Summary
About 30 percent of patients diagnosed with myelodysplastic syndromes (MDS) progress to acute myeloid leukemia (AML). The senescence of bone marrow-derived mesenchymal stem cells (BMSCs) seems to be one of the determining factors in inducing this drift. Research is continuously looking for new methodologies and technologies that can use bioelectric signals to act on senescence and cell differentiation towards the phenotype of interest. The Radio Electric Asymmetric Conveyer (REAC) technology, aimed at reorganizing the endogenous bioelectric activity, has already shown to be able to determine direct cell reprogramming effects and counteract the senescence mechanisms in stem cells. Aim of the present study was to prove if the anti-senescence results previously obtained in different kind of stem cells with the REAC Tissue optimization – regenerative (TO-RGN) treatment, could also be observed in BMSCs, evaluating cell viability, telomerase activity, p19ARF, P21, P53, and hTERT gene expression. The results show that the REAC TO-RGN treatment may be a useful tool to counteract the BMSCs senescence which can be the basis of AML drift. Nevertheless, further clinical studies on humans are needed to confirm this hypothesis.
Keywords: REAC, Myelodysplastic syndromes, Acute myeloid leukemia, Senescence, Stem cells, Cellular mechanism
Introduction
Myelodysplastic syndromes (MDS) are a heterogeneous group of hematopoietic stem cell disorders and hematological malignancies. About 30 percent of patients who are diagnosed with MDS progress to acute myeloid leukemia (AML) [1]. MDS are characterized by dysplastic morphology in the bone marrow (BM) in association with the transformation of hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs), leading to unsuccessful hematopoiesis.
Most of mature blood cell compartments need to be continuously replenished by HPCs and HSCs. Evidently, in MDS, cell senescence has a primary role in BMSCs ‘loss of self-renewal capability, loss of function, and BM suppression [2–4].
The underlying etiopathogenesis of MDS recognizes several causes, including genetic abnormalities in myeloid progenitors and epigenetic factors affecting bone marrow (BM) [5,6]. Concurrently, the transition to AML is also conditioned by epigenetic causes [7,8] that can modify endogenous voltage potentials, endogenous bioelectric fields (EBF), and cell polarity behavior, thus playing a decisive role in MDS and its transformation into AML [9].
In turn, epigenetic and transcriptional cascades are conditioned by endogenous bioelectric phenomena that can determine radical changes in cellular properties and behavior, such as proliferation, differentiation, migration, morphology, and apoptosis [10,11], which can be the basis of tumor drifts [12].
Moreover, some studies investigating endogenous bioelectric phenomena have unveiled important advances in our understanding of leukemia, with potential therapeutic implications [13].
Aging, also interpreted as the summation of epigenetic modifications, is another important risk factor in the development of these diseases, as most patients with MDS are over 50 years old (median age range of 65 to 70 years) [14].
Tissue optimization-regenerative (TO-RGN) treatments are specific treatment protocols with radio electric asymmetric conveyer (REAC) technology. The mechanism of action on which the REAC technology is based to create a non-uniform radio electric field, which can interact with particles even if not electrically charged, to optimize their endogenous bioelectric characteristics. Adequate manipulation of endogenous bioelectric activity can epigenetically reprogram cell fate. The REAC-RGN protocol was designed for this purpose. TO-RGN, which aims to reorganize endogenous bioelectric activity, has been shown to be capable of determining direct cell reprogramming effects [15–17], thus counteracting senescence in stem cells [18,19] by modulating both dependent and independent telomerase mechanisms [20].
The ability of these specific REAC treatments to counteract aging in stem cells is one of the assumptions that motivate this study. Onco-hematological diseases (OHD) represent a group of clonal diseases whose pathogenesis is fueled by the presence of acquired genetic defects that compromise the functionality of hematopoietic medullary precursors, such as hematopoietic stem cells (HSCs) and BMSCs. One of the factors that mainly condition the regenerative capability of BMSCs is linked to the effects of senescence on self-renewal processes and differentiation potential. Senescence is a complex process involving a continuous decline in cell proliferation, changes in cell morphology and differentiation, and abnormalities in signaling pathways [4]. In addition, replicative senescence is often associated with altered expression of key molecules involved in the interaction between hematopoietic stem and progenitor cells and the BM microenvironment [2,21]. An aged microenvironment may also be responsible for the occurrence of somatic mutations and the onset of acute myeloid leukemia (AML) [22]. Based on these findings, in the present study, we aimed to assess how a specific REAC treatment that is capable of reorganizing endogenous bioelectric activity can interfere with the senescent phenotype of BMSCs. This could lead to the identification of its preventive effects in the transformation of MDS into AML by inducing the most functional epigenetic response toward the exposome and counteracting the aging process. For this reason, BMSCs obtained from low-risk MDS patients were selected to evaluate the effect of REAC TO-RGN treatment on the expression of the following senescence-associated genes: 1) p19ARF, which regulates self-renewal and cellular senescence programs; 2) p21, the cyclin-dependent kinase inhibitor 1; 3) p53, which regulates cell cycle progression and apoptosis; and 4) telomerase reverse transcriptase (hTERT), which is the catalytic subunit of the enzyme telomerase that is responsible for telomere elongation and integrity. The activity of this enzyme was also assessed to confirm the gene expression analysis results.
Methods
Ethics statement
This study was conducted according to the guidelines of the Declaration of Helsinki of 1975, revised in 2013 and approved by the Institutional Review Board of the Centre for developmental biology and reprogramming (CEDEBIOR) of University of Sassari, Italy. Protocol code N.01/2021, date of approval 18 January 2021. Informed consent was obtained from all subjects involved in the study.
Radio electric asymmetric conveyer (REAC) technology
The REAC technology was designed to carry out neurobiological stimulation treatments through the manipulation of EBF. To achieve this effect, it is necessary that a particular type of electrode, called asymmetric conveyer probe (ACP) connected with the REAC device, is put in contact with the cell culture or specific areas of the body. This manipulation is possible because this technology considers the bioelectric asymmetricity at the base of the ionic flows that generates the EBF. REAC technology can modulate the current flows at both cellular and body levels by acting on EBF and exerting a therapeutic effect. Unlike other technologies that impose their electrical, magnetic, or electromagnetic stimulus on cell cultures or tissues to be treated, and consequently the effect depends on their stimulation strength, REAC technology generates a very weak radio electric field, which at the origin is lower than 2 milliwatts. This radio electric field, once emitted, is dispersed in the environment undergoing a dispersion that decreases with the square of the distance from the emitter. The effect of this dispersion is extremely important to further decrease the strength of the REAC radio electric field so that it does not impose itself on the endogenous bioelectrical characteristics of cell cultures but generates in them a gradient such as to modulate the activation of ionic flows. The treatment used in this study was TO-RGN. The REAC device used in this study was BENE Mod. 110 (ASMED S.r.l., Florence, Italy).
