Abstract
Background
Carbapenems are the last-line therapy for multidrug-resistant (MDR) infections caused by Enterobacterales, including those caused by Enterobacter species. However, the recent emergence of carbapenem-resistant (CR) and extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae pathogens, which are resistant to nearly all antibiotics, has raised concerns among international healthcare organizations. Hence, because there is no comprehensive data in Iran, the current study aimed to evaluate the prevalence of antibiotic resistance among Enterobacter species, especially CR and ESBL-producing strains, in Iran.
Methods
The literature search was performed up to June 21, 2021, in national and international databases using MeSH-extracted keywords, i.e., Enterobacter, antibiotic resistance, carbapenem, ESBL, and Iran. Study selection was done based on the predefined inclusion and exclusion criteria, and data analysis was carried out using the Comprehensive Meta-Analysis (CMA) software.
Results
The pooled prevalence of Enterobacter species resistant to various antibiotics is as follows: imipenem 16.6%, meropenem 16.2%, aztreonam 40.9%, ciprofloxacin 35.3%, norfloxacin 31%, levofloxacin 48%, gentamicin 42.1%, amikacin 30.3%, tobramycin 37.2%, tetracycline 50.1%, chloramphenicol 25.7%, trimethoprim/sulfamethoxazole 52%, nalidixic acid 49.1%, nitrofurantoin 43%, ceftriaxone 49.3%, cefixime 52.4%, cefotaxime 52.7%, ceftazidime 47.9%, cefepime 43.6%, and ceftizoxime 45.5%. The prevalence rates of MDR and ESBL-producing Enterobacter species in Iran were 63.1% and 32.8%, respectively.
Conclusion
In accordance with the warning of international organizations, our results revealed a high prevalence of ESBL-producing Enterobacter species in Iran, which is probably associated with the high prevalence of Enterobacter species resistant to most of the assessed antibiotics, especially MDR strains. However, the resistance rate to carbapenems was relatively low, and these drugs can still be considered as drugs of choice for the treatment of Enterobacter infections in Iran. Nevertheless, continuous monitoring of drug resistance along with antibiotic therapy based on the local data and evaluation of the therapeutic efficacy of new antibiotics or combination therapeutic strategies, such as ceftazidime/avibactam, meropenem/vaborbactam, plazomicin, and eravacycline, is recommended.
1. Introduction
The genus Enterobacter includes three medically important species, i.e., Enterobacter cloacae complex, Enterobacter aerogenes complex, and Enterobacter sakazakii [1, 2]. These enteric Gram-negative rods belong to the Enterobacteriaceae family and rarely cause infection in immunocompetent patients, but they are commonly associated with nosocomial infections, especially by the Enterobacter cloacae complex, in neonates and immunocompromised patients [1–6]. The most common nosocomial infections associated with these lactose-fermenting Enterobacter species include pneumonia, urinary tract infection, septicemia, and wound infection, as well as device-associated infections [1, 2]. Like many bacterial infections, in which an increasing trend of antibiotic resistance has led to the emergence of public health problems and imposed economic costs on healthcare, such an increasing trend of antibiotic resistance has also been reported for Enterobacter species [3, 6]. Among different mechanisms of resistance to various antibiotics in these Gram-negative rods, the intrinsic or acquired production of antibiotic-inactivating enzymes such as β-lactamases is very important [1]. Enterobacter species producing AmpC chromosomal cephalosporins are intrinsically resistant to ampicillin as well as first- and second-generation cephalosporins [2]. Plasmid-encoded extended-spectrum β-lactamase (ESBL) genes are involved in Enterobacter species' resistance to most β-lactam antibiotics, including second- and third-generation cephalosporins and aztreonam [6]. On the other hand, acquired resistance to quinolones, aminoglycosides, and carbapenems has been identified in hospital-acquired strains, which is highly important because these antibiotics are the last line of treatment [2, 4].
Recently, based on the World Health Organization (WHO) report, CR and ESBL-producing Enterobacteriaceae have been identified as one of the greatest threats to human health [5]. Although Escherichia and Klebsiella species are two main threats among CR and ESBL-producing Enterobacteriaceae [3], in the United States, CR Enterobacter species are considered the second most common CR Enterobacteriaceae [6].
However, there is no comprehensive data on antibiotic resistance patterns of Enterobacter species, especially CR strains, and ESBL-mediated resistance mechanisms in Iran. Therefore, the current systematic review and meta-analysis were designed to determine the prevalence of antibiotic resistance patterns of Enterobacter species, especially carbapenem-resistant strains, along with the frequency of ESBL-producing strains in Iran.
2. Methods
2.1. Literature Search and Study Selection
International databases including PubMed, Scopus, and Google Scholar, along with national databases including Scientific Information Database (https://www.sid.ir/) and Magiran (https://www.magiran.com/), were searched independently by two investigators to find studies conducted on the prevalence of antibiotic resistance and ESBL-producing Enterobacter species in Iran. The search was performed from 1996 to June 21, 2021. The most common Medical Subject Headings (MeSH)-extracted keywords used for the literature search were as follows: Enterobacter, antibiotic resistance, carbapenem, ESBL, and Iran. We defined the inclusion and exclusion criteria for the studies retrieved in the search and selected studies that met our criteria after a review of the titles, abstracts, and full text of the articles. The following studies were removed from the meta-analysis: studies reporting antibiotic resistance and ESBL-positive isolates published in languages other than English or Persian, studies conducted in other countries, studies reporting other bacteria in the Enterobacteriaceae family, studies with a small sample size (less than 10 bacterial isolates), studies with insufficient data, and nonoriginal articles, abstracts, and duplicates. Reference lists of the included articles were checked in order to find any possible missed studies. The current systematic review and meta-analysis were designed according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines [7].
2.2. Data Extraction
Two different investigators extracted the data, and a third investigator tabulated the required information in Table 1 after resolving possible disagreements in the results of the search and reaching a consensus. Required data were as follows: first author's surname, study location, study enrollment date, the number of isolates, antibiotic susceptibility testing methods, the prevalence of Enterobacter species resistance to different drugs, the prevalence of multidrug-resistant (MDR) Enterobacter species, and the frequency of ESBL-positive isolates. It is noteworthy that Enterobacter species have intrinsic resistance to β-lactam antibiotics including ampicillin, amoxicillin-clavulanate, ampicillin-sulbactam, cephalosporins I (cefazolin and cephalothin), cephamycins (cefoxitin and cefotetan), and cephalosporin II (cefuroxime). According to the Clinical and Laboratory Standards Institute (CLSI) guideline, susceptibility testing is unnecessary for the above-mentioned antibiotics [8]. For this reason, these antibiotics are not included in Table 1.
Table 1.
