in 50 U.S. brands (Pazo et al., 2016) | ||||||||||
| 1.6 – 4.9 (modified ISO) or 3.2 – 12 (CI) μg/cig in 10 U.S. popular cigarettes (Junco et al., 2021) | ||||||||||
| 2-Nitropropane (79-46-9) | 2B | Inade. | Suffic. | 71 | 1999 | × | × | nd – 18.7 μg/cig (Hecht, 2012) | ||
| Barely detectable (Oldham et al., 2014) | ||||||||||
| 0.18 – 1.5 (ISO) μg/cig in 10 Chinese cigarette brands (Wang et al., 2015) | ||||||||||
| Nitrobenzene (98-95-3) | 2B | Inade. | Suffic. | 65 | 1996 | × | × | 18 – 38 ng/cig in 13 U.S. popular cigarettes (Chapman et al., 2018) | ||
| Phenolic compounds (2 compounds) | ||||||||||
| Catechol (120-80-9) | 2B | ND | Suffic. | 71 | 1999 | × | × | 5.1 – 89.9 μg/cig (Hecht, 2012) | <0.7 – 50.5 μg/g in unheated waterpipe tobacco solid waste (Hsieh et al., 2021) | |
| 22.7 – 51.1 (ISO) or 49.6 – 118 (CI) μg/cig in 10 cigarette brands (Wu et al., 2012a) | ||||||||||
| Present (Oldham et al., 2014) | ||||||||||
| 25.0 – 54.8 (ISO) and 86.3 – 137.0 (CI) μg/cig in 3 commercial brands (Eldridge et al., 2015) | ||||||||||
| Caffeic acid (331-39-5) | 2B | ND | Suffic. | 56 | 1993 | × | × | Detectable (Oldham et al., 2014) | ||
| Miscellaneous compounds (5 compounds) | ||||||||||
| Acetamide (60-35-5) | 2B | ND | Suffic. | 71 | 1999 | × | × | 2.2 – 111 μg/cig (Hecht, 2012) | ||
| Present (Oldham et al., 2014) | ||||||||||
| Acrylamide (79-06-1) | 2A | Inade. | Suffic. | 60 | 1994 | × | × | 2.3 μg/cig (Hecht, 2012) | ||
| Present (Oldham et al., 2014) | ||||||||||
| Ethyl carbamate (51-79-6) | 2A | Inade. | Suffic. | 96 | 2010 | × | × | 10 – 35 ng/cig (Hecht, 2012) | Barely detectable (Oldham et al., 2014) | |
| Barely detectable (Oldham et al., 2014) | ||||||||||
| Vinyl acetate (108-05-4) | 2B | Inade. | Limited | 63 | 1995 | × | × | 1.6 – 4 μg/cig (Hecht, 2012) | ||
| Present (Oldham et al., 2014) | ||||||||||
| 0.056 – 0.497 (ISO) or 0.670 – 1.272 (CI) μg/cig in 50 U.S. cigarette brands (Pazo et al., 2016) | ||||||||||
| Acrylonitrile (107-13-1) | 2B | Inade. | Suffic. | 71 | 1999 | × | × | 0.9 – 19.6 μg/cig (Hecht, 2012) | ||
| Present (Oldham et al., 2014) | ||||||||||
| 6.1 – 12.6 (ISO) and 32.4 – 37.4 (CI) μg/cig in 3 commercial brands (Eldridge et al., 2015) | ||||||||||
| 0.90 – 15.34 μg/cig (ISO); 19.7 – 37.7 μg/cig (CI) in 50 U.S. brands (Pazo et al., 2016) | ||||||||||
| 28 ± 3.6 (ISO) or 69 ± 6.6 (CI) μg/little cigar (mean ± SD) in 60 U.S. brands (Vu et al., 2021) | ||||||||||
| Inorganic compounds (8 compounds) | ||||||||||
| Arsenic | 1 | Suffic. | Suffic. | 100C | 2012 | × | × | Present (Oldham et al., 2014) | 0.22 – 0.36 μg/g dry weight in the tobacco of 50 U.S. cigarette brands (Fresquez et al., 2013) | |
| <0.5 – 5.8 (ISO) or 5.7 – 15.2 (CI) ng/cig in 50 U.S. cigarette brands (Pappas et al., 2014) | Present (Oldham et al., 2014) | |||||||||
| <2.7 – 2.8 (ISO) or <8.8 – 9.6 (CI) ng/cig in 3 commercial brands (Eldridge et al., 2015) | 0.12 – 0.66 μg/g dry weight in the tobacco of 17 U.S. litter cigar brands (Pappas et al., 2015) | |||||||||
| 0.105 – 0.177 μg/g in the tobacco of SPECTRUM cigarettes (Richter et al., 2016) | ||||||||||
| 0.48 – 0.64 μg/g in 13 Natural American Spirit cigarettes and 0.42 – 0.67 μg/g in 5 U.S. popular cigarette brands (Jain et al., 2019) | ||||||||||
| Beryllium | 1 | Suffic. | Suffic. | 100C | 2012 | × | × | nd – 0.5 ng (Hecht, 2012) | 0.015 – 0.049 μg/g dry weight in the tobacco of 50 U.S. cigarette brands (Fresquez et al., 2013) | |
| Barely detectable (Oldham et al., 2014) | Detectable (Oldham et al., 2014) | |||||||||
| 0.020 – 0.075 μg/g dry weight in the tobacco of 17 U.S. litter cigar brands (Pappas et al., 2015) | ||||||||||
| 13 – 20 ng/g in the tobacco of SPECTRUM cigarettes (Richter et al., 2016) | ||||||||||
| Cadmium | 1 | Suffic. | Suffic. | 100C | 2012 | × | × | Present (Oldham et al., 2014) | 1.0 – 1.7 μg/g dry weight in the tobacco of 50 U.S. cigarette brands (Fresquez et al., 2013) | |
| <5.0 – 80 (ISO) and 81 – 200 ng/cig in 50 U.S. cigarette brands (Pappas et al., 2014) | Present [Oldham et al, 2014](Oldham et al., 2014) | |||||||||
| 13.2 – 30.0 (ISO) and 77.8 – 96.7 (CI) ng/cig in 3 commercial brands (Eldridge et al., 2015) | 0.752 – 1.69 μg/g dry weight in the tobacco of 17 U.S. litter cigar brands (Pappas et al., 2015) | |||||||||
| 0.91 – 1.11 μg/g in the tobacco of SPECTRUM cigarettes (Richter et al., 2016) | ||||||||||
| 0.90 – 1.74 μg/g in 13 Natural American Spirit cigarettes and 1.01 – 1.11 μg/g in 5 U.S. popular cigarette brands (Jain et al., 2019) | ||||||||||
| Chromium(VI) | 1 | Suffic. | Suffic. | 100C | 2012 | × | × | Barely detectable (Oldham et al., 2014) | 1.4 – 3.1 μg/g dry weight in the tobacco of 50 U.S. cigarette brands (Fresquez et al., 2013) | |
| <0.88 (ISO) or <1.1 (CI) ng/cig in 50 U.S. cigarette brands (Pappas et al., 2014) | Present (Oldham et al., 2014) | |||||||||
| <3.7 (ISO) or <14.0 (CI) ng/cig in 3 commercial brands (Eldridge et al., 2015) | 0.88 – 6.46 μg/g dry weight in the tobacco of 17 U.S. litter cigar brands (Pappas et al., 2015) | |||||||||
| 0.6 – 1.03 ng/cig in U.S. commercial cigarettes (Fresquez et al., 2017) | 0.89 – 1.34 μg/g in the tobacco of SPECTRUM cigarettes (Richter et al., 2016) | |||||||||