Cell isolation and culturing
Bone marrow-derived mesenchymal stem cells (BMSCs) were isolated from bone marrow of 10 low-risk MDS onco-hematological adult female and male patients (age=50± 15 years) collected during tests carried out for clinical reasons. The samples were immediately processed using Ficoll-Paque PLUS density gradient media (GE Healthcare Life Sciences, United Kingdom) according to the manufacturer’s protocol. The cells obtained were immediately counted and seeded in a tissue culture dish measuring 100 mm at a concentration of 8x105-1x106 cells/plate in a medium composed of Dulbecco’s modified Eagle’s medium (DMEM, Thermo Fisher Scientific, USA), 10% fetal bovine serum (FBS, Thermo Fisher Scientific, USA), 200 mM L-glutamine (Euroclone, Milano, Italy) and 200 U/ml penicillin–0.1 mg/ml streptomycin (Euroclone, Milano, Italy). The REAC-RGN treatment is a pre-programmed treatment. In this case cells were cultured for 24, 48, or 72h in the absence or presence of REAC- RGN treatment.
Trypan Blue exclusion test of cell viability
The dye exclusion test was used to evaluate the number of viable cells cultured in the presence or absence of REAC treatment. The living cells possess intact cell membranes that excluded Trypan Blue dye, while the dead cells appear with a blue cytoplasm. The cell suspension was mixed with 0.4 % dye solution (Thermo Fisher Scientific, USA) in a proportion of 1:1 and then counted using a LUNA-II™ Automated Cell Counter to obtain information about the cell viability and cell size distributions.
Gene expression analysis of real-time PCR
RNA extraction was performed using ChargeSwitch total RNA cell kits (Thermo Fisher Scientific, USA) at 0, 24, 48, and 72h for the cells cultured with electromagnetic fields and the untreated control. Approximately 1 μg of total isolated mRNA was reverse transcribed into cDNA using the Superscript Vilo cDNA synthesis kit (Thermo Fisher Scientific, USA), for a final volume of 20 μl, according to the manufacturer’s protocol. Quantitative polymerase chain reaction was performed in triplicate according to the protocol specified in the Platinum® Quantitative PCR SuperMix-UDG Kit (Thermo Fisher Scientific, USA) using a CFX-96 Thermal Cycler (Bio-Rad) (Applied Biosystems), as previously described [19]. The standard qRT-PCR conditions were 50 °C for 2 min, 95 °C for 2 min, and then cycled at 95 °C for 15 s, 55–59 °C for 30 s and 60 °C for 1 min for 40 cycles. The total volume of each reaction was 25 μl, composed of 2X SuperMix with SYBR Green I, 0.1 μM of each primer, and 3 μl cDNA generated from 1 μg of the total RNA template. The target Ct values were normalized on hGAPDH, considered as a reference gene, while the mRNA levels of the BMSCs cultured in the presence or absence of REAC were expressed as fold of change (2−ΔΔCt) relative to the mRNA levels observed in the BMSCs at time 0 before starting treatment. Each experiment included a distilled water control. The qRT-PCR analysis was performed for the following genes: telomerase reverse transcriptase (TERT), RNA silencing suppressor p19 ARF (p19ARF), cyclin-dependent kinase inhibitor 1 (p21), tumor suppressor protein p53 (p53), octamer-binding transcription factor 4 (Oct-4); Sex determining region Y-box 2 (Sox2) and homeobox protein Nanog (NANOG). All primers were highly specific and designed with Primer3 [15] and were obtained from Invitrogen (Primer: hGAPDH, Forward: GAGTCAACGGAATTTGGTCGT, Reverse: GACAAGCTTCCCGTTCTCAG; Primer: TERT, Forward: GACGTGGAAGATGAGCGTG, Reverse: GACGACGTACACACTCATC; Primer: p19ARF, Forward: GCCTTCGGCTGACTGGCTGG, Reverse: TCGTCCTCCAGAGTCGCCCG; Primer: p21, Forward: CAAAGGCCCGCTCTACATCTT, Reverse: AGGAACCTCTCATTCACCCGA; Primer: p53, Forward: TGGCCTTGAAACCACCTTTT, Reverse: AACTACCAACCCACCAGCCAA; Primer: Oct-4, Forward: GAGGAGTCCCAGGCAATCAA, Reverse: CATCGGCCTGTGTATATCCC; Primer: Sox2, Forward: CCGTTCATGTAGGTCTCGGAGCTG, Reverse: CAACGGCAGCTACAGCTAGATGC; Primer: NANOG, Forward: CATGAGTGTGGATCCAGCT, Reverse: CCTGAATAAGCAGATCCAT).
Telomerase activity detection
To evaluate the telomerase activity, the TRAPeze® Kit RT Telomerase Detection Kit (Millipore, MA, USA) was used. It directly measures the fluorescence emission of telomerase activity through real-time PCR. The BMSCs were seeded in a TC dish measuring 100 mm and treated or not treated with REAC for 24, 48, and 72h. Then, they were pelleted for the performance of the assay in accordance with the manufacturer’s instructions. All samples underwent the procedure in triplicate using a CFX-96 Thermal Cycler (Bio-Rad). Briefly, the cells were lysed with a CHAPS lysis buffer included in the kit; the protein concentrations were determined using Nanodrop (Thermo Fisher Scientific, USA). The master mix was composed of 5X TRAPeze® RT reaction mix, Taq polymerase (5 units/μl), nuclease free water, and samples, for a final volume of 20 μl. The PCR amplification conditions were 30 °C for 30 min, 95 °C for 2 min, and then cycled at 94 °C for 15 s, 59 °C for 60 s, and 45 °C for 10 s for 45 cycles. Each sample included a negative and a positive control. The telomerase activity of each sample was normalized to the Ct of the standard curve generated from the control reaction mix included in the kit.