Required data were extracted from included articles in the meta-analysis.
| Author (Ref) | City | Year | Isolate (n) | AST | Resistance rate (n) | ESBL-positive (n) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IPM | MEM | ATM | CIP | NOR | LVX | GEN | AMK | TOB | TET | CHL | SXT | NAL | NIT | CRO | CFM | CTX | CAZ | CEP | ZOX | MDR | ||||||
| Amin et al. [9] | Ahvaz | 2015-2016 | 152 | Disk diffusion | 80 | 53 | ND | 84 | 70 | 63 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Afrugh et al. [10] | Ahvaz | 2013-2014 | 17 | Disk diffusion | 15 | 15 | ND | 15 | ND | ND | 14 | 16 | ND | 17 | ND | 12 | 16 | 13 | 16 | ND | 17 | 15 | 14 | 16 | ND | ND |
| Mousavian et al. [11] | Ahvaz | 2012 | 65 | Disk diffusion | 0 | ND | ND | 6 | ND | ND | 5 | 3 | ND | ND | ND | ND | ND | ND | 10 | ND | 11 | 9 | ND | 11 | ND | 27 |
| Khosravi et al. [12] | Ahvaz | 2009–2012 | 156 | Disk diffusion | 98 | ND | ND | 91 | ND | ND | 102 | 60 | ND | 64 | ND | 108 | 94 | 75 | 121 | 110 | ND | ND | ND | ND | ND | ND |
| Khosravi et al. [13] | Ahvaz | 2009-2010 | 209 | Disk diffusion | 124 | ND | ND | 88 | ND | ND | 143 | 117 | ND | 124 | ND | 146 | 119 | 117 | 143 | 148 | ND | ND | ND | ND | ND | ND |
| Didgar [14] | Arak | 2010–2012 | 47 | Disk diffusion | 6 | ND | ND | 19 | ND | ND | 19 | 18 | ND | ND | ND | 20 | ND | ND | 29 | ND | ND | 40 | 25 | 19 | ND | ND |
| Ghasemi et al. [15] | Babol | 2020 | 30 | Disk diffusion | 6 | ND | ND | 6 | ND | ND | 4 | 6 | ND | ND | ND | ND | ND | 9 | ND | ND | 10 | ND | 28 | ND | 28 | ND |
| Bayani et al. [16] | Babol | 2011-2012 | 30 | Disk diffusion | 2 | ND | ND | 2 | ND | ND | ND | 2 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 5 | 4 | ND | 0 | ND |
| Ghafouri et al. [17] | Bojnurd | 2013 | 12 | Disk diffusion | 3 | 5 | ND | 8 | ND | ND | 8 | 2 | ND | 3 | 0 | 6 | ND | 1 | 6 | 0 | 0 | 1 | ND | ND | ND | ND |
| Peymani et al. [18] | Different cities | 2014 | 49 | Disk diffusion | 2 | 2 | 27 | 16 | ND | ND | 20 | 11 | ND | ND | ND | 25 | ND | ND | 28 | ND | 34 | 27 | ND | ND | 26 | ND |
| Peymani et al. [19] | Different cities | 2011-2012 | 137 | Disk diffusion | 2 | 1 | 67 | 22 | 16 | ND | 59 | ND | ND | ND | ND | 83 | ND | ND | 78 | ND | 80 | 71 | ND | ND | 83 | ND |
| Poorabbas et al. [20] | Different cities | 2008-2009 | 38 | Disk diffusion | 38 | ND | ND | 33 | ND | 35 | 24 | 26 | 0 | ND | ND | 22 | ND | 22 | 18 | 13 | 19 | 20 | 24 | ND | ND | ND |
| Molazade et al. [21] | Fasa | 2012-2013 | 28 | Disk diffusion | ND | ND | ND | 11 | ND | ND | 8 | 0 | ND | 11 | ND | 14 | 11 | 11 | 11 | 11 | 0 | ND | ND | ND | ND | ND |
| Esmaeili et al. [22] | Hamadan | 2011 | 15 | Disk diffusion | ND | ND | ND | 13 | ND | ND | 13 | ND | 13 | ND | ND | 10 | 6 | 4 | 11 | ND | ND | ND | ND | ND | ND | ND |
| Yasemi et al. [23] | Ilam | 2007–2009 | 20 | Disk diffusion | ND | ND | ND | ND | ND | ND | 3 | ND | ND | ND | ND | 9 | ND | 6 | ND | ND | ND | ND | ND | ND | ND | ND |
| Fatemi et al. [24] | Isfahan | 2014-2015 | 135 | Disk diffusion | 13 | 16 | 93 | 58 | ND | ND | 54 | 46 | 53 | 96 | 26 | 23 | ND | ND | ND | ND | 89 | 87 | 90 | ND | 98 | ND |
| Shokri et al. [25] | Isfahan | 2012-2013 | 35 | Disk diffusion | 3 | 3 | ND | 21 | ND | ND | 22 | 10 | ND | ND | ND | ND | ND | 6 | ND | ND | 24 | 22 | 12 | ND | 35 | ND |
| Kargar et al. [26] | Jahrom | 2011-2012 | 25 | Disk diffusion | ND | ND | ND | ND | ND | ND | 17 | 7 | 11 | 24 | ND | 20 | 24 | ND | ND | ND | 10 | 10 | ND | ND | ND | 1 |
| Shajari et al. [27] | Kashan | 2005-2006 | 35 | Disk diffusion | 10 | ND | ND | 13 | ND | ND | 14 | 12 | 11 | ND | ND | 21 | 28 | ND | ND | 18 | 22 | 22 | ND | ND | ND | ND |
| Sepehri et al. [28] | Kerman | 1996, 2000 | 72 | Disk diffusion | ND | ND | ND | ND | ND | ND | 43 | ND | ND | ND | ND | 46 | 12 | 28 | ND | ND | ND | ND | ND | ND | ND | ND |
| Mortazavi et al. [29] | Kermanshah | 2016-2017 | 72 | Disk diffusion | 7 | ND | 29 | 35 | 30 | ND | 36 | 35 | ND | ND | ND | 49 | 31 | 16 | ND | 40 | 37 | 38 | ND | ND | 54 | ND |
| Amini et al. [30] | Kermanshah | 2015 | 18 | Disk diffusion | ND | ND | ND | 7 | ND | ND | 8 | 9 | ND | ND | ND | 13 | 7 | 5 | 6 | 6 | ND | 10 | ND | ND | ND | ND |
| Karambin and Zarkesh [31] | Rasht | 2008–2010 | 50 | Disk diffusion | ND | ND | ND | 0 | ND | ND | 15 | 41 | ND | ND | ND | 40 | ND | ND | ND | ND | 43 | ND | ND | ND | ND | ND |
| Yaghubi et al. [32] | Rasht | 2013–2015 | 147 | Disk diffusion | 79 | 61 | ND | 80 | ND | ND | 81 | 66 | 78 | 0 | ND | 108 | 105 | 102 | 102 | 119 | 109 | 92 | ND | 80 | ND | ND |
| Rouhi et al. [33] | Sanandaj | 2013-2014 | 10 | Disk diffusion | 2 | ND | ND | 2 | 0 | ND | 0 | 5 | ND | 2 | ND | ND | 0 | 0 | 3 | ND | 5 | 4 | ND | 5 | ND | ND |
| Nikkhoo et al. [34] | Sanandaj | 2009-2010 | 11 | Disk diffusion | ND | ND | ND | 3 | ND | ND | 7 | 6 | ND | 7 | ND | 2 | ND | ND | 6 | 5 | 5 | ND | ND | ND | ND | ND |
| Ramazanzadeh et al. [35] | Sanandaj | 2007-2008 | 28 | Disk diffusion | ND | ND | ND | 14 | 22 | ND | 3 | 6 | ND | ND | ND | 13 | 23 | ND | 9 | ND | 7 | 7 | ND | 5 | ND | ND |
| Afkhamzadeh et al. [36] | Sanandaj | 2007-2008 | 15 | Disk diffusion | ND | ND | ND | 10 | ND | ND | 11 | 7 | ND | 10 | ND | 10 | ND | ND | 13 | ND | 13 | 4 | ND | ND | ND | ND |