| 0.42 – 0.95 μg/g in 13 Natural American Spirit cigarettes and 1.07 – 2.15 μg/g in 5 U.S. popular cigarette brands (Jain et al., 2019) | ||||||||||
| Cobalt | 2B | Inade. | Suffic./limited/Inade. | 52 | 1991 | × | × | Barely detectable (Oldham et al., 2014) | 0.44 – 1.11 μg/g dry weight in the tobacco of 50 U.S. cigarette brands (Fresquez et al., 2013) | |
| <0.025 – 0.151 (ISO) or 0.09 – 0.30 (CI) ng/cig in 50 U.S. cigarette brands (Pappas et al., 2014) | 0.65 – 1.00 μg/g dry weight in the tobacco of 17 U.S. litter cigar brands (Pappas et al., 2015) | |||||||||
| 0.36 – 0.58 μg/g in the tobacco of SPECTRUM cigarettes (Richter et al., 2016) | ||||||||||
| Lead (inorganic) | 2A | Limited | Suffic. | 87 | 2006 | × | × | Present (Oldham et al., 2014) | 0.60 – 1.16 μg/g dry weight in the tobacco of 50 U.S. cigarette brands (Fresquez et al., 2013) | |
| <5.0 – 23 (ISO) or 29 – 58 (CI) ng/cig in 50 U.S. cigarette brands (Pappas et al., 2014) | Present (Oldham et al., 2014) | |||||||||
| <10.3 – 12.7 (ISO) or 26.3 – 33.3 (CI) ng/cig in 3 commercial brands (Eldridge et al., 2015) | 0.40 – 0.82 μg/g in the tobacco of SPECTRUM cigarettes (Richter et al., 2016) | |||||||||
| 0.59 – 0.78 μg/g in 13 Natural American Spirit cigarettes and 0.66 – 1.03 μg/g in 5 U.S. popular cigarette brands (Jain et al., 2019) | ||||||||||
| Nickel | 1 | Suffic. | Suffic. | 100C | 2012 | × | × | Present (Oldham et al., 2014) | 2.1 – 3.9 μg/g dry weight in the tobacco of 50 U.S. cigarette brands (Fresquez et al., 2013) | |
| <0.38 – 0.48 (ISO) or < 0.50 – 1.09 (CI) ng/cig in 50 U.S. cigarette brands (Pappas et al., 2014) | Present (Oldham et al., 2014) | |||||||||
| <30.9 (ISO) and <25.5 (CI) ng/cig in 3 commercial brands (Eldridge et al., 2015) | 1.58 – 4.37 μg/g dry weight in the tobacco of 17 U.S. litter cigar brands (Pappas et al., 2015) | |||||||||
| 1.35 – 1.79 μg/g in SPECTRUM cigarettes (Richter et al., 2016) | ||||||||||
| 0.56 – 1.65 μg/g in 13 Natural American Spirit cigarettes and 2.11 – 3.24 μg/g in 5 U.S. popular cigarette brands (Jain et al., 2019) | ||||||||||
| Polonium-210 | 1 | Inade. | Suffic. | 78 | 2001 | × | × | 0.03 – 1.0 pCi/cig (Hecht, 2012) | Present (Oldham et al., 2014) | |
| Present (Oldham et al., 2014) | ||||||||||
ND: no adequate data; Inade.: inadequate evidence; Limited: limited evidence; Suffic.: sufficient evidence.
Hecht, SS. Nicotine Tob. Res. 2012, 14 (1), 18.
In the U.S. FDA list of harmful and potentially harmful constitutes in tobacco and tobacco smoke, a total of 79 carcinogens is included. However, some compounds including 1-aminonaphthalene (group 3), dibenzo[a,e]pyrene (also in the Hecht list, group 3), mercury and inorganic mercury compounds (group 3) are not considered carcinogenic to humans by IARC. The compounds cresols and uranium-235 and -238 have not been classified. Aflatoxin B1 is generally considered as the contaminant from the preparation and storage of tobacco products.
ISO: ISO 3308 non-intense smoking regimen; CI: ISO 20778 Canadian intense smoking regimen.
nd: not detected.
For the description of the reported detection of tobacco carcinogens by Oldham et al, 2014, Regul. Toxicol. Pharmacol. 70 (1), 138 – 148: “Present” indicates the analyte levels were above limit of quantitation in multiple batches of samples; “Detectable” indicates the analyte levels were less than limit of quantitation in at least some batches of samples; “Barely detectable” indicates the analyte levels were less than limit of detection.
Figure:
Chemical structures of IARC-classified carcinogens identified in unburned tobacco and tobacco smoke.
It is noteworthy that IARC-classified carcinogens only comprise a portion of the potentially toxic compounds in tobacco and tobacco smoke. There are multiple compounds that cause human health problems other than cancer (Talhout et al., 2011). For example, carbon monoxide causes cardiotoxicity; ammonia leads to respiratory effects; manganese has been linked to lung fibrosis and neurotoxicity. Their risks to humans have been evaluated by environmental protection agencies (Talhout et al., 2011). The toxicants proposed to be mandated for lowering by the World Health Organization Study Group on Tobacco Product Regulation are not limited to tobacco carcinogens NNK, NNN, benzene, benzo[a]pyrene (B[a]P), 1,3-butadiene, formaldehyde, acrolein, acetaldehyde, but also include carbon monoxide (World Health Organization, 2019).
3. Occurrence trends of selected tobacco carcinogens in recent years
3.1. Polycyclic aromatic hydrocarbons (PAH)
The formation of PAH results primarily from incomplete combustion of tobacco components during smoking. They are also present at relatively low levels in unburned tobacco. Of the ~510 PAH reported in cigarette mainstream smoke (Rodgman and Perfetti, 2013), 16 have been classified as carcinogenic to humans by the IARC as summarized in the Table. One study analyzed 14 PAH from selected cigarettes purchased from the U.S. market between 2002 and 2011(Hearn et al., 2018). The overall average occurrence of these compounds in the mainstream smoke using the ISO smoking regimen showed no significant change during the multiple year study period. Naphthalene, benz[a]anthracene and chrysene appear to be the 3 most abundant among the 16 carcinogenic PAH in the mainstream smoke of cigarettes (Jeffery et al., 2018).