Senescence-Associated β-Galactosidase Staining
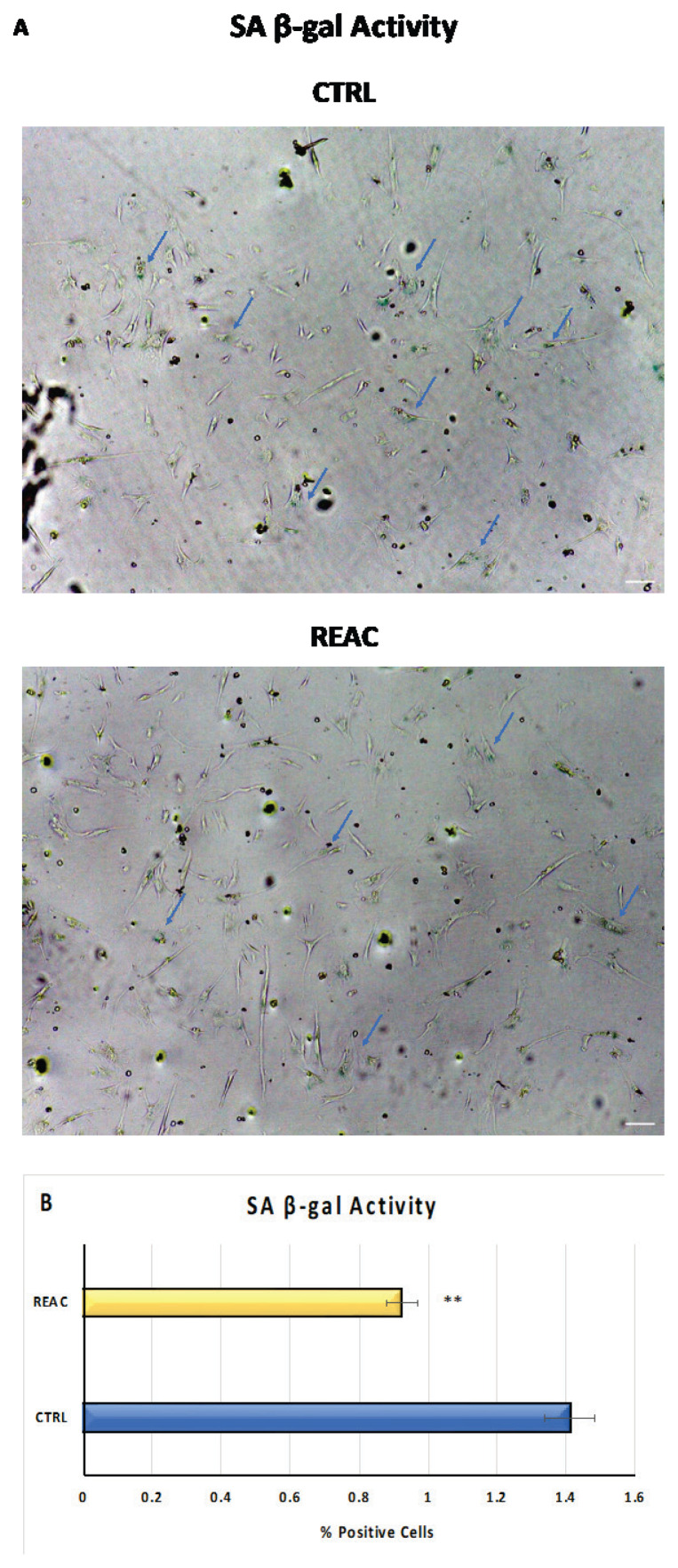
Cell senescence was evaluated in BMSCs treated or not treated with REAC for 72h by Senescence-associated (SA) β-Galactosidase Staining Kit (Cell Signaling Technology, Euroclone, Milan, Italy). After treatment, cells were fixed and processed according to the manufacturer’s instructions. Detection of SA-β-Galactosidase activity, visible by blue staining, was performed by inverted light microscope (10x magnification in bright field). The number of positively blue-stained cells was calculated as the percentage of total number of cells with an image software analysis (ImageJ, version 1.8.0, National Institutes of Health, Bethesda, MD, USA) (mean ±SD; n=6) (*p≤0.05; **p≤0.01).
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Science Version 13 (SPSS Inc., Chicago, IL, USA). For this study, assuming a p value <0.05 as statistically significant, Wilcoxon signed-rank test was used to evaluate the congruity of the observed set, while Kruskal-Wallis rank sum was used to assess the values found in the different observation topics. The experiments were performed two times with three technical replicates for each sample (n=10).
Results
Cell viability
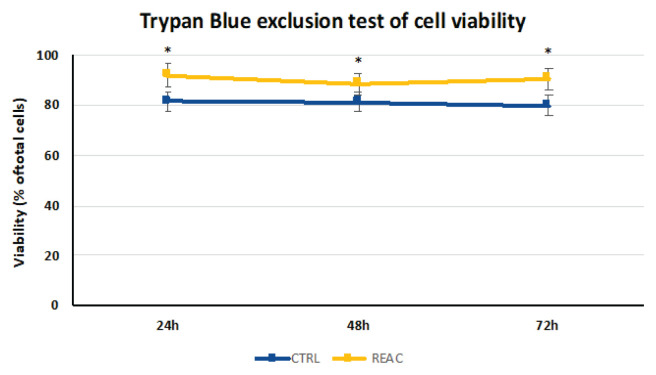
After plating (1 × 106 cells/plate), the cells were cultured in the absence or presence of REAC TO-RGN treatment for 24, 48, and 72h. Then, the BMSCs were collected and counted using an automatic cell counter. The percentage of vital cells was calculated as the number of positive cells divided by the total number of counted cells. The REAC TO-RGN treatment significantly increased the BMSC viability and proliferation at each time point compared with the untreated control cells (Fig. 1, Table 1).
Fig. 1.
Increase of viability in REAC TO-RGN-treated cells. After plating (1 × 106 cells/plate), the cells were cultured in the absence or presence of REAC TO-RGN treatment for 24, 48, and 72h. Then, BMSCs were collected and counted using an automatic cell counter. The percentage of vital cells was calculated as the number of positive cells divided by the total number of counted cells (mean ± SE; n = 6) (* p≤0.05).
Table 1.