| Jazayeri [37] | Semnan | 1999 | 11 | Disk diffusion | ND | ND | ND | 5 | ND | ND | 5 | ND | ND | ND | ND | 10 | 10 | 10 | ND | 8 | ND | ND | ND | ND | ND | ND |
| Khashei et al. [38] | Shiraz | 2016-2017 | 96 | Disk diffusion | 21 | ND | ND | 31 | ND | ND | 39 | 22 | ND | ND | ND | 45 | ND | 68 | ND | ND | ND | 70 | ND | ND | 93 | 35 |
| Malekzadegan et al. [39] | Shiraz | 2015-2016 | 61 | Disk diffusion | 26 | ND | ND | 30 | ND | ND | 46 | 61 | ND | ND | ND | 21 | 3 | 4 | ND | ND | ND | 55 | ND | ND | 56 | ND |
| Nematolahi et al. [40] | Shiraz | 2005–2014 | 90 | Disk diffusion | 4 | 1 | 44 | 14 | ND | ND | 33 | 27 | ND | ND | 30 | ND | ND | ND | 52 | 62 | 55 | 47 | 34 | ND | 10 | 14 |
| Mardaneh et al. [41] | Shiraz | 2013 | 33 | Disk diffusion | 2 | 7 | 14 | 5 | ND | ND | 11 | 7 | 14 | 24 | 8 | 9 | ND | ND | 14 | 19 | 15 | 13 | 13 | ND | ND | 13 |
| Rezaee and Abdinia [42] | Tabriz | 2010–2014 | 40 | Disk diffusion | ND | ND | ND | 0 | ND | ND | 20 | 0 | ND | ND | ND | 40 | 40 | 40 | 20 | ND | 40 | ND | ND | 10 | 40 | ND |
| Hamishehkar et al. [43] | Tabriz | 2010–2012 | 282 | Disk diffusion | 61 | ND | ND | 89 | ND | ND | 84 | 61 | ND | ND | 78 | 109 | ND | 25 | 79 | ND | 41 | 55 | ND | 49 | ND | ND |
| Azimi et al. [44] | Tehran | 2013–2018 | 45 | Disk diffusion | 8 | 23 | 11 | 9 | ND | 7 | 26 | 15 | 36 | 45 | ND | 20 | 9 | 45 | 30 | ND | 38 | 31 | 32 | 39 | 12 | ND |
| Akhavizadegan et al. [45] | Tehran | 2016-2017 | 18 | Disk diffusion | 0 | ND | ND | 7 | ND | 7 | ND | 0 | ND | ND | ND | 8 | 6 | 2 | 5 | 7 | ND | 4 | 2 | ND | ND | ND |
| Sohrabi fard et al. [46] | Tehran | 2014-2015 | 12 | Disk diffusion | ND | ND | ND | ND | ND | ND | ND | ND | ND | 4 | ND | ND | ND | ND | 5 | ND | ND | ND | ND | ND | ND | ND |
| Ghanavati et al. [47] | Tehran | 2013-2014 | 57 | Disk diffusion | 8 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 28 | 21 | ND | ND | 10 | 30 |
| Salimian rizi et al. [48] | Tehran | 2012-2013 | 45 | Disk diffusion | 0 | ND | 14 | 6 | ND | ND | 10 | 6 | 10 | 26 | 8 | 28 | ND | ND | ND | ND | 13 | 15 | 7 | ND | 45 | ND |
| Mahmoudi et al. [49] | Tehran | 2011–2016 | 100 | Disk diffusion | 4 | ND | ND | ND | ND | ND | 27 | 30 | ND | ND | ND | 20 | ND | ND | ND | ND | 69 | ND | 36 | ND | 46 | ND |
| Rajabi et al. [50] | Tehran | 2011–2012 | 17 | Disk diffusion | ND | ND | ND | 2 | ND | ND | ND | ND | ND | 2 | ND | 2 | ND | 9 | ND | ND | ND | ND | ND | ND | ND | ND |
| Afsharpaiman et al. [51] | Tehran | 2011 | 33 | Disk diffusion | 5 | 0 | ND | ND | ND | ND | 17 | 14 | ND | ND | 16 | 18 | 11 | 30 | 14 | 14 | 30 | 22 | ND | 9 | ND | ND |
| Rahbar et al. [52] | Tehran | 2010-2011 | 101 | Disk diffusion | 2 | ND | 14 | ND | ND | ND | 15 | 3 | 19 | 19 | 19 | 20 | ND | ND | ND | ND | 20 | 23 | 5 | ND | ND | 33 |
| Ranjbar et al. [53] | Tehran | 2006-2007 | 83 | Disk diffusion | ND | ND | ND | 32 | ND | ND | 41 | 49 | 44 | ND | 22 | 47 | 47 | 60 | 45 | 50 | ND | 54 | ND | 53 | ND | ND |
| Taheri et al. [54] | Tehran | 2004–2012 | 14 | Disk diffusion | ND | ND | ND | ND | 0 | ND | 9 | 4 | ND | ND | ND | 6 | 1 | 5 | 2 | ND | ND | 0 | ND | 2 | ND | ND |
| Haghi et al. [55] | Tehran | 2003-2004 | 39 | Disk diffusion | ND | ND | ND | ND | ND | ND | 13 | 5 | 11 | ND | ND | 17 | 23 | 23 | 13 | 16 | ND | 16 | ND | 12 | ND | ND |
| Navidinia et al. [56] | Tehran | NA | 69 | Disk diffusion | 1 | 1 | ND | 12 | ND | ND | 5 | 2 | 4 | ND | ND | ND | ND | ND | 39 | ND | ND | ND | 43 | 67 | 4 | ND |
| Sadeghi bojd et al. [57] | Zahedan | 2013–2015 | 32 | Disk diffusion | ND | ND | ND | 3 | ND | ND | 2 | 3 | ND | ND | ND | 14 | 9 | 2 | 6 | 6 | 6 | ND | ND | ND | ND | ND |
IPM-imipenem; MEM-meropenem; ATM-aztreonam; CIP-ciprofloxacin; NOR-norfloxacin; LVX-levofloxacin; GEN-gentamicin; AMK-amikacin; TOB-tobramycin; TET-tetracycline; CHL-chloramphenicol; SXT-trimethoprim/sulfamethoxazole; NAL-nalidixic acid; NIT-nitrofurantoin; CRO-ceftriaxone; CFM-cefixime; CTX-cefotaxime; CAZ-ceftazidime; CEP-cefepime; ZOX-ceftizoxime; MDR-multidrug-resistant; ESBL-extended-spectrum β-lactamase; AST-antimicrobial susceptibility testing; ND-not determined.
2.3. Data Analysis
In the current study, Cochrane's Q test (chi-squared, χ2) and Higgins I2 statistics were used to assess heterogeneity across the included studies. For this purpose, if the p value was less than 0.1 for the χ2 test and the I2 value was higher than 25%, the presence of heterogeneity was considered and a random-effects model was applied for the meta-analysis. Extracted data on the prevalence of Enterobacter species' antibiotic resistance and ESBL-producing species in Iran were expressed as a percentage and 95% confidence intervals (95% CIs). Additionally, a subgroup analysis was performed based on the location of the study. A funnel plot-based method was used for reporting the presence or absence of publication bias in the meta-analyses, and it was considered a potential sign of publication bias if the graph showed an asymmetric shape. The Comprehensive Meta-Analysis (CMA) software (Biostat, Englewood, NJ) was used for the meta-analysis.