Among these 16 PAH, B[a]P is the most extensively investigated compound due to its strong carcinogenicity, early identification, and common occurrence as a combustion product. Quantitation of B[a]P in cigarette smoke and unburned tobacco has been consistently reported (Soleimani et al., 2022). Statistical analysis for both single-year and multiple-year sampling of PAH emissions in U.S. cigarette mainstream smoke suggested a strong correlation between B[a]P and other PAH (Hearn et al., 2018; Vu et al., 2015). These results supported the use of a limited set of PAH, or even a single PAH such as B[a]P, to predict overall PAH exposure in cigarette smokers. However, precautions are required when using B[a]P data collected in the presence of other combustion sources in addition to cigarette smoking (Gao et al., 2015).
3.2. Tobacco-specific N-nitrosamines (TSNAs)
N-Nitrosamines are one of the most important groups of carcinogenic components in unburned tobacco and tobacco smoke. NDMA, NSAR, and NPYR were consistently detected in tobacco products at generally low levels; NEMA, NDEA, NDBA, NDELA, NPIP and NMOR were only reported in limited numbers of recent reports at very low concentrations. However, TSNAs including NNN, NNK, N'-nitrosoanabasine (NAB) and N'-nitrosoanatabine (NAT) (the latter two being Group 3 compounds that are “not classifiable as to their carcinogenicity to humans”), occur in relatively high amounts in both unburned tobacco and tobacco smoke. The level of total TSNAs in tobacco filler of U.S. commercial cigarettes was (mean ± SD) 3.88 ± 0.69 μg/g. Levels of total TSNAs in the mainstream smoke of the same cigarettes were 232 ± 80 and 521 ± 122 ng/cigarette under the ISO machine-smoking regimen and the Canadian intense machine-smoking regimen, respectively (Edwards et al., 2017).
Convincing evidence in laboratory animals in combination with an understanding of their carcinogenic mechanisms support the Group 1 classification of NNN and NNK by the IARC (Hecht, 1998; International Agency for Research on Cancer, 2007; Li and Hecht, 2022). Exposure to NNN and NNK in smokers of the Shanghai Cohort study showed a remarkable coherence with the risk of developing esophageal and lung cancer, respectively (Stepanov et al., 2014b; Yuan et al., 2011). Smokeless tobacco has been established as a cause of oral cancer; NNN is consistently found to be the most abundant oral carcinogen in these products (Hecht, 2020). It is thus critical to regulate the levels of NNN and NNK in tobacco products to reduce potential cancer risks in tobacco users who are unwilling to quit.
Unfortunately, there is no clear trend suggesting a continuing significant decrease of NNN and NNK in U.S. cigarette products (Gunduz et al., 2016; Stepanov et al., 2012). For example, in the tobacco filler of 50 U.S. commercial brands of cigarettes purchased in 2011, the mean ± SD levels of NNN and NNK were 1.90 ± 0.36 and 0.52 ± 0.16 μg/g, respectively (Edwards et al., 2017). These numbers are fairly close to the values reported in 1979, with NNN and NNK amounting to 1.4 and 0.7 μg/g, respectively, in an unidentified filtered U.S. commercial cigarette (Hoffmann et al., 1979). However, the overall mean values of NNN and NNK (n = 1809) in tobacco fillers of 1809 brands of cigarettes sold in Canada from 2005 to 2012 were dramatically lower, amounting to mean ± SD concentrations of 92.8 ± 230.4 and 29.5 ± 79.6 ng/cig, respectively (Czoli and Hammond, 2018).
The same trend with no obvious decrease of NNN and NNK levels in smokeless tobacco products also holds true in the U.S. market. Considerable variability of NNN and NNK levels (n = 5008) in 9 smokeless tobacco products from 2008 to 2017 was observed, ranging from approximately 2 – 10 and 0.5 – 3.5 μg/g dry weight, respectively. No decreasing trend was noted in these products over the tested time period (Oldham et al., 2020). The median values of NNN and NNK yields in 31 top-selling brands of smokeless tobacco purchased in 2011 from the U.S. market were 0.48 – 11.11 and 0.15 – 3.44 μg/g wet weight (Hatsukami et al., 2015). The numbers remained in the same range in 8 U.S. snus products purchased in 2013 – 2014, with the mean ± SD levels of NNN and NNK being 1.05 ± 1.68 and 0.32 ± 0.07 μg/g wet weight (Lawler et al., 2020). The concentrations of NNN and NNK in 6 common U.S. brands of snuff purchased in 2016 were 1.9 – 3.9 and 0.65 – 1.9 μg/g wet weight, respectively (Nasrin et al., 2020). More strikingly higher concentrations of NNN and NNK were found in some smokeless tobacco products such as Zarda and Gul in South-East Asia, in which NNN and NNK reached 59 and 34 μg/g wet powder (Nasrin et al., 2020; Stanfill et al., 2018).
It is possible to reduce the levels of NNN and NNK in smokeless tobacco products, as has been demonstrated in some northern European snus products that contained significantly lower levels of these carcinogenic N-nitrosamines. For example, the sum of NNN and NNK in 56 northern Europe snus products purchased in 2013 – 2014 was ~2.1 times lower than that in the U.S. snus (Lawler et al., 2020). The average content of NNN plus NNK in snus manufactured by Swedish Match in 2021 was 0.51 μg/g (Swedish Match AB, 2021). The U.S. FDA has proposed a product standard requirement of a maximum mean level of NNN at 1.0 μg/g (on a dry weight basis) in any batch of finished smokeless tobacco products in 2017 (U.S. Food and Drug Administration, 2017). The application of the proposed standard should hopefully decrease the levels of NNN in smokeless tobacco in the U.S. market, resulting in a decrease in the incidence of oral cancers and other associated health damage in smokeless tobacco users.
3.3. Aldehydes
Aldehydes identified in tobacco smoke that are classified as carcinogenic by IARC as of 2022 include formaldehyde (Group 1), acrolein (Group 2A), acetaldehyde and crotonaldehyde (Group 2B). They generally occur in much higher levels than PAH and TSNAs, ranging from μg/cigarette to mg/cigarette. The formation of aldehydes primarily results from tobacco combustion and pyrolysis, since these compounds were barely detected in unburned tobacco. The yields of aldehydes in cigarette smoke are dependent on the sugar and humectant content in tobacco (Pennings et al., 2020; Talhout et al., 2006). Cigarette filter ventilation as well as smoking protocol also affect the yields (Pauwels et al., 2018). Among the aldehydes, smoke levels of acetaldehyde and acrolein were strongly correlated, and can be used as representatives for other volatile aldehydes (Pauwels et al., 2018). As shown in the Table, no clear declining trend has been observed in the occurrence of these carcinogenic aldehydes in tobacco smoke in recent reports.