Cell concentration in control and REAC TO-RGN-treated cells (mean ± SE; n = 6).
| T0 | 24h CTRL | 24h REAC | 48h CTRL | 48h REAC | 72h CTRL | 72h REAC | |
|---|---|---|---|---|---|---|---|
| Total cell concentration | 6.20 ± 2.9 × 106 | 2.47 ± 0.42 × 106 | 2.74 ± 0.7 × 106 | 3.46 ± 0.38 × 106 | 3.23 ± 0.43 × 106 | 2.63 ± 0.47 × 106 | 2.07 ± 0.54 × 106 |
| Live cell concentration | 4.27 ± 1.8 × 106 | 2.17 ± 0.36 × 106 | 2.53 ± 0.6 × 106 | 2.69 ± 0.38 × 106 | 2.74 ± 0.46 × 106 | 2.06 ± 0.38 × 106 | 1.89 ± 0.49 × 106 |
| Dead cell concentration | 1.94 ± 1.1 × 106 | 3.04 ± 0.10 × 105 | 2.03 ± 0.8 × 105 | 7.61 ± 0.21 × 105 | 4.90 ± 0.22 × 105 | 4.74 ± 0.19 × 105 | 1.85 ± 0.062 × 105 |
| Viability | 68.87 % | 87.85 % | 93.13 % | 77.74 % | 84.82 % | 78.32 % | 91.30 % |
Telomerase activity
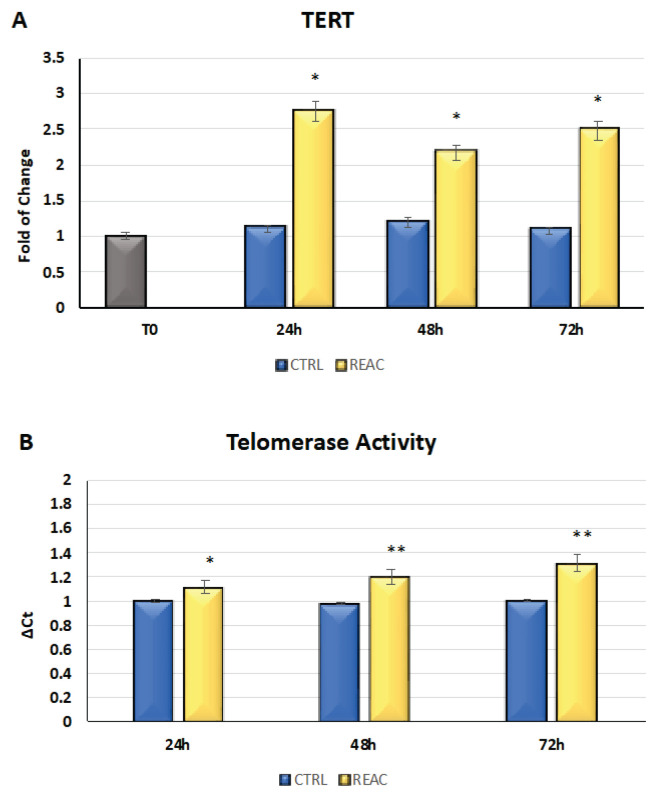
The cells were cultured for 24, 48, or 72h in the absence or presence of REAC. The mRNA levels of TERT from the control (blue bars) or REAC-treated (yellow bars) cells were normalized to GAPDH and plotted as fold changes relative to the mRNA expression at time 0, defined as 1 (gray bar). All the REAC-treated cells after 24, 48 and 72h were significantly different from the untreated control cells. The REAC TO-RGN treatment modulates hTERT gene expression by determining its upregulation (Fig. 2A).
Fig. 2.
A) Effect of REAC TO-RGN in increasing the level of expression of TERT in the REAC TO-RGN-treated cells compared with the untreated control cells. For each timepoint, the mRNA level of the cells exposed to REAC TO-RGN treatment was significantly upregulated compared with the untreated controls (mean ± SE; n=6) (* p≤0.05) B) REAC TO-RGN treatment increases telomerase activity. The REAC TO-RGN-treated cells showed an increase in telomerase activity compared with the untreated control cells for each time of observation (mean ± SE; n=6; *p≤0.05; **p≤0.001).
The TRAPeze-RT assay was performed in the untreated control cells and in the cells exposed to REAC TO-RGN after 24, 48, and 72h in culture. The telomerase activity of the REAC-treated cells was compared with that of the untreated control cells. Telomerase activity was calculated by comparing the average Ct values from the sample wells against the standard curve generated by the TSR8 control template. The REAC TO-RGN treated-cells exhibited a significant increase in telomerase activity compared with the untreated control cells for each time of observation (Fig. 2B).
P19ARF, P21, P53 gene expression
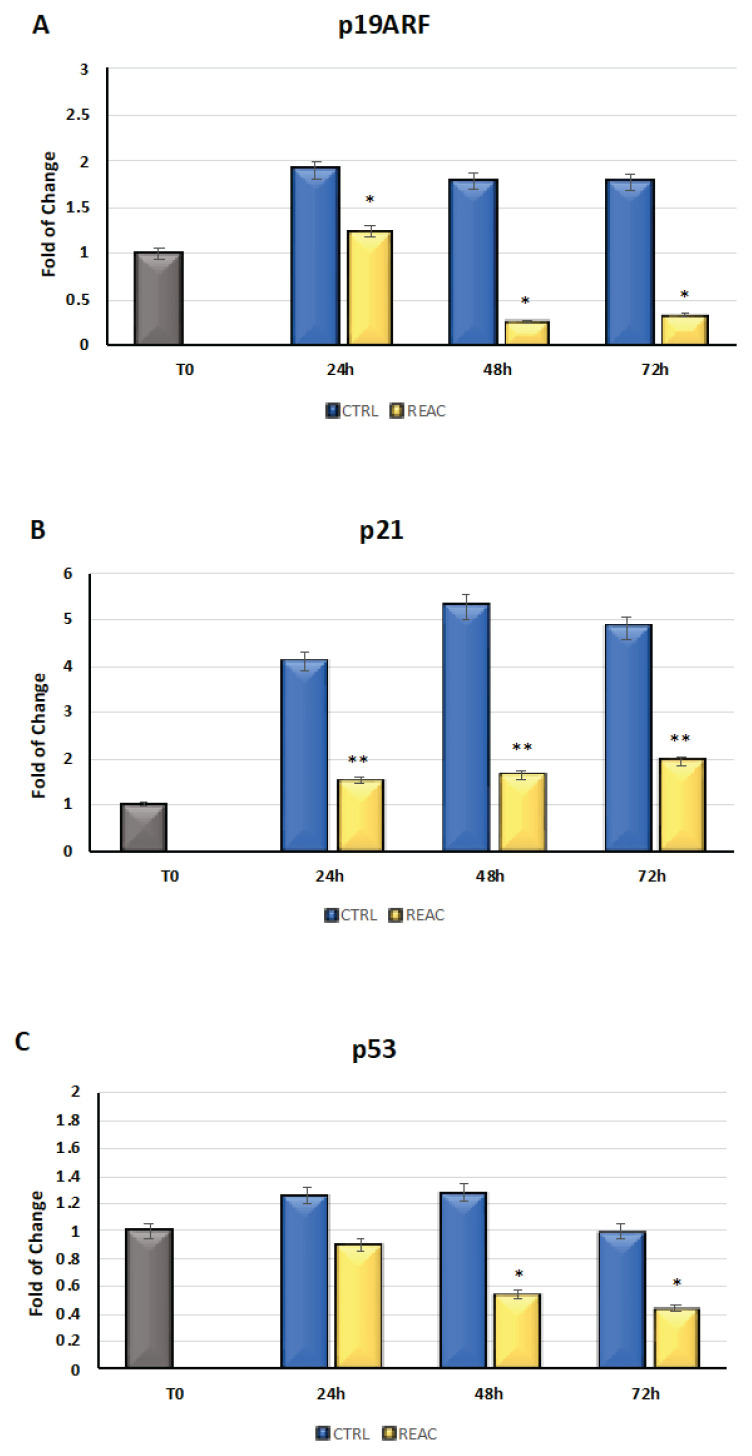
The cells were cultured for 24, 48, or 72h in the absence or presence of REAC TO-RGN treatment. As showed in Fig. 3, the mRNA levels of p19ARF (Panel A), p21 (Panel B), and p53 (Panel C) from the control (blue bars) or REAC-treated (yellow bars) cells were normalized to GAPDH and plotted as fold changes relative to the mRNA expression at time 0, defined as 1 (gray bar). All of the REAC TO-RGN-treated cells at each time point were significantly different from each untreated control cell for P19ARF and for p21. REAC TO-RGN-treated cells at were significantly different from each untreated control cell at 48 and 72 hours for p53. The REAC TO-RGN treatment modulates p19ARF, p21, p53 gene expression by determining their downregulation.