3. Results
Among 19,669 eligible studies published from 1996 until June 21, 2021, 49 articles (20 in Persian and 29 in English) met the inclusion criteria and were included in the meta-analysis (Figure 1). As shown in Table 1, data were obtained from 19 cities (Ahvaz (n = 5), Arak (n = 1), Babol (n = 2), Bojnurd (n = 1), Fasa (n = 1), Hamadan (n = 1), Ilam (n = 1), Isfahan (n = 2), Jahrom (n = 1), Kashan (n = 1), Kerman (n = 1), Kermanshah (n = 2), Rasht (n = 2), Sanandaj (n = 4), Semnan (n = 1), Shiraz (n = 4), Tabriz (n = 2), Tehran (n = 13), and Zahedan (n = 1)) in Iran. All studies used the disk diffusion method for antimicrobial susceptibility testing. The pooled prevalence of Enterobacter species' resistance to various antibiotics was as follows: imipenem 16.6% (95% CI: 11–24.1; I2 = 93.1%; Q = 439.9; p ≤ 0.001) (Figure 2), meropenem 16.2% (95% CI: 8.9–27.9; I2 = 89.8%; Q = 117.8; p ≤ 0.001), aztreonam 40.9% (95% CI: 29.6–53.2; I2 = 89.3%; Q = 75; p ≤ 0.001), ciprofloxacin 35.3% (95% CI: 29.5–41.6; I2 = 86.1%; Q = 273.6; p ≤ 0.001), norfloxacin 31% (95% CI: 14.3–54.7; I2 = 91.6%; Q = 59.9; p ≤ 0.001), levofloxacin 48% (95% CI: 21.3–75.9; I2 = 90.7%; Q = 32.4; p ≤ 0.001), gentamicin 42.1% (95% CI: 36.2–48.3; I2 = 87.2%; Q = 328.5; p ≤ 0.001), amikacin 30.3% (95% CI: 24.5–36.8; I2 = 86.9%; Q = 298.8; p ≤ 0.001), tobramycin 37.2% (95% CI: 26.3–49.5; I2 = 88.3%; Q = 103.1; p ≤ 0.001), tetracycline 50.1% (95% CI: 37.3–62.9; I2 = 88%; Q = 134; p ≤ 0.001), chloramphenicol 25.7% (95% CI: 20.5–31.6; I2 = 61.1%; Q = 20.5; p ≤ 0.001), trimethoprim/sulfamethoxazole 52% (95% CI: 45.4–58.6; I2 = 87.5%; Q = 304.9; p ≤ 0.001), nalidixic acid 49.1% (95% CI: 38.8–59.4; I2 = 87.6%; Q = 177.4; p ≤ 0.001), nitrofurantoin 43% (95% CI: 32.4–54.2; I2 = 91.7%; Q = 328.8; p ≤ 0.001), ceftriaxone 49.3% (95% CI: 41.8–56.9; I2 = 87.1%; Q = 226.1; p ≤ 0.001), cefixime 52.4% (95% CI: 43.7–61; I2 = 83.4%; Q = 102.5; p ≤ 0.001), cefotaxime 52.7% (95% CI: 42.4–62.7; I2 = 91.9%; Q = 359.3; p ≤ 0.001), ceftazidime 47.9% (95% CI: 39.8–56.2; I2 = 89.7%; Q = 302; p ≤ 0.001), cefepime 43.6% (95% CI: 31.3–56.8; I2 = 90.1%; Q = 142.2; p ≤ 0.001) and ceftizoxime 45.5% (95% CI: 30.6–61.3; I2 = 92.7%; Q = 178.4; p ≤ 0.001).
Figure 1.
A schematic view of the study selection process.
Figure 2.
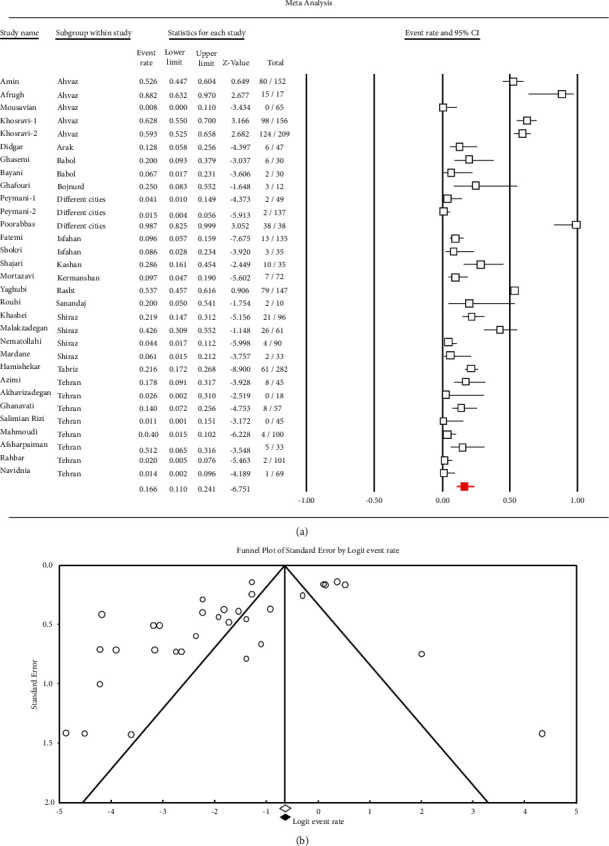
Forest plots (a) and funnel plots (b) illustrate the prevalence of imipenem-resistant Enterobacter species in Iran.
In addition, Table 2 shows the antibiotic resistance profiles of Enterobacter species in different cities of Iran. The rate of MDR Enterobacter species in Iran was 63.1% (95% CI: 45.2–78; I2 = 93.9%; Q = 249.1; p ≤ 0.001).
Table 2.
Antibiotic resistance profile of Enterobacter isolates from cities in Iran.