It is important to note that exposure to aldehydes in smokers may also result from other pathways (Esterbauer et al., 1991). For example, acrolein exposure may also result from dietary sources such as fried food, and exogenous sources such as secondhand cigarette smoke and incomplete combustion of fuels, and endogenous sources such as lipid peroxidation, polyamine and hydroxyl-amino acid (such as threonine) metabolism (Stevens and Maier, 2008). Oxidative ring opening of certain drugs such as cyclophosphamide and ifosfamide also leads to the release of acrolein in patients (Brock et al., 1979). Lipid peroxidation is an additional source of formaldehyde and crotonaldehyde (Esterbauer et al., 1991). Endogenous sources of formaldehyde include amino acid metabolism, methanol metabolism and demethylation of nucleic acids and histone (Zhang, 2018).
3.4. Others
Tobacco carcinogens occurring in relatively high abundance (μg/g or μg/cigarette) also include the volatile hydrocarbons 1,3-butadiene, isoprene, styrene, benzene, ethylbenzene and cumene; the ether furan; nitro compounds such as nitromethane and 2-nitropropane; phenolic compounds such as catechol, also a powerful co-carcinogen (Hecht et al., 1981; Melikian et al., 1986; Van Duuren et al., 1973); and the miscellaneous compounds acetamide, acrylamide, vinyl acetate and acrylonitrile. Some carcinogens with relatively low concentrations are of great interest with respect to regulation due to their strong carcinogenicity. The Group 1 carcinogens detected in tobacco and tobacco smoke also include ortho-toluidine, 4-aminobiphenyl, 2-naphthylamine from the class of aromatic amines; ethylene oxide from the class of ethers; vinyl chloride, 2,3,4,7,8-pentachlorodibenzofuran from the class of halogenated compounds; inorganic compounds containing arsenic, beryllium, cadmium, chromium(VI), nickel and polonium-210. Similar to the occurrence trends of the carcinogens discussed above, no clear decreases were noticed in recent reports on their concentrations in tobacco products as suggested by the Table.
4. Concluding remarks
Reductions in smoking have contributed significantly to an overall decrease of 32% in cancer deaths in the U.S. compared to nearly 30 years ago (Siegel et al., 2022). However, tobacco use remains the leading preventable cause of death in the United States and worldwide. While current smoking in U.S. adults decreased to 14% in 2019 (Centers for Disease Control and Prevention, 2019), the prevalence of tobacco use globally remains high, with 36.7% of men and 7.8% of women having used tobacco in 2020 (World Health Organization, 2021). The highest tobacco use rate was reported among men from the Western Pacific Region which largely reflects the high prevalence of current tobacco use among males in China (47.8%) (World Health Organization, 2021).
Tobacco and tobacco smoke contain a very complex mixture of over 9500 compounds (Rodgman and Perfetti, 2013). Carcinogens identified in tobacco and tobacco smoke have drawn much attention by the U.S. FDA as well as other authorities including the World Health Organization. There are 93 harmful and potentially harmful constituents included in the U.S. FDA list established in 2012, 79 of which are considered to be carcinogens (U.S. Food and Drug Administration, 2012). The total number of IARC-classified carcinogens in tobacco and tobacco smoke has increased to 83 as of 2022, which is mainly due to the increased number of compounds reviewed by the IARC and possibly also to better methods for the analysis of tobacco components. It is noted that some chemicals included in the FDA carcinogen list such as 1-aminonaphthalene, dibenzo[a,e]pyrene (classified to be “reasonably anticipated to be a human carcinogen” by the U.S. National Toxicology Program)(NTP (National Toxicology Program), 2021) and mercury and inorganic mercury are “not classifiable as to their carcinogenicity to humans” by the IARC. Cresols and uranium-235 and −238 have not been evaluated by the IARC regarding their carcinogenicity to humans. The Group 1 carcinogen aflatoxin B1 is generally considered as a contaminant of tobacco products, and is not discussed here as a tobacco carcinogen.
As reflected by the levels of tobacco carcinogens summarized in the Table, no clear decreasing trends were observed in any of them in recent years. A very high variation was observed regarding total nicotine content, product pH which affects the percentage of un-ionized nicotine, and TSNAs in oral tobacco products (Stanfill et al., 2011). Similar results were also noted in TSNAs and PAH levels of cigarette products (Czoli and Hammond, 2018; Hearn et al., 2018). Surveillance of the levels of carcinogens discussed above along with nicotine and other factors such as pH should be conducted on a regular basis to provide quality-controlled products (Lawler et al., 2017).
In summary, we updated the list of IARC-classified carcinogens identified in tobacco and tobacco smoke and provided a summary of recent data on their levels in tobacco products since 2012. There were no clear decreasing trends in levels of these tobacco carcinogens such as PAH and TSNAs in recent years. Surveillance of the quality of tobacco products as well as regulatory actions are needed to ensure relatively low levels of carcinogens so that potential reduced risks of cancer and other diseases may be achieved.
Highlights:
Carcinogens in tobacco and tobacco smoke are proposed to be closely monitored by the U.S. Food and Drug Administration.
Many new data have been published since 2012, increasing our understanding of carcinogen levels in tobacco products.
A total of 83 carcinogens have been identified with their occurrence levels reported since 2012.
No clear decreasing trends were observed for any of these carcinogens in recent years.
ACKNOWLEDGMENTS
The authors thank Robert (Bob) Carlson for his editorial assistance.
Funding
This work was supported by grant CA-81301 from the National Cancer Institute.
ABBREVIATIONS
- B[a]P
benzo[a]pyrene
- COPD
chronic obstructive pulmonary disease
- FDA
Food and Drug Administration
- IARC
International Agency for Research on Cancer
- NAB
N'-nitrosoanabasine
- NAT
N'-nitrosoanatabine
- NDBA
N-nitrosodi-n-butylamine
- NDEA
N-nitrosodiethylamine
- NDELA
N-nitrosodiethanolamine
- NDIPA
N-nitrodiisopropylamine
- NDMA
N-nitrosodimethylamine
- NDPA
N-nitrosodi-n-propylamine
- NMEA
N-nitrosomethylethylamine
- NMOR
N-nitrosomorpholine
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- NNN
N2-nitrosonornicotine
- NPIP
N-nitrosopiperidine
- NPYR
N-nitrosopyrrolidine
- NSAR
N-nitrososarcosine
- PAH
polycyclic aromatic hydrocarbons
- SD
standard deviation
- TSNAs
tobacco-specific N-nitrosamines
Footnotes
CRediT authorship contribution statement
Yupeng Li: Conceptualization, Writing – original draft, Writing – review & editing. Stephen S. Hecht: Conceptualization, Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare no competing financial interest.