Fig. 3.
Gene expression of p19ARF (Panel A), p21 (Panel B), and p53 (Panel C) in BMSCs that were treated or not treated with REAC TO-RGN for 24, 48, or 72h. The expression of each gene increased for each time point in the untreated control cells, while it was significantly downregulated in the REAC TO-RGN-treated cells (mean ± SD; n=6) (* p≤0.05; ** p≤0.01).
Expression of stemness-related genes
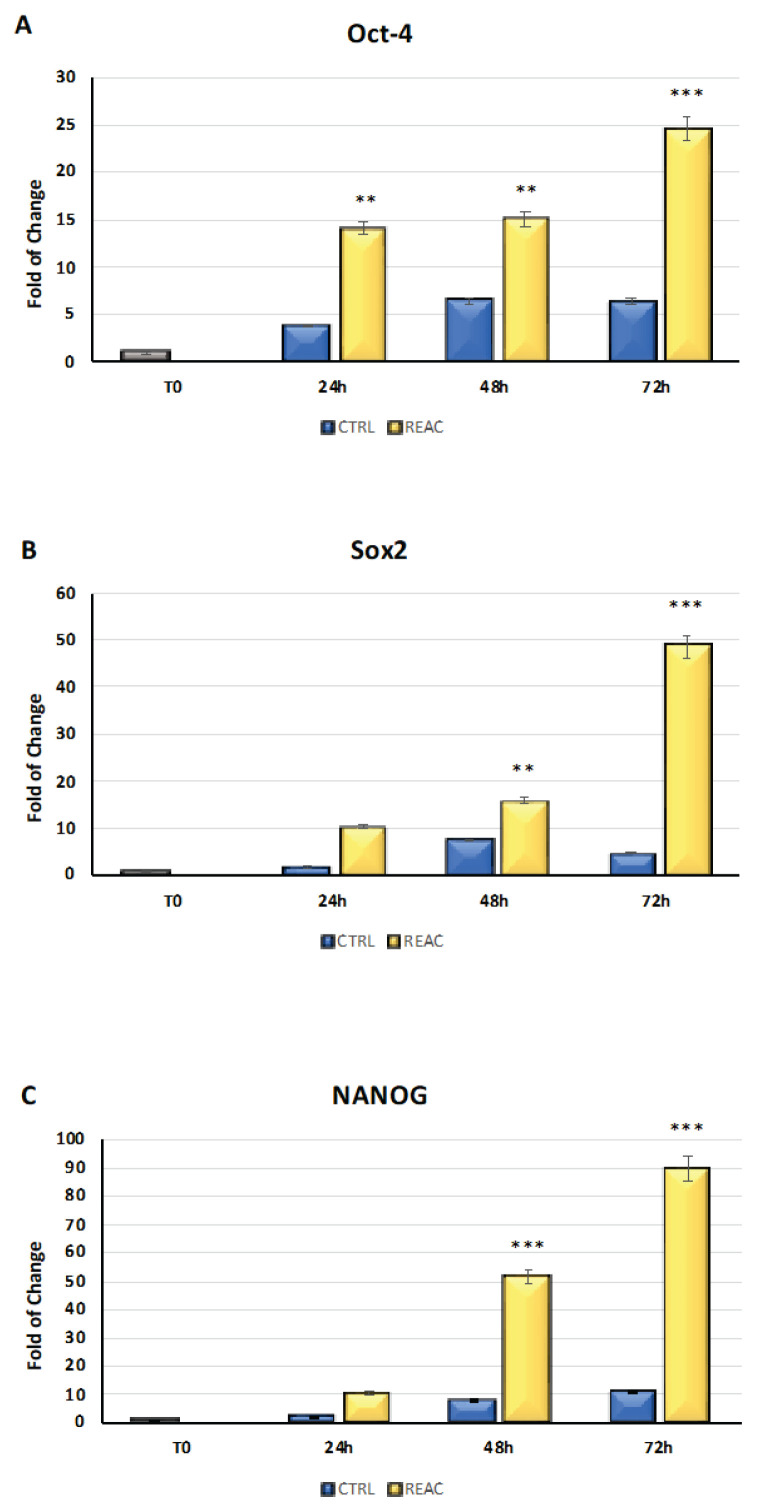
The cells were cultured for 24, 48, or 72h in the absence or presence of REAC. As showed in Fig. 4, the mRNA levels of Oct-4 (Panel A), Sox2 (Panel B), and NANOG (Panel C) from the control (blue bars) or REAC-treated (yellow bars) cells were normalized to GAPDH and plotted as fold changes relative to the mRNA expression at time 0, defined as 1 (gray bar). All the REAC-treated cells after 48 and 72h were significantly different from the untreated control cells. The REAC TO-RGN treatment modulates stemness-related gene expression by determining its upregulation.
Fig. 4.
Effect of REAC TO-RGN in increasing the level of expression of Oct-4 (Panel A), Sox2 (Panel B), and NANOG (Panel C) in the REAC TO-RGN-treated cells compared with the untreated control cells. For each timepoint, the mRNA level of the cells exposed to REAC TO-RGN treatment was significantly upregulated compared with the untreated controls (mean ± SD; n=6) (* p≤0.05, **p≤0.001, ***p≤0.001).