| City | Percentage resistance (%) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IPM | MEM | ATM | CIP | NOR | LVX | GEN | AMK | TOB | TET | CHL | SXT | NAL | NIT | CRO | CFM | CTX | CAZ | CEP | ZOX | |
| Ahvaz | 58 | 64.4 | NA | 46.8 | 46.1 | 41.4 | 53.1 | 40.9 | NA | 56.7 | NA | 69.6 | 61.2 | 55.1 | 63.6 | 70.7 | 68.8 | 50.9 | 82.4 | 61.8 |
| Arak | 12.8 | NA | NA | 40.4 | NA | NA | 40.4 | 38.3 | NA | NA | NA | 42.6 | NA | NA | 61.7 | NA | NA | 85.1 | 53.2 | 40.4 |
| Babol | 13.2 | NA | NA | 13.2 | NA | NA | 13.3 | 13.2 | NA | NA | NA | NA | NA | 30 | NA | NA | 33.3 | 16.7 | 58.8 | NA |
| Bojnurd | 25 | 41.7 | NA | 66.7 | NA | NA | 66.7 | 16.7 | NA | 25 | 3.8 | 50 | NA | 8.3 | 50 | 3.8 | 3.8 | 8.3 | NA | NA |
| Fasa | NA | NA | NA | 39.3 | NA | NA | 28.6 | 1.7 | NA | 39.3 | NA | 50 | 39.3 | 39.3 | 39.3 | 39.3 | 1.7 | NA | NA | NA |
| Hamadan | NA | NA | NA | 86.7 | NA | NA | 86.7 | NA | 86.7 | NA | NA | 66.7 | 40 | 26.7 | 73.3 | NA | NA | NA | NA | NA |
| Ilam | NA | NA | NA | NA | NA | NA | 15 | NA | NA | NA | NA | 45 | NA | 30 | NA | NA | NA | NA | NA | NA |
| Isfahan | 9.4 | 11.3 | 68.9 | 49.9 | NA | NA | 50.3 | 33 | 39.3 | 71.1 | 19.3 | 17 | NA | 17.1 | NA | NA | 66.5 | 64.1 | 51.4 | NA |
| Jahrom | NA | NA | NA | NA | NA | NA | 68 | 28 | 44 | 96 | NA | 80 | 96 | NA | NA | NA | 40 | 40 | NA | NA |
| Kashan | 28.6 | NA | NA | 37.1 | NA | NA | 40 | 34.3 | 31.4 | NA | NA | 60 | 80 | NA | NA | 51.4 | 62.9 | 62.9 | NA | NA |
| Kerman | NA | NA | NA | NA | NA | NA | 59.7 | NA | NA | NA | NA | 63.9 | 16.7 | 38.9 | NA | NA | NA | NA | NA | NA |
| Kermanshah | 9.7 | NA | 40.3 | 46.7 | 41.7 | NA | 48.9 | 48.9 | NA | NA | NA | 68.9 | 42.2 | 23.4 | 33.3 | 46.8 | 51.4 | 53.3 | NA | NA |
| Rasht | 53.7 | 41.5 | NA | 11.8 | NA | NA | 42.8 | 65.1 | 53.1 | 0.3 | NA | 75 | 71.4 | 69.4 | 69.4 | 81 | 79.4 | 62.6 | NA | 54.4 |
| Sanandaj | 20 | NA | NA | 43.2 | 34.2 | NA | 33.5 | 40.5 | NA | 51.6 | NA | 45.2 | 36.4 | 4.5 | 50.7 | 45.5 | 51.2 | 28.6 | NA | 30.7 |
| Semnan | NA | NA | NA | 45.5 | NA | NA | 45.5 | NA | NA | NA | NA | 90.9 | 90.9 | 90.9 | NA | 72.7 | NA | NA | NA | NA |
| Shiraz | 15.1 | 5.9 | 47.2 | 26.9 | NA | NA | 46.8 | 34.7 | 42.4 | 72.7 | 31.1 | 37.5 | 4.9 | 29.9 | 51.7 | 65.1 | 54.8 | 66.5 | 38.2 | NA |
| Tabriz | 21.6 | NA | NA | 9.1 | NA | NA | 38.3 | 7.5 | NA | NA | 27.7 | 85.3 | 98.8 | 70.8 | 37.4 | NA | 75.9 | 19.5 | NA | 19 |
| Tehran | 6.3 | 6.7 | 22 | 22.9 | 3.3 | 25 | 33.1 | 18.9 | 31.2 | 42.8 | 26.4 | 39.4 | 36.3 | 63.1 | 45.3 | 47.6 | 58.6 | 41.8 | 28.8 | 59.2 |
| Zahedan | NA | NA | NA | 9.4 | NA | NA | 6.3 | 9.4 | NA | NA | NA | 43.8 | 28.1 | 6.3 | 18.8 | 18.8 | 18.8 | NA | NA | NA |
NA-not available.
In addition, the prevalence of ESBL-producing Enterobacter species was 32.8% (95% CI: 23.3–44; I2 = 79.4%; Q = 29.1; p ≤ 0.001) in Iran.
It should be noted that a random-effects model was applied for the meta-analysis due to the existence of high heterogeneity across the included studies in this study.
4. Discussion
The emergence of MDR- and ESBL-producing Enterobacteriaceae, including Enterobacter species, has increased the necessity to deal with these organisms [5, 6]. The Centers for Disease Control and Prevention (CDC) estimated 197,400 cases of ESBL-producing Enterobacteriaceae along with 9,100 deaths among hospitalized patients in the United States in 2017 [58]. The antibiotic of choice to treat infections caused by MDR and ESBL-producing Enterobacteriaceae is carbapenem [3, 58, 59]. However, the widespread use of carbapenem antibiotics has led to the emergence of CR bacteria [3, 59]. According to the CDC report for 2019, increased prevalence of CR Enterobacteriaceae, especially CR Enterobacter cloacae complex, has become a public health issue in the United States [58].
In Iran, the prevalence of MDR (63.1%) and ESBL-producing Enterobacter species (32.8%) was high. This is an alarming rate despite the relatively low frequency of imipenem- and meropenem-resistant Enterobacter species in Iran. The results suggest that carbapenems are still the drugs of choice for the treatment of infections caused by MDR and ESBL-producing Enterobacter species in Iran. The distribution of ESBL-producing Enterobacter species in other countries was as follows: Pakistan 14.9%, Nigeria 37.5%, and Ethiopia 50% [60, 61].
The CDC has reported that CR Enterobacteriaceae-associated infections frequently occur in patients using medical devices, including catheters (intravenous and urinary) and ventilators, and some of these microorganisms are resistant to all available antibiotics, hence their infections are difficult to treat [58]. Currently, the available antimicrobial agents for the treatment of CR Enterobacteriaceae are limited [62]. Historically, aminoglycosides, tigecycline, polymyxins, and fosfomycin have been used as therapeutic options for this purpose [62]. However, according to the included articles in this study, there is insufficient data on the prevalence of tigecycline-, polymyxins-, and fosfomycin-resistant Enterobacter species in Iran. Hence, the evaluation of Enterobacter species resistance rates to these antibiotics is recommended. In the present study, the rate of tetracycline-resistant Enterobacter species was high (50.1%).
On the other hand, aminoglycosides, including gentamicin, amikacin, and tobramycin, are also recommended as anti-CR Enterobacteriaceae therapies [62]. However, based on the present study, the prevalence of gentamicin-, amikacin-, and tobramycin-resistant Enterobacter species was high in Iran. It is recommended that older antibiotics such as trimethoprim/sulfamethoxazole and chloramphenicol may be effective for the treatment of infections caused by CR Enterobacteriaceae pathogens [62]. Our results showed that the prevalence of Enterobacter species resistant to chloramphenicol was higher than those resistant to trimethoprim/sulfamethoxazole (25.7% vs. 52%). Other treatment options for infections caused by CR Enterobacteriaceae include combination strategies (high-dose tigecycline, high-dose carbapenem, and double-carbapenem therapy), new antibiotics (ceftazidime/avibactam, meropenem/vaborbactam, plazomicin, and eravacycline), and new antibiotics in development (imipenem/cilastatin, relebactam, and cefiderocol) [62]. However, information on the therapeutic efficacy of these drugs against CR Enterobacter species is not available in Iran (according to the included articles in this study). Based on the current study, the frequency of meropenem and ceftazidime-resistant Enterobacter species was 16.2% and 47.9%, respectively. Enterobacter species' drug resistance rates to the third-generation cephalosporins and aztreonam were high in Iran. Considering the prevalence of ESBL-producing Enterobacter species in this study (32.8%), it seems that these ESBLs are involved in resistance to third-generation cephalosporins and aztreonam in Iran. The CDC estimated the rate of quinolone-resistant Enterobacter species as 30% [3]; however, the prevalence of Enterobacter species resistant to quinolones was higher in this study.