References:
- American Cancer Society, 2019. Cancer Facts & Figures 2019. American Cancer Society,, Atlanta, GA. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html [Google Scholar]
- Baker RR, Bishop LJ, 2004. The pyrolysis of tobacco ingredients. J. Anal. Appl. Pyrol 71, 223–311. [Google Scholar]
- Baker RR, Pereira da Silva JR, Smith G, 2004a. The effect of tobacco ingredients on smoke chemistry. Part I: Flavourings and additives. Food. Chem. Toxicol 42 Suppl, S3–37. [DOI] [PubMed] [Google Scholar]
- Baker RR, Pereira da Silva JR, Smith G, 2004b. The effect of tobacco ingredients on smoke chemistry. Part II: casing ingredients. Food. Chem. Toxicol 42 Suppl, S39–52. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Bernert JT, Foulds J, Hecht SS, Jacob P, Jarvis MJ, Joseph A, Oncken C, Piper ME, 2020. Biochemical verification of tobacco use and abstinence: 2019 update. Nicotine Tob. Res 22, 1086–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgerding MF, Bodnar JA, Curtin GM, Swauger JE, 2012. The chemical composition of smokeless tobacco: A survey of products sold in the United States in 2006 and 2007. Regul. Toxicol. Pharmacol 64, 367–387. [DOI] [PubMed] [Google Scholar]
- Brock N, Stekar J, Pohl J, Niemeyer U, Scheffler G, 1979. Acrolein, the causative factor of urotoxic side-effects of cyclophosphamide, ifosfamide, trofosfamide and sufosfamide. Arzneimittelforschung 29, 659–661. [PubMed] [Google Scholar]
- Cai B, Li Z, Wang R, Geng Z, Shi Y, Xie S, Wang Z, Yang Z, Ren X, 2019. Emission level of seven mainstream smoke toxicants from cigarette with variable tobacco leaf constituents. Regul. Toxicol. Pharmacol 103, 181–188. [DOI] [PubMed] [Google Scholar]
- Cecil TL, Brewer TM, Young M, Holman MR, 2017. Acrolein yields in mainstream smoke from commercial cigarette and little cigar tobacco products. Nicotine Tob. Res 19, 865–870. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2006. History of the Surgeon General's Reports on Smoking and Health, [Google Scholar]
- Centers for Disease Control and Prevention, 2019. Current Cigarette Smoking Among Adults in the United States, [Google Scholar]
- Chapman GM, Bravo R, Stanelle RD, Watson CH, Valentín-Blasini L, 2018. Sensitive and selective gas chromatography-tandem mass spectrometry method for the detection of nitrobenzene in tobacco smoke. J. Chromatogr. A 1565, 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoli CD, Hammond D, 2018. Trends over time in tobacco-specific nitrosamines (TSNAs) in whole tobacco and smoke emissions from cigarettes sold in Canada. Nicotine Tob. Res 20, 649–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YS, Richter P, Hearn B, Zhang L, Bravo R, Yan X, Perez JJ, Chan M, Hughes J, Chen P, Chen W, Wong J, Holmberg S, Smith S, Larango M, Valentin-Blasini L, Watson CH, 2017. Chemical characterization of mainstream smoke from SPECTRUM variable nicotine research cigarettes. Tob. Regul. Sci 3, 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YS, Yan X, Wong J, Chan M, Watson CH, 2016. In situ derivatization and quantification of seven carbonyls in cigarette mainstream smoke. Chem. Res. Toxicol 29, 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll R, Hill AB, 1954. The mortality of doctors in relation to their smoking habits; a preliminary report. Br. Med. J 1, 1451–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube MF, Green C, 1982. Methods of collection of smoke for analytical purposes. Recent Adv. Tob. Sci 8, 42–102. [Google Scholar]
- Edwards SH, Hassink MD, Taylor KM, Watson CH, Kuklenyik P, Kimbrell B, Wang L, Chen P, Valentín-Blasini L, 2021. Tobacco-specific nitrosamines in the tobacco and mainstream smoke of commercial little cigars. Chem. Res. Toxicol 34, 1034–1045. [DOI] [PubMed] [Google Scholar]
- Edwards SH, Rossiter LM, Taylor KM, Holman MR, Zhang L, Ding YS, Watson CH, 2017. Tobacco-specific nitrosamines in the tobacco and mainstream smoke of U.S. commercial cigarettes. Chem. Res. Toxicol 30, 540–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge A, Betson TR, Gama MV, McAdam K, 2015. Variation in tobacco and mainstream smoke toxicant yields from selected commercial cigarette products. Regul. Toxicol. Pharmacol 71, 409–427. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H, 1991. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med 11, 81–128. [DOI] [PubMed] [Google Scholar]
- Fresquez MR, Gonzalez-Jimenez N, Gray N, Valentin-Blasini L, Watson CH, Pappas RS, 2017. Electrothermal vaporization-QQQ-ICP-MS for determination of chromium in mainstream cigarette smoke particulate. J. Anal. Toxicol 41, 307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresquez MR, Pappas RS, Watson CH, 2013. Establishment of toxic metal reference range in tobacco from U.S. cigarettes. J. Anal. Toxicol 37, 298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Du X, Wang X, Tang J, Ding X, Zhang Y, Bi X, Zhang G, 2015. Parent, alkylated, and sulfur/oxygen-containing polycyclic aromatic hydrocarbons in mainstream smoke from 13 brands of Chinese cigarettes. Environ. Sci. Technol 49, 9012–9019. [DOI] [PubMed] [Google Scholar]
- Gunduz I, Kondylis A, Jaccard G, Renaud JM, Hofer R, Ruffieux L, Gadani F, 2016. Tobacco-specific N-nitrosamines NNN and NNK levels in cigarette brands between 2000 and 2014. Regul. Toxicol. Pharmacol 76, 113–120. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Stepanov I, Severson H, Jensen JA, Lindgren BR, Horn K, Khariwala SS, Martin J, Carmella SG, Murphy SE, Hecht SS, 2015. Evidence supporting product standards for carcinogens in smokeless tobacco products. Cancer Prev. Res. (Phila) 8, 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearn BA, Ding YS, Watson CH, Johnson TL, Zewdie G, Jeong-Im JH, Walters MJ, Holman MR, Rochester CG, 2018. Multi-year study of PAHs in mainstream cigarette smoke. Tob. Regul. Sci 4, 96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearn BA, Renner CC, Ding YS, Vaughan-Watson C, Stanfill SB, Zhang L, Polzin GM, Ashley DL, Watson CH, 2013. Chemical analysis of Alaskan Iq'mik smokeless tobacco. Nicotine Tob. Res 15, 1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS, 1998. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem. Res. Toxicol 11, 559–603. [DOI] [PubMed] [Google Scholar]
- Hecht SS, 2003. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat. Rev. Cancer 3, 733–744. [DOI] [PubMed] [Google Scholar]