β-galactosidase activity
Fig. 5 shows the results from β-galactosidase staining assay in BMSCs treated or not with REAC for 72h. Results have revealed that REAC significantly counteract the senescence process, as compared to control untreated BMSCs (Panel A). The number of blue positive senescent cells was significantly reduced after REAC treatment (Panel B). Data are expressed as mean ±SD (n=6) (**p≤0,001).
Fig. 5.
A) Senescence-associated β-galactosidase activity. B-galactosidase was evaluated in BMSCs treated or not with REAC for 72h. Blue arrows indicate the blue-positive cells. Scale bar=100 μm. B) The number of blue positive cells was calculated using ImageJ. Data are expressed as mean ± SD (n=6) (**p≤0,001).
Discussion
OHD represents a group of clonal diseases whose pathogenesis is characterized by the presence of acquired genetic defects that compromise the functionality of the hematopoietic precursors [23]. In recent years, the demonstration that the BM microenvironment plays a substantial role in supporting or inhibiting the evolution of tumor clones has attracted extensive interest [24–26]. The growing knowledge on the mechanisms that mediate the hematopoietic function of the medullary precursors has made it possible to elaborate on the concept of hematopoietic niche [27], which is designated as the most relevant functional unit in this context. BMSCs represent a subfamily of stem cells with lineage-oriented features in the connective sense [28]. In addition to their tissue regeneration capability that is common to other stem cell subtypes, they present two other functional attributes, namely, the ability to support hematopoiesis [29–31] and specific immunomodulation properties [32].
The regenerative capability of BMSCs is not only linked to the original tissue, the medullary microenvironment in which they are found, and the age of the donor, but also to the effects that senescence exerts on the self-renewal processes and on the differentiation potential [33,34].
Alongside conventional methodologies represented by chemical stimuli, innovative strategies, methods, and devices are sought and applied that would allow the control of differentiation and of the various physiological processes through the transmission of the signals necessary to direct the differentiation potential toward the phenotype of interest [35].
Bioelectric signaling plays an important role in the induction and maintenance of differentiation processes as it is a functional regulator of the differentiation process in various cells and tissues [12,36,37]. To attain maximum effectiveness in inducing cell proliferation, differentiation, migration, morphology, apoptosis, and anti-senescence, it is crucial that bioelectric signals are not imposed from the outside but are endogenously induced. The REAC technology was designed to obtain this type of endogenous induction in order to recover and optimize the endogenous bioelectric potentials and the functions that depend on them [15–20,38–46].
The objective of the present study was to determine whether the previous anti-senescence results obtained on different stem cell lines could also be observed in BMSCs. For this reason, we evaluated the effects of REAC TO-RGN treatment on cell viability and on the expression of the senescence-associated genes p19ARF, p21, and p53. Moreover, the expression of hTERT, which is the product of the telomerase gene, was analyzed, together with the telomerase activity. The expression of the stemness-related genes and β-galactosidase activity were also evaluated.
The results show that the REAC TO-RGN treatment can induce a significant increase in cell viability, as showed by increased number of live cells, as compared to control untreated cells, after trypan blue exclusion test. It also acts on the upregulation of hTERT gene expression in telomerase activity. BMSCs have the capability to maintain some basal level of telomerase activity that increases upon differentiation and proliferation. Nevertheless, other authors have described a negative correlation between hTERT activity, MDS [47], and AML prognosis [48].
It is important to consider that in this study, the upregulation of telomerase activity agrees with the observed increase in cell viability; it is also essential in preserving chromosome integrity in newborn cells. On the other hand, the REAC TO-RGN treatment was able to contemporarily downregulate the expression of the genes responsible for the establishment of a senescent phenotype, namely, p19ARF, p21, and p53, and increase the expression of stemness-related genes, Oct-4, Sox2 and NANOG. Based on the gene expression profiling of circulating BMSCs, we demonstrate that REAC TO-RGN endogenous bioelectric manipulation can improve the molecular pattern of cell senescence, ultimately inducing a higher telomerase activity.
The perspectives opened by this study can be easily translated to the clinic, because the specific therapeutic treatments of REAC neurobiological stimulation have already been in use for years to promote reparative and regenerative effects, with already standardized methods of administration.
Conclusions
These results provide insights into the possibility of reversing or counteracting the functional impairments observed in low-risk MDS patients and open the chance to reactivate a compromised hematopoiesis. Future translational studies are warranted to determine if the REAC TO-RGN treatment is a useful tool to counteract the senescence mechanism that gives rise to AML. Moreover, it may constitute a valuable tool for traditional therapy, such as in regular blood transfusions for MDS patients.
Acknowledgements
This work was supported by funds of institutional research (TA 29) FVM UVS Brno. The authors would like to acknowledge Dr. Alessandra Cappelli and Dr. Eng. Matteo Lotti Margotti for their valuable cooperation.
Footnotes
Conflict of Interest
SR and VF are the authors of REAC patent. The other authors declare no conflict of interest.