Such a high antibiotic resistance of Enterobacter species, especially MDR, in this study can be attributed to the indiscriminate use of antibiotics and easy, without a prescription, access to antibiotics and self-medication in Iran [63, 64]. On the other hand, since Enterobacter species are responsible for nosocomial infections, using appropriate infection control programs and practices of hygiene such as hand decontamination, glove use, sterilization, and disinfection practices can play an important role in preventing the spread of resistant strains in healthcare settings.
One of the limitations of the current study was the inability to compare the obtained results with other countries, particularly adjacent countries, which needs to be addressed in future multicenter and international studies.
5. Conclusion
This study is the first systematic review and meta-analysis reporting Enterobacter species antibiotic resistance in Iran. The results of this meta-analysis indicated the high prevalence of Enterobacter species resistant to the majority of assessed antibiotics in the included studies, i.e., quinolones, aminoglycosides, third- and fourth-generation cephalosporins, aztreonam, tetracycline, chloramphenicol, trimethoprim/sulfamethoxazole, and nitrofurantoin. In addition, the prevalence rates of ESBL-producing Enterobacter species (32.8%) and MDR (63.1%) strains were high in Iran. Such an increasing trend of antibiotic resistance in Enterobacter species can impose more economic costs on healthcare systems in Iran due to prolonged periods of hospitalization, increased drug consumption, poor patient outcomes, and higher mortality and morbidity. In total, we suggest the management of antibiotic prescription, launching and developing health education and infection control programs, continuous monitoring of drug resistance, and evaluation of the therapeutic efficacy of new antimicrobial agents (herbal medicine and new antimicrobial peptides) or combination therapeutic strategies are required to control Enterobacter species-associated infections and antibiotic resistance in Iran. Finally, in comparison with the above-mentioned antibiotics, the prevalence of CR Enterobacter species was relatively low in Iran, and it seems that carbapenems can still be considered as drugs of choice for the treatment of MDR and ESBL-producing Enterobacter species.
Data Availability
No data were used to support this study.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Ruppé É, Woerther P. L., Barbier F. Mechanisms of antimicrobial resistance in gram-negative bacilli. Annals of Intensive Care . 2015;5(1):21–25. doi: 10.1186/s13613-015-0061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carroll K. C., Butel J. S., Morse S. A. Jawetz Melnick & Adelbergs Medical Microbiology . New York, NY, USA: McGraw Hill Professional; 2016. [Google Scholar]
- 3.Morehead M. S., Scarbrough C. Emergence of global antibiotic resistance. Primary Care: Clinics in Office Practice . 2018;45(3):467–484. doi: 10.1016/j.pop.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Anju V. T., Siddhardha B., Dyavaiah M. Model Organisms for Microbial Pathogenesis, Biofilm Formation and Antimicrobial Drug Discovery 2020 . Berlin, Germany: Springer; 2020. Enterobacter infections and antimicrobial drug resistance. [Google Scholar]
- 5.World Health Organization. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed . Geneva, Switzerland: WHO; 2017. [Google Scholar]
- 6.Annavajhala M. K., Gomez-Simmonds A., Uhlemann A. C. Multidrug-resistant Enterobacter cloacae complex emerging as a global, diversifying threat. Frontiers in Microbiology . 2019;10:p. 44. doi: 10.3389/fmicb.2019.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liberati A., Altman D. G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine . 2009;6(7) doi: 10.1371/journal.pmed.1000100.e1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing-Twenty-Eighth Informational Supplement. CLSI Document M100 . Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2021. [Google Scholar]
- 9.Amin M., Dibachi S., Shahin M. Prevalence of class 1 integrons and plasmid-mediated qnr-genes among Enterobacter isolates obtained from hospitalized patients in Ahvaz, Iran. Informe Medico . 2017;25(4):351–357. [PubMed] [Google Scholar]
- 10.Afrugh P., Mardaneh J., Kaidani A., Serajian A. A., Abbasi P., Yahyavi M. Distribution and antimicrobial susceptibility pattern of gram negative bacteria causing urinary tract infection (UTI) and detection New Delhi metallo-beta-lactamase-1 (NDM-1) producing isolates in ahwaz. Iranian South Medical Journal . 2016;19(1):15–26. [Google Scholar]
- 11.Mousavian S. M., Ahmad Khosravi N., Shoja S. Survey of frequency in extended-spectrum beta lactamase producing Enterobacteriaceae and determination of the antibiotic resistant pattern in clinical specimens in teaching hospitals of Ahvaz Jundishapur university of medical sciences. Jundishapur Scientific Medical Journal (JSMJ) . 2014;13(2):1–9. [Google Scholar]
- 12.Khosravi A. D., Abasi Montazeri E., Ghorbani A., Parhizgari N. Bacterial urinary tract infection in renal transplant recipients and their antibiotic resistance pattern: a four-year study. Iranian Journal of Microbiology . 2014;6(2):74–78. [PMC free article] [PubMed] [Google Scholar]
- 13.Khosravi A. D., Parhizgari N., Montazeri E., Mozaffari A., Abbasi F. The prevalence of bacteria isolated from endotracheal tubes of patients in Golestan Hospital, Ahvaz, Iran, and determination of their antibiotic susceptibility patterns. Jundishapur Journal of Microbiology . 2012;6(1):67–71. doi: 10.5812/jjm.4583. [DOI] [Google Scholar]
- 14.Didgar F., Sarmadian H., Ghasemikhah R. Antimicrobial resistance pattern of gram–negative bacilli isolated of Vali-Asr Hospital wards in Arak. Iranian South Medical Journal . 2014;17(5):938–947. [Google Scholar]
- 15.Ghasemi E., Ferdosi-Shahandashti E., Rajabnia M., Sabbagh P., Maali A., Ferdosi-Shahandashti A. Class I integrons among multidrug resistant Enterobacter spp. isolates from hospitalized patients in Babol, North of Iran. Caspian Journal of Internal Medicine . 2021;12(1):65–69. doi: 10.22088/cjim.12.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bayani M., Siadati S., Rajabnia R., Taher A. A. Drug resistance of Pseudomonas aeruginosa and Enterobacter cloacae isolated from ICU, Babol, northern Iran. International Journal of Molecular and Cellular Medicine . 2013;2(4):204–209. [PMC free article] [PubMed] [Google Scholar]
- 17.Ghafouri M., Hashemi S. A., Azimian A., Garevani T., SeyedSharifi S. H. Evaluation of antibiotic resistance to bacteria isolated from patients with nosocomial infections hospitalized in Imam Reza in Bojnurd City in 2013. Journal of Rafsanjan University of Medical Sciences . 2015;14(7):599–610. [Google Scholar]