- Hecht SS, 2012. Research opportunities related to establishing standards for tobacco products under the Family Smoking Prevention and Tobacco Control Act. Nicotine Tob. Res 14, 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS, 2020. Metabolism and DNA adduct formation of carcinogenic tobacco-specific nitrosamines found in smokeless tobacco products, in: Pickworth WB (Ed.), Smokeless Tobacco Products, doi: 10.1016/b978-0-12-818158-4.00007-8. Elsevier, Amsterdam, pp. 151–166. [DOI] [Google Scholar]
- Hecht SS, Carmella S, Mori H, Hoffmann D, 1981. A study of tobacco carcinogenesis. XX. Role of catechol as a major cocarcinogen in the weakly acidic fraction of smoke condensate. J Natl Cancer Inst 66, 163–169. [PubMed] [Google Scholar]
- Hoffmann D, Adams JD, Brunnemann KD, Hecht SS, 1979. Assessment of tobacco-specific N-nitrosamines in tobacco products. Cancer Res. 39, 2505–2509. [PubMed] [Google Scholar]
- Hoffmann D, Hoffmann I, 2001. The changing cigarette: chemical studies and bioassays. In: Risks Associated with Smoking Cigarettes with Low Machine-Measured Yields of Tar and Nicotine (Smoking and Tobacco Control Monograph No. 13; NIH Publ. No. 02-5074). doi, 159–191. [Google Scholar]
- Hoffmann D, Hoffmann I, El-Bayoumy K, 2001. The less harmful cigarette: A controversial issue. a tribute to Ernst L. Wynder. Chem. Res. Toxicol 14, 767–790. [DOI] [PubMed] [Google Scholar]
- Hsieh JR, Mekoli ML, Edwards RL Jr., 2021. Levels of chemical toxicants in waterpipe tobacco and waterpipe charcoal solid waste. J Environ. Prot. (Irvine, Calif) 12, 913–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer, Monographs Available: IARC Monographs on the Identification of Carcinogenic Hazards to Humans, [Google Scholar]
- International Agency for Research on Cancer, 1986. Tobacco Smoking. IARC, Lyon, FR. [Google Scholar]
- International Agency for Research on Cancer, 2004. Tobacco Smoke and Involuntary Smoking. IARC, Lyon, FR. [Google Scholar]
- International Agency for Research on Cancer, 2007. Smokeless Tobacco and Some Tobacco-Specific N-Nitrosamines. IARC, Lyon, FR. [Google Scholar]
- International Agency for Research on Cancer, 2012a. 2-Methylimidazole, Some Chemicals Present in Industrial and Consumer Products, Food and Drinking-Water: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 101. IARC, Lyon, FR, p. 437. [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer, 2012b. 4-Methylimidazole, Some Chemicals Present in Industrial and Consumer Products, Food and Drinking-Water: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 101. IARC, Lyon, FR, p. 452. [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer, 2012c. Methyleugenol, Some Chemicals Present in Industrial and Consumer Products, Food and Drinking-Water: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 101. IARC, Lyon, FR, p. 415. [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer, 2012d. Some Chemicals Present in Industrial and Consumer Products, Food and Drinking-Water. IARC, Lyon, FR. [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer, 2019a. Pyridine, Some Chemicals that Cause Tumours of the Urinary Tract in Rodents: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 119. IARC, Lyon, FR, p. 177. [PubMed] [Google Scholar]
- International Agency for Research on Cancer, 2019b. Quinoline, Styrene, Styrene-7,8-oxide, and Quinoline: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 121. IARC, Lyon, FR, p. 304. [Google Scholar]
- International Agency for Research on Cancer, 2021a. Acrolein, Acrolein, Crotonaldehyde, and Arecoline: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 128. IARC, Lyon, FR, p. 55; Table 51.53. [Google Scholar]
- International Agency for Research on Cancer, 2021b. Aniline and Aniline Hydrochloride, Some Aromatic Amines and Related Compounds: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 127. IARC, Lyon, FR, p. 116. [Google Scholar]
- Islami F, Goding Sauer A, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, McCullough ML, Patel AV, Ma J, Soerjomataram I, Flanders WD, Brawley OW, Gapstur SM, Jemal A, 2018. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin 68, 31–54. [DOI] [PubMed] [Google Scholar]
- Jain V, Alcheva A, Huang D, Caruso R, Jain A, Lay M, O'Connor R, Stepanov I, 2019. Comprehensive chemical characterization of Natural American Spirit cigarettes. Tob. Regul. Sci 5, 381–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery J, Carradus M, Songin K, Pettit M, Pettit K, Wright C, 2018. Optimized method for determination of 16 FDA polycyclic aromatic hydrocarbons (PAHs) in mainstream cigarette smoke by gas chromatography-mass spectrometry. Chem. Cent. J 12, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone RAW, Plimmer JR, 1959. The chemical constituents of tobacco and tobacco smoke. Chem. Rev 59, 885–936. [Google Scholar]
- Junco JG, Chapman GM, Bravo Cardenas R, Watson CH, Valentín-Blasini L, 2021. Quantification of nitromethane in mainstream smoke using gas chromatography and tandem mass spectrometry. Toxicol Rep. 8, 405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler TS, Stanfill SB, deCastro BR, Lisko JG, Duncan BW, Richter P, Watson CH, 2017. Surveillance of nicotine and ph in cigarette and cigar filler. Tob. Regul. Sci 3, 101–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler TS, Stanfill SB, Tran HT, Lee GE, Chen PX, Kimbrell JB, Lisko JG, Fernandez C, Caudill SP, deCastro BR, Watson CH, 2020. Chemical analysis of snus products from the United States and northern Europe. PLoS One 15, e0227837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hecht SS, 2022. Metabolism and DNA adduct formation of tobacco-specific N-nitrosamines. Int. J. Mol. Sci 23, 5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv F, Guo J, Yu F, Zhang T, Zhang S, Cui H, Liu X, Chen L, Liu L, Liu S, Xie F, 2016. Determination of nine volatile N-nitrosamines in tobacco and smokeless tobacco products by dispersive solid-phase extraction with gas chromatography and tandem mass spectrometry. J. Sep. Sci 39, 2123–2128. [DOI] [PubMed] [Google Scholar]
- Marcilla A, Martínez I, Berenguer D, Gómez-Siurana A, Beltrán MI, 2012. Comparative study of the main characteristics and composition of the mainstream smoke of ten cigarette brands sold in Spain. Food. Chem. Toxicol 50, 1317–1333. [DOI] [PubMed] [Google Scholar]