References
- 1.Disperati P, Ichim CV, Tkachuk D, Chun K, Schuh AC, Wells RA. Progression of myelodysplasia to acute lymphoblastic leukaemia: implications for disease biology. Leuk Res. 2006 Feb;30(2):233–239. doi: 10.1016/j.leukres.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Mattiucci D, Maurizi G, Leoni P, Poloni A. Aging- and senescence-associated changes of mesenchymal stromal cells in myelodysplastic syndromes. Cell Transplant. 2018 May;27(5):754–764. doi: 10.1177/0963689717745890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ernst P, Heidel FH. Molecular mechanisms of senescence and implications for the treatment of myeloid malignancies. Cancers (Basel) 2021 Feb 4;13(4):612. doi: 10.3390/cancers13040612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X, Li N, Weng J, Du X. Senescent mesenchymal stem cells in myelodysplastic syndrome: Functional alterations, molecular mechanisms, and therapeutic strategies. Front Cell Dev Biol. 2021 Feb 11;:8617466. doi: 10.3389/fcell.2020.617466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Issa JP. Epigenetic changes in the myelodysplastic syndrome. Hematol Oncol Clin North Am. 2010 Apr;24(2):317–30. doi: 10.1016/j.hoc.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heuser M, Yun H, Thol F. Epigenetics in myelodysplastic syndromes. Semin Cancer Biol. 2018 Aug;51:170–179. doi: 10.1016/j.semcancer.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehdipour P, Santoro F, Minucci S. Epigenetic alterations in acute myeloid leukemias. FEBS J. 2015 May;282(9):1786–800. doi: 10.1111/febs.13142. [DOI] [PubMed] [Google Scholar]
- 8.Goldman SL, Hassan C, Khunte M, Soldatenko A, Jong Y, Afshinnekoo E, Mason CE. Epigenetic modifications in acute myeloid leukemia: Prognosis, treatment, and heterogeneity. Front Genet. 2019 Mar 1;10:133. doi: 10.3389/fgene.2019.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prebet T, Lhoumeau AC, Arnoulet C, Aulas A, Marchetto S, Audebert S, Puppo F, Chabannon C, Sainty D, Santoni MJ, et al. The cell polarity ptk7 receptor acts as a modulator of the chemotherapeutic response in acute myeloid leukemia and impairs clinical outcome. Blood. 2010 Sep 30;116(13):2315–23. doi: 10.1182/blood-2010-01-262352. [DOI] [PubMed] [Google Scholar]
- 10.Levin M. Molecular bioelectricity: How endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo. Mol Biol Cell. 2014 Dec 1;25(24):3835–50. doi: 10.1091/mbc.e13-12-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruciani S, Garroni G, Ventura C, Danani A, Necas A, Maioli M. Stem cells and physical energies: Can we really drive stem cell fate? Physiol Res. 2019 Dec 30;68(Suppl 4):S375–S384. doi: 10.33549/physiolres.934388. [DOI] [PubMed] [Google Scholar]
- 12.McLaughlin KA, Levin M. Bioelectric signaling in regeneration: Mechanisms of ionic controls of growth and form. Dev Biol. 2018 Jan 15;433(2):177–189. doi: 10.1016/j.ydbio.2017.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gruszka AM, Valli D, Alcalay M. Wnt signalling in acute myeloid leukaemia. Cells. 2019 Nov 7;8(11):1403. doi: 10.3390/cells8111403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ria R, Moschetta M, Reale A, Mangialardi G, Castrovilli A, Vacca A, Dammacco F. Managing myelodysplastic symptoms in elderly patients. Clin Interv Aging. 2009;4:413–23. doi: 10.2147/CIA.S5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maioli M, Rinaldi S, Santaniello S, Castagna A, Pigliaru G, Gualini S, Fontani V, Ventura C. Radiofrequency energy loop primes cardiac, neuronal, and skeletal muscle differentiation in mouse embryonic stem cells: A new tool for improving tissue regeneration. Cell Transplant. 2012;21(6):1225–33. doi: 10.3727/096368911X600966. [DOI] [PubMed] [Google Scholar]
- 16.Maioli M, Rinaldi S, Santaniello S, Castagna A, Pigliaru G, Gualini S, Cavallini C, Fontani V, Ventura C. Radio electric conveyed fields directly reprogram human dermal skin fibroblasts toward cardiac, neuronal, and skeletal muscle-like lineages. Cell Transplant. 2013;22(7):1227–35. doi: 10.3727/096368912X657297. [DOI] [PubMed] [Google Scholar]
- 17.Maioli M, Rinaldi S, Santaniello S, Castagna A, Pigliaru G, Delitala A, Bianchi F, Tremolada C, Fontani V, Ventura C. Radioelectric asymmetric conveyed fields and human adipose-derived stem cells obtained with a nonenzymatic method and device: A novel approach to multipotency. Cell Transplant. 2014;23(12):1489–500. doi: 10.3727/096368913X672037. [DOI] [PubMed] [Google Scholar]
- 18.Rinaldi S, Maioli M, Santaniello S, Castagna A, Pigliaru G, Gualini S, Margotti ML, Carta A, Fontani V, Ventura C. Regenerative treatment using a radioelectric asymmetric conveyor as a novel tool in antiaging medicine: An in vitro beta-galactosidase study. Clin Interv Aging. 2012;7:191–4. doi: 10.2147/CIA.S33312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maioli M, Rinaldi S, Santaniello S, Castagna A, Pigliaru G, Delitala A, Lotti Margotti M, Bagella L, Fontani V, Ventura C. Anti-senescence efficacy of radio-electric asymmetric conveyer technology. Age (Dordr) 2014 Feb;36(1):9–20. doi: 10.1007/s11357-013-9537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rinaldi S, Maioli M, Pigliaru G, Castagna A, Santaniello S, Basoli V, Fontani V, Ventura C. Stem cell senescence. Effects of reac technology on telomerase-independent and telomerase-dependent pathways. Sci Rep. 2014 Sep 16;4:6373. doi: 10.1038/srep06373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geyh S, Oz S, Cadeddu RP, Frobel J, Bruckner B, Kundgen A, Fenk R, Bruns I, Zilkens C, Hermsen D, et al. Insufficient stromal support in mds results from molecular and functional deficits of mesenchymal stromal cells. Leukemia. 2013 Sep;27(9):1841–51. doi: 10.1038/leu.2013.193. [DOI] [PubMed] [Google Scholar]
- 22.Ruiz-Aparicio PF, Vernot JP. Bone marrow aging and the leukaemia-induced senescence of mesenchymal stem/stromal cells: Exploring similarities. J Pers Med. 2022 Apr 29;12(5):716. doi: 10.3390/jpm12050716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bao EL, Cheng AN, Sankaran VG. The genetics of human hematopoiesis and its disruption in disease. EMBO Mol Med. 2019 Aug;11(8):e10316. doi: 10.15252/emmm.201910316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu Y, Gong Z, Zhang S, Zhou J, Cao K, et al. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017 Feb 25;8(5):761–773. doi: 10.7150/jca.17648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou J, Mauerer K, Farina L, Gribben JG. The role of the tumor microenvironment in hematological malignancies and implication for therapy. Front Biosci. 2005 May 1;10:1581–96. doi: 10.2741/1642. [DOI] [PubMed] [Google Scholar]