- 18.Peymani A., Yeylagh Beigi M., Mohammadi Ghanbarlou M., Najafipour R., Samimi R. Multidrug resistance in Pseudomonas aeruginosa and Enterobacter cloacae isolated from intensive care units of Qazvin and Tehran hospitals. Journal of Clinical Research in Paramedical Sciences . 2014;3(1):16–24. [Google Scholar]
- 19.Peymani A., Farivar T. N., Ghoraiian P., Najafipour R. Association between class 1 integrons and multidrug resistance pattern among Enterobacter spp. isolated from Qazvin and Tehran teaching hospitals. Journal of Qazvin University of Medical Sciences . 2014;18(2):30–38. [Google Scholar]
- 20.Poorabbas B., Mardaneh J., Rezaei Z., et al. Nosocomial infections: multicenter surveillance of antimicrobial resistance profile of Staphylococcus aureus and gram negative rods isolated from blood and other sterile body fluids in Iran. Iranian Journal of Microbiology . 2015;7(3):127–135. [PMC free article] [PubMed] [Google Scholar]
- 21.Molazade A., Gholami M. S., Shahi A., et al. Evaluation of antibiotic resistance pattern of isolated gram-negative bacteria from urine culture of hospitalized patients in different wards of vali-asr hospital in Fasa during the years 2012 and 2013. Journal of Fasa University of Medical Science . 2014;4(3):275–283. [Google Scholar]
- 22.Esmaeili R., Hashemi H., Moghadam Shakib M., Alikhani M., Sohrabi Z. Bacterial etiology of urinary tract infections and determining their antibiotic resistance in adults hospitalized in or referred to the Farshchian hospital in Hamadan. Journal of Ilam University of Medical Sciences . 2013;21:281–287. [Google Scholar]
- 23.Yasemi M., Peyman H., Asadollahi K., et al. Frequency of bacteria causing urinary tract infections and their antimicrobial resistance patterns among pediatric patients in Western Iran from 2007–2009. Journal of Biological Regulators & Homeostatic Agents . 2014;28(3):443–448. [PubMed] [Google Scholar]
- 24.Fatemi S. M., Shokri D., Mohammadi S., Koupahi H. Investigation of NDM metallo-beta-lactamase and CMY-2 AmpC β-lactamase production in Escherichia coli and Enterobacter spp. isolated from human. Comparative Clinical Pathology . 2018;27(4):1007–1015. doi: 10.1007/s00580-018-2694-5. [DOI] [Google Scholar]
- 25.Shokri D., Mobasherizadeh S., Fatemi S. M., Moayednia R., Sadeghi Naeeni M. Hospital based surveillance of carbapenem resistance in multidrug-resistant (MDR) strains of Enterobacter and Escherichia coli in Isfahan. Journal of Microbial World . 2015;8(22):64–75. [Google Scholar]
- 26.Kargar M., Jahromi M., Kargar M., Najafi A., Ghorbani-Dalini S. Molecular detection of ESBLs production and antibiotic resistance patterns in gram negative bacilli isolated from urinary tract infections. Indian Journal of Pathology & Microbiology . 2014;57(2):244–248. doi: 10.4103/0377-4929.134688. [DOI] [PubMed] [Google Scholar]
- 27.Shajari G. H., Khorshidi A., Moosavi G. H. Bacterial isolation and antibiotic resistance of nosocomial pneumonia in hospitalaized patients-Kashan, Iran. Hormozgan Medical Journal . 2009;13(3):197–205. [Google Scholar]
- 28.Sepehri G. R., Dabiri S., Vosoogh M. R. Comparison the sensitivity of microbial agents causing urinary tract infections to commonly used antibiotics in kerman in the years 1996 and 2000. Journal of Rafsanjan University of Medical Sciences . 2004;3(4):216–224. [Google Scholar]
- 29.Mortazavi S. H., Mansouri F., Azizi M., et al. Prevalence of class I and II integrons among MDR Enterobacter cloacae isolates obtained from clinical samples of children in Kermanshah, Iran. Journal of Clinical and Diagnostic Research . 2018;12(12):13–16. [Google Scholar]
- 30.Amini F., Vaziri S., Karimpour H. A., Hasani S., Mohamadi S., Azizi M. Antibiotic resistance pattern of urinary tract infection pathogens in children of Kermanshah in 2015. Razi Journal of Medical Sciences . 2017;24(155):20–27. [Google Scholar]
- 31.Karambin M., Zarkesh M. Entrobacter, the most common pathogen of neonatal septicemia in rasht, Iran. Iranian Journal of Pediatrics . 2011;21(1):83–87. [PMC free article] [PubMed] [Google Scholar]
- 32.Yaghubi T., Pourkazemi A., Farashbandi H., Balu H. Epidemiological study of nosocomial infections and antibiotic resistance patterns in Guilan. Yafteh . 2019;21(1):52–62. [Google Scholar]
- 33.Rouhi S., Mohammadi B., Ramazanzadeh R., Mohammadi S., Zandi S. Prevalence of isolated bacterial and antibiotic resistant pattern of them in positive blood cultures isolated from patients admitted to different parts of Toohid Hospital of Sanandaj city (2013-2014) Navid No . 2015;18(60):34–41. [Google Scholar]
- 34.Nikkhoo B., Lahurpur F., Delpisheh A., Rasouli M. A., Afkhamzadeh A. Neonatal blood stream infections in tertiary referral hospitals in Kurdistan, Iran. Italian Journal of Pediatrics . 2015;41(1):43–44. doi: 10.1186/s13052-015-0136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramazanzadeh R., Chitsaz M., Bahmani N. Prevalence and antimicrobial susceptibility of extended-spectrum beta-lactamase-producing bacteria in intensive care units of Sanandaj general hospitals (Kurdistan, Iran) Chemotherapy . 2009;55(4):287–292. doi: 10.1159/000224656. [DOI] [PubMed] [Google Scholar]
- 36.Afkhamzadeh A., Lahoorpour F., Delpisheh A., Janmardi R. Incidence of ventilator associated pneumonia (VAP) and bacterial resistance pattern in adult patients hospitalised at the intensive care unit of Besat Hospital in Sanandaj. SJKU . 2011;16(1):20–26. [Google Scholar]
- 37.Jazayeri M. A. Frequency of the bacterial agents in urinary tract infection and their antibiotic susceptibility pattern in Semnan. Journal of Semnan University Medical Science . 2000;1(4):1–16. [Google Scholar]
- 38.Khashei R., Edalati Sarvestani F., Malekzadegan Y., Motamedifar M. The first report of Enterobacter gergoviae carrying blaNDM-1 in Iran. Iranian Journal of Basic Medical Sciences . 2020;23(9):1184–1190. doi: 10.22038/ijbms.2020.41225.9752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malekzadegan Y., Hadadi M., Ebrahim-Saraie H. S., Heidari H., Motamedifar M. Antimicrobial resistance pattern and frequency of multiple-drug resistant Enterobacter spp. at a tertiary care hospital in Southwest of Iran. Journal of Krishna Institute of Medical Sciences University . 2017;6(2):33–39. [Google Scholar]
- 40.Nematolahi S., Mosadegh A., Mardaneh J., Poorabbas B. Identification of ESBL-producing and blaSHV gene harboring Enterobacter spp. isolated from bloodstream infections of hospitalized patients during 10 Years in south of Iran (Shiraz) IIranian South Medical Journal . 2016;19(4):536–548. doi: 10.18869/acadpub.ismj.19.4.536. [DOI] [Google Scholar]
- 41.Mardaneh J., Anvarinejad M., Abbasian A., et al. Emergence of multi-drug resistant ESBL producing strains among Enterobacteriaceae members isolated from patients blood samples in south of Iran. Iranian South Medical Journal . 2015;18(5):970–981. [Google Scholar]