- Melikian AA, Leszczynska JM, Hecht SS, Hoffmann D, 1986. Effects of the co-carcinogen catechol on benzo[a]pyrene metabolism and DNA adduct formation in mouse skin. Carcinogenesis 7, 9–15. [DOI] [PubMed] [Google Scholar]
- Moir D, Rickert WS, Levasseur G, Larose Y, Maertens R, White P, Desjardins S, 2008. A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem. Res. Toxicol 21, 494–502. [DOI] [PubMed] [Google Scholar]
- Nasrin S, Chen G, Watson CJW, Lazarus P, 2020. Comparison of tobacco-specific nitrosamine levels in smokeless tobacco products: High levels in products from Bangladesh. PLoS One 15, e0233111–e0233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Toxicology Program, U.S. Department of Health and Human Services, 2016. NTP Report on Carcinogens (14th Edition): N-nitrosamines: 15 listings. Rep. Carcinog doi, Page 23. [Google Scholar]
- NTP (National Toxicology Program), 2021. Report on Carcinogens, Fifteenth Edition. U.S. Department of Health and Human Services, Public Health Service, Research Triangle Park, NC. http://ntp.niehs.nih.gov/go/roc14 [Google Scholar]
- Oladipupo OA, Dutta D, Chong NS, 2019. Analysis of chemical constituents in mainstream bidi smoke. BMC Chem. 13, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham MJ, DeSoi DJ, Rimmer LT, Wagner KA, Morton MJ, 2014. Insights from analysis for harmful and potentially harmful constituents (HPHCs) in tobacco products. Regul. Toxicol. Pharmacol 70, 138–148. [DOI] [PubMed] [Google Scholar]
- Oldham MJ, Lion KE 3rd, Phillips DJ, Morton MJ, Lusso MF, Harris EA, Jordan JL, Franke JE, Strickland JA, 2020. Variability of TSNA in U.S. tobacco and moist smokeless tobacco products. Toxicol Rep. 7, 752–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas RS, Fresquez MR, Martone N, Watson CH, 2014. Toxic metal concentrations in mainstream smoke from cigarettes available in the USA. J. Anal. Toxicol 38, 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas RS, Martone N, Gonzalez-Jimenez N, Fresquez MR, Watson CH, 2015. Determination of toxic metals in little cigar tobacco with 'triple quad' ICP-MS. J. Anal. Toxicol 39, 347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels C, Klerx WNM, Pennings JLA, Boots AW, van Schooten FJ, Opperhuizen A, Talhout R, 2018. Cigarette filter ventilation and smoking protocol influence aldehyde smoke yields. Chem. Res. Toxicol 31, 462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazo DY, Moliere F, Sampson MM, Reese CM, Agnew-Heard KA, Walters MJ, Holman MR, Blount BC, Watson CH, Chambers DM, 2016. Mainstream smoke levels of volatile organic compounds in 50 U.S. domestic cigarette brands smoked with the ISO and Canadian intense protocols. Nicotine Tob. Res 18, 1886–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennings JLA, Cremers J, Becker MJA, Klerx WNM, Talhout R, 2020. Aldehyde and volatile organic compound yields in commercial cigarette mainstream smoke are mutually related and depend on the sugar and humectant content in tobacco. Nicotine Tob. Res 22, 1748–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter P, Steven PR, Bravo R, Lisko JG, Damian M, Gonzalez-Jimenez N, Gray N, Keong LM, Kimbrell JB, Kuklenyik P, Lawler TS, Lee GE, Mendez M, Perez J, Smith S, Tran H, Tyx R, Watson CH, 2016. Characterization of SPECTRUM variable nicotine research cigarettes. Tob. Regul. Sci 2, 94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DL, 1988. Natural tobacco flavor. Recent Adv. Tob. Sci 14, 49–81. [Google Scholar]
- Rodgman A, Perfetti TA, 2008. The Chemical Components of Tobacco and Tobacco Smoke, 1st Edition. CRC Press, Boca Raton, FL. [Google Scholar]
- Rodgman A, Perfetti TA, 2013. The Chemical Components of Tobacco and Tobacco Smoke, 2nd Edition. CRC Press, Boca Raton, FL. [Google Scholar]
- Saha S, Mistri R, Ray BC, 2010. Determination of pyridine, 2-picoline, 4-picoline and quinoline from mainstream cigarette smoke by solid-phase extraction liquid chromatography/electrospray ionization tandem mass spectrometry. J. Chromatogr. A 1217, 307–311. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Fuchs HE, Jemal A, 2022. Cancer statistics, 2022. CA Cancer J. Clin. 72, 7–33. [DOI] [PubMed] [Google Scholar]
- Slade J, 1997. Historical Notes on Tobacco, in: B. CT, F. KC (Eds.), The Tobacco Epidemic, vol. 28. Karger, Basel, Switzerland. [Google Scholar]
- Smith CJ, Livingston SD, Doolittle DJ, 1997. An international literature survey of "IARC Group I carcinogens" reported in mainstream cigarette smoke. Food. Chem. Toxicol 35, 1107–1130. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Perfetti TA, Mullens MA, Rodgman A, Doolittle DJ, 2000a. "IARC group 2B Carcinogens" reported in cigarette mainstream smoke. Food. Chem. Toxicol 38, 825–848. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Perfetti TA, Rumple MA, Rodgman A, Doolittle DJ, 2000b. "IARC group 2A Carcinogens" reported in cigarette mainstream smoke. Food. Chem. Toxicol 38, 371–383. [DOI] [PubMed] [Google Scholar]
- Soleimani F, Dobaradaran S, De-la-Torre GE, Schmidt TC, Saeedi R, 2022. Content of toxic components of cigarette, cigarette smoke vs cigarette butts: A comprehensive systematic review. Sci. Total. Environ 813, 152667. [DOI] [PubMed] [Google Scholar]
- Stanfill SB, Connolly GN, Zhang L, Jia LT, Henningfield JE, Richter P, Lawler TS, Ayo-Yusuf OA, Ashley DL, Watson CH, 2011. Global surveillance of oral tobacco products: total nicotine, unionised nicotine and tobacco-specific N-nitrosamines. Tob. Control 20, e2. [DOI] [PubMed] [Google Scholar]
- Stanfill SB, Croucher RE, Gupta PC, Lisko JG, Lawler TS, Kuklenyik P, Dahiya M, Duncan B, Kimbrell JB, Peuchen EH, Watson CH, 2018. Chemical characterization of smokeless tobacco products from South Asia: Nicotine, unprotonated nicotine, tobacco-specific N'-nitrosamines, and flavor compounds. Food. Chem. Toxicol 118, 626–634. [DOI] [PubMed] [Google Scholar]
- Stanfill SB, Oliveira da Silva AL, Lisko JG, Lawler TS, Kuklenyik P, Tyx RE, Peuchen EH, Richter P, Watson CH, 2015. Comprehensive chemical characterization of Rapé tobacco products: Nicotine, un-ionized nicotine, tobacco-specific N'-nitrosamines, polycyclic aromatic hydrocarbons, and flavor constituents. Food. Chem. Toxicol 82, 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman RL, 1968. The chemical composition of tobacco and tobacco smoke. Chem. Rev 68, 153–207. [DOI] [PubMed] [Google Scholar]