- 26.Zhang P, Zhang C, Li J, Han J, Liu X, Yang H. The physical microenvironment of hematopoietic stem cells and its emerging roles in engineering applications. Stem Cell Res Ther. 2019 Nov 19;10(1):327. doi: 10.1186/s13287-019-1422-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei Q, Frenette PS. Niches for hematopoietic stem cells and their progeny. Immunity. 2018 Apr 17;48(4):632–648. doi: 10.1016/j.immuni.2018.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Augello A, De Bari C. The regulation of differentiation in mesenchymal stem cells. Hum Gene Ther. 2010 Oct;21(10):1226–38. doi: 10.1089/hum.2010.173. [DOI] [PubMed] [Google Scholar]
- 29.Lucas D, Frenette PS. Stem cells: Reprogramming finds its niche. Nature. 2014 Jul 17;511(7509):301–2. doi: 10.1038/nature13516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Resnick I, Stepensky P, Elkin G, Barkatz C, Gurevich O, Prigozhina T, Pikarsky E, Waldman E, Amar A, Samuel S, et al. Msc for the improvement of hematopoietic engraftment. Bone Marrow Transplant. 2010 Mar;45(3):605–6. doi: 10.1038/bmt.2009.199. [DOI] [PubMed] [Google Scholar]
- 31.Poon Z, Dighe N, Venkatesan SS, Cheung AMS, Fan X, Bari S, Hota M, Ghosh S, Hwang WYK. Bone marrow mscs in mds: Contribution towards dysfunctional hematopoiesis and potential targets for disease response to hypomethylating therapy. Leukemia. 2019 Jun;33(6):1487–1500. doi: 10.1038/s41375-018-0310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang M, Yuan Q, Xie L. Mesenchymal stem cell-based immunomodulation: Properties and clinical application. Stem Cells Int. 2018 Jun 14;2018:3057624. doi: 10.1155/2018/3057624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fafian-Labora JA, Morente-Lopez M, Arufe MC. Effect of aging on behaviour of mesenchymal stem cells. World J Stem Cells. 2019 Jun 26;11(6):337–346. doi: 10.4252/wjsc.v11.i6.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Wu Q, Wang Y, Li L, Bu H, Bao J. Senescence of mesenchymal stem cells (review) Int J Mol Med. 2017 Apr;39(4):775–782. doi: 10.3892/ijmm.2017.2912. [DOI] [PubMed] [Google Scholar]
- 35.Maioli M, Basoli V, Santaniello S, Cruciani S, Delitala AP, Pinna R, Milia E, Grillari-Voglauer R, Fontani V, Rinaldi S, et al. Osteogenesis from dental pulp derived stem cells: A novel conditioned medium including melatonin within a mixture of hyaluronic, butyric, and retinoic acids. Stem Cells Int. 2016;2016:2056416. doi: 10.1155/2016/2056416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silver BB, Nelson CM. The bioelectric code: Reprogramming cancer and aging from the interface of mechanical and chemical microenvironments. Front Cell Dev Biol. 2018 Mar 6;6:21. doi: 10.3389/fcell.2018.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levin M. Molecular bioelectricity: How endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo. Mol Biol Cell. 2014 Dec 1;25(24):3835–50. doi: 10.1091/mbc.e13-12-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rinaldi S, Mura M, Castagna A, Fontani V. Long-lasting changes in brain activation induced by a single reac technology pulse in wi-fi bands. Randomized double-blind fmri qualitative study. Sci Rep. 2014 Jul 11;4:5668. doi: 10.1038/srep05668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maioli M, Rinaldi S, Migheli R, Pigliaru G, Rocchitta G, Santaniello S, Basoli V, Castagna A, Fontani V, Ventura C, Serra PA. Neurological morphofunctional differentiation induced by reac technology in pc12. A neuro protective model for parkinson’s disease. Sci Rep. 2015 May 15;5:10439. doi: 10.1038/srep10439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zippo AG, Rinaldi S, Pellegata G, Caramenti GC, Valente M, Fontani V, Biella GE. Electrophysiological effects of non-invasive radio electric asymmetric conveyor (reac) on thalamocortical neural activities and perturbed experimental conditions. Sci Rep. 2015 Dec 11;5:18200. doi: 10.1038/srep18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lorenzini L, Giuliani A, Sivilia S, Baldassarro VA, Fernandez M, Lotti Margotti M, Giardino L, Fontani V, Rinaldi S, Calza L. Reac technology modifies pathological neuroinflammation and motor behaviour in an alzheimer’s disease mouse model. Sci Rep. 2016 Oct 24;6:35719. doi: 10.1038/srep35719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maioli M, Rinaldi S, Pigliaru G, Santaniello S, Basoli V, Castagna A, Fontani V, Ventura C. Reac technology and hyaluron synthase 2, an interesting network to slow down stem cell senescence. Sci Rep. 2016 Jun 24;6:28682. doi: 10.1038/srep28682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panaro MA, Aloisi A, Nicolardi G, Lofrumento DD, De Nuccio F, La Pesa V, Cianciulli A, Rinaldi R, Calvello R, Fontani V, et al. Radio electric asymmetric conveyer technology modulates neuroinflammation in a mouse model of neurodegeneration. Neurosci Bull. 2018 Apr;34(2):270–282. doi: 10.1007/s12264-017-0188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rinaldi S, Meloni MA, Galleri G, Maioli M, Pigliaru G, Cugia G, Santaniello S, Castagna A, Fontani V. Radio electric asymmetric conveyer (reac) technology to obviate loss of t cell responsiveness under simulated microgravity. PLoS One. 2018 Jul 6;13(7):e0200128. doi: 10.1371/journal.pone.0200128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Basoli V, Santaniello S, Rinaldi S, Fontani V, Pigliaru G, Wieser M, Strajeriu A, Castagna A, Redl H, Ventura C, et al. Physical stimulation by reac and bmp4/wnt-1 inhibitor synergistically enhance cardiogenic commitment in iPCSs. PLoS One. 2019 Jan 23;14(1):e0211188. doi: 10.1371/journal.pone.0211188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collodel G, Fioravanti A, Pascarelli NA, Lamboglia A, Fontani V, Maioli M, Santaniello S, Pigliaru G, Castagna A, Moretti E, et al. Effects of regenerative radioelectric asymmetric conveyer treatment on human normal and osteoarthritic chondrocytes exposed to il-1beta. A biochemical and morphological study. Clin Interv Aging. 2013;8:309–16. doi: 10.2147/CIA.S42229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grandjenette C, Schnekenburger M, Gaigneaux A, Gerard D, Christov C, Mazumder A, Dicato M, Diederich M. Human telomerase reverse transcriptase depletion potentiates the growth-inhibitory activity of imatinib in chronic myeloid leukemia stem cells. Cancer Lett. 2020 Jan 28;469:468–480. doi: 10.1016/j.canlet.2019.11.017. [DOI] [PubMed] [Google Scholar]
- 48.Hartmann U, Brümmendorf T, Balabanov S, Thiede C, Illme T, Schaich M. Telomere length and hTERT expression in patients with acute myeloid leukemia correlates with chromosomal abnormalities. Haematologica. 2005 Mar;90(3):307–16. [PubMed] [Google Scholar]