- 42.Rezaee M. A., Abdinia B. Etiology and antimicrobial susceptibility pattern of pathogenic bacteria in children subjected to UTI: a referral hospital-based study in Northwest of Iran. Medicine . 2015;94(39) doi: 10.1097/md.0000000000001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamishehkar H., Shadmehr P., Mahmoodpoor A., Mashayekhi S. O., Entezari-Maleki T. Antimicrobial susceptibility patterns among bacteria isolated from intensive care units of the largest teaching hospital at the northwest of Iran. Brazilian Journal of Pharmaceutical Sciences . 2016;52(3):403–412. doi: 10.1590/s1984-82502016000300006. [DOI] [Google Scholar]
- 44.Azimi T., Maham S., Fallah F., Azimi L., Gholinejad Z. Evaluating the antimicrobial resistance patterns among major bacterial pathogens isolated from clinical specimens taken from patients in Mofid Children’s Hospital, Tehran, Iran: 2013–2018. Infection and Drug Resistance . 2019;12:2089–2102. doi: 10.2147/idr.s215329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akhavizadegan H., Hosamirudsar H., Pirroti H., Akbarpour S. Antibiotic resistance: a comparison between inpatient and outpatient uropathogens. Eastern Mediterranean Health Journal . 2021;27(2):124–130. doi: 10.26719/emhj.20.085. [DOI] [PubMed] [Google Scholar]
- 46.Sohrabi fard M., Noorbakhsh F., Elikaei A. Studying the prevalence of TetA and TetB in Kelebsiella, Proteus and Enterobacter isolated from urinary tract infection. Medical Sciences . 2016;26(4):222–228. [Google Scholar]
- 47.Ghanavati R., Emaneini M., Kalantar-Neyestanaki D., et al. Clonal relation and antimicrobial resistance pattern of extended-spectrum β-lactamase-and AmpC β-lactamase-producing Enterobacter spp. isolated from different clinical samples in Tehran, Iran. Journal of the Brazilian Society of Tropical Medicine . 2018;51(1):88–93. doi: 10.1590/0037-8682-0227-2017. [DOI] [PubMed] [Google Scholar]
- 48.Salimian Rizi K., Najar Peerayeh S., Bakhshi B., Rahbar M. Prevalence of integrons and antimicrobial resistance genes among clinical isolates of Enterobacter spp. from hospitals of Tehran. International Journal of Enteric Pathogen . 2015;3(1) doi: 10.17795/ijep22531.e22531 [DOI] [Google Scholar]
- 49.Mahmoudi S., Mahzari M., Banar M., et al. Antimicrobial resistance patterns of gram-negative bacteria isolated from bloodstream infections in an Iranian referral paediatric hospital: a 5.5-year study. Journal of Global Antimicrobial Resistance . 2017;11:17–22. doi: 10.1016/j.jgar.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 50.Rajabi Z., Soltan Dallal M. M. Study on bacterial strains causing blood and urinary tract infections in the neonatal Intensive care unit and determination of their antibiotic resistance pattern. Jundishapur Journal of Microbiology . 2015;8(8) doi: 10.5812/jjm.19654v2.e19654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Afsharpaiman S., Bairaghdar F., Torkaman M., et al. Bacterial pathogens and resistance patterns in children with community-acquired urinary tract infection: a cross sectional study. Journal of Comprehensive Pediatrics . 2012;3(1):16–20. doi: 10.5812/jcp.7078. [DOI] [Google Scholar]
- 52.Rahbar M., Azimi L., Mohammad-Zadeh M., et al. The prevalence of nosocomial infections caused by Enterobacter cloacae and antibiotic resistant patterns in samples isolated from patients in two hospitals in Tehran. Tehran University Medical Journal . 2012;70(3):183–187. [Google Scholar]
- 53.Ranjbar R. E., Haghi A. M., Joneydi J. N., Abedini M. The prevalence and antimicrobial susceptibility of bacterial uropathogens isolated from pediatric patients. Iranian Journal of Public Health . 2009;38(2):134–138. [Google Scholar]
- 54.Taheri P. A., Navabi B., Khatibi E. Frequency and susceptibility of bacteria caused urinary tract infection in neonates: eight-year study at neonatal division of Bahrami children’s hospital, Tehran Iran. Iranian Journal of Public Health . 2013;42(10):1126–1133. [PMC free article] [PubMed] [Google Scholar]
- 55.Haghi A. M., Abedini M., Sadeghifard N. K., Soroush S., Taheri K. M. Etiology and antibacterial resistance of bacterial urinary tract infections in children’s medical center, Tehran, Iran. Acta Medica Iranica . 2007;45(2):153–157. [Google Scholar]
- 56.Navidinia M., Goudarzi M., Rameshe S. M., et al. Molecular characterization of resistance genes in MDR-ESKAPE pathogens. Journal of Pure and Applied Microbiology . 2017;11(2):779–792. doi: 10.22207/jpam.11.2.17. [DOI] [Google Scholar]
- 57.Sadeghi Bojd S., Soleimani G., Teimouri A., Zarifi E., Rashidi S. Evaluation of antibiotic sensitivity of urinary tract pathogens among children in zahedan, south east of Iran. International Journal of Pediatrics . 2017;5(10):5965–5974. [Google Scholar]
- 58.The Centers for Disease Control and Prevention. Antibiotic resistance threats in the united states. 2019. https://www.cdc.gov/DrugResistance/Biggest-Threats.html .
- 59.Elshamy A. A., Aboshanab K. M. A review on bacterial resistance to carbapenems: epidemiology, detection and treatment options. Future Science OA . 2020;6(3) doi: 10.2144/fsoa-2019-0098.FSO438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amin H., Zafar A., Ejaz H., Jameel N. U. A. Phenotypic characterization of ESBL producing Enterobacter cloacae among children. Pakistan Journal of Medical Sciences . 2013;29(1):144–147. doi: 10.12669/pjms.291.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teklu D. S., Negeri A. A., Legese M. H., Bedada T. L., Woldemariam H. K., Tullu K. D. Extended-spectrum beta-lactamase production and multi-drug resistance among Enterobacteriaceae isolated in Addis Ababa, Ethiopia. Antimicrobial Resistance and Infection Control . 2019;8(1) doi: 10.1186/s13756-019-0488-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sheu C. C., Chang Y. T., Lin S. Y., Chen Y. H., Hsueh P. R. Infections caused by carbapenem-resistant Enterobacteriaceae: an update on therapeutic options. Frontiers in Microbiology . 2019;10:p. 80. doi: 10.3389/fmicb.2019.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bazghandi S. A., Arzanlou M., Peeridogaheh H., Vaez H., Sahebkar A., Khademi F. Prevalence of virulence genes and drug resistance profiles of Pseudomonas aeruginosa isolated from clinical specimens. Jundishapur Journal of Microbiology . 2021;14(8) doi: 10.5812/jjm.118452.e118452 [DOI] [Google Scholar]
- 64.Ghalehnoo Z., Vaez H., Salehi-Abargouei A., Khademi F. Multidrug resistant Pseudomonas aeruginosa in Iran: a systematic review and metaanalysis. Journal of Global Infectious Diseases . 2018;10(4):212–217. doi: 10.4103/jgid.jgid_113_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used to support this study.