- Stepanov I, Abrams J, Jain V, Walter K, Kittner DL, 2017a. Variations of toxic and carcinogenic constituents in nasvai: call for systematic research and regulation. Tob. Control 26, 355–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I, Biener L, Yershova K, Nyman AL, Bliss R, Parascandola M, Hatsukami DK, 2014a. Monitoring tobacco-specific N-nitrosamines and nicotine in novel smokeless tobacco products: Findings from round II of the new product watch. Nicotine Tob. Res 16, 1070–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I, Gupta PC, Dhumal G, Yershova K, Toscano W, Hatsukami D, Parascandola M, 2015. High levels of tobacco-specific N-nitrosamines and nicotine in Chaini Khaini, a product marketed as snus. Tob. Control 24, e271–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I, Gupta PC, Parascandola M, Yershova K, Jain V, Dhumal G, Hatsukami DK, 2017b. Constituent variations in smokeless tobacco purchased in Mumbai, India. Tob. Regul. Sci 3, 305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I, Knezevich A, Zhang L, Watson CH, Hatsukami DK, Hecht SS, 2012. Carcinogenic tobacco-specific N-nitrosamines in U.S. cigarettes: Three decades of remarkable neglect by the tobacco industry. Tob. Control 21, 44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I, Sebero E, Wang R, Gao YT, Hecht SS, Yuan JM, 2014b. Tobacco-specific N-nitrosamine exposures and cancer risk in the Shanghai Cohort Study: Remarkable coherence with rat tumor sites. Int. J. Cancer 134, 2278–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JF, Maier CS, 2008. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol. Nutr. Food Res 52, 7–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedish Match AB, 2021. GOTHIATEK limits for undesired components, [Google Scholar]
- Talhout R, Opperhuizen A, van Amsterdam JG, 2006. Sugars as tobacco ingredient: Effects on mainstream smoke composition. Food. Chem. Toxicol 44, 1789–1798. [DOI] [PubMed] [Google Scholar]
- Talhout R, Schulz T, Florek E, van Benthem J, Wester P, Opperhuizen A, 2011. Hazardous compounds in tobacco smoke. Int J Environ Res Public Health 8, 613–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration, 2012. Harmful and potentially harmful constituents in tobacco products and tobacco smoke. Fed. Reg doi, 20034–20037. [Google Scholar]
- U.S. Food and Drug Administration, 2017. Tobacco Product Standard for N-Nitrosonornicotine Level in Finished Smokeless Tobacco Products. Fed. Reg 82, 8004–8053. [Google Scholar]
- Van Duuren BL, Katz C, Goldschmidt BM, 1973. Cocarcinogenic agents in tobacco carcinogenesis. J. Natl. Cancer Inst 51, 703–705. [PubMed] [Google Scholar]
- Vu AT, Hassink MD, Taylor KM, McGuigan M, Blasiole A, Valentin-Blasini L, Williams K, Watson CH, 2021. Volatile organic compounds in mainstream smoke of sixty domestic little cigar products. Chem. Res. Toxicol 34, 704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu AT, Taylor KM, Holman MR, Ding YS, Hearn B, Watson CH, 2015. Polycyclic aromatic hydrocarbons in the mainstream smoke of popular U.S. cigarettes. Chem. Res. Toxicol 28, 1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Guo J, Shang J, Ding L, Zhao G, Xie F, Jia Y, Qin Y, Yu Y, Chen L, Zhang S, 2015. Determination of nitroalkanes in mainstream cigarette smoke by heart-cutting multidimensional gas chromatography system coupled with mass spectrometry detection. J. Chromatogr. A 1424, 118–126. [DOI] [PubMed] [Google Scholar]
- Wang X, Qin Y, Nie C, Guo J, Pan L, Xie F, Wang S, Wang B, Zhao X, Wang B, Jia G, 2021. Smokeless tobacco analysis: Simultaneous extraction and purification of alkaloids, volatile N-nitrosamines, and polycyclic hydrocarbons for GC-MS/MS. J. Sep. Sci 44, 2642–2654. [DOI] [PubMed] [Google Scholar]
- Werneth M, Pani J, Hofbauer L, Pummer S, Weber MT, Pour G, Kahlig H, Mayer-Helm B, Stepan H, 2021. Stereospecific response of E/Z-isomers of N-nitrososarcosine in LC-ESI-MS/MS. J. Chromatogr. Sci 59, 813–822. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2019. WHO study group on tobacco product regulation: report on the scientific basis of tobacco product regulation: seventh report of a WHO study group. WHO, Geneva. https://apps.who.int/iris/handle/10665/329445 [Google Scholar]
- World Health Organization, 2021. WHO global report on trends in prevalence of tobacco use 2000-2025, fourth edition. WHO, Geneva. https://www.who.int/publications/i/item/9789240039322 [Google Scholar]
- Wu J, Rickert WS, Masters A, 2012a. An improved high performance liquid chromatography-fluorescence detection method for the analysis of major phenolic compounds in cigarette smoke and smokeless tobacco products. J. Chromatogr. A 1264, 40–47. [DOI] [PubMed] [Google Scholar]
- Wu JC, Rickert WS, Masters A, Joza P, 2012b. Determination of N-nitrososarcosine in tobacco and smokeless tobacco products using isotope dilution liquid chromatography tandem mass spectrometry. Anal. Methods 4, 3448–3452. [Google Scholar]
- Wynder EL, Graham EA, 1950. Tobacco smoking as a possible etiologic factor in bronchiogenic carcinoma; a study of 684 proved cases. J. Am. Med. Assoc 143, 329–336. [DOI] [PubMed] [Google Scholar]
- Yershova K, Yuan JM, Wang R, Valentin L, Watson C, Gao YT, Hecht SS, Stepanov I, 2016. Tobacco-specific N-nitrosamines and polycyclic aromatic hydrocarbons in cigarettes smoked by the participants of the Shanghai Cohort Study. Int. J. Cancer 139, 1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JM, Knezevich AD, Wang R, Gao YT, Hecht SS, Stepanov I, 2011. Urinary levels of the tobacco-specific carcinogen N'-nitrosonornicotine and its glucuronide are strongly associated with esophageal cancer risk in smokers. Carcinogenesis 32, 1366–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Bai R, Zhou Z, Liu X, Zhou J, 2017. Simultaneous analysis of nine aromatic amines in mainstream cigarette smoke using online solid-phase extraction combined with liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem 409, 2993–3005. [DOI] [PubMed] [Google Scholar]
- Zhang L, 2018. CHAPTER 1. Introduction to Formaldehyde, Formaldehyde: Exposure, Toxicity and Health Effects, doi: 10.1039/9781788010269-00001. The Royal Society of Chemistry, pp. 1–19. [DOI] [Google Scholar]
- Zhang L, Ashley DL, Watson CH, 2011. Quantitative analysis of six heterocyclic aromatic amines in mainstream cigarette smoke condensate using isotope dilution liquid chromatography-electrospray ionization tandem mass spectrometry. Nicotine Tob. Res 13, 120–126. [DOI] [PubMed] [Google Scholar